Abstract
A growing body of evidence suggests that alterations of the stress response system may be a mechanism by which childhood maltreatment alters risk for psychopathology. FK506 binding protein 51 (FKBP5) binds to the glucocorticoid receptor and alters its ability to respond to stress signaling. The aim of the present study was to examine methylation of the FKBP5 gene (FKBP5), and the role of an FKBP5 genetic variant, in relation to childhood maltreatment in a sample of impoverished preschool-aged children. One hundred seventy-four families, including n=69 with child welfare documentation of moderate-severe maltreatment in the past six months, participated in this study. Children ranged in age from 3 to 5 years, and were racially and ethnically diverse. Structured record review and interviews in the home were used to assess a history of maltreatment, other traumas, and contextual life stressors, and a composite variable assessed the number exposures to these adversities. Methylation of two CpG sites in intron 7 of FKBP5 was measured via sodium bisulfite pyrosequencing. Maltreated children had significantly lower levels of methylation at both CpG sites (p<.05). Lifetime contextual stress exposure showed a trend for lower levels of methylation at one of the sites, and a trend for an interaction with the FKBP5 polymorphism. A composite adversity variable was associated with lower levels of methylation at one of the sites as well (p<.05). FKBP5 alters glucocorticoid receptor responsiveness and FKBP5 gene methylation may be a mechanism of the bio-behavioral effects of adverse exposures in young children.
Introduction
Childhood maltreatment significantly increases risk for behavior problems (Carr et al., 2013; Hickman et al., 2013; Kim-Spoon, Cicchetti & Rogosch, 2013; Mills et al., 2013; Norman et al., 2012) which can be identified as early as 12-48 months of age and are strongly correlated with the development of significant psychopathology (Briggs-Gowan et al., 2006; Mesman & Koot, 2001; Roza et al., 2003). Childhood maltreatment is also significantly associated with adult psychopathology (Brown & Anderson, 1991; Bryer et al., 1987; Kendler et al., 1993; Lewinsohn, Hoberman & Rosenbaum, 1988) and among those with disorders, a history of maltreatment predicts greater severity, worse prognosis, and multiple comorbidities (Nanni, Uher & Danese, 2012; Widom, DuMont & Czaja, 2007). A number of biological mechanisms have been proposed to mediate risk for psychopathology following exposure to maltreatment. Alterations of neural structure and function and other biological changes have been observed in children and adults with a history of maltreatment (Cicchetti, 2015; Cicchetti, Handley & Rogosch, 2015; Hart & Rubia, 2012; Philip et al., 2015; Ridout et al., 2014; Ridout et al., 2015; Toth et al., 2013; Tyrka et al., 2013; Tyrka et al., 2015b). Identifying these mediating factors is critical for understanding how early childhood maltreatment contributes to risk of psychopathology in order to prevent long-term sequelae.
Epigenetic modifications to the genome are of major interest in this effort because they can stably alter gene expression in response to environmental exposures. There is a growing body of literature to support the importance of epigenetic changes in mediating the effects of childhood maltreatment on psychopathology and on biological systems involved in the development of psychopathology (Guintivano & Kaminsky, 2014; Januar, Saffery & Ryan, 2015; Lutz et al., 2015). Epigenetic modifications alter gene expression but do not change the DNA sequence and thus allow for elaboration of the genome beyond what is determined by the DNA (Szyf, 2015). Accumulating evidence indicates that childhood maltreatment may exert long-lasting effects through epigenetic changes (Lutz & Turecki, 2014; Szyf, 2011; Tyrka et al., 2015a). DNA methylation, involving the addition of a methyl group at sites where a cytosine nucleotide occurs next to a guanine nucleotide (CpG dinucleotides), is thought to be the most stable form of epigenetic alteration (Reik, 2007). Methylation can block the binding of transcription factors or interfere with gene expression through other mechanisms (Klengel et al., 2014; Lee et al., 2011; Yang et al., 2014). Consistent with this, genes that are highly expressed typically have low levels of promoter methylation (Sasaki, de Vega & McGowan, 2013; Wagner et al., 2014; Zhang et al., 2013).
Maltreatment and the development of the physiologic stress response
Research examining the biologic underpinnings of the associations between childhood maltreatment and psychopathology highlights the importance of the physiologic stress response system, and in particular, the hypothalamic pituitary adrenal (HPA) axis. In response to stressful stimuli, glucocorticoids are released and exert cellular responses by binding at the intracellular glucocorticoid receptor (GR) (Tyrka et al., 2013). Glucocorticoid receptors are distributed throughout the body and brain where they regulate basal physiologic function and effect changes in various organ systems and tissues that promote adaptive responding to acute stressors (de Kloet, Joels & Holsboer, 2005; Kadmiel & Cidlowski, 2013). Activation of the GR through cortisol binding also involves a negative feedback mechanism that inhibits release of cortisol and prevents damaging effects of chronic activation (Herman et al., 2012; Laryea et al., 2015). Thus, changes in GR number or function can influence the activity of the stress response system and consequently, the biological adaptation to stressful or traumatic experiences.
Studies examining the development of the stress response system reveal that early environment is linked to abnormalities in HPA axis function, with exaggerated or attenuated basal cortisol and stress-induced cortisol concentrations (Carpenter et al., 2007; Carpenter et al., 2009; Dozier et al., 2006; Elzinga et al., 2008; Gonzalez, 2013; Gunnar & Vazquez, 2001; Heim et al., 2001; Heim et al., 2000b; Klaassens et al., 2009; McCrory, De Brito & Viding, 2010; Tyrka et al., 2009; Tyrka et al., 2010). Excessive activation of the HPA axis over time or in response to extreme stress may result in down-regulation of this system (Fries et al., 2005; Heim, Ehlert & Hellhammer, 2000a; McEwen, 2007; Pryce et al., 2005; Tyrka et al., 2008). For example, in comparison with children who were not maltreated, maltreated children have greater variance in cortisol levels at baseline, and those with high baseline cortisol levels are more likely than nonmaltreated children to have blunted cortisol levels over time (Doom, Cicchetti & Rogosch, 2014). Several studies of adults with a history of early life stress show attenuated cortisol response to stress (Carpenter et al., 2007; Carpenter et al., 2011; Carpenter et al., 2009; Elzinga et al., 2008; Klaassens et al., 2009), suggesting that the physiologic effects of maltreatment are long lasting.
