Abstract
The development and application of stereo- and site-selective catalytic methods that directly convert lower alcohols to higher alcohols are described. These processes merge the characteristics of transfer hydrogenation and carbonyl addition, exploiting alcohols and π-unsaturated reactants as redox pairs, which upon hydrogen transfer generate transient carbonyl-organometal pairs en route to products of C-C coupling. Unlike classical carbonyl additions, stoichiometric organometallic reagents and discrete alcohol-to-carbonyl redox reactions are not required. Additionally, due to a kinetic preference for primary alcohol dehydrogenation, the site-selective modification of glycols and higher polyols is possible, streamlining or eliminating use of protecting groups. The total syntheses of several iconic type I polyketide natural products were undertaken using these methods. In each case, the target compounds were prepared in significantly fewer steps than previously achieved.
Graphical Abstract
Introduction to Ideal Chemical Synthesis
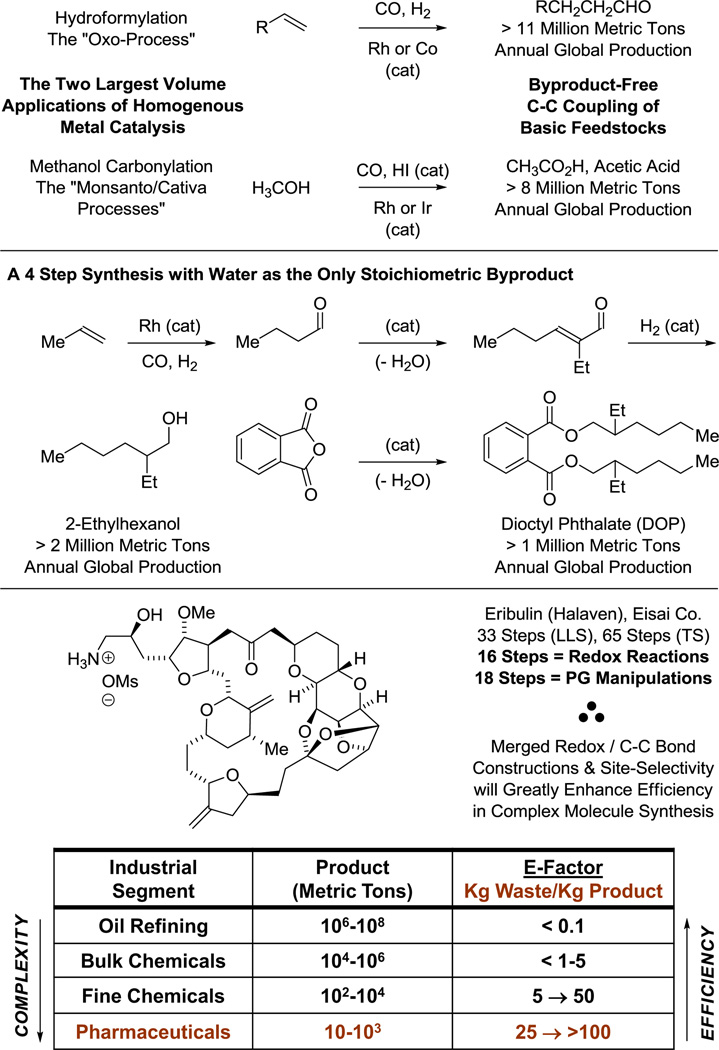
The most authentic displays of efficient chemical synthesis are evident where economic selective pressure is greatest: the realm of commodity chemical manufacture. Thus, it is instructive to consider the two largest volume applications of homogenous metal catalysis (Figure 1), hydroformylation (the oxo-process)1 and methanol carbonylation (the Monsanto/Cativa processes).2 Both are C-C bond formations, both employ noble metal catalysts (rhodium and iridium), and both are byproduct-free. These processes speak to the fundamental significance of C-C bond construction in chemical synthesis, and reveal that a principal characteristic of a “process-relevant” method is the ability to transform an abundant, ideally renewable feedstock to a value-added product in the absence of stoichiometric byproducts.3
Figure 1.
Economic selective pressure as a driver for synthetic efficiency and the technological gap vis-à-vis methods for complex molecule construction.
In multi-step chemical synthesis, an inverse correlation between complexity and efficiency is observed and may be quantified by E-factor analysis.4 For example, dioctyl phthalate, a simple industrial plasticizer, is prepared through a 4-step linear sequence in which water is the only stoichiometric byproduct (Figure 1).5 In contrast, the commercial manufacturing route for eribulin (Halaven), a highly complex polyketide-based therapeutic agent, comprises a total of 65 steps.6 While the synthesis of eribulin represents an heroic milestone, half of the transformations are oxidation level adjustments and protecting group manipulations and nearly every step generates stoichiometric byproducts (Figure 1). The juxtaposition of these two syntheses reveals a technological gap and, more importantly, defines an opportunity for innovation: the development of stereo- and site-selective C-C bond formations accompanied by the addition, re-distribution or removal of hydrogen.
Redox-economic methods7 for site-selective8 skeletal assembly should dramatically impact synthetic efficiency, as they bypass discrete oxidation level adjustments and use of protecting groups.9 As demonstrated by the benchmark provided by eribulin, in complex settings such transformations may represent over half the steps of a typical synthetic route even after intensive process optimization.10 Hendrickson’s view on synthetic efficiency11 tacitly recognizes the significance of merged redox-construction events7 and isomer-selective transformations,12 including site-selective processes8 for protecting group-free chemical synthesis.9 Considerations of “process relevance,” including atom-economy,3 the minimization of preactivation (the degree of separation between reagent and feed-stock)13 and the principles of green chemistry14 provide a more complete perspective.
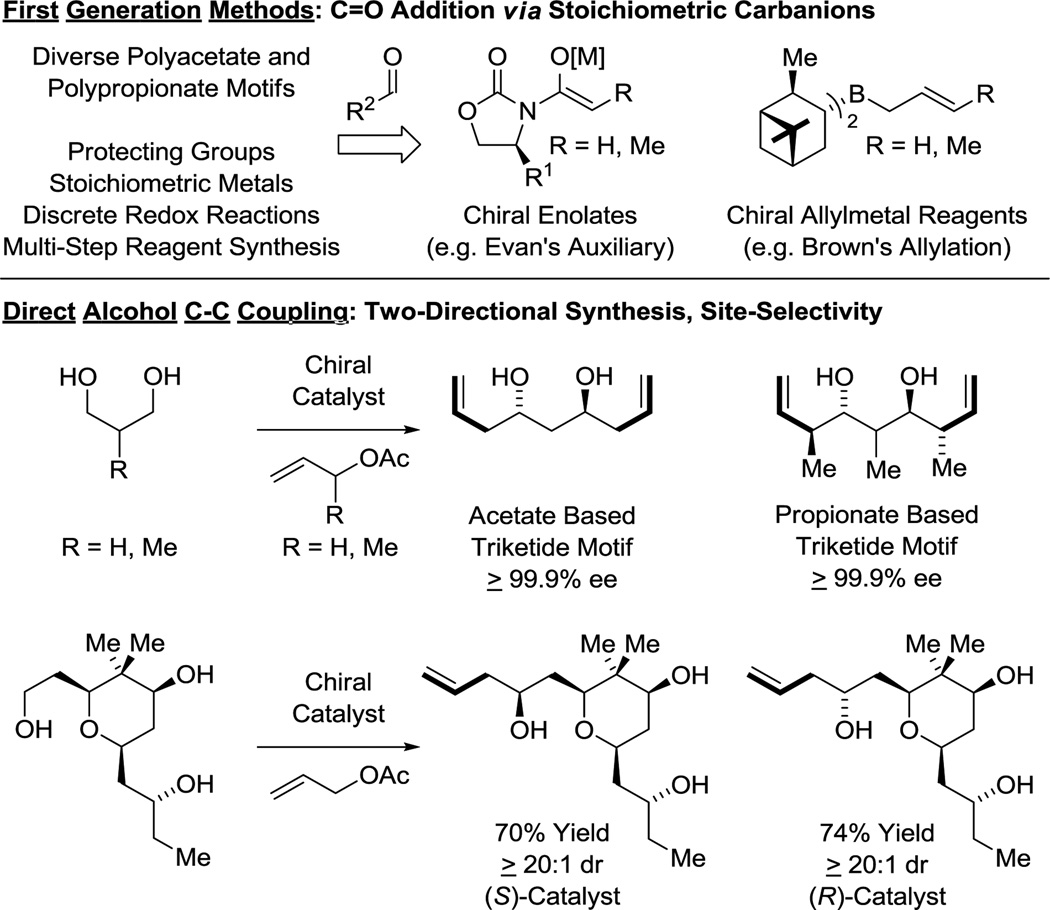
Guided by these concepts, we have developed a broad, new suite of catalytic methods for the direct stereo- and site-selective conversion of lower alcohols to higher alcohols.15 These processes merge the characteristics of transfer hydrogenation with carbonyl addition, exploiting the native reducing ability of alcohols to drive generation of transient carbonyl-organometal pairs. Unlike classical synthetic sequences involving carbonyl addition, discrete alcohol-to-carbonyl redox reactions and use of premetalated C-nucleophiles are not required. Most remarkably, due to a kinetic preference for primary alcohol dehydrogenation, these methods may be applied to the site-selective (protecting group-free) modification of glycols and higher polyols.16 In this perspective, we describe how this new technology has streamlined type I polyketide construction. Iconic type I polyketide natural products were targeted in order to obtain the highest number of benchmarks. As any given transformation is amenable to optimization, the fundamental metric of step count is applied as the primary indicator of strategic efficiency.17
Alcohol C-C Coupling for Polyketide Construction
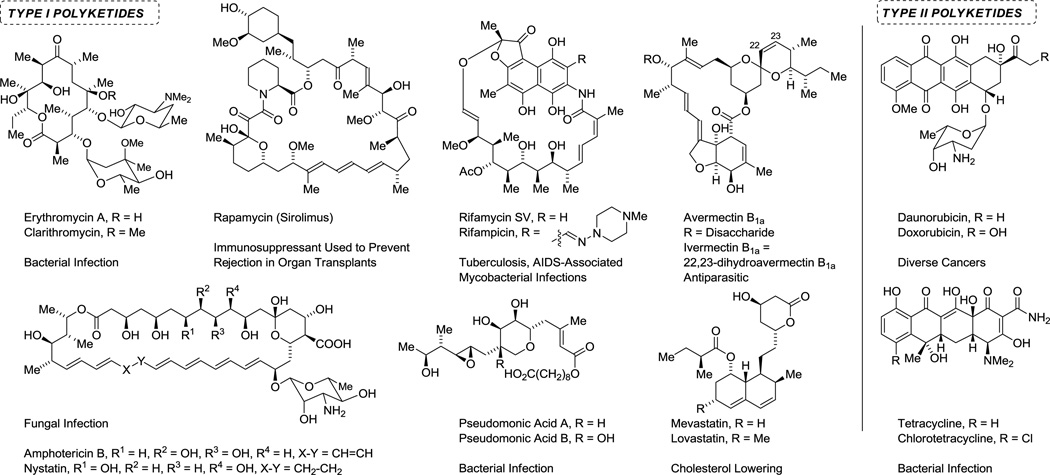
The commercialization of erythromycin A (1952)18 and subsequent discoveries of amphotericin B (1955)19 and rifamycin B (1957)20 comprise a turning point in both human medicine and synthetic organic chemistry. These natural products belong to the “polyketide” class of secondary metabolites - a broad and structurally diverse family of compounds that are used to treat a variety of indications (Figure 2).21 Approximately 20% of the top-selling small molecule drugs are polyketides.22 Remarkably, soil bacteria are the principal source of these compounds, yet less than 5% of soil bacteria are amenable to culture with many phyla having eluded culture completely.23 Hence, one may assume that as methods for bacterial cultivation improve, polyketides will play an even more pervasive role in human medicine.
Figure 2.
Selected polyketide natural products and semi-synthetic congeners used in human medicine.
