Summary
Francisella tularensis can cause fatal respiratory tularemia in humans and animals and is increasingly being isolated in the US and several European countries. The correlates of protective immunity against this intracellular bacterium are not known and currently there are no licensed vaccines available for human use. Cell-mediated immunity has long been believed to be critical for protection and the importance of humoral immunity is also now recognized. Furthermore, synergy between antibodies, T cell derived cytokines, and phagocytes appears to be critical to achieve sterilizing immunity against F. tularensis. Thus, novel vaccine approaches should be designed to induce robust antibody and cell-mediated immune responses to this pathogen.
Keywords: bacterial, lung, immunotherapies, phagocytosis, Fc receptors, antibodies
Introduction
The genus Francisella consists of two species: F. tularensis and F. philomiragia. F. tularensis is further classified into four subspecies: F. tularensis subspecies tularensis (type A), F. tularensis subspecies holarctica (type B), F. tularensis subspecies novicida, and F. tularensis subspecies mediasiatica (1). Types A and B are the major causes of human disease, while F. novicida is virulent in mice but avirulent for humans (2). Nevertheless, this strain has been used as an experimental model, because until recently, genetic studies were difficult to perform using the other subspecies (3, 4).
Wild rodents are the natural hosts for F. tularensis, but the bacteria can also survive in ticks and fresh water amoeba (5, 6). Humans are accidental hosts for the bacterium, and thus, only sporadic cases of human disease are reported. However, a recent surge in the incidence of human tularemia at Martha's Vineyard as well as in Eastern Europe is a cause for concern (7, 8). Cutaneous tularemia is the most common form of the disease but is rarely fatal (3). Typhoidal and respiratory forms of the disease, which are contracted by drinking contaminated water and by exposure to infected animal carcasses, respectively, can result however in >30% mortality if not treated with antibiotics (3). The extreme infectious nature of F. tularensis is exemplified by the fact that as few as 10 colony-forming units (cfu) of F. tularensis can cause fatal disease in humans and animals. Since this bacterium can be easily cultured, aerosolized, and rendered antibiotic resistant, it was developed into a biological weapon by many nations including the US and the former Soviet Union (9-11). Although stockpiles of weaponized F. tularensis were destroyed by the US military in the 1970s, it is unclear whether some former Soviet Union stockpiles remain (6). Thus, this bacterium is a major candidate for bioterrorism use.
F. tularensis is a typical intracellular pathogen with a high predilection for growth in macrophages (12). However, it is increasingly being recognized that many other cell types, such as alveolar epithelial cells, hepatocytes, and neutrophils, can also support the replication of F. tularensis (10, 13-15). Furthermore, recent reports have indicated that the bacteria can survive outside of host cells, but it is unknown if they can replicate in such an environment (16, 17). It has been shown that the bacterium utilizes receptors such as scavenger, mannose, and Fc receptors for its cellular entry (13, 18, 19). Serum components such as complement have also been shown to facilitate bacterial uptake (19-22). Once inside the host cell, the bacterium escapes from the phagosome and replicates within the cytoplasm (13, 23). F. tularensis modulates host defense mechanisms, perhaps by downregulating nuclear factor κB (NFκB)-mediated signaling pathway and attenuating inflammatory responses (12). In addition, even though F. tularensis is a Gram-negative bacterium, its lipopolysaccharide (LPS) is relatively inert (24-26). These features are typical of many other highly virulent intracellular bacteria such as Legionella and Burkholderia and extracellular pathogens such as Yersinia (27-29). Hence, an understanding of the host immune response to F. tularensis could lead to the design of novel platform strategies to treat and prevent a variety of bacterial infections.
Little is known about the host immune response to F. tularensis, particularly in the pulmonary tract. Nevertheless, the renewed interest in the bacterium following the 9/11 terrorist attacks has recently yielded valuable new information. F. holarctica and F. tularensis share 99% gene sequence homology and cause similar disease in animal models (30). Since there is available an attenuated strain of F. holarctica, the live vaccine strain (LVS) that is pathogenic to animals but not humans, many investigators have used LVS as an attractive surrogate model to study the biology and immunology of F. tularensis. Furthermore, LVS induces protective immunity against virulent strains of F. tularensis and F. holarctica in humans and animals (31-34). Although many laboratory animals including rabbits, rats, and guinea pigs, have been used for experimental studies of F. tularensis infection, the bulk of the available information has been obtained from mouse models of the disease (35). This review focuses primarily on the LVS mouse infection model, in which a clear picture of host-pathogen interaction is emerging. However, where applicable, we also refer to studies utilizing virulent strains of F. tularensis, F. holarctica, and F. novicida.
Humoral immunity to F. tularensis
The role of antibodies in protection against intracellular pathogens has been controversial. The prevailing dogma in the field has been that humoral immunity plays a critical role in protection against extracellular pathogens, whereas cell-mediated immunity (CMI) is more important for protection against intracellular pathogens. This notion in turn has had a profound influence on studies related to immune protection against F. tularensis. It has become generally believed that CMI is primarily responsible for the protection against F. tularensis, despite the fact that in the pre-antibiotic age, patients suffering from tularemia were successfully treated with xenogeneic immune serum (36-39). However, it is unclear from the early reports if this treatment was successful against the respiratory form of the disease. Recently, several studies have shown that antibodies are active in clearing infections with intracellular pathogens such as Salmonella and Ehrlichia (40) as well as influenza virus (41) and can aid in recovery from disease, thus challenging the previous dogma. Several groups have now demonstrated that antibodies also play an important role in protection against F. tularensis, including pulmonary tularemia.
Serum antibody responses
Many laboratories have reported that a robust anti-Francisella antibody response is generated in humans within two weeks of vaccination or actual infection (36, 42-45). The antibodies are directed primarily against the LPS component, but reactivity has also been detected against outer membrane proteins such as FopA and OmpA, and against intracellular proteins such as GroEL and KatG (46). In fact, one of the criteria for diagnosing human tularemia is the detection of F. tularensis-specific serum antibodies (47). Mouse studies of LVS infection have found a similar profile of antibody responses (48, 49). We have demonstrated that antibody responses are detected as early as seven days following infection of mice with a sublethal dose of LVS by the intranasal route (50). The antibody response reaches a peak at seven weeks following infection and consists of both T-helper 1 (Th1) and Th2 type antibody isotypes. Although BALB/c and C57BL/6 mice differ somewhat in their susceptibility to pulmonary LVS infection, antibody responses are similar in the two strains of mice. Western blot analysis of mouse immune sera has demonstrated that the majority of serum antibodies are directed against LPS (51). Similarly, a study reporting the generation of monoclonal antibodies against LVS indicated that most of the clones recovered were specific for LPS (51).
