Abstract
Parental care increases parental fitness through improved offspring condition and survival but comes at a cost for the caretaker(s). To increase life‐time fitness, caring parents are, therefore, expected to adjust their reproductive investment to current environmental conditions and parental capacities. The latter is thought to be signaled via ornamental traits of the bearer. We here investigated whether pre‐ and/or posthatching investment of blue tit (Cyanistes caeruleus) parents was related to ornamental plumage traits (UV crown coloration and carotenoid‐based plumage coloration) expressed by either the individual itself (i.e. “good parent hypothesis”) or its partner (i.e. “differential allocation hypothesis”). Our results show that neither prehatching (that is clutch size and offspring begging intensity) nor posthatching parental investment (provisioning rate, offspring body condition at fledging) was related to an individual's UV crown coloration or to that of its partner. Similar observations were made for carotenoid‐based plumage coloration, except for a consistent positive relationship between offspring begging intensity and maternal carotenoid‐based plumage coloration. This sex‐specific pattern likely reflects a maternal effect mediated via maternally derived egg substances, given that the relationship persisted when offspring were cross‐fostered. This suggests that females adjust their offspring's phenotype toward own phenotype, which may facilitate in particular mother‐offspring co‐adaptation. Overall, our results contribute to the current state of evidence that structural or pigment‐based plumage coloration of blue tits are inconsistently correlated with central life‐history traits.
Keywords: Differential allocation, good parent hypothesis, male ornament, parental care, parental investment, sexual selection
Introduction
Parental care is a widespread behavior within the animal kingdom, because it increases parental fitness through improved offspring condition and survival (Clutton‐Brock 1991; Kölliker et al. 2012). Providing care, however, is costly, for example in terms of time, energy, and a potentially increased predation risk (e.g. Reguera and Gomendio 1999; Milonoff et al. 2004; de Heij et al. 2006), rendering care an investment (Trivers 1974). Thus, parents are expected to trade‐off the amount of care directed toward current offspring against their own future reproductive capacity and survival to maximize lifetime fitness (Stearns 1992).
A factor impinging on these reproductive decisions is partner quality, as this is likely to affect brood value due to direct (e.g. a high level of parental care) and/or indirect (“good genes” for attractiveness or viability, e.g. Kempenaers 2007) benefits for offspring. Thus, it is important for each individual parent to reliably assess the quality of a mate. It has been hypothesized that this can be done based on the expression of (conspicuous) ornamental traits, which are costly to produce or maintain and thus should be honest signals of quality (Zahavi 1975; Hamilton and Zuk 1982; Andersson 1986). These considerations were originally employed to explain conspicuous male ornaments (such as a peacock's tail fan) (Zahavi 1975), but females may also show condition‐dependent phenotypic traits, which may play a role in mate choice and female competition (Amundsen 2000; Kraaijeveld et al. 2007; Clutton‐Brock 2009). Thus, one may expect to find a positive relationship between an individual's ornament and the amount of care it provides (“good parent hypothesis”, Hoelzer 1989; Price et al. 1993). If true, this enables an individual to adjust its investment into current offspring in relation to the quality of its partner (indicating offspring quality) in order to maximize life‐time fitness. More specifically, an individual can increase its investment in current offspring when mated to a high quality partner because of the higher genetic/phenotypic value of the offspring (“differential allocation”, Burley 1986, 1988; Sheldon 2000). However, individuals may also increase investment when mated to a partner, whose traits indicate low quality, thus, compensating via their own investment (“reproductive compensation”, Ratikainen and Kokko 2010). This may be the case when the individual preference is not the same as the general consensus of preference within the species, e.g. when ornaments exploit a sensory bias rather than predicting offspring value (Gowaty 2008).
The signaling function of plumage coloration, in particular UV crown coloration, has been extensively studied in blue tits (Cyanistes caeruleus) (reviewed in Parker 2013). Blue tits represent an excellent model system in this context as they provide substantial amounts of biparental care and possess plumage coloration that seems to signal quality. Previous studies have suggested that blue tit UV crown coloration is positively linked to survival (Sheldon et al. 1999; Griffith et al. 2003; Doutrelant et al. 2008), genetic quality (García‐Navas et al. 2009; Ferrer et al. 2015), reproductive success (Parker et al. 2011; Henderson et al. 2013), and sexual attractiveness (Andersson et al. 1998; Hunt et al. 1999). Furthermore, there is evidence that mates alter their investment in function of the UV crown coloration of their partner (Limbourg et al. 2004, 2012, 2013). Females had higher provisioning rates when mated to males with bright UV crown coloration, whereas males provided less food to offspring when mated to bright UV females. This either indicates sex differences in preference or sex differences in reproductive strategies according to UV crown coloration. However, in a recent study males provided less food when paired with an experimentally UV‐reduced female (Mahr et al. 2012). Finally, male UV crown coloration has also been shown to affect prenatal (maternal) investment in terms of yolk androgen deposition (Kingma et al. 2009). Interestingly, yolk androgens have been shown to influence begging behavior (e.g. Schwabl 1996; Eising and Groothuis 2003; Groothuis et al. 2005) and such maternal effects may link postnatal care and UV crown coloration via begging.
However, the role of UV crown coloration as signal of quality in blue tits has been called into question in a recent meta‐analysis (Parker 2013). In fact, Parker (2013) found only evidence for a sex‐difference in plumage coloration (with males reflecting more light in the UV than females) and a weak age‐effect (with birds in their second adult plumage being more intensely colored than birds in their first adult plumage), but no quality trait could be linked to plumage coloration. One of the main conclusions of Parker's review (2013) was to investigate the temporal and spatial consistency and thus significance of blue tit UV crown coloration as a quality signal. Here, we focus on temporal consistency of UV crown coloration in the context of parental care, and additionally investigated these questions for carotenoid based plumage coloration, given the evidence that it may also indicate blue tit quality (Senar et al. 2002; Hidalgo‐Garcia 2006; Doutrelant et al. 2008; García‐Navas et al. 2012; Midamegbe et al. 2013; Ferrer et al. 2015).
