abstract
Solid-organ transplantation (SOT) has become the preferred strategy to treat a number of end-stage organ disease, because a continuous improvement in survival and quality of life. While preventive strategies has decreased the risk for classical opportunistic infections (such as viral, fungal and parasite infections), bacterial infections, and particularly bloodstream infections (BSIs) remain the most common and life-threatening complications in SOT recipients. The source of BSI after transplant depends on the type of transplantation, being urinary tract infection, pneumonia, and intraabdominal infections the most common infections occurring after kidney, lung and liver transplantation, respectively. The risk for candidemia is higher in abdominal-organ than in thoracic-organ transplantation. Currently, the increasing prevalence of multi-drug resistant (MDR) Gram-negative pathogens, such as extended-spectrum betalactamase-producing Enterobacteriaciae and carbapenem-resistant Klebsiella pneumoniae, is causing particular concerns in SOT recipients, a population which presents several risk factors for developing infections due to MDR organisms. The application of strict preventive policies to reduce the incidence of post transplant BSIs and to control the spread of MDR organisms, including the implementation of specific stewardship programs to avoid the overuse of antibiotics and antifungal drugs, are essential steps to reduce the impact of post transplant infections on allograft and patient outcomes.
Keywords: bloodstream infections, Candida infection, immunosuppression, multi-drug resistant organisms, surgical complications
Introduction
Solid-organ transplantation (SOT) has been established as an accepted therapy for a large variety of end-stage organ conditions.1 The number of SOT procedures is steadily increasing for all organs over these last years.2 As compared to 2012, an increase on the number of transplantations of 2.1% in kidney, 3.2% in liver, 9.1% in lung and 6.1% in heart transplant recipients was observed in 2013 in the US.2 In addition, allograft survival, mostly in the short-term post-transplant period, has significantly improved over the past few decades.2,3
However, the potential for surgical and technical complications combined with the impact of immunosuppression predisposes SOT recipients to infectious complications.1 In particular, bloodstream infections (BSIs) remain a major cause of mortality after transplantation.4-7 Reported BSIs-associated mortality ranges from 3% to 33% in heart, 10%–52% in liver, 6%–25% in lung, 6%–44.4% in pancreas, and 2.5%–11% in kidney transplant recipients.7-13 Mortality can reach up to 50% when bacteremia is accompanied with septic shock.7,14 Analysis of the data from a large US registry between 1987 and 2000 showed that post-transplant infections were the leading cause of hospitalization up to 24 months post-transplant.15 Also, as a consequence of BSIs, patients require longer hospital stays with increased hospitalization and therapy-associated costs. It has been estimated that the cost for care in the US of a BSI episode in the post renal transplant setting is approximately $48,400.7,16
In this article, we will review the epidemiology, risk factors and outcomes of BSIs occurring in SOT recipients, focusing on the impact of multidrug resistance on allograft and patient outcomes.
Risk of Infection in solid-organ transplant recipients
Predisposing factors for infection after transplantation include those being present before transplant in the recipient or the donor and those secondary to intraoperative and post transplant events.1
The type of organ transplant is an important determinant of the location of infection, especially during the early period following transplantation, due to local ischemic injury and bleeding, as well as potential contamination. The chest, abdomen, and urinary tract are the most common sites of infection occurring in thoracic-organ, liver, and kidney transplant recipients, respectively. Particular underlying diseases may increase the risk for post transplant infections; patients with cystic fibrosis who undergo lung transplantation are at higher risk for Pseudomonas and fungal infections as compared to patients with other conditions.17,18 Also, chronic diseases already present before transplant may persist after transplantation and increase the risk of infection. For example, diabetes mellitus predisposes to the development of soft-tissue and urinary tract infections also in SOT recipients.19
Donor-derived disease transmissions are defined as any disease present in the organ donor that is transmitted to at least one of the recipients. Bacterial contamination of organs or bacterial infections and colonization in the donor occurs frequently but rarely results in transmission of infection.20,21 However, donor-derived infections are generally associated with poor outcomes. As such, all early bacterial infections in the recipient should prompt a careful review of donor cultures and consideration of the donor as a potential source of infection. Of great concern is the increasing incidence of multidrug resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE) and multidrug-resistant Gram-negative rods. The problem is particularly serious with carbapenemase-producing Gram-negative Enterobacteriaceae, which usually exhibit extended-drug resistant phenotypes and remain susceptible to only a few antibiotics. There have been only a few reports assessing the optimal evaluation and risk mitigation management related to these infections caused by extensively resistant bacteria.22-25
Surgical factors have a major contribution of early post transplant infection. Technical problems affecting the vascular supply and functional integrity of the allograft are major risk factors for infectious complications that manifest after transplantation. There is a correlation between the duration of surgery and the mean number of episodes of infection per patient.26 The duration of these operations is the manifestation of many individual risk factors, including surgical stress, loss of blood and body fluids, and direct tissue damage. Examples of specific technical problems associated with infection include thrombosis of the hepatic artery after liver transplantation,27 vesicoureteral reflux after kidney transplantation,28 and mediastinal bleeding requiring re-exploration in thoracic-organ transplantation. These complications have been associated with hepatic abscesses and bloodstream infection,27 allograft pyelonephritis 28 and mediastinitis,27 respectively.
A recent meta-analysis found that severe hypogammaglobulinemia during the first year post transplantation significantly increased the risk of respiratory fungal and bacterial infections, and was associated with higher one-year all-cause mortality.29 Bacterial infections have been traditionally associated with hypogammaglobulinemia, predominantly IgG2 subclass.29-31 It is also suggested that the potential side effects of immunosuppressive drugs interfere with several neutrophil functions, which may explain the enhanced susceptibility for bacterial infections in patients after transplantation.32
Epidemiology and risk factors for bloodstream infections in transplantation
Conventional bacteria remain the main cause of life-threatening infections after transplantation. In a large Spanish cohort of SOT recipients, incidence rates of BSIs ranged from 7.3% in kidney to 20% in pancreas transplant recipients.7 In this study, around 60% of all BSIs occurred within one month post-transplantation and late-onset BSIs (more than 180 days post transplant) represented 6% of all BSIs. Risk factors for the development of BSIs have been reported in several studies. Most frequent risk factors that are common to all SOT recipients include intensive care unit (ICU) stay, previous exposure to antibiotics, donor or recipient age, and retransplantation.33-35 Additional risk factors have been identified in specific organ transplantation and will be discussed in the next section.