The HPA axis and risk for psychopathology and other medical conditions
Converging lines of evidence indicate that excessive exposure to glucocorticoid activity may be involved in the pathogenesis of depressive and anxiety disorders. Stimulation of the HPA system through chronic glucocorticoid treatment produces anxiety- and depressive-like behaviors and causes dendritic atrophy, pyramidal cell loss, and suppression of hippocampal neurogenesis (Cameron & Gould, 1994; Dachir et al., 1993; Gould, Woolley & McEwen, 1991; Magarinos, Orchinik & McEwen, 1998; Watanabe, Gould & McEwen, 1992; Woolley, Gould & McEwen, 1990). Altered cortisol responses are associated with internalizing behaviors (Beauchaine, Crowell & Hsiao, 2015; Hartman et al., 2013), externalizing behaviors (Hartman et al., 2013; Marsman et al., 2008), suicidal ideation (Beauchaine et al., 2015), and post-traumatic stress disorder (PTSD) (de Kloet et al., 2008; de Kloet et al., 2006; Golier et al., 2014), and depression (Ciufolini et al., 2014; Colich et al., 2015) (de Kloet et al., 2008; Suzuki et al., 2013). The HPA axis directs the secretion of glucocorticoids, which potentiate systemic inflammation, oxidative stress, and advance cellular aging; factors that may underlie the association of stress, psychopathology, and somatic conditions (Bauer, Jeckel & Luz, 2009; Wolkowitz et al., 2010). Evidence suggests that children with certain genetic alleles that alter the function of the HPA axis may be at a higher risk of developing psychopathology after experiencing maltreatment and that childhood maltreatment exerts its effects on HPA axis function through epigenetic modifications of key regulatory genes (Klengel & Binder, 2015a; Nugent et al., 2011; Turecki & Meaney, 2014; Tyrka et al., 2012).
Genetic and epigenetic factors influencing the HPA axis
There are multiple levels of regulation and control that can help determine the function of the HPA axis in response to stress (Booij et al., 2013; Stankiewicz, Swiergiel & Lisowski, 2013). These include genetic variants such as single nucleotide polymorphisms (SNPs) that influence levels of gene expression as well as epigenetic regulation of HPA axis-involved genes (Booij et al., 2013; Gillespie et al., 2009; Lutz & Turecki, 2014; Stankiewicz et al., 2013). For example, the GR is regulated by a number of mechanisms allowing for tissue-specific control of its expression and adaptation to stress exposure (Binder, 2009; Lutz et al., 2015; Spijker & van Rossum, 2012; Vandevyver, Dejager & Libert, 2014), altering the feedback on and function of the HPA axis. Early environmental experiences are associated with epigenetic changes to the promoter region of the GR gene, NR3C1, that influence GR expression and potentially alter HPA-axis set points and responses to stress (Turecki & Meaney, 2014; van der Knaap et al., 2015a). NR3C1 methylation is associated with a greater risk of internalizing symptoms and disorders (Radtke et al., 2015; van der Knaap et al., 2015b). Recently, our group reported work with the present sample showing that higher NR3C1 methylation is associated with childhood maltreatment and other adversities in preschool-aged children (Tyrka et al., 2015a).
The role of FKBP5 in GR regulation and the HPA axis
An important regulator of the GR is the FK506 binding protein 51 (FKBP5). In response to stressors, cortisol is released, travels systemically through the blood and moves into the cytoplasm of cells (Kadmiel & Cidlowski, 2013). Cortisol binding to the GR in the cytosol induces translocation of the GR into the nucleus, where it binds to specific regions of the genome called glucocorticoid response elements. The bound GR serves as a transcription factor at cortisol-responsive genes, and thereby activates systems necessary to cope with stressors (Magee et al., 2006). The GR also provides negative feedback to the brain to shut down the HPA stress response. FKBP5 mediates an additional negative feedback loop on glucocorticoids. GR activation results in rapid induction of FKBP5 which binds to the GR and decreases its ability to bind cortisol and to translocate to the nucleus. Thus, FKBP5 decreases systemic sensitivity to cortisol and reduces GR-mediated negative feedback modulation of the HPA axis (Binder, 2009; Cioffi, Hubler & Scammell, 2011; Schmidt et al., 2015; Tatro et al., 2009).
Genetic variation in the FKBP5 gene confers altered GR function and a poorly regulated neuroendocrine response to stress (Zannas & Binder, 2014). A single nucleotide polymorphism (SNP) in FKBP5 (C to T SNP in intron 2, rs1360780) increases the ability of the GR to bind to the glucocorticoid response elements and induce FKBP5 expression (Zannas & Binder, 2014). This “risk” T allele is associated with GR resistance (Hohne et al., 2015; Ising et al., 2008; Menke et al., 2013) and has been linked with PTSD, depressive and anxiety symptoms and disorders, and suicide (Leszczynska-Rodziewicz et al., 2014; Suzuki et al., 2014; Szczepankiewicz et al., 2014; VanZomeren-Dohm et al., 2015; Zannas & Binder, 2014).
