Abstract
Objective
High-volume centers have better outcomes than low-volume centers when managing complex conditions including subarachnoid hemorrhage (SAH). We aimed to quantify the SAH volume-outcome association and determine the extent to which this association is influenced by aggressiveness of care.
Methods
Serial cross-sectional retrospective study using Nationwide Inpatient Sample for 2002–2010. Included were all adult (>18 years) discharged patients with primary diagnosis of SAH admitted through emergency department or transferred to discharging hospital; cases of trauma or arteriovenous malformation were excluded. Survey-weighted descriptive statistics estimated temporal trends. Multi-level logistic regression estimated volume-outcome associations for inpatient mortality and discharge home. Models were adjusted for demographics, year, transfer status, insurance status, all individual Charlson comorbidities, intubation, and APR-DRG mortality. Analyses were repeated excluding cases where aggressive care was not pursued.
Results
A total 32,336 discharges were included; 13,398 patients were clipped (59.1%) or coiled (40.9%). Inpatient mortality declined from 32.2% in 2002 to 22.2% in 2010; discharge home increased from 28.5% to 40.8% during the same period. As SAH volume decreased from 100/year, mortality increased from 18.7% to 19.8% at 80/year, 21.7% at 60/year, 24.5% at 40/year, 28.4% at 20/year. As SAH patient volume decreased, probability of discharge home decreased from 40.3% at 100/year to 38.7% at 60/year, and 35.3% at 20/year. Better outcomes persisted in patients receiving aggressive care and in those not receiving aggressive care.
Conclusions
Short-term SAH outcomes have improved. High-volume hospitals have more favorable outcomes than low-volume hospitals. This effect is substantial, even for hospitals conventionally classified as high-volume.
Keywords: High-volume hospitals, low-volume hospitals, outcomes, subarachnoid hemorrhage
Introduction
Considerable evidence exists in supporting the notion that high-volume centers have better outcomes for complex surgical procedures. For example, between 1999 and 2008, mortality decreased between 8% to 36% for multiple complex surgical procedures when care for these procedures was regionalized to high-volume centers.1 The emergence of such evidence led healthcare purchasing organizations, such as The Leapfrog Group, to begin requiring referral of patients with complex conditions to high-volume centers. More recently, the Joint Commission developed a number of recognized disease-specific certification programs that require minimal-volume standards. For example, to obtain Comprehensive Stroke Center (CSC) certification, the Joint Commission requires that institutions manage at least 20 cases of subarachnoid hemorrhage (SAH) per year, and perform at least 15 coiling and/or clipping procedures in managing cerebral aneurysms.1
Compared to ischemic stroke, SAH is relatively uncommon but results in extremely high morbidity and mortality.2 Approximately 35,000 patients suffering from nontraumatic SAH are treated yearly in the United States (US), with the majority requiring coiling or microsurgical clip ligation to prevent repeat SAH.3 When comparing SAH-related clinical outcomes, high SAH volume hospitals historically perform better than lower SAH volume hospitals. This trend is similar in the United Kingdom.3–6 Since publication of these data, a greater proportion of SAH patients are being managed at high-volume centers than in the past.5
In this study, we had 2 primary goals. First, we sought to quantify the shape of the SAH volume-outcome association to report the optimal SAH patient volume thresholds needed to maximize quality of care. Second, we explored whether this relationship holds broadly across SAH patient populations or whether it is impacted by aggressiveness of care.
Methods
We performed a serial cross-sectional retrospective study on prospectively obtained data from the Nationwide Inpatient Sample (NIS) to address 3 specific questions: 1) Does a volume-outcome association still exist for SAH, given the redistribution of patients to more high-volume centers over time? 2) If so, how does the volume-outcome association vary across hospital volumes? 3) If a volume-outcome association exists, is it limited to specific SAH patient populations, or is the effect more broad-based?
Data Source
The NIS, managed by the Healthcare Cost and Utilization Project (HCUP) under the guidance of the Agency of Healthcare Research and Quality, is a sample of hospital discharge abstracts including data on approximately 20% of all hospital discharges in a given year, from which a nationally representative estimate of all discharges in the US can be generated.7 For included hospitals, all discharges at that hospital within a given year are included in the sample. NIS includes information on patient demographics, hospital characteristics, primary diagnoses, and procedures via ICD-9-CM procedure codes for discharges.
Patient Selection
All adults (>18 years of age) discharged with a primary ICD-9-CM diagnosis of SAH (ICD-9-CM 430), admitted from the emergency department or transferred to the discharging hospital during the period 2002–2010 were included in this analysis.8 Patients were excluded if they had any diagnoses resulting from traumatic injuries so as to exclude cases of traumatic SAH (ICD-9-CM 800 to 909.2, 909.4, 909.9, 910 to 994.9, 995.5 to 995.59, and 995.80 to 995.85).8, 9 Discharges with cerebral arteriovenous malformation (ICD-9-CM 747.81) were also excluded to ensure only patients with aneurismal SAH were evaluated.
