Abstract
GH is considered necessary for the proper development and maintenance of several tissues, including the heart. Studies conducted in both GH receptor null and bovine GH transgenic mice have demonstrated specific cardiac structural and functional changes. In each of these mouse lines, however, GH-induced signaling is altered systemically, being decreased in GH receptor null mice and increased in bovine GH transgenic mice. Therefore, to clarify the direct effects GH has on cardiac tissue, we developed a tamoxifen-inducible, cardiac-specific GHR disrupted (iC-GHRKO) mouse line. Cardiac GH receptor was disrupted in 4-month-old iC-GHRKO mice to avoid developmental effects due to perinatal GHR gene disruption. Surprisingly, iC-GHRKO mice showed no difference vs controls in baseline or postdobutamine stress test echocardiography measurements, nor did iC-GHRKO mice show differences in longitudinal systolic blood pressure measurements. Interestingly, iC-GHRKO mice had decreased fat mass and improved insulin sensitivity at 6.5 months of age. By 12.5 months of age, however, iC-GHRKO mice no longer had significant decreases in fat mass and had developed glucose intolerance and insulin resistance. Furthermore, investigation via immunoblot analysis demonstrated that iC-GHRKO mice had appreciably decreased insulin stimulated Akt phosphorylation, specifically in heart and liver, but not in epididymal white adipose tissue. These changes were accompanied by a decrease in circulating IGF-1 levels in 12.5-month-old iC-GHRKO mice. These data indicate that whereas the disruption of cardiomyocyte GH-induced signaling in adult mice does not affect cardiac function, it does play a role in systemic glucose homeostasis, in part through modulation of circulating IGF-1.
Growth hormone (GH) is a principal mediator of growth, development, and metabolism. Traditionally, GH is thought to exert most its metabolic effects on liver, skeletal muscle, and adipose tissue and its growth effects on bone; however, several studies have demonstrated that the myocardium is also a target of GH action. That is, GH and its downstream mediator, IGF-1, have been shown to trigger molecular changes in the heart including the promotion of hypertrophy (1) and the regulation of contractility through ion channel regulation and myosin isoform switching (2–4).
In humans with GH-related pathologies, unique cardiovascular phenotypes are associated with too much or too little GH action. Acromegaly, a disease caused by hypersecretion of GH secondary to a pituitary adenoma, results in several cardiovascular derangements including cardiomyopathy, hypertension, dyslipidemia, and vascular dysfunction (5). The main cause of death in untreated acromegaly is cardiac failure related to biventricular hypertrophy, with resulting diastolic dysfunction and eventual systolic failure (6). On the opposite end of the spectrum is GH deficiency (GHD), which results from a lack of GH production due to a genetic or physical cause such as brain radiation or traumatic brain injury. Patients with GHD often exhibit decreased heart dimensions with thinned ventricular walls, increased systolic wall stress, and decreased exercise tolerance (7). Likewise, patients with Laron syndrome (LS), a disease resulting from mutations in the GH receptor (GHR) gene or downstream intracellular signaling intermediates, exhibit decreased heart size and decreased cardiac function (8, 9). In all of these clinical scenarios, GH/IGF-1-induced signaling is altered systemically, being high in acromegaly and low in GHD and LS. Therefore, determining the aspects of the cardiovascular changes caused specifically by myocardial GH action is difficult because many of the observed changes may be the result of altered GH and/or IGF-1 effects in other tissues.
Studies conducted using bovine GH (bGH) transgenic mice and global GHR null or knockout (GHRKO) mice, which are viewed as models for acromegaly and LS, respectively, have shown a distinct effect of GH status on cardiovascular function. Results of systolic blood pressure (BP) measurements in the short-lived (10) bGH transgenic mice are mixed. Some studies report normal (11–13) and others report increased systolic BP (SBP) (14, 15). Interestingly, we recently demonstrated that the elevation of SBP in bGH mice depends on age (16). Functionally, bGH mice have been shown to have decreased (11) to normal cardiac function (14) and increased fractional shortening of isolated cardiomyocytes (17). However, cardiac hypertrophy and fibrosis are prominent features in bGH mice (11, 12, 16). On the other hand, GHRKO mice are long lived (18) and have demonstrated decreased baseline cardiac function. However, when the results are normalized to body size, GHRKO mice have normal cardiac function (19). Similar to the aforementioned clinical studies, these studies in both bGH and GHRKO mice are not able to differentiate the myocardial effects between GH and/or IGF-1.
To study the direct role of GH on the myocardium in the absence of developmental deficiencies due to lack of GH action in early life, we produced an adult-inducible, cardiac-specific GHRKO mouse line (iC-GHRKO). We report that the loss of GHR-induced signaling, specifically in murine adult cardiomyocytes, does not alter baseline or dobutamine stressed cardiac function in the first year of life. Whereas the loss of cardiac GH action did not affect cardiac weight, function, or dimensions, surprisingly, it did affect body composition and systemic insulin sensitivity with a concomitant decrease in circulating IGF-1 in later life. Interestingly, insulin-stimulated Akt phosphorylation was decreased in the liver and heart of these mice but not in epididymal white adipose tissue. Our results indicate that in adult mice, the cardiac GHR is involved in mediating systemic glucose homeostasis and points to a tissue specific mechanism.
Materials and Methods
Animals
Mice, hemizygous for the myosin heavy chain 6 (Myh6)-driven MerCreMer (MCM) gene (20), were purchased from Jackson Laboratories (number 005657) and crossed with GHR floxed mice (GHRfl/fl) (21) to produce Myh6-MerCreMer+/−/GHR+/fl offspring. These offspring were crossed to produce the experimental mouse line Myh6-MerCreMer+/−/GHRfl/fl, which were maintained through matings, yielding half Myh6-MerCreMer+/−/GHRfl/fl and half GHRfl/fl mice. We refer to tamoxifen-injected Myh6-MerCreMer+/−/GHRfl/fl mice as iC-GHRKO mice (tamoxifen induced, cardiac specific, GHR knockout). All mice were housed up to four mice per cage in a temperature-controlled (23°C) vivarium and exposed to a 14-hour light, 10-hour dark cycle. All mice were allowed ab libitum access to water and food (ProLab RMH 3000; PMI Nutrition International.). All procedures performed with the mice were approved by the Institutional Animal Care and Use Committee at Ohio University and are in accordance with all standards set forth by federal, state, and local authorities.
Tamoxifen induction of Cre recombinase
Tamoxifen (Sigma-Aldrich) was prepared as previously described (22). Briefly, 100 mg tamoxifen free base was dissolved in 500 μL absolute ethanol and then diluted to 10 mL with peanut oil (Fisher). The solution was then sonicated for 30 minutes and subsequently filter sterilized using a vacuum filter (<0.5 μm pore size). Aliquots were stored at −20°C for up to 1 month. All tamoxifen injections began at 4 months of age. Tamoxifen-injected mice received a total dose of 80 mg/kg body weight, administered as two ip injections, one per day for 2 days.