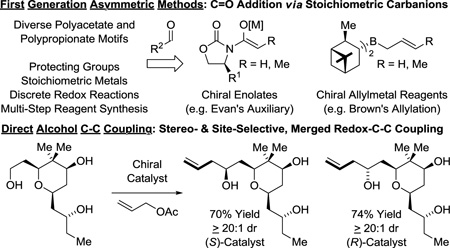
The impact of polyketides on synthetic organic chemistry has been profound. The challenges posed by these complex structures drove enormous advances in the development of stereoselective methods for carbonyl addition, culminating in a “first generation lexicon” largely centered on the use of asymmetric aldol reactions,24 as exemplified by Evans’ reagents,25 and carbonyl allylmetalations,26 as exemplified by Brown’s allyl-/crotylboron reagents (Figure 3).27 Despite the availability of this technology, all commercial polyketide-based drugs, with the exception of eribulin, are prepared by fermentation or semi-synthesis. De novo syntheses of polyketide-related structures that rely on these first-generation technologies are generally too lengthy for commercial application due in large part to (a) the separation of redox and skeletal construction events, and (b) the persistent requirement of protecting groups. As shown in this perspective, new capabilities inherent to direct alcohol C-H functionalization, namely, C-C coupling in the absence of protecting groups or discrete alcohol-to-carbonyl redox reactions (Figure 3), not only streamline polyketide construction, but evoke a shift in retrosynthetic paradigm.
Figure 3.
First-generation methods for polyketide construction and new capabilities availed by direct alcohol C-C coupling.
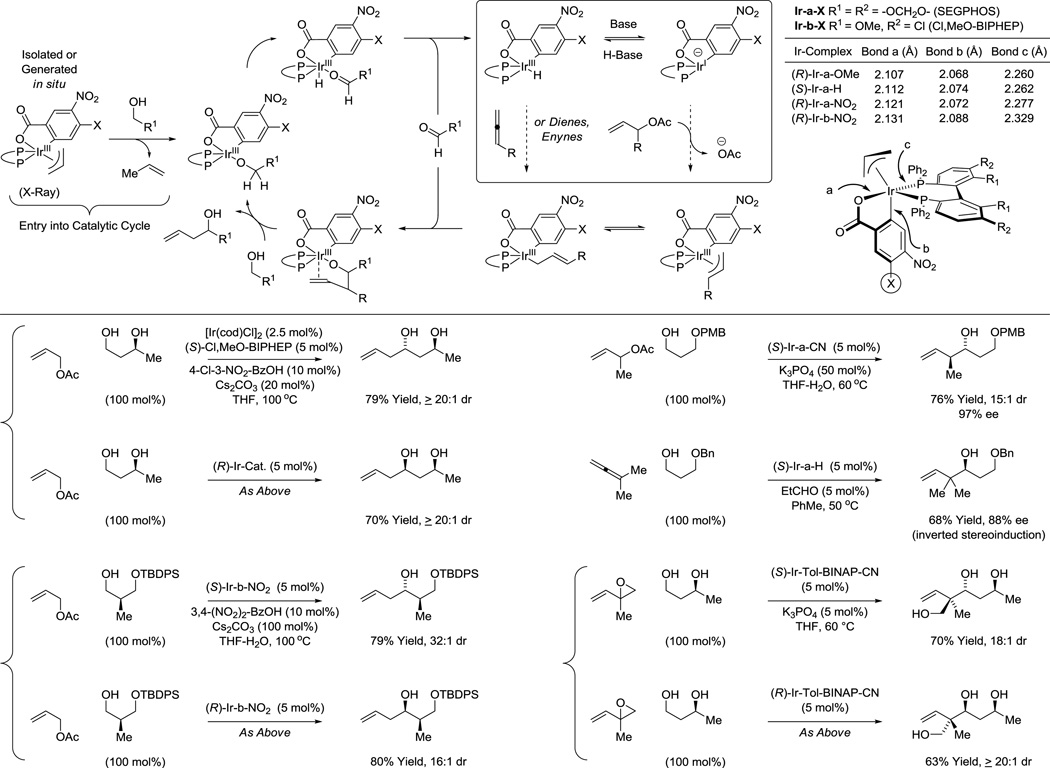
Iridium28 and ruthenium29 complexes have found the greatest use in metal catalyzed transfer hydrogenation, and in our own asymmetric alcohol C-C couplings.15 Novel cyclometalated π-allyliridium ortho-C,O-benzoate complexes derived from [Ir(cod)Cl]2, allyl acetate, various 4-substituted-3-nitrobenzoic acids and axially chiral bis(phosphine) ligands have proven especially effective (Figure 4). These robust air stable iridium(III) complexes can be generated in situ from their components or can be isolated by precipitation or even conventional flash silica gel chromatography. As illustrated in the indicated catalytic mechanism, these complexes promote C-C coupling through one of two distinct reaction pathways wherein alcohol oxidation is balanced by (a) C-X reductive cleavage or (b) C=C π-bond hydrometalation. The former pathway is rendered more efficient upon use of catalysts that embody more electron deficient ortho-C,O-benzoate moieties, which enhance Lewis acidity at iridium and, in turn, accelerate turn-over limiting carbonyl addition. Consistent with this interpretation, single crystal X-ray diffraction analysis of a series of π-allyliridium ortho-C,O-benzoate complexes reveals a lengthening of the C-Ir, O-Ir, and P-Ir bonds for more inductive 4-substituents, suggesting enhanced Lewis acidity at iridium.30e
Figure 4.
General mechanism for iridium catalyzed C-C coupling of primary alcohols and selected transformations applicable to polyketide construction: allylation, crotylation, tert-prenylation and tert-(hydroxy)prenylation.
Hydrometalative pathways are promoted by more electron rich ortho-C,O-benzoate moieties, which may be attributed to stabilization of the intermediate iridium hydride with respect to deprotonation.
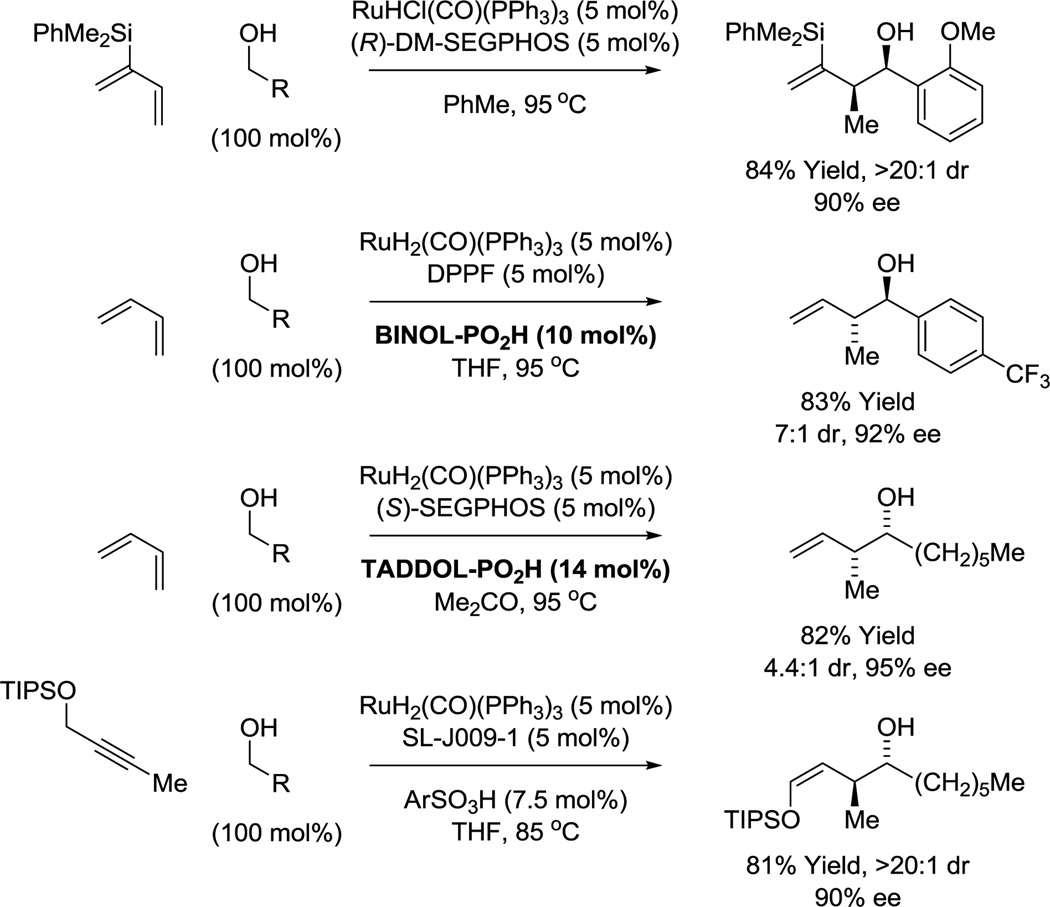
Numerous enantioselective iridium catalyzed C-C couplings based on these mechanisms have been developed.15 Those most relevant to polyketide construction include alcohol C-H allylation30 (site-selective,16,30f,g bidirectional30c), anti-crotylation31 (bidirectional31c), methallylation,32 propargylation,33a α-(methyl)propargylation,33b tert-prenylation34a,b and tert-(hydroxy)prenylation (Figure 4).34c Additionally, enantioselective ruthenium catalyzed alcohol C-C couplings for polyketide construction have been developed. The ruthenium catalyzed reactions are based solely on hydrometalative pathways, and include methods for alcohol C-H syn-crotylation35a,c and anti-crotylation (Scheme 1).35b,d
Scheme 1.
Enantioselective ruthenium catalyzed alcohol C-H crotylation.
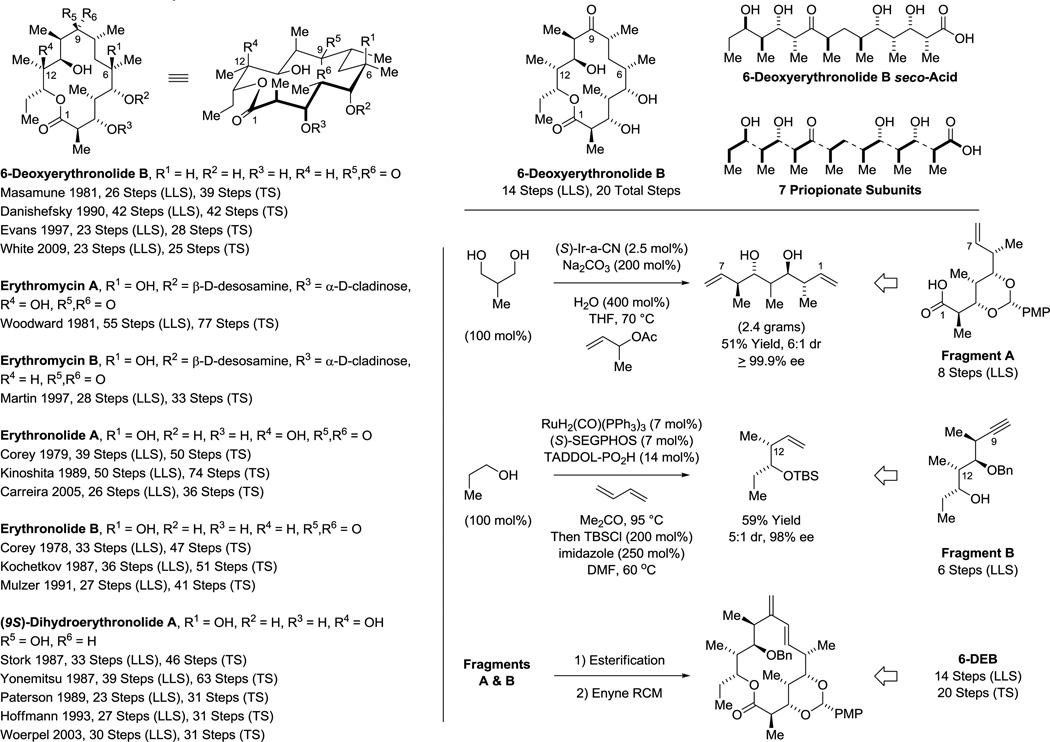
While additional process optimization will be required to realize the full potential of these methods, these protocols nevertheless define a distinct “second generation lexicon” for type I polyketide construction. 6-Deoxy-erythronolide B,36 the “Proteus” of polyketides, biogenic precursor to all erythromycin family members, served as an ideal testing ground. When first discovered, de novo construction of the erythromycins was deemed insurmountable.42 However, beginning with Corey’s landmark synthesis of erythronolide B in 1978,40a over 18 total syntheses of erythromycin family members are now reported (Scheme 2).36–41 This large body of prior art provided a unique opportunity to benchmark the impact of our methods on synthetic efficiency. As 6-deoxy-erythronolide B comprises 7 propionate subunits, our synthesis36f manifested as an exposition in carbonyl crotylation technology. The propionate-based triketide motif spanning C1–C6, a stereoquintet, was directly assembled via iridium catalyzed anti-diastereo- and enantioselective iridium double crotylation of 2-methyl-1,3-propane diol.31c As the minor enantiomer of the mono-adduct is converted to the meso-diastereomer,43 the double crotylation delivers a single enantiomer. The C10–C13 propionate-based diketide stereoquartet was prepared through ruthenium catalyzed C-C coupling of butadiene and propanol to form the indicated product of syn-crotylation.35c These fragments were combined through esterification and ring-closing enyne metathesis44 to form the 14-membered macrolide, enabling access to 6-deoxy-erythronolide B in only 14 steps (LLS), nearly 10 steps shorter than the prior shortest routes, the most concise construction of any erythronolide reported, to date (Scheme 2).