Although immune serum has been used to successfully treat human tularemia, several clinical studies performed in Europe in the middle of the last century found that killed vaccines, which were predicted to induce only antibody responses but little CMI, had no beneficial effect on the clinical course of systemic disease (52-54). This finding prompted many investigators to believe that antibodies had no role in protection, particularly against type A strains. An initial report addressing the importance of serum antibodies suggested that B cells, but not antibodies, were important for protection against LVS (55). In this study, it was found that B-cell-deficient μMT mice were able to clear primary intradermal infections with LVS. However, the mice were defective in mounting a secondary response against a lethal intraperitoneal LVS challenge. This defect could be overcome by transfer of naive B cells but not serum antibodies. The authors suggested that B cells were critical in dampening the infiltration of neutrophils to the spleen by an unknown mechanism. However, the titers of LVS-specific antibodies in recipient mice inoculated with serum antibodies before challenge were extremely low. Additionally, this study did not address the possibility that naive B cells, which were transferred a week before challenge, produced antibody in response to the challenge infection. Several later reports demonstrated that passive transfer of sufficient amounts of immune serum can protect mice against cutaneous forms of disease, including one report that specifically demonstrated the protective properties of LPS-specific immunoglobulin G (IgG) (56-58). Interestingly, intradermal vaccination with a sublethal dose of LVS protected wildtype but not μMT mice against subsequent lethal intraperitoneal challenge with LVS only two to three day after the initial challenge (59). The authors suggested that these results might be due to induction of LPS-specific antibodies in wildtype mice. A recent report also indicated that O-antigen specific antibodies are necessary for passive protection against lethal LVS challenge (60). In summary, apart from a few early reports, antibodies have been shown to provide protection against intradermal and intraperitoneal LVS infection.
The aerosol route of infection that results in fatal pulmonary tularemia is the most likely strategy to be used for bioterrorism. Therefore, we addressed the potential role of serum antibodies in protection against pulmonary tularemia in a mouse model (50). Passive intraperitoneal transfer of immune serum provided complete protection against intranasal challenge with lethal doses of LVS. Importantly, transfer of serum 24 to 48 h post-infection still provided a significant degree of protection against pulmonary tularemia. These results indicated that not only can serum antibodies contribute to protection in the lung, but they can also be used for therapeutic purposes. This finding is especially critical in the context of a terrorism threat in which an antibiotic resistant bacterium is likely to be used. Consistent with our results, immune serum generated by vaccinating mice with inactivated LVS rather than live LVS, as used in our studies, also afforded protection against lethal intranasal LVS challenge (61). Furthermore, others showed that monoclonal antibodies against LPS could be successfully used to treat pulmonary tularemia caused by LVS (51). Monoclonal antibodies against FopA have also been found to be protective against cutaneous and pulmonary forms of the disease (A.L. Savitt, personal communication). Taken together, these data show that antibodies can provide protection against pulmonary tularemia caused by LVS. Recently, similar observations were made in a mouse model of F. novicida infection (62).
Since LVS has been shown to exist in an extracellular form in mice, it is not surprising that antibodies can access and clear the bacteria. In fact, it is generally thought that F. tularensis spreads from the lungs via the hematogenous route to systemic organs such as the liver and spleen, possibly extracellularly (16). Thus, it is conceivable that antibodies are able to prevent this systemic spread of bacteria. Our data support this hypothesis, since very few bacteria were recovered from the blood, livers, and spleens of immune serum-treated mice following infection with LVS (50). Nevertheless, bacteria were also rapidly cleared from the lungs, presumably due to the exudation of serum antibodies into the lung environment. Further experiments designed to examine the immune correlates of protection revealed that serum antibody-mediated bacterial clearance was complement-independent. These data are consistent with earlier reports indicating that F. tularensis is protected from complement-mediated killing through expression of the O-antigen (60, 63). However, Fc receptors were necessary for the observed antibody-mediated protection, suggesting that phagocytosis is a key process involved in this protection (50). Indeed, Fc receptor-bearing phagocytes, including both macrophages and neutrophils, were found to be involved in bacterial clearance. Interestingly, these cells types also support growth of F. tularensis (13). A possible explanation for this apparent paradox is discussed below.
There are few data addressing the possible contribution of serum antibodies in protection against tularemia caused by F. tularensis type A strains. We investigated this by passively transferring serum anti-LVS antibodies into naive mice before intranasal challenge with F. tularensis SchuS4. A modest but statistically insignificant increase in median time to death was observed in mice treated with immune serum compared to mice treated with normal serum (Fig. 1). The reason for the failure of anti-LVS antibodies to protect mice against a Type A strain, even though the antibodies could bind in vitro to SchuS4, is not currently known and is an area of active study in our laboratory. One possible explanation is that a highly virulent F. tularensis strain completely shuts down the inflammatory responses that are required for efficient antibody-mediated bacterial clearance. In fact, recent reports and our own unpublished observations indicate that no significant inflammation is detected in mice until 72 to 96 h after infection with a type A strain of F. tularensis (64, 65, C.S. Bakshi and D.W. Metzger, unpublished observations). However, in LVS-infected mice, a dramatic inflammatory response is observed as early as 24 to 48 h post-infection (66, 67). Early clinical studies in North America reported that inoculation of inactivated LVS protected against type A strains of F. tularensis, suggesting that antibodies can indeed contribute to protection against serious forms of tularemia (37, 68, 69).
Fig. 1. Serum antibodies fail to protect mice against type A F. tularensis.
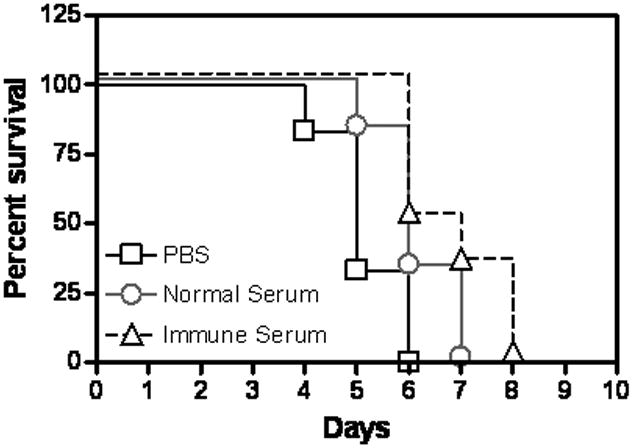
Groups of 4-5-week-old BALB/c mice (8/group) were treated intraperitoneal on days −1, 0, and +1 with 250 μl of normal serum or immune serum that was derived from mice immunized with LVS. The mice were challenged intranasal with 20 cfu of F. tularensis SchuS4 on day 0. Survival of the mice was observed for 10 days. (P=0.1676)
Mucosal antibody responses
Both human and animal studies have demonstrated that serum IgA is produced following vaccination or infection (42, 43). Mouse studies have also shown that IgA is detected in bronchioalveolar lavage (BAL) fluids following pulmonary vaccination (61, 70). However, the precise role of mucosal antibodies such as IgA in protection in the lung remains to be explored, since most of the early reports employed intradermal and intraperitoneal routes of infection. This response is particularly relevant for aerosol models of infection, where mucosal IgA may prevent bacterial adherence and augment clearance. In fact, indirect evidence for the protective role of mucosal antibody responses was obtained in vaccination studies against F. tularensis type A strains. As mentioned above, intradermal vaccination with LVS has been demonstrated to provide protection against intradermal but not intranasal challenge with Type A strains (33, 34). However, intranasal vaccination with LVS was found to induce complete protection against both routes of challenge. These results suggest that intranasal vaccination induces a local mucosal immune response that aids in protection against pulmonary F. tularensis. We have specifically addressed the contribution of IgA in protection against pulmonary tularemia using gene knockout mice. Our data have shown that IgA plays a critical role in protection against lethal LVS challenge in mice previously vaccinated with inactivated LVS and interleukin-12 (IL-12) as an adjuvant (71). Furthermore, a novel vaccination strategy of directed targeting of inactivated LVS to Fc receptor-bearing cells has also been found to be dependent on IgA (70). Interestingly, mice lacking the polymeric Ig receptor, which is necessary for transport of IgA across mucosal surfaces, survived lethal challenges with LVS or a type A strain following immunization with live LVS (Fig. 2), suggesting that live vaccine-induced protection is not dependent upon secretory IgA. The difference in the apparent need for IgA following vaccination with inactivated versus live bacteria could be due to the degree of inflammation induced in the lung following vaccination, i.e. an increased amount of inflammation induced by inoculation of live vaccine compared to inactivated bacteria may have allowed a greater amount of serum IgG transudation into the lung, thus masking a role for locally produced, secretory IgA (72). Further analysis of IgA-dependent and -independent mechanisms of protection is currently in progress.