Covariate Definition
ICD-9 procedure codes were used to determine patient aneurysm treatment type (clipping, ICD-9-CM 39.51; coiling, ICD-9-CMs 39.52, 39.72, 39.79). We also included a number of potentially important outcome predictors in adjusted mortality models. First, we tabulated stroke-modified Charlson comorbidity indices for all discharges using the methodology of Quan.10, 11 In addition, all patient-refined diagnosis-related groups (APR-DRG) risk-of-mortality indices were used to predict risk of mortality for individual patients.12 Based on secondary diagnosis and procedure codes, these indices assign a mortality category to each patient, ranging from minor to moderate, major, or extreme.2
Defining Volume and Outcome
The primary exposure of interest was patient SAH volume—the number of SAH discharges per year per hospital, calculated by summing the number of SAH cases per hospital per year. Our primary outcome was inpatient mortality. We also explored 2 secondary outcomes to determine whether hospitals select treatment strategies that reduce mortality while increasing the proportion of patients with significant disability. We defined a “bad outcome” as either inpatient mortality, discharge to hospice (DISPUB05 = 50 or DISPUB92 = 51), or discharge to a long-term acute care facility (DISPUB04 or DISPUB92 = 63). “Good outcome” was defined as discharge to home (DISPUB04 or DISPUB92 = 1).
Primary Analysis
Descriptive statistics were used to describe the patient population and temporal trends in outcomes, to cross-tabulate the relationship between volume and outcomes, and to determine the proportion of patients treated in hospitals by volume quintile after applying NIS survey weights. To study the volume-outcome association while accounting for differential distribution of patient characteristics by hospital, we developed models to predict mortality. The baseline model predicted the independent variable, inpatient mortality, in a multi-level logistic regression model with volume and year of admission as the only dependent variables while including a random hospital-level intercept. Based on the prior conceptual argument that volume-outcome associations, if they exist, likely reach a plateau at some level of volume, we explored adding a quadratic volumes term to our primary models. Including this term improved model fit, reduced mean model errors, and reduced the Akaike and Bayesian information criteria. We also explored whether the volume-outcomes association may be better modeled using higher order polynomials. Using the systematic approach of Royston et al, we found that spline models that included only linear and quadratic terms best fit the data and were similar to models that directly included linear and quadratic terms. Thus, we included linear and quadratic terms in all subsequent models as this approach appeared to maximize model fit while remaining relatively parsimonious. To determine whether and to what extent volume-outcome associations may be explained by patient characteristics, we developed a fully adjusted model by including age, sex, race/ethnicity (including a missing indicator for states that do not report race/ethnicity data), year of admission, transfer status (whether a patient was transferred from another hospital), insurance, all individual Charlson comorbidities, intubation status, and APR-DRG mortality category. To characterize the volume-outcome association in the context of higher level terms, we estimated average marginal effects across hospital volume quintiles.13 All analyses were performed using Stata statistical software, Release 12 (StataCorp, College Station, TX).
Secondary Analyses
Secondary analyses repeated the primary analysis in subsets of the overall patient population. Since some mortality may be concordant with patient values in the context of severe illness and to account for patients where aggressive care may be futile, we repeated our primary analysis excluding patients where aggressive care was not pursued. We applied 2 different definitions of “less aggressive care”: 1) patients that were not intubated or did not receive a coiling or clipping procedure, and 2) patients that were not intubated, did not receive clip/coiling, and who died or were discharged to hospice within 48 hours. Next, we examined whether a volume-outcome association existed in patients who did receive aggressive care, and quantified this association by repeating our primary analysis in patients who were intubated and clipped or coiled.
Results
A total of 32,336 discharges were included in the weighted sample who matched the inclusion and exclusion criteria, thus representing the nontraumatic SAH patient population. Demographic characteristics of the nontraumatic SAH patient population as well as the subdivided groups of those within the clip/coil group (13,398 patients; 59.1% clipped and 40.9% coiled) and the non-procedure (not clipped or coiled) group (18,938 patients) are reported in Table 1. Of the entire population, 62.7% were females, mean age was 58.3 years, and 47.2% were identified as being white. There were more females in the clip/coil group (69.0%) as opposed to the non-procedure group (58.3%). Medicare and private insurance were the medical insurers for 74.4% of the total patient population. Of note, private insurance was the provider for 47.3% of the clip/coil group as opposed to 36.2% of the non-procedure group. Within the entire study population, the most common comorbidities were tobacco use (17.8%), hypertension (56.4%), and diabetes mellitus (13.1%). Tobacco use was more common in the clip/coil group (24.8%) as compared to the non-procedure group (12.8%). Higher incidence of peripheral vascular disease was observed within the clip/coil group (12.2%) as compared to the non-procedure group (5.9%). Ventriculostomies and hemicraniectomies were more common in the clip/coil group (15.4% and 7.1%, respectively) as compared to the non-procedure group (6.3% and 2.4%, respectively). While intubation was equally frequent in the clip/coil group (38.9%) and the non-procedure group (36.6%), a greater proportion of the clip/coil group (13.0%) required a tracheostomy compared to the non-procedure group (4.0%).
Table 1.