Echocardiography and dobutamine stress test
Mice were shipped to the University of Massachusetts Mouse Phenotyping Core at which M-mode echocardiography was performed on 12.5-month-old iC-GHRKO (n = 8) and control (peanut oil injected Myh6-MerCreMer+/−/GHRfl/fl) littermates (n = 8) using a VisualSonics Vevo2100 imaging system and a 40-Mhz linear transducer collecting data at a rate of 279 frames/sec. Mice were placed on a heating pad to avoid hypothermia and put under light anesthesia and maintained with a minimal amount of isoflurane (1%–2%), while keeping the heart rate greater than 400 bpm. The parasternal short-axis view at the level of the papillary muscles was used to obtain measurements. Three sets of replicate measurements were performed on each mouse and average values were used to calculate ejection fraction (EF), fractional shortening (FS), and left ventricular (LV) mass. A dobutamine stress test was subsequently performed by collecting baseline M-mode measurements, injecting each mouse with dobutamine (1.5 μg/g body weight, ip), waiting 5 minutes, and collecting postadministration M-mode measurements.
Body composition measurements
Body composition was measured in iC-GHRKO (n = 8) and control littermates (n = 24) monthly at 3 and 4.5–12.5 months of age. Measurements were collected using a desktop nuclear magnetic resonance Bruker LF50 Minispec as previously described (23).
Noninvasive BP measurement
SBP was measured monthly from 4.5 to 12.5 months of age in iC-GHRKO (n = 8) and control littermates (n = 24). SBP measurements were made using a noninvasive BP tail-cuff system (number IN125/M; ADInstruments) connected to a PowerLab system (number PL3508; ADInstruments). Training, acclimation procedures, and data collection were done as previously described (16).
Real-time quantitative PCR (RTqPCR)
A two-step RTqPCR was performed using total RNA isolated from samples of heart, kidney, or brain of iC-GHRKO (n = 5) and control (n = 15) mice as previously described (16). All data were analyzed using qBase Plus version 2.4 (Biogazelle), and all RTqPCR assays and analyses were performed in compliance with Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines (24). Please see Supplemental Materials and Methods for specific primer sequences (Supplemental Table 1) and methodology details.
Plasma measurements
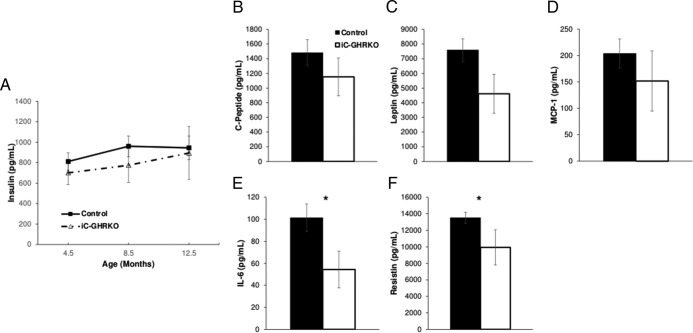
Whole blood was collected from the tail tips of iC-GHRKO (n = 8) and littermate controls (n = 24) after a 12-hour overnight fast by clipping approximately 1 mm of the tail tip and collecting approximately 250 μL of blood using heparinized capillary tubes. Plasma was collected after centrifugation for 10 minutes at 7000 × g at 4°C. Circulating levels of insulin were determined using the mouse insulin ELISA (number 80-INSMS-E01; ALPCO) following the manufacturer's protocol. Levels of circulating IGF-1 were measured using the mouse IGF-1 ELISA (number 22-IG1MS-E01; ALPCO) following the manufacturer's directions. C-peptide, leptin, resistin, monocyte chemoattractant protein 1 (MCP-1), and IL-6 were measured in samples from 12.5-month-old mice using a Milliplex mouse metabolic panel (number MMHMAG-44K; Millipore) according to the manufacturer's instructions.
Intraperitoneal insulin/glucose tolerance testing (ITT/GTT)
For the GTT, iC-GHRKO (n = 8) and littermate controls (n = 24) were fasted for 12 hours prior to measurements. Each mouse received an ip injection of 10% glucose at 0.01 mL/g body weight. For the ITT, iC-GHRKO (n = 8) and control littermates (n = 24) were nonfasted; however, food was not provided during glucose measurements after the insulin injection. Each mouse received an ip injection of 1 U/kg body weight insulin (Humilin R; Lilly). For both the GTT and ITT, blood glucose measurements were collected using OneTouch Ultra (Lifescan) test strips and glucometers before glucose or insulin injection (0 min) and at 20, 40, 60, and 90 minutes (GTT) or 15, 30, 45, 60, and 90 minutes (ITT) after injection.
Immunoblots
For the insulin-induced intracellular signaling intermediate immunoblots, 12.5-month-old iC-GHRKO (n = 3) and oil-injected Myh6-MerCreMer+/−/GHRfl/fl (control) littermates (n = 3) were injected with 1 U/kg body weight insulin (Humulin R; Lilly). Mice were killed 15 minutes after insulin injection. For the signal transducer and activator of transcription 5 (STAT5) phosphorylation immunoblots, 12.5-month-old iC-GHRKO and control mice (n = 2) were injected with recombinant bGH (5 μg/g body weight) and dissected 15 minutes later. Full details regarding the antibodies used can be found in the Supplemental Materials and Methods.
Statistics
All values are reported as mean ± SEM. Statistics were performed using SPSS version 17.0 (IBM). Unless otherwise noted, due to no statistical differences based on an ANOVA analysis with Tukey's honestly significant difference pairwise comparison among control groups, the control mice in all experiments are the combined results from Myh6-MerCreMer+/−/GHRfl/fl mice injected with oil (vehicle) and GHRfl/fl mice injected with either tamoxifen or oil. All time-dependent analyses were performed using a two-way ANOVA with repeated measures, using genotype as the main factor. Maulchy's test of sphericity was performed on repeated-measures data. The Green-House Geisser correction was applied for significant Maulchy's tests. For single time point measurements between two groups, equality of variance was tested using Levene's test, and group means were compared using an independent Student's t test or a Welch's t test for unequal variances. For single time point measurements between more than two groups, the values were analyzed using an ANOVA with orthogonal contrasts for tamoxifen treatment and genotype. For all tests, statistical significance was defined as P < .05.