In this study, we examined whether parental plumage traits indicate aspects of an individual's quality. We expect that parental investment in the current brood (in terms of clutch size and rate of parental provisioning) is positively linked to parental crown UV chroma and breast plumage carotenoid chroma (“good parent hypothesis”). Parental investment, in turn, should be directly reflected by offspring body condition at fledging (via postnatal care). But also the offspring behavioral phenotype, i.e. begging intensity, may be positively related to the expression of parental ornamental traits, if mothers adjust offspring phenotype to current environmental and social conditions (such as the degree of parental care) via prenatal maternal effects (Mousseau and Fox 1998; Kingma et al. 2009). Lastly, we expect that an individual's investment is not only linked to its own quality but also to that of the partner (“differential allocation hypothesis”, Burley 1986, 1988; Sheldon 2000; and “reproductive compensation hypothesis”, Ratikainen and Kokko 2010). Previous studies on parental investment in relation to the expression of ornamental traits of the partner have revealed opposing strategies between sexes (Limbourg et al. 2013, 2004, 2012; but see Mahr et al. 2012). We performed our study over two consecutive years, which enabled us to study the temporal consistency of any observed pattern. We also cross‐fostered full clutches in 1 year, allowing us to partition pre‐ and postnatal effects, and thus differences in resource allocation at different time points during reproduction.
Material and Methods
Study area and general methods
We conducted our experiments in a nest‐box population of blue tits breeding in Peerdsbos, a mature oak‐beech forest near Antwerp (51°16′N, 4°29′E, Belgium) in spring (March – May) 2013 and 2014. By checking nest‐boxes daily we determined clutch size, onset of incubation and hatch date. All clutches of this population were cross‐fostered in 2013 as part of the general experimental procedures on this study population. In 2014, clutches were not cross‐fostered between nests. However, none of our behavioral measures (i.e. begging) appears to be affected by cross‐fostering, so data were pooled (see also Hinde et al. 2009, 2010; Estramil et al. 2013; Lucass et al. 2016b). When clutches were cross‐fostered, full clutches were reciprocally exchanged between two nests of similar clutch size and laying date 3 days prior to the estimated hatch date (see also Lucass et al. 2016a,b).
Day of hatching was defined as day 1. On day 15 we measured tarsus length (to the nearest 0.01 mm, further referred to as offspring size) and body mass (to the nearest 0.01 g) of individual chicks to calculate offspring body condition (by taking the residuals of body mass regressed against tarsus length). Subsequently, each individual was provided with a uniquely numbered metal ring. Parents were caught on day 9 while feeding chicks using nest‐box traps. They obtained a unique color ring combination facilitating further sex identification and we measured their plumage coloration (see below). All experiments were approved by the Ethical Committee for Animal experiments (ECD) of the University of Antwerp (license number 2011‐10).
Begging behavior
On day 7, the 2nd and 4th chick in a descending weight ranking were individually placed in a warmed artificial nest‐box to record their begging behavior in a food deprivation gradient. We chose the 2nd and 4th chick in order to standardize the begging protocol between broods and thus avoid rank effects on begging (Kilner 1995). Prior to the begging test, chicks were fed with defrosted blue bottle maggots to equalize hunger levels among chicks. We opened the artificial nest‐box (see Estramil et al. 2014 for more information) after 60, 90, and 120 min and videotaped the nestling's begging behavior until it ceased begging, with a video camera (Sony, DCR‐SX 30, Minato, Tokio, Japan). Besides the visual stimulus of a change from darkness to natural daylight, we simultaneously presented an acoustic stimulus, that is a playback of two parental feeding calls, recorded in 2011. Nestlings were immediately returned to their nest after testing.
We analysed the chick's begging behavior from the videotapes, according to a rating scale, modified from Kilner (2002), ranging from 0 (chick is not begging) to 5 (the chick's beak is open, the head is leant back at an angle of 90° and the back of the chick is in a vertical position) (for details see Lucass et al. 2016b,a). For each begging test, scores were applied every second and then summed. For the statistical analysis we used the average values for the begging scores of the two chicks (i.e. the mean of the scores for the two chicks for each of the three measurements taken at 60, 90, and 120 min).
Provisioning behavior
Between 8 and 9 am in the morning of day 10 we placed an infrared camera (420TVL) underneath the lid of the nest‐boxes, facing downwards into the nest. We discarded the first 30 min of video recordings to avoid a potential influence of this disturbance on our measurements (Kölliker et al. 1998). The following 2 h of the recordings were used for later analysis. Here, provisioning behavior was scored as the number of individual feeding visits per minute (= provisioning rate) using “The Observer XT” software (version 10.0.526, 2010; Noldus Information Technology, Wageningen, The Netherlands).
Color measurements
When catching adults on day 9 posthatching, we measured coloration of their crown and breast plumage (2013: 28 females and 21 males, 2014: 28 females and 26 males), using a portable Ocean Optics Jaz spectrophotometer with a built‐in pulsed xenon lamp as light source (see Figures S1 and S2 for average reflectance curves of blue tit crown and breast plumage). The spectrophotometer was connected to a bifurcated encased fiber optic probe. Three replicate measures were taken perpendicularly to the feathers relative to a white standard (WS‐1‐SL; Ocean Optics Inc. Dunedin, Florida, USA) and reference measurements were made for each bird. We averaged reflectance curves, covering 320–700 nm, which is the full spectral range a bird can detect (Cuthill et al. 2000; Hart et al. 2000), respectively, for crown and breast plumage of an individual. From this, we calculated crown UV chroma (∑R320–400/∑R320–700), which represents the purity of UV coloration, and breast plumage carotenoid chroma [(R700–R450)/R700], which represents the relative reflectance around peak absorbance of carotenoids (mainly lutein and zeaxanthin, see Hill 2006). We decided to focus on crown UV chroma and breast plumage carotenoid chroma, as these indices have previously been postulated as important indicators of individual quality in blue tits (Andersson et al. 1998; Hunt et al. 1999; Sheldon et al. 1999; Griffith et al. 2003; Doutrelant et al. 2008; García‐Navas et al. 2009, 2012; Ferrer et al. 2015).