Patient population demographics (N = 32,336).
| Total Patient Population | No Clip/Coil Procedure (n = 13,398) |
Clip/Coil Procedure (n = 18,938) |
|
|---|---|---|---|
|
Demographics
| |||
| Mean age, yrs | 58.3 (16.0) | 61.3 (17.0) | 54.2 (13.5) |
| Female | 20,287 (62.7%) | 11,039 (58.3%) | 9,248 (69.0%) |
| White | 15,263 (47.2%) | 9,266 (48.9%) | 5,997 (44.8%) |
| Black | 3,592 (11.1%) | 1,985 (10.5%) | 1,607 (12.0%) |
| Hispanic | 3,076 (9.5%) | 1,714 (9.1%) | 1,362 (10.2%) |
| Asian | 1,005 (3.1%) | 615 (3.2%) | 390 (2.9%) |
| Other/missing race | 9,400 (29.1%) | 5,358 (28.3%) | 4,042 (30.2%) |
| Medicare | 10,856 (33.6%) | 7,857 (41.5%) | 2,999 (22.4%) |
| Medicaid | 3,715 (11.5%) | 1,827 (9.6%) | 1,888 (14.1%) |
| Private Insurance | 13,198 (40.8%) | 6,861 (36.2%) | 6,337 (47.3%) |
|
| |||
|
Comorbidities
| |||
| Smoking | 5,755 (17.8%) | 2,429 (12.8%) | 3,326 (24.8%) |
| Hypertension | 18,232 (56.4%) | 10,982 (58.0%) | 7,250 (54.1%) |
| Myocardial infarction | 1,822 (5.6%) | 1,117 (5.9%) | 705 (5.3%) |
| PVD | 2,753 (8.5%) | 1,124 (5.9%) | 1,629 (12.2%) |
| Congestive heart failure | 2,193 (6.8%) | 1,403 (7.4%) | 790 (5.9%) |
| Dementia | 428 (1.3%) | 409 (2.2%) | 19 (0.1%) |
| COPD | 3,820 (11.8%) | 2,091 (11.0%) | 1,729 (12.9%) |
| Rheumatologic condition | 552 (1.7%) | 337 (1.8%) | 215 (1.6%) |
| Pulmonary disease | 196 (0.6%) | 104 (0.5%) | 92 (0.7%) |
| Mild liver disease | 648 (2.0%) | 424 (2.2%) | 224 (1.7%) |
| Diabetes (uncomplicated) | 3,914 (12.1%) | 2,754 (14.5%) | 1,160 (8.7%) |
| Diabetes (complicated) | 311 (1.0%) | 264 (1.4%) | 47 (0.4%) |
| Hemiplegia | 2,935 (9.1%) | 1,455 (7.7%) | 1,480 (11.0%) |
| Renal disease | 1,239 (3.8%) | 1,034 (5.5%) | 205 (1.5%) |
| Cancer | 562 (1.7%) | 474 (2.5%) | 88 (0.7%) |
| Moderate/severe liver disease | 84 (0.3%) | 59 (0.3%) | 25 (0.2%) |
| Metastases | 232 (0.7%) | 202 (1.1%) | 30 (0.2%) |
| AIDS | 83 (0.3%) | 54 (0.3%) | 29 (0.2%) |
|
| |||
|
Aneurysm Treatment
| |||
| Clip | 7,918 (24.5%) | 0 (0.0%) | 7,918 (59.1%) |
| Coil | 5,719 (17.7%) | 0 (0.0%) | 5,719 (42.7%) |
|
| |||
|
Procedures
| |||
| Ventriculostomy | 3,252 (10.1%) | 1,193 (6.3%) | 2,059 (15.4%) |
| PEG | 2,547 (7.9%) | 942 (5.0%) | 1,605 (12.0%) |
| Tracheostomy | 2,503 (7.7%) | 758 (4.0%) | 1,745 (13.0%) |
| Hemicraniectomy | 1,401 (4.3%) | 450 (2.4%) | 951 (7.1%) |
| Intubation | 12,136 (37.5%) | 6,927 (36.6%) | 5,209 (38.9%) |
| Hemodialysis | 347 (1.1%) | 275 (1.5%) | 72 (0.5%) |
| Cardiopulmonary resuscitation | 436 (1.3%) | 354 (1.9%) | 82 (0.6%) |
|
| |||
|
Discharge Location
| |||
| Died as inpatient | 8,583 (26.5%) | 6,710 (35.4%) | 1,873 (14.0%) |
| Hospice | 608 (1.9%) | 484 (2.6%) | 124 (0.9%) |
| Long-term acute care | 616 (1.9%) | 208 (1.1%) | 408 (3.0%) |
| Any bad outcome | 9,807 (30.3%) | 7,402 (39.1%) | 2,405 (18.0%) |
| Discharge home | 11,702 (36.2%) | 6,490 (34.3%) | 5,212 (38.9%) |
Temporal Trend in Clinical Outcome
Inpatient mortality, bad outcome, and discharge to home for the period 2002–2010 are graphically represented in Figure 1. Inpatient mortality declined considerably from 32.2% (95% CI, 30.1%–33.9%) to 22.2% (95% CI, 20.8%–23.6%), while discharge to home (good outcome) increased substantially during the same time interval from 28.5% (95% CI, 27.0%–30.03%) to 40.8% (95% CI, 39.1%–42.4%).
Figure 1.