Scheme 2.
Prior total syntheses of selected erythromycin family members and total synthesis of 6-deoxyerythronolide B via enantioselective alcohol C-H crotylation.a
aFor graphical summaries of prior total syntheses, see Supporting Information. LLS = Longest Linear Sequence; TS = Total Steps.
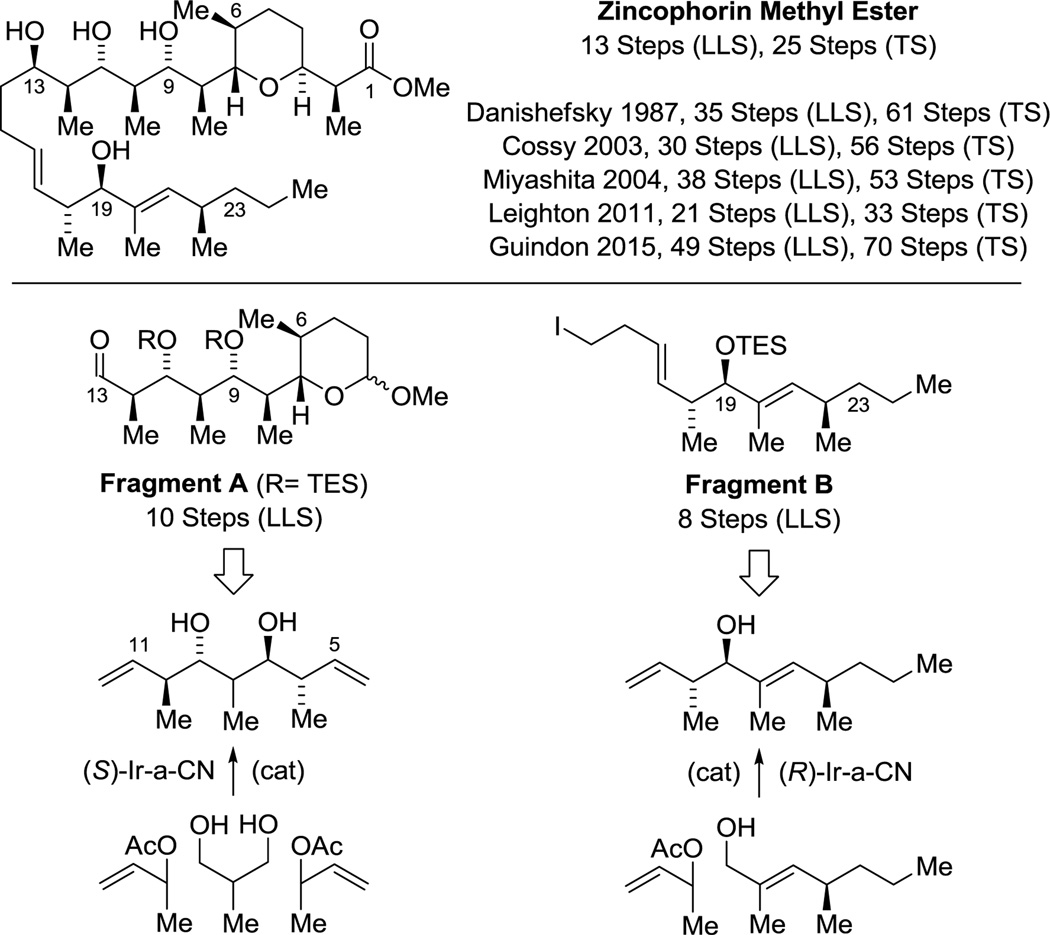
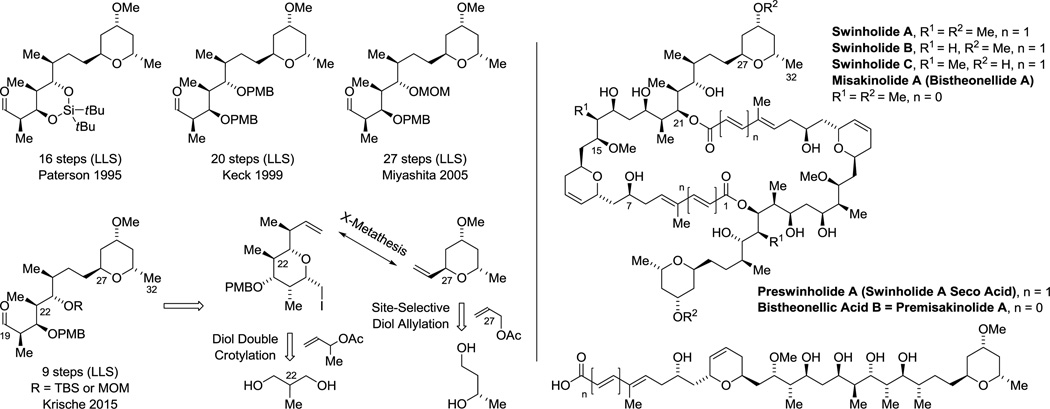
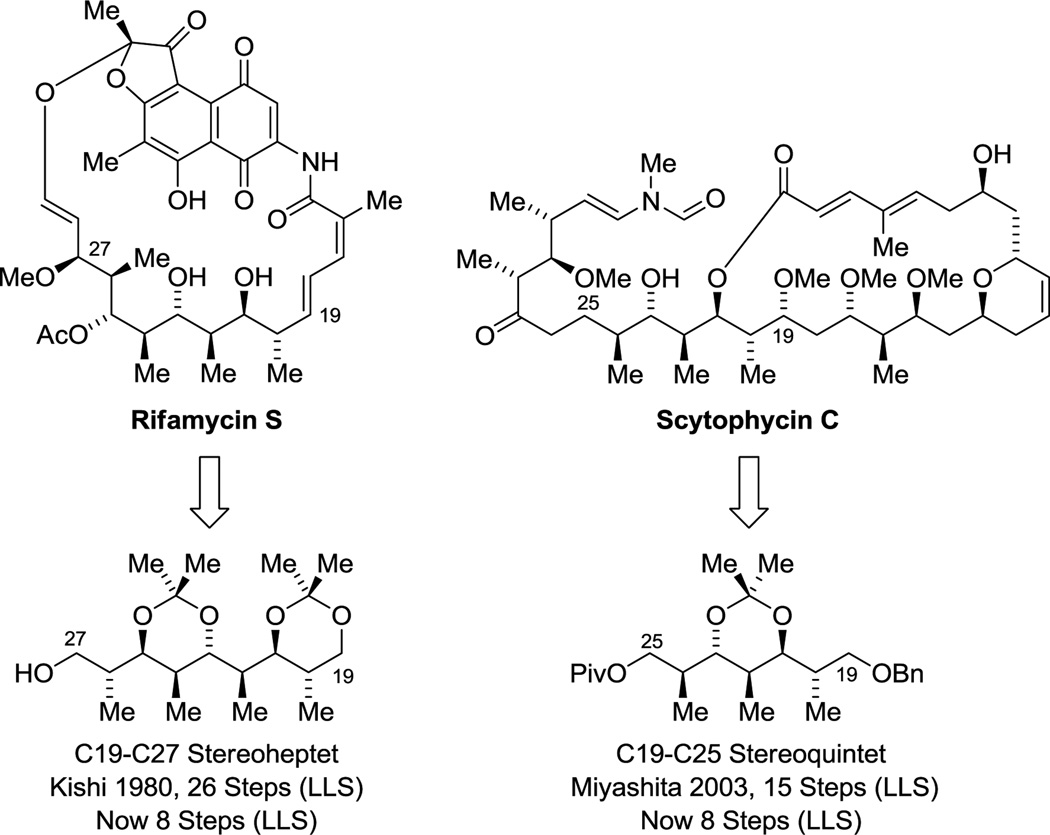
The enantioselective bidirectional bis(anti-crotylation) of 2-methyl-1,3-propane diol31c provided a concise means of preparing diverse type I polyketide natural products. For example, application of this method to the construction of (+)-zincophorin methyl ester,45 which bears 13 stereogenic centers, enabled a remarkably concise 13 step (LLS) synthesis.46i The 5 previously reported syntheses range between 21–49 steps in length (LLS) (Scheme 3).46a–h Similarly, the C19–C27 stereoheptet of rifamycin S20,47 and the C19–C25 stereoquintet of scytophycin C48,49 could be assembled in a fraction of the steps previously required, representing formal syntheses of these compounds.31c Finally, using diol double crotylation31c in combination with site-selective diol allylation,30g a stereopolyad common to the swinholides50–54 and numerous other type I polyketides,52i,54,57 including saliniketal,55 reidispongiolide56 and premisakinolide,57 could be prepared in a relatively short number of steps, constituting a formal synthesis of the latter (Scheme 4).58
Scheme 3.
Total synthesis of (+)-zincophorin methyl ester via enantioselective alcohol C-H crotylation.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
Scheme 4.
Formal synthesis of premisakinolide A and C(19)–C(32) of swinholide A via site-selective C-H allylation and crotylation of unprotected diols.a
aFor graphical summaries of prior syntheses, see Supporting Information.
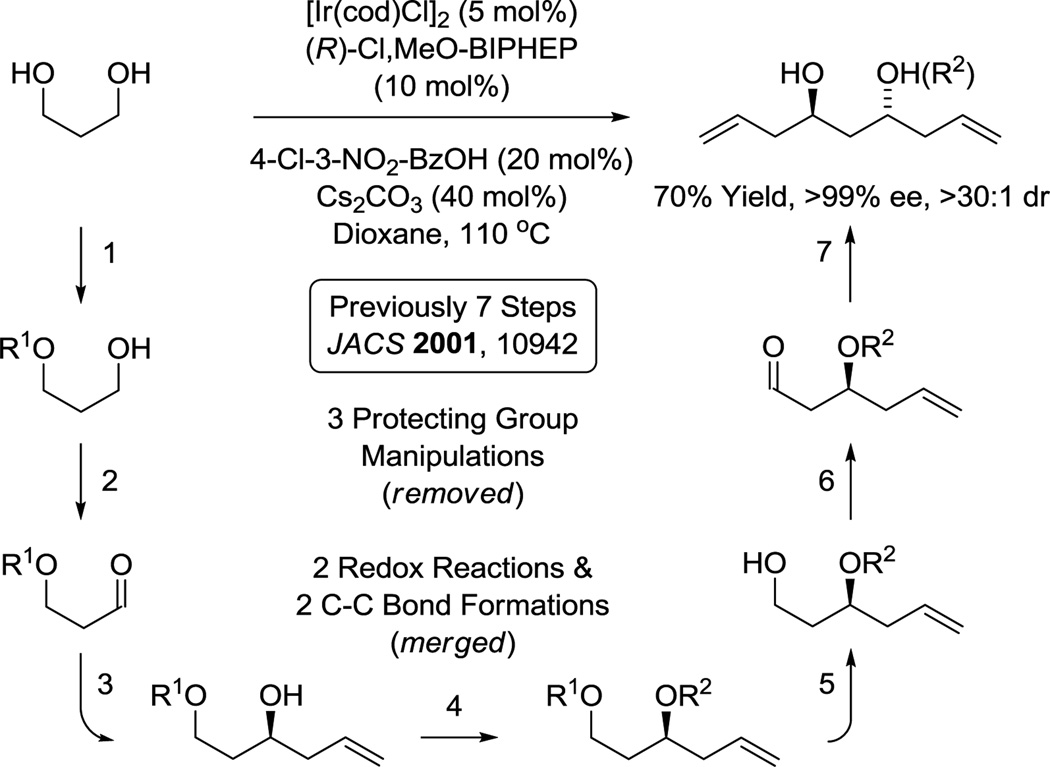
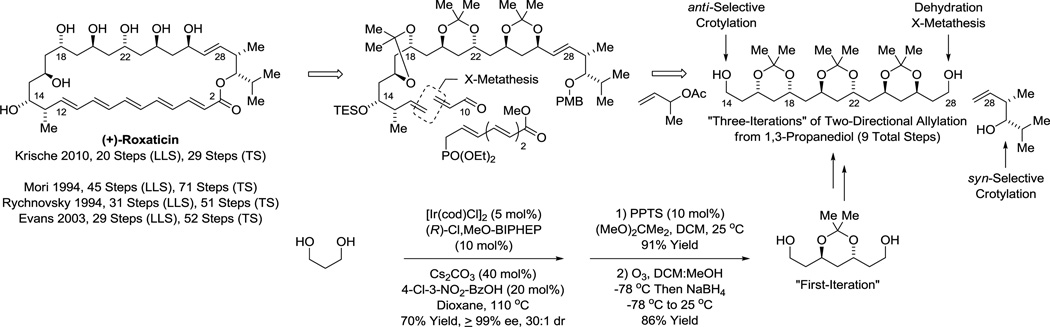
Polyacetate substructures in the form of 1,3-polyols also represent an important motif in type I polyketide natural products. Double C-H allylation of 1,3-propane diol enables direct formation of an acetate-based triketide motif,30c which forms as a single enantiomer due to the aforementioned amplification effect.43 Notably, the same C2-symmetric diol was prepared through a 7 step sequence involving 3 protecting group manipulations, two alcohol-to-aldehyde redox reactions and two carbonyl allylboration reactions (Scheme 5).59 This bidirectional chain elongation can be deployed iteratively to form 1,3-polyol substructures evident in numerous oxo-polyene macrolides.30c For example (+)-roxaticin60 incorporates a C2-symmetric polyol substructure that is readily formed using three iterations of bidirectional alcohol C-H allylation. Functionalization of the enantiotopic primary alcohol termini via dehydration-cross-metathesis and alcohol C-H crotylation, respectively, set the stage for installation of the polyene motif using a second cross-metathesis followed by Horner-Wadsworth-Emmons olefination. Finally, macrolactonization followed by global deprotection delivered (+)-roxaticin in 20 steps (LLS) from 1,3-propanediol.61f Nine of ten C-C bonds formed in the longest linear sequence are made via metal catalysis with 6 C-C bonds formed via iridium catalyzed alcohol C-H allylation. This synthesis of (+)-roxaticin is 9–25 steps shorter than previous routes that employ conventional carbanion chemistry (Scheme 6).61
Scheme 5.
