Abstract
There is an urgent need for a new and improved vaccine against tuberculosis for controlling this disease that continues to pose a global health threat. The current research strategy is to replace the present BCG vaccine or boost BCG-immunity with subunit vaccines such as viral vectored- or protein-based vaccines. The use of recombinant proteins holds a number of production advantages including ease of scalability, but requires an adjuvant inducing cell-mediated immune responses. A number of promising novel adjuvant formulations have recently been designed and show evidence of induction of cellular immune responses in humans. A common trait of effective TB adjuvants including those already in current clinical testing is a two-component approach combining a delivery system with an appropriate immunomodulator. This review summarises the status of current TB adjuvant research with a focus on the division of labor between delivery systems and immunomodulators.
Keywords: Tuberculosis, BCG boost vaccine, dual-component adjuvants, immunomodulators, delivery systems, long-term memory, mucosal adjuvants, PAMPs
Graphical Abstract
1. Introduction
The etiological agent of tuberculosis (TB), Mycobacterium tuberculosis (M.tb.), is the most successful pathogen on earth with an estimated one third of the world’s population harboring the bacteria. Although we have witnessed a decrease in TB annual death rates during the last 15 years, there is still an alarming 1.5 million deaths/year according to the latest Global Tuberculosis Report from 2014 [1]. The BCG vaccine, developed by an attenuation of the virulent Mycobacterium bovis, remains the only vaccine against TB and is the world’s most widely used vaccine with a coverage rate of more than 80% in areas endemic for TB. BCG has a documented effect against the childhood manifestations of TB in children but neonatal administration of BCG has very limited protection against pulmonary TB which is the most common clinical form of TB in adults [2]. Developing a novel and effective vaccination strategy is a high priority area and is together with new treatment modalities possibly the only way of reaching the target of eliminating TB by 2050.
Two different TB vaccination strategies are being pursued; replacing the current BCG with an improved BCG or an attenuated M.tb., or alternatively boosting BCG-immunity either shortly after BCG administration in infants or more predominantly as a booster in adolescence when there is an increase in TB incidence. Currently, there are sixteen novel vaccines in clinical development, of which the majority are BCG booster vaccines [3]. The booster vaccines are based on one or more selected M.tb. antigens expressed by a live viral vector or produced recombinantly as a fusion of the proteins. Fusion protein constructs hold a number of production advantages including ease of scalability, low extent of batch-to-batch variation, high level of safety, and many quality control parameters are relatively uncomplicated e.g. performing full characterization. On the down side, proteins are not inherently immunogenic and need adjuvants to strengthen the immune response.
At the dawn of clinical testing in the TB vaccine field, there were only two adjuvants approved for use in humans and very few available for human testing; Alum was used successfully in vaccines for many decades with the key feature being induction of antibodies. Similarly, MF59® mainly induce antibody responses and is in particularly a very effective adjuvant in influenza vaccines [4]. For these adjuvants, there was no evidence of induction of the cell-mediated immune (CMI) response needed for combating an intracellular pathogen like M.tb., and indeed preclinical studies showed no protective potential of using Alum in TB vaccines [5]. Thus, with the first TB fusion proteins getting ready for clinical testing just after the millennium, an important task of developing suitable CMI-inducing adjuvants was running in parallel.
2. What type of immune response should we aim for?
Control of M.tb. infection is correlated with the induction of a Th1 response and in particularly the crucial role of CD4 T cells has been acknowledged for many years. The evidence is abundant and comes from classical animal studies showing protection by adoptive transfer of CD4 T cells or antibody depletion of the same subset [6] [7] and from humans where reduced CD4 T cell counts, e.g. during HIV infection, is associated with increased risk of developing TB. The CD4 T cells secrete interferon-γ (IFN-γ) that is capable of activating bacteriacidal effector mechanisms in the infected macrophage, and for many years TB vaccine research including adjuvant screening was focused on identifying the most vigorous Th1-inducing construct or vaccination strategy using IFN-γ as a marker of success [8] [9].
Today it is evident that immunity against TB is much more complex and involves several cell subsets, cytokines, and effector mechanisms (for a recent review e.g. [10] [11]). Tumor necrosis factor alpha (TNF-α) secreted by CD4 T cells, but also by macrophages, is important for differentiating monocytes into macrophages and in granuloma formation, and mice lacking TNF-α receptors are highly susceptible to TB [12]. Re-activation of latent M.tb. infection is a considerable risk upon initiation of TNF-α inhibitor therapy used to treat chronic immune-mediated diseases. CD8 T cells take part in TB immunity by a direct cytolytic activity through secretion of perforin and granulysin and/or by secreting IFN-γ and adoptive transfer of CD8 T cell can provide protection in murine models [13] [14]. Interleukin (IL)-17 secreted by Th17 CD4 T cells is important for recruitment and initiation of the Th1 response in the lung, and more recently IL-17 has also been suggested a more direct protective role against virulent clinical strains of M.tb. [15]. Adoptive transfer of BCG-or M.tb.-specific IL-17-secreting T cells showed significant protection against TB challenge even in the absence of IFN-γ secretion [16]. In fact, co-secretion of IFN-γ seem to be detrimental for the long-term protection afforded by the Th17 cells [17]. A number of unconventional cell types like mucosa-associated invariant T cells (MAIT cells), CD1 and γ/δ T cells have also been implicated in protective immunity, although at this stage we have limited knowledge on how to exploit this using vaccine technologies, including adjuvants. Finally, it is important to bear in mind that correlates of protection as identified in individuals not developing TB disease after infection is not necessarily the same as vaccine-induced correlates of protection. In this regard, a recent paper showed that TNF-α/IFN-γ secreting CD4 T cells in fact were redundant for vaccine-protection by ID93/GLA-SE [18] despite the abundant evidence on the importance of these two cytokines in natural control of M.tb. infection. Conversely, the Khader lab have convincingly shown that Th17 cells play a key role in vaccine-induced protection against TB whereas their role during natural M.tb. immunity is more debated [17] [19].
3. Dual component adjuvant systems – a key to better TB protection
As evident from above there is no single unique correlate of immunity to M.tb., rendering adjuvant research and development highly challenging. Despite this, there are a number of highly promising adjuvant candidates in clinical testing that have solid evidence of protection in various preclinical animal models including non-human primates (NHPs), and three of the candidates (IC31®, AS01, CAF01) have demonstrated the ability to induce CMI responses in human TB clinical trials (Table 1).
Table 1.
Adjuvant systems in TB clinical trials
| Adjuvant | Vaccine antigen | Delivery | Immunomodulator | Signalling pathwaya |
|---|---|---|---|---|
| GLA-SE | ID93 | Emulsion | GLA; glucopyranosyl lipid adjuvant |
TLR4 |
| AS01 | M72 | Liposomes | MPL; 3-O-desacyl-4’- monophosphoryl lipid A |
TLR4 |
| IC31® | H4/H56 | Polypeptide | ODN1a; oligodeoxynucleotide |
TLR9 |
| CAF01 | H1 | Liposomes | TDB; a synthetic variant of mycobacterial cord factor |
Mincle; |
Signalling pathway of the immunomodulator
Older experimental TB adjuvants were highly complex preparations but with the quite consisting finding that optimal activity was obtained by combining e.g. an extract of cell wall in liposomes or oil droplets [20]. Pioneering studies from Edgar Ribi showed that protection of a heat-killed fraction of BCG was dependent on co-delivering the material in an oil paste [21]. Accelerated by modern regulatory demands, there was a wish to use more defined adjuvant preparations than bacterial fractions that could potentially fulfill quality requirements for human use but still with a CMI-inducing profile. This attested into the widespread use of liposomes prepared of the quaternary ammonium compound dimethyl dioctadecyl ammonium (DDA). DDA induced a CMI-response with secretion of IFN-γ and combining DDA with mixture of mycobacterial proteins, e.g. culture filtrate proteins, provided significant protection against both M.tb. and M. bovis [22] [23]. Later, it became apparent that in order to effectively adjuvant highly refined recombinantly produced protein antigens an additional immune-stimulator was needed. Thus, whereas DDA was effective with a highly immunogenic protein like Ag85, proteins of lower inherent immunogenicity required the addition of the TLR4 ligand monophosphoryl lipid A (MPL) for maximizing T cell responses and protective immunity [24]. Today, the common denominator of most adjuvants effective in TB animal models including the ones in clinical development rely on the dual activity of an immunomodulator stimulating through a pathogen-recognition receptor (PRR) and a delivery system for efficient vaccine targeting.