Kidney transplant recipients
Studies assessing the incidence of BSIs after kidney transplantation have consistently identified the urinary tract as the most common infection site in kidney transplant recipients. Urinary tract infections (UTI) are favored by several anatomical considerations, such as the disruption of the urinary tract during surgery, the presence of ureteral catheters during the first weeks post transplant, and preexistent urinary tract abnormalities (vesico-ureteral reflux, prostatic conditions).36,37 Among 1400 kidney transplant recipients included in the Spanish RESITRA cohort, urinary tract infection (UTI)-associated bacteremia was seen in 39%, followed by catheter-related BSIs (21%) and surgical wound infections (4%).7 Similarly, other cohorts have reported rates of BSI originating from the urinary tract ranging from 37.8% to 55.2%.38,39 Risk factors for BSI in kidney transplant recipients reported in several studies include ABO incompatibility, previous cytomegalovirus (CMV) infection, pre-transplant dialysis, acute rejection, urologic disease, presence of a ureteral stent, and high post transplant serum creatinine levels.40,41 Table 1 summarizes the most common risk factors of BSIs reported in the literature according to the type of organ transplant.
Table 1.
Risk factors for the development of BSI in SOT recipients in selected publications.
| Organ | Reference | Type of study | Number of patients | Risk factors | Summary of main results |
|---|---|---|---|---|---|
| Liver | 52 | Retrospective | 242 | - Diabetes mellitus - Albumine level <2.4g/dl | Hypoalbuminemia (p = 0.006), catheterization for more than 3 weeks (p = 0.009) and post-transplant hemodialysis (p = 0.001) independently predicted BSI. Hyperglycemia (p = 0.03 to p = 0.002 according to the studies) and previous antibiotic therapy (OR 11.15, p = 0.005 if exposure to more than 3 antibiotics) were additionally associated with the development of candidemia. Reoperation or biliary complications (p < 0.001) as well as retransplantation (p = 0.014) were the most important risk factors not only for BSI but also for BSI due to MDR Gram-negative pathogens. Preoperative S. aureus nasal carriage also increased the risk for post transplant BSI (p = 0.007). |
| 9 | Retrospective | 144 | - Catheterization for more than 22 days- Post transplant hemodialysis - Recipient age >55 years | ||
| 53 | Retrospective matched case-control study | 26 with candidemia and 52 controls | - Hyperglycemia treated with insulin up to 2 weeks before candidemia - Exposure to > 3 different intravenous antibiotics | ||
| 54 | Prospective | 704 | - Preoperative S. aureus nasal carriage - Kidney transplantation- Intraoperative transfusions - Return to surgery - Retransplantation- Biliary complications | ||
| 55 | Prospective | 475 | - Post transplant abdominal infection - Reoperation - One or more episodes of acute rejection- Prolonged endotracheal intubation - Tracheostomy - Length of ICU stay after transplantation | ||
| Kidney | 40 | Retrospective | 33′479 | - Female recipient - Older recipient age >65 years - Diabetes- Urologic disease - Dialysis in the first week after transplant - Duration of pre-transplant dialysis - Rejection | Female gender (OR 1.49), older age (OR 1.44) and diabetes (OR 2.05) were independent risk factors associated with bacteremia after kidney transplantation. Both studies emphasized the role of renal insufficiency (adjusted OR 2.55, p = 0.045) and dialysis before (OR 1.17) as well as after transplantation (OR 1.28 if dialysis in the first week post transplant) |
| 41 | Retrospective | 99 | - Immunosuppression with tacrolimus - Baseline serum creatinine level >1.3 mg/dL | ||
| Heart | 5 | Prospective | 309 | - Hemodialysis - Prolonged ICU stay - Viral infection (mainly cytomegalovirus) | Independent risk factors for BSI after heart transplantation were hemodialysis (OR 6.5; 95% CI 3.2–13, p < 0.001), prolonged ICU stay (OR 3.6; 95% CI 1.6–8.1, p = 0.002), and viral infection (OR 2.1; 95% CI 1.1–4, p = 0.01). |
| Lung | 62 | Prospective | 176 | - Cystic fibrosis - Pretransplant mechanical ventilation - Younger age | BSIs were significantly more common in patients with cystic fibrosis (p = 0.001), and with the use of pre transplant mechanical ventilation (p = 0.007) |
Notes: BSI: Bloodstream infection
ICU: intensive care unit
MDR: multidrug resistant
OR: odds ratio
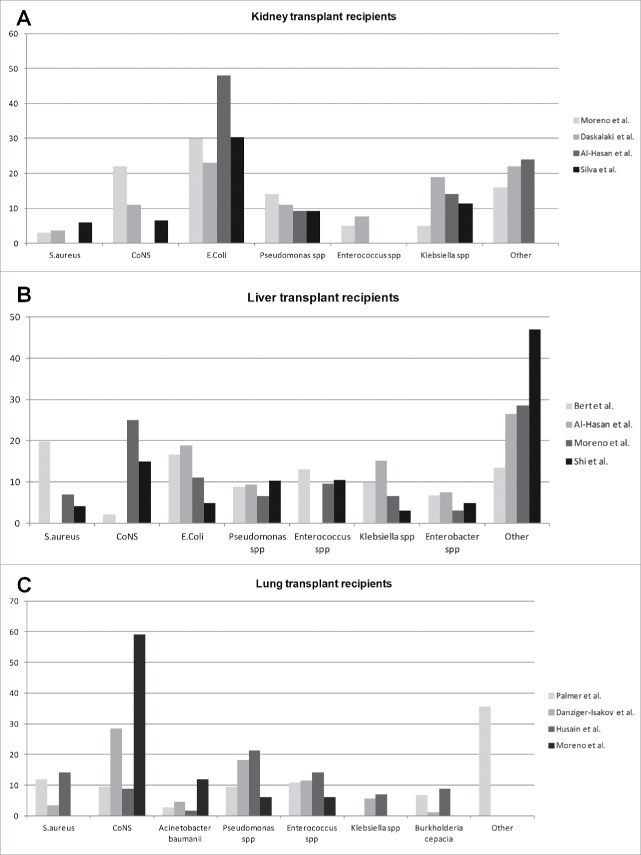