Fig. 2. Secretory IgA is not required live vaccine-induced immunity.
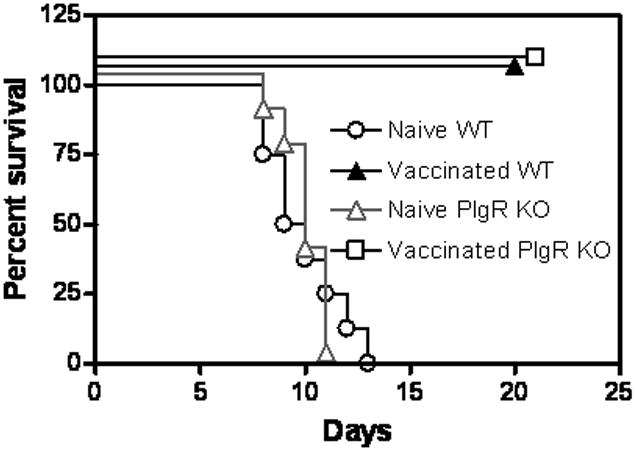
Groups of 4-5-week-old wildtype or polymeric Ig receptor KO mice (8/group) were vaccinated intranasally with a sublethal dose (1000 cfu) of LVS. The mice were challenged intranasally with an LD100 dose of LVS (104 cfu) on day 49, and survival was monitored for 21 days.
Challenges for antibody therapy of tularemia
Serum antibodies alone are clearly not sufficient to provide protection against highly virulent F. tularensis type A strains following pulmonary challenge (Fig. 1). However, as noted, in the early 20th century, xenogeneic immune serum was the drug of choice for treating human tularemia. It is possible that antibodies are effective for treating milder cutaneous or typhoidal forms of the disease but not for treating pulmonary tularemia. Additionally, monoclonal antibodies may be more efficacious than polyclonal antibodies for treatment of type A strain infections. It has been demonstrated that particular monoclonal antibodies are highly effective in treating infections caused by Cryptococcus, Ehrlichia, and Salmonella (40), because there is an absence of other antibodies that are typically present in polyclonal serum and that compete for binding to the cognate antigen(s), thus masking the beneficial effect of protective antibodies. A final possibility is that antibodies alone will never be sufficient to clear type A F. tularensis infection. As discussed below, a combinatorial approach is likely to be needed for effective therapy against F. tularensis type A strains.
CMI
Due to the intracellular nature of F. tularensis, CMI has been thought to play the major role in protection against this bacterium. Accumulating evidence indeed suggests that T cells are important for immunity against type A strains of F. tularensis (34, 73). Studies related to cell-mediated adaptive immunity have focused on the contribution of CD4+ and CD8+ T cells. However, phagocytic cells that are involved in traditional innate immune responses are also emerging as key players in limiting disease progression, shaping adaptive immunity, and effectively clearing the bacteria.
Macrophages, dendritic cells, and neutrophils
Macrophages are considered to be the primary host cells for F. tularensis infection (2, 3, 13, 74). However, depletion of alveolar macrophages (AMs) using liposomal chlodronate in an intranasal model of infection did not affect disease progression (50). This outcome could be explained by the fact that a variety of cell types, including neutrophils, dendritic cells (DCs), hepatocytes, and alveolar epithelial cells, have also been found to support bacterial replication (13).
The contribution of macrophages to protective immunity against F. tularensis is difficult to determine with certainty due to the relative paucity of macrophage-deficient animal models. Since macrophages are by far the predominant cell type in the alveoli of normal mice, it is likely that they play a critical role in innate defense against pulmonary bacterial infections, and hence, it is not surprising that F. tularensis has found ways to skew macrophage responses to favor its survival (12, 75). However, adaptive immunity in the form of cytokines and antibodies could reverse this modulation and allow macrophages to become effective in clearing bacteria. We have shown that macrophages play a key role antibody-mediated protection against LVS, since depletion of these cells resulted in abrogation of passive antibody-mediated protection (50). Our in vitro data demonstrated that AMs are capable of phagocytosing opsonized bacteria via Fc receptors and killing intracellularly via a nitric oxide-dependent mechanism (G.S. Kirimanjeswara and D.W. Metzger, unpublished observations). However, for efficient bactericidal effects, the macrophages needed to be activated with interferon-γ (IFN-γ). These data suggest that macrophages are a critical cell type for effective bacterial clearance and their activity can be augmented by B cells that produce antibodies and cells such as T or natural killer (NK) cells that produce IFN-γ.
DCs, regarded as another professional antigen-presenting cell, can also phagocytose and aid in the elimination of bacteria. These cells, upon phagocytosis, secrete cytokines such as IL-12 and upregulate expression of several costimulatory molecules such as CD80 and CD86 to aid in initiation of adaptive immune responses (76). It has been recently reported that LVS can invade and replicate in DCs. This process was found to be followed by an aberrant activation of DCs in which CD40, CD80, and CD86 expression was upregulated but no cytokines were produced (64, 77). In the case of type A strain of F. tularensis, responses of DCs were completely inhibited following infection (64). The authors concluded that LVS invades DCs but interferes with their activation and thus resists intracellular bactericidal mechanisms. Simultaneously, spread of bacterial infection to systemic sites via the migrating DCs would be enhanced. However, depletion of DCs using chlodronate resulted in only a marginal (one day) increase in the median time to death following lethal intranasal LVS challenge. Furthermore, vigilance needs to be taken in the phenotyping of pulmonary DCs; CD11c, usually taken as a selective marker of DCs, is also expressed on resident AMs, which, conversely, do not express the characteristic CD11b marker of peripheral monocytic populations.