Statistical analyses
Mixed models were used to test whether individual and partner plumage coloration is predictive for parental investment and offspring phenotype. To explore variability in individual parental provisioning rates, provisioning rates of the partner, (genetic) offspring begging intensity and offspring body condition at fledging, we used brood size at day 10, hatch date (as standardized Julian date), year, parental sex, and plumage coloration (crown UV and breast carotenoid chroma in separate parallel analyses), as well as all two‐way and three‐way interactions with the latter three variables (see Tables 1 and 2) as explanatory variables. Given that we cross‐fostered offspring between nests in 2013 but not in 2014, fledgling condition was analysed once considering the genetic link between parents and offspring (although the latter were raised by foster parents in 2013) and once considering a potential link between offspring and the actual caring parents (i.e. foster parents in 2013, but genetic parents in 2014). We used a generalized linear mixed model with Poisson error distribution and a log link function, to test for effects on clutch size. In this analysis we used the same explanatory variables as above, except that we replaced the hatch date with the lay date of the first egg (again as standardized Julian date). Obviously, brood size was not included in these analyses. All models were adjusted for a bias in statistical independence by including unique nest box number as random effect (provisioning rate) or repeated measures (clutch size and offspring phenotype). Furthermore, five females and three males were measured in two succeeding years. Therefore, all the above analyses were repeated, excluding the data of 2014 for these specific individuals. However, this did not yield different results, hence we considered these successive data points as independent and consistently reported the statistical outcome based on the full dataset. Assumptions for normality were met for all variables (Shapiro‐Wilk: all W ≥ 0.92). The outcome of each analysis is reported for the full statistical model, as well as the significant outcome after model reduction. The minimal model was obtained through stepwise backwards elimination by sequentially deleting terms with a P‐value higher than 0.05, starting with the least significant interaction. Values of both color indices were standardized within sex and year using z‐scores, which minimizes potential methodological artifacts caused by the use of different spectrophotometers in both years (although units were identical). Sample sizes vary slightly among analyses as we were not able to collect data for all variables at all times. All analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC).
Table 1.
Results of the mixed model approach explaining variation in parental investment and offspring phenotype in relation to crown UV chroma. The table represents the outcome of the full models and the final outcome of reduced models is given in parentheses. Numerator degrees of freedom is 1 in cases b) to e) and df in the table refers to the denominator degrees of freedom. Values for the main effect “Sex” and the interaction “Sex × Year” are not presented in subtable a, b, and e, as those effects are just required for statistical modeling (i.e. the three‐way interaction), but biologically irrelevant. Significant results are indicated in bold
| Effect | df | χ²/F | P |
|---|---|---|---|
| (a) Clutch size | |||
| Standardized Julian date (lay date 1st egg) | 1 (1) | 7.04 (7.29) | 0.008 (0.007) |
| UV chroma | 1 | 1.01 | 0.315 |
| Year | 1 | 1.37 | 0.242 |
| UV chroma × Sex | 1 | 1.13 | 0.288 |
| UV chroma × Year | 1 | 0.62 | 0.430 |
| UV chroma × Sex × Year | 1 | 0.59 | 0.444 |
| (b) Begging intensity of genetic offspring | |||
| Standardized Julian date (hatch date) | 52 | 0.25 | 0.621 |
| Brood size | 52 | 0.44 | 0.509 |
| UV chroma | 36 | 1.84 | 0.183 |
| Year | 52 (54) | 6.07 (8.17) | 0.017 (0.006) |
| UV chroma × Sex | 36 | 0.65 | 0.427 |
| UV chroma × Year | 36 | 1.20 | 0.282 |
| UV chroma × Sex × Year | 36 | 0.32 | 0.577 |
| (c) Provisioning rate | |||
| Standardized Julian date (hatch date) | 28 | 2.02 | 0.166 |
| Brood size | 28 | 0.56 | 0.459 |
| UV chroma | 28 | 0.02 | 0.898 |
| Sex | 28 | 0.02 | 0.880 |
| Year | 28 | 2.42 | 0.131 |
| UV chroma × Sex | 28 | 0.07 | 0.795 |
| UV chroma × Year | 28 | 1.25 | 0.273 |
| Sex × Year | 28 | 0.09 | 0.764 |
| UV chroma × Sex × Year | 28 | 0.19 | 0.665 |
| (d) Partner provisioning rate | |||
| Standardized Julian date (hatch date) | 27 | 3.06 | 0.092 |
| Brood size | 27 | 1.17 | 0.289 |
| UV chroma | 27 | 0.01 | 0.915 |
| Sex | 27 | 0.01 | 0.907 |
| Year | 27 | 5.46 | 0.027 |
| UV chroma × Sex | 27 | 0.01 | 0.911 |
| UV chroma × Year | 27 | 0.43 | 0.519 |
| Sex × Year | 27 | 0.01 | 0.920 |
| UV chroma × Sex × Year | 27 | 1.87 | 0.183 |
| (e) Body condition of genetic offspring | |||
| Standardized Julian date (hatch date) | 49 | 0.15 | 0.700 |
| Brood size | 49 (50) | 4.82 (4.71) | 0.033 (0.035) |
| UV chroma | 36 | 1.66 | 0.206 |
| Year | 49 (50) | 2.72 (6.29) | 0.105 (0.015) |
| UV chroma × Sex | 36 | 0.19 | 0.666 |
| UV chroma × Year | 36 | 1.05 | 0.313 |
| UV chroma × Sex × Year | 36 | 0.10 | 0.759 |
Table 2.