Results
Validation of GHR gene disruption
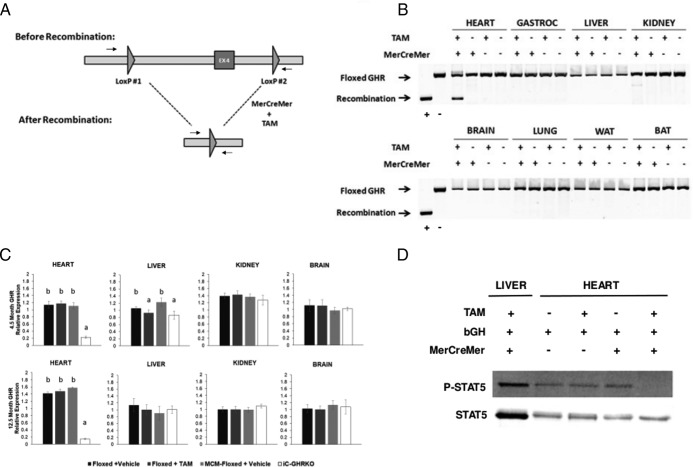
Disruption of the GHR gene in cardiac tissue was accomplished using the inducible MerCreMer-LoxP system (Figure 1A). PCR analysis of genomic DNA from eight tissues revealed that recombination bordering exon 4 of the GHR gene occurred specifically in cardiac tissue in a tamoxifen-dependent manner (Figure 1B). Because the recombination did not appear to be complete at the level of DNA, we performed RTqPCR to quantify GHR RNA transcript levels in heart, liver, kidney, and brain from 4.5-month-old and 12.5-month-old mice. The tamoxifen treatment protocol yielded an approximately 80% decrease in GHR mRNA levels in the heart (Figure 1C, top panel) at 4.5 months of age (2 wk after tamoxifen injection). There did, however, appear to be a (∼10%-20%) change in GHR mRNA levels in the 4.5-month-old liver, which occurred in a tamoxifen-dependent manner. Evaluation of GHR transcript levels at 12.5 months demonstrated an approximately 95% decrease in the GHR mRNA level in the heart and resolution of the tamoxifen-dependent decrease in liver (Figure 1C, bottom panel). To evaluate the disruption of GH-induced signaling, 12-month-old iC-GHRKO and control mice were injected with recombinant bGH, and protein homogenates from the heart and liver (iC-GHRKO) were assayed for STAT5 phosphorylation. Figure 1D demonstrates that mice injected with tamoxifen and coexpressing both the floxed GHR locus and the Myh6-MerCreMer transgene (iC-GHRKO mice) failed to show bGH-induced STAT5 phosphorylation in cardiac tissue. In the liver, however, iC-GHRKO mice responded to the injection of bGH with robust STAT5 phosphorylation.
Figure 1.
Verification of inducible GHR gene disruption. A, Diagram showing exon 4 of GHR flanked by LoxP sites before and after recombination when in the presence of both MerCreMer expression and tamoxifen. Arrows denote primer positions used to distinguish floxed locus from recombined locus. B, PCR results of the floxed GHR exon 4 locus using genomic DNA isolated from eight different tissues (heart, gastrocnemius, liver, kidney, brain, lung, epididymal WAT, and brown adipose tissue) in GHRfl/fl mice without or without MerCreMer expression and with or without tamoxifen injection. C, RTqPCR analysis of GHR in heart, liver, kidney, and whole brain at 4.5 and 12.5 months of age. D, Bovine GH-stimulated STAT5 phosphorylation in 12.5-month-old heart from GHRfl/fl mice without or without MerCreMer expression and with or without tamoxifen injection and in liver from Myh6-MerCreMer+/−/GHRfl/fl (iC-GHRKO) mice. Different letters denote a difference between groups of mice (P < .05). BAT, brown adipose tissue; EX4, exon 4 of GHR; TAM, tamoxifen.
Body composition analysis and tissue weights
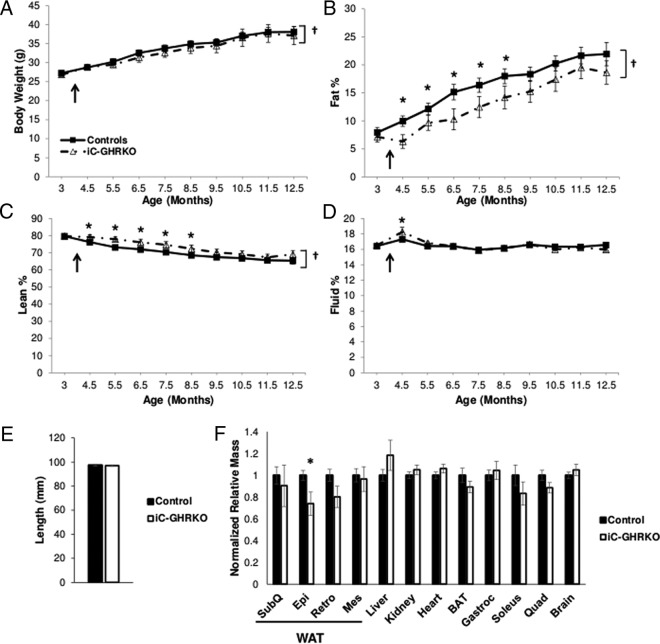
There was a significant effect of age on body weight, fat, and lean percentage in both iC-GHRKO and control mice (P < .05) (Figure 2, A–D). Both the iC-GHRKO and control mice had similar body weights at all time points tested (Figure 2A). Body composition analysis revealed that the iC-GHRKO mice gained less fat mass from 4.5 to 8.5 months of age (Figure 2B, P < .05). This failure to gain fat mass was accompanied by an increase in lean body mass from 4.5 to 8.5 months of age (Figure 2C, P < .05). There was also a relatively small increase in percent fluid mass at 4.5 months of age in the iC-GHRKO vs control mice (Figure 2D, P < .05).
Figure 2.
Body composition analysis, length, and dissected tissue weights. A, Body weight in grams, percentage fat mass (B), percentage lean mass (C), and percentage fluid mass (D) in iC-GHRKO (n = 8) mice and littermate controls (n = 24) from 3 to 12.5 months of age. The black arrow denotes the injection of tamoxifen at 16 weeks of age. E, Length in millimeters of iC-GHRKO (n = 8) and control mice (n = 24) at dissection (12.5 mo of age). F, Tissue mass normalized to body mass and graphed relative to controls (n = 24) in iC-GHRKO mice (n = 8) at dissection (12.5 mo of age). *, Significant difference between genotypes (P < .05); †, significant difference with age (P < .05). BAT, brown adipose tissue; Epi, epididymal; Mes, mesenteric; Quad, quadricep muscle; Retro, retroperitoneal; SubQ, subcutaneous (inguinal).
Along with no change in body weight, the iC-GHRKO and control mice showed similar nose-to-anus lengths at dissection (Figure 2E). With the exception of epididymal white adipose tissue (WAT), discussed below, dissected tissue masses did not vary significantly between iC-GHRKO and control mice. Tissue weights at dissection indicate that iC-GHRKO mice have similar sized hearts compared with control littermates (Figure 2F). In terms of absolute tissue mass, the heart of iC-GHRKO mice was 163 ± 12 mg, whereas the heart of control mice was 155 ± 4 mg (P = .279). Surprisingly, there was an approximately 26% decrease in epididymal WAT mass between iC-GHRKO and control mice (P = .014).