3.1 Immunomodulators
The discovery of PPRs and their signalling pathways has had a major impact on adjuvant research, rendering this area a very high-profiled research discipline. The deliberate activation of the innate immune system through triggering of appropriate PRRs by natural purified compounds as well as synthetic derivatives has gained intense interest [25]. Several families of PRRs recognize mycobacterial pathogen-associated molecular patterns (PAMPs) including membrane-bound C-type lectin receptors, membrane-bound and cytosolic Toll-like receptors (TLRs), and cytosolic NOD-like receptors (for a review see [26]). Compared to old generation crude adjuvant preparations, increased knowledge on receptor-mediated recognition allows us to design adjuvants which stimulate signalling pathways that serve to promote mycobacterial killing while at the same time avoid PAMPs that could be exploited by the mycobacteria to downregulate such responses.
3.1.1 Novel generation Freund Adjuvant-inspired immunomodulators
The existence of biologically active components in the mycobacterial cell wall has been known for years and is illustrated by the fact that inactivated mycobacteria constitute a key ingredient in the archetypical adjuvant Freund’s Complete Adjuvant (FCA) [27]. Over the years, considerable efforts have been devoted towards identifying single components in FCA with adjuvant activity. Derivatives from the peptidoglycan cell wall layer and in particular the minimal active structure muramyl dipeptide (MDP) signal through NOD2 [28] and have a number of immune stimulating effects through activation of the NF-kB pathway with the subsequent induction of proinflammatory cytokines [29]. In vivo, MDP has primarily been used for various cancer therapeutic applications and has also demonstrated anti-influenza activity, whereas the use in TB vaccines has been sparse and not very successful. Testing MDP in combination with DDA liposomes as an adjuvant for the M.tb. antigen ESAT-6 showed no enhanced IFN-γ responses compared to mice receiving DDA alone and no protective effect in the conventional prophylactic aerosol mouse challenge model [8].
In contrast, it has been extensively documented that another mycobacterial cell wall component, trehalose dimycolate (TDM), also known as cord factor, is highly efficacious in TB adjuvant formulations [30] [8]. TDM is the most abundant glycolipid in the mycobacterial cell wall and it has been known for many years that TDM can induce cellular responses and provide protection against TB. Dating back to 1969, the lab of Edgar Lederer showed that i.v. injection with small amounts of cord factor derived from different mycobacteria could induce an immune response in the lungs of mice very similar to that seen in mice infected with live mycobacteria, and that cord-factor treated mice were protected against a subsequent challenge with M. tb. H37Rv [31]. Accelerated by the potent activity of TDM including e.g. for tumor regression also led to the realization that TDM was too toxic for human use. A series of analogues was synthesized and trehalose dibehenate (TDB) identified as maintaining a stimulatory effect but with a more acceptable toxicity profile [32] [33].
Later, the oil was substituted with DDA and the combination of DDA and TDB has been tested extensively in different TB animal models including mice [8] [34], guinea pigs [35] (Olsen, IAI), and non-human primates (JoAnne Flynn, personal communication). When TDB is incorporated into the unstable DDA liposomes in a ratio of 1:5, the formulation is highly stable with a shelf life at 4 °C of more than 2 years [36] (Lars Vibe Andreasen, personal communication) and designated CAF01 (cationic adjuvant formulation). The key trait of this adjuvant system is the induction of a long-lived and balanced Th1/Th17 response with a high frequency of central memory T cells (TCM) producing TNF-α and IL-2 in combination [30] [37] [38]. At this stage, CAF01 has been tested in three clinical Phase I trials showing an excellent safety profile and a remarkable maintenance of the TNF-α/IL-2 co-producers also in humans [39].
The receptors and downstream intracellular pathways activated by TDM and TDB have recently been under intense investigations and it has been shown that these glycolipids bind to the C-type lectin receptor Mincle, and that depletion of Mincle in mice abrogated granuloma formation and the Th17 response seen upon injection with TDM [40] [41]. More recently, the macrophage C-type lectin (MCL) was also found to sense TDM [42]. Importantly, a recent study describes that the human Mincle receptor also responds to stimulation with TDM/TDB and that human stimulated antigen-presenting cells (APCs) produce G-CSF and IL-6, known to contribute to Th1 differentiation in humans [43].
More recently, other mycobacterial cell wall components have been used as immunomodulators in vaccines. This includes phosphoglycolipid phosphatidylinositol mannosides (PIMs), that have been tested in both the native formats and as synthetic analogues and shown to be efficient inducers of CMI responses [44, 45]. In a murine efficacy study, only the synthetic PIM2 construct resulted in significant protection indicating that the adjuvant efficacy of PIMs are highly influenced by their structure [46]. Similarly, the mycobacterial mycolate, glycerol monomycolate (GroMM or MMG), has been shown to stimulate innate responses and has been used in TB adjuvants either as a natural purified component or a synthetic variant [47] [48]. This molecule is also a ligand for Mincle [49] and its discovery emphasizes that the mycobacterial cell wall contains a wealth of immunostimulatory components that potentially can be exploited for use in TB adjuvants.
3.2.1 The use of TLR ligands in TB adjuvants
TLRs are also involved in recognition of M.tb. and initiation of cellular responses to mycobacterial products with ligands identified for TLR2, TLR4, TLR6, TLR9 and possibly also TLR8 (for a review see [50]). TLR2 recognizes a number of different mycobacterial glycolipids e.g. lipoarabinomannan but this pathway has not been exploited in adjuvants. TLR9 recognizes unmethylated CpG motifs in bacterial DNA leading to IL-12 production by dendritic cells (DCs); a cytokine that is pivotal for induction and maintenance of Th1 responses [51]. Soluble CpG has been used for adjuvanting different TB-derived antigens but with limited protective effect in most mouse models and with most vaccine antigens like Ag85 and culture filtrate proteins [52] [53] whereas the use of CpG as adjuvant in HLA-DR3 transgenic mice has been more successful [54] [55]. CpG has also been combined with various delivery systems for improving on the immunogenecity of e.g. nanoparticles and polymers. In IC31®, the single-stranded DNA-phosphodiesther oligo-d(IC)13 (ODN1a) is combined with a polypeptide KLKL5KLK (referred to as KLK) [56]. IC31® acts through the TLR9/MyD88 pathway with induction of a long-term Th1 response that is highly protective in mice [5] and NHPs [57]. In humans, IC31® has been tested with three different fusion proteins (H1, H4, H56) in 11 different clinical trials and is currently in Phase II testing with H4 and H56 (Ingrid Kromann, personal communication). Vaccination with IC31® caused minimal adverse effects even in HIV-positive individuals and induced strong Th1 responses that persisted throughout the 2.5 year follow-up and consisted primarily of IFN-γ/IL-2/TNF-α triple positive CD4 T cells or IL-2/TNF-α double positives [58] [59] [60] [61] [62]. Most recently, IC31® was tested in healthy adults already harboring a M.tb. infection with induction of Th1 responses and with no safety considerations [63]. At this stage, IC31® has not been found to induce CD8 T cell responses when used as an adjuvant in TB vaccines. This is in contrast with a study using cationic liposomes, where the addition of a TLR9 (and TLR3) agonist was particularly effective in generating strong CD8 T cell responses, whereas TLR2, TLR4, and TLR7 agonists complexed to the liposomes elicited marginal CD8 T cell responses [64]. Vaccination with the liposome TLR9-agonist complex (designated LANAC) gave rise to significant protection when combined with ESAT-6, although the protective effect was most likely mediated by CD4 T cells.
Although the role of TLR4 during M.tb. infection is not clear, it has been known for many years that ligands for this PAMP can be used successfully in TB vaccines. Many research groups have used MPL in combination with a delivery system e.g. DDA, and TLR4 ligands are key ingredients in two of the most promising TB adjuvants (GLA-SE, AS01). In a direct comparison in cattle, DDA/MPL was superior in terms of enhancing BCG-induced protection against M. bovis compared to DDA with a synthetic phosphatidylinositol mannoside-2 (PIM2) and DDA/Pam3Cys, respectively [65]. The heparin-binding haemagglutinin (HBHA) was also protective in DDA/MPL whereas HBHA encapsulated in nanoparticles with CpG failed to induce protective responses despite the induction of strong IFN-γ responses [66].
