Abstract
Context:
C1q/TNF-related protein-9 (CTRP9) is a novel adipokine that has beneficial metabolic and cardiovascular effects in various animal models. Alterations in circulating CTRP9 have also been observed in patients with cardiovascular disease and diabetes, but little is known about the impact of obesity and bariatric surgery on CTRP9 concentrations.
Objective:
The aim of this study was to compare CTRP9 levels in obese and lean subjects and to determine whether circulating CTRP9 levels in morbidly obese patients are altered by bariatric surgery.
Design, Setting, and Participants:
Fifty-nine obese bariatric surgical patients and 62 lean controls were recruited to participate in a cross-sectional study at an academic medical center. The obese patients were further invited to participate in a cohort study, and 21 returned for analysis at 3 and 6 months postsurgery.
Intervention:
Bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) was the intervention for this study.
Main Outcome Measures:
Fasting serum was obtained from all subjects on entry to the study and was analyzed in the core laboratory for hemoglobin A1c, glucose, aspartate aminotransferase, alanine aminotransferase, total cholesterol, high- and low-density lipoprotein cholesterol, and triglycerides; CTRP9, insulin, adiponectin, and leptin were measured by ELISA. Serum from the patients in the cohort study was also analyzed at 3 and 6 months.
Results:
Serum CTRP9 was significantly higher in the obese group compared to the lean group. CTRP9 was associated with obesity, even after controlling for age, gender, and ethnicity. Following bariatric surgery, there was a significant decrease in weight at 3 and 6 months postprocedure, accompanied by decreases in CTRP9, hemoglobin A1c and leptin, and an increase in serum adiponectin.
Conclusions:
CTRP9 levels are elevated in obesity and significantly decrease following weight loss surgery. Our data suggest that CTRP9 may play a compensatory role in obesity, similar to that of insulin, and is down-regulated following weight loss surgery.
The study compared CTRP9 levels in obese and lean subjects, and in morbidly obese patients after bariatric surgery. CTRP9 levels are elevated in obesity and decrease following weight loss surgery.
Obesity remains a major public health problem in the United States, affecting an estimated one-third of adults (1). These individuals are at higher risk of metabolic syndrome, type II diabetes, hyperlipidemia, and heart disease (2). Management of obesity with diet, exercise, and pharmacologic agents has been largely unsuccessful in producing long-term weight loss (3). In contrast, bariatric surgery has achieved sustained weight loss in patients with severe obesity (3) as well as improvement in metabolic parameters (4). However, the mechanism by which this occurs is not well-understood (5).
Research has focused on adipose tissue–derived hormones, or adipokines, and how their role in metabolism and inflammation is altered in obesity (6, 7). Adiponectin, the most widely studied adipokine, is a multifunctional, insulin-sensitizing adipokine known to regulate many aspects of glucose and lipid homeostasis. Circulating adiponectin is reduced in obesity, and increases following bariatric surgery and weight loss (8–10). In contrast, leptin is increased in obesity and decreases with weight loss. We have previously demonstrated that a novel family of secreted plasma proteins, the C1q/TNF-related proteins (CTRP1–15), play important roles in regulating glucose and/or lipid metabolism (11–24). Both adiponectin and CTRPs belong to the larger C1q family and share common structural features. Of the 15 family members, CTRP9 shares the highest amino acid sequence identity (approximately 54%) with adiponectin, and similarly improves glucose metabolism and promotes insulin sensitivity in mouse models (11, 20, 25). CTRP9 is also known to have beneficial cardiovascular effects by causing vascular relaxation (26), modulating vascular smooth muscle cell proliferation (27), and attenuating adverse cardiac remodeling following ischemic injury in mice (28). CTRP9 is also reported to be a protective factor for coronary artery disease (29).