Because of the frequent urinary origin of BSIs in kidney transplant recipients, Gram-negative bacteria are usually identified as the main pathogens responsible for BSI, with rates ranging from 62% to 70% of all episodes. Figure 1 shows the rates of specific pathogens causing bloodstream infections in SOT recipients, according to the organ transplant. In the RESITRA cohort, Escherichia coli was the responsible for up to 30% of episodes of BSI, followed by Pseudomonas aeruginosa in 14%, Klebsiella spp. in 5%, Enterobacter spp in 4% and Acinetobacter baumanii in 3%. Gram-positive pathogens were seen in 32% of the episodes of BSI, being coagulase-negative Staphylococci (CNS) in 22% of cases, Enterococcus spp in 5% and S. aureus in 3%, the most common pathogens.7 The incidence of candidemia after kidney transplantation is overall low, and it has been estimated to be between 5–6% of all episodes of BSIs.7,39 Most common species of Candida found in these studies include C. albicans, C. parapsilosis and C. glabrata, although major differences were observed according to the region of transplantation.7,39 Specific risk factors for the development of candidemia after kidney transplantation were similar to non-SOT recipients (antimicrobial use, sepsis, neutropenia, parenteral nutrition, recent surgery, diabetes) except for corticosteroid therapy (71% in SOT recipients vs. 29% in non-SOT patients, p < 0.01).42
Figure 1.
Rates of specific pathogens in selected publications causing bloodstream infections in SOT recipients, according to the organ transplant. (A) kidney transplant recipients; (B) Liver transplant recipients; (C) Lung transplant recipients. X-axis represents the percentage of pathogens at each publication. The study from Al-Hasan et al (38), included only episodes of bacteremia by Gram-negative pathogens. CoNS: Coagulase negative staphylococci.
Kidney transplant recipients are at significant risk for developing infection by multidrug -resistant (MDR) pathogens, and some recent studies have assessed the incidence of bacteremia due to rESKAPE pathogens (VRE, MRSA, extended-spectrum betalactamase [ESBL]-producing K. pneumoniae, carbapenem-resistant A. baumannii, carbapenem-resistant P. aeruginosa, and ESBL-producing Enterobacter spp). In a single-center study from Greece, 22/108 (20%) kidney transplant recipients presented 26 episodes of bacteremia,43 and 7 (26.9%) of these episodes were due to rESKAPE strains.43 In Spain, Bodro et al. identified rESKAPE pathogens in up to 19.6% of BSI in a cohort of 190 SOT recipients.34 In this study, most of the pathogens were ESBL-producing K. pneumoniae and multidrug-resistant Pseudomonas which was associated with an increase of mortality. Patients with rESKAPE BSI more often received inappropriate empirical antibiotic therapy than in case of BSI due to other organisms (41% vs. 21.6%; p = 0.01). Persistence of bacteremia, respiratory failure, ICU admission, and invasive mechanical ventilation were more frequent in rESKAPE bacteremic episodes and outcomes were poorer compared with other etiologies, with a higher overall case-fatality rate (35.2% vs. 14.4%; p=0.001).34 Reported rates of infection by ESBL-producing organisms ranged from 26% to 45% in Spain and Brazil, particularly in case of relapsing episodes of UTI.44,45 In a retrospective cohort from Brazil, infection by carbapenemase resistant-Klebsiella pneumoniae (CR-KP) in kidney transplant recipients was particularly prevalent in patients with ureteral stents and it was associated with high mortality.46 Of note, the incidence of resistant bacterial infection in SOT recipients is lower in other countries,47 although a significant trend toward an increase of infections due to resistant bacteria is universally observed.
In patients receiving cotrimoxazole prophylaxis for UTI, up to 62% of infections have been reported as caused by cotrimoxazole-resistant organisms,48,49 although this has to be balanced with the high efficacy of cotrimoxazole for preventing other opportunistic infections. Use of fluoroquinolones as prophylaxis for kidney transplant recipients has been linked to surges in fluoroquinolone-resistant P. aeruginosa.48,50 Frequent use of antibiotics for treatment of asymptomatic bacteriuria also has been associated with antimicrobial resistance, and current guidelines generally do not recommend treating patients without symptoms.48 In a study of patients with asymptomatic E. coli bacteriuria, treatment led to selection of resistant organisms in 78% of cases.51 Empiric coverage for resistant organisms should therefore be considered according to the local epidemiology, use of prophylactic antimicrobial drugs, and severity of clinical presentation, particularly in the early post-transplant period.38 Of note, outbreaks of organisms resistant to all commonly available antibiotics have occurred in kidney transplant recipients; treatment options may be restricted to nephrotoxic agents such as colistin or aminoglycosides,48 which can be associated with impaired allograft outcomes.