Recent groundbreaking work by Klengel and colleagues (Klengel et al., 2013) examined the rs1360780 SNP and methylation of functional glucocorticoid response elements in FKBP5 in blood in relation to childhood maltreatment. In comparison with controls, adults with a history of childhood trauma had lower levels of methylation in regulatory regions of intron 7 of FKBP5 in those with the rs1360780 risk allele, and this was associated with decreased GR sensitivity. Moreover, glucocorticoid teratment of human hippocampal progenitor cells also induced long-lasting demethylation of this regulatory region (Klengel et al., 2013). One other study assessed FKBP5 methylation and found effects of maltreatment in saliva DNA from children ages 5-14 (Weder et al., 2014), but this involved a 450K methylation array which did include the region studied by Klengel and colleagues. No prior work has examined early childhood, and this may be a sensitive period for the development of these epigenetic effects (Klengel et al., 2013). The aim of the present study was to examine FKBP5 methylation, and the role of the FKBP5 genetic variant, in relation to childhood maltreatment in a sample of impoverished preschool-aged children.
Methods
One-hundred and seventy-four children were included in this study, including sixty-nine with child welfare documentation of moderate-severe maltreatment in the past six months. Children ranged in age from 3 to 5 years (M = 49.87 months; SD = 8.4 months), were racially and ethnically diverse (38 White non-Hispanic, 81 Hispanic, 29 Black, 26 other races), and 84 were male. Most caregivers (n=161) were biological mothers. Thirty-one caregivers had less than a high school degree, 65 completed high school, 61 had some post-secondary education, 16 had a bachelor’s degree, and one did not provide education information. Ninety-eight caregivers were unemployed and 156 of the families qualified for public assistance. No more than one child per family was included.
Procedure
Families with a maltreated child were identified from the local child welfare agency and an emergency maltreatment assessment service via record review. Families of children with no indicated case of maltreatment within the past six months were recruited at a pediatric medical clinic during a well-child visit and at childcare centers. Based on review of available medical records and parent report, children with a chronic medical or neurologic condition, medication use, obesity, and failure-to-thrive were excluded. Those with acute illness or medication use were included no less than 2 weeks following resolution of illness and medication use.
Families completed two home visits and questionnaires between the visits. The first home visit, during which caregivers completed interviews on child stress exposure and a saliva sample for DNA isolation was collected from the children, is the focus of the current report.
Measures
Socioeconomic adversity
Indicators of socioeconomic adversity (parental education ≤ high school degree, parental unemployment, and single parenthood) were summed.
Child maltreatment status
All families consented to examination of child welfare records to determine maltreatment status. Trained research staff coded the records using the System for Coding Subtype and Severity of Maltreatment in Child Protective Records (Barnett, Manly & Cicchetti, 1993). Five maltreatment subtypes and severity scores ranging from 1 (least severe) to 5 (most severe) were derived. Children with a case of moderate to severe levels of maltreatment (score of 3-5) within the last six months were considered as part of the maltreated group (n=69). Eight children had substantiated cases of physical abuse, 13 sexual abuse, 8 physical neglect/failure to provide, 21 physical neglect/lack of supervision, and 47 emotional maltreatment. Three of the maltreated children were removed from the home and were in the care of their maternal grandmother. The comparison group included children who had never had a substantiated case of maltreatment. In addition five children had an episode of moderate maltreatment that occurred at least 18 months prior to participation. Results were consistent whether these children were in the maltreatment or comparison group. Because n=5 is insufficient for a separate analysis, these children were included in the comparison group.
Contextual stress interview
Caregivers completed a semi-structured interview developed in our laboratory to assess the child’s experience of contextual stressors in the past month and in the child’s lifetime. Categories were: death of a caregiver, separation from a caregiver, frequent change of residence or homelessness, inadequate food or clothing, and other events including witnessing neighborhood violence or parental arrest. Each domain was scored positive if at least one episode occurred, and domains were summed for past month and lifetime. Possible scores ranged from 0 (no stressors) to 5 (stressors in all five domains) for each summary scale. Past month contextual stressors ranged from 0 to 3, with mean of 0.64 and SD of 0.84, and lifetime contextual stressors ranged from 0 to 4 with mean of 1.39 and SD of 1.24.
Traumatic life events
The Diagnostic Infant and Preschool Assessment (Scheeringa & Haslett, 2010) interview was conducted with caregivers to assess child experiences of traumatic life events. Interviews were conducted by trained clinical social workers and PhD level psychologists, reviewed in a group supervision format, and scored based upon group consensus. The number of types of traumas experienced in the child’s lifetime was summed. Categories were: experiencing an accident, animal attack, man-made disaster, natural disaster, witnessing violence, accidental burning, medical emergency/hospitalization/invasive medical procedure, kidnapping, and other events such as a near drowning. Physical and sexual abuse were not included in this variable because they were assessed as maltreatment (above). Possible scores ranged from 0 to 9, and in the present sample ranged from 0 to 4 with mean of 0.93 and SD of 1.03.
Adversity Composite
The number of types of maltreatment experienced, the number of lifetime contextual stressors, and the number of other traumatic life events were summed to create an adversity composite. Possible scores ranged from 0 to 18, and in the sample ranged from 0 to 9 with a mean of 2.93 and a SD of 2.47.
Genotyping
Saliva samples were obtained using the Oragene DISCOVER kits (OGR-575) for Assisted Collections (DNA Genotek, Kanata, Ontario, Canada), and DNA was isolated following the manufacturer’s instructions. Samples were genotyped for the SNP rs1360780 through an allelic discrimination assay using predesigned Taqman primers (part #C_8852038_10, Life Technologies) and Taqman universal master mix (Life Technologies) via established protocols as directed by the manufacturer on a Bio-Rad CFX connect. Genotype data were available for 156 children for this report.