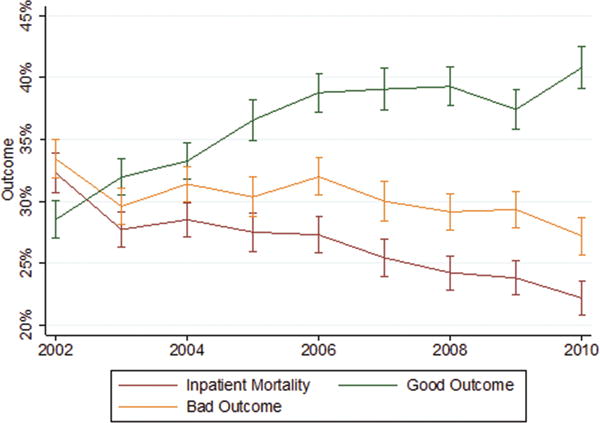
Outcome trends by time. Proportion of all SAH cases resulting in inpatient mortality (red line), bad outcomes (orange line), or good outcomes/discharge to home (green line) dichotomized by year. Vertical lines represent 95% confidence intervals.
Unadjusted Volume-Outcome Association
High-volume centers had lower proportions of patients who died as inpatients or who experienced bad outcomes (Table 2). Hospitals in the highest volume quintile (quintile 5; greater than 14 discharges per year) had an unadjusted inpatient mortality of 22.5% (95% CI, 21.9%–23.0%), while hospitals in quintile 4 (5–13 discharges/year) had an unadjusted inpatient mortality of 32.4% (95% CI, 31.1%–33.6%). Similar trends were seen when focusing on the subsets of patients that received aggressive care or when excluding those patients that did not receive aggressive care.
Table 2.
Unadjusted association of outcomes with subarachnoid hemorrhage patient volume.
| Quintile 1 (95% CI) |
Quintile 2 (95% CI) |
Quintile 3 (95% CI) |
Quintile 4 (95% CI) |
Quintile 5 (95% CI) |
|
|---|---|---|---|---|---|
| SAH Patient Volume | 1–1 | 2–2 | 3–4 | 5–13 | 14–230 |
|
| |||||
|
All Cases
| |||||
| Inpatient Mortality | 52.0% (48.8%–55.0%) | 43.3% (40.0%–46.6%) | 41.0% (38.5%–43.4%) | 32.4% (31.1%–33.6%) | 22.5% (21.9%–23.0%) |
| Good Outcome | 16.2% (14.0%–18.6%) | 20.9% (18.3%–23.7%) | 24.4% (22.3%–26.6%) | 31.0% (29.7%–32.2%) | 39.5% (38.8%–40.1%) |
| Bad Outcome | 57.4% (54.3%–60.4%) | 48.8% (45.4%–52.1%) | 45.6% (43.1%–48.0%) | 36.7% (35.3%–38.0%) | 26.0% (25.3%–26.5%) |
|
| |||||
|
Intubated
| |||||
| Inpatient Mortality | 84.7% (80.2%–88.0%) | 80.2% (75.2%–84.3%) | 76.9% (73.2%–80.1%) | 65.3% (63.1%–67.0%) | 47.4% (46.3%–48.4%) |
| Good Outcome | 3.1% (1.6%–5.6%) | 2.3% (1.1%–4.7%) | 4.2% (2.8%–6.1%) | 5.2% (4.2%–6.0%) | 11.4% (10.7%–12.0%) |
| Bad Outcome | 88.8% (84.7%–91.9%) | 85.8% (81.3%–89.3%) | 80.8% (77.4%–83.8%) | 70.3% (68.2%–72.2%) | 52.9% (51.8%–53.9%) |
|
| |||||
|
Clip Coil
| |||||
| Inpatient Mortality | 16.9% (5.5%–41.3%) | 27.5% (15.9%–43.2%) | 27.8% (21.6%–34.8%) | 18.1% (16.0%–20.2%) | 13.3% (12.7%–13.9%) |
| Good Outcome | 32.7% (15.3%–56.4%) | 26.0% (14.5%–41.9%) | 30.5% (24.1%–37.5%) | 32.4% (29.9%–34.9%) | 39.6% (38.7%–40.4%) |
| Bad Outcome | 34.0% (16.1%–57.8%) | 32.3% (19.7%–48.1%) | 30.3% (23.9%–37.4%) | 22.9% (20.6%–25.1%) | 17.2% (16.4%–17.8%) |
|
| |||||
|
Excluding No Aggressive Care
| |||||
| Inpatient Mortality | 82.8% (78.2%–86.5%) | 75.4% (70.3%–79.8%) | 67.9% (64.2%–71.2%) | 48.4% (46.5%–50.2%) | 28.0% (27.3%–28.6%) |
| Good Outcome | 4.5% (2.7%–7.3%) | 5.1% (3.1%–8.1%) | 10.2% (8.1%–12.7%) | 17.3% (15.9%–18.7%) | 31.0% (30.3%–31.7%) |
| Bad Outcome | 87.1% (82.9%–90.3%) | 80.8% (76.0%–84.7%) | 71.2% (67.7%–74.4%) | 52.7% (50.8%–54.5%) | 31.9% (31.1%–32.6%) |
|
| |||||
|
Excluding No Aggressive Care in Early Deaths
| |||||
| Inpatient Mortality | 43.1% (39.8%–46.4%) | 38.2% (34.8%–41.6%) | 37.1% (34.6%–39.5%) | 30.1% (28.8%–31.3%) | 21.1% (20.5%–21.0%) |
| Good Outcome | 19.9% (17.3%–22.8%) | 23.3% (20.4%–26.3%) | 26.5% (24.2%–28.0%) | 32.3% (31.0%–33.5%) | 40.3% (39.7%–40.9%) |
| Bad Outcome | 47.6% (44.2%–50.9%) | 43.0% (39.5%–46.5%) | 41.0% (38.5%–43.5%) | 34.1% (32.7%–35.3%) | 24.4% (23.8%–24.9%) |
CI = confidence interval
High-volume centers also had a higher proportion of patients with favorable outcomes. Discharge to home was most common in the highest volume hospitals; 39.5% (95% CI, 38.8%–40.1%) in quintile 5 vs. 31.0% (95% CI, 29.7%–32.2%) in quintile 4. Again, this trend persisted in subsets of patients that received aggressive care or when excluding those that did not receive aggressive care.