Liver transplant recipients
BSIs are frequent complications in liver transplant recipients. This is related to the risk of relative immunosuppression of cirrhotic patients previous to transplantation, and to the prolonged surgical procedure of transplantation. Identified risk factors for BSIs after liver transplantation include diabetes mellitus, hypoproteinemia, catheterization, preoperative massive effusion or ascites, preoperatively S. aureus carriage, post-transplant hemodialysis, operative blood loss, reoperation, need for mechanical ventilation, and bile duct complications.9,52-55
Because improvement in surgical techniques and in preventive antimicrobial strategies, some differences in the types of organisms isolated from blood cultures after liver transplantation have been reported over time. In the 1980s, BSIs were predominantly caused by Enterobacteriaceae species and were mostly associated with an intra-abdominal source.26,54 The emergence of Gram-positive cocci was subsequently reported in studies conducted in the 1990s, with MRSA being the leading causative agent and intravascular catheters the most frequent source of BSIs, respectively.54,56 More recent cohorts have reported a similar incidence between Gram-positive and Gram-negative organisms as the cause of BSI after liver transplantation.7
In a prospective single-center study from France, 259 episodes of BSI in 205 patients were documented resulting in an incidence rate of 36.8 episodes per 100 transplanted patients (259/704), including Gram-negative bacilli in 52% and Gram-positive organisms in 37% of cases.54 Enterobacteriaceae members represented the vast majority of Gram-negative bacilli, and the most common species was E. coli. Among Gram-positive organisms, S. aureus was the most common pathogen. In this study, sources of BSIs were intra-abdominal infections (27.7%), catheter-related infections (15.1%), urinary tract infections (12.9%), pulmonary infections (9.4%), biliary infections (8.2%), and wound infections (3.1%). The primary focus remained unknown in 26.6% of the episodes. Candida infection is a significant concern in liver transplant recipients and can be a major factor associated with poor prognosis. Prior studies have shown that between 5% and 42% of liver transplant patients develop at least one fungal infection after transplantation with an associated mortality ranging from 25% to 71%.57,58
Antibiotic resistance was frequently reported in these studies. Infection due to ESBL-producing Enterobacteriaciae has been reported to be as frequent as 8% of all BSI.38 Other series have shown an increased incidence of infection by MDR Gram-negative organisms, such as Stenotrophomonas maltophilia, Ochrobactrum anthropi, Pseudomonas spp, and A. baumanii. Infection by these organisms reached up to 56% of all episodes of BSIs.55 In a cohort of liver transplant recipients in Italy, infection by CR-KP was seen in up to 8.4% of patients, being bacteremic the majority of these infections.59 Crude mortality in these patients was 45%, as compared to 7.3% in non-infected patients. Specific risk factors for BSI by CR-KP included renal replacement therapy, mechanical ventilation >48 hours, histological recurrence of HCV and rectal carriage by CR-KP. Infection by other MDR organisms has also been associated with significant morbidity and mortality. Pre-transplant colonization with VRE doubles the risk of mortality after transplant.60,61 Patients who underwent a liver transplantation with MRSA nasal colonization had a significant higher risk of infection by MRSA associated with inferior outcomes.54,61
Lung transplant recipients
There are fewer studies assessing the epidemiology of BSI in lung transplant recipients than in other transplant types. In a single-center study from the US, BSI occurred in 25% (44/176) of all lung transplant recipients,62 being S. aureus, P. aeruginosa, and Candida spp. the most common bloodstream isolates. The epidemiology varied considerably between early and late post transplant time periods and also differed between cystic fibrosis (CF) and non-CF patients. In pediatric lung transplantation, the most commonly isolated organism from BSIs was also CNS, reflecting the use of intravenous catheters in CF patients.63 In a prospective multicenter study involving 305 patients, pulmonary (46%) and vascular catheter infections (41%) accounted for a majority of the episodes of BSIs after lung transplantation.6 Fifty percent of BSIs in the first post transplant year were of pulmonary origin. After one year, the proportion of BSIs that were due to pulmonary infections declined to 26.7% and vascular catheters emerged as the leading source of BSI (53.3%). A total of 57% of the Gram-negative BSIs were due to MDR bacteria; these included 50% of P. aeruginosa, 100% of Burkholderia cepacia group and 50% of K. pneumoniae isolates. Pulmonary infection was the most common source of BSI caused by resistant Gram-negative pathogens (71%). In all, 35% of the patients with CF compared to 8% with other underlying lung diseases had resistant Gram-negative bacteremia, and this was mostly due to infection by B. cepacia group in CF patients.
Bacteremia by B. cepacia group, and particularly B. cenocepacea, is an important concern in CF lung transplant recipients.64,65 Patients colonized by B. cenocepacea have an increased mortality after transplantation, reaching up to 80% in the first year post transplant.66-68 Cause of death is usually due to the so-called cepacia syndrome, consisting in progressive necrotizing pneumonia with persistent bacteremia.69 Because B. cenocepacia is virtually panresistant to all antibiotics, therapy usually consists in the administration of a combination of high doses of antibiotics with suboptimal in vitro susceptibility. Antibiotics that may be used in this situation include tygecyclin, chloramphenicol, temocillin, ceftazidime, meropenem and tobramycin.70 Only some case reports of successful therapy for cepacia syndrome after lung transplantation have been reported, so that colonization by B. cenocepacia is considered as a contra-indication for lung transplantation in most lung transplant programs.71
Heart transplant recipients
In the study from the RESITRA cohort from Spain the incidence of BSI in heart transplant recipients was approximately 11%.7 Gram-positive organisms were the most frequent etiologic agents with CNS isolated in around 27% of all the bacteremias, followed by S. aureus in 21% of cases. Central venous catheters were by far the most frequent source for BSIs (44%), followed by pulmonary (9%) and surgical wound (9%).