Since no cytokines can be detected in the lungs for up to 96 h following infection with SchuS4, it is reasonable to believe that almost all pro-inflammatory host responses are actively inhibited by type A strains (64, 65). This finding could, as mentioned above, explain the relative ineffectiveness of serum antibodies to protect mice. LVS also induces little inflammatory response 24 to 48 h following infection, but neutrophils do arrive at the site of infection by 72 hr (66, 67). Nevertheless, the importance of neutrophils in innate immunity to F. tularensis is controversial. In a cutaneous model of disease, neutrophils were reported to be critical for innate immunity, as their depletion resulted in increased sensitivity of mice to LVS (78). However, in a secondary intraperitoneal LVS challenge model employing μMT mice, neutrophils accumulating in the spleen were believed to contribute to death rather than protection (79). In a pulmonary model of disease, neutrophils were reported to not play a significant role in protection (80). In fact, similar to the findings in μMT mice, it was suggested that they could even be harmful. It is known that activation of matrix metalloproteinase-9 (MMP-9) breaks down tissue collagen, which releases neutrophil attracting peptides. In the absence of MMP-9, LVS infection results in significantly reduced neutrophil recruitment into the lung and mice survive a normally lethal intranasal challenge (66). A similar result has been observed with type A strain infection (66). Consistent with these data, histopathological studies have revealed a massive infiltration of neutrophils into the lungs of mice before they succumb to LVS or SchuS4 infection. Our results, in contrast, have implicated a beneficial role of early recruitment of neutrophils (Fig. 3). Treatment of mice intranasally with IL-12 resulted in an early IFN-γ response and recruitment of neutrophils to the lungs within 24 h following infection. This early IFN-γ response appeared to be dependent on recruitment of NK cells (S. Olmos and D.W. Metzger, unpublished observations). The result was rapid clearance of bacteria after pulmonary LVS challenge and complete protection from death. Consistent with these observations, various avirulent mutants of LVS induce a rapid recruitment of neutrophils into the lung during infection and the bacteria are rapidly cleared (C.S. Bakshi and D.W. Metzger, unpublished observations). Similarly, an early recruitment of neutrophils is observed in IFN-αβR−/− mice, which are resistant to lethal challenge with LVS or F. novicida (S. O'Connell and D.W. Metzger, unpublished observations). Finally, we have shown that upon depletion of neutrophils, normal mice succumb to LVS challenge at a rate slightly faster than that of the neutrophil-sufficient animals and that the protective effects of passive antibody treatment against LVS infection are abrogated by such depletion (50). Interestingly, it has been reported that LVS can replicate in neutrophils (10). However, it is not known whether a similar replication can occur in IFN-γ-activated neutrophils. In summary, it is reasonable to conclude that early neutrophil recruitment into an IFN-γ-rich environment is beneficial to the host in controlling infection, while a delayed neutrophil response is actually counter productive.
Fig. 3. IL-12 treatment results in an early recruitment of neutrophils to the lungs and rapid clearance of bacteria.
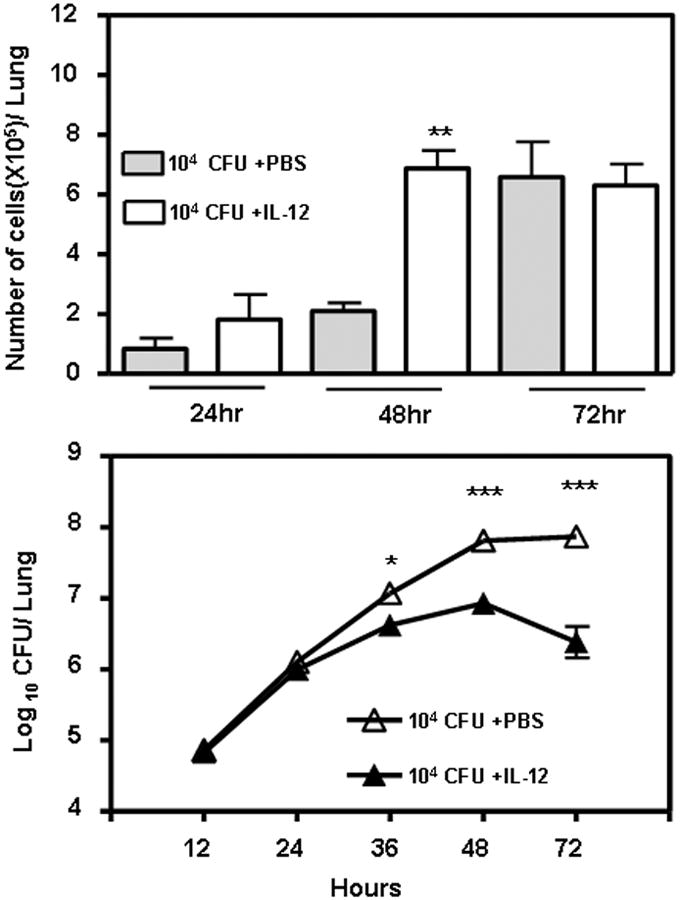
Groups of 4-5-week-old wildtype mice were treated intranasally with 0.5μg of IL-12 or phosphate buffered serum (PBS). The mice were challenged with an LD100 dose of LVS (104 cfu) 24 h later. A subset of 4 mice from each group were sacrificed at the indicated time points, and the lungs were homogenized and plated on chocolate agar plates for bacterial enumeration (lower panel), or their BAL fluids were collected and the number of neutrophils were enumerated by Geimsa's staining (upper panel). There were no significant differences in the numbers of macrophages or lymphocytes between the two groups. (* P<0.05, ** P<0.01, ***P<0.001 by student's t test)
NK and NKT cells
NK cells are the first cells to be recruited into the lungs following intranasal infection with LVS (81). The majority of these NK cells are IFN-γ+. Likewise, in human peripheral blood, NK cells are the primary cells that respond after in vitro exposure to inactivated LVS (82). Following intradermal LVS challenge, hepatic NK cells have been shown to be induced within 48 h to secrete IFN-γ and are the key cells responsible for granuloma formation, which may limit bacterial spread (83, 84). Depletion of NK cells results in a significant reduction in the median survival time of mice infected with LVS, suggesting that NK cells contribute to the innate immune response (72). However, in mice vaccinated with inactivated LVS, infiltration of NK cells into the lungs following bacterial challenge is significantly reduced compared to unvaccinated animals, suggesting that these cells may not be critical for vaccine-mediated protection (71). Similarly, transfer of immune serum to severe combined immunodeficient (SCID) mice, which harbor normal or even elevated levels of NK cells, does not result in protection against lethal intranasal LVS challenge, suggesting that NK cells are not sufficient for antibody-mediated protection (50). With regard to the contribution of NKT cells to immunity against F. tularensis, NKT-deficient CD1 knockout mice have been found to be more resistant to intranasal LVS challenge compared to wildtype animals (81). Thus, NK and NKT cells appear to play some roles in innate resistance and/or susceptibility to LVS infection, but their precise contributions remain to be defined.
T cells
As mentioned above, CD4+ and CD8+ T cells are considered to be the major cells responsible for immunity against F. tularensis. However, their relative involvement in immunity against LVS and type A strains appears to differ. Following intranasal or intradermal LVS infection of naive mice, the absence of CD4+ or CD8+ T cells had no significant effect on bacterial clearance (34). Similarly, during a secondary immune response induced by either vaccination or infection, depletion of either CD4+ or CD8+ T cells did not result in any appreciable loss of protection (85). In fact, in our studies, antibodies maintained their protective efficacy in CD4 knockout (KO) mice and CD8 KO mice, suggesting that either population of these cells is sufficient for humoral immune response-mediated protection (Fig. 4). However, depletion of both CD4+ and CD8+ T cells abrogated the beneficiary effect of serum antibodies against intranasal LVS challenge, indicating that T cells are required for antibody-mediated protection. This observation is consistent with our previous findings that SCID mice fail to be protected by serum antibody treatment (50). However, depletion of either CD4+ or CD8+ T cells results in the complete abrogation of immunity against type A strains of F. tularensis (33, 34). Although these latter results suggest that CD4+ and CD8+ T cells are essential for immunity against type A strains, it is not clear if they are sufficient, i.e. it is possible that they contribute to other arms of immunity as discussed below. For example, CD4+ T cells have been shown to limit the growth of LVS inside macrophages in an IFN-γ-dependent manner (86, 87). Interestingly, CD4−CD8− splenic T cells have been reported to be sufficient for protection against intradermal LVS challenge and have been reported to inhibit intracellular growth of LVS in an IFN-γ independent manner (88). A recent study attributed this bactericidal effect to membrane-bound tumor necrosis factor-α (TNF-α)(89). In support of these observations, both TNF-α– and IFN-γ-deficient mice are extremely susceptible to LVS challenge (90, 91). The relative contributions and interplay of CD4+, CD8+, and CD4−CD8− T cells in protection against LVS remains to be fully elucidated.