FKBP5 and NR3C1 Methylation
Sodium bisulfite modification was performed with 500 ng of DNA using the EZ DNA methylation Kit (Zymo Research, Irvine, CA, USA). PyroMark Assay Design software version 2.0.1.15 (Qiagen) was used to design the pyrosequencing assays. The PyroMark (Qiagen) PCR kit and forward and reverse primers were used to amplify FKBP5 intron 7 and regions of the NR3C1 promoter, and PCR products were sequenced using a PyroMark MD system (Qiagen) as previously described (Paquette et al., 2014; Tyrka et al., 2015a). Percent DNA methylation at each CpG locus was quantified with the PyroMark CpG software, version 1.0.11 (Qiagen). All procedures were performed following manufacturer’s protocols. Each sequencing run contained no template and genomic DNA negative controls, as well as methylated and unmethylated controls (Epitect). A bisulfite conversion control within the assay sequence was used to assess conversion efficiency. PCR, sequencing primers (Integrated DNA Technologies, Inc, Coralville, IA) and pyrosequencing assay sequences are available from the corresponding author upon request.
Our main focus was on FKBP5, but because we previously found effects of maltreatment on NR3C1 methylation, we also examined the association of NR3C1 and FKBP5 methylation. The percent of alleles that were methylated was used in statistical analyses. Consistent with other studies on NR3C1 methylation in a variety of cell types, methylation levels were low for NR3C1. For CpG sites in NR3C1 region 1D, percent methylation ranged from 0 to 7.02 with mean of 1.10 and SD of 0.19 across the whole region. Region 1F methylation varied from 0 to 6.42. The mean and SD of the whole region was 1.47 and 0.26. For 1H, the range of methylation was 0 to 7.11, and the mean across the region was 1.24 and SD of 0.20. For FKBP5, two CpGs in intron 7 (Chr 6: 35558488, CpG 1 and 35558514, CpG2) were studied based on findings of Klengel and colleagues (Klengel et al., 2013); percent methylation ranged from 79.00 to 98.00 with mean of 88.02 and SD of 2.76 for CpG 1 and mean of 88.61 and SD of 3.73 for CpG 2.
Results
Preliminary Analyses
Child age, race, sex, and socioeconomic adversity were not associated with FKBP5 methylation at CpG 1 or CpG 2. Child age, race, and socioeconomic adversity were not associated with NR3C1 methylation, but males had greater mean methylation at 1F than females (p =.006), so child sex was included in models testing associations with NR3C1 methylation. Child age, sex, and race did not differ according to FKBP5 genotype.
FKBP5 Methylation in Association with Childhood Maltreatment and Other Adversities
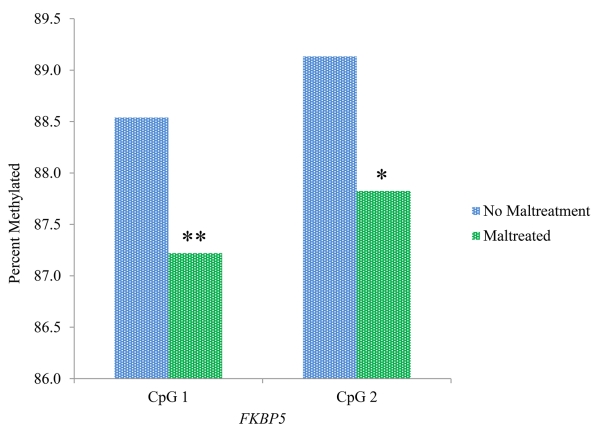
As illustrated in Figure 1, maltreated children had lower levels of FKBP5 methylation at CpG 1 and CpG 2 (t = 3.16, p = .002 and t = 2.29, p = .023, respectively).
Figure 1.
Association of child maltreatment with methylation of FKBP5
Note. ** p <.005; * p < .05.
With respect to other forms of adversity, there was a trend level association of lifetime contextual stress and methylation of FKBP5 at CpG 1 (r = −.14, p = .064) but not CpG 2 (p = .54). In contrast, past month contextual stress and the number of traumatic life events were not associated with FKBP5 methylation at either CpG site.
The adversity composite was negatively associated with methylation of FKBP5 at CpG 1 (r = −.17, p = .026) but not CpG 2 (p = .36).
FKBP5 Methylation and NR3C1 Methylation
Methylation of FKBP5 at CpG 1 was negatively associated with mean methylation of NR3C1 at Exon 1F (r = −.17, p = .023), but not exons 1D or 1H. This association remained after controlling for maltreatment (p = .04) or the adversity composite (p = .051). No significant associations were observed between methylation of FKBP5 at CpG 2 and methylation of NR3C1 at any of the alternate first exons. The associations of the adversity variables and FKBP5 methylation described above were consistent when methylation of NR3C1 at exons 1D, 1F, and 1H were covaried in the models.
Associations of FKBP5 Genotype, Adversity, and FKBP5 Methylation
Seventy-seven children were homozygotes for the C allele, 68 were CT heterozygotes, and 11 were homozygotes for the T allele. The minor allele frequency (MAF) was .29 for the sample, and the distribution conformed to the Hardy-Weinberg equilibrium (p = .44). Our overall sample MAF was not statistically different from the National Center for Biotechnology Information (NCBI) SNP database (dbSNP)-reported MAF of .33 (p = .44). Sample age, sex, and race did not differ according to genotype. Calculated MAF for males and females were .34 and .27 respectively; the distribution conformed to the Hardy-Weinberg equilibrium (p = .65 and .35, respectively). Calculated MAFs for Whites, Blacks and Hispanics were .3, .29, and .28 respectively; the distribution conformed to the Hardy-Weinberg equilibrium (p = .36, .23, and .40 respectively). These did not significantly differ from the MAF reported in the NCBI dbSNP for these populations of .21, .40, and .23 respectively (p = .36, .23, and .40, respectively).