In another study, 60 episodes of BSI in 49 out of 309 patients (15.8%) were diagnosed.5 Most episodes of BSI were nosocomially-acquired (66%), especially those occurring earlier after transplantation. Lower respiratory tract infection (23%), urinary tract infection (20%) and catheter-related-BSI (16%) were the most frequent sources of bacteremia. Gram-negative microorganisms predominated (55.3%) (including E.coli, P. aeruginosa, K. pneumoniae, S. marcescens), followed by Gram-positive microorganisms (44.6%) (S.aureus, S. epidermidis, E.faecalis), 6 (10%) episodes were polymicrobial and there was one case of fungemia (C.albicans). Independent risk factors for BSI in this study were hemodialysis, prolonged intensive care unit stay and previous CMV infection.5
Over the last years, an increasing number of heart transplant candidates are transplanted with a ventricular assist device (VAD) in place. Patients with a pre-transplant VAD had a higher incidence of local infection (mediastinitis) at the time of transplant. Some studies have also associated pre-transplant VAD infection with an increased risk for bacteremia following transplantation, and a decreased survival,72,73 particularly in case of Candida infection.74
Pancreas transplant recipients
Pancreas and kidney-pancreas transplant recipients are at significant risk for bacterial infectious complications. This is due to the high rate of surgical complications in this type of transplantation and the higher net state of immunosuppression (with increased rates of acute rejection). Main infectious complications include intraabdominal infections, duodenal leaks, recurrent UTIs (in case of bladder-drained allograft) and wound infections. A retrospective analysis of kidney-pancreas and solitary pancreas transplantation with enteric drainage showed that bacteremia occurred in 29/110 (26%) patients with a 17% of recurrent infections.75 Most of the BSIs were seen during the first 3 months after transplantation. In this series, the most common organisms included CNS, Enterobacteriaciae, VRE, and Acinetobacter spp Bacteremia was associated with coexisting site infection in the majority of the cases: deep abdominal wound (31%); catheter (31%); urinary tract (34%); and pulmonary (7%). In a prospective study by Kawecki et al. in simultaneous pancreas-kidney transplantation recipients with BSIs, the most common isolates were Gram-positive bacteria (73.9%) with predominance of Staphylococci strains (81.8%) (76). Gram-negative bacteria comprised 17.4% of positive cultures, whereas yeast-like fungi were seen in 8.7% of cases, with a predominance of C. glabrata.
Intestinal transplant recipients
Small bowel transplantation is one of the least commonly performed SOT procedures worldwide and one of the most technically challenging, so that there are few studies that have comprehensively assessed the burden of BSIs in this setting. Of note, a significant numbers of these procedures are performed in the pediatric population. The incidence of BSIs after small bowel and/or combined transplantation appears to be higher than in other types of transplant. Among pediatric intestinal transplant recipients a total of 39/62 intestinal transplant recipients had 133 bloodstream infections (2.1 episodes/patient) including 121 episodes of bacteremia and 12 of fungemia. Enteric organisms were the most frequently recovered pathogens, with 76 episodes due to Gram-negative rods and 36 episodes due to Enterococci.77 Other studies have found a higher prevalence of Gram-positive pathogens. For example, in a retrospective study of adult and pediatric small-bowel transplant recipients, from a total of 85 BSI episodes, 66% were due to Gram-positive organisms, 34% to Gram-negative organisms, and 2.4% due to fungi. The most common isolates were Enterococcus spp., Enterobacter spp, Klebsiella spp., and CNS, reflecting the most frequent sources of infection, namely intraabdominal and catheter-related infection.78
Prevention strategies of BSIs in SOT recipients
Infection control measures to reduce the incidence of specific BSIs that have shown efficacy in the general population should also be implemented in SOT recipients. These include the prevention of catheter-associated BSIs,79 prevention of catheter-associated UTI (removal of unnecessary bladder or ureteral catheters) 80 or ventilator associated pneumonia.81
Recent guidelines for the prevention of catheter-associated BSIs recommend ultrasound guidance for CVC placement to reduce the number of cannulation attempts and mechanical complications, and that only staff fully trained in this technique should undertake this procedure.82 Other major areas of emphasis are the use of maximal sterile barrier precautions during insertion, use of a 2% chlorhexidine preparation for skin antisepsis, avoiding routine replacement of catheters and use of chlorhexidine-impregnated sponge dressings.82 Although prevention of catheter-associated BSIs has been explored mainly in ICU patients, there is no reason to believe that those general recommendations should not be appropriate for SOT recipients.83
Perioperative antibacterial prophylaxis has been shown to be effective in preventing wound infections in kidney transplant recipients,84 but literature on the efficacy of antibiotic prophylaxis on other transplant recipients is scarce. Duration of antibiotic prophylaxis should be as short as possible (24–48 hours), except in patients with pre transplant infections (CF patients, infection of a VAD) or with suspected donor-derived infection (aspiration pneumonia in a lung donor). The choice of the antibiotic should be given the local epidemiology, but even centers with a high incidence of MDR Gram negative pathogens usually do not expand the antibiotic spectrum to cover colonizing pathogens. However, in patients that are colonized by MRSA, topical decolonization may decrease the risk of infection in SOT recipients. In a study involving liver transplant recipients with nasal carriage of MRSA, the rates of infection decreased from 40.4% to 4.1% and the rate of BSI from 25.5% to 4.1%, after decolonization with topical mupirocin.60,85 Selective bowel decontamination (SBD) is used in liver, pancreas and intestinal transplant recipients to prevent colonization of the gastrointestinal tract by aerobic gram-negative bacilli and fungi while sparing the anaerobic gut flora.86,87 The use of SBD has been shown to result in a reduction of bacterial infections in 2 randomized controlled trials in liver transplant recipients.88,89 The impact of SBD on the rising incidence of infections with VRE and MRSA has been noted at some liver transplant centers but has not been fully characterized.90
Pulmonary infections are common in lung transplant recipients, because of impaired mucociliary clearance and abolition of the cough reflex distal to the tracheal or bronchial anastomosis.91 To reduce the risk of infection in patients with CF, perioperative antibiotics are chosen on the basis of sputum culture results obtained preoperatively, and the antibiotics are administered for a longer period postoperatively (14 days). Routine sinus surgery is advocated by some centers.