Fig. 4. T cells are necessary and CD4+ or CD8+ T cells are sufficient for antibody-mediated protection against LVS.
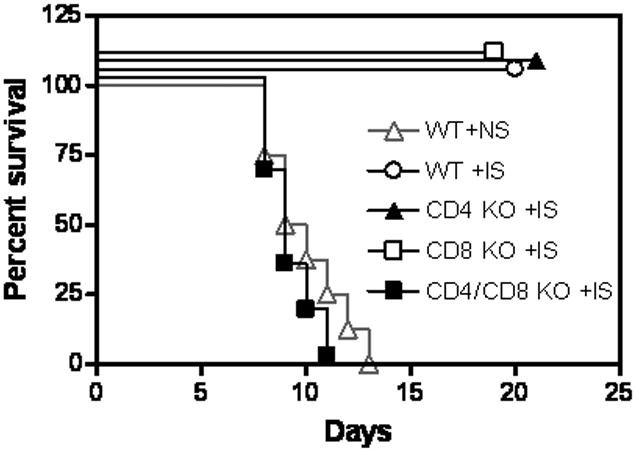
Groups of 4-5-week-old wildtype, CD4 KO mice, CD8 KO mice, and CD8 KO mice treated with anti-CD4 antibodies (8/group) were inoculated intraperitoneally with 250 μl of normal serum (NS) or immune serum (IS). The mice were challenged intranasally with 104 cfu of LVS 24 h later. Survival of the mice was monitored for 21 days. Normal serum-treated CD4 KO mice, CD8 KO mice, and CD4-depleted CD8 KO mice succumbed to infection in a manner identical to that of normal serum-treated wildtype mice.
Synergy between CMI and humoral immunity: an integrated model
It is being increasingly recognized that close and critical interactions between humoral immunity and CMI are required for effective protection against infectious agents. This holds true for both intracellular and extracellular pathogens. For instance, antibodies and neutrophils are critical for protection against the extracellular bacterium, Bordetella pertussis. However, this pathogen elaborates a toxin that prevents recruitment of neutrophils. To overcome this, antigen-specific CD4+ T cells secrete IFN-γ, which helps neutrophil recruitment. Similarly, protection against Yersinia pestis has been demonstrated to be dependent on IFN-γ, TNF-α, and inducible nitric oxide synthase (iNOS), and interactions between CMI and humoral immunity (92-94). In the case of Salmonella and Listeria, CMI plays a critical role in antibody-dependent bacterial clearance (40). In the case of F. tularensis, a similar interaction was found to be necessary for antibody-mediated protection (50). However, in all of these examples, the specific mechanisms by which CMI augments bacterial clearance have not been elucidated.
In vitro experiments employing primary AMs or an AM cell line, MH-S, showed that anti-LVS antibodies facilitated bacterial uptake and enhanced bacterial replication (Fig. 5). However, when the macrophages were first activated with IFN-γ, the antibody-opsonized bacteria were rapidly killed. IFN-γ-activated AMs, however, had only a bacteriostatic effect on non-opsonized bacteria. These data indicate that antibodies and IFN-γ, a component of CMI, work in concert to mediate rapid bacterial clearance.
Fig. 5. IFN-γ-activated macrophages rapidly kill an opsonized type A strain of F. tularensis.
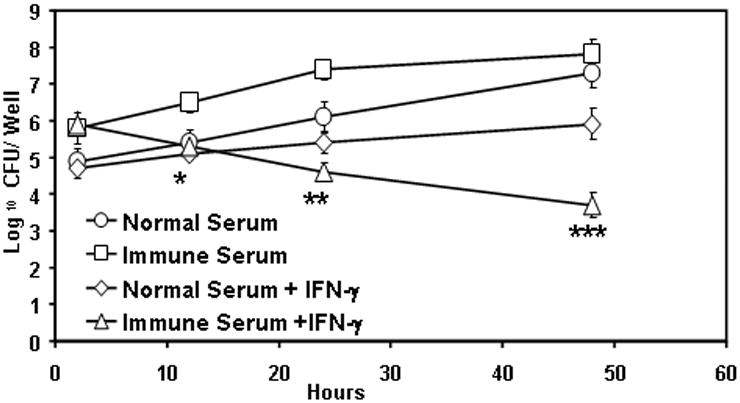
AMs were isolated from the BAL fluid of wildtype mice and were incubated in 24-well plates with 50 U/ml of IFN-γ or PBS for 18 h. Serum antibody opsonized or non-opsonized F. tularensis SchuS4 was then added at an MOI of 100 bacteria to each macrophage. After two hours of infection, the cells were treated with 50 μg/ml of gentamicin to kill any extracellular bacteria. The growth of the bacteria was monitored for 48 h by lysing the cells and plating on chocolate agar plates. (* P<0.05, ** P<0.01, ***P<0.001 by student's t test)
The cellular source of IFN-γ for antibody-mediated bacterial clearance is not known. Since a large number of IFN+ NK cells are recruited into the lung within 24 to 48 h following a lethal challenge with LVS, it is conceivable that these cells are the source of IFN-γ. Furthermore, antibodies are effective in CD4−/− and CD8−/− mice, suggesting that the presence of either CD4 or CD8 population is sufficient for antibody-mediated protection against LVS (Fig. 4). It is yet uncertain whether these T cells are Francisella-specific and how these T cells get activated and recruited so early in the infection. However, serum antibodies fail to protect SCID mice, which harbor slightly higher number of NK cells than wildtype mice. We have reported that neutrophils are also required for antibody-mediated protection. Interestingly, it was shown that neutrophils are recruited into the lungs following NK cell recruitment. Furthermore, early induction of IFN-γ by IL-12 results in a rapid recruitment of neutrophils (Fig. 3). These data suggest that IFN-γ secreted by NK cells aids in the recruitment of neutrophils to the lungs; however, this process is not sufficient for antibody-mediated protection.
We suggest a model in which LVS induces the recruitment of NK cells to the lungs within 24 to 48 h of infection (Fig. 6). These NK cells secrete IFN-γ, perhaps through Toll-like receptor 2 (TLR2) activation. IFN-γ in turn regulates the recruitment of neutrophils and activates both AMs and neutrophil inflammatory activities. Serum antibodies may transudate into the lung environment due to this inflammation. Such an environment would mimic in vitro experimental conditions in which IFN-γ-activated macrophages phagocytose and rapidly kill opsonized bacteria. These events lead to enhanced bacterial clearance in the lungs and prevent bacterial spread to other organs. In fact, in vivo data support this model, since serum antibody treatment has no effect on bacterial burden in the lungs during the first 24 to 48 h, at a time when no IFN-γ or neutrophils can be detected. By 72 h, however, increased levels of IFN-γ and neutrophils are observed, and bacterial numbers decrease.
Fig. 6. A model for synergy between humoral immunity and CMI in clearing pulmonary tularemia.