Results of the mixed model approach explaining variation in parental investment and offspring phenotype in relation to breast carotenoid chroma. The table represents the outcome of the full models and the final outcome of reduced models is given in parentheses. Numerator degrees of freedom is 1 in cases b) to e) and df in the table refers to the denominator degrees of freedom. Values for the main effect “Sex” and the interaction “Sex × Year” are not presented in subtable a, b, and e, as those effects are just required for statistical modeling (i.e. the three‐way interaction), but biologically irrelevant. Significant results are indicated in bold
| Effect | df | χ²/F | P |
|---|---|---|---|
| (a) Clutch size | |||
| Standardized Julian date (lay date 1st egg) | 1 (1) | 7.04 (7.29) | 0.008 (0.007) |
| Breast chroma | 1 | 0.68 | 0.414 |
| Year | 1 | 1.37 | 0.242 |
| Breast chroma × Sex | 1 | 0.12 | 0.726 |
| Breast chroma × Year | 1 | 2.50 | 0.114 |
| Breast chroma × Sex × Year | 1 | 0.43 | 0.513 |
| (b) Begging intensity of genetic offspring | |||
| Standardized Julian date (hatch date) | 52 | 0.44 | 0.508 |
| Brood size | 52 | 0.01 | 0.906 |
| Breast chroma | 37 (40) | 0.97 (0.49) | 0.332 (0.486) |
| Year | 52 (54) | 7.17 (7.67) | 0.010 (0.008) |
| Breast chroma × Sex | 37 (40) | 6.49 (7.48) | 0.015 (0.009) |
| Breast chroma × Year | 37 | 1.86 | 0.181 |
| Breast chroma × Sex × Year | 37 | 0.14 | 0.706 |
| (c) Provisioning rate | |||
| Standardized Julian date (hatch date) | 29 | 1.75 | 0.196 |
| Brood size | 29 | 1.23 | 0.276 |
| Breast chroma | 29 | 1.41 | 0.245 |
| Sex | 29 | 0.01 | 0.913 |
| Year | 29 | 2.48 | 0.126 |
| Breast chroma × Sex | 29 | 0.71 | 0.405 |
| Breast chroma × Year | 29 | 0.03 | 0.858 |
| Sex × Year | 29 | 0.11 | 0.741 |
| Breast chroma × Sex × Year | 29 | 0.01 | 0.919 |
| (d) Partner provisioning rate | |||
| Standardized Julian date (hatch date) | 29 | 6.57 | 0.016 |
| Brood size | 29 | 1.58 | 0.219 |
| Breast chroma | 29 | 0.80 | 0.378 |
| Sex | 29 | 0.00 | 0.983 |
| Year | 29 | 6.19 | 0.019 |
| Breast chroma × Sex | 29 | 1.89 | 0.180 |
| Breast chroma × Year | 29 | 0.17 | 0.679 |
| Sex × Year | 29 | 0.04 | 0.850 |
| Breast chroma × Sex × Year | 29 | 1.87 | 0.182 |
| (e) Body condition of genetic offspring | |||
| Standardized Julian date (hatch date) | 49 | 0.25 | 0.617 |
| Brood size | 49 (50) | 4.59 (4.71) | 0.037 (0.035) |
| Breast chroma | 37 | 0.07 | 0.794 |
| Year | 49 (50) | 2.51 (6.29) | 0.120 (0.015) |
| Breast chroma × Sex | 37 | 0.00 | 0.959 |
| Breast chroma × Year | 37 | 0.67 | 0.418 |
| Breast chroma × Sex × Year | 37 | 1.26 | 0.269 |
Results
The full outcome of the statistical models is presented in Tables 1 and 2. Crown UV chroma never significantly explained variation in parental or partner investment (see Fig. 1A,C), offspring phenotype, or clutch size neither as a main effect, nor in interaction with year, sex or their combination, indicating consistency across years and in sex differences (see Table 1).
Figure 1.
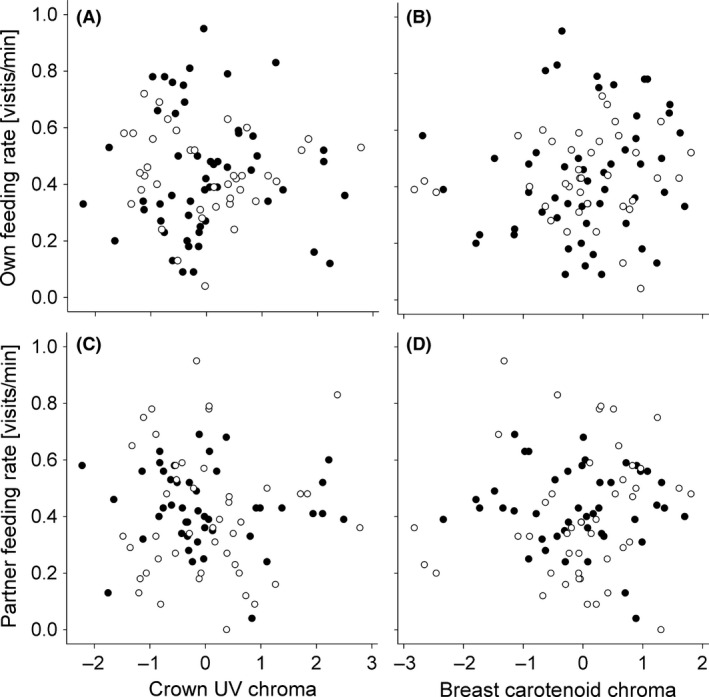
An individual's UV crown coloration (z‐transformed) does not explain variation in own (A), respectively, partner provisioning rate (C). Similarly, an individual's breast carotenoid chroma (z‐transformed) does not explain variation in own (B), respectively, partner provisioning rate (D). Filled circles represent mothers and open circles represent fathers.
Breast carotenoid chroma had no significant effect on clutch size (Table 2a), or individual or partner provisioning rates (Table 2c and d, Fig. 1B,D). However, interestingly, parental breast carotenoid chroma affected genetic offspring begging intensity, but this effect differed significantly between sexes (breast chroma × sex interaction; Table 2b; Fig. 2). This pattern was consistent across years (breast chroma × sex × year interaction: F 1,37 = 0.14; P = 0.706), and reflects a strong positive relationship in females (Posthoc covariance test for equal slopes: t 39 = 2.56; P = 0.014; estimate ± SE = 0.875 ± 0.342; effect size: r = 0.146), while there was no such relationship in males (t 39 = −1.37; P = 0.179; estimate ± SE = −0.517 ± 0.378; effect size: r = 0.094). As we cross‐fostered full clutches between nests in 2013, we also investigated whether the begging intensity of offspring was influenced by breast chroma of individual foster parents in the respective year. However, this was not the case (breast chroma of foster parents × sex interaction: F 1,17 = 0.57; P = 0.460).
Figure 2.
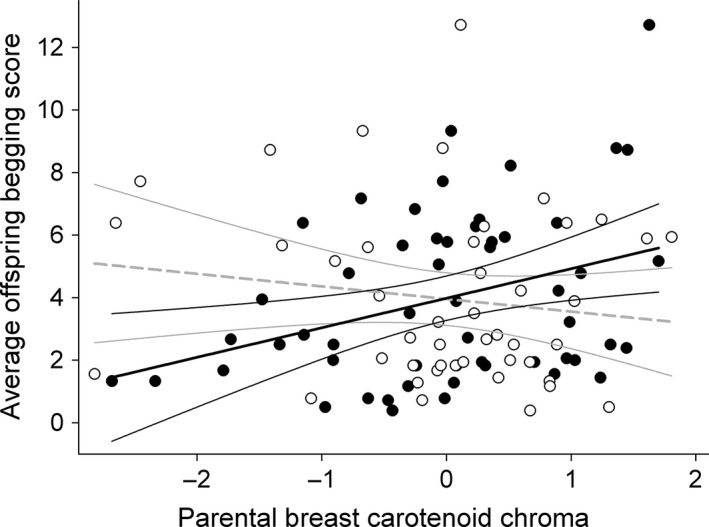
Begging intensity of genetic chicks plotted against parental breast carotenoid chroma (z‐transformed). Filled circles and significant solid black regression line (with 95% confidence bands) represent mothers. Open circles and nonsignificant striped gray regression fit represent fathers.