The best strategy for the prevention of UTI in kidney and in kidney-pancreas transplant recipients remains to be established. Prophylaxis with cotrimoxazole has been shown to be effective for the prevention of UTIs and bacteremia after kidney transplantation in randomized clinical trials,92,93 and it is highly recommended during the first months post transplant to additionally reduce the incidence of opportunistic infections, such as Pneumocystis jirovecii pneumonia, toxoplasmosis and listeriosis, among other infections. However, a significant number of patients may develop positive urinary cultures despite cotrimoxazole prophylaxis. It is debated whether treating asymptomatic bacteriuria may prevent cases of pyelonephritis and BSIs, and may preserve allograft function. Because of the serious concern of the acquisition of antibiotic resistance, and the discordance literature about the impact of untreated asymptomatic bacteriuria, screening and therapy of asymptomatic bacteriuria it is generally not recommended after the first weeks post transplant.94
Antifungal prophylaxis against Candida infection is based on both risk and epidemiologic factors. In kidney and in heart transplant recipients, administration of antifungal prophylaxis is not routinely recommended, and should be based on individual risk factors (such as the use of extracorporeal membrane oxygenation (ECMO), or renal replacement therapy (RRT).95 Lung transplant recipients are a lower risk for developing Candida infection, but usually receive anti-mold prophylaxis, which appropriately covers most Candida species. Liver transplant recipients that are at higher risk for developing of invasive candidiasis early after transplant (i.e. retransplantation, RRT) should receive antifungal prophylaxis with fluconazole or an echinocandin.96 Although there is little data specifically in pancreas and in intestinal transplantation, given the higher incidence of invasive candidiasis, universal antifungal prophylaxis is usually recommended in these populations. Duration of antifungal prophylaxis varies among centers (between 2–14 days) and caution should be taken regarding the potential interaction between azoles and calcineurin inhibitors.97
Conclusion
Bacterial infections, including BSIs, have become the most significant infectious threat in SOT recipients, currently overtaking classical opportunistic infections as the most common infectious complications in terms of morbidity and mortality. In addition, the increasing prevalence of MDR bacteria worldwide (especially MDR Gram-negative pathogens) is causing particular concerns in SOT recipients, a population which presents several risk factors for developing infections due to MDR organisms. This is especially important due to the potential increased toxicity and suboptimal efficacy of antibiotic therapy used to treat infections due to MDR pathogens in SOT recipients. The application of strict preventive policies to reduce the incidence of post transplant BSIs and to control the spread of MDR organisms, such as the administration of a short duration of pre transplant antibiotic prophylaxis, a timely removal of unnecessary catheters, and the implementation of specific stewardship programs to avoid the overuse of antibiotics and antifungal drugs (for example, for treating asymptomatic bacteriuria in kidney transplant recipients) is an essential step to reduce the impact of BSI on allograft and patient outcomes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Green M. Introduction: Infections in solid organ transplantation. Am J Transplant. 2013; 13 Suppl 4:3-8; http://dx.doi.org/ 10.1111/ajt.12093 [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, et al.. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015; 15 Suppl 2:1-28; http://dx.doi.org/ 10.1111/ajt.13197 [DOI] [PubMed] [Google Scholar]
- 3.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, et al.. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015; 15 Suppl 2:1-34; http://dx.doi.org/ 10.1111/ajt.13195 [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transplant 2000; 6(1):54-61. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez C, Munoz P, Rodriguez-Creixems M, Yanez JF, Palomo J, Bouza E. Bloodstream infections among heart transplant recipients. Transplantation. 2006; 81(3):384-91; http://dx.doi.org/ 10.1097/01.tp.0000188953.86035.2d [DOI] [PubMed] [Google Scholar]
- 6.Husain S, Chan KM, Palmer SM, Hadjiliadis D, Humar A, McCurry KR, Wagener MM, Singh N. Bacteremia in lung transplant recipients in the current era. Am J Transplant 2006; 6(12):3000-7; http://dx.doi.org/ 10.1111/j.1600-6143.2006.01565.x [DOI] [PubMed] [Google Scholar]
- 7.Moreno A, Cervera C, Gavalda J, Rovira M, de la Camara R, Jarque I, Montejo M, de la Torre-Cisneros J, Miguel Cisneros J, Fortún J, et al.. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7(11):2579-86; http://dx.doi.org/ 10.1111/j.1600-6143.2007.01964.x [DOI] [PubMed] [Google Scholar]
- 8.Shao M, Wan Q, Xie W, Ye Q. Bloodstream infections among solid organ transplant recipients: epidemiology, microbiology, associated risk factors for morbility and mortality. Transplant Rev 2014; 28(4):176-81; http://dx.doi.org/ 10.1016/j.trre.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Kim SI, Kim YJ, Jun YH, Wie SH, Kim YR, Choi JY, Yoon SK, Moon IS, Kim DG, Lee MD, et al.. Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med J 2009; 50(1):112-21; http://dx.doi.org/ 10.3349/ymj.2009.50.1.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iida T, Kaido T, Yagi S, Yoshizawa A, Hata K, Mizumoto M, Mori A, Ogura Y, Oike F, Uemoto S. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transplant 2010; 16(12):1379-85; http://dx.doi.org/ 10.1002/lt.22165 [DOI] [PubMed] [Google Scholar]