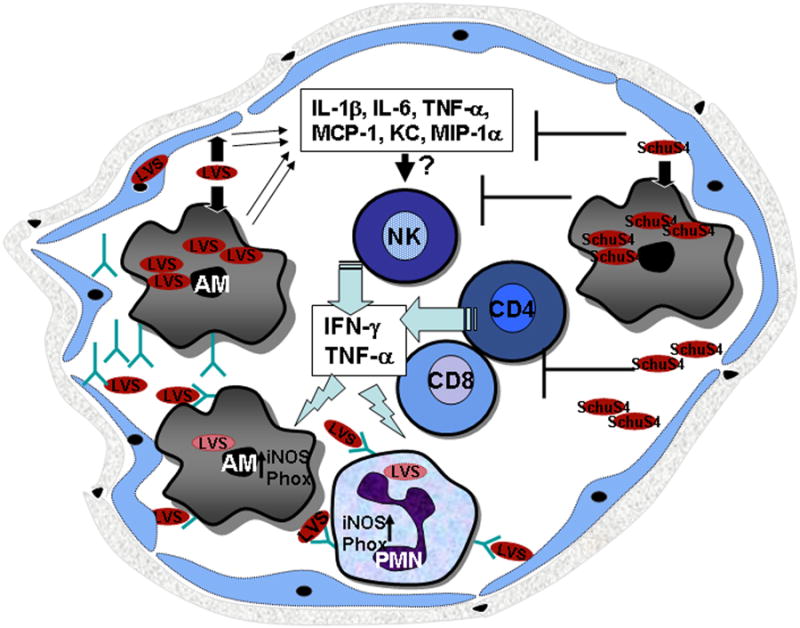
(Left) Attenuated LVS enters the alveolar space and is taken up by resident macrophages and epithelial cells. While the bacteria grow in such an intracellular environment early during infection, few inflammatory changes occur, and AMs are unable to control the growth of bacteria. However, by 24 to 48 h, LVS induces pro-inflammatory cytokines, which in turn recruit NK cells to the alveoli. The NK cells produce IFN-γ, which results in the recruitment of monocytes and neutrophils to the site of infection. Newly recruited CD4+ and CD8+ T cells also produce IFN-γ and TNF-α. This inflammatory environment results in the exudation of serum components including antibodies into the alveolar space. IFN-γ and TNF-α also prime FcγR-bearing neutrophils and macrophages. Opsonized bacteria trigger the activation of primed phagocytes via FcγR and enhance bactericidal activity, resulting in the killing of opsonized bacteria. However, highly virulent type A strains of F. tularensis such as SchuS4 (Right) suppresses all proinflammtroy responses at least for 72 h following infection. After 96 h, inflammatory changes are detected in the lungs but are not sufficient to control the high bacterial numbers.
How can this model of synergy between CMI and humoral immunity explain the failure of antibodies to protect against F. tularensis type A strains? As few as 20 cfu of F. tularensis SchuS4 have been shown to severely suppress host inflammatory responses for at least 72-96 h post-infection. In the meantime, the bacteria escape to peripheral organs such as the spleen and liver while the bacterial numbers in the lungs increase to ∼106 cfu. By 96 h, the bacterial burden in the lungs, spleen, and liver reaches to ∼108/organ. Although IFN-γ can be detected in the lungs 96 h after infection, it is likely too late for antibodies to have any effect, since the mice succumb to infection within the next 24 to 48 h. Thus, we predict that if inflammatory responses can be upregulated early during the infection, it will allow adoptively transferred antibodies to mediate bacterial clearance and allow the host to survive infection. Indeed, in vitro experiments have demonstrated that IFN-γ-activated macrophages are fully capable of phagocytosing and killing antibody-opsonized F. tularensis SchuS4 (G.S. Kirimanjeswara and D.W. Metzger, unpublished observations).
Several reports have indicated that following LVS vaccination, either CD4+ or CD8+ T cells are sufficient to protect mice against lethal LVS challenge. However, these reports have not addressed the contribution of antibodies in augmenting bacterial clearance. Since μMT and IFN-γ−/− mice do not develop protective immunity against LVS, it is conceivable that CD4+ and CD8+ T cells help antibody-mediated bacterial clearance in an IFN-γ-dependent fashion, particularly in primed hosts. Interestingly, antibodies and IFN-γ have also been implicated to play a major role in protection of mice against F. novicida. In the case of F. tularensis type A strains, both CD4+ and CD8+ T cells have been found to be required for protection following LVS vaccination. However, in this case, the role of antibodies in protection against F. tularensis has not been determined. A synergy between CMI and humoral immune response is likely to be involved in protective immunity against F. tularensis type A strains, suggesting an opportunity for prophylactic measures.
Conclusion
The recent emphasis on studying the biology and immunology of F. tularensis due to potential bioterror threats has significantly increased our current understanding of the host response to this pathogen. However, we still lack a unified and clear understanding of protective immunity against F. tularensis. This lack is partly due to the presence of the diversity of this group of bacteria and the various routes of infections and immunizations that have been utilized by individual investigators. It is further complicated by the extreme pathogenicity of type A strains, to which the host appears to mount no protective innate or adaptive immunity, although this latter complication can now be addressed due to the recent availability of procedures for generating attenuated type A strains through genetic manipulation.
Despite these assorted difficulties, we have been able to formulate a model for induction of protective immune responses to Francisella. Although many of our assumptions still need to be tested, synergy between humoral and CMI responses appears to be a critical requirement for inducing effective protection. In fact, a novel vaccination strategy that involves utilization of antibodies to target killed LVS to Fc receptors of antigen-presenting cells and thereby initiate protective mucosal and CMI responses has now been shown to provide protection against pulmonary SchuS4 infection (70). Furthermore, a superoxide dismutase mutant of LVS, which appears to enhance CMI, provides better protection against F. tularensis SchuS4 infection than wild type LVS, even in the highly susceptible C57BL/6 background (Fig. 7). In both cases, mucosal and serum antibody responses have been implicated in contributing to protection. Elucidation of the mechanisms responsible for rapid antibody-mediated clearance of F. tularensis has provided insights into the design of vaccination and immunotherapeutic strategies to achieve sterilizing immunity. Since there are no licensed vaccines available for human use, continued study of the immunobiology of F. tularensis host-pathogen interactions will provide important information that will be useful for the design of vaccine platforms against many intracellular biothreats.
Fig. 7. Vaccination with a superoxide dismutase mutant of LVS (sodBFt) confers protection against F. tularensis SchuS4 challenge.