The adjuvant effect of the archetypical TLR4 ligand, lipopolysaccharide (LPS), adheres to its ability to induce two signalling pathways; MyD88 and TRIF/TRAM that results in induction of various proinflammatory cytokines but also production of type I interferons and these two signalling pathways act synergistically to induce potent Th1 responses [67]. MPL is a chemically modified derivative of the toxic LPS component from Salmonella minnesota retaining much of the stimulatory effect of the parent molecule but with a highly acceptable safety profile. MPL together with QS21 constitute the core of the Adjuvant Systems from GSK either in an oil in water emulsion (AS02) or with liposomes (AS01) (for a review see [68]). AS01E (with the E indicating reduced dose for pediatric use) has been used for adjuvanting the GSK antigen candidate M72 and tested in several clinical trials with induction of very potent CD4 T cell response with multiple Th1 cytokine combinations, CD8 T cells and activation of NK cells, and without serious adverse events [69, 70].
Glucopyranosyl lipid adjuvant (GLA) is a synthetic TLR4 agonist which have retained the stimulatory profile of naturally-derived MPL and with an even more vigorous stimulatory effect on human DCs characterized by increased levels of DC maturations markers and higher levels of MyD88 and TRIF-dependent gene transcripts [71]. When administered in vivo in a stable-emulsion (SE), MPL and GLA generated similar immune profiles with the only difference being lower TNF-α levels with GLA. The combination of GLA and SE (GLA-SE) is used in combination with the vaccine candidate ID93 and a series of preclinical studies have shown potent Th1 responses with a predominant contribution of IFN-γ/IL-2/TNF-α triple positive CD4 T cells as well as IFN-γ/TNF-α co-producers [72]. Additionally, GLA-SE/ID93 induces significant protection in mice and guinea pigs and has also been used in NHPs as a therapeutic intervention in combination with standard isoniazid/rifampicin treatment [73]. GLA-SE/ID93 is currently in Phase I testing.
3.2 Delivery systems
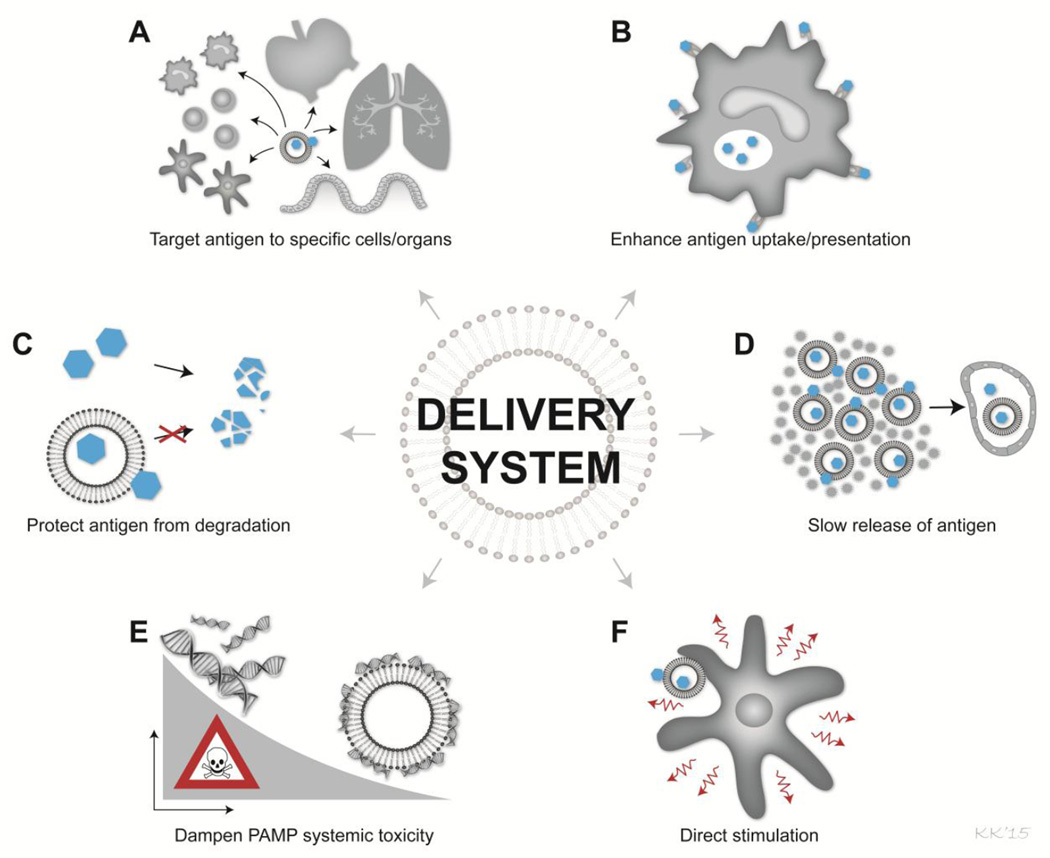
Traditionally, delivery systems have been considered inert carriers of the vaccine components, basically serving to ensure that the vaccine antigen is not excreted immediately after injection [74]. A classical example is Alum-containing adjuvants where the adjuvant activity mainly has been ascribed the ability to capture antigen; a so-called depot effect (for a review see [75]. However, a number of recent publications have shown that in addition to their carrier activities, delivery systems also can serve a number of important functions in relation to targeting the vaccine components to specific cells of the immune system, enhancing antigen presentation, prolonging immune responses and reducing unwanted adverse effects of PAMPs (Figure 1). In addition, delivery systems previously regarded as inert material have been shown to have many direct immunostimulatory properties.
Figure 1. Characteristics of delivery systems for optimal adjuvant effect in TB vaccines.
Delivery systems have numerous effects that can enhance the activity of the adjuvant system including; A) targeting the antigen to specific organs e.g. lymph nodes or cells e.g. DCs, B) enhance the antigen uptake and antigen presentation in the APC, C) protecting the vaccine antigen from degradation and rapid excretion from the system, D) ensure slow release of antigen from the injection site, E) dampen systemic toxicity of PAMPs by avoiding global activation of all APCs, F) direct stimulatory effect of the target APC or adjacent cells e.g. NK cells.
There is a range of different delivery systems including mineral salts, emulsions, liposomes, virosomes, and biodegradable polymer microspheres of which liposomes and emulsions are included in the most promising TB adjuvants. The IDRI research group has tested the effect of combining GLA with different delivery formulations and noted that even the Th2-inducing Alum can be re-directed to induce IFN-γ by adding GLA [76]. In a direct comparison, however, Alum/GLA was found less protective against TB compared to GLA delivered in an oil-in-water emulsion, and in particular the combination of emulsions composed of squalene oils and GLA was effective in terms of inducing potent Th1 responses and protection in the standard TB mouse model. The group also tested different liposomes for their Th1-inducing capability when combined with GLA and found that anionic liposomes gave rise to the strongest enhancement of Th1 responses. The superiority of anionic liposomes is in contrast with data using DDA liposomes where replacing highly cationic DDA liposomes with the zwitterionic distearoylphosphatidylcholine caused the surface charge to decrease from approximately 70 mV down to 15 mV and by that also a significant reduction of the Th1 response [77].
The immunostimulatory effect of Alum-, liposome- and emulsion-based adjuvants has also been compared in the GSK series of AS adjuvants with a clear benefit of using liposome-based systems for TB vaccines. Preclinical studies have shown that although both emulsion and liposomes-based adjuvants provided protection from a mycobacterial challenge, the liposome-based (AS01) triggered higher T-cell responses as monitored by a stronger IFN-γ response, but also a contribution by CD8 T cells, although the underlying mechanism was not elucidated [78]. This hierarchy was also seen in human volunteers and AS01 was choosen as the preferred adjuvant candidate for the vaccine antigen M72 [79].
In addition to directing the right Th1 type of immune response, it has become clear that the type of delivery system and its physicochemical properties can be pivotal in two key aspects of optimal adjuvant activity; avoiding that CMI-inducing PAMPs display overt toxicity and maintaining long-term memory responses of the vaccine.