- 11.Hsu J, Andes DR, Knasinski V, Pirsch J, Safdar N. Statins are associated with improved outcomes of bloodstream infection in solid-organ transplant recipients. Eur J Clin Microbiol Infect Dis 2009; 28(11):1343-51; http://dx.doi.org/ 10.1007/s10096-009-0787-4 [DOI] [PubMed] [Google Scholar]
- 12.Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am J Infect Control. 1992; 20(5):239-47; http://dx.doi.org/ 10.1016/S0196-6553(05)80197-X [DOI] [PubMed] [Google Scholar]
- 13.Wade JJ, Rolando N, Hayllar K, Philpott-Howard J, Casewell MW, Williams R. Bacterial and fungal infections after liver transplantation: an analysis of 284 patients. Hepatology. 1995; 21(5):1328-36; http://dx.doi.org/ 10.1002/hep.1840210517 [DOI] [PubMed] [Google Scholar]
- 14.Candel FJ, Grima E, Matesanz M, Cervera C, Soto G, Almela M, Martínez JA, Navasa M, Cofán F, Ricart MJ, et al.. Bacteremia and septic shock after solid-organ transplantation. Transplant Proc 2005; 37(9):4097-9; http://dx.doi.org/ 10.1016/j.transproceed.2005.09.181 [DOI] [PubMed] [Google Scholar]
- 15.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: a report of the NAPRTCS. Am J Transplant. 2004; 4(3):384-9; http://dx.doi.org/ 10.1111/j.1600-6143.2004.00350.x [DOI] [PubMed] [Google Scholar]
- 16.Kutinova A, Woodward RS, Ricci JF, Brennan DC. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am J Transplant. 2006; 6(1):129-39; http://dx.doi.org/ 10.1111/j.1600-6143.2005.01156.x [DOI] [PubMed] [Google Scholar]
- 17.Bonvillain RW, Valentine VG, Lombard G, LaPlace S, Dhillon G, Wang G. Post-operative infections in cystic fibrosis and non-cystic fibrosis patients after lung transplantation. J Heart Lung Transplant 2007; 26(9):890-7; http://dx.doi.org/ 10.1016/j.healun.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 18.Gavalda J, Meije Y, Fortun J, Roilides E, Saliba F, Lortholary O, Muñoz P, Grossi P, Cuenca-Estrella M; ESCMID Study Group for Infections in Compromised Hosts . Invasive fungal infections in solid organ transplant recipients. Clin Microbiol Infect 2014; 20 Suppl 7:27-48; http://dx.doi.org/ 10.1111/1469-0691.12660 [DOI] [PubMed] [Google Scholar]
- 19.Tolkoff-Rubin NE, Rubin RH. The infectious disease problems of the diabetic renal transplant recipient. Infect Dis Clin North Am 1995; 9(1):117-30. [PubMed] [Google Scholar]
- 20.Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant. 2011; 11(6):1123-30; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03493.x [DOI] [PubMed] [Google Scholar]
- 21.Len O, Gavalda J, Blanes M, Montejo M, San Juan R, Moreno A, Carratalà J, de la Torre-Cisneros J, Bou G, Cordero E, et al.. Donor infection and transmission to the recipient of a solid allograft. Am J Transplant. 2008; 8(11):2420-5; http://dx.doi.org/ 10.1111/j.1600-6143.2008.02397.x [DOI] [PubMed] [Google Scholar]
- 22.Ison MG, Grossi P, Practice ASTIDCo . Donor-derived infections in solid organ transplantation. Am J Transplant. 2013; 13 Suppl 4:22-30; http://dx.doi.org/ 10.1111/ajt.12095 [DOI] [PubMed] [Google Scholar]
- 23.Sifri CD, Ison MG. Highly resistant bacteria and donor-derived infections: treading in uncharted territory. Transplant Infect Dis 2012; 14(3):223-8; http://dx.doi.org/ 10.1111/j.1399-3062.2012.00752.x [DOI] [PubMed] [Google Scholar]
- 24.Goldberg E, Bishara J, Lev S, Singer P, Cohen J. Organ transplantation from a donor colonized with a multidrug-resistant organism: a case report. Transplant Infect Dis 2012; 14(3):296-9; http://dx.doi.org/ 10.1111/j.1399-3062.2011.00697.x [DOI] [PubMed] [Google Scholar]
- 25.Martins N, Martins IS, de Freitas WV, de Matos JA, Magalhaes AC, Girao VB, Dias RC, de Souza TC, Pellegrino FL, Costa LD, et al.. Severe infection in a lung transplant recipient caused by donor-transmitted carbapenem-resistant Acinetobacter baumannii. Transplant Infect Dis 2012; 14(3):316-20; http://dx.doi.org/ 10.1111/j.1399-3062.2011.00701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusne S, Dummer JS, Singh N, Iwatsuki S, Makowka L, Esquivel C, Tzakis AG, Starzl TE, Ho M. Infections after liver transplantation. An analysis of 101 consecutive cases. Medicine 1988; 67(2):132-43; http://dx.doi.org/ 10.1097/00005792-198803000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unal B, Gonultas F, Aydin C, Otan E, Kayaalp C, Yilmaz S. Hepatic artery thrombosis-related risk factors after living donor liver transplantation: single-center experience from Turkey. Transplant Proc 2013; 45(3):974-7; http://dx.doi.org/ 10.1016/j.transproceed.2013.02.070 [DOI] [PubMed] [Google Scholar]
- 28.Golebiewska JE, Debska-Slizien A, Rutkowski B. Urinary tract infections during the first year after renal transplantation: one center's experience and a review of the literature. Clin Transplant 2014; 28(11):1263-70; http://dx.doi.org/ 10.1111/ctr.12465 [DOI] [PubMed] [Google Scholar]
- 29.Florescu DF, Kalil AC, Qiu F, Schmidt CM, Sandkovsky U. What is the impact of hypogammaglobulinemia on the rate of infections and survival in solid organ transplantation? A meta-analysis. Am J Transplant. 2013; 13(10):2601-10; http://dx.doi.org/ 10.1111/ajt.12401 [DOI] [PubMed] [Google Scholar]
- 30.Mathiesen T, Brattstrom C, Andersson J, Linde A, Ljungman P, Wahren B. Immunoglobulin G subclasses and lymphocyte stimulatory responses to cytomegalovirus in transplant patients with primary cytomegalovirus infections. J Med Virol 1992; 36(1):65-9; http://dx.doi.org/ 10.1002/jmv.1890360113 [DOI] [PubMed] [Google Scholar]