There was no main effect of FKBP5 genotype on methylation of FKBP5. Child maltreatment status, past month contextual stress, and the number of traumatic life events did not moderate links between FKBP5 genotype and methylation of FKBP5. There was a trend-level interaction of FKBP5 genotype and lifetime contextual stress; Children with no lifetime contextual stress with the protective CC genotype had the highest levels of FKBP5 CpG 1 methylation, but this did not reach significance (F(1,152) = 3.07, p = .082). The interaction of lifetime contextual stress and FKBP5 genotype was not associated with methylation of CpG 2 (p = .321).
Discussion
The central finding of this study is that childhood maltreatment was associated with lower levels of methylation of FKBP5 at two sites within a regulatory region in intron 7 of this gene. These sites were found by Klengel and colleagues (Klengel et al., 2013) to exhibit lower levels of methylation in adults with a history of childhood adversity, with a moderating effect of rs1360870 variation. FKBP5 regulates GR sensitivity and function, so FKBP5 demethylation may be a mechanism of the effect of maltreatment on HPA axis dysfunction and associated risk for psychiatric and other stress-associated conditions.
FKBP5 plays a dynamic role in the regulation of the HPA axis. Prolonged glucocorticoid exposure increases FKBP5 levels, which in turn limit the activation and translocation of GRs, creating a negative feedback loop that ultimately downregulates FKBP5 activation (Klengel & Binder, 2015b; Klengel et al., 2013; Lee et al., 2011). Methylation of FKBP5 decreases the induction of FKBP5 that occurs following glucocorticoid binding to GR, and demethylation can be induced by prolonged glucocorticoid exposure (Klengel et al., 2013; Lee et al., 2011). Thus, demethylation at glucocorticoid response elements in FKBP5 allows for another level of feedback-loop control in response to glucocorticoids. Our finding in the present study that methylation of FKBP5 and NR3C1 1F were negatively correlated with each other and were associated with maltreatment in different directions suggests that both of these negative regulators of HPA axis activation are at work. These epigenetic changes may serve to establish a steady-state level and counteract elevated levels of glucocorticoids (Lee et al., 2011).
Converging lines of evidence indicate that methylation of glucocorticoid response elements in FKBP5 in DNA from the periphery may have functional effects in the body and may reflect changes to GR sensitivity in the brain. Treatment with glucocorticoids was associated with lower levels of methylation of a key regulatory locus in Fkbp5 in blood in mice, which in turn was associated with important physiological changes in glucocorticoid target tissues, including atrophy of the thymus, spleen, and the adrenal glands (Lee et al., 2011). Although epigenetic modifications to DNA are often tissue-specific, glucocorticoid-induced demethyation of regulatory regions of Fkbp5 in blood was associated with anxiety-like behavior (Lee et al., 2011) and levels of Fkbp5 methylation in blood were significantly correlated with both methylation and gene expression in hippocampus (Ewald et al., 2014). Demethylation of the regulatory region examined in the present study was also shown in a human hippocampal progenitor cell line following glucocorticoid exposure (Klengel et al., 2013). Taken together, these findings suggest that studies using peripheral markers in humans may have direct implications for brain function. It is important to note that we utilized saliva DNA, which may originate from a combination of blood leukocytes (Endler et al., 1999) (Thiede et al., 2000) and buccal epithelial cells. Some evidence indicates that saliva DNA may actually more closely reflect methylation patterns in the brain than DNA from leukocytes (Smith et al., 2015).
This system is complex, and the literature regarding the functional and dynamic effects on systemic cortisol levels and psychopathology remains at an early stage. There is evidence that lower levels of FKBP5 methylation are associated with higher FKBP5 expression (Lee et al., 2011; Sarapas et al., 2011), and reduced GR sensitivity (Klengel et al., 2013). FKBP5 expression is positively associated with cortisol levels (Lee et al., 2011; Sarapas et al., 2011). However, early stress and PTSD are often associated with low levels of cortisol (Morris, Compas & Garber, 2012; Tyrka et al., 2013) and increased sensitivity of the GR has been shown in some studies (Rohleder et al., 2004; Daskalakis, Lehrner & Yehuda, 2013). There is also evidence of lower FKBP5 expression in PTSD (Yehuda et al., 2009). One report indicated that the FKBP5 risk allele that confers higher FKBP5 expression is linked to blunted cortisol response to dexamethasone challenge in adults over age 50, consistent with elevated GR sensitivity (Fujii et al., 2014). Thus, it is not clear how findings of lower methylation and reduced GR sensitivity are related to some of these other findings, and more work is needed to clarify the role of FKBP5 in regulating glucocorticoid signaling in the wake of childhood maltreatment.
Most of the families in our sample were impoverished and exposed to a variety of contextual stressors, though we did not observe significant effects with our measure of socioeconomic adversity, and we only saw trend-level effects of contextual stressors. There may be effects of early-life or in utero exposures that we did not measure. It is also possible that undocumented cases of maltreatment, such as neglect, may have occurred in the non-maltreated group and this would have diminished the magnitude of the effect. Moreover, maltreatment characteristics, such as type, timing of onset, and chronicity may influence DNA methylation patterns and this should be examined in future studies.
In summary, this study shows that maltreatment in preschool-aged children is associated with lower levels of methylation of a functional glucocorticoid response element of FKBP5. Methylation of this region decreases the induction of FKBP5 that occurs when the GR is activated by glucocorticoids. Future work should examine associations of diurnal and provoked cortisol concentrations in relation to FKBP5 and NR3C1 methylation in maltreated children. Lower levels of methylation of FKBP5 and associated increases in FKBP5 induction by activated glucocorticoid receptor may explain some of the neuroendocrine dysfunction and associated psychiatric and medical morbidity that result from childhood maltreatment.