Investigating whether offspring condition at fledging is linked to plumage coloration of the genetic parents yielded strikingly similar results to the same analysis but with plumage coloration of the actual caring parents (i.e. foster parents in 2013 and genetic parents in 2014) instead of genetic parents. We only report, therefore, on the analysis between fledgling condition and plumage coloration of the genetic parents (see Tables 1e and 2e). Specifically, fledgling condition was only negatively affected by brood size (estimate: −0.039 ± 0.018; effect size: r = 0.24) and was higher in 2013 (0.083 ± 0.020) compared to 2014 (−0.100 ± 0.052).
Discussion
We investigated whether the pre‐ and/or posthatching parental investment of blue tits was related to ornamental plumage traits, expressed by either the individual itself or its partner. In general, most aspects of parental investment were unrelated to plumage coloration, with one exception. Offspring begging intensity was affected by female breast carotenoid coloration in both years. These data contrast a number of previous studies, but confirm the recent view that most previously reported relationships of plumage coloration, in particular of UV crown coloration, with life‐history traits in blue tits are still uncertain (Parker 2013).
UV crown coloration
We found that an individual's UV crown coloration was not predictive for its level of parental investment neither pre‐ nor posthatching. Prehatching investment was measured here in terms of clutch size and begging behavior, with begging reflecting among others maternal effects such as maternally derived yolk androgens (e.g. Schwabl 1996; Eising and Groothuis 2003; reviewed in Groothuis et al. 2005). But despite the fact that prehatching investment is strongly dependent on females (Mousseau and Fox 1998), no relationships with female UV crown coloration were found. Maternal resource allocation was also unaffected by the UV crown coloration of its partner. The lack of an effect of partner coloration on clutch size contrasts with a previous meta‐analysis dissecting the evidence for differential allocation (Horváthová et al. 2012). This meta‐analysis revealed that females of biparental species have larger clutches when exposed to (or paired with) attractive males.
Furthermore, it has been shown that blue tit mothers modulate egg yolk androgen concentrations in relation to male UV crown coloration (Kingma et al. 2009; see also Table 1 in the same paper for an overview of relationships in other species), which in turn should lead to changes in offspring phenotype. However, it may be that changes in terms of yolk androgens are too limited to become functionally significant, which could explain why we did not find that begging intensity varies with male UV crown coloration. Alternatively and also potentially more likely given the clutch size data, there is no relationship between male attractiveness, here UV crown coloration, and female prehatching investment, at least not in our study.
Similar patterns were found for parental investment posthatching, measured in terms of parental provisioning rates, which were unrelated to UV crown coloration of the focal individual. This (absent relationship) corresponds with the observation that offspring body condition at fledging was unrelated to UV crown coloration. Body condition at fledging can be interpreted as an integrative measure of provisioning over the entire nestling period, it is, however, impossible to unravel the different contributions from the sexes. These results are also in line with a study by Limbourg et al. (2012) on the same species. However, Limbourg et al. (2004, 2012, 2013) found convincing evidence, both in correlative and experimental studies, that females increase provisioning with increasing male UV crown coloration, whereas males decreased provisioning with increasing female UV. We, however, found that provisioning is not adjusted toward UV crown coloration of the partner, although our study is in fact almost identical with respect to the set‐up of the correlative study by Limbourg et al. (2012), which was only performed in a different study population in different years and we did not distinguish the age of the parents (yearling or older). The results of our study, together with a recent experimental study in yet another blue tit population (Mahr et al. 2012), revealing a pattern that contrasts with the one reported by Limbourg et al. (2012, 2013), indicate thus a high level of inconsistency on the spatial level.
Breast carotenoid coloration
Carotenoid‐dependent plumage traits in blue tits have received considerably less attention compared to other species. For example, house‐finch (Carpodacus mexicanus) females preferentially mate with males that display carotenoid‐based bright red plumage (Hill 1990), as these males have a higher overwinter survival (Hill 1991), are in better nutritional condition (Hill and Montgomerie 1994) and they feed the incubating female more than pale yellow males do (Hill 1991). But also in blue tits recent experimental and correlative evidence indicates that carotenoid‐based coloration may act as a signal, reflecting individual quality (Senar et al. 2002; Hidalgo‐Garcia 2006; Doutrelant et al. 2008; García‐Navas et al. 2012; Midamegbe et al. 2013; Ferrer et al. 2015).
We found that an individual's breast carotenoid coloration was unrelated to clutch size, but we found a consistent positive association between offspring begging intensity and their mother's carotenoid coloration. This intriguing finding likely reflects a maternal effect, potentially mediated via yolk androgens, given that the relationship between offspring begging intensity and maternal breast carotenoid chroma is consistent between years, although offspring were cross‐fostered in 1 year (2013), and the fact that yolk androgens are known to have long‐lasting effects on the phenotype (Eising and Groothuis 2003; Groothuis et al. 2005; Müller et al. 2007). It has been previously shown that carotenoid‐supplemented mothers lay eggs with higher carotenoid concentrations (e.g. Blount et al. 2002a,b; Bortolotti et al. 2003; Biard et al. 2005), which may result in more intense begging offspring (Helfenstein et al. 2008). If females adjust their offspring's phenotype toward her own phenotype, it may facilitate in particular mother‐offspring co‐adaptation, as has been previously found (see Kölliker et al. 2000).
We then focused on the relationship between prehatching maternal investment and partner carotenoid plumage coloration. Such a relationship had been previously shown, for example, for blue‐footed booby mothers that adjusted their prehatching reproductive investment to a carotenoid‐based male trait (Velando et al. 2006), a pattern, that we could not confirm in blue tits. However, the trait measured in the latter study (foot skin color) varies rapidly with nutritional status (Velando et al. 2006), which is in contrast with a rather static plumage trait as measured in this study (see below for a more extensive discussion on the meaning of signals in feathers). Being a static trait may be one explanation as to why male carotenoid‐based plumage coloration did not affect maternal investment prehatching.
However, females did not adjust their prehatching investment in relation to male breast carotenoid coloration. This likely related to our observation that breast carotenoid coloration is not predictive for provisioning or offspring body condition at fledging.
