Dear Editor,
The problem of whether some systemic pharmacological adjuncts confer significant benefit to the healing process of chronic venous ulcers has been widely discussed in several narrative surveys and in a number of consensus conferences, systematic reviews and meta-analyses.
The most relevant are the extensive and detailed survey by Nelson1 and the multi-society consensus on the management of venous ulcers just published.2 These outstanding analyses of the available data showed that only a few oral agents have supporting evidence as most likely effective adjuvants in this condition: namely pentoxifylline, micronized flavonoids and sulodexide, a natural sulphated glycosaminoglycan with a number of biological and clinical activities on endothelial cell functions.3
Just before the publication of the recent multi-society consensus,2 a systematic review appeared in your Journal reconsidering most of the agents previously surveyed by other groups.4 The authors concluded that, among the many systemic pharmacological agents studied to manage venous leg ulcers, pentoxifylline “… is currently the only drug that has promising evidence to support its use.”
One of us, coordinator of the largest trial on sulodexide in venous ulcers,5 that was preceded by a pilot study with similar design and equivalent results,6 noticed with great surprise that, although each of the two trials resulted largely positive (OR for complete healing at 60 days: 2.04 (1.13–3.69) and 2.45 (1.0–5.67), respectively), the authors of the review stated that “Pooled results from both these trials, however, have shown that sulodexide in comparison to placebo may not have beneficial effects as an adjunct to ulcer healing in addition to conventional therapy (RR 0.16 95% CI 0.06 to 0.26).”
We wondered whether there was any logical or statistical explanation for the fact that pooling the data of two similar studies, both significantly positive for the same drug, the result could be inverted, reversing the benefit shown in both individual studies into a pooled loss of benefit. Since a similar phenomenon (the Simpson’s paradox) may rarely occur under special conditions,7 we re-examined the statistics underlying the conclusion of the published review.
Unfortunately, it was immediately apparent that the authors of the review made a critical and macroscopic statistical error. The figures given in the Varatharajan’s paper on the pooled data from the two sulodexide trials were the absolute risk difference (RD: 0.16 (0.06; 0.26)), that they reported as relative risk (or risk ratio; RR), whereas the true RR is 1.65 (1.20; 2.27). If the authors would have correctly read and reported the statistical output of the meta-analysis, the pooled effect of sulodexide would have resulted superimposed to that of pentoxifylline (RR as reported in the Cochrane meta-analysis: 1.70 (1.30–2.24)).8 Further, the outcome analysed for sulodexide was complete healing, while that for pentoxifylline was “complete ulcer healing or … significant improvement,” with all the problems relating to leg ulcer measurement (another point the authors overlooked).
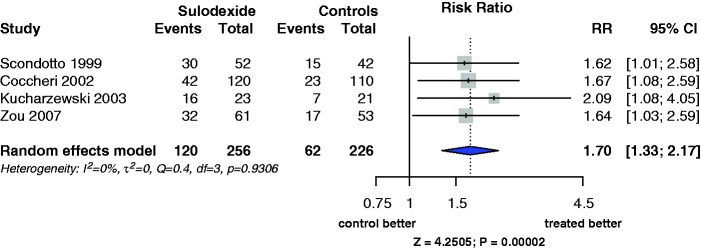
In addition, by simply browsing into the literature,1,9 two more papers reporting the effect of sulodexide in venous leg ulcers could easily have been retrieved.10,11 Including these papers into the meta-analysis would allow a more precise estimate of the pooled effect of sulodexide. The result of the meta-analysis (random effects model) of the four studies did not differ from that of the two studies mentioned in the Varatharajan’s paper, without evidence of heterogeneity (I2 = 0%; Figure 1). The risk ratio (RR) was 1.70 (1.33; 2.17), and the absolute risk difference was 0.19 (0.11; 0.27).
Figure 1.
Forest plot of the four published randomised controlled trials testing the effect of oral sulodexide plus compression vs. placebo or compression alone at two months, analysed according to the random effects model. The effect is reported as RR (risk ratio) with the relevant 95% confidence interval. The outcomes of the Zou study were monitored at one month.
It is therefore evident that considering a risk difference as a risk ratio, brought the authors to completely wrong conclusions about the effect of sulodexide, conclusions that were the opposite of those estimated by other important systematic reviews.1,2 Writing – wrongly as in the Varatharajan’s paper – “RR 0.16 95% CI 0.06 to 0.26” meant that the authors estimated that treated patients had a chance of healing, which was 16% of that of controls. Writing instead, correctly, “RD 0.16 95% CI 0.06 to 0.26” would mean that for each 6 patients (95% CI: 4 to 17) treated with the addition of sulodexide for two months, one more would experiences complete healing of venous leg ulcers. The two statements are quite substantially different. Using the pooled estimate from the four papers, changes the statement by a little amount: for each five patients (95% CI: 4 to 9) treated with the addition of sulodexide for two months, one more would experience complete healing of venous leg ulcers.
We are afraid that, in view of this macroscopic mistake, the paper must be corrected for all the considerations relevant to sulodexide, including the relevant statements in the abstract (and the record in Medline) as well as in the “What’s already known” paragraph, all – as well as the text – strongly biased by the error made in calculations.
References
- 1.Nelson EA. Venous leg ulcers. BMJ Clin Evid 2011; 2011: 1902–1902. [PMC free article] [PubMed] [Google Scholar]
- 2.Mosti G, De Maeseneer M, Cavezzi A, et al. Society for Vascular Surgery and American Venous Forum Guidelines on the management of venous leg ulcers: the point of view of the International Union of Phlebology. Int Angiol 2015; 34: 202–218. [PubMed] [Google Scholar]
- 3.Coccheri S, Mannello F. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des Devel Ther 2013; 8: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varatharajan L, Thapar A, Lane T, et al. Pharmacological adjuncts for chronic venous ulcer healing: a systematic review. Phlebology. 2016; 31: 356-365. [DOI] [PubMed]
- 5.Coccheri S, Scondotto G, Agnelli G, et al. Venous arm of the SUAVIS (Sulodexide Arterial Venous Italian Study) Group. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb Haemost 2002; 87: 947–952. [PubMed] [Google Scholar]
- 6.Scondotto G, Aloisi D, Ferrari P, et al. Treatment of venous leg ulcers with sulodexide. Angiology 1999; 50: 883–889. [DOI] [PubMed] [Google Scholar]
- 7.Norton HJ, Divine G. Simpson’s paradox and how to avoid it. Significance 2015; 12: 40–43. [Google Scholar]
- 8.Jull AB, Arroll B, Parag V, et al. Pentoxifylline for treating venous leg ulcers. Cochrane Database System Rev 2012; 12: CD001733–CD001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreozzi GM. Sulodexide in the treatment of chronic venous disease. Am J Cardiovasc Drugs 2012; 12: 73–81. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y-X, Feng X, Jing Z-P. Efficacy and safety of sulodexide in the treatment of venous ulcers of leg. Pharm Care Res (Yaoxue Fuwu Yu Yanjiu) 2007; 7: 22–24. [Google Scholar]
- 11.Kucharzewski M, Franek A, Koziolek H. Treatment of venous leg ulcers with sulodexide. Phlebologie 2003; 32: 115–120. [Google Scholar]