Insulin/glucose tolerance testing
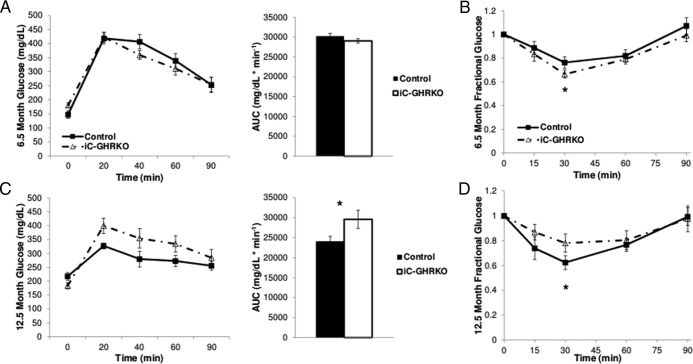
Intraperitoneal glucose and insulin tolerance tests were performed at both 6.5 and 12.5 months of age in iC-GHRKO and control littermates. At 6.5 months of age, there was no difference in GTT (Figure 3A); however, there was an increase in insulin sensitivity in iC-GHRKO mice at 30 minutes (Figure 3B, P < .05). Unexpectedly, at 12.5 months of age, the iC-GHRKO mice were significantly more glucose intolerant than control mice, having an area under the curve of 29 575 ± 723 mg/dL·min−1 vs 23 962 ± 578 mg/dL·min−1 in control mice (Figure 3C, P < .05). In line with this observation, iC-GHRKO mice were significantly more insulin resistant than control littermates based on ITT results at 30 minutes (Figure 3D, P < .05).
Figure 3.
Insulin and glucose tolerance testing. IGTT was performed in 6.5-month-old (A) and 12.5-month-old (C) iC-GHRKO (n = 8) and control littermates (n = 24) after a 12-hour fast and an injection of 10 μL/g body weight of a 10% glucose solution. Area under the curve of the glucose tolerance testing was calculated for both groups. Intraperitoneal ITT was performed in 6.5-month-old (B) and 12.5-month-old (D) nonfasted iC-GHRKO (n = 8) and control littermates (n = 24) after an injection of 1 U/kg body weight insulin. *, Significant difference between genotypes (P < .05). AUC, area under curve.
Immunoblots for insulin responsiveness
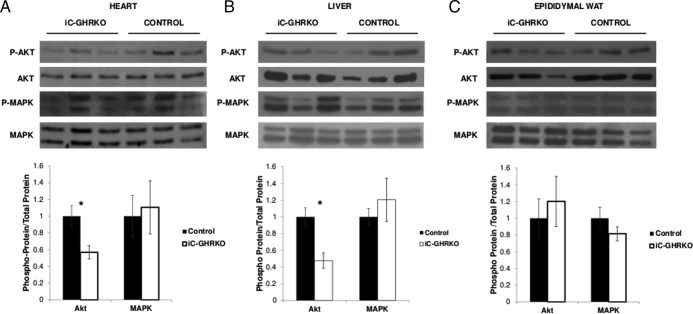
To determine whether the observed insulin resistance was tissue specific at 12.5 months of age, both iC-GHRKO and peanut oil-injected MerCreMer+/−/GHRfl/fl control mice were injected with 1 U/kg ip insulin and dissected 15 minutes later. As shown in Figure 4A, the ratio of phosphorylated Akt to total Akt was decreased by approximately 44% in the heart of iC-GHRKO mice vs control littermates (P = .045). Likewise, in liver (Figure 4B), the ratio of phosphorylated Akt to total Akt was decreased by approximately 53% vs control littermates (P = .022). The ratio of phosphorylated MAPK to total MAPK was not different between the two groups of mice in either the heart or liver. Interestingly, there was no difference between groups of mice in either ratio of phosphorylated Akt or MAPK in epididymal WAT (Figure 4C).
Figure 4.
Insulin-stimulated Akt and MAPK phosphorylation. Immunoblot quantification of insulin-stimulated phosphorylation of Akt (Ser473) and p42/44 MAPK from dissected heart (A), liver (B), and epididymal WAT tissue (C) 15 minutes after 1 U/kg ip injection of insulin in iC-GHRKO (n = 3) and peanut oil-injected MerCreMer+/−/GHRfl/fl (control) littermates at 12.5 months of age (n = 3). *, Significant difference between genotypes (P < .05).
Plasma parameters and IGF-1 RNA expression
To help explain the increase in glucose intolerance and insulin resistance with age, we examined several metabolically related circulating proteins. Circulating insulin levels in iC-GHRKO were similar to controls at 4.5, 8.5, and 12.5 months of age (Figure 5A). Levels of circulating IL-6 (iC-GHRKO: 50.5 ± 14.6 pg/mL vs control: 101.3 ± 12.4 pg/mL, Figure 5E, P = .038) and resistin (iC-GHRKO: 9921 ± 2131 pg/mL vs control: 13 524 ± 676 pg/mL, Figure 5F, P = .041) in 12.5-month-old iC-GHRKO mice were significantly lower than control littermates. There was no significant difference in the C-peptide, leptin, or MCP-1 levels between iC-GHRKO and control mice.
Figure 5.
Circulating metabolic peptides. A, Circulating levels of insulin at 4.5, 8.5, and 12.5 months of age in iC-GHRKO (n = 8) and control (n = 24) littermates. Circulating levels of C-peptide (B), leptin (C), MCP-1 (D), IL-6 (E), and resistin (F) in 12.5-month-old iC-GHRKO (n = 8) and control (n = 24) littermates are shown. *, Significant difference between genotypes (P < .05).
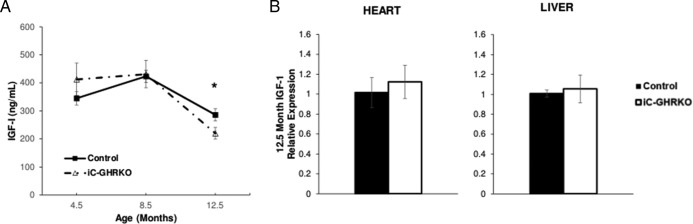
Circulating levels of IGF-1 were similar in both iC-GHRKO and control littermates at 4.5 and 8.5 months of age but were significantly decreased at 12.5 months of age, with iC-GHRKO mice having 220.2 ± 20.5 ng/mL and controls having 285.9 ± 21.8 ng/mL (Figure 6A, P = .039). However, there were no differences in RNA levels of IGF-1 in either heart or liver tissue between the two groups (Figure 6B).
Figure 6.
Circulating IGF-1 and IGF-1 mRNA quantification. A, Circulating levels of IGF-1 at 4.5, 8.5, and 12.5 months of age in iC-GHRKO (n = 8) and control (n = 24) littermates. B, RTqPCR analysis of IGF-1 in heart and liver of iC-GHRKO (n = 5) and control (n = 15) littermates at 12.5 months of age. *, Significant difference between genotypes (P < .05).
Echocardiography and SBP
To examine cardiac functional changes, baseline and postdobutamine injection M-mode echocardiography was performed. At baseline, iC-GHRKO mice had similar cardiac dimensions compared with peanut oil-injected control mice (Table 1, top). Relative wall thickness was significantly (P = .04) decreased in iC-GHRKO mice (0.36 ± 0.01) vs control mice (0.42 ± 0.02) at baseline. However, there was no change in LV mass, EF, FS, or cardiac output. After stressing the mice with the β1-adrenergic agonist dobutamine, iC-GHRKO showed no difference when compared with vehicle-injected control mice in dimensions or calculated functional parameters.
Table 1.