Plumage coloration and parental investment in blue tits
Initially, accumulated evidence pointed toward blue tit plumage coloration acting as a signal of individual quality (Andersson et al. 1998; Hunt et al. 1999; Sheldon et al. 1999; Griffith et al. 2003; Doutrelant et al. 2008; García‐Navas et al. 2009, 2012; Henderson et al. 2013; Ferrer et al. 2015). However, a recent meta‐analysis revealed that our gain in knowledge even after more than 10 years of studying functional aspects of in this case UV crown coloration is particular limited (Parker 2013). By the time of this review the relationship of UV crown coloration and parental care had not been published, while these studies showed a high level of consistency across years within a population in The Netherlands (Limbourg et al. 2004, 2012, 2013). We therefore set out to test the robustness of these findings, implementing the temporal (by investigating 2 years) consistency of aspects in our study. As advocated among others by Parker (2013), research benefits from studies that focus on within‐population consistency of previously reported patterns by replicating. Having detected (in)consistencies between populations may on one hand reflect (the lack of) an overall relationship. Or it may, on other hand, stimulate studies investigating those ecological and/or social factors that drive such temporal and spatial variation.
One important aspect that should always be kept in mind is that the expression of (pigment‐based) plumage coloration is determined at molt (which takes place between July and September, Cramp and Perrins 1993) and, therefore, most strongly reflects an individual's quality in that (relatively short time) period (Hill 2006). That is to say that carotenoid‐based plumage critically depends on the amount of ingested carotenoids during molt (Saks et al. 2003). But we currently lack knowledge on whether carotenoids are actually limited during that period or not (Olson and Owens 1998), a central aspect for its signaling function.
As UV crown coloration depends on the nano‐structural arrangements of feathers (Shawkey et al. 2003), its expression may again depend on an individual's condition during molt. But numerous other processes will impinge on its expression before mating/caring for offspring, such as feather abrasion, bleaching and accumulation of dirt (Figuerola and Senar 2005; Delhey et al. 2006). These processes are thought to be responsible for the changes in UV crown coloration, that have been observed with the time of the season (Figuerola and Senar 2005; Delhey et al. 2006), and are likely to introduce additional noise on the signal. Thus, it remains to be shown how individual and territory quality at molt relate to the ability to forage caterpillars (the main diet of dependent nestlings) and territory quality during the breeding season, also given the high level of stochasticity in for example environmental conditions.
Conclusions
We investigated the relationship between blue tit plumage coloration and parental investment – stimulated by previously reported intriguing patterns of parental investment adjusted to (changes in) partner UV crown coloration. However, our results do not confirm that individual investment of blue tits into current offspring varies with plumage coloration, neither of the individual itself nor of its partner. An exception to this is maternal breast carotenoid coloration that was positively linked to offspring begging intensity, likely reflecting a maternal effect. Thus, observed patterns of investment in relation to partner plumage coloration appear to be less consistent than previously thought, at least across years and populations. This study adds to the uncertainty of the signaling function of UV crown coloration in blue tits, but potentially also in other bird species. Furthermore, our results suggest that such inconsistency could also apply for relationships with carotenoid‐based plumage coloration.
Conflict of Interest
None declared.
Supporting information
Figure S1. Mean reflectance curves of crown plumage of male (N = 47) and female (N = 56) blue tits.
Figure S2. Mean reflectance curves of carotenoid based breast plumage of male (N = 47) and female (N = 56) blue tits.
Acknowledgments
The authors wish to thank William and Petra Van Dieren and the Agentschap Natuur en Bos (ANB) for providing electricity and facilities for the begging tests. We thank Erik Matthysen for giving us access to his field site and Joris Elst and Nolwenn Fresneau for help with the practical work and Josie Meaney‐Ward helped to improve the English. All experiments were conducted under license from the animal experiment committee of the University of Antwerp (license number 2011‐10). The authors declare that they have no conflict of interest. This work was financially supported by the Fonds Wetenschappelijk Onderzoek – Vlaanderen (FWO) (11F4314N to CL; 1517815N and 12I1916N to AI) and the University of Antwerp (BOF NOI UA).
References
- Amundsen, T. 2000. Why are female birds ornamented? Trends Ecol. Evol. 15:149–155. [DOI] [PubMed] [Google Scholar]
- Andersson, M. 1986. Evolution of condition‐dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution 40:804–816. [DOI] [PubMed] [Google Scholar]
- Andersson, S. , Örnborg J., and Andersson M.. 1998. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. B Biol. Sci. 265:445–450. [Google Scholar]
- Biard, C. , Surai P. F., and Møller A. P.. 2005. Effects of carotenoid availability during laying on reproduction in the blue tit. Oecologia 144:32–44. [DOI] [PubMed] [Google Scholar]
- Blount, J. D. , Surai P. F., Nager R. G., Houston D. C., Møller A. P., Trewby M. L., et al. 2002a. Carotenoids and egg quality in the lesser blackbacked gull Larus fuscus: a supplemental feeding study of maternal effects. Proc. R. Soc. B Biol. Sci. 269:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount, J. D. , Surai P. F., Houston D. C., and Møller A. P.. 2002b. Patterns of yolk enrichment with dietary carotenoids in gulls: the roles of pigment acquisition and utilization. Funct. Ecol. 16:445–453. [Google Scholar]
- Bortolotti, G. R. , Negro J. J., Surai P. F., and Prieto P.. 2003. Carotenoids in eggs and plasma of red‐legged partridges: effects of diet and reproductive output. Physiol. Biochem. Zool. 76:367–374. [DOI] [PubMed] [Google Scholar]