Adjusted Volumes-Outcome Association
To allow for a more flexible representation of the volume-outcome relationship and to account for the effect of differences in patient characteristics on outcomes, we developed a series of models to measure the volume-outcome association. These models identified that higher volume hospitals were associated with lower mortality, fewer bad outcomes, and more favorable outcomes than lower volume hospitals, as depicted in Figure 2. For all 3 outcomes, the magnitude of this association was attenuated after full adjustment; however, a strong volume-outcome association persisted. The fully adjusted models had excellent predictive value (primary fully adjusted model c-statistic 0.86; intra-class correlation coefficient 0.06). Of note, within the highest volume quintile, a robust volume-outcome association persisted. For example, as SAH volume decreased from 100/year, mortality increased from 18.7% (95% CI, 17.5%–20.0%) to 19.8% (95% CI, 18.6%–21.0%) at 80/year, to 21.7% (95% CI, 20.7%–22.7%) at 60/year, to 24.5% (95% CI, 23.7%–25.3%) at 40/year, to 28.4% (95% CI, 27.8%–29.1%) at 20/year. Conversely, as SAH volume decreases, the probability of discharge home decreased to 40.3% (95% CI, 37.6%–43.0%) at 100/year, to 38.7% (95% CI, 37.1%–40.4%) at 60/year, to 35.3% (95% CI, 34.4%–36.2%) at 20/year.
Figure 2.
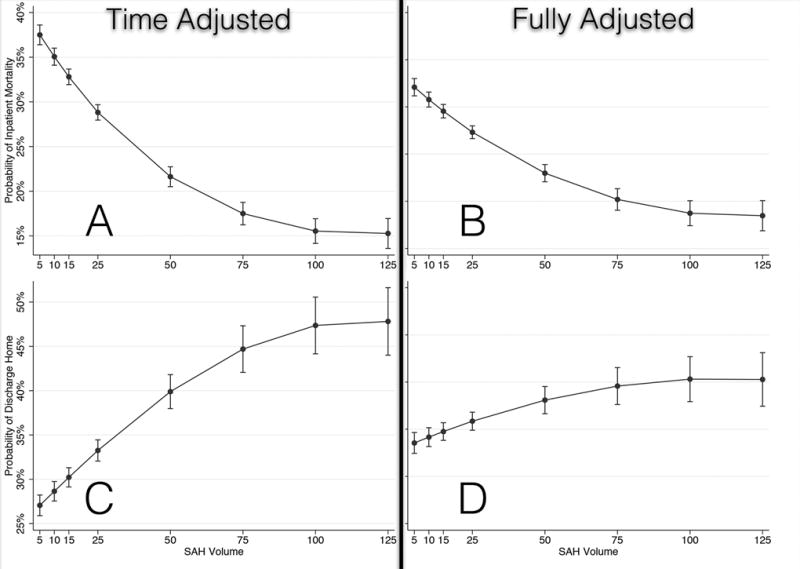
Volume-outcome associations. Each row of panels demonstrates volume-outcome associations for each of the 3 outcome studies (inpatient mortality, bad outcomes, and good outcomes/discharge to home. The left-hand column of panels demonstrates the volume-outcome association after adjusting for the temporal trend only, and the right-hand column shows the fully adjusted association—age, sex, race/ethnicity (including a missing indicator for states that do not report race/ethnicity data), year of admission, transfer status (whether a patient was transferred from another hospital), insurance, all individual Charlson comorbidities, intubation status, and APR-DRG mortality category. A) Time-adjusted mortality. B) Fully adjusted mortality. C) Time-adjusted bad outcomes. D) Fully adjusted bad-outcomes. E) Time-adjusted good outcomes/discharge to home. F) Fully adjusted good outcomes/discharge to home.
Secondary Analyses
An adjusted volume-outcome association persisted across all subpopulations studied, as summarized in Table 3. Of note, when focusing exclusively on the population that received clipping or coiling, while volume-outcomes associations persisted across all outcomes, they were considerably attenuated. (Supplemental Figure 1).
Table 3.