Echocardiography Measurements at 12.5 Months
| 12.5 Month Baseline |
12.5 Month Dobutamine Test |
Change From Baseline |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | iC-GHRKO | P Value | Control | iC-GHRKO | P Value | Control | iC-GHRKO | |
| IVSd, mm | 0.76 ± 0.04 | 0.73 ± 0.08 | .71 | 0.88 ± 0.04 | 0.80 ± 0.04 | .14 | 0.12 ± 0.06 | 0.07 ± 0.09 |
| IVSs, mm | 1.16 ± 0.05 | 1.10 ± 0.11 | .62 | 1.51 ± 0.06 | 1.31 ± 0.08 | .08 | 0.35 ± 0.08 | 0.21 ± 0.14 |
| LVIDd, mm | 3.75 ± 0.12 | 3.95 ± 0.12 | .26 | 3.40 ± 0.10 | 3.50 ± 0.19 | .63 | −0.35 ± 0.16 | −0.45 ± 0.22 |
| LVIDs, mm | 2.48 ± 0.09 | 2.74 ± 0.10 | .08 | 1.48 ± 0.09 | 1.71 ± 0.16 | .22 | −1.00 ± 0.13 | −1.03 ± 0.19 |
| LVPWd, mm | 0.79 ± 0.03 | 0.71 ± 0.02 | .08 | 0.87 ± 0.05 | 0.84 ± 0.03 | .62 | 0.08 ± 0.06 | 0.13 ± 0.04 |
| LVPWs, mm | 1.18 ± 0.06 | 1.01 ± 0.06 | .06 | 1.52 ± 0.06 | 1.40 ± 0.06 | .23 | 0.34 ± 0.08 | 0.39 ± 0.08 |
| Heart rate, bpm | 510 ± 23 | 471 ± 13 | .20 | 540 ± 16 | 542 ± 18 | .96 | 30 ± 28 | 71 ± 22 |
| EF, % | 63.2 ± 1.8 | 58.4 ± 2.0 | .10 | 87.4 ± 1.5 | 82.8 ± 3.2 | .18 | 24.2 ± 2.3 | 24.4 ± 3.7 |
| FS, % | 33.7 ± 1.4 | 30.5 ± 1.4 | .12 | 56.7 ± 2.2 | 51.5 ± 3.3 | .20 | 23 ± 2.6 | 21 ± 3.6 |
| LV mass, mg | 100.9 ± 6.0 | 100.8 ± 12.2 | .99 | 103.9 ± 7.5 | 99.0 ± 9.6 | .69 | 3 ± 9.6 | −1.8 ± 15.5 |
| SV, μL | 38.2 ± 3.1 | 40.0 ± 3.5 | .72 | 41.9 ± 2.9 | 43.0 ± 5.5 | .85 | 3.7 ± 4.2 | 3 ± 6.5 |
| CO, mL/min | 19.2 ± 1.2 | 18.8 ± 1.8 | .86 | 22.6 ± 1.6 | 23.2 ± 3.0 | .85 | 3.4 ± 2.0 | 4.4 ± 3.5 |
| RWT | 0.42 ± 0.02 | 0.36 ± 0.01 | .04 | 0.52 ± 0.03 | 0.49 ± 0.03 | .57 | 0.10 ± 0.04 | 0.13 ± 0.03 |
Abbreviations: CO, cardiac output; d, diastole; IVS, interventricular septum; LVID, LV internal diameter; LVPW, LV posterior wall; RWT, relative wall thickness; s, systole; SV, stroke volume.
SBP showed a significant increase throughout age in both iC-GHRKO and control mice (P < .05). However, there were no differences in BP at any time point between the two groups (Supplemental Figure 1).
Cardiac calcium channel RNA expression
Levels of RNA for the four major cardiac channels were assessed at 2 weeks and 12 months after the GHR gene disruption, 4.5 months (Supplemental Figure 1A) and 12.5 months (Supplemental Figure 1B) of age. Interestingly, 2 weeks after the GHR gene disruption, there was a decrease (∼28%) in sarcoendoplasmic reticulum Ca2+-ATPase 2 (SERCA2) (Supplemental Figure 1A) and an increase (∼30%) in Na+/Ca2+ exchanger 1 (NCX1) (Supplemental Figure 2A) mRNA, with no change in ryanodine channel 2 or L-type calcium channel RNA in iC-GHRKO mice vs controls (P < .05). However, by 12.5 months of age, the decrease in SERCA2 expression (Supplemental Figure 2B) in iC-GHRKO mice had slightly recovered, with only an approximately 11% decrease in expression (P < .05). The level of NCX1 expression returned to that of controls at 12.5 months of age.
Discussion
The results of this study reveal several interesting insights into the role of GH induced signaling in adult murine cardiomyocytes. First, GH action through the cardiac GHR does not appear to be necessary to maintain cardiac mass, nor does it appear to be necessary for the maintenance of baseline or dobutamine-stressed cardiac function throughout the first year of life in mice. Evaluation of SBP over age also revealed no effect of cardiac GHR gene disruption. Surprisingly, cardiac GHR gene disruption was associated with an age-dependent decline in glucose tolerance and insulin sensitivity. Immunoblot analysis demonstrated that the iC-GHRKO mice had lower levels of insulin-stimulated Akt phosphorylation in the heart and liver but not the epididymal WAT, indicating a decrease in tissue-specific insulin sensitivity. Along with the decrease in insulin sensitivity, iC-GHRKO mice had lower circulating IGF-1 levels at 12.5 months of age but not earlier in life. This change in plasma IGF-1 was not associated with changes in IGF-1 RNA expression in the liver or heart of the iC-GHRKO mice. There was no change in circulating insulin at any of the three age points examined. Finally, the iC-GHRKO mice experienced a shift in body composition with a tendency to have lower fat mass and increased lean mass, which was most prominent from 4.5 to 8.5 months of age but without a change in body weight throughout life. Together these data indicate adult cardiac GHR-mediated action in mice plays a metabolic role in later life.
Animal studies of transgenic GH and GHRKO mice have established possible roles for GH action in the development, maintenance, and function of cardiac tissue. Studies of transgenic mice overexpressing bGH have shown disparate results with regard to cardiovascular function. An echocardiographic study of 8-month-old female bGH mice shows that EF and FS is decreased in bGH vs wild-type controls (11). These changes are related to a decreased phosphocreatine to ATP ratio and enlarged, disorganized cardiac mitochondria (11). A later study, however, using direct ventricular cannulation and isoprenaline stimulation reports no difference in LV function (14). Reports of BP in bGH mice are also mixed, with some studies reporting no difference (11–13) and others reporting increased BP (14–16). Indeed, our laboratory recently showed that bGH transgenic mice develop increased SBP and cardiac fibrosis as a function of age and that this change is associated with an increase in circulating inflammatory cytokines (MCP-1 and IL-6) throughout the first year of life (16). Thus, age of the bGH mice likely accounts for some of the variance seen in the literature.