A recent study of CTRP9 in patients with type 2 diabetes showed a positive correlation with insulin resistance and body mass index (BMI). Further, CTRP9 levels were positively associated with increased arterial stiffness, a correlate of atherosclerosis (30). In conjunction with our previous observations that young (8-week-old), leptin-deficient obese (ob/ob) mice have increased CTRP9 expression in adipose tissue, this raises the possibility that elevated CTRP9 levels in obesity and insulin resistance are a compensatory response. To explore this, we investigated whether serum CTRP9 concentrations were altered by obesity and how CTRP9 was related to other metabolic parameters. We also studied the effects of bariatric surgery on circulating CTRP9 in morbidly obese patients.
Research Design and Methods
Study design and participants
A cross-sectional study was conducted from October 2013 to March 2015 at the Johns Hopkins Medical Institutions. One hundred and twenty-one (n = 62 lean and n = 59 obese) subjects were recruited. Lean controls (BMI ≤26 kg/m2) with no history of diabetes or cardiovascular disease were recruited from students and staff at the Johns Hopkins Hospital. Obese patients (BMI >40 kg/m2 or >35 kg/m2 with one or more obesity-associated comorbidities) undergoing bariatric surgery (Roux-en-Y gastric bypass [RYGB] or vertical sleeve gastrectomy) were recruited from the Johns Hopkins Center for Bariatric Surgery. Patients with previous weight loss surgery were excluded.
All 59 bariatric patients were invited to participate in a prospective cohort study. Of these, 21 patients completed both 3- and 6-month follow-up visits. Patients completing the 6-month follow-up were comparable to those who did not.
Participants were verbally briefed about the study and signed written informed consent. All human studies were approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Clinical and laboratory measurements
BMI was calculated as weight/height2 (kg/m2). All blood samples were obtained in the morning following an overnight fast. Blood samples were centrifuged at 3200 × g for 7 minutes and serum was aliquoted and stored at –80°C for subsequent assays. Fasting glucose, aspartate aminotransferase, alanine aminotransferase, cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and hemoglobin A1c (HbA1c) were performed by the Johns Hopkins Pathology Core Laboratory. Serum insulin levels were measured by ELISA (Millipore Human Insulin). Insulin resistance was calculated with the homeostasis model assessment of insulin resistance (HOMA-IR) (31). Human adiponectin was measured by ELISA (AdipoGen). Human CTRP9 was measured by ELISA (USCN Life Science). Leptin was measured by quantikine ELISA (R&D Systems).
Intra-assay coefficients of variation were 3.1 ± 0.1 (leptin), 3.4 ± 0.4 (adiponectin). Interassay coefficients of variation were 4.3 ± 0.9 (leptin) and 4.3 ± 1.2 (adiponectin). For the CTRP9 ELISA, per the manufacturer, intra-assay variation was less than 10% and interassay variation was less than 12%.
Statistical analysis
Continuous variables were normally distributed and are presented as mean +/- standard error of the mean with ranges. Categorical variables are expressed as proportions (percentage). Student's t tests (two-tailed) were used to compare demographic and biochemical variables between the obese and lean groups, and CTRP9 between genders. CTRP9 levels among ethnicities were compared using a one-way ANOVA. Correlations between CTRP9 and continuous variables (BMI, alanine aminotransferase, aspartate aminotransferase, HbA1c, insulin, glucose, HOMA-IR, and cholesterol levels) were analyzed using Pearson's correlations and linear regression. Linear regression analysis was performed for each independent variable with CTRP9. If variables reached statistical significance on univariate analysis, they were included in multivariate analysis with CTRP9 as a dependent variable. Paired t tests were used to analyze differences in patient characteristics and metabolic variables pre- and postbariatric surgery. Linear mixed effects models were fit to capture the effects of weight on CTRP9 taking into account the multiple visits per patient (using the R statistical software package, version 3.2). All other statistical analyses were performed using STATA statistical software 11.0 (StataCorp LP). A P value of <.05 was considered significant.