Hospital volume categories within top quintile.
| 14–50 Patients (95% CI) |
51–100 Patients (95% CI) |
101–150 Patients (95% CI) |
150+ Patients (95% CI) |
|
|---|---|---|---|---|
| Patient admissions in 2010 | 6852 (6489 – 7215) | 1838 (1647 – 2030) | 2596 (2369 – 2822) | 2510 (2288 – 2732) |
| Total admissions in category | 49.7% (47.8% – 51.6%) | 13.3% (12.1% – 14.7%) | 18.8% (17.4% – 20.4%) | 18.2% (16.8% – 19.7%) |
| Died | 21.3% (19.2% – 23.6%) | 16.0% (12.5% – 20.2%) | 18.7% (15.5% – 22.4%) | 18.9% (15.7% – 22.7%) |
| Bad Outcome | 25.8% (23.5% – 28.2%) | 17.9% (14.3% – 22.3%) | 25.3% (21.7% – 29.3%) | 22.1% (18.6% – 26.0%) |
| Good Outcome | 41.5% (38.9% – 44.2%) | 51.2% (45.9% – 56.4%) | 43.1% (38.8% – 47.6%) | 44.5% (40.1% – 49.0%) |
CI = confidence interval
Discharge Distribution by Volume Quintile
In 2010, a substantial majority of all SAH discharges were cared for in the quintile of hospitals with the highest volume; 80.7% of all discharges were in these 20% of hospitals. Among patients that underwent clipping or coiling in this sample, an even larger proportion (90.9%) was cared for in the highest volume quintile (Table 4). Within the highest quintile, patients treated at larger SAH volume centers had improved clinical outcome as compared to those treated at smaller SAH volume centers (Table 5). The greatest benefit was seen when comparing the centers treating 14–50 SAH patients vs. those treating 51–100 SAH patients (eg, mortality 21.3% vs. 16.0%). The same was true of percentage of patients having bad outcome or good outcome. Out of the 725 hospitals in the sample that performed at least 1 clippling or coiling procedure, 125 hospitals averaged more than 20 per year.
Table 4.
Distribution of SAH cases by hospital volume.
| Quintile 1 (95% CI) |
Quintile 2 (95% CI) |
Quintile 3 (95% CI) |
Quintile 4 (95% CI) |
Quintile 5 (95% CI) |
|
|---|---|---|---|---|---|
| No. patients | 291 (220 – 363) |
323 (245 – 402) |
832 (707 – 958) |
1,876 (1,690 – 2,063) |
13,869 (13,360 – 14,378) |
|
| |||||
| No. intubated patients | 96 (55 – 137) |
112 (65 – 159) |
330 (251 – 410) |
800 (677 – 922) |
5,574 (5,244 – 5,903) |
|
| |||||
| No. patients, excluding those receiving no aggressive care |
258 (191 – 326) |
292 (217 – 366) |
782 (661 – 904) |
1,825 (1,640 – 2,009) |
13,672 (13,166 – 14,178) |
|
| |||||
| No. patients undergoing clip/coiling | 14 (−2 – 30) |
10 (−4 – 24) |
176 (118 – 234) |
541 (441 – 642) |
7,411 (7,033 – 7,789) |
CI = confidence interval; SAH = subarachnoid hemorrhage
Table 5.
Hospital volume categories within top quintile.
| 14–50 Patients (95% CI) |
51–100 Patients (95% CI) |
101–150 Patients (95% CI) |
150+ Patients (95% CI) |
|
|---|---|---|---|---|
| Patient admissions in 2010 | 6852 (6489 – 7215) | 1838 (1647 – 2030) | 2596 (2369 – 2822) | 2510 (2288 – 2732) |
| Total admissions in category | 49.7% (47.8% – 51.6%) | 13.3% (12.1% – 14.7%) | 18.8% (17.4% – 20.4%) | 18.2% (16.8% – 19.7%) |
| Died | 21.3% (19.2% – 23.6%) | 16.0% (12.5% – 20.2%) | 18.7% (15.5% – 22.4%) | 18.9% (15.7% – 22.7%) |
| Bad Outcome | 25.8% (23.5% – 28.2%) | 17.9% (14.3% – 22.3%) | 25.3% (21.7% – 29.3%) | 22.1% (18.6% – 26.0%) |
| Good Outcome | 41.5% (38.9% – 44.2%) | 51.2% (45.9% – 56.4%) | 43.1% (38.8% – 47.6%) | 44.5% (40.1% – 49.0%) |
CI = confidence interval
Discussion
Consistent with prior work, we found that SAH management at a high nontraumatic SAH volume facility is associated with reduced inpatient mortality compared to management at a low-volume facility.4–6, 14–16 This, the largest and most recent nationally representative hospital sample study of its kind, adds to the literature by demonstrating that this finding was robust both to adjustment for patient characteristics as well as to aggressiveness of treatment. Given that survival with disability is common in this population, it is conceivable that high-volume hospitals may only reduce mortality while increasing the proportion of patients that live with severe disability. Reassuringly, we did not find this to be the case, as high nontraumatic SAH volume was not only associated with reduced mortality, but also with an increased probability of discharge to home. These findings demonstrate that even with the increased distribution of SAH patients to higher volume hospitals over the last decade, the SAH volume-outcome relationship persists and suggests that, in general, SAH systems of care should seek to distribute patients to high SAH volume facilities.