Direct generation of an acetate-based triketide motif via bidirectional enantioselective C-H allylation of 1,3-propane diol.a
aFor further experimental details, see references 30c, 61f, 67e, 70h.
Scheme 6.
Total synthesis of the oxo-polyene macrolide (+)-roxaticin via enantioselective alcohol C-H allylation and crotylation.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
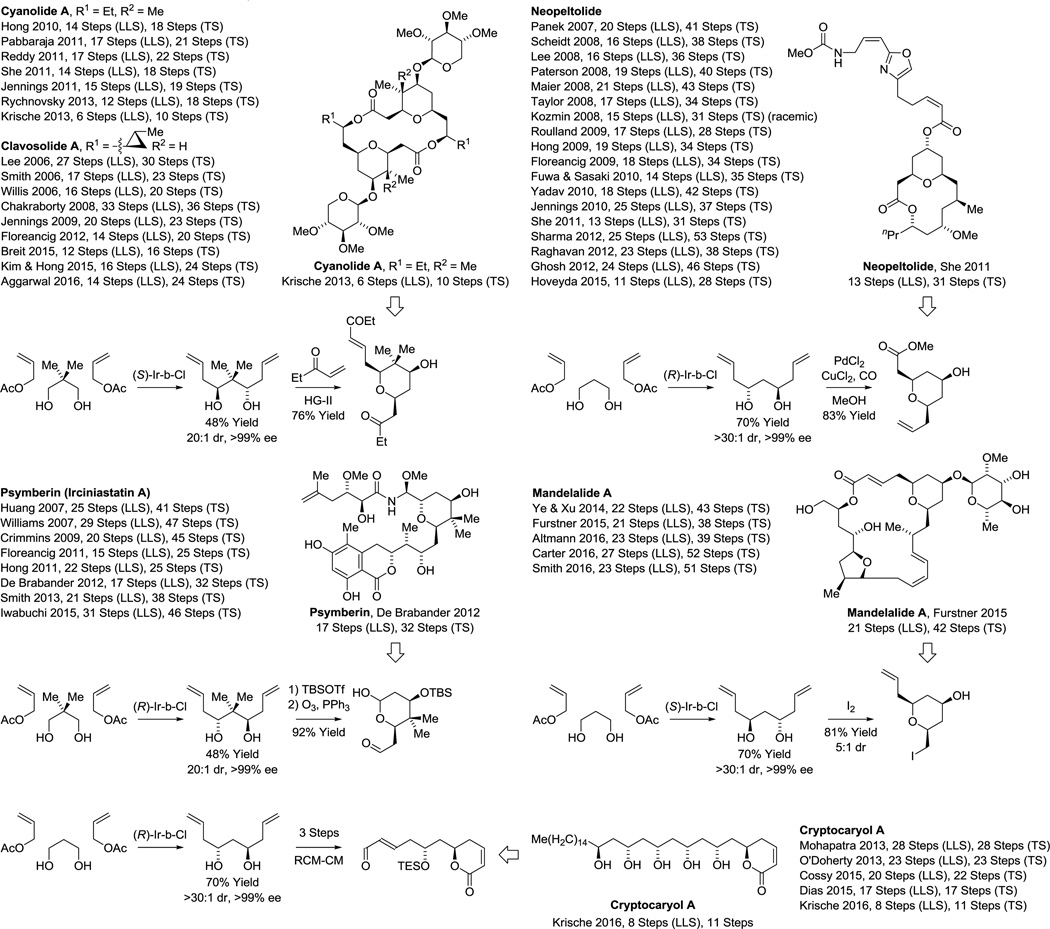
The bidirectional C-H allylation of 1,3-diols has proven effective in total syntheses of several other type I polyketides (Scheme 7). In the total synthesis of the macrodiolide cyanolide A,63g double C-H allylation of neopentyl glycol30c followed by tandem cross-metathesis-oxa-Michael cyclization provides rapid access to the highly substituted pyran core with complete control of diastereo- and enantioselectivity, enabling access to the natural product in less than half the steps previously required.63 Bidirectional C-H allylation of neopentyl glycol also was used by De Brabander in the total synthesis of psymberin (irciniostatin A).64a,i The Floreancig synthesis of this compound employs a strategically related iridium catalyzed mono-allylation reaction.64g These two routes to psymberin (irciniostatin A) are the shortest reported, to date (Scheme 7).64 In She’s total synthesis of neopeltolide,65q the C2-symmetric diol derived upon bidirectional C-H allylation of 1,3-propane diol is subjected to palladium catalyzed oxypalladation-alkoxycarbonylation to form the trisubstituted pyran core as a single diastereo- and enantiomer. A total of 18 total syntheses of neopeltolide have been reported.65 Hence, it is remarkably that She’s route,65q which employs bidirectional double C-H allylation is the second shortest (Scheme 7). Fürstner’s total synthesis of mandelalide A66a,c showcases yet another means of elaborating the C2-symmetric diol derived upon bidirectional C-allylation. Here, iodoetherification differentiates the olefin termini to define the structure of a trisubstituted pyran (Scheme 7). Finally, in the total synthesis of cryptocaryol A,67f the product of enantioselective diol double C-H allylation is subjected to ring-closing metathesis-cross metathesis to form an α-pyrone, which is converted to the natural product via aldol addition. This 8-step (LLS) synthesis of cryptocaryol A is fewer than half the steps of any prior approach.67
Scheme 7.
Total syntheses of cyanolide A, neopeltolide, psymberin (irciniastatin A), mandelalide and cryptocaryol A via bidirectional enantioselective alcohol C-H allylation.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
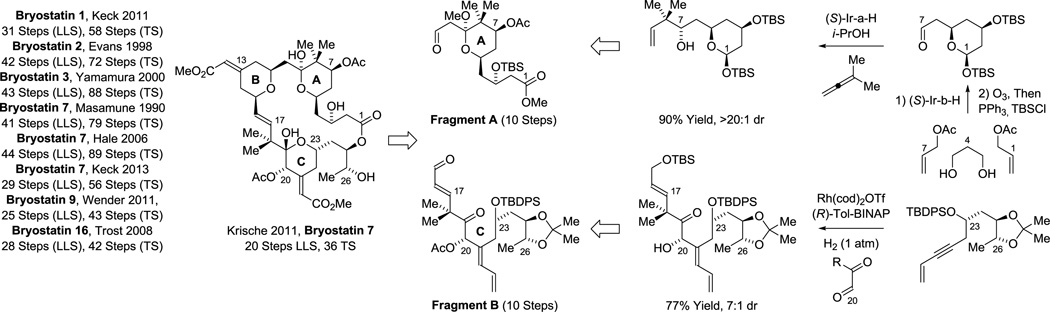
From these data it can be seen that the direct assembly of triketide motifs via bidirectional enantioselective diol C-H allylation can be applied across diverse contexts, evoking especially efficient strategies. For polyketides that embody higher degrees of structural complexity, streamlining or eliminating redox reactions and protecting groups becomes more challenging, and the nuanced marriage of strategy and methodology plays an increasingly important role. The bryostatins,68 a broad family of marine macrolides with impressive biological properties,69 offered a more stringent testing ground for our methods, as well as numerous benchmarks in the form of 8 previous syntheses.70a–g,i–k In our total synthesis of bryostatin 7,70h the natural product is convergently assembled through the union of the indicated fragments, which are of roughly equal complexity (Scheme 8). Fragment A is prepared through bidirectional C-H allylation of 1,3-propane diol, which assembles the polyacetate substructure spanning C1-C7.30c,71a The gem-dimethyl moiety at C8, a common motif in type I polyketides typically generated through the agency of SAM-dependent C-methyltransferases, is formed through asymmetric Ir-catalyzed tert-prenylation.34a Fragment B is prepared through hydrogen-mediated alkyne-carbonyl reductive coupling.71b Using these methods, bryostatin 7 was prepared in 20 steps (LLS), the most concise route to any bryostatin family member reported to date (Scheme 8).70h This strategy also enabled concise entry to bryostatin analogues.71c
Scheme 8.
Total synthesis of bryostatin 7 via hydrogenative and transfer hydrogenative carbonyl addition.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
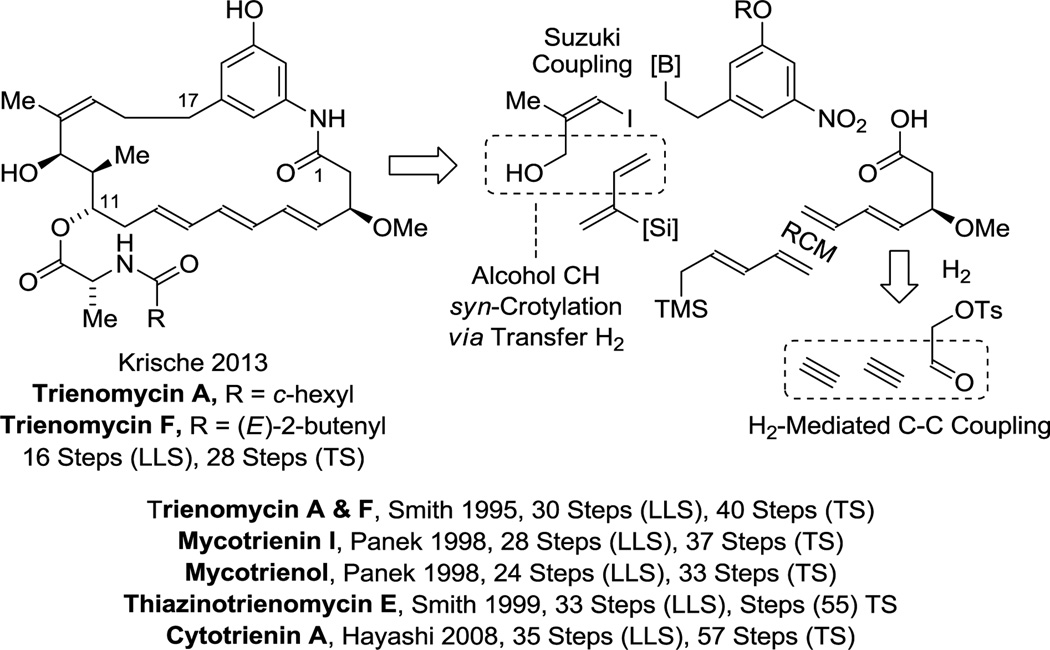
Merged redox and C-C bond construction events in the form of both hydrogenative and transfer hydrogenative methods were used in the total synthesis of trienomycins A and F.72 Specifically, enantioselective ruthenium catalyzed alcohol C-H-syn-crotylation35a followed by chelation-controlled carbonyl dienylation was used to prepare the C11–C13 stereotriad. Enantioselective rhodium catalyzed acetylene-aldehyde reductive coupling73 mediated by gaseous hydrogen forms a diene that ultimately is subjected to diene-diene ring closing metathesis to form the macrocycle. This approach is 14 steps shorter (LLS) than the prior syntheses of trienomycins A and F, and 8 steps shorter (LLS) than any prior synthesis of a triene-containing C17-benzene ansamycin (Scheme 9).72
Scheme 9.