Results
Patient demographics and baseline clinical characteristics
Our cohort included 121 patients (22 men and 89 women) with a mean age of 39.2 ± 1.0 years (range, 22–66). BMI of the lean group (n = 62) was 22.1 ± 0.3 kg/m2 (range, 17.6–26) and the obese group (n = 59) was 46.2 ± 0.9 kg/m2 (range, 35.6–68.6). The lean group included 43 (69%) Caucasian, 5 (8%) Black, 5 (8%) Hispanic, and 9 (15%) Asian individuals. The obese patient group included 42 (71%) Caucasians, 14 (24%) Blacks, and 3 (5%) Asians. Baseline metabolic parameters were within the normal range for all laboratory testing in the lean group. The baseline characteristics of all study subjects are summarized in Table 1. The obese group showed a significantly higher mean BMI, fasting glucose, HbA1c, insulin, HOMA, alanine aminotransferase, triglyceride, and leptin when compared to the lean control group. HDL cholesterol and adiponectin were significantly lower in the obese group compared to the lean group. We found significantly higher circulating CTRP9 levels in the obese group than the lean controls (115.3 ± 4.7 vs 76.5 ± 2.7 ng/ml, P < .0001). A subset analysis was performed on obese patients with a previous diagnosis of diabetes, hypertension, and hypercholesterolemia. In the obese group, 17 had a diagnosis of type 2 diabetes, 37 had hypertension, and 13 had hypercholesterolemia. There was no difference in CTRP9 levels by metabolic diagnosis, but when we looked at patients with a blood glucose higher than 100 mg/dl, CTRP9 levels were also significantly elevated (P < .001) compared to patients with a blood glucose lower than 100 mg/dl. There was no difference in CTRP9 by ethnicity, but there was a trend for women to have higher CTRP9 levels compared to men (98.8 ng/mL ± 3.8 vs 85.9 ng/mL ± 5.5, P = .07).
Table 1.
Baseline Characteristics of Lean and Obese Study Subjects
| Control Group (n = 62) | Obese Group (n = 59) | P Value | |
|---|---|---|---|
| Sex, female n (%) | 41 (66) | 48 (81) | |
| Age (y) | 37 (22–64) | 41.4 (23–66) | .03 |
| BMI (kg/m2) | 22.1 (17.6–26) | 45.2 (35.6–68.6) | <.0001 |
| AST (IU/liter) | 19.6 (12–47) | 18.6 (7–43) | .46 |
| ALT (IU/liter) | 16.2 (8–42) | 24.4 (7–81) | <.0001 |
| Total cholesterol (mg/dL) | 175.5 (120–258) | 174.7 (112–239) | .87 |
| Triglycerides (mg/dL) | 72.7 (34–165) | 150.3 (40–651) | <.0001 |
| HDL cholesterol (mg/dL) | 67.2 (30–97) | 45.5 (24–78) | <.0001 |
| LDL cholesterol (mg/dL) | 92.2 (16–151) | 100.2 (38–151) | .11 |
| Glucose (mg/dL) | 76.8 (56–106) | 110.2 (75–259) | <.0001 |
| Insulin (uU/mL) | 2.9 (0.1–13.0) | 7.5 (0.4–19.6) | <.0001 |
| HOMA-IR | 0.57 (0.02–3.18) | 1.99 (0.1–5.0) | <.0001 |
| HbA1c (%) | 5.2 (4.5–5.8) | 6.3 (4.7–10.4) | <.0001 |
| Adiponectin (ng/ml) | 15 936 (4622–37 197) | 7415 (3214–17 304) | <.0001 |
| CTRP9 (ng/ml) | 76.5 (22.4–120.5) | 115.3 (60.9–254.5) | <.0001 |
| Leptin (pg/ml) | 10 034 (658–52 585) | 59 156 (1825–163 543) | <.0001 |
Data are means, with range. P values calculated by two-tailed t test. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Association between CTRP9 levels and metabolic parameters
CTRP9 levels were positively correlated with BMI (P < .01), age (P < .01), glucose (P < .01), insulin (P < .01), HOMA-IR (P < .01), HbA1c (P < .01), triglycerides (P < .01), and leptin (P = .01), and inversely correlated with HDL cholesterol (P < .01) and adiponectin (P < .01). As shown in Table 2, univariate analysis confirmed these results. Multiple regression analysis revealed that BMI retained its significance as a predictor of CTRP9 when controlling for age, gender, ethnicity, and all metabolic variables that were significant on univariate analysis. Age, ethnicity, and insulin also retained significance as predictors of CTRP9, whereas the metabolic parameters of triglycerides, HDL cholesterol, glucose, HbA1c, HOMA-IR, adiponectin, and leptin did not retain significance as predictors of CTRP9 when all variables were entered into a multivariate model.