3.2.1 Long term memory – a key role for delivery systems
Independent of vaccination strategy and type of adjuvant, it is the goal of TB vaccination to establish long-lived memory immunity. It has for many years been widely accepted that non-live subunit vaccines stimulated poor memory immunity compared to live vaccines. Orme and co-workers showed that vaccination with culture filtrate proteins in Freund’s incomplete adjuvant gave rise to levels of protection comparable to BCG but that this protection waned over time; experiments that led to the assumption that antigen has to persist in order to provide long-lived immunity but also that additional immunomodulation was required [80]. The additional immunomodulation is clearly represented by various PAMPs in modern generation adjuvant formulations, and importantly an optimal delivery system ensures prolonged retention of the vaccine antigen at the injection site and thereby mimicking the live conditions of antigen persistency. A study on the longevity of the immune response promoted by CAF01 showed that when both of these key molecules are present (DDA and TDB), subunit vaccines are indeed capable of inducing vaccine memory responses that are comparable to or even better than BCG [38]. CAF01 in combination with the fusion protein H1 induced very robust CD4 T cell responses that are maintained for more than one year in the mouse model. Strikingly, a similar longevity of immune response is seen in humans, with both CAF01 and IC31®, with both adjuvants showing no waning of vaccine-induced CD4 T cells during the 2–3 years of follow-up of the clinical trials [59] [39].
A common denominator of the delivery systems in IC31® (KLK) and CAF01 (DDA) is a highly positive charge which seem to be crucial for retaining the vaccine components at the injection site [56]. As most recombinant antigens are negatively charged, the positively charged delivery systems readily adsorbs antigen and thereby creates an antigen depot for a prolonged period [81] [82]. Using radiolabeling of vaccine components, adjuvants based on cationic liposomes were characterized by a better retention of vaccine at the site-of-injection and a superior uptake of vaccine antigen into murine DCs and subsequent improved antigen presentation when compared with adjuvants based on neutral liposomes [83]. Further studies using cationic liposomes showed that in addition to charge, the rigidity of the delivery system is also a critical determinant for antigen depot formation. When comparing two liposome delivery systems that only differed in their degree of saturation of acyl chains (rendering DDA rigid and DODA fluid at physiological temperature), long-term retention and slow release of both liposome and vaccine antigen from the injection site was most optimally achieved with the rigid DDA system and was favorable for sustained Th1 responses [84]. This clearly emphasize that the antigen release kinetics is highly decisive for the immunological outcome, and that the kinetics is controlled by the physicochemical properties of the delivery system.
Memory CD4 T cells are a heterogeneous population composed of different subtypes co-expressing different chemokines, cytokines, and surface markers. In terms of TB vaccine development and using IFN-γ as a correlate of a “good” TB vaccine, there has been a clear bias towards strategies that generates effector memory T cells (TEM). It has however become increasingly clear that central memory T cells (TCM) are important mediators of long-term immune responses. These cells are characterized by their expression of the chemokine receptor 7 (CCR7) and memory markers CD62L/CD45RA and their functional ability to secrete IL-2 [85]. In a heroic series of adoptive transfer experiments, the Kaufmann lab showed that the protection afforded by recombinant BCG primarily resided with the TCM population [86]. Conventional BCG is indeed characterized by its preferential induction of TEM and with poor levels TCM; a characteristic that has been associated with waning of BCG-induced immunity over time (reviewed in [87]). Boosting BCG-immunity with a H1/CAF01 vaccine expanded the TCM population, and a significant proportion of IL-2 positive CD4 T cells with a strong proliferative potential was still present almost two years after vaccination in mice [37]. Although it is clear that subunit vaccines are superior than live vaccines like BCG in generating TCM, the underlying mechanism is not known. It is tempting to speculate that an optimal delivery system has an antigen release kinetics than balances between sufficient antigen initially available for induction of a T cell response but without overt antigenic presence and stimulation ultimately leading to exhausted vaccine responses. Deciphering how to induce various memory subsets by vaccination and what influence different delivery systems has on the magnitude of the TCM population could be very valuable for designing future generation TB delivery systems.
3.2.2 Delivery systems for taming PAMPs
There has been considerable safety concern with adjuvants that could stimulate potent CMI-responses illustrated by the toxicity of FCA but also when using soluble Th1-stimulating PAMPs, and several clinical trials using soluble TLR agonist have been halted due to safety issues (reviewed in [88]). Recent data suggest that delivery systems can be used to dampen the toxicity of PAMPs by limiting the systemic exposure. The Siegrist lab showed that vaccination with IC31®/H1 was associated with a specific targeting of vaccine components to a minute proportion of DCs (less than 0.3% of the total DC population) and that only the cells taking up the IC31® component were activated and reflected an enhanced expression of surface maturation markers [89]. As only the small population of adjuvant-bearing DCs were activated, systemic toxicity was avoided whereas global stimulation of all DCs by injection of soluble CpG gave rise to significant levels of proinflammatory cytokines in the serum. Further, in vitro experiments showed that the delivery system KLK created aggregates with ODN1a that readily associated with DCs and that ODN1a co-localized with TLR9 positive compartments following KLK-mediated uptake [90]. This targeted delivery was due to unique conformation properties of KLK and not the positive charge of KLK as changing a single amino acid in KLK with no net charge change abolished the effect. The same specific targeting to a minute population of DCs has been seen for CAF01 where two immunizations with H1/CAF01 resulted in only a few hundred vaccine-bearing DCs in the draining lymph node despite leading to the induction of prominent Th1/Th17 responses [91].
The reduced toxicity profile seen when delivering the immunomodulator exclusively to the relevant vaccine-antigen bearing APCs has also been achieved by designing insoluble TLR agonists that stay at injection site or for intranasal vaccination purposes by coating the delivery system/TLR ligand (PLGA/TLR7) with a muco-adhesive chitosan layer [92] [93]. Co-targeting of intracellular TLR ligands to DCs using nanoparticles coated with antibodies that recognizes DC-specific receptors has also been shown to strongly enhance CTL responses [94]. This not only reflects that it is indeed possible to design CMI-inducing adjuvants with no or limited overt toxicity but also that strong systemic inflammatory responses are not necessary for their efficacy.
4. Preclinical adjuvant candidates
The majority of the current TB vaccines in late-stage preclinical development are various vector-based regimens [95]. In addition to these more official portfolio candidates, there are also several experimental adjuvants or novel immunization strategies involving adjuvants with potential in TB vaccines.
A very attractive strategy is aimed at more simple immunization procedures in a format of an immunogen with so-called inherent adjuvant effect. Mycobacterial antigens have been directly conjugated to adjuvants to obtain a convenient single product. One example is the novel adjuvant Lipokel which is the lipid Pam2Cys TLR2 ligand linked to a chelating entity 3NTA [96]. In mice, this construct provided significant protection against challenge although not to the same extent as the control BCG vaccine. Similarly, antigen multimerization has been used for enhancing the immunogenicity of proteins and a fusion of Ag85A to IMX313, a hybrid avian oligomerization domain, showed enhanced CD4 and CD8 T cell responses when used in DNA and MVA vaccine constructs [97]. In order to induce combined CD4 and CD8 T cell responses, a formulation of IL-12 delivered by the heamagglutinin virus of Japan-envelope and –liposomes has also been used. When combined with a DNA vaccine expressing the heat shock protein 65, this vaccine was capable of prolonging the survival of mice infected with MDR-TB although the lack of comparisons to other vaccines, e.g. BCG, renders it difficult to assess the full potential of this vaccine [98] [99].
In addition to enhancing the immune response of proteins and DNA vaccines, adjuvants have also been used for augmenting the effect of BCG. Directly admixing BCG with the iron-binding and immunstimulatory glycoprotein lactoferrin resulted in decreased pulmonary pathology upon M.tb. challenge compared to BCG alone, although no effect was observed when monitoring organ CFUs [100]. BCG incorporating α-galactosylceramide was found to activate Natural Killer T cells and showed enhanced CD8 T cell responses compared to BCG alone; an effect that correlated with decreased bacterial burden in the lungs of M.tb. challenged mice although the protection seemed to wane over time [101]. DDA/TDB has also been used to enhance the immunogenicity of BCG or the mutant BCG ΔmmaA4 and this led to significantly higher frequencies of CD4 T cells co-secreting TNF-α and IL-2. Although this study did not analyze induction of TCM, it is possible that the addition of DDA/TDB can re-direct BCG-induced T cells to a less differentiated state [102]. Co-delivery of BCG and an adjuvanted protein has also shown potential presumably by a synergistic effect where BCG serves as an additional immunomodulator to enhance the effect of the subunit vaccine [103]. There was no requirement of formulating the BCG and subunit vaccine together and the vaccines could be delivered in separate syringes rendering it a more realistic scenario to introduce an add-on subunit vaccine than introducing a novel BCG vaccine into the childhood vaccination program. With the recent focus on adjuvants and delivery strategies also from important funding bodies including EU Horizon2020 and NIH, it is very likely that we will see several novel adjuvants for augmenting CMI responses of relevance for TB vaccines in the coming years.