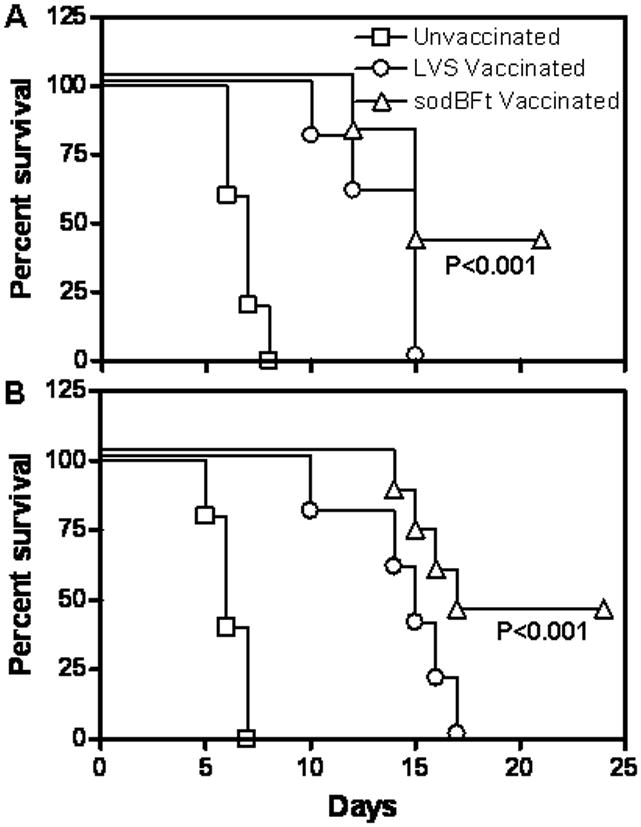
(A) C57BL/6 mice were vaccinated intranasal with 5×103 cfu of sodBFt or LVS and challenged with 10 CFU of F. tularensis SchuS4 on day 21 post-vaccination. (B) C57BL/6 were vaccinated intranasally with 5×102 cfu of F. tularensis LVS or sodBFt, boosted at day 21 with 1×103 cfu of the respective strain, and challenged with 100 CFU of F. tularensis SchuS4 on day 42 post-primary vaccination. Unvaccinated mice were used as controls. The mice were monitored for morbidity and mortality for a period of 21-30 days post-challenge. The results are expressed as Kaplan-Meier curves, and P values were determined using log-rank test.
Acknowledgments
We sincerely thank Michelle Wyland-O'Brien, Sherie O'Connell, and Sharon Salmon for excellent technical assistance and Bhuvana Katkere for the illustrations. We also thank the Center for Immunology and Microbial Disease Immunology Core Laboratory for assistance with experimental procedures. This work was supported by NIH grants PO1 AI056320 and U54 AI057158.
References
- 1.Sjostedt AB. Francisella. In: Boone DR, Castenholz RW, Brenner DJ, Garrity GM, Krieg NR, Staley JT, editors. The Proteobacteria, Part B, Bergey's Manual of Systematic Bacteriology. 2nd. Vol. 2. New York: Springer; 2005. pp. 200–210. [Google Scholar]
- 2.Ellis J, et al. Tularemia. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 4.Kawula TH, et al. Use of transposon-transposase complexes to create stable insertion mutant strains of Francisella tularensis LVS. Appl Environ Microbiol. 2004;70:6901–6904. doi: 10.1128/AEM.70.11.6901-6904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinclair R, et al. Persistence of category A select agents in the environment. Appl Environ Microbiol. 2008;74:555–563. doi: 10.1128/AEM.02167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keim P, Johansson A, Wagner DM. Molecular epidemiology, evolution, and ecology of Francisella. Ann NY Acad Sci. 2007;1105:30–66. doi: 10.1196/annals.1409.011. [DOI] [PubMed] [Google Scholar]
- 7.Vorou RM, Papavassiliou VG, Tsiodras S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol Infect. 2007;135:1231–1247. doi: 10.1017/S0950268807008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliasson H, et al. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20:289–311. doi: 10.1016/j.idc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Cronquist SD. Tularemia: the disease and the weapon. Dermatol Clin. 2004;22:313–320, vi. doi: 10.1016/j.det.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–1230. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson N, Cerdeno-Tarraga A, Bentley S. Massive attack. Nat Rev Microbiol. 2005;3:586–587. doi: 10.1038/nrmicro1212. [DOI] [PubMed] [Google Scholar]
- 12.Sjostedt A. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 2006;8:561–567. doi: 10.1016/j.micinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Clemens DL, Horwitz MA. Uptake and intracellular fate of Francisella tularensis in human macrophages. Ann NY Acad Sci. 2007;1105:160–186. doi: 10.1196/annals.1409.001. [DOI] [PubMed] [Google Scholar]
- 14.Hall JD, et al. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect Immun. 2007;75:1034–1039. doi: 10.1128/IAI.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen JW, et al. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect Immun. 2006;74:6590–6598. doi: 10.1128/IAI.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forestal CA, et al. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis. 2007;196:134–137. doi: 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- 17.Yu JJ, R E, Murthy AK, Guentzel MN, Klose KE, Arulanandam BP. The presence of infectious extracellular Francisella tularensis subsp. novicida in murine plasma after pulmonary challenge. Eur J Clin Microbiol Infect Dis. 2008;27:323–325. doi: 10.1007/s10096-007-0434-x. [DOI] [PubMed] [Google Scholar]
- 18.Balagopal A, et al. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierini LM. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 2006;8:1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 20.Schulert GS, Allen LA. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukoc Biol. 2006;80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemens DL, Lee BY, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Nasr A, et al. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leukoc Biol. 2006;80:774–786. doi: 10.1189/jlb.1205755. [DOI] [PubMed] [Google Scholar]
- 23.Santic M, et al. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 2006;14:37–44. doi: 10.1016/j.tim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Rahhal RM, et al. Differential effects of Francisella tularensis lipopolysaccharide on B lymphocytes. J Leukoc Biol. 2007;82:813–820. doi: 10.1189/jlb.1206765. [DOI] [PubMed] [Google Scholar]
- 25.Kanistanon D, et al. A Francisella Mutant in Lipid A Carbohydrate Modification Elicits Protective Immunity. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann N Y Acad Sci. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heesemann J, Sing A, Trulzsch K. Yersinia's stratagem: targeting innate and adaptive immune defense. Curr Opin Microbiol. 2006;9:55–61. doi: 10.1016/j.mib.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Molofsky AB, Swanson MS. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol Microbiol. 2004;53:29–40. doi: 10.1111/j.1365-2958.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 29.Peacock SJ. Melioidosis. Curr Opin Infect Dis. 2006;19:421–428. doi: 10.1097/01.qco.0000244046.31135.b3. [DOI] [PubMed] [Google Scholar]
- 30.Matyas BT, Nieder HS, Telford SR., 3rd Pneumonic tularemia on Martha's Vineyard: clinical, epidemiologic, and ecological characteristics. Ann NY Acad Sci. 2007;1105:351–377. doi: 10.1196/annals.1409.013. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, et al. Low dose aerosol infection of mice with virulent type A Francisella tularensis induces severe thymus atrophy and CD4+CD8+ thymocyte depletion. Microb Pathog. 2005;39:189–196. doi: 10.1016/j.micpath.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KuoLee R, et al. Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis Vaccine. 2007;25:3781–3791. doi: 10.1016/j.vaccine.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu TH, et al. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73:2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayne Conlan J, Oyston PC. Vaccines against Francisella tularensis. Ann NY Acad Sci. 2007;1105:325–350. doi: 10.1196/annals.1409.012. [DOI] [PubMed] [Google Scholar]
- 35.Rick Lyons C, Wu TH. Animal models of Francisella tularensis infection. Ann NY Acad Sci. 2007;1105:238–265. doi: 10.1196/annals.1409.003. [DOI] [PubMed] [Google Scholar]
- 36.Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 37.Foshay L. Tularemia. Annu Rev Microbiol. 1950;4:313–330. doi: 10.1146/annurev.mi.04.100150.001525. [DOI] [PubMed] [Google Scholar]
- 38.Francis E, Felton LD. Antitularemic serum. Public Health Rep. 1942;57:44–55. [Google Scholar]
- 39.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann NY Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- 40.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 41.Huber VC, et al. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 42.Koskela P, Salminen A. Humoral immunity against Francisella tularensis after natural infection. J Clin Microbiol. 1985;22:973–979. doi: 10.1128/jcm.22.6.973-979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koskela P, Herva E. Cell-mediated and humoral immunity induced by a live Francisella tularensis vaccine. Infect Immun. 1982;36:983–989. doi: 10.1128/iai.36.3.983-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janovska S, et al. Proteomic analysis of antibody response in a case of laboratory-acquired infection with Francisella tularensis subsp. tularensis. Folia Microbiol (Praha) 2007;52:194–198. doi: 10.1007/BF02932159. [DOI] [PubMed] [Google Scholar]