Total synthesis of trienomycin A and F via hydrogenative and transfer hydrogenative carbonyl addition.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
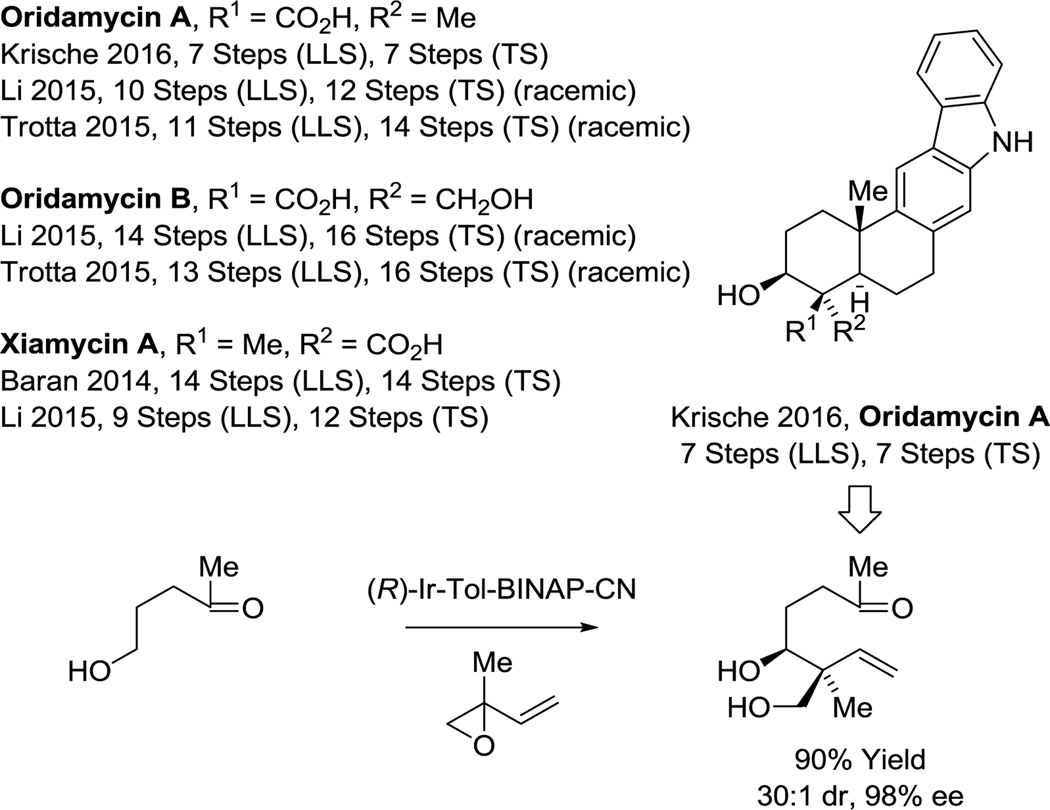
Finally, application of our methodology beyond the testing ground of type I polyketides is now underway. As illustrated in the total synthesis of the terpene alkaloid oridamycin A (Scheme 10),74d diastereo- and enantioselective iridium catalyzed tert-(hydroxy)prenylation34c converts a simple γ-hydroxy ketone to the indicated diol bearing an all-carbon quaternary stereocenter with exceptional control of relative and absolute stereochemistry. Using this transformation, oridamycin A was prepared in 7 steps (LLS), representing the first asymmetric synthesis of oridamycin A and shortest route to any member of this compound class.74
Scheme 10.
Total synthesis of oridamycin A via enantioselective alcohol C-H tert-(hydroxy)prenylation.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
Conclusions
As organic molecules are compounds composed of carbon and hydrogen, C-C bond formations accompanied by the addition, redistribution or removal of hydrogen represent a natural endpoint in the evolution of methods for efficient chemical synthesis. This concept initially led us to develop hydrogen-mediated reductive couplings of carbonyl compounds75 and, there-from, transfer hydrogenative C-C couplings that directly convert lower alcohols to higher alcohols:15 two new classes of catalytic C-C bond formations that bypass use of preformed carbanions. To benchmark the utility of these methods, total syntheses of several iconic type I polyketide natural products were undertaken. As shown in this perspective, a uniform increase in step-economy was observed in each case. Thus, by harnessing the native reducing ability of alcohols for the generation of transient carbonyl-organometal pairs, stereo- and site-selective skeletal assembly may be achieved in a manner that streamlines or eliminates the use of protecting groups and discrete alcohol-to-carbonyl redox reactions. These methods reinvent the chemistry of polyketide construction and, more broadly, the chemistry of carbonyl addition.
Supplementary Material
Figure 5.
Formal syntheses of rifamycin S and scytophycin C via enantioselective C-H crotylation of 2-methyl-1,3-propane diol.a
aFor graphical summaries of prior total syntheses, see Supporting Information.
Acknowledgments
The Robert A. Welch Foundation (F-0038), the NIH-NIGMS (RO1-GM093905) and the ACS-DOC graduate fellowship program (ZAK) are acknowledged for partial support of this research.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Graphical summaries of prior total syntheses of neopeltolide, psymberin (irciniastatin A), mandelalide, oridamycins, xiamycin A, trienomycins, roxaticin, bryostatins, swinholide, erythromycins, cyanolide A, clavosolide A, zincophorin and cryptocaryol A. This material is available free of charge via the internet at http://pubs.acs.org
REFERENCES
- 1.(a) Beller M, Cornils B, Frohning CD, Kohlpaintner CW. J. Mol. Catal. A. 1995;104:17. [Google Scholar]; (b) Frohning CD, Kohlpaintner CW, Bohnen H-W. In: Applied Homogeneous Catalysis with Organometallic Compounds. Cornils B, Herrmann WA, editors. Vol. 1. Weinheim: Wiley-VCH; 1996. pp. 29–104. [Google Scholar]; (c) van Leeuwen PWNM, Claver C, editors. Rhodium Catalyzed Hydroformylation. Norwell, MA: Kluwer Academic Publishers; 2000. [Google Scholar]; (d) Breit B, Seiche W. Synthesis. 2001:1. [Google Scholar]; (e) Weissermel K, Arpe H-J. Industrial Organic Chemistry. 4th. Weinheim: Wiley-VCH; 2003. pp. 127–144. [Google Scholar]; (f) van Leeuwen PWNM, editor. Homogeneous Catalysis: Understanding the Art. Dordrecht: Kluwer Academic Publishers; 2004. [Google Scholar]
- 2.(a) Jones JH. Platinum Met. Rev. 2000;44:94. [Google Scholar]; (b) Thomas CM, Suss-Fink G. Coord. Chem. Rev. 2003;243:125. [Google Scholar]; (c) Haynes A. Top. Organomet. Chem. 2006;18:179. [Google Scholar]; (d) Haynes A. Adv. Catal. 2010;53:1. [Google Scholar]
- 3.(a) Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; (b) Trost BM. Angew. Chem. Int. Ed. Engl. 1995;34:259. [Google Scholar]
- 4.(a) Sheldon RA. Chem. Ind. 1997:12. [Google Scholar]; (b) Sheldon RA. Green Chem. 2007;9:1273. [Google Scholar]; (c) Sheldon RA. Chem. Soc. Rev. 2012;41:1437. doi: 10.1039/c1cs15219j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Slezak Z. Chemik. 2003;56:153. [Google Scholar]; (b) Cadogan DF, Howick CJ. In: Kirk-Othmer Encyclopedia of Chemical Technology. 4th. Howe-Grant M, editor. Vol. 19. New York: Wiley & Sons; 1992. pp. 258–290. [Google Scholar]
- 6.Eribulin is roughly 200 times more potent than taxol, which compensates for its relatively high manufacturing costs: Yu MJ, Zheng W, Seletsky BM. Nat. Prod. Rep. 2013;30:1158. doi: 10.1039/c3np70051h.
- 7.Burns NZ, Baran PS, Hoffmann RW. Angew. Chem. Int. Ed. 2009;48:2854. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 8.(a) Site-Selective Catalysis; Kawabata T, editor. Topics in Current Chemistry. Vol. 372. Cham, Switzerland: Springer; 2016. Colby Davie EA, Mennen SM, Xu Y, Miller SJ. Chem. Rev. 2007;107:5759. doi: 10.1021/cr068377w.
- 9. Hoffmann RW. Synthesis. 2006:3531. Young IS, Baran PS. Nature Chem. 2009;1:193. doi: 10.1038/nchem.216. Saicic RN. Tetrahedron. 2014;70:8183. (d) Addition-Correction: Saicic RN. Tetrahedron. 2015;71:2777.
- 10.Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- 11.“The ideal synthesis creates a complex skeleton… in a sequence only of successive construction reactions involving no intermediary refunctionalizations, and leading directly to the structure of the target, not only its skeleton but also its correctly placed functionality.” Hendrickson JB. J. Am. Chem. Soc. 1975;97:5784.
- 12.Trost BM. Science. 1983;219:245. doi: 10.1126/science.219.4582.245. [DOI] [PubMed] [Google Scholar]
- 13.Han SB, Kim IS, Krische MJ. Chem. Commun. 2009:7278. doi: 10.1039/b917243m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn PJ. Chem. Soc. Rev. 2012;41:1452. doi: 10.1039/c1cs15041c. [DOI] [PubMed] [Google Scholar]
- 15.For a recent review, see: Ketcham JM, Shin I, Montgomery TP, Krische MJ. Angew. Chem. Int. Ed. 2014;53:9142. doi: 10.1002/anie.201403873.
- 16.Shin I, Krische MJ. Top. Curr. Chem. 2016;372:85. doi: 10.1007/128_2015_651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.“Given the fact that every reaction may be optimized… the total number of chemical transformations is the only variable in the determination of strategic efficiency. Obviously, the fewer the total number of reactions steps in a synthetic design, the higher the level of strategic efficiency.” Qiu F. Can. J. Chem. 2008;86:903. In our analysis, a step is defined as an operation that does not involve any intervening purification/separation, including removal of solvent, commencing with compounds that are over $50/gram.
- 18.For isolation of erythromycin A, see: McGuire JM, Bunch RL, Anderson RC, Boaz HE, Flynn EH, Powell HM, Smith JW. Antibiot. Chemother. 1952;2:281.
- 19.For isolation of amphotericin B, see: Vandeputte J, Watchtel JL, Stiller ET. Antibior. Annu. 1956:587. (b) X-ray structure: Mechinski W, Shaffner CP, Ganis P, Avitabile G. Tetrahedron Lett. 1970;11:3873. Ganis P, Avitabile G, Mechinski W, Shaffner CP. J. Am. Chem. Soc. 1971;93:4560. doi: 10.1021/ja00747a037.
- 20.For a review, see: Sensi P. Rev. Infect. Dis. 1983;5:S402. doi: 10.1093/clinids/5.supplement_3.s402.
- 21.For selected reviews, see: O’Hagan D. The Polyketide Metabolites. Chichester: Ellis Horwood; 1991. Rimando AM, Baerson SR. ACS Symp. Ser. 2007;955:1. Itoh T, Dechert-Schmitt A-MR, Schmitt DC, Gao X, Krische MJ. Nat. Prod. Rep. 2014;31:504. doi: 10.1039/c3np70076c.
- 22.(a) Newman DJ, Cragg GM. J. Nat. Prod. 2007;70:461. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]; (b) Newman DJ, Grothaus PG, Cragg GM. Chem. Rev. 2009;109:3012. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]; (c) Rohr J. Angew. Chem. Int. Ed. 2000;39:2847. doi: 10.1002/1521-3773(20000818)39:16<2847::aid-anie2847>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23. Sait M, Hugenholtz P, Janssen PH. Environ. Microbiol. 2002;4 doi: 10.1046/j.1462-2920.2002.00352.x. 654 and references cited therein.