4. Adjuvants and immunization strategies for mucosal use
The failure of the first novel TB vaccine to reach efficacy testing in many years, MVA85A, has accelerated the search for new innovative methods of vaccine delivery with the respiratory tract being an increasingly popular site to target. By matching the route of vaccination to the site of infection, it is believed that we can obtain a better and not least earlier control of infection. This has even led to testing the MVA85A vaccine administered by a nebulizer to the mucosal tissue instead of the previous intradermal route [104]. The potential of using established parenteral adjuvants by various mucosal routes has also been tested. MPL administered by the oral route gave rise to strong CMI responses and highly significant levels of protection but only when used as a booster for responses primed subcutaneously with DDA/MPL [105]. A comparative testing of CpG and MPL delivered into the lungs together with Ag85A showed induction of Th1 responses and a Th17 response with MPL but only marginal levels of protection, indicating that also for mucosal delivery it is important to include a delivery system for better targeting and induction of T cells in the mucosal tissues [106]. Tissue-resident memory T cells (TRM) is a relatively newly identified subset of cells that resides at various mucosal tissues including the lungs and there is accumulating evidence for their protective function [107] [108]. Experiments in mice have shown that lung-resident T cells generated by BCG vaccination provide protection against mycobacterial challenge without the need for further recruitment of T cells from the lymph nodes or periphery [109]. In addition, it has been shown that vaccination through mucosal routes can promote more IL-17 biased immune response compared to conventional parenteral routes. Mucosal vaccination with e.g. BCG or Ag85B conjugated to nanoparticles enhanced IL-17 responses and gave rise to superior protection compared to intradermal delivery of the same vaccines [110] [52]. Given the emergence of Th17 cells as a key player in protection against TB, the selective induction of IL-17 warrants further investigation of using mucosal routes for vaccination.
The existence and definition of TRM is novel and there are very few attempts to design subunit vaccination strategies and/or adjuvants for specific induction of this subset. In contrast, it is known that specific classes of mucosal adjuvants selectively induce Th17 responses e.g. Cholera toxin (CT) [111]. In a recent study, Th17 responses induced by the mucosal model adjuvant, Escherichia coli enterotoxin (LT), gave rise to early control of M.tb. through the formation of lymphoid structures with intense macrophage activation; a protective signature that was independent of the IFN-γ pathway [112]. Boosting BCG vaccination with CT also increased numbers of IL-17 secreting CD4 T cells and showed superior protection [110]. Both CT and LT are very potent and toxic mucosal adjuvants and due to their toxic activity not rendered possible to use in humans. There has been several attempts on designing non-toxic variants during the last decade. LTK63 is a LT mutant that was designed to be devoid of a toxic effect but retaining the strong adjuvant effect. In preclinical models, vaccination with H1/LTK63 through the intranasal route led to protective levels comparable to H1 delivered subcutaneously in DDA/MPL and importantly these responses were sustained and gave rise to high levels of protection even 24 weeks post infection [113]. Very unfortunately, two cases of transient facial paralysis was later reported in human clinical trials and the use of LTK63 has not been further pursued [114].
More recently, novel adjuvant formulations for specific delivery to the mucosa have been designed. This includes a delivery construct for specific targeting of the extracellular heparin sulfate-containing matrix located in the lung alveoli [115]. This construct of wax nanoparticles adsorbed with Ag85B-HBHA was found to bind to immobilized heparin in vitro and provide significant protection when used as a BCG booster vaccine intranasally. Although the mechanism including the importance of IL-17 production was not addressed in detail, it clearly shows that it is possible to use a strategy for deliberate targeting of the lung. Bacillus subtilis spores has also been used to deliver antigens to mucosal surfaces and are known to induce secretory IgA and also strong Th1 responses [116]. In the context of TB, inert bacillus spores coated with MPT64 and Ag85B have shown protection in the mouse model at BCG levels [117]. Overall, there is a shortage of adjuvants approved for human use and this is even more evident when it comes to adjuvants approved for mucosal use where safety requirements would be extremely rigorous. Currently, most attempts to vaccinate through the mucosal surfaces uses parenterally-designed adjuvants or more crude preparations like killed mycobacteria. If we are to fully exploit and investigate the potential of targeting the respiratory tract, it is necessary to allocate specific resources in mucosal adjuvant research and discovery.
5. Future scientific perspectives
There are currently four different adjuvants in TB vaccine clinical development. Although our insights on their mechanism of action continues to improve, these adjuvants have been developed in a period when IFN-γ was used as the primary screening tool. It is therefore beyond doubt that by using updated knowledge on correlates of protection and vaccine-mechanisms combined with novel state-of-the-art adjuvant technology, we will be able to bring adjuvant research into a new era of rationally designed adjuvants that can improve TB vaccine efficacy.
There are multiple novel adjuvant technologies and vaccine strategies emerging that could potentially lead to increased efficacy. So far, only a handful of immunomodulators have been used in TB vaccines and numerous more possibilities exists or will be discovered and tested in the years to come. Combining PAMPs and thereby creating synergy between different signalling modes is also an attractive approach. In this regard, GLA-SE was formulated with an additional TLR agonist (CpG) leading to enhanced protection compared to GLA-SE alone using the standard small animal short-term protection model [118]. Although it is important to dissect to longevity of this enhancement including whether increased signalling strength leads to generation of more TEM and thereby potentially a more rapid exhaustion of the vaccine-induced T cells, this clearly illustrates that it is possibly to obtain PAMP synergy. The induction of T cell lineages apart from CD4 and CD8 T cells e.g. MAITs, CD1, and γ/δ T cells is still a relatively unexplored area, and we will hopefully see a number of novel compounds in testing in the coming years including immunomodulators for specific targeting of cells in the respiratory tract. As we have very limited knowledge relating to induction of non-conventional T cells, I believe this area of research and development will require substantial investment of funding and resources in the years to come in order to translate basic scientific knowledge into technologies for use in vaccines. The benefits of using particulate delivery systems have also just recently been fully appreciated and more sophisticated technologies for controlled antigen/immunomodulator release is starting to emerge. Serial boosting to induce sufficient levels of long-lived immune response is a major hurdle for vaccine compliance and it is possible that technologies mimicking repeated vaccinations in a single syringe would eliminate the need for repeated immunizations.
TB vaccine research and development faces enormous challenges and perhaps the greatest is the lack of a correlate of protection that can guide the rational development of novel adjuvants. Studies in NHPs, considered the optimal animal model for predicting TB outcome, have so far mostly been allocated to study mechanism of disease or testing different vaccines antigens whereas studies on adjuvants including their mechanism have been non-existent in NHPs. Comparative studies where different adjuvants including those already in clinical testing are tested are rarely performed and, if so, only in the mouse model. In order to improve our knowledge on adjuvant candidates, their mechanism/safety in higher species, and their likelihood of success I believe we could benefit from more focus on adjuvant research to be performed in NHP models. Early exploratory clinical trials testing novel adjuvants can also provide important new information on adjuvants and should be designed to address aspects of adjuvant safety, mechanism, and immune profile. Systematic investigations from clinical trials can provide important feedback that is pivotal in informing us how to design the novel TB adjuvants and thereby maximize the impact of adjuvants in future generation TB vaccines.
Acknowledgements
Supported by the European Union’s Seventh Framework Programme (EU FP7) ADITEC (HEALTH-F4-2011-280873), the European Union’s Research and Innovation Programme Horizon 2020 (EU H2020) TBVAC2020 (H2020-PHC-2014-2015/H2020-PHC-2014, Grant # 643381), Innovation Fund Denmark (060-2009-3), and National Institutes of Health (AI 105422). I thank Joshua S. Woodworth for critical reading of the manuscript as well as valuable input and Karen Smith Korsholm for the excellent figures.