- Burley, N. 1986. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127:415–445. [Google Scholar]
- Burley, N. 1988. The differential‐allocation hypothesis: an experimental test. Am. Nat. 132:611–628. [Google Scholar]
- Clutton‐Brock, T. H. 1991. The evolution of parental care. Princeton Univ. Press, Princeton, NJ. [Google Scholar]
- Clutton‐Brock, T. 2009. Sexual selection in females. Anim. Behav. 77:3–11. [Google Scholar]
- Cramp, S. , and Perrins C. M.. 1993. Blue tit Pp. 225–248 in Cramp S. and Perrins C. M., eds. The birds of the Western Palearctic Vol. 7 Oxford Univ. Press, Oxford, U.K. [Google Scholar]
- Cuthill, I. C. , Partridge J. C., Bennet A. T. D., Church S. C., Hart N. S., Hunt S.. 2000. Ultraviolet vision in birds Pp. 159–214 in Slater P. J. B., et al., eds. Advances in the study of behavior Vol. 29 Elsevier Academic Press Inc, San Diego, CA. [Google Scholar]
- Delhey, K. , Peters A., Johnsen A., and Kempenaers B.. 2006. Seasonal changes in blue tit crown color: do they signal individual quality? Behav. Ecol. 17:790–798. [Google Scholar]
- Doutrelant, C. , Grégoire A., Grnac N., Gomez D., Lambrechts M. M., and Perret P.. 2008. Female coloration indicates female reproductive capacity in blue tits. J. Evol. Biol. 21:226–233. [DOI] [PubMed] [Google Scholar]
- Eising, C. M. , and Groothuis T. G. G.. 2003. Yolk androgens and begging behaviour in black‐headed gull chicks: an experimental field study. Anim. Behav. 66:1027–1034. [Google Scholar]
- Estramil, N. , Eens M., and Müller W.. 2013. Coadaptation of offspring begging and parental provisioning ‐ an evolutionary ecological perspective on avian family life. PLoS ONE 8:e70463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estramil, N. , Eens M., and Müller W.. 2014. On the coadaptation of offspring begging and parental supply—a within‐individual approach across life stages. Behav. Ecol. Sociobiol. 68:1481–1491. [Google Scholar]
- Ferrer, E. S. , García‐Navas V., Bueno‐Enciso J., Sanz J. J., and Ortego J.. 2015. Multiple sexual ornaments signal heterozygosity in male blue tits. Biol. J. Linn. Soc. 115:362–375. [Google Scholar]
- Figuerola, J. , and Senar J. C.. 2005. Seasonal changes in carotenoid‐ and melanin‐based plumage coloration in the Great Tit Parus major . The Ibis 147:797–802. [Google Scholar]
- García‐Navas, V. , Ortego J., and Sanz J. J.. 2009. Heterozygosity‐based assortative mating in blue tits (Cyanistes caeruleus): implications for the evolution of mate choice. Proc. Biol. Sci. R. Soc. 276:2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Navas, V. , Ferrer E. S., and Jose Sanz J.. 2012. Plumage yellowness predicts foraging ability in the blue tit Cyanistes caeruleus . Biol. J. Linn. Soc. 106:418–429. [Google Scholar]
- Gowaty, P. A. 2008. Reproductive compensation. J. Evol. Biol. 21:1189–1200. [DOI] [PubMed] [Google Scholar]
- Griffith, S. C. , Ornborg J., Russell A. F., Andersson S., and Sheldon B. C.. 2003. Correlations between ultraviolet coloration, overwinter survival and offspring sex ratio in the blue tit. J. Evol. Biol. 16:1045–1054. [DOI] [PubMed] [Google Scholar]
- Groothuis, T. , Müller W., von Engelhardt N., Carere C., and Eising C.. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29:329–352. [DOI] [PubMed] [Google Scholar]
- Hamilton, W. D. , and Zuk M.. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218:384–387. [DOI] [PubMed] [Google Scholar]
- Hart, N. S. , Partridge J. C., Cuthill I. C., and Bennett A. T.. 2000. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A. 186:375–387. [DOI] [PubMed] [Google Scholar]
- de Heij, M. E. , van den Hout P. J., and Tinbergen J. M.. 2006. Fitness cost of incubation in great tits (Parus major) is related to clutch size. Proc. R. Soc. B Biol. Sci. 273:2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfenstein, F. , Berthouly A., Tanner M., Karadas F., and Richner H.. 2008. Nestling begging intensity and parental effort in relation to prelaying carotenoid availability. Behav. Ecol. 19:108–115. [Google Scholar]
- Henderson, L. J. , Heidinger B. J., Evans N. P., and Arnold K. E.. 2013. Ultraviolet crown coloration in female blue tits predicts reproductive success and baseline 9 corticosterone. Behav. Ecol.. doi:10.1093/beheco/art066. [Google Scholar]
- Hidalgo‐Garcia, S. 2006. The carotenoid‐based plumage coloration of adult Blue Tits Cyanistes caeruleus correlates with the health status of their brood. The Ibis 148:727–734. [Google Scholar]
- Hill, G. E. 1990. Female house finches prefer colorful males ‐ sexual selection for a condition‐dependent trait. Anim. Behav. 40:563–572. [Google Scholar]
- Hill, G. E. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350:337–339. [Google Scholar]
- Hill, G. E. 2006. Environmental regulation of ornamental coloration Pp. 507–560 in Hill G. E. and McGraw K. J., eds. Bird coloration, volume 1: mechanisms and measurements. Harvard Univ. Press, Cambridge, MA. [Google Scholar]
- Hill, G. E. , and Montgomerie R.. 1994. Plumage colour signals nutritional condition in the house finch. Proc. R. Soc. Lond. B Biol. Sci. 258:47–52. [Google Scholar]
- Hinde, C. A. , Buchanan K. L., and Kilner R. M.. 2009. Prenatal environmental effects match offspring begging to parental provisioning. Proc. R. Soc. B Biol. Sci. 276:2787–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde, C. A. , Johnstone R. A., and Kilner R. M.. 2010. Parent‐offspring conflict and coadaptation. Science 327:1373–1376. [DOI] [PubMed] [Google Scholar]
- Hoelzer, G. A. 1989. The good parent process of sexual selection. Anim. Behav. 38:1067–1078. [Google Scholar]
- Horváthová, T. , Nakagawa S., and Uller T.. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. Lond. B Biol. Sci. 279:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, S. , Cuthill I. C., Bennet A. T. D., and Griffiths R.. 1999. Preferences for ultraviolet partners in the blue tit. Anim. Behav. 58:809–815. [DOI] [PubMed] [Google Scholar]
- Kempenaers, B. 2007. Mate choice and genetic quality: a review of the heterozygosity theory Pp. 189–278 in Brockmann H. J., et al., eds. Advances in the study of behavior Vol. 37. Elsevier Academic Press Inc, San Diego, CA. [Google Scholar]
- Kilner, R. M. 1995. When do canary parents respond to nestling signals of need? Proc. R. Soc. Lond. B Biol. Sci., 260:343–348. [Google Scholar]