Unlike the mixed reports of bGH mice, GHRKO mice (25) (which systemically lack GH signaling and have severely decreased circulating IGF-1 levels) have been shown to have cardiac function that develops in line with their small body size and remains normal at 9 months of age (14). Egecioglu et al (19) show that GHRKO mice have decreased systolic function using echocardiography, but cardiac output is sufficient to meet the metabolic demands of the smaller sized mouse. Opposite of what is described in bGH mice, GHRKO mice are reported to have lower SBP and enhanced vessel response to norepinephrine and nitric oxide (19). It has also been shown that there is increased insulin stimulated Akt phosphorylation in the heart of GHRKO mice, which may provide an enhanced cardioprotective effect (26). This is contrary to what we observed in the cardiac tissue of the iC-GHRKO mice, which had decreased insulin-stimulated Akt phosphorylation. However, a direct comparison with the GHRKO mouse and our iC-GHRKO mouse is difficult due to the fact that GHRKO mice have an absence of GH signaling during development and throughout life.
In both bGH and GHRKO mice, the gain or loss of GH signaling is present throughout development, and downstream IGF-1 is concomitantly affected. Therefore, it is difficult to separate the specific effects of GH from IGF-1 in these mouse lines. Studies using cardiac-specific IGF-1 receptor (IGF-1R) knockout mice have demonstrated that the loss of IGF-1R signaling does not change cardiac growth but does blunt exercise-induced hypertrophy, perhaps through an AMPK-driven mechanism (27). Adult disruption of cardiac-specific IGF-1R in mice using the same inducible Myh6-driven MerCreMer transgene used in our current study results in different phenotypes depending on the age of induction (28). Specifically, when IGF-1R gene disruption is induced in adult (11 mo old) mice, there is a decrease in diastolic cardiac function, indicating that cardiac IGF-1R signaling is important in maintaining cardiac function late in life (28). Taken together with our results showing no change in baseline or dobutamine-stressed cardiac function when cardiac GHR is disrupted at 4 months of age, it appears that cardiac IGF-1R but not GHR-mediated signaling is required for proper cardiac structure and function in adult mice.
Along with GH and IGF-1 action, insulin also plays a role in cardiac development and function. Constitutive cardiac-specific insulin receptor knockout mice are reported to have a small heart phenotype, impaired substrate use, switched myosin expression to the fetal β-form (29), and increased cardiac mitochondrial uncoupling and oxidative stress (30). In the diabetic state, the cardiac-specific insulin receptor knockout mice show decreased cardiac efficiency (31). Double-cardiac-specific knockout of both insulin receptor and IGF-1R results in dramatic 100% mortality by the fourth week of life due to heart failure from dilated cardiomyopathy (32). Death in these mice is preceded by the down-regulation of genes involved in the mitochondrial electron transport and β-oxidation of fatty acid and distinct structural changes to cardiomyocytes including increased mitochondria and disrupted sarcomere organization (32). Given the molecular cross talk inherent among GH-, IGF-1-, and insulin-induced intracellular signaling, which includes p42/44 MAPK and Akt signaling, it may be that when a given signaling pathway is lost, the others respond to compensate for the deficit, and therefore, only when several of the pathways are disrupted (32) is an overt phenotype observed.
Circulating IGF-1 is derived mainly from hepatocytes. Estimates of liver derived circulating IGF-1 range from approximately 50% (33) to 75% (34, 35) and 90% in liver-specific GHR gene-deleted mice (36, 37). The approximately 22% decrease in plasma IGF-1 we observe in 12.5-month-old iC-GHRKO mice implies that the heart may modulate circulating IGF-1 in adult mice. However, we did not observe a difference in IGF-1 RNA expression in either the heart or liver of iC-GHRKO vs controls. This indicates that rather than a direct reduction of IGF-1 expression from the heart or liver, there must be another indirect mechanism. The lower circulating IGF-1 level in iC-GHRKO may, in part, explain the decreased level of Akt and MAPK phosphorylation we observe in the heart and liver. It is well known that IGF-1 acts through both of these pathways through its interaction with the IGF-1R and with the insulin/IGF-1 hybrid receptors (38). However, this does not fully explain why Akt and MAPK phosphorylation was unaffected in the WAT of iC-GHRKO mice. Indeed, we show that iC-GHRKO mice had normal GTT and slightly improved ITT results at 6.5 months but by 12.5 months experienced decreased glucose tolerance and decreased insulin sensitivity. This decrease in insulin sensitivity was supported by immunoblots demonstrating that iC-GHRKO mice had decreased insulin stimulated Akt phosphorylation in the heart and liver, but not in the epididymal WAT. Therefore, we cannot discount the idea that another circulating mediator is acting in a tissue-dependent manner to regulate insulin stimulated Akt and MAPK activation.
One intriguing possibility is that a cardiokine is being released from the cardiomyocytes lacking GHR, leading to systemic alterations in insulin signaling. The emerging role of the heart as a metabolic mediator was demonstrated in 2012 when Grueter et al (39) discovered another protein, cardiac Mediator Complex Subunit 13, which can control systemic energy homeostasis. Since then, additional cardiokines have been reported. A recent review of cardiokines describes more than 10 secreted factors that are recognized to be released from the heart, supporting the idea of the heart being viewed as an endocrine organ (40). Another possibility is that secondary to decreased circulating IGF-1, there may be increased GH action at other tissues. GH is an established lipolytic hormone (41), and increased circulating free fatty acids can act to cause decreased insulin sensitivity via the cycle by Randle et al (42). Finally, the decrease we observe in insulin-stimulated Akt phosphorylation could be linked to increased basal activation of the pathway, similar to what has been observed in the hearts of ob/ob mice (43). However, this would not fully account for the differences we observe in the liver and WAT. Future studies with the iC-GHRKO mice will be required to examine possible changes in heart-derived secreted factors and the possible changes in circulating lipids.
Of the cytokines we assayed known to mediate insulin signaling, IL-6 and resistin showed significant differences, with iC-GHRKO mice having decreased circulating levels vs controls at 12.5 months of age. IL-6 is often regarded as a proinflammatory cytokine and is known to play a dual role in the regulation of insulin resistance by inducing insulin resistance in hepatocytes and decreasing insulin resistance in skeletal muscle (44). IL-6 is mainly derived from adipose tissue (44) and our observation that iC-GHRKO mice had decreased the percentage of fat mass and decreased the relative epididymal fat pad size is in line with this fact. IL-6 also is known to play an important role in cardiac remodeling, with circulating levels positively correlating with severity of heart failure (45). Rats infused with IL-6 will develop concentric hypertrophy and increased collagen deposition similar to hypertensive cardiac remodeling (46). Therefore, given the lower level of IL-6 we observe in iC-GHRKO mice, it is unlikely to be contributing to the change in insulin-stimulated Akt and MAPK phosphorylation we observe in the liver and muscle. Resistin, in rodents, is also primarily an adipocyte-derived cytokine, and elevated levels are associated with increased insulin resistance (47). Elevated levels of circulating resistin are also known to be correlated with decreased LV mass and FS in humans (48), and increased cardiomyocyte expression of resistin in rodents leads to increased cardiac hypertrophy and decreased contractility (49). However, given that we observed lower levels of circulating resistin in iC-GHRKO mice, it is an unlikely candidate for the change in insulin-stimulated Akt and MAPK phosphorylation we observe.