Acknowledgments
This research was supported by the National Institute of Mental Health through grant numbers R01 MH083704 and R25 MH101076. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIMH. We thank the families who participated, and the Rhode Island Department of Children, Youth, and Families, Hasbro Children’s Hospital, and Rhode Island Head Start for assisting in recruitment of study participants.
References
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Ablex Publishing Corp.; Norwood, NJ: 1993. pp. 7–73. [Google Scholar]
- Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. Annals of the New York Academy of Sciences. 2009;1153:139–52. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Crowell SE, Hsiao RC. Post-dexamethasone cortisol, self-inflicted injury, and suicidal ideation among depressed adolescent girls. Journal of Abnormal Child Psychology. 2015;43(4):619–32. doi: 10.1007/s10802-014-9933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Booij L, Wang D, Levesque ML, Tremblay RE, Szyf M. Looking beyond the DNA sequence: the relevance of DNA methylation processes for the stress-diathesis model of depression. Philososophical Transactions of the Royal Society of London. Series B, Biological Sciencesi. 2013;368(1615):20120251. doi: 10.1098/rstb.2012.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Bosson-Heenan J, Guyer AE, Horwitz SM. Are infant-toddler social-emotional and behavioral problems transient? Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(7):849–58. doi: 10.1097/01.chi.0000220849.48650.59. [DOI] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. The American Journal of Psychiatry. 1991;148(1):55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Bryer JB, Nelson BA, Miller JB, Krol PA. Childhood sexual and physical abuse as factors in adult psychiatric illness. The American Journal of Psychiatry. 1987;144(11):1426–30. doi: 10.1176/ajp.144.11.1426. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61(2):203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214(1):367–75. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biological Psychiatry. 2009;66(1):69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. The Journal fo Nervous and Mental Disease. 2013;201(12):1007–20. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Neural plasticity, sensitive periods, and psychopathology. Development and Psychopathology. 2015;27(2):319–20. doi: 10.1017/S0954579415000012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Handley ED, Rogosch FA. Child maltreatment, inflammation, and internalizing symptoms: Investigating the roles of C-reactive protein, gene variation, and neuroendocrine regulation. Development and Psychopathology. 2015;27(2):553–66. doi: 10.1017/S0954579415000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi DL, Hubler TR, Scammell JG. Organization and function of the FKBP52 and FKBP51 genes. Current Opinion in Pharmacology. 2011;11(4):308–13. doi: 10.1016/j.coph.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciufolini S, Dazzan P, Kempton MJ, Pariante C, Mondelli V. HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies. Neuroscience and Biobehavioral Reviews. 2014;47:359–68. doi: 10.1016/j.neubiorev.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Colich NL, Kircanski K, Foland-Ross LC, Gotlib IH. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. 2015;55:94–101. doi: 10.1016/j.psyneuen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behavioral and Neural Biology. 1993;60(2):103–9. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinology and Metabolism Clinics. 2013;42(3):503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- de Kloet C, Vermetten E, Lentjes E, Geuze E, van Pelt J, Manuel R, Heijnen C, Westenberg H. Differences in the response to the combined DEX-CRH test between PTSD patients with and without co-morbid depressive disorder. Psychoneuroendocrinology. 2008;33(3):313–20. doi: 10.1016/j.psyneuen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research. 2006;40(6):550–67. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews: Neuroscience. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA. Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(11):1206–15. doi: 10.1016/j.jaac.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children’s diurnal production of cortisol: an exploratory study. Child Maltreatment. 2006;11(2):189–97. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–37. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Endler G, Greinix H, Winkler K, Mitterbauer G, Mannhalter C. Genetic fingerprinting in mouthwashes of patients after allogeneic bone marrow transplantation. Bone Marrow Transplantation. 1999;24(1):95–8. doi: 10.1038/sj.bmt.1701815. [DOI] [PubMed] [Google Scholar]
- Ewald ER, Wand GS, Seifuddin F, Yang X, Tamashiro KL, Potash JB, Zandi P, Lee RS. Alterations in DNA methylation of Fkbp5 as a determinant of blood-brain correlation of glucocorticoid exposure. Psychoneuroendocrinology. 2014;44:112–22. doi: 10.1016/j.psyneuen.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–6. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Fujii T, Hori H, Ota M, Hattori K, Teraishi T, Sasayama D, Yamamoto N, Higuchi T, Kunugi H. Effect of the common functional FKBP5 variant (rs1360780) on the hypothalamic-pituitary-adrenal axis and peripheral blood gene expression. Psychoneuroendocrinology. 2014;42:89–97. doi: 10.1016/j.psyneuen.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26(11):984–92. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golier JA, Caramanica K, Makotkine I, Sher L, Yehuda R. Cortisol response to cosyntropin administration in military veterans with or without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:151–8. doi: 10.1016/j.psyneuen.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. The impact of childhood maltreatment on biological systems: Implications for clinical interventions. Paediatrics & Child Health. 2013;18(8):415–8. [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. Journal of Comparative Neurology. 1991;313(3):479–85. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Guintivano J, Kaminsky ZA. Role of epigenetic factors in the development of mental illness throughout life. Neuroscience Research. 2014 doi: 10.1016/j.neures.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, Ormel J. Self- or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: a systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypothesis. Biological Psychology. 2013;94(1):175–84. doi: 10.1016/j.biopsycho.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000a;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. The American Journal of Psychiatry. 2001;158(4):575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000b;284(5):592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian Journal of Medical and Biological Research. 2012;45(4):292–8. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman LJ, Jaycox LH, Setodji CM, Kofner A, Schultz D, Barnes-Proby D, Harris R. How much does “how much” matter? Assessing the relationship between children’s lifetime exposure to violence and trauma symptoms, behavior problems, and parenting stress. Journal of Interpersonal Violence. 2013;28(6):1338–62. doi: 10.1177/0886260512468239. [DOI] [PubMed] [Google Scholar]