Table 2.
Univariate and Multivariate Analysis of the Relationship Between CTRP9 and Metabolic Parameters
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Sex | 12 895 | .07 | 6642 | .4 |
| Age (y) | 941 | .001 | 789 | <.01 |
| Ethnicity | 1407 | .67 | 6071 | .03 |
| BMI | 1598 | <.001 | 1111 | .01 |
| AST | −719 | .13 | ||
| ALT | 299 | .31 | ||
| Total cholesterol | 165 | .15 | ||
| Triglycerides | 176 | <.001 | 61 | .2 |
| LDL cholesterol | 234 | .05 | ||
| HDL cholesterol | −736 | <.001 | 57 | .8 |
| Fasting glucose | 304 | .001 | 262 | .2 |
| Insulin | 3582 | <.001 | 5939 | .03 |
| HbA1c | 10 779 | .001 | 788 | .9 |
| HOMA-IR | 12 455 | <.001 | −20 244 | .07 |
| Adiponectin | −1.23 | .002 | −0.12 | .7 |
| Leptin | 0.004 | .01 | 0.03 | .6 |
r2 of the multivariate model was 0.48 (P < .01). Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; β, unstandardized coefficient.
Effects of bariatric surgery on serum CTRP9 levels
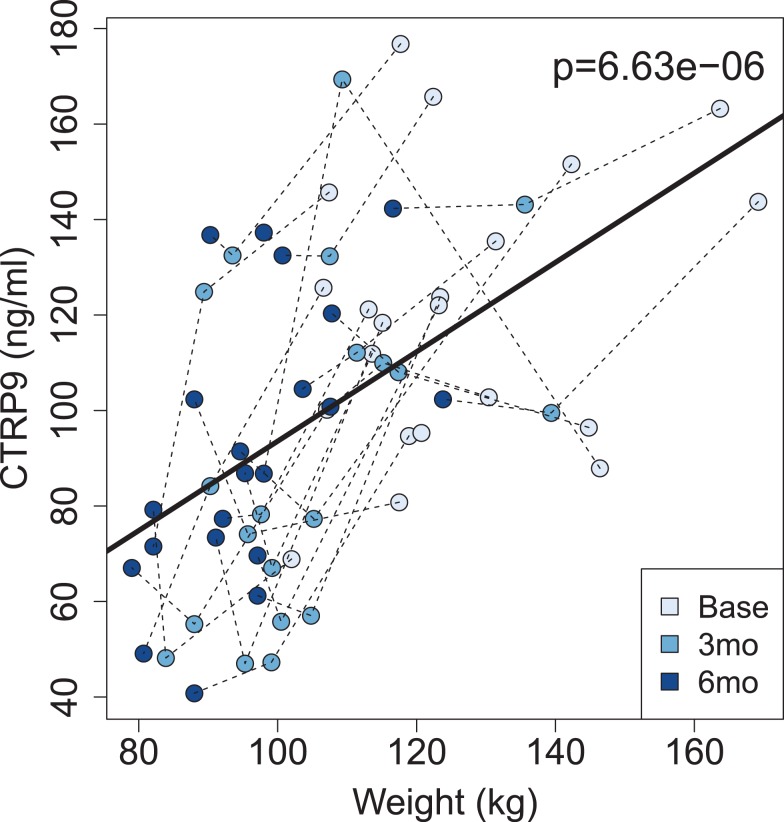
Demographic and baseline metabolic characteristics of the 21 bariatric patients who completed the follow-up at 3 and 6 months after surgery were similar to those of the initial 59 subjects in the cross-sectional study. Of the 21 patients, 3 were men (14%) and 18 were women (86%), with 15 Caucasian (71%), and 6 Black (29%) individuals. Fourteen (67%) patients underwent vertical sleeve gastrectomy and 7 (33%) had RYGB. Table 3 shows the clinical and metabolic data on all patients before and after surgery. There was no significant difference in any variable by surgery type, and thus all patients were analyzed together (Supplemental Table 1). BMI was 44.4 ± 3.9 kg/m2 (range, 37.6–68.6) before bariatric surgery, 35.2 ± 3.2 kg/m2 (29.8–56.5) at 3 months, and 33.9 ± 2.5 kg/m2 (28–50.2) at 6 months after surgery. There was a significant decrease in CTRP9 from baseline (120.6 ng/mL [68–176]) to 3 months (92.3 ng/mL [47–169], P < .01) and 6 months (92 ng/mL [40.8–142], P < .01) postprocedure. There were also significant decreases