This study further adds understanding of the SAH volume-outcome relationship by clarifying the shape of the volume-outcome curve—an essential step in defining volume requirements for SAH systems of care. The Joint Commission’s CSC program presently requires that hospitals care for at least 20 SAH patients and treat at least 15 cerebral aneurysms annually in order to gain CSC certification. Our findings suggest this threshold may be relatively low. For example, as SAH volume decreases from 100/year, mortality increases from 18.7% (95% CI, 17.5%–20.0%) to 19.8% (95% CI, 18.6%–21.0%) at 80/year, to 21.7% (95% CI, 20.7%–22.7%) at 60/year, to 24.5% (95% CI, 23.7%–25.3%) at 40/year, to 28.4% (95% CI, 27.8%–29.1%) at 20/year. A similar volume-outcome relationship exists for the clipped/coiled patient population. Thus, it is possible that SAH systems of care that redistributed patients to the highest volume hospitals (eg, 100 patients/year) may represent an opportunity to improve clinical outcome. A realistic threshold for SAH systems of care needs to consider not just the shape of the volume-outcome association, but also the geographic distribution of SAH cases and high-volume centers. As it stands, the threshold of 20 nontraumatic SAH patients per center is unlikely to substantially alter treatment patterns, as currently 90% of nontraumatic SAH patients that are clipped and/or coiled are already treated in hospitals exceeding this volume threshold.
While it is not clear what mediates the volume-outcome association, these data may help answer that question. First, this effect does not appear to be substantially explained by low-volume hospitals only retaining patients with particularly dire prognoses, as we found that volume-outcome associations persist even after excluding patients that do not receive aggressive care. In additional, the residual volume-outcome association is most likely explained by a phenomenon that applies to the entire nontraumatic SAH patient population. For example, while we found a persistent volume-outcome association in the clipped/coiled population, the magnitude of this association was similar to the overall association in the entire population. A number of factors may explain why higher volume centers have better outcomes, including increased access to neurointensivists, increased availability of complex procedures that may improve outcomes (eg, cerebral angioplasty), improved nursing care due to increased experience, better surgical outcomes due to increased experience with microsurgical/endovascular procedures, and lower failure-to-rescue (FTR) rates following medical and surgical complications.
The addition of neurointensivists to neurosurgical intensive care units has led to improvement in clinical outcomes for stroke patients.17 A similar effect may be seen in SAH patients and may contribute to the observed volume-outcome association, given that patients are more likely to receive critical care from neurointensivists in higher volume centers. Standardization of care as well as multidisciplinary disease-based care has produced better clinical outcomes in ischemic stroke patients and thus, similar care models could also explain better outcomes for SAH patients at high-volume centers.18 The leading cause of death and disability in SAH patients is associated with development of clinically significant vasospasm; thus, centers that offer comprehensive mechanisms for diagnosing and managing this complication likely reduce SAH-related mortality. A recent report by Khatri et al.16 showed that hospitals offering cerebral angioplasty tended to be high-volume centers and had an improved mortality rate compared to hospitals not offering cerebral angioplasty, which tended to be lower volume centers. FTR, defined as the probability of death after medical or surgical complications, seems to be partially responsible for why lower volume centers have higher mortality rates.19 In 2014, Gonzalez et al.20 utilized the Centers for Medicare and Medicaid Services Medicare Provider Analysis and Review Files to evaluate clinical outcomes of patients undergoing complex cardiovascular procedures. They reported FTR rates of 10.9% at high-volume centers and 13.3% at low volume centers. While similar studies are lacking in the neurosurgical literature, it is plausible that SAH-related complication FTR ratios are lower at high-volume centers and may, in part, be responsible for higher rates of SAH-related mortalities at lower volume centers.
The use of nationally representative hospital sample data leads to both the primary strengths and limitations of this study. By using administrative data, we estimated the volume-outcome relationship over a large number of representative hospitals in the US using a systematic approach to identify all SAH cases at the hospital level. Limitations to this approach include potentially inaccurate identification of SAH cases and limited clinical detail. To the extent cases are inaccurately identified, conclusions about the optimal volume-outcome thresholds should be advanced cautiously. In our patient population, 58.6% did not receive clipping and/or coiling and most likely consisted of perimesencephalic SAH patients, severely ill patients who were not deemed candidates for treatment, or traumatic SAH patients who were unable to be excluded secondary to coding errors. In addition, lack of detailed clinical information (eg, neurologic condition at presentation, Hunt and Hess grade, Fisher score) may, in general, lead to inadequate adjustment for differences in patient characteristics at the hospital level. Finally, the lack of granular clinical data regarding limitations of care suggests that some patients may have poor outcomes as a consequence of limitations of care that we were unable to measure. These omissions are unlikely to significantly bias the observed volume-outcome association, given the excellent predictive value of our models and robustness of the primary associations.
Conclusions
High SAH patient volume is robustly and strongly associated with lower inpatient mortality, fewer poor outcomes, and more discharges to home. The observed SAH patient volume association does not plateau until facilities are treating over 100 SAH patients per year. This is a considerably higher patient volume threshold than the 20 SAH/year/facility set forth by the Joint Commission for CSC certification.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS:
Drafting/revising the manuscript for content, including medical writing content:
Study concept or design:
Analysis or interpretation of data:
DISCLOSURES:
Dr. Pandey reports no disclosures.