- 24.(a) Arya P, Qin H. Tetrahedron. 2000;56:917. [Google Scholar]; (b) Otera J, editor. Modern Carbonyl Chemistry. Chapter 10. Weinheim: Wiley-VCH; 2000. p. 11. [Google Scholar]; (c) Mahrwald R, editor. Modern Methods in Stereoselective Aldol Reactions. Chapter 1. Weinheim: Wiley-VCH; 2013. pp. 5–7. [Google Scholar]; (d) Knochel P, Molander GA, editors. Comprehensive Organic Synthesis. 2nd. Vol. 2. Amsterdam: Elsevier; 2014. [Google Scholar]
- 25.Evans DA, Bartroli J, Shih TL. J. Am. Chem. Soc. 1981;103:2127. [Google Scholar]
- 26.For selected reviews on enantioselective carbonyl allylation, crotylation and related processes, see: Ramachandran PV. Aldrichim. Acta. 2002;35:23. Denmark SE, Fu J. Chem. Rev. 2003;103:2763. doi: 10.1021/cr020050h. Yu C-M, Youn J, Jung H-K. Bull. Korean Chem. Soc. 2006;27:463. Marek I, Sklute G. Chem. Commun. 2007:1683. doi: 10.1039/b615042j. Hall DG. Synlett. 2007:1644. Hargaden GC, Guiry PJ. Adv. Synth. Catal. 2007;349:2407. Lachance H, Hall DG. Org. React. 2008;73:1. Yus M, González-Gómez JC, Foubelo F. Chem. Rev. 2011;111:7774. doi: 10.1021/cr1004474.
- 27.For Brown’s chiral allyl-/crotyboron reagents, see: Brown HC, Jadhav PK. J. Am. Chem. Soc. 1983;105:2092. Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:293. doi: 10.1021/ja00279a042. Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:5919. doi: 10.1021/ja00279a042.
- 28.Suzuki T. Chem. Rev. 2011;111:1825. doi: 10.1021/cr100378r. [DOI] [PubMed] [Google Scholar]
- 29.(a) Noyori R, Hashiguchi S. Acc. Chem. Res. 1997;30:97. [Google Scholar]; (b) Clapham SE, Hadzovic A, Morris RH. Coord. Chem. Rev. 2004;248:2201. [Google Scholar]; (c) Warner MC, Casey CP, Baeckvall J-E. Top. Organomet. Chem. 2011;37:85. [Google Scholar]
- 30.For allylation, see: Kim IS, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2008;130:6340. doi: 10.1021/ja802001b. Kim IS, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2008;130:14891. doi: 10.1021/ja805722e. Lu Y, Kim IS, Hassan A, Del Valle DJ, Krische MJ. Angew. Chem. Int. Ed. 2009;48:5018. doi: 10.1002/anie.200901648. Hassan A, Lu Y, Krische MJ. Org. Lett. 2009;11:3112. doi: 10.1021/ol901136w. Schmitt DC, Dechert-Schmitt A-MR, Krische MJ. Org. Lett. 2012;14:6302. doi: 10.1021/ol3030692. Dechert-Schmitt A-MR, Schmitt DC, Krische MJ. Angew. Chem. Int. Ed. 2013;52:3195. doi: 10.1002/anie.201209863. Shin I, Wang G, Krische MJ. Chem. Eur. J. 2014:13382. doi: 10.1002/chem.201404065.
- 31.For crotylation, see: Kim IS, Han SB, Krische MJ. J. Am. Chem. Soc. 2009;131:2514. doi: 10.1021/ja808857w. Gao X, Townsend IA, Krische MJ. J. Org. Chem. 2011;76:2350. doi: 10.1021/jo200068q. Gao X, Han H, Krische MJ. J. Am. Chem. Soc. 2011;133:12795. doi: 10.1021/ja204570w.
- 32.For methallylation, see: Hassan A, Townsend IA, Krische MJ. Chem. Comm. 2011:10028. doi: 10.1039/c1cc14392a.
- 33.For propargylation and α-(methyl)propargylation, see: Geary LM, Woo SK, Leung JC, Krische MJ. Angew. Chem. Int. Ed. 2012;51:2972. doi: 10.1002/anie.201200239. Woo SK, Geary LM, Krische MJ. Angew. Chem. Int. Ed. 2012;51:7830. doi: 10.1002/anie.201203334.
- 34.For tert-prenylation and tert-(hydroxy)prenylation, see: Han SB, Kim IS, Han H, Krische MJ. J. Am. Chem. Soc. 2009;131:6916. doi: 10.1021/ja902437k. Han SB, Kim IS, Han H, Krische MJ. J. Am. Chem. Soc. 2010;132:12517. Feng J, Garza VJ, Krische MJ. J. Am. Chem. Soc. 2014;136:8911. doi: 10.1021/ja504625m.
- 35.For crotylation (Ru), see: Zbieg JR, Moran J, Krische MJ. J. Am. Chem. Soc. 2011;133:10582. doi: 10.1021/ja2046028. Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Science. 2012;336:324. doi: 10.1126/science.1219274. McInturff EL, Yamaguchi E, Krische MJ. J. Am. Chem. Soc. 2012;134:20628. doi: 10.1021/ja311208a. Liang T, Zhang W, Chen T-Y, Nguyen KD, Krische MJ. J. Am. Chem. Soc. 2015;137:13066. doi: 10.1021/jacs.5b08019.
- 36.For total syntheses of 6-deoxyerythronolide B see: Masamune S, Hirama M, Mori S, Ali SA, Garvey DS. J. Am. Chem. Soc. 1981;103:1568. Myles DC, Danishefsky SJ. J. Org. Chem. 1990;55:1636. Evans DA, Kim AS. Tetrahedron Lett. 1997;38:53. Evans DA, Kim AS, Metternich R, Novack VJ. J. Am. Chem. Soc. 1998;120:5921. Stang EM, White MC. Nat. Chem. 2009;1:547. doi: 10.1038/nchem.351. Gao X, Woo SK, Krische MJ. J. Am. Chem. Soc. 2013;135:4223. doi: 10.1021/ja4008722. (g) Formal Synthesis: Crimmins MT, Slade DJ. Org. Lett. 2006;8:2191. doi: 10.1021/ol0607241.
- 37.For total syntheses of erythromycin A, see: Woodward RB, Logusch E, Nambiar KP, Sakan K, Ward DE, Au-Yeung B-W, Balaram P, Browne LJ, Card PJ, Chen CH, Chênevert RB, Fliri A, Froble K, Gais HJ, Garratt DG, Hayakawa K, Heggie W, Hesson DP, Hoppe D, Hoppe I, Hyatt JA, Ikeda D, Jacobi PA, Kim KS, Kobuke Y, Kojima K, Krowicki K, Lee VJ, Leutert T, Malchenko S, Martens J, Matthews RS, Ong BS, Press JB, Rajan Babu TV, Rousseau G, Sauter HM, Suzuki M, Tatsuta K, Tolbert LM, Truesdales EA, Uchida I, Ueda Y, Uyehara T, Vasella AT, Vladuchick WC, Wade PA, Williams RM, Wong HN-C. J. Am. Chem. Soc. 1981;103 3215 and references cited therein. (b) Formal Syntheses: Bernet B, Bishop PM, Caron M, Kawamata T, Roy BL, Ruest L, Sauvé G, Soucy P, Deslongchamps P. Can. J. Chem. 1985;63 2818 and references cited therein. Nakata T, Fukui M, Oishi T. Tetrahedron Lett. 1988;29 2223 and references cited therein.
- 38.For total syntheses of erythromycin B, see: Martin SF, Hida T, Kym PR, Loft M, Hodgson A. J. Am. Chem. Soc. 1997;119:3193. Hergenrother PJ, Hodgson A, Judd AS, Lee W-C, Martin SF. Angew. Chem. Int. Ed. 2003;42:3278. doi: 10.1002/anie.200351136. Breton P, Hergenrother PJ, Hida T, Hodgson A, Judd AS, Kraynack E, Kym PR, Lee W-C, Loft MS, Yamashita M, Martin SF. Tetrahedron. 2007;63:5709.
- 39.For total syntheses of erythronolide A, see: Corey EJ, Hopkins PB, Kim S, Yoo S-E, Nambiar KP, Falck JR. J. Am. Chem. Soc. 1979;101 7131 and references cited therein. Nakata M, Arai M, Tomooka K, Ohsawa N, Kinoshita M. Bull. Chem. Soc. Jpn. 1989;62:2618. Muri D, Lohse-Fraefel N, Carreira EM. Angew. Chem. Int. Ed. 2005;117:4036. doi: 10.1002/anie.200500172. Muri D, Carreira EM. J. Org. Chem. 2009;74:8695. doi: 10.1021/jo901817b. (e) Formal Synthesis: Hikota M, Tone H, Horita K, Yonemitsu O. Tetrahedron. 1990;46:4613. Hikota M, Tone H, Horita K, Yonemitsu O. J. Org. Chem. 1990;55:7. Sviridov AF, Borodkin VS, Ermolenko MS, Yashunsky DV, Kochetkov NK. Tetrahedron. 1991;47 2317 and references cited therein.
- 40.For total syntheses of erythronolide B, see: Corey EJ, Kim S, Yoo S, Nicolaou KC, Melvin LS, Brunelle DJ, Falck JR, Trybulski EJ, Lett R, Sheldrake PW. J. Am. Chem. Soc. 1978;100:4620. and references cited therein. Sviridov AF, Ermolenko MS, Yashunsky DV, Borodkin VS, Kochetkov NK. Tetrahedron Lett. 1987;28:3839. and references cited therein. Mulzer J, Kirstein HM, Buschmann J, Lehmann C, Luger P. J. Am. Chem. Soc. 1991;113:910. (d) Formal Synthesis: Chandra B, Fu D, Nelson SG. Angew. Chem. Int. Ed. 2010;49:2591. doi: 10.1002/anie.200906245.
- 41.For total syntheses of (9S)-dihydroerythronolide A, see: Stork G, Rychnovsky SD. J. Am. Chem. Soc. 1987;109:1565. Stork G, Rychnovsky SD. Pure Appl. Chem. 1987;59:345. Tone H, Nishi T, Oikawa Y, Hikota M, Yonemitsu O. Tetrahedron Lett. 1987;28:4569. Tone H, Nishi T, Oikawa Y, Hikota M, Yonemitsu O. Chem. Pharm. Bull. 1989;37:1167. Paterson I, Rawson DJ. Tetrahedron Lett. 1989;30:7463. Stürmer R, Ritter K, Hoffmann RW. Angew. Chem. Int. Ed. 1993;32:101. Peng Z-H, Woerpel KA. J. Am. Chem. Soc. 2003;125:6018. doi: 10.1021/ja034865z.
- 42.“Erythromycin, with all of our advantages, looks at present time quite hopelessly complex, particularly in view of its plethora of asymmetric centers.” Woodward RB. In: Perspectives in Organic Chemistry. Todd A, editor. New York: Wiley-Interscience; 1956. p. 160.
- 43.(a) Kogure T, Eliel EL. J. Org. Chem. 1984;49:576. [Google Scholar]; (b) Midland MM, Gabriel J. J. Org. Chem. 1985;50:1143. [Google Scholar]; (c) Poss CS, Schreiber SL. Acc. Chem. Res. 1994;27:9. [Google Scholar]
- 44.For an authoritative review of enyne metathesis, see: Diver ST, Giessert AJ. Chem. Rev. 2004;104:1317. doi: 10.1021/cr020009e. For a related enone-olefin cross-metathesis, see: Xuan R, Oh H-S, Lee Y, Kang H-Y. J. Org. Chem. 2008;73:1456. doi: 10.1021/jo702384d.
- 45.For the isolation and structure determination of (+)-zincophorin, also known as M144255 or griseocholin, see: Gräfe U, Schade W, Roth M, Radics L, Incze M, Ujszászy K. J. Antibiot. 1984;37:836. doi: 10.7164/antibiotics.37.836. Radics L. J. Chem. Soc. Chem. Commun. 1984:599. Brooks HA, Gardner D, Poyser JP, King TJ. J. Antibiot. 1984;37:1501. doi: 10.7164/antibiotics.37.1501. Radics L, Kajtár-Peredy M. J. Chem. Soc., Perkin Trans. 2. 1986:1471. Tonew E, Tonew M, Graefe U, Zopel P. Pharmazie. 1988;43:717. Scharfenberg-Pfeiffer D, Czugler M. Pharmazie. 1991;46:781.
- 46.For total syntheses of (+)-zincophorin and its methyl ester, see: Danishefsky SJ, Selnick HG, DeNinno MP, Zelle RE. J. Am. Chem. Soc. 1987;109:1572. Danishefsky SJ, Selnick HG, Zelle RE, DeNinno MP. J. Am. Chem. Soc. 1988;110:4368. Defosseux M, Blanchard N, Meyer C, Cossy J. Org. Lett. 2003;5:4037. doi: 10.1021/ol035177n. Defosseux M, Blanchard N, Meyer C, Cossy J. J. Org. Chem. 2004;69:4626. doi: 10.1021/jo0496042. Komatsu K, Tanino K, Miyashita M. Angew. Chem. Int. Ed. 2004;43:4341. doi: 10.1002/anie.200460434. Cossy J, Meyer C, Defosseux M, Blanchard N. Pure Appl. Chem. 2005;77:1131. Harrison TJ, Ho S, Leighton JL. J. Am. Chem. Soc. 2011;133:7308. doi: 10.1021/ja201467z. Godin F, Mochirian P, St-Pierre G, Guindon Y. Tetra-hedron. 2015;71:709. Kasun ZA, Gao X, Lipinski RM, Krische MJ. J. Am. Chem. Soc. 2015;137:8900. doi: 10.1021/jacs.5b05296.