in weight, BMI, HbA1c, and leptin, and a significant increase in serum adiponectin. Linear mixed model regression demonstrated that weight loss was associated with decreased CTRP9. In most patients (17/21), CTRP9 also decreased with weight loss (Figure 1). There was no association between baseline CTRP9 levels and degree of weight loss after surgery.
Table 3.
Clinical, Hormonal, and Metabolic Features of Patients Before and After Bariatric Surgery
| Variable | Presurgery | 3 Months Postsurgery | Significance | 6 months Postsurgery | Significance |
|---|---|---|---|---|---|
| BMI (kg/m2) | 44.4 (37.6 to − 68.6) | 36.9 (29.8–56.5) | <.0001 | 33.9 (28–50.2) | <.0001 |
| Weight (kg) | 125.6 (102–169.2) | 103.9 (83.9–139.4) | <.0001 | 95.9 (79–123.8) | <.0001 |
| AST (IU/liter) | 18.9 (10–42) | 19.4 (10–56) | .8 | 20.8 (12–77) | .7 |
| ALT (IU/liter) | 27.1 (7–81) | 21.4 (7–70) | .3 | 22.6 (7–133) | .4 |
| Total cholesterol (mg/dl) | 167.4 (129–213) | 157.1 (110–200) | .1 | 165.5 (109–213) | .9 |
| Triglycerides (mg/dl) | 135.3 (40–305) | 108.3 (54–204) | .1 | 104.7 (43–217) | .06 |
| HDL cholesterol (mg/dl) | 50.1 (27–78) | 46.4 (29–90) | .6 | 52.9 (34–98) | .2 |
| LDL cholesterol (mg/dl) | 90.8 (46–140) | 87.6 (32–142) | .7 | 91.0 (28–125) | .9 |
| Glucose (mg/dl) | 108.8 (77–247) | 104.8 (66–199) | .6 | 98.6 (73–181) | .14 |
| Insulin (uU/ml) | 7.4 (0.4–17.9) | 10 (1–30.9) | .2 | 7.4 (0.9–24) | .9 |
| HOMA-IR | 1.98 (0.1–5) | 2.6 (0.4–7.8) | .3 | 1.4 (0.3–3.3) | .12 |
| HbA1c (%) | 6.3 (4.7–9.9) | 5.5 (4.8–6.1) | .6 | 5.4 (4.7–6.3) | .01 |
| Adiponectin (ng/ml) | 6021 (3394–10 202) | 12 739 (7258–26 778) | <.0001 | 16 404 (7623–30 928) | <.0001 |
| CTRP9 (ng/ml) | 120.6 (68–176) | 92.3 (47–169) | .002 | 92 (40.8–142) | <.0001 |
| Leptin (pg/ml) | 84 899 (1826–490 825) | 24 347 (4756–58 356) | .005 | 19 775 (3875–44 729) | .004 |
Data are means, with range. P values calculated by paired t test, in comparison to baseline.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Figure 1.