- Hohne N, Poidinger M, Merz F, Pfister H, Bruckl T, Zimmermann P, Uhr M, Holsboer F, Ising M. FKBP5 genotype-dependent DNA methylation and mRNA regulation after psychosocial stress in remitted depression and healthy controls. The International Journal of Neuropsychopharmacology. 2015;18(4) doi: 10.1093/ijnp/pyu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. The European Journal Neuroscience. 2008;28(2):389–98. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Januar V, Saffery R, Ryan J. Epigenetics and depressive disorders: a review of current progress and future directions. International Journal of Epidemiology. 2015 doi: 10.1093/ije/dyu273. [DOI] [PubMed] [Google Scholar]
- Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences. 2013;34(9):518–30. doi: 10.1016/j.tips.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. The American Journal of Psychiatry. 1993;150(8):1139–48. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Cicchetti D, Rogosch FA. A longitudinal study of emotion regulation, emotion lability-negativity, and internalizing symptomatology in maltreated and nonmaltreated children. Child Development. 2013;84(2):512–27. doi: 10.1111/j.1467-8624.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuropsychopharmacology & Biological Psychiatry. 2009;33(5):889–94. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Klengel T, Binder EB. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron. 2015a;86(6):1343–57. doi: 10.1016/j.neuron.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Klengel T, Binder EB. FKBP5 allele-specific epigenetic modification in gene by environment interaction. Neuropsychopharmacology. 2015b;40(1):244–6. doi: 10.1038/npp.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–32. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Laryea G, Muglia L, Arnett M, Muglia LJ. Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice. Frontiers in Neuroendocrinology. 2015;36:150–64. doi: 10.1016/j.yfrne.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Huo Y, Rongione M, Potash JB, Wand GS. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacology (Berl) 2011;218(1):303–12. doi: 10.1007/s00213-011-2307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynska-Rodziewicz A, Szczepankiewicz A, Narozna B, Skibinska M, Pawlak J, Dmitrzak-Weglarz M, Hauser J. Possible association between haplotypes of the FKBP5 gene and suicidal bipolar disorder, but not with melancholic depression and psychotic features, in the course of bipolar disorder. Neuropsychiatric Disease and Treatment. 2014;10:243–8. doi: 10.2147/NDT.S54538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Hoberman HM, Rosenbaum M. A prospective study of risk factors for unipolar depression. Journal of Abnormal Psychology. 1988;97(3):251–64. doi: 10.1037//0021-843x.97.3.251. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Almeida D, Fiori LM, Turecki G. Childhood maltreatment and stress-related psychopathology: the epigenetic memory hypothesis. Current Pharmaceutical Design. 2015;21(11):1413–7. doi: 10.2174/1381612821666150105124928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Turecki G. DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience. 2014;264:142–56. doi: 10.1016/j.neuroscience.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Research. 1998;809(2):314–8. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147(1):590–8. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- Marsman R, Swinkels SH, Rosmalen JG, Oldehinkel AJ, Ormel J, Buitelaar JK. HPA-axis activity and externalizing behavior problems in early adolescents from the general population: the role of comorbidity and gender The TRAILS study. Psychoneuroendocrinology. 2008;33(6):789–98. doi: 10.1016/j.psyneuen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51(10):1079–95. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Menke A, Klengel T, Rubel J, Bruckl T, Pfister H, Lucae S, Uhr M, Holsboer F, Binder EB. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes, Brain, and Behavior. 2013;12(3):289–96. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- Mesman J, Koot HM. Early preschool predictors of preadolescent internalizing and externalizing DSM-IV diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(9):1029–36. doi: 10.1097/00004583-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Mills R, Scott J, Alati R, O’Callaghan M, Najman JM, Strathearn L. Child maltreatment and adolescent mental health problems in a large birth cohort. Child Abuse & Neglect. 2013;37(5):292–302. doi: 10.1016/j.chiabu.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clinical Psychology Review. 2012;32(4):301–15. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. The American Journal of Psychiatry. 2012;169(2):141–51. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl) 2011;214(1):175–96. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lester BM, Koestler DC, Lesseur C, Armstrong DA, Marsit CJ. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PLoS One. 2014;9(8):e104913. doi: 10.1371/journal.pone.0104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Carpenter SL, Albright SE, Price LH, Carpenter LL. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging and Behavior. 2015 doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):649–74. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Radtke KM, Schauer M, Gunter HM, Ruf-Leuschner M, Sill J, Meyer A, Elbert T. Epigenetic modifications of the glucocorticoid receptor gene are associated with the vulnerability to psychopathology in childhood maltreatment. Translational Psychiatry. 2015;5:e571. doi: 10.1038/tp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–32. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Ridout KK, Parade SH, Seifer R, Price LH, Gelernter J, Feliz P, Tyrka AR. Interleukin 1B gene (IL1B) variation and internalizing symptoms in maltreated preschoolers. Development and Psychopathology. 2014;26(4 Pt 2):1277–87. doi: 10.1017/S0954579414001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout SJ, Ridout KK, Kao HT, Carpenter LL, Philip NS, Tyrka AR, Price LH. Telomeres, early-life stress and mental illness. Advances in Psychosomatic Medicine. 2015;34:92–108. doi: 10.1159/000369088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biological Psychiatry. 