Dr. Gemmete reports no disclosures.
Dr. Wilson reports no disclosures.
Dr. Chaudhary reports no disclosures.
Dr. Thompson reports no disclosures.
Dr. Morganstern reports no disclosures.
Dr. Burke reports no disclosures.
References
- 1.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahs AL, Nishioka H, Torner JC, Graf CJ, Kassell NF, Goettler LC. Cooperative study of intracranial aneurysms and subarachnoid hemorrhage: a long-term prognostic study. I Introduction Archives of neurology. 1984;41:1140–1141. doi: 10.1001/archneur.1984.04050220034010. [DOI] [PubMed] [Google Scholar]
- 3.Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73:217–223. doi: 10.1227/01.neu.0000430290.93304.33. [DOI] [PubMed] [Google Scholar]
- 4.Cross DT, 3rd, Tirschwell DL, Clark MA, et al. Mortality rates after subarachnoid hemorrhage: variations according to hospital case volume in 18 states. Journal of neurosurgery. 2003;99:810–817. doi: 10.3171/jns.2003.99.5.0810. [DOI] [PubMed] [Google Scholar]
- 5.Leake CB, Brinjikji W, Kallmes DF, Cloft HJ. Increasing treatment of ruptured cerebral aneurysms at high-volume centers in the United States. Journal of neurosurgery. 2011;115:1179–1183. doi: 10.3171/2011.7.JNS11590. [DOI] [PubMed] [Google Scholar]
- 6.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: Population-based study and systematic review. Neurology. 2010;74:1494–1501. doi: 10.1212/WNL.0b013e3181dd42b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HCUP. Nationwide Inpatient Sample [online] Available at: www.hcup-us.ahrq.gov/nisoverview.jsp Accessed Sept. 1.
- 8.McDonald RJ, Cloft HJ, Kallmes DF. Impact of admission month and hospital teaching status on outcomes in subarachnoid hemorrhage: evidence against the July effect. Journal of neurosurgery. 2012;116:157–163. doi: 10.3171/2011.8.JNS11324. [DOI] [PubMed] [Google Scholar]
- 9.Greenspan AI, Coronado VG, Mackenzie EJ, Schulman J, Pierce B, Provenzano G. Injury hospitalizations: using the nationwide inpatient sample. The Journal of trauma. 2006;61:1234–1243. doi: 10.1097/01.ta.0000231558.71696.1a. [DOI] [PubMed] [Google Scholar]
- 10.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge R, Osler T. The ICD-9-based illness severity score: a new model that outperforms both DRG and APR-DRG as predictors of survival and resource utilization. The Journal of trauma. 1998;45:791–799. doi: 10.1097/00005373-199810000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Hatlem T, Jones C, Woodard EK. Reducing mortality and avoiding preventable ICU utilization: analysis of a successful rapid response program using APR DRGs. Journal for healthcare quality: official publication of the National Association for Healthcare Quality. 2011;33:7–16. doi: 10.1111/j.1945-1474.2011.00084.x. [DOI] [PubMed] [Google Scholar]
- 13.Almond D, Doyle JJ, Jr, Kowalski AE, Williams H. The role of hospital heterogeneity in measuring marginal returns to medical care: a reply to Barreca, Guldi, Lindo, and Waddell. The quarterly journal of economics. 2011;126:2125–2131. doi: 10.1093/qje/qjr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald RJ, McDonald JS, Bida JP, Kallmes DF, Cloft HJ. Subarachnoid hemorrhage incidence in the United States does not vary with season or temperature. AJNR Am J Neuroradiol. 2012;33:1663–1668. doi: 10.3174/ajnr.A3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vespa P, Diringer MN, Bleck TP, et al. High-volume centers. Neurocritical care. 2011;15:369–372. doi: 10.1007/s12028-011-9602-z. [DOI] [PubMed] [Google Scholar]
- 16.Khatri R, Tariq N, Vazquez G, Suri MF, Ezzeddine MA, Qureshi AI. Outcomes after nontraumatic subarachnoid hemorrhage at hospitals offering angioplasty for cerebral vasospasm: a national level analysis in the United States. Neurocritical care. 2011;15:34–41. doi: 10.1007/s12028-010-9423-5. [DOI] [PubMed] [Google Scholar]
- 17.Varelas PN, Schultz L, Conti M, Spanaki M, Genarrelli T, Hacein-Bey L. The impact of a neuro-intensivist on patients with stroke admitted to a neurosciences intensive care unit. Neurocritical care. 2008;9:293–299. doi: 10.1007/s12028-008-9050-6. [DOI] [PubMed] [Google Scholar]
- 18.Koton S, Schwammenthal Y, Merzeliak O, et al. Effectiveness of establishing a dedicated acute stroke unit in routine clinical practice in Israel. The Israel Medical Association journal: IMAJ. 2005;7:688–693. [PubMed] [Google Scholar]
- 19.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg. 2014;149:119–123. doi: 10.1001/jamasurg.2013.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