Scatterplot of circulating CTRP9 levels vs weight at the time of bariatric surgery, and at 3 (3mo) and 6 months (6mo) after surgery.
Discussion
Obesity contributes to a state of chronic low-grade inflammation, with altered production and function of adipokines and cytokines (32). It is well-known that diet-induced obese mice have higher levels of leptin and lower levels of adiponectin (33). Similarly, in humans, we have previously shown that obese patients have higher blood levels of leptin and lower levels of adiponectin compared to nonobese controls (9). In this study, we have demonstrated that the novel adipokine CTRP9 is associated with obesity and falls following bariatric surgery.
Our laboratory has demonstrated that the CTRPs, a novel family of secreted plasma proteins, play important roles in regulating whole-body glucose and/or lipid metabolism (11–24). CTRP9, the closest paralog of adiponectin, regulates glucose metabolism and insulin sensitivity. CTRP9 is predominantly expressed by adipose tissue and is up-regulated in young (8 weeks old), but not old (12 weeks old), leptin-deficient ob/ob mice, a model of severe obesity and diabetes. A modest short-term overexpression of CTRP9 in mice using an adenoviral vector leads to a significant reduction in blood glucose as well as lower insulin levels (17). A chronic long-term overexpression of CTRP9 in transgenic mice resulted in a lean phenotype (11). When challenged with a calorie-dense, high-fat diet, CTRP9 transgenic mice are remarkably resistant to body weight gain, having reduced adiposity, lower fasting glucose levels, and a significant improvement in glucose tolerance compared to wild-type control mice (11). Conversely, targeted deletion of the CTRP9 gene results in insulin resistance and impaired metabolic homeostasis compared to wild-type littermate controls (20). CTRP9-deficient mice also have reduced insulin-stimulated AKT phosphorylation (a metric of insulin signaling) in liver, suggesting that this adipokine is important for optimum hepatic insulin action (20). Taken together, our previous studies using gain- and loss-of-function mouse models indicate that CTRP9 is a beneficial hormone with glucose lowering and insulin sensitizing properties, and its up-regulation likely reflects a compensatory response to severe obesity and insulin resistance.
Here, we have shown that CTRP9 concentrations are significantly higher in obese individuals, suggesting a similar CTRP9 compensatory response in humans. Further, BMI is a predictor of CTRP9, even after controlling for age, gender, and ethnicity. Circulating CTRP9 is also associated with triglycerides, glucose, insulin, and HbA1c, and inversely associated with HDL and adiponectin, all of which is consistent with the typical metabolic abnormalities of obesity and metabolic syndrome.
This is the first study investigating CTRP9 that includes a multiethnic patient cohort, but we did not find any differences between CTRP9 and ethnic background. Our previous studies of 8-week-old C57BL/6 mice showed a sexually dimorphic pattern with female mice expressing higher levels of CTRP9 mRNA relative to male mice (17). This finding has also been demonstrated in humans, with higher CTRP9 concentrations in women compared to men (30), and our cohort showed a similar trend, although this did not reach statistical significance (P = .07). We also observed a significant increase in CTRP9 with increasing age, which remained true when controlling for gender and ethnicity. However, the change in CTRP9 levels in obese patients after bariatric surgery shows a stronger association of CTRP9 with weight than age.