Abbreviations
- AS
Adjuvant Systems
- CAF
Cationic adjuvant formulation
- CMI
Cell-mediated immune
- TCM
central memory T cell
- DDA
dimethyl dioctadecyl ammonium
- TEM
effector memory T cell
- FCA
Freunds complete adjuvant
- GLA
glucopyranosyl lipid adjuvant
- GRoMM, MMG
glycerol monomycolate
- IFN-γ
Interferon-γ
- IL-17
interleukin-17
- LPS
lipopolysaccharide
- MCL
macrophage C-type lectin
- MPL
monophosphoryl lipid A
- MAIT
mucosa-associated invariant T cells
- MDP
muramyl dipeptide
- M.tb
Mycobacterium tuberculosis
- NHPs
non-human primates
- ODN1a
oligo-d(IC)13
- PAMP
pathogen-asscoiated molecular pattern
- PRR
pattern-recognition receptor
- SE
stable emulsion
- TLR
toll-like receptor
- TDB
trehalosedibehenate
- TDM
trehalose dimycolate
- TB
tuberculosi
- TNF-a
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Global Tuberculosis Report. 2014
- 2.Shann F. Commentary: BCG vaccination halves neonatal mortality. The Pediatric infectious disease journal. 2012;31:308–309. doi: 10.1097/INF.0b013e3182465be8. [DOI] [PubMed] [Google Scholar]
- 3.Frick M. The Tuberculosis Vaccines Pipeline: A New Path to the Same Destination? HIV, HCV & TB Pipeline Report (Treatment Action Group) 2015 [Google Scholar]
- 4.Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–1416. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 5.Agger EM, Rosenkrands I, Olsen AW, Hatch G, Williams A, Kritsch C, Lingnau K, von Gabain A, Andersen CS, Korsholm KS, Andersen P. Protective immunity to tuberculosis with Ag85B–ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 2006;24:5452–5460. doi: 10.1016/j.vaccine.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 6.Orme IM. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988;140:3589–3593. [PubMed] [Google Scholar]
- 7.Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun. 2004;72:1608–1617. doi: 10.1128/IAI.72.3.1608-1617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freidag BL, Melton GB, Collins F, Klinman DM, Cheever A, Stobie L, Suen W, Seder RA. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect Immun. 2000;68:2948–2953. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orme IM, Robinson RT, Cooper AM. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol. 2015;16:57–63. doi: 10.1038/ni.3048. [DOI] [PubMed] [Google Scholar]
- 11.Andersen P, Urdahl KB. TB vaccines; promoting rapid and durable protection in the lung. Curr Opin Immunol. 2015;35:55–62. doi: 10.1016/j.coi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Feng CG, Britton WJ. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guerin. J Infect Dis. 2000;181:1846–1849. doi: 10.1086/315466. [DOI] [PubMed] [Google Scholar]
- 14.Behar SM, Woodworth JS, Wu Y. Next generation: tuberculosis vaccines that elicit protective CD8+ T cells. Expert Rev Vaccines. 2007;6:441–456. doi: 10.1586/14760584.6.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, Ramos-Payan R, Stallings CL, Reinhart TA, Kolls JK, Kaushal D, Nagarajan U, Rangel-Moreno J, Khader SA. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS pathogens. 2014;10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun. 2010;78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monin L, Griffiths KL, Slight S, Lin Y, Rangel-Moreno J, Khader SA. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal immunology. 2015;8:1099–1109. doi: 10.1038/mi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr MT, Windish HP, Beebe EA, Argilla D, Huang PW, Reese VA, Reed SG, Coler RN. Interferon gamma and tumor necrosis factor are not essential parameters of CD4+ T-Cell responses for vaccine control of tuberculosis. J Infect Dis. 2015;212:495–504. doi: 10.1093/infdis/jiv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 20.Ribi E, Granger DL, Milner KC, Yamamoto K, Strain SM, Parker R, Smith RW, Brehmer W, Azuma I. Induction of resistance to tuberculosis in mice with defined components of mycobacteria and with some unrelated materials. Immunology. 1982;46:297–305. [PMC free article] [PubMed] [Google Scholar]
- 21.Ribi E, Larson C, Wicht W, List R, Goode G. Effective nonliving vaccine against experimental tuberculosis in mice. Journal of bacteriology. 1966;91:975–983. doi: 10.1128/jb.91.3.975-983.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect.Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosio CM, Orme IM. Effective, nonsensitizing vaccination with culture filtrate proteins against virulent Mycobacterium bovis infections in mice. Infect Immun. 1998;66:5048–5051. doi: 10.1128/iai.66.10.5048-5051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt L, Elhay MJ, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis . Infect Immun. 2000;68:791–795. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci U S A. 2014;111:12294–12299. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killick KE, Ni Cheallaigh C, O’Farrelly C, Hokamp K, MacHugh DE, Harris J. Receptor-mediated recognition of mycobacterial pathogens. Cell Microbiol. 2013;15:1484–1495. doi: 10.1111/cmi.12161. [DOI] [PubMed] [Google Scholar]
- 27.Ribi E, Meyer TJ, Azuma I, Parker R, Brehmer W. Biologically active components from mycobacterial cell walls. IV. Protection of mice against aerosol infection with virulent mycobacterium tuberculosis. Cell Immunol. 1975;16:1–10. doi: 10.1016/0008-8749(75)90180-x. [DOI] [PubMed] [Google Scholar]
- 28.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 30.Werninghaus K, Babiak A, Gross O, Holscher C, Dietrich H, Agger EM, Mages J, Mocsai A, Schoenen H, Finger K, Nimmerjahn F, Brown GD, Kirschning C, Heit A, Andersen P, Wagner H, Ruland J, Lang R. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekierkunst A, Levij IS, Yarkoni E, Vilkas E, Adam A, Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. Journal of bacteriology. 1969;100:95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pimm MV, Baldwin RW, Polonsky J, Lederer E. Immunotherapy of an ascitic rat hepatoma with cord factor (trehalose-6, 6'-dimycolate) and synthetic analogues. Int J Cancer. 1979;24:780–785. doi: 10.1002/ijc.2910240614. [DOI] [PubMed] [Google Scholar]
- 33.Parant M, Audibert F, Parant F, Chedid L, Soler E, Polonsky J, Lederer E. Nonspecific immunostimulant activities of synthetic trehalose-6,6'-diesters (lower homologs of cord factor) Infect Immun. 1978;20:12–19. doi: 10.1128/iai.20.1.12-19.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen AW, Pinxteren LAHv, Okkels L, P.B. R, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001;69:2773–2778. doi: 10.1128/IAI.69.5.2773-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004;72:6148–6150. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, Agger EM, Andersen P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6’-dibehenate) - a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718:22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Lindenstrom T, Agger EM, Andersen P. Control of chronic M. tuberculosis infection by CD4 KLRG1- IL-2 secreting memory cells. J Immunol. 2013;190:6311–19. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 38.Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 39.van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA, O’Dee DM, Graves A, Thierry-Carstensen B, Andreasen LV, Ruhwald M, de Visser AW, Agger EM, Ottenhoff TH, Kromann I, Andersen P. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32:7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, Brown GD, Wells C, Lang R. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, Yamada H, Ono K, Suyama M, Iwakura Y, Yoshikai Y, Yamasaki S. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. 2013;38:1050–1062. doi: 10.1016/j.immuni.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Ostrop J, Jozefowski K, Zimmermann S, Hofmann K, Strasser E, Lepenies B, Lang R. Contribution of MINCLE-SYK Signaling to activation of primary human APCs by mycobacterial cord factor and the novel adjuvant TDB. J Immunol. 2015;195:2417–2428. doi: 10.4049/jimmunol.1500102. [DOI] [PubMed] [Google Scholar]
- 44.Denis M, Ainge GD, Larsen DS, Severn WB, Painter GF. A synthetic analogue of phosphatidylinositol mannoside is an efficient adjuvant. Immunopharmacol Immunotoxicol. 2009;31:577–582. doi: 10.3109/08923970902824862. [DOI] [PubMed] [Google Scholar]