2004;55(7):745–51. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. The American Journal of Psychiatry. 2003;160(12):2116–21. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Uhr M, Holsboer F, Buxbaum JD, Yehuda R. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Disease Markers. 2011;30(2-3):101–10. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, de Vega WC, McGowan PO. Biological embedding in mental health: an epigenomic perspective. Biochemistry and Cell Biology. 2013;91(1):14–21. doi: 10.1139/bcb-2012-0070. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Haslett N. The reliability and criterion validity of the Diagnostic Infant and Preschool Assessment: a new diagnostic instrument for young children. Child Psychiatry and Human Development. 2010;41(3):299–312. doi: 10.1007/s10578-009-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U, Buell DR, Ionescu IA, Gassen NC, Holsboer F, Cox MB, Novak B, Huber C, Hartmann J, Schmidt MV, Touma C, Rein T, Herrmann L. A role for synapsin in FKBP51 modulation of stress responsiveness: Convergent evidence from animal and human studies. Psychoneuroendocrinology. 2015;52:43–58. doi: 10.1016/j.psyneuen.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, Ressler KJ, Binder EB. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2015;168B(1):36–44. doi: 10.1002/ajmg.b.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker AT, van Rossum EF. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology. 2012;95(3):179–86. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AM, Swiergiel AH, Lisowski P. Epigenetics of stress adaptations in the brain. Brain Research Bulletin. 2013;98:76–92. doi: 10.1016/j.brainresbull.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Matsumoto Y, Sadahiro R, Enokido M, Goto K, Otani K. Relationship of the FKBP5 C/T polymorphism with dysfunctional attitudes predisposing to depression. Comprehensive Psychiatry. 2014;55(6):1422–5. doi: 10.1016/j.comppsych.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Belden AC, Spitznagel E, Dietrich R, Luby JL. Blunted stress cortisol reactivity and failure to acclimate to familiar stress in depressed and sub-syndromal children. Psychiatry Research. 2013;210(2):575–83. doi: 10.1016/j.psychres.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepankiewicz A, Leszczynska-Rodziewicz A, Pawlak J, Narozna B, Rajewska-Rager A, Wilkosc M, Zaremba D, Maciukiewicz M, Twarowska-Hauser J. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. Journal of Affective Disorders. 2014;164:33–7. doi: 10.1016/j.jad.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Szyf M. The early life social environment and DNA methylation: DNA methylation mediating the long-term impact of social environments early in life. Epigenetics: Offical Journal of the DNA Methylation Society. 2011;6(8):971–8. doi: 10.4161/epi.6.8.16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Molecular Medicine. 2015;21(2):134–44. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Everall IP, Kaul M, Achim CL. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: implications for major depressive disorder. Brain Research. 2009;1286:1–12. doi: 10.1016/j.brainres.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede C, Prange-Krex G, Freiberg-Richter J, Bornhauser M, Ehninger G. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone Marrow Transplantation. 2000;25(5):575–7. doi: 10.1038/sj.bmt.1702170. [DOI] [PubMed] [Google Scholar]
- Toth SL, Gravener-Davis JA, Guild DJ, Cicchetti D. Relational interventions for child maltreatment: past, present, and future perspectives. Development Psychopathology. 2013;25(4 Pt 2):1601–17. doi: 10.1017/S0954579413000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, Meaney MJ. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatrica Scandinavica. 2013;128(6):434–47. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Eslinger NM, Marsit CJ, Lesseur C, Armstrong DA, Philip NS, Josefson B, Seifer R. Methylation of exons 1D, 1F, and 1H of the glucocorticoid receptor gene promoter and exposure to adversity in preschool-aged children. Development and Psychopathology. 2015a;27(2):577–85. doi: 10.1017/S0954579415000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of Mitochondrial DNA Copy Number and Telomere Length with Early Adversity and Psychopathology. Biological Psychiatry. 2015b doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biological Psychiatry. 2009;66(7):681–5. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67(6):531–4. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological Psychiatry. 2008;63(12):1147–54. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap LJ, Oldehinkel AJ, Verhulst FC, van Oort FV, Riese H. Glucocorticoid receptor gene methylation and HPA-axis regulation in adolescents. The TRAILS study. Psychoneuroendocrinology. 2015a;58:46–50. doi: 10.1016/j.psyneuen.2015.04.012. [DOI] [PubMed] [Google Scholar]
- van der Knaap LJ, van Oort FV, Verhulst FC, Oldehinkel AJ, Riese H. Methylation of NR3C1 and SLC6A4 and internalizing problems. The TRAILS study. Journal of Affective Disorders. 2015b;180:97–103. doi: 10.1016/j.jad.2015.03.056. [DOI] [PubMed] [Google Scholar]
- Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocrine Reviews. 2014;35(4):671–93. doi: 10.1210/er.2014-1010. [DOI] [PubMed] [Google Scholar]
- VanZomeren-Dohm AA, Pitula CE, Koss KJ, Thomas K, Gunnar MR. FKBP5 moderation of depressive symptoms in peer victimized, post-institutionalized children. Psychoneuroendocrinology. 2015;51:426–30. doi: 10.1016/j.psyneuen.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JR, Busche S, Ge B, Kwan T, Pastinen T, Blanchette M. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biology. 2014;15(2):R37. doi: 10.1186/gb-2014-15-2-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588(2):341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weder N, Zhang H, Jensen K, Yang BZ, Simen A, Jackowski A, Lipschitz D, Douglas-Palumberi H, Ge M, Perepletchikova F, O’Loughlin K, Hudziak JJ, Gelernter J, Kaufman J. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(4):417–24. e5. doi: 10.1016/j.jaac.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64(1):49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depression and Anxiety. 2010;27(4):327–38. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Research. 1990;531(1-2):225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26(4):577–90. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biological Psychiatry. 2009;66(7):708–11. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes, Brain, and Behavior. 2014;13(1):25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Labonte B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38(1):111–23. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]