Although we did not see a significant difference in CTRP9 levels between patients with and without type 2 diabetes, CTRP9 levels in patients with impaired fasting blood glucose (>100 mg/dl) were significantly higher (P < .001) than patients with normal blood glucose. Similarly, CTRP9 was positively correlated with insulin resistance, consistent with the findings by Jung et al (30). In contrast, the study by Hwang et al found that CTRP9 concentrations were associated with a favorable glucose and metabolic phenotype (34). The discrepancy in findings may be due to differences in the study population, specifically the degree of obesity and glucose tolerance. The study by Hwang et al evaluated patients with a wide range of glucose tolerance and intolerance, whereas the study by Jung et al looked at patients with longstanding type 2 diabetes. The Hwang et al study investigated patients with a BMI range up to 30 kg/m2, whereas our population included obese patients with BMI > 35 kg/m2.
Our data in bariatric surgery patients demonstrating that CTRP9 levels significantly decrease after weight loss surgery also support the compensatory nature of CTRP9. Bariatric surgery leads to significant weight loss in addition to reducing inflammation (35), insulin resistance, cardiovascular disease, and associated mortality from obesity-associated comorbidities. It is considered the most effective long-term treatment for morbid obesity. Many of the benefits of bariatric surgery seem to occur independently of weight loss (36). For instance, in obese patients with diabetes, up to 80% experience diabetes remission following gastric bypass surgery (4, 36). This remission has been shown to be independent of weight loss, but the mechanism by which this occurs is not well-understood (37). Many mechanisms have been proposed to explain decreased insulin resistance. Some suggest that the increase in adiponectin following bariatric surgery contributes to improved insulin sensitivity, whereas others suggest that the improved inflammatory milieu reduces insulin resistance. Still others have examined the altered gastric anatomy or changes in gut hormones as contributing to the resolution of diabetes. Likely several of these factors are involved in the remission of diabetes and other comorbidities following bariatric surgery. Our study confirms that following bariatric surgery, leptin levels significantly decrease and adiponectin levels significantly increase with weight loss. This is the first study to investigate changes in CTRP9 levels following bariatric surgery and here we have shown that circulating CTRP9 levels significantly decrease following weight loss surgery. This decrease, and the change in metabolic parameters, was not different by surgery type. Additionally, although CTRP9 and adiponectin are structurally very similar, and they can form hetero-oligomers when coexpressed in vitro, the changes in CTRP9 levels after surgery were independent of adiponectin levels (P < .0.001) (17).
Multiple studies have shown early improvements in glycemic control following bariatric surgery. A meta-analysis by Buchwald et al in 2009 showed improvement in glycemic control and remission of diabetes in the first 1–2 years of follow-up after bariatric surgery (38). Other studies have examined diabetes remission several years following bariatric surgery, with current estimates ranging from 37–80% remission rates (39). Although diabetes remission was not a primary outcome in our study, we did note significantly decreased HbA1c values 6 months after surgery in our cohort (P < .01). Further, there were seven morbidly obese patients receiving medical therapy for diabetes before surgery, of which four discontinued all antidiabetic treatment by 6 months following surgery. Diabetes remission seems to occur shortly after surgical weight loss intervention (especially so for patients undergoing RYGB), again raising the possibility of involvement of adipokines and other hormones as drivers of diabetes remission, independent of weight loss.
One of the strengths of our study is that the patient population was demographically diverse and therefore more likely to be generalizable to the general population. Although the number of subjects followed as a cohort was small, the characteristics of these subjects were similar to the entire obese study population. Further, the participants in the cohort study serve as their own controls, thereby allowing us to determine the relative metabolic improvements and changes in adipokines after bariatric surgery. Because the patients were followed at two time points after surgery, this allowed us to confirm that the observed changes in circulating CTRP9 levels persist over time. We also used the same commercially available CTRP9 ELISA assay as several previous studies, thereby increasing the comparability of our results to that reported by other groups.