- 45.Sprott GD, Dicaire CJ, Gurnani K, Sad S, Krishnan L. Activation of dendritic cells by liposomes prepared from phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette-Guerin and adjuvant activity in vivo. Infect Immun. 2004;72:5235–5246. doi: 10.1128/IAI.72.9.5235-5246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parlane NA, Compton BJ, Hayman CM, Painter GF, Basaraba RJ, Heiser A, Buddle BM. Phosphatidylinositol di-mannoside and derivates modulate the immune response to and efficacy of a tuberculosis protein vaccine against Mycobacterium bovis infection. Vaccine. 2012;30:580–588. doi: 10.1016/j.vaccine.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 47.Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, Gilleron M. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol. 2009;16:82–92. doi: 10.1016/j.chembiol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Andersen CS, Agger EM, Rosenkrands I, Gomes JM, Bhowruth V, Gibson KJ, Petersen RV, Minnikin DE, Besra GS, Andersen P. A simple mycobacterial monomycolated glycerol lipid has potent immunostimulatory activity. J Immunol. 2009;182:424–432. doi: 10.4049/jimmunol.182.1.424. [DOI] [PubMed] [Google Scholar]
- 49.Hattori Y, Morita D, Fujiwara N, Mori D, Nakamura T, Harashima H, Yamasaki S, Sugita M. Glycerol monomycolate is a novel ligand for the human, but not mouse macrophage inducible C-type lectin, Mincle. J Biol Chem. 2014;289:15405–15412. doi: 10.1074/jbc.M114.566489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mortaz E, Adcock IM, Tabarsi P, Masjedi MR, Mansouri D, Velayati AA, Casanova JL, Barnes PJ. Interaction of pattern recognition receptors with Mycobacterium tuberculosis. J Clin Immunol. 2014 doi: 10.1007/s10875-014-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 52.Ballester M, Nembrini C, Dhar N, de Titta A, de Piano C, Pasquier M, Simeoni E, van der Vlies AJ, McKinney JD, Hubbell JA, Swartz MA. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine. 2011;29:6959–6966. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 53.Fonseca DM, Silva CL, Paula MO, Soares EG, Marchal G, Horn C, Bonato VL. Increased levels of interferon-gamma primed by culture filtrate proteins antigen and CpG-ODN immunization do not confer significant protection against Mycobacterium tuberculosis infection. Immunology. 2007;121:508–517. doi: 10.1111/j.1365-2567.2007.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geluk A, van den Eeden SJ, van Meijgaarden KE, Dijkman K, Franken KL, Ottenhoff TH. A multistage-polyepitope vaccine protects against Mycobacterium tuberculosis infection in HLA-DR3 transgenic mice. Vaccine. 2012;30:7513–7521. doi: 10.1016/j.vaccine.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 55.Coppola M, van den Eeden SJ, Wilson L, Franken KL, Ottenhoff TH, Geluk A. Synthetic long peptide derived from Mycobacterium tuberculosis latency antigen Rv1733c protects against tuberculosis. Clinical and vaccine immunology, CVI. 2015;22:1060–1069. doi: 10.1128/CVI.00271-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schellack C, Prinz K, Egyed A, Fritz JH, Wittmann B, Ginzler M, Swatosch G, Zauner W, Kast C, Akira S, von Gabain A, Buschle M, Lingnau K. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 57.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. 2012;122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Dissel JT, Soonawala D, Joosten SA, Prins C, Arend SM, Bang P, Tingskov PN, Lingnau K, Nouta J, Hoff ST, Rosenkrands I, Kromann I, Ottenhoff TH, Doherty TM, Andersen P. Ag85B–ESAT-6 adjuvanted with IC31(R) promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine. 2011;29:2100–2109. doi: 10.1016/j.vaccine.2010.12.135. [DOI] [PubMed] [Google Scholar]
- 59.van Dissel JT, Arend SM, Prins C, Bang P, Tingskov PN, Lingnau K, Nouta J, Klein MR, Rosenkrands I, Ottenhoff TH, Kromann I, Doherty TM, Andersen P. Ag85B–ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 2010;28:3571–3581. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 60.Reither K, Katsoulis L, Beattie T, Gardiner N, Lenz N, Said K, Mfinanga E, Pohl C, Fielding KL, Jeffery H, Kagina BM, Hughes EJ, Scriba TJ, Hanekom WA, Hoff ST, Bang P, Kromann I, Daubenberger C, Andersen P, Churchyard GJ. Safety and immunogenicity of H1/IC31(R), an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PloS one. 2014;9:e114602. doi: 10.1371/journal.pone.0114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lenz N, Schindler T, Kagina BM, Zhang JD, Lukindo T, Mpina M, Bang P, Kromann I, Hoff ST, Andersen P, Reither K, Churchyard GJ, Certa U, Daubenberger CA. Antiviral innate immune activation in HIV-infected adults negatively affects H1/IC31-induced vaccine-specific memory CD4+ T Cells. Clinical and vaccine immunology, CVI. 2015;22:688–696. doi: 10.1128/CVI.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geldenhuys H, Mearns H, Miles DJ, Tameris M, Hokey D, Shi Z, Bennett S, Andersen P, Kromann I, Hoff ST, Hanekom WA, Mahomed H, Hatherill M, Scriba TJ, Group HITS, van Rooyen M, Bruce McClain J, Ryall R, de Bruyn G, Groupa HITS. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: A randomized controlled trial. Vaccine. 2015;33:3592–3599. doi: 10.1016/j.vaccine.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 63.Luabeya AK, Kagina BM, Tameris MD, Geldenhuys H, Hoff ST, Shi Z, Kromann I, Hatherill M, Mahomed H, Hanekom WA, Andersen P, Scriba TJ, Group HTS, Schoeman E, Krohn C, Day CL, Africa H, Makhethe L, Smit E, Brown Y, Suliman S, Hughes EJ, Bang P, Snowden MA, McClain B, Hussey GD. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine. 2015;33:4130–4140. doi: 10.1016/j.vaccine.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 64.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 65.Wedlock DN, Denis M, Painter GF, Ainge GD, Vordermeier HM, Hewinson RG, Buddle BM. Enhanced protection against bovine tuberculosis after coadministration of Mycobacterium bovis BCG with a mycobacterial protein vaccine-adjuvant combination but not after coadministration of adjuvant alone. CVI. 2008;15:765–772. doi: 10.1128/CVI.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verwaerde C, Debrie AS, Dombu C, Legrand D, Raze D, Lecher S, Betbeder D, Locht C. HBHA vaccination may require both Th1 and Th17 immune responses to protect mice against tuberculosis. Vaccine. 2014;32:6240–6250. doi: 10.1016/j.vaccine.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Shen H, Tesar BM, Walker WE, Goldstein DR. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol. 2008;181:1849–1858. doi: 10.4049/jimmunol.181.3.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 69.Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, Moris P, Hatherill M, Ofori-Anyinam O, Hanekom W, Vaccine Study T, Bollaerts A, Demoitie MA, Kany Luabeya AK, De Ruymaeker E, Tameris M, Lapierre D, Scriba TJ. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine. 2015;33:4025–4034. doi: 10.1016/j.vaccine.2015.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, Erasmus M, Makhethe L, Hughes EJ, Gelderbloem S, Bollaerts A, Bourguignon P, Cohen J, Demoitie MA, Mettens P, Moris P, Sadoff JC, Hawkridge A, Hussey GD, Mahomed H, Ofori-Anyinam O, Hanekom WA. Induction and regulation of T Cell immunity by the novel TB vaccine M72/AS01 in South African adults. American journal of respiratory and critical care medicine. 2013;188:492–502. doi: 10.1164/rccm.201208-1385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PloS one. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol. 2012;188:2189–2197. doi: 10.4049/jimmunol.1102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coler RN, Bertholet S, Pine SO, Orr MT, Reese V, Windish HP, Davis C, Kahn M, Baldwin SL, Reed SG. Therapeutic immunization against Mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis. 2012;207:1242–1252. doi: 10.1093/infdis/jis425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 75.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hussain MJ, Wilkinson A, Bramwell VW, Christensen D, Perrie Y. Th1 immune responses can be modulated by varying dimethyldioctadecylammonium and distearoyl-sn-glycero-3-phosphocholine content in liposomal adjuvants. J Pharm Pharmacol. 2014;66:358–366. doi: 10.1111/jphp.12173. [DOI] [PubMed] [Google Scholar]