- 47.For total syntheses of rifamycin S, see: Nagaoka H, Rutsch W, Schmid G, Iio H, Johnson MR, Kishi Y. J. Am. Chem. Soc. 1980;102:7962. Iio H, Nagaoka H, Kishi Y. J. Am. Chem. Soc. 1980;102:7965. Kishi Y. Pure Appl. Chem. 1981;53:1163. Nagaoka H, Kishi Y. Tetrahedron. 1981;37:3873. Nagaoka H, Schmid G, Iio H, Kishi Y. Tetrahedron Lett. 1981;22:899. Rifamycin W, Nakata M, Akiyama N, Kojima K, Masuda H, Kinoshita M, Tatsuta K. Tetrahedron Lett. 1990;31:1585. Nakata M, Akiyama N, Kamata J, Kojima K, Masuda H, Kinoshita M, Tatsuta K. Tetrahedron. 1990;46:4629.
- 48.For isolation and structure determination of the scytophycins, see: (a) Isolation: Ishibashi M, Moore RE, Paterson GML, Xu C, Clardy J. J. Org. Chem. 1986;51:5300. Moore RE, Paterson GML, Mynderse JS, Barchi J, Jr, Norton TR, Furusawa E, Furusawa S. Pure Appl. Chem. 1986;58:263. Carmeli S, Moore RE, Paterson GML. J. Nat. Prod. 1990;53:1533. doi: 10.1021/np50072a021. Jung JH, Moore RE, Paterson GML. Phytochemistry. 1991;30:3615.
- 49.For total syntheses of the scytophycins, see: Paterson I, Watson C, Yeung K-S, Wallace PA, Ward RA. J. Org. Chem. 1997;62:452. doi: 10.1021/jo962189w. Paterson I, Yeung K-S, Watson C, Ward RA, Wallace PA. Tetrahedron. 1998;54:11935. Paterson I, Watson C, Yeung K-S, Ward RA, Wallace PA. Tetrahedron. 1998;54:11955. Nakamura R, Tanino K, Miyashita M. Org. Lett. 2003;5:3579. doi: 10.1021/ol035227o. Nakamura R, Tanino K, Miyashita M. Org. Lett. 2003;5:3583. doi: 10.1021/ol0352299.
- 50.For isolation and structure determination of swinholide A, see: Carmely S, Kashman Y. Tetrahedron Lett. 1985;26:511. Kobayashi M, Tanaka J, Katori T, Matsuura M, Kitagawa I. Tetrahedron Lett. 1989;30:2963. Kitagawa I, Kobayashi M, Katori T, Yamashita M, Tanaka J, Doi M, Ishida T. J. Am. Chem. Soc. 1990;112:3710. Kobayashi M, Tanaka J, Katori T, Matsuura M, Yamashita M, Kitagawa I. Chem. Pharm. Bull. 1990;38:2409. doi: 10.1248/cpb.38.2960. Doi M, Ishida T, Kobayashi M, Kitagawa I. J. Org. Chem. 1991;56:3629. Bewley CA, Holland ND, Faulkner DJ. Experientia. 1996;52:716. doi: 10.1007/BF01925581.
- 51.For isolation and structure determination of swinholides B–K see: Kobayashi M, Tanaka J-i, Katori T, Kitagawa I. Chem. Pharm. Bull. 1990;38:2960. doi: 10.1248/cpb.38.2960. Tsukamoto S, Ishibashi M, Sasaki T, Kobayashi J-i. J. Chem. Soc., Perkin Trans. 1. 1991:3185. Dumdei EJ, Blunt JW, Munro MHG, Pannell LK. J. Org. Chem. 1997;62:2636. doi: 10.1021/jo961745j. Youssef DTA, Mooberry SL. J. Nat. Prod. 2006;69:154. doi: 10.1021/np050404a. De Marino S, Festa C, D’Auria MV, Cresteil T, Debitus C, Zampella A. Mar. Drugs. 2011;9:1133. doi: 10.3390/md9061133. Sinisi A, Calcinai B, Cerrano C, Dien HA, Zampella A, D’Amore C, Renga B, Fiorucci S, Taglialatela-Scafati O. Bioorg. Med. Chem. 2013;21:5332. doi: 10.1016/j.bmc.2013.06.015. Andrianasolo EH, Gross H, Goeger D, Musafija-Girt M, McPhail K, Leal RM, Mooberry SL, Gerwick WH. Org. Lett. 2005;7:1375. doi: 10.1021/ol050188x.
- 52.For Paterson’s total synthesis of swinholide A, see: Paterson I. Pure Appl. Chem. 1992;64:1821. Paterson I, Cumming JG. Tetrahedron Lett. 1992;33:2847. Paterson I, Smith JD. J. Org. Chem. 1992;57:3261. Paterson I, Smith JD. Tetrahedron Lett. 1993;34:5351. Paterson I, Cumming JG, Smith JD, Ward RA. Tetrahedron Lett. 1994;35:441. Paterson I, Cumming JG, Smith JD, Ward RA, Yeung K-S. Tetrahedron Lett. 1994;35:3405. Paterson I, Smith JD, Ward RA, Cumming JG. J. Am. Chem. Soc. 1994;116:2615. Paterson I, Yeung K-S, Ward RA, Cumming JG, Smith JD. J. Am. Chem. Soc. 1994;116:9391. Paterson I, Cumming JG, Ward RA, Lamboley S. Tetrahedron. 1995;51:9393. Paterson I, Smith JD, Ward RA. Tetrahedron. 1995;51:9413. Paterson I, Ward RA, Smith JD, Cumming JG, Yeung K-S. Tetrahedron. 1995;51:9437. Paterson I, Yeung K-S, Ward RA, Smith JD, Cumming JG, Lamboley S. Tetrahedron. 1995;51:9467.
- 53.For Nicolaou’s total synthesis of swinholide A, see: Patron AP, Richter PK, Tomaszewski MJ, Miller RA, Nicolaou KC. J. Chem. Soc., Chem. Commun. 1994:1147. Richter PK, Tomaszewski MJ, Miller RA, Patron AP, Nicolaou KC. J. Chem. Soc., Chem. Commun. 1994:1151. Nicolaou KC, Patron AP, Ajito K, Richter PK, Khatuya H, Bertinato P, Miller RA, Tomaszewski MJ. Chem. Eur. J. 1996;2:847. Nicolaou KC, Ajito K, Patron AP, Khatuya H, Richter PK, Bertinato P. J. Am. Chem. Soc. 1996;118:3059.
- 54.Keck GE, Lundquist GD. J. Org. Chem. 1999;64:4482. [Google Scholar]
- 55.For isolation and total syntheses of saliniketals A and B, see: (a) Isolation: Williams PG, Asolkar RN, Kondratyuk T, Pezzuto JM, Jensen PR, Fenical W. J. Nat. Prod. 2007;70:83. doi: 10.1021/np0604580. (b) Total Syntheses: Paterson I, Razzak M, Anderson EA. Org. Lett. 2008;10:3295. doi: 10.1021/ol801148d. Liu J, De Brabander JK. J. Am. Chem. Soc. 2009;131:12562. doi: 10.1021/ja9061757. Yadav JS, Hossain SkS, Madhu M, Mohapatra DK. J. Org. Chem. 2009;74:8822. doi: 10.1021/jo901913h.
- 56.For isolation and total synthesis of (−)-reidispongiolide A, see: (a) Isolation: D'Auria MV, Gomez-Paloma L, Minale L, Zampella A, Verbist J-F, Roussakis C, Dibitus C, Patissou J. Tetrahedron. 1994;50:4829. (b) Total Synthesis: Paterson I, Ashton K, Britton R, Cecere G, Chouraqui G, Florence GJ, Stafford J. Angew. Chem. Int. Ed. 2007;46:6167. doi: 10.1002/anie.200702178.
- 57.Nakamura R, Tanino K, Miyashita M. Org. Lett. 2005;7:2929. doi: 10.1021/ol050864v. [DOI] [PubMed] [Google Scholar]
- 58.Shin I, Krische MJ. Org. Lett. 2015;17:4686. doi: 10.1021/acs.orglett.5b02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AB, III, Minbiole KP, Verhoest PR, Schelhaas M. J. Am. Chem. Soc. 2001;123:10942. doi: 10.1021/ja011604l. [DOI] [PubMed] [Google Scholar]
- 60.For isolation of (+)-roxaticin, see: Maehr H, Yang R, Hong L-N, Liu C-M, Hatada MH, Todaro LJ. J. Org. Chem. 1992;57:4793.
- 61.For total syntheses of (+)-roxaticin, see: Rychnovsky SD, Hoye RC. J. Am. Chem. Soc. 1994;116:1753. Mori Y, Asai M, Kawade J-i, Okumura A, Furukawa H. Tetrahedron Lett. 1994;35:6503. Mori Y, Asai M, Okumura A, Furukawa H. Tetrahedron. 1995;51:5299. Mori Y, Asai M, Kawade J-i, Furukawa H. Tetrahedron. 1995;51:5315. Evans DA, Connell BT. J. Am. Chem. Soc. 2003;125:10899. doi: 10.1021/ja027638q. Han SB, Hassan A, Kim I-S, Krische MJ. J. Am. Chem. Soc. 2010;132:15559. doi: 10.1021/ja1082798.
- 62.For total and formal syntheses of clavosolide A, see: Son JB, Kim SN, Kim NY, Lee DH. Org. Lett. 2006;8:661. doi: 10.1021/ol052851n. Smith AB, III, Simov V. Org. Lett. 2006;8:3315. doi: 10.1021/ol0611752. Barry CS, Elsworth JD, Seden PT, Bushby N, Harding JR, Alder RW, Willis CL. Org. Lett. 2006;8:3319. doi: 10.1021/ol0611705. Chakraborty TK, Reddy VR, Gajula PK. Tetrahedron. 2008;64:5162. Carrick JD, Jennings MP. Org. Lett. 2009;11:769. doi: 10.1021/ol8028302. Peh GR, Floreancig PE. Org. Lett. 2012;14:5614. doi: 10.1021/ol302744t. Baker JB, Kim H, Hong J. Tetrahedron Lett. 2015;56:3120. doi: 10.1016/j.tetlet.2014.11.135. Haydl AM, Breit B. Angew. Chem. Int. Ed. 2015;54:15530. doi: 10.1002/anie.201506618. Millán A, Smith JR, Aggarwal VK. Angew. Chem. Int. Ed. 2016;55:2498. doi: 10.1002/anie.201511140.
- 63.For total and formal syntheses of cyanolide A, see: Kim H, Hong J. Org. Lett. 2010;12:2880. doi: 10.1021/ol101022z. Hajare AK, Ravikumar V, Khaleel S, Bhuniya D, Reddy DS. J. Org. Chem. 2011;76:963. doi: 10.1021/jo101782q. Yang Z, Xie X, Jing P, Zhao G, Zheng J, Zhao C, She X. Org. Biomol. Chem. 2011;9:984. doi: 10.1039/c0ob00971g. Pabbaraja S, Satyanarayana K, Ganganna B, Yadav JS. J. Org. Chem. 2011;76:1922. doi: 10.1021/jo102401v. Gesinski MR, Rychnovsky SD. J. Am. Chem. Soc. 2011;133:9727. doi: 10.1021/ja204228q. Sharpe RJ, Jennings MP. J. Org. Chem. 2011;76:8027. doi: 10.1021/jo201210u. Waldeck AR, Krische MJ. Angew. Chem. Int. Ed. 2013;52:4470. doi: 10.1002/anie.201300843.