- Kilner, R. M. 2002. Sex differences in canary (Serinus canaria) provisioning rules. Behav. Ecol. Sociobiol. 52:400–407. [Google Scholar]
- Kingma, S. A. , Komdeur J., Vedder O., von Engelhardt N., Korsten P., and Groothuis T. G. G.. 2009. Manipulation of male attractiveness induces rapid changes in avian maternal yolk androgen deposition. Behav. Ecol. 20:172–179. [Google Scholar]
- Kölliker, M. , Richner H., Werner I., and Heeb P.. 1998. Begging signals and biparental care: nestling choice between parental feeding locations. Anim. Behav. 55:215–222. [DOI] [PubMed] [Google Scholar]
- Kölliker, M. , Brinkhof M. W. G., Heeb P., Fitze P. S., and Richner H.. 2000. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. R. Soc. B Biol. Sci. 267:2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker, M. , Royle N. J., and Smiseth P. T.. 2012. Parent‐offspring co‐adaptation Pp. 285–303 in Royle N. J., Smiseth P. T., Kölliker M., eds. The evolution of parental care. Oxford Univ. Press, Oxford; USA. [Google Scholar]
- Kraaijeveld, K. , Kraaijeveld‐Smit F. J. L., and Komdeur J.. 2007. The evolution of mutual ornamentation. Anim. Behav. 74:657–677. [Google Scholar]
- Limbourg, T. , Mateman A. C., Andersson S., and Lessells C. M.. 2004. Female blue tits adjust parental effort to manipulated male UV attractiveness. Proc. R. Soc. Lond. B Biol. Sci. 271:1903–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg, T. , Mateman A. C., and Lessells C. M.. 2012. Parental care and UV coloration in blue tits: opposite correlations in males and females between provisioning rate and mate's coloration. J. Avian Biol., 44:017–026. [Google Scholar]
- Limbourg, T. , Mateman A. C., and Lessells C. M.. 2013. Opposite differential allocation by males and females of the same species. Biol. Lett. 9:20120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucass, C. , Stöwe M., Eens, M. , and Müller W.. 2016a. Favored parent–offspring trait combinations? On the interplay of parental and offspring traits. Behav. Ecol. 27:134–140. [Google Scholar]
- Lucass, C. , Korsten P., Eens, M. , and Müller W.. 2016b. Within‐family parent–offspring co‐adaptation in a wild bird: on static traits, behavioural reaction norms, and sex differences. Funct. Ecol. 30:274–282. [Google Scholar]
- Mahr, K. , Griggio M., Granatiero M., and Hoi H.. 2012. Female attractiveness affects paternal investment: experimental evidence for male differential allocation in blue tits. Front. Zool. 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midamegbe, A. , Grégoire A., Staszewski V., Perret P., Lambrechts M. M., Boulinier T., and Doutrelant C.. 2013. Female blue tits with brighter yellow chests transfer more carotenoids to their eggs after an immune challenge. Oecologia 173:387–397. [DOI] [PubMed] [Google Scholar]
- Milonoff, M. , Pöysä H., Runko P., and Ruusila V.. 2004. Brood rearing costs affect future reproduction in the precocial common goldeneye Bucephala clangula . J. Avian Biol. 35:344–351. [Google Scholar]
- Mousseau, T. A. , and Fox C. W.. 1998. Maternal effects as adaptations. Oxford Univ. Press, New York, NY. [Google Scholar]
- Müller, W. , Deptuch K., López‐Rull I., and Gil D.. 2007. Elevated yolk androgen levels benefit offspring development in a between‐clutch context. Behav. Ecol. 18:929–936. [Google Scholar]
- Olson, V. A. , and Owens I. P. F.. 1998. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 13:510–514. [DOI] [PubMed] [Google Scholar]
- Parker, T. H. 2013. What do we really know about the signalling role of plumage colour in blue tits? A case study of impediments to progress in evolutionary biology. Biol. Rev. 88:511–536. [DOI] [PubMed] [Google Scholar]
- Parker, T. H. , Wilkin T. A., Barr I. R., Sheldon B. C., Rowe L., and Griffith S. C.. 2011. Fecundity selection on ornamental plumage colour differs between ages and sexes and varies over small spatial scales. J. Evol. Biol. 24:1584–1597. [DOI] [PubMed] [Google Scholar]
- Price, T. , Schluter D., and Heckman N. E.. 1993. Sexual selection when the female directly benefits. Biol. J. Linn. Soc. 48:187–211. [Google Scholar]
- Ratikainen, I. I. , and Kokko H.. 2010. Differential allocation and compensation: who deserves the silver spoon? Behav. Ecol. 21:195–200. [Google Scholar]
- Reguera, P. , and Gomendio M.. 1999. Predation costs associated with parental care in the golden egg bug Phyllomorpha laciniata (Heteroptera: Coreidae). Behav. Ecol. 10:541–544. [Google Scholar]
- Saks, L. , McGraw K., and Hõrak P.. 2003. How feather colour reflects its carotenoid content. Funct. Ecol. 17:555–561. [Google Scholar]
- Schwabl, H. 1996. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. A Physiol. 114:271–276. [DOI] [PubMed] [Google Scholar]
- Senar, J. C. , Figuerola J., and Pascual J.. 2002. Brighter yellow blue tits make better parents. Proc. R. Soc. Lond. B Biol. Sci., 269:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey, M. D. , Estes A. M., Siefferman L. M., and Hill G. E.. 2003. Nanostructure predicts intraspecific variation in ultraviolet–blue plumage colour. Proc. R. Soc. Lond. B Biol. Sci. 270:1455–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, B. C. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15:397–402. [DOI] [PubMed] [Google Scholar]
- Sheldon, B. C. , Andersson S., Griffith S. C., Örnborg J., and Sendecka J.. 1999. Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877. [Google Scholar]
- Stearns, S. C. 1992. The Evolution of Life Histories. Oxford Univ. Press, Oxford: Available at: https://global.oup.com/academic/product/the-evolution-of-life-histories-9780198577416?cc=be&lang=en&. [Google Scholar]
- Trivers, R. L. 1974. Parent‐offspring conflict. Integr. Comp. Biol. 14:249–264. [Google Scholar]
- Velando, A. , Beamonte‐Barrientos R., and Torres R.. 2006. Pigment‐based skin colour in the blue‐footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149:535–542. [DOI] [PubMed] [Google Scholar]
- Zahavi, A. 1975. Mate selection—A selection for a handicap. J. Theor. Biol. 53:205–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean reflectance curves of crown plumage of male (N = 47) and female (N = 56) blue tits.
Figure S2. Mean reflectance curves of carotenoid based breast plumage of male (N = 47) and female (N = 56) blue tits.