In conclusion, our study demonstrates that CTRP9 levels are associated with obesity and that bariatric surgery decreases CTRP9 levels in morbidly obese patients. In addition to its association with obesity, CTRP9 levels are also positively associated with metabolic parameters related to the comorbidities of obesity, including insulin resistance, HbA1c, triglyceride, and leptin. For the first time, we have demonstrated that bariatric surgery and weight loss decrease circulating CTRP9 levels, supporting the notion that CTRP9 exhibits a compensatory response in the setting of obesity and insulin resistance. Further work will help pinpoint whether this effect is specific to bariatric surgical patients or to weight loss itself.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK084171), the Pediatric Endocrine Society, and the Endocrine Society.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CTRP
- C1q/TNF-related protein
- RYGB
- Roux-en-Y bypass
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- HbA1c
- hemoglobin A1c
- HOMA
- homeostasis model assessment.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 2. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 3. Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:S89–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schauer PR, Bhatt DL, Kirwan JP, et al. , Investigators S. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med. 2014;370:2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–2311. [DOI] [PubMed] [Google Scholar]
- 6. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. [DOI] [PubMed] [Google Scholar]
- 7. Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. [DOI] [PubMed] [Google Scholar]
- 9. Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower circulating C1q/TNF-related protein-3 (CTRP3) levels are associated with obesity: a cross-sectional study. PLoS One. 2015;10:e0133955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wroblewski E, Swidnicka-Siergiejko A, Hady HR, et al. Variation in blood levels of hormones in obese patients following weight reduction induced by endoscopic and surgical bariatric therapies. Cytokine. 2016;77:56–62. [DOI] [PubMed] [Google Scholar]
- 11. Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2013;305:R522–R533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei Z, Peterson JM, Lei X, et al. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287:10301–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem. 2011;286:15652–15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem. 2013;288:10214–10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seldin MM, Lei X, Tan SY, Stanson KP, Wei Z, Wong GW. Skeletal muscle-derived myonectin activates the mammalian target of rapamycin (mTOR) pathway to suppress autophagy in liver. J Biol Chem. 2013;288:36073–36082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, et al. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab. 2014;306:E779–E790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285:39691–39701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterson JM, Aja S, Wei Z, Wong GW. CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem. 2012;287:1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol. 2013;305:G214–G224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peterson JM, Seldin MM, Tan SY, Wong GW. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS One. 2014;9:e88535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson JM, Wei Z, Wong GW. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem Biophys Res Commun. 2009;388:360–365. [DOI] [PubMed] [Google Scholar]
- 26. Zheng Q, Yuan Y, Yi W, et al. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol. 2011;31:2616–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uemura Y, Shibata R, Ohashi K, et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. [DOI] [PubMed] [Google Scholar]
- 28. Sun Y, Yi W, Yuan Y, et al. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation. 2013;128:S113–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Hang T, Cheng XM, et al. Associations of C1q/TNF-related protein-9 levels in serum and epicardial adipose tissue with coronary atherosclerosis in humans. Biomed Res Int. 2015;2015:971683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jung CH, Lee MJ, Kang YM, et al. Association of serum C1q/TNF-related protein-9 concentration with arterial stiffness in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E2477–E2484. [DOI] [PubMed] [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 32. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hwang YC, Woo Oh S, Park SW, Park CY. Association of serum C1q/TNF-related protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. Int J Obes (Lond). 2014;38:1207–1212. [DOI] [PubMed] [Google Scholar]
- 35. Sams VG, Blackledge C, Wijayatunga N, et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc. In press.. [DOI] [PubMed] [Google Scholar]
- 36. Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery: it's all gastrointestinal factors versus it's all food restriction. Diabetes Care. 2013;36:S287–S291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 38. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. [DOI] [PubMed] [Google Scholar]
- 39. Panunzi S, Carlsson L, De Gaetano A, et al. Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care. 2016;39:166–174. [DOI] [PubMed] [Google Scholar]