- 64.For total and formal syntheses of psymberin (irciniastatin A), see: Jiang X, García-Fortanet J, De Brabander JK. J. Am. Chem. Soc. 2005;127:11254. doi: 10.1021/ja0537068. Huang X, Shao N, Palani A, Aslanian R, Buevich A. Org. Lett. 2007;9:2597. doi: 10.1021/ol071068n. Shangguan N, Kiren S, Williams LJ. Org. Lett. 2007;9:1093. doi: 10.1021/ol063143k. Smith AB, III, Jurica JA, Walsh SP. Org. Lett. 2008;10:5625. doi: 10.1021/ol802466t. Crimmins MT, Stevens JM, Schaaf GM. Org. Lett. 2009;11:3990. doi: 10.1021/ol901655e. Watanabe T, Imaizumi T, Chinen T, Nagumo Y, Shibuya M, Usui T, Ka-noh N, Iwabuchi Y. Org. Lett. 2010;12:1040. doi: 10.1021/ol1000389. Wan S, Wu F, Rech JC, Green ME, Balachandran R, Horne WS, Day BW, Floreancig PE. J. Am. Chem. Soc. 2011;133:16668. doi: 10.1021/ja207331m. Byeon SR, Park H, Kim H, Hong J. Org. Lett. 2011;13:5816. doi: 10.1021/ol2024289. Feng Y, Jiang X, De Brabander JK. J. Am. Chem. Soc. 2012;134:17083. doi: 10.1021/ja3057612. An C, Jurica JA, Walsh SP, Hoye AT, Smith AB., III J. Org. Chem. 2013;78:4278. doi: 10.1021/jo400260m. Uesugi S-I, Watanabe T, Imaizumi T, Ota Y, Yoshida K, Ebisu H, Chinen T, Nagumo Y, Shibuya M, Kanoh N, Usui T, Iwabuchi Y. J. Org. Chem. 2015;80:12333. doi: 10.1021/acs.joc.5b02256.
- 65.For total and formal syntheses of neopeltolide, see: Youngsaye W, Lowe JT, Pohlki F, Ralifo P, Panek JS. Angew. Chem. Int. Ed. 2007;46:9211. doi: 10.1002/anie.200704122. Custar DW, Zabawa TP, Scheidt KA. J. Am. Chem. Soc. 2008;130:804. doi: 10.1021/ja710080q. Woo SK, Kwon MS, Lee E. Angew. Chem. Int. Ed. 2008;47:3242. doi: 10.1002/anie.200800386. Paterson I, Miller NA. Chem. Commun. 2008:4708. doi: 10.1039/b812914b. Vintonyak VV, Maier ME. Org. Lett. 2008;10:1239. doi: 10.1021/ol8001255. Vintonyak VV, Kunze B, Sasse F, Maier ME. Chem. Eur. J. 2008;14:11132. doi: 10.1002/chem.200801398. Kartika R, Gruffi TR, Taylor RE. Org. Lett. 2008;10:5047. doi: 10.1021/ol802254z. Ulanovskaya OA, Janjic J, Suzuki M, Sabharwal SS, Schumacker PT, Kron SJ, Kozmin SA. Nat. Chem. Biol. 2008;4:418. doi: 10.1038/nchembio.94. Custar DW, Zabawa TP, Hines J, Crews CM, Scheidt KA. J. Am. Chem. Soc. 2009;131:12406. doi: 10.1021/ja904604x. Guinchard X, Roulland E. Org. Lett. 2009;11:4700. doi: 10.1021/ol902047z. Tu W, Floreancig PE. Angew. Chem. Int. Ed. 2009;48:4567. doi: 10.1002/anie.200901489. Kim H, Park Y, Hong J. Angew. Chem. Int. Ed. 2009;48:7577. doi: 10.1002/anie.200903690. Cui Y, Tu W, Floreancig PE. Tetrahedron. 2010;66:4867. doi: 10.1016/j.tet.2010.03.066. Fuwa H, Saito A, Sasaki M. Angew. Chem. Int. Ed. 2010;49:3041. doi: 10.1002/anie.201000624. Yadav JS, Kumar GGKSN. Tetrahedron. 2010;66:480. Martinez-Solorio D, Jennings MP. J. Org. Chem. 2010;75:4095. doi: 10.1021/jo100443h. Yang Z, Zhang B, Zhao G, Yang J, Xie X, She X. Org. Lett. 2011;13:5916. doi: 10.1021/ol2025718. Sharma GVM, Reddy SV, Ramakrishna KVS. Org. Biomol. Chem. 2012;10:3689. doi: 10.1039/c2ob25151e. Raghavan S, Samanta PK. Org. Lett. 2012;14:2346. doi: 10.1021/ol3007698. Athe S, Chandrasekhar B, Roy S, Pradhan TK, Ghosh S. J. Org. Chem. 2012;77:9840. doi: 10.1021/jo301425c. Yu M, Schrock RR, Hoveyda AH. Angew. Chem. Int. Ed. 2015;54:215. doi: 10.1002/anie.201409120.
- 66.For total syntheses of the initially proposed and revised structures assigned to mandelalide, see: Willwacher J, Fürstner A. Angew. Chem. Int. Ed. 2014;53:4217. doi: 10.1002/anie.201400605. Lei H, Yan J, Yu J, Liu Y, Wang Z, Xu Z, Ye T. Angew. Chem. Int. Ed. 2014;53:6533. doi: 10.1002/anie.201403542. Willwacher J, Heggen B, Wirtz C, Thiel W, Fürstner A. Chem. Eur. J. 2015;21:10416. doi: 10.1002/chem.201501491. Brütsch TM, Bucher P, Altmann K-H. Chem. Eur. J. 2016;22:1292. doi: 10.1002/chem.201504230. Veerasamy N, Ghosh A, Li J, Watanabe K, Serrill JD, Ishmael JE, McPhail KL, Carter RG. J. Am. Chem. Soc. 2016;138:770. doi: 10.1021/jacs.5b12318. Nguyen MH, Imanishi M, Kurogi T, Smith AB., III J. Am. Chem. Soc. 2016;138:3675. doi: 10.1021/jacs.6b01731.
- 67.For total syntheses of the initially proposed and revised structures assigned to cryptocaryol A, see: Reddy DS, Mohapatra DK. Eur. J. Org. Chem. 2013:1051. Wang Y, O’Doherty GA. J. Am. Chem. Soc. 2013;135:9334. doi: 10.1021/ja404401f. Brun E, Bellosta V, Cossy J. J. Org. Chem. 2015;80:8668. doi: 10.1021/acs.joc.5b01323. Dias LC, Kuroishi PK, de Lucca EC. Org. Biomol. Chem. 2015;13:3575. doi: 10.1039/c5ob00080g. Perez F, Waldeck AR, Krische MJ. Angew. Chem. Int. Ed. 2016;55 doi: 10.1002/anie.201600591. In Press.
- 68.For isolation and structural assignment of bryostatin 1, see: Pettit GR, Day JF, Hartwell JL, Wood HB. Nature. 1970;227:962. doi: 10.1038/227962a0. Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. J. Am. Chem. Soc. 1982;104:6846. (c) It is believed that the natural product actually derives from a bacterial symbiont of B. neritina: Sudek S, Lopanik NB, Waggoner LE, Hildebrand M, Anderson C, Liu H, Patel A, Sherman DH, Haygood MG. J. Nat. Prod. 2007;70:67. doi: 10.1021/np060361d.
- 69.For selected reviews of the chemistry and biology the bryostatins and their analogues, see: Pettit GR. J. Nat. Prod. 1996;59:812. doi: 10.1021/np9604386. Mutter R, Wills M. Bioorg. Med. Chem. Lett. 2000;8:1841. doi: 10.1016/s0968-0896(00)00150-4. Yu L, Krische MJ. In: Total Synthesis: At the Frontier of Organic Chemistry. Li JJ, Corey EJ, editors. Springer: Heidelberg, Germany; 2013. pp. 103–130.
- 70.For total and formal syntheses of the bryostatins, see: (a) Bryostatin 1: Keck GE, Poudel YB, Cummins TJ, Rudra A, Covel JA. J. Am. Chem. Soc. 2011;133:744. doi: 10.1021/ja110198y. (b) Bryostatin 2: Evans DA, Carter PH, Carreira EM, Prunet JA, Charette AB, Lautens M. Angew. Chem. Int. Ed. 1998;37:2354. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2354::AID-ANIE2354>3.0.CO;2-9. Evans DA, Carter PH, Carreira EM, Charette AB, Prunet JA, Lautens M. J. Am. Chem. Soc. 1999;121:7540. (d) Bryostatin 3: Ohmori K, Ogawa Y, Obitsu T, Ishikawa Y, Nishiyama S, Yamamura S. Angew. Chem. Int. Ed. 2000;39:2290. Ohmori K. Bull. Chem. Soc. Jpn. 2004;77:875. (f) Bryostatin 7: Kageyama M, Tamura T, Nantz M, Roberts JC, Somfai P, Whritenour DC, Masamune S. J. Am. Chem. Soc. 1990;112:7407. Manaviazar S, Frigerio M, Bhatia GS, Hummersone MG, Aliev AE, Hale KJ. Org. Lett. 2006;8:4477. doi: 10.1021/ol061626i. Lu Y, Woo SK, Krische MJ. J. Am. Chem. Soc. 2011;133:13876. doi: 10.1021/ja205673e. Kedei N, Lewin NE, Geczy T, Selezneva J, Braun DC, Chen J, Herrmann MA, Heldman MR, Lim L, Mannan P, Garfield SH, Poudel YB, Cummins TJ, Rudra A, Blumberg PM, Keck GE. ACS Chem. Biol. 2013;8:767. doi: 10.1021/cb300671s. (j) Bryostatin 9: Wender PA, Schrier AJ. J. Am. Chem. Soc. 2011;133:9228. doi: 10.1021/ja203034k. (k) Bryostatin 16: Trost BM, Dong G. Nature. 2008;456:485. doi: 10.1038/nature07543.
- 71.(a) Lu Y, Krische MJ. Org. Lett. 2009;11:3108. doi: 10.1021/ol901096d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cho C-W, Krische MJ. Org. Lett. 2006;8:891. doi: 10.1021/ol052976s. [DOI] [PubMed] [Google Scholar]; (c) Andrews IP, Ketcham JM, Blumberg PM, Kedei N, Lewin NE, Krische MJ. J. Am. Chem. Soc. 2014;136:13209. doi: 10.1021/ja507825s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.For total and formal syntheses of triene-containing C17-benzene ansamycins trienomycins A and F and thiazinotrienomycin E, mycotrienol I and mycotrienin I and cytotrienin A, see: Smith AB, III, Barbosa J, Wong W, Wood JL. J. Am. Chem. Soc. 1995;117:10777. Smith AB, III, Barbosa J, Wong W, Wood JL. J. Am. Chem. Soc. 1996;118:8316. Smith AB, III, Wan Z. Org. Lett. 1999;1:1491. doi: 10.1021/ol991049g. Smith AB, III, Wan Z. J. Org. Chem. 2000;65:3738. doi: 10.1021/jo991958j. Del Valle DJ, Krische MJ. J. Am. Chem. Soc. 2013;135:10986. doi: 10.1021/ja4061273. Panek JS, Masse CE. J. Org. Chem. 1997;62:8290. doi: 10.1021/jo971793j. Masse CE, Yang M, Solomon J, Panek JS. J. Am. Chem. Soc. 1998;120:4123. Hayashi Y, Shoji M, Ishikawa H, Yamaguchi J, Tamura T, Imai H, Nishigaya Y, Takabe K, Kakeya H, Osada H. Angew. Chem. Int. Ed. 2008;47:6657. doi: 10.1002/anie.200802079.
- 73.(a) Kong J-R, Krische MJ. J. Am. Chem. Soc. 2006;128:16040. doi: 10.1021/ja0664786. [DOI] [PubMed] [Google Scholar]; (b) Skucas E, Kong J-R, Krische MJ. J. Am. Chem. Soc. 2007;129:7242. doi: 10.1021/ja0715896. [DOI] [PubMed] [Google Scholar]; (c) Han SB, Kong J-R, Krische MJ. Org. Lett. 2008;10:4133. doi: 10.1021/ol8018874. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Williams VM, Kong J-R, Ko B-J, Mantri Y, Brodbelt JS, Baik M-H, Krische MJ. J. Am. Chem. Soc. 2009;131:16054. doi: 10.1021/ja906225n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.For total syntheses of oridamycin A and B and xiamycin A, see: Rosen BR, Werner EW, O’Brien AG, Baran PS. J. Am. Chem. Soc. 2014;136:5571. doi: 10.1021/ja5013323. Meng Z, Yu H, Li L, Tao W, Chen H, Wan M, Yang P, Edmonds DJ, Zhong J, Li A. Nat. Commun. 2015;6:6096. doi: 10.1038/ncomms7096. Trotta AH. Org. Lett. 2015;17:3358. doi: 10.1021/acs.orglett.5b01629. Feng J, Noack F, Krische MJ. Manuscript in Preparation.
- 75.For a review on reductive C-C coupling via catalytic hydrogenation, see: Hassan A, Krische MJ. Org. Proc. Res. Devel. 2011;15:1236. doi: 10.1021/op200195m.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.