Calcium channels in the myocardium are principal mediators of cardiac function. A possible positive effect of GH stimulation on cardiac calcium handling is indicated in a study showing improved contractility of isolated ventricular myocytes from bGH mice (17). However, IGF-1 likely also plays a role by sensitizing the contractile apparatus to calcium (4). Our mRNA analysis of four of the cardiac calcium channels in iC-GHRKO mice does allude to an acute change in cardiac calcium channel expression with a decrease in SERCA2 and an increase in NCX1 expression, a pattern often observed in heart failure (50). SERCA2 is responsible for the reuptake of calcium ions from cytoplasm into the sarcoplasmic endoreticulum and subsequent relaxation of the cardiomyocyte. This causes decreased myosin-actin cross-bridge formation and a decrease in contraction (50). Increased levels of NCX1 can compensate for decreased SERCA2 activity by leaking calcium out of the cell, resulting in decreased contractile force (50). This pattern of decreased SERCA2 and increased NCX1, however, is attenuated in the older iC-GHRKO mice and may be due to induction of the Cre recombinase in cardiomyocytes, which has previously been shown to acutely alter cardiac gene expression (51), with changes that resolve within 1 month of induction.
Although our current study is the first to report specific disruption of cardiac GHR, three other studies have reported constitutive disruption of both cardiac and skeletal muscle GHR using the creatine kinase (52, 53) and mef-2c (54) promoters. All three studies show significant alterations in glucose homeostasis. Results from Vijayakumar et al (52) and our laboratory (53) report improved glucose tolerance at 5 and 6 months of age, respectively, whereas Mavalli et al (54) report decreased glucose tolerance at 6 months of age. The three studies also report differences in body composition of the muscle-specific GHRKO mice. Vijayakumar et al (52) observe mice that have lower absolute fat mass but similar percentage adiposity compared with controls. Similarly, our laboratory reports decreased body weight and fat mass in male mice at 12 and 14 months of age, with a similar decrease in fat mass (53). Mavalli et al (54) report increased adiposity in muscle specific GHRKO up to 26 weeks of age. A likely explanation for these disparate results is the difference in promoters driving Cre recombinase expression. Whereas creatine kinase is accepted to be muscle specific, the mef-2c promoter is known to be active outside the striated muscle, with demonstrated activity in endothelium, brain, and lymphocytes (55), which may potentially impact systemic glucose homeostasis.
The iC-GHRKO mice in our current study have decreased adiposity and increased insulin sensitivity at 6.5 months of age, observations that are in line with findings from two of the three muscle-specific GHRKO studies (52, 53). Unfortunately, glucose tolerance data from aged muscle-specific GHRKO mice are not available, and therefore, we cannot compare the study with our observation of impaired insulin sensitivity in iC-GHRKO mice at 12.5 months of age. Vijayakumar et al (52) posit that an improved metabolic efficiency may explain the decreased adiposity in muscle-specific GHRKO mice because they observe an increased respiratory exchange ratio when the mice are fed a high-fat diet (52). Although we do not believe an altered metabolic efficiency is responsible for the decrease in adiposity observed in the iC-GHRKO mice because there was no change in body weight, future studies are needed on the topic to rule out this possibility.
Our study has several limitations. We examined only a single time point for cardiomyocyte GHR gene disruption. Studies with the iCM-IGFIRKO mice have shown changes, depending on the age of gene disruption (28). Therefore, a future study would be to induce disruption at several time points to give a more complete picture of the role of cardiac GH signaling in cardiac development. Furthermore, we examined only male mice due to the possible confounding effects of tamoxifen, high endogenous estrogen levels, and GH signaling in female mice. Finally, we did not explore lipid metabolism or the effect of exercise on cardiac function in the iC-GHRKO mice, both of which may play a role in increased insulin resistance.
In conclusion, we report on the first cardiomyocyte-specific, GHR gene-disrupted mouse line. Our data allude to tissue cross talk, with adult cardiac GH signaling being important in the regulation of systemic metabolic homeostasis: iC-GHRKO developed insulin resistance and glucose intolerance at 12.5 months of age and had decreased fat mass from 4.5 to 8.5 months of age. Surprisingly, our results do not support a prominent role of adult cardiac GH signaling in the maintenance of baseline cardiac function in male mice with no differences in unstressed echocardiographic measurements at baseline or after a dobutamine challenge. In the future, it would be worthwhile to use the iC-GHRKO mice to provide answers to several interesting questions: what is the nature of the cardiac secretome, and how is it altered by cardiac GH action? Is cardiac GH signaling important in postinfarction recovery? Does cardiac GH action play a role in exercise-induced hypertrophy? Do the iC-GHRKO mice respond differently from control mice to dietary challenges such as high-fat feeding or caloric restriction? And finally, what are the effects of cardiac GHR action on longevity?
Acknowledgments
We acknowledge Yoshihiro Azuma, PhD (Cardiovascular Core, National Mouse Metabolic Phenotyping Center, University of Massachusetts Medical School) for his assistance in performing and interpreting the results from the echocardiography experiment; Kevin Funk, MS (Ohio University Edison Biotechnology Institute) for his assistance in designing a PCR-based genotyping assay; and Lauren Volpe, MEd (Ohio University Patton College of Education) for her careful editing of the manuscript.
This work was supported by the Gates Millennium Scholars Graduate Fellowship Program; National Institutes of Health Grants P01AG031736 and U24-DK093000; the State of Ohio's Eminent Scholar Program, which includes a gift from Milton and Lawrence Goll; the Scholarly Enhancement Award; the Provost Undergraduate Research Fund; and the Diabetes Institute at Ohio University.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bGH
- bovine GH
- BP
- blood pressure
- EF
- ejection fraction
- FS
- fractional shortening
- GHD
- GH deficiency
- GHR
- GH receptor
- GHRKO
- GHR null or knockout
- GTT
- glucose tolerance testing
- iC-GHRKO
- tamoxifen-inducible, cardiac-specific GHR disrupted
- IGF-1R
- IGF-1 receptor
- ITT
- insulin tolerance testing
- LS
- Laron syndrome
- LV
- left ventricular
- MCP-1
- monocyte chemoattractant protein 1
- Myh6
- myosin heavy chain 6
- NCX1
- Na+/Ca2+ exchanger 1
- RTqPCR
- real-time quantitative PCR
- SBP
- systolic BP
- SERCA2
- sarcoendoplasmic reticulum Ca2+-ATPase 2
- STAT5
- signal transducer and activator of transcription 5
- WAT
- white adipose tissue.