- 78.Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, Campos-Neto A, Lobet Y, Dalemans W, Orme IM, Reed SG. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 79.Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitie MA, Mettens P, Moris P, Ledent E, Leroux-Roels G, Ofori-Anyinam O, The MSG. Improved CD4(+) T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: A randomized trial. Vaccine. 2012;31:2196–2206. doi: 10.1016/j.vaccine.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 80.Roberts AD, Sonnenberg MG, Ordway DJ, Furney SK, Brennan PJ, Belisle JT, Orme IM. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 81.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P, Perrie Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release. 2010;145:102–108. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 82.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger EM, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142:180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 83.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121:216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christensen D, Henriksen-Lacey M, Kamath AT, Lindenstrom T, Korsholm KS, Christensen JP, Rochat AF, Lambert PH, Andersen P, Siegrist CA, Perrie Y, Agger EM. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release. 2012;160:468–476. doi: 10.1016/j.jconrel.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 85.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 86.Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, Kaufmann SH. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine's superior protection against tuberculosis. J Infect Dis. 2014;210:1928–1937. doi: 10.1093/infdis/jiu347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orme IM. The Achilles heel of BCG. Tuberculosis (Edinb) 2010;90:329–332. doi: 10.1016/j.tube.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Horscroft NJ, Pryde DC, Bright H. Antiviral applications of Toll-like receptor agonists. J Antimicrob Chemother. 2012;67:789–801. doi: 10.1093/jac/dkr588. [DOI] [PubMed] [Google Scholar]
- 89.Kamath AT, Valenti MP, Rochat AF, Agger EM, Lingnau K, von Gabain A, Andersen P, Lambert PH, Siegrist CA. Protective anti-mycobacterial T cell responses through exquisite in vivo activation of vaccine-targeted dendritic cells. Eur J Immunol. 2008;38:1247–1256. doi: 10.1002/eji.200737889. [DOI] [PubMed] [Google Scholar]
- 90.Aichinger MC, Ginzler M, Weghuber J, Zimmermann L, Riedl K, Schutz G, Nagy E, von Gabain A, Schweyen R, Henics T. Adjuvating the adjuvant: facilitated delivery of an immunomodulatory oligonucleotide to TLR9 by a cationic antimicrobial peptide in dendritic cells. Vaccine. 2011;29:426–436. doi: 10.1016/j.vaccine.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Kamath AT, Rochat AF, Christensen D, Agger EM, Andersen P, Lambert PH, Siegrist CA. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PloS one. 2009;4:e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu TY, Singh M, Miller AT, De Gregorio E, Doro F, D’Oro U, Skibinski DA, Mbow ML, Bufali S, Herman AE, Cortez A, Li Y, Nayak BP, Tritto E, Filippi CM, Otten GR, Brito LA, Monaci E, Li C, Aprea S, Valentini S, Calabromicron S, Laera D, Brunelli B, Caproni E, Malyala P, Panchal RG, Warren TK, Bavari S, O'Hagan DT, Cooke MP, Valiante NM. Rational design of small molecules as vaccine adjuvants. Science translational medicine. 2014;6:263ra160. doi: 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- 93.Primard C, Poecheim J, Heuking S, Sublet E, Esmaeili F, Borchard G. Multifunctional PLGA-based nanoparticles encapsulating simultaneously hydrophilic antigen and hydrophobic immunomodulator for mucosal immunization. Mol Pharm. 2013;10:2996–3004. doi: 10.1021/mp400092y. [DOI] [PubMed] [Google Scholar]
- 94.Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 95.Aeras. http://www.aeras.org/candidates#preclinical.
- 96.Tyne AS, Chan JG, Shanahan ER, Atmosukarto I, Chan HK, Britton WJ, West NP. TLR2-targeted secreted proteins from Mycobacterium tuberculosis are protective as powdered pulmonary vaccines. Vaccine. 2013;31:4322–4329. doi: 10.1016/j.vaccine.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 97.Spencer AJ, Hill F, Honeycutt JD, Cottingham MG, Bregu M, Rollier CS, Furze J, Draper SJ, Sogaard KC, Gilbert SC, Wyllie DH, Hill AV. Fusion of the Mycobacterium tuberculosis antigen 85A to an oligomerization domain enhances its immunogenicity in both mice and non-human primates. PloS one. 2012;7:e33555. doi: 10.1371/journal.pone.0033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kita Y, Hashimoto S, Nakajima T, Nakatani H, Nishimatsu S, Nishida Y, Kanamaru N, Kaneda Y, Takamori Y, McMurray D, Tan EV, Cang ML, Saunderson P, Dela Cruz EC, Okada M. Novel therapeutic vaccines [(HSP65 + IL-12)DNA-, granulysin-and Ksp37-vaccine] against tuberculosis and synergistic effects in the combination with chemotherapy. Human vaccines & immunotherapeutics. 2013;9:526–533. doi: 10.4161/hv.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Nakatani H, Takao K, Kishigami C, Inoue Y, Matsumoto M, McMurray DN, Dela Cruz EC, Tan EV, Abalos RM, Burgos JA, Saunderson P, Sakatani M. Novel prophylactic and therapeutic vaccine against tuberculosis. Vaccine. 2009;27:3267–3270. doi: 10.1016/j.vaccine.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 100.Hwang SA, Welsh KJ, Boyd S, Kruzel ML, Actor JK. Comparing efficacy of BCG/lactoferrin primary vaccination versus booster regimen. Tuberculosis (Edinb) 2011;91(Suppl 1):S90–S95. doi: 10.1016/j.tube.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, Reddington F, Besra GS, Jacobs WR, Jr, Porcelli SA. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Derrick SC, Dao D, Yang A, Kolibab K, Jacobs WR, Morris SL. Formulation of a mmaA4 gene deletion mutant of Mycobacterium bovis BCG in cationic liposomes significantly enhances protection against tuberculosis. PloS one. 2012;7:e32959. doi: 10.1371/journal.pone.0032959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dietrich J, Billeskov R, Doherty TM, Andersen P. Synergistic effect of bacillus calmette guerin and a tuberculosis subunit vaccine in cationic liposomes: increased immunogenicity and protection. J Immunol. 2007;178:3721–3730. doi: 10.4049/jimmunol.178.6.3721. [DOI] [PubMed] [Google Scholar]
- 104.White AD, Sibley L, Dennis MJ, Gooch K, Betts G, Edwards N, Reyes-Sandoval A, Carroll MW, Williams A, Marsh PD, McShane H, Sharpe SA. An evaluation of the safety and immunogenicity of a candidate TB vaccine, MVA85A, delivered by aerosol to the lungs of macaques. Clinical and vaccine immunology : CVI. 2013 doi: 10.1128/CVI.00690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doherty TM, Olsen AW, van Pinxteren L, Andersen P. Oral vaccination with subunit vaccines protects animals against aerosol infection with Mycobacterium tuberculosis. Infect Immun. 2002;70:3111–3121. doi: 10.1128/IAI.70.6.3111-3121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Todoroff J, Lemaire MM, Fillee C, Jurion F, Renauld JC, Huygen K, Vanbever R. Mucosal and systemic immune responses to Mycobacterium tuberculosis antigen 85A following its co-delivery with CpG, MPLA or LTB to the lungs in mice. PloS one. 2013;8:e63344. doi: 10.1371/journal.pone.0063344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal immunology. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, Barber DL. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192:2965–2969. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Le Gros G, Kirman JR. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur J Immunol. 2010;40:2482–2492. doi: 10.1002/eji.200940279. [DOI] [PubMed] [Google Scholar]
- 110.Griffiths KL, Stylianou E, Poyntz HC, Betts GJ, Fletcher HA, McShane H. Cholera toxin enhances vaccine-induced protection against Mycobacterium tuberculosis challenge in mice. PloS one. 2013;8:e78312. doi: 10.1371/journal.pone.0078312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PloS one. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, Khader SA. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal immunology. 2013 doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177:6353–6360. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 114.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PloS one. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stylianou E, Diogo GR, Pepponi I, van Dolleweerd C, Arias MA, Locht C, Rider CC, Sibley L, Cutting SM, Loxley A, Ma JK, Reljic R. Mucosal delivery of antigen-coated nanoparticles to lungs confers protective immunity against tuberculosis infection in mice. Eur J Immunol. 2014;44:440–449. doi: 10.1002/eji.201343887. [DOI] [PubMed] [Google Scholar]
- 116.Huang JM, Hong HA, Van Tong H, Hoang TH, Brisson A, Cutting SM. Mucosal delivery of antigens using adsorption to bacterial spores. Vaccine. 2010;28:1021–1030. doi: 10.1016/j.vaccine.2009.10.127. [DOI] [PubMed] [Google Scholar]
- 117.Reljic R, Sibley L, Huang JM, Pepponi I, Hoppe A, Hong HA, Cutting SM. Mucosal vaccination against tuberculosis using inert bioparticles. Infect Immun. 2013;81:4071–4080. doi: 10.1128/IAI.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PloS one. 2014;9:e83884. doi: 10.1371/journal.pone.0083884. [DOI] [PMC free article] [PubMed] [Google Scholar]