- 45.Waag DM, et al. Cell-mediated and humoral immune responses after vaccination of human volunteers with the live vaccine strain of Francisella tularensis. Clin Diagn Lab Immunol. 1995;2:143–148. doi: 10.1128/cdli.2.2.143-148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundaresh S, et al. From protein microarrays to diagnostic antigen discovery: a study of the pathogen Francisella tularensis. Bioinformatics. 2007;23:i508–i518. doi: 10.1093/bioinformatics/btm207. [DOI] [PubMed] [Google Scholar]
- 47.Splettstoesser WD, et al. Diagnostic procedures in tularaemia with special focus on molecular and immunological techniques. J Vet Med B Infect Dis Vet Public Health. 2005;52:249–261. doi: 10.1111/j.1439-0450.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- 48.Eyles JE, et al. Immunodominant Francisella tularensis antigens identified using proteome microarray. Crown Copyright 2007 Dstl Proteomics. 2007;7:2172–2183. doi: 10.1002/pmic.200600985. [DOI] [PubMed] [Google Scholar]
- 49.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirimanjeswara GS, et al. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 51.Lu Z, et al. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol Lett. 2007;112:92–103. doi: 10.1016/j.imlet.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saslaw S, Carhart S. Studies with tularemia vaccines in volunteers. III. Serologic aspects following intracutaneous or respiratory challenge in both vaccinated and nonvaccinated volunteers. Am J Med Sci. 1961;241:689–699. [PubMed] [Google Scholar]
- 53.Saslaw S, et al. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 54.Saslaw S, et al. Tularemia vaccine study. I. Intracutaneous challenge. Arch Intern Med. 1961;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- 55.Elkins KL, Bosio CM, Rhinehart-Jones TR. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fulop M, et al. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 57.Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenmark S, et al. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb Pathog. 2003;35:73–80. doi: 10.1016/s0882-4010(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 59.Culkin SJ, Rhinehart-Jones T, Elkins KL. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1997;158:3277–3284. [PubMed] [Google Scholar]
- 60.Sebastian S, et al. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect Immun. 2007;75:2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavine CL, et al. Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol. 2007;37:3007–3020. doi: 10.1002/eji.200737620. [DOI] [PubMed] [Google Scholar]
- 62.Pammit MA, et al. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun. 2006;74:2063–2071. doi: 10.1128/IAI.74.4.2063-2071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raynaud C, et al. Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect Immun. 2007;75:536–541. doi: 10.1128/IAI.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol. 2007;178:4538–4547. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- 65.Conlan JW, et al. Molecular immunology of experimental primary tularemia in mice infected by respiratory or intradermal routes with type A Francisella tularensis. Mol Immunol. 2008;45:2962–2969. doi: 10.1016/j.molimm.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malik M, et al. Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J Immunol. 2007;178:1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- 67.Malik M, et al. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–3662. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadull PJ, et al. Studies on tularemia. V. Immunization of man. J Immunol. 1950;65:425–435. [PubMed] [Google Scholar]
- 69.Foshay L, Hesselbrock WG, Wittenberg HJ. Vaccine prophylaxis against tularemia in man. Am J Public Health Nations Health. 1942;32:1131–1145. doi: 10.2105/ajph.32.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rawool D, Bitsaktsis C, Li Y, Gosselin DR, Metzger DW, Gosselin E. Utilization of Fc Receptors as a Mucosal Vaccine Strategy against an Intracellular Bacterium, Francisella tularensis. J Immuol. 2008;180:5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect Immun. 2007;75:2152–2162. doi: 10.1128/IAI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Metzger DW. IgA and respiratory immunity. In: Kaetzel CS, editor. Mucosal Immune Defense: Immunoglobulin A. New York: Springer Publishers; 2007. pp. 269–290. [Google Scholar]
- 73.Griffin KF, Oyston PC, Titball RW. Francisella tularensis vaccines. FEMS Immunol Med Microbiol. 2007;49:315–323. doi: 10.1111/j.1574-695X.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 74.Fortier AH, et al. Life and death of an intracellular pathogen: Francisella tularensis and the macrophage. Immunol Ser. 1994;60:349–361. [PubMed] [Google Scholar]
- 75.Woolard MD, et al. Francisella tularensis-infected macrophages release prostaglandin E2 that blocks T cell proliferation and promotes a Th2-like response. J Immunol. 2007;178:2065–2074. doi: 10.4049/jimmunol.178.4.2065. [DOI] [PubMed] [Google Scholar]
- 76.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 77.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol. 2005;175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 78.Sjostedt A, Conlan JW, North RJ. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bosio CM, Elkins KL. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect Immun. 2001;69:194–203. doi: 10.1128/IAI.69.1.194-203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conlan JW, et al. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb Pathog. 2002;32:127–134. doi: 10.1006/mpat.2001.0489. [DOI] [PubMed] [Google Scholar]
- 81.Lopez MC, et al. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell Immunol. 2004;232:75–85. doi: 10.1016/j.cellimm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Gosselin EJ, Gosselin DR, Lotz SA. Natural killer and CD8 T cells dominate the response by human peripheral blood mononuclear cells to inactivated Francisella tularensis live vaccine strain. Hum Immunol. 2005;66:1039–1049. doi: 10.1016/j.humimm.2005.08.240. [DOI] [PubMed] [Google Scholar]
- 83.Bokhari SM, et al. NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect Immun. 2008;76:1379–1389. doi: 10.1128/IAI.00745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butchar JP, et al. IFNgamma enhances IL-23 production during Francisella infection of human monocytes. FEBS Lett. 2008;582:1044–1048. doi: 10.1016/j.febslet.2008.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yee D, Rhinehart-Jones TR, Elkins KL. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157:5042–5048. [PubMed] [Google Scholar]
- 86.Cowley SC, Elkins KL. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon gamma receptors. J Exp Med. 2003;198:379–389. doi: 10.1084/jem.20030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elkins KL, et al. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993;61:823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cowley SC, et al. CD4-CD8- T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med. 2005;202:309–319. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cowley SC, Sedgwick JD, Elkins KL. Differential requirements by CD4+ and CD8+ T cells for soluble and membrane TNF in control of Francisella tularensis live vaccine strain intramacrophage growth. J Immunol. 2007;179:7709–7719. doi: 10.4049/jimmunol.179.11.7709. [DOI] [PubMed] [Google Scholar]
- 90.Chen W, et al. Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb Pathog. 2004;36:311–318.S. doi: 10.1016/j.micpath.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Sjostedt A, North RJ, Conlan JW. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology. 1996;142:1369–1374. doi: 10.1099/13500872-142-6-1369. [DOI] [PubMed] [Google Scholar]
- 92.Smiley ST. Cell-mediated defense against Yersinia pestis infection. Adv Exp Med Biol. 2007;603:376–386. doi: 10.1007/978-0-387-72124-8_35. [DOI] [PubMed] [Google Scholar]
- 93.Parent MA, et al. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006;74:3381–3386. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parent MA, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005;73:7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