References
- 1. Bruel A, Oxlund H. Biosynthetic growth hormone increases the collagen deposition rate in rat aorta and heart. Eur J Endocrinol. 1995;132(2):195–199. [DOI] [PubMed] [Google Scholar]
- 2. Rubin SA, Buttrick P, Malhotra A, Melmed S, Fishbein MC. Cardiac physiology, biochemistry and morphology in response to excess growth hormone in the rat. J Mol Cell Cardiol. 1990;22(4):429–438. [DOI] [PubMed] [Google Scholar]
- 3. Xu XP, Best PM. Decreased transient outward K+ current in ventricular myocytes from acromegalic rats. Am J Physiol. 1991;260(3 Pt 2):H935–H942. [DOI] [PubMed] [Google Scholar]
- 4. Solem ML, Thomas AP. Modulation of cardiac Ca2+ channels by IGF1. Biochem Biophys Res Commun. 1998;252(1):151–155. [DOI] [PubMed] [Google Scholar]
- 5. Colao A. The GH-IGF-1 axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf). 2008;69(3):347–358. [DOI] [PubMed] [Google Scholar]
- 6. Castellano G, Affuso F, Conza PD, Fazio S. The GH/IGF-1 axis and heart failure. Curr Cardiol Rev. 2009;5(3):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colao A, Somma CD, Savanelli MC, Leo MD, Lombardi G. Beginning to end: cardiovascular implications of growth hormone (GH) deficiency and GH therapy. Growth Horm IGF Res. 2006;16(suppl A):S41–S48. [DOI] [PubMed] [Google Scholar]
- 8. Feinberg MS, Scheinowitz M, Laron Z. Echocardiographic dimensions and function in adults with primary growth hormone resistance (Laron syndrome). Am J Cardiol. 2000;85(2):209–213. [DOI] [PubMed] [Google Scholar]
- 9. Ben-Dov I, Gaides M, Scheinowitz M, Wagner R, Laron Z. Reduced exercise capacity in untreated adults with primary growth hormone resistance (Laron syndrome). Clin Endocrinol (Oxf). 2003;59(6):763–767. [DOI] [PubMed] [Google Scholar]
- 10. Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36(2–3):201–208. [DOI] [PubMed] [Google Scholar]
- 11. Bollano E, Omerovic E, Bohlooly-y M, et al. Impairment of cardiac function and bioenergetics in adult transgenic mice overexpressing the bovine growth hormone gene. Endocrinology. 2000;141(6):2229–2235. [DOI] [PubMed] [Google Scholar]
- 12. Dilley RJ, Schwartz SM. Vascular remodeling in the growth hormone transgenic mouse. Circ Res. 1989;65(5):1233–1240. [DOI] [PubMed] [Google Scholar]
- 13. Peten EP, Striker LJ, Fogo A, Ichikawa I, Patel A, Striker GE. The molecular basis of increased glomerulosclerosis after blockade of the renin angiotensin system in growth hormone transgenic mice. Mol Med. 1994;1(1):104–115. [PMC free article] [PubMed] [Google Scholar]
- 14. Izzard AS, Emerson M, Prehar S, et al. The cardiovascular phenotype of a mouse model of acromegaly. Growth Horm IGF Res. 2009;19(5):413–419. [DOI] [PubMed] [Google Scholar]
- 15. Bohlooly-y M, Carlson L, Olsson B, et al. Vascular function and blood pressure in GH transgenic mice. Endocrinology. 2001;142(8):3317–3323. [DOI] [PubMed] [Google Scholar]
- 16. Jara A, Benner CM, Sim D, et al. Elevated systolic blood pressure in male GH transgenic mice is age dependent. Endocrinology. 2014;155(3):975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colligan PB, Brown-Borg HM, Duan J, Ren BH, Ren J. Cardiac contractile function is enhanced in isolated ventricular myocytes from growth hormone transgenic mice. J Endocrinol. 2002;173(2):257–264. [DOI] [PubMed] [Google Scholar]
- 18. Bonkowski MS, Rocha JS, Masternak MM, Regaiey KAA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103(20):7901–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egecioglu E, Andersson IJ, Bollano E, et al. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Metab. 2007;292(5):E1418–E1425. [DOI] [PubMed] [Google Scholar]
- 20. Sohal DS, Nghiem M, Crackower MA, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89(1):20–25. [DOI] [PubMed] [Google Scholar]
- 21. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol (Baltimore, Md). 2013;27(3):524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersson KB, Winer LH, Mork HK, Molkentin JD, Jaisser F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res. 2010;19(4):715–725. [DOI] [PubMed] [Google Scholar]
- 23. Palmer AJ, Chung MY, List EO, et al. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150(3):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. [DOI] [PubMed] [Google Scholar]
- 25. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94(24):13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giani JF, Bonkowski MS, Munoz MC, et al. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: effects of calorie restriction. J Gerontol Biol Sci Med Sci. 2008;63(8):788–797. [DOI] [PubMed] [Google Scholar]
- 27. Kim J, Wende AR, Sena S, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol (Baltimore, Md). 2008;22(11):2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moellendorf S, Kessels C, Peiseler L, et al. IGF-1R signaling attenuates the age-related decline of diastolic cardiac function. Am J Physiol Metab. 2012;303(2):E213–E222. [DOI] [PubMed] [Google Scholar]
- 29. Belke DD, Betuing S, Tuttle MJ, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109(5):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boudina S, Bugger H, Sena S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119(9):1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bugger H, Riehle C, Jaishy B, et al. Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J Mol Cell Cardiol. 2012;52(5):1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laustsen PG, Russell SJ, Cui L, et al. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol. 2007;27(5):1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci USA. 2008;105(49):19378–19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96(13):7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sjogren K, Liu JL, Blad K, et al. Liver-derived insulin-like growth factor I (IGF-1) is the principal source of IGF-1 in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96(12):7088–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. List EO, Berryman DE, Funk K, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-1, increased local IGF-1, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937–19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slaaby R, Schäffer L, Lautrup-Larsen I, et al. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-1R) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem. 2006;281(36):25869–25874. [DOI] [PubMed] [Google Scholar]
- 39. Grueter CE, van Rooij E, Johnson BA, et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149(3):671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shimano M, Ouchi N, Walsh K. Cardiokines: recent progress in elucidating the cardiac secretome. Circulation. 2012;126(21):e327–e332. [DOI] [PubMed] [Google Scholar]
- 41. Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. [DOI] [PubMed] [Google Scholar]
- 42. Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem. 1994;55(suppl):1–11. [DOI] [PubMed] [Google Scholar]
- 43. Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31(1):100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim J-H, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm. 2009;80:613–633. [DOI] [PubMed] [Google Scholar]
- 45. Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(2):391–398. [DOI] [PubMed] [Google Scholar]
- 46. Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56(2):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barnes KM, Miner JL. Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Pept Sci. 2009;10(1):96–107. [DOI] [PubMed] [Google Scholar]
- 48. McManus DD, Lyass A, Ingelsson E, et al. Relations of circulating resistin and adiponectin and cardiac structure and function: the Framingham Offspring Study. Obes (Silver Spring, Md). 2012;20(9):1882–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim M, OH JK, Sakata S, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45(2):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93(6):487–490. [DOI] [PubMed] [Google Scholar]
- 51. Koitabashi N, Bedja D, Zaiman AL, et al. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res. 2009;105(1):12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vijayakumar A, Wu Y, Sun H, et al. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61(1):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. List EO, Berryman DE, Ikeno Y, et al. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR−/− mice. Aging. 2015;7(7):500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mavalli MD, DiGirolamo DJ, Fan Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120(11):4007–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development (Cambridge, England). 2007;134(23):4131–4140. [DOI] [PubMed] [Google Scholar]