- 31.Tarzi MD, Grigoriadou S, Carr SB, Kuitert LM, Longhurst HJ. Clinical immunology review series: An approach to the management of pulmonary disease in primary antibody deficiency. Clin Exp Immunol 2009; 155(2):147-55; http://dx.doi.org/ 10.1111/j.1365-2249.2008.03851.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmaldienst S, Horl WH. Bacterial infections during immunosuppression - immunosuppressive agents interfere not only with immune response, but also with polymorphonuclear cell function. Nephrol, Dialysis, Transplant 1996; 11(7):1243-5; http://dx.doi.org/ 10.1093/ndt/11.7.1243 [DOI] [PubMed] [Google Scholar]
- 33.Linares L, Garcia-Goez JF, Cervera C, Almela M, Sanclemente G, Cofan F, Ricart MJ, Navasa M, Moreno A. Early bacteremia after solid organ transplantation. Transplant Proc 2009; 41(6):2262-4; http://dx.doi.org/ 10.1016/j.transproceed.2009.06.079 [DOI] [PubMed] [Google Scholar]
- 34.Bodro M, Sabe N, Tubau F, Llado L, Baliellas C, Roca J, Cruzado JM, Carratalà J. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation. 2013; 96(9):843-9; http://dx.doi.org/ 10.1097/TP.0b013e3182a049fd [DOI] [PubMed] [Google Scholar]
- 35.Johnson LE, D'Agata EM, Paterson DL, Clarke L, Qureshi ZA, Potoski BA, Peleg AY. Pseudomonas aeruginosa bacteremia over a 10-year period: multidrug resistance and outcomes in transplant recipients. Transplant Infect Dis 2009; 11(3):227-34; http://dx.doi.org/ 10.1111/j.1399-3062.2009.00380.x [DOI] [PubMed] [Google Scholar]
- 36.Chuang P, Parikh CR, Langone A. Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin Transplant 2005; 19(2):230-5; http://dx.doi.org/ 10.1111/j.1399-0012.2005.00327.x [DOI] [PubMed] [Google Scholar]
- 37.Lapchik MS, Castelo Filho A, Pestana JO, Silva Filho AP, Wey SB. Risk factors for nosocomial urinary tract and postoperative wound infections in renal transplant patients: a matched-pair case-control study. J Urol 1992; 147(4):994-8. [DOI] [PubMed] [Google Scholar]
- 38.Al-Hasan MN, Razonable RR, Eckel-Passow JE, Baddour LM. Incidence rate and outcome of Gram-negative bloodstream infection in solid organ transplant recipients. Am J Transplant. 2009; 9(4):835-43; http://dx.doi.org/ 10.1111/j.1600-6143.2009.02559.x [DOI] [PubMed] [Google Scholar]
- 39.Silva M Jr., Marra AR, Pereira CA, Medina-Pestana JO, Camargo LF. Bloodstream infection after kidney transplantation: epidemiology, microbiology, associated risk factors, and outcome. Transplantation 2010; 90(5):581-7; http://dx.doi.org/ 10.1097/TP.0b013e3181e8a680 [DOI] [PubMed] [Google Scholar]
- 40.Abbott KC, Oliver JD 3rd, Hypolite I, Lepler LL, Kirk AD, Ko CW, Hawkes CA, Jones CA, Agodoa LY. Hospitalizations for bacterial septicemia after renal transplantation in the united states. Am J Nephrol 2001; 21(2):120-7; http://dx.doi.org/ 10.1159/000046234 [DOI] [PubMed] [Google Scholar]
- 41.Wu SW, Liu KS, Lin CK, Hung TW, Tsai HC, Chang HR, Lian JD. Community-acquired urinary tract infection in kidney transplantation: risk factors for bacteremia and recurrent infection. J Formosan Medical Association = Taiwan yi zhi 2013; 112(3):138-43; http://dx.doi.org/ 10.1016/j.jfma.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 42.van Hal SJ, Marriott DJ, Chen SC, Nguyen Q, Sorrell TC, Ellis DH, Slavin MA; Australian Candidaemia Study . Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transplant Infect Dis 2009; 11(2):122-7; http://dx.doi.org/ 10.1111/j.1399-3062.2009.00371.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daskalaki E, Koukoulaki M, Bakalis A, Papastamopoulos V, Belesiotou E, Perivolioti E, Skoutelis A, Drakopoulos S. Blood stream infections in renal transplant recipients: a single-center study. Transplant Proc 2014; 46(9):3191-3; http://dx.doi.org/ 10.1016/j.transproceed.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 44.Vidal E, Torre-Cisneros J, Blanes M, Montejo M, Cervera C, Aguado JM, Len O, Carratalá J, Cordero E, Bou G, et al.. Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transplant Infect Dis 2012; 14(6):595-603; http://dx.doi.org/ 10.1111/j.1399-3062.2012.00744.x [DOI] [PubMed] [Google Scholar]
- 45.Pinheiro HS, Mituiassu AM, Carminatti M, Braga AM, Bastos MG. Urinary tract infection caused by extended-spectrum beta-lactamase-producing bacteria in kidney transplant patients. Transplant Proc 2010; 42(2):486-7; http://dx.doi.org/ 10.1016/j.transproceed.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 46.Freire MP, Abdala E, Moura ML, de Paula FJ, Spadao F, Caiaffa-Filho HH, David-Neto E, Nahas WC, Pierrotti LC. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients. Infection. 2015; 43(3):315-23; http://dx.doi.org/ 10.1007/s15010-015-0743-4 [DOI] [PubMed] [Google Scholar]
- 47.Bucheli E, Kralidis G, Boggian K, Cusini A, Garzoni C, Manuel O, Meylan PR, Mueller NJ, Khanna N, van Delden C, et al.. Impact of enterococcal colonization and infection in solid organ transplantation recipients from the Swiss transplant cohort study. Transplant Infect Dis 2014; 16(1):26-36; http://dx.doi.org/ 10.1111/tid.12168 [DOI] [PubMed] [Google Scholar]
- 48.Parasuraman R, Julian K, Practice ASTIDCo . Urinary tract infections in solid organ transplantation. Am J Transplant. 2013; 13 Suppl 4:327-36; http://dx.doi.org/ 10.1111/ajt.12124 [DOI] [PubMed] [Google Scholar]
- 49.Green H, Rahamimov R, Gafter U, Leibovitci L, Paul M. Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: a systematic review and meta-analysis. Transplant Infect Dis 2011; 13(5):441-7; http://dx.doi.org/ 10.1111/j.1399-3062.2011.00644.x [DOI] [PubMed] [Google Scholar]
- 50.Rafat C, Vimont S, Ancel PY, Xu-Dubois YC, Mesnard L, Ouali N, Denis M, Vandewalle A, Rondeau E, Hertig A. Ofloxacin: new applications for the prevention of urinary tract infections in renal graft recipients. Transplant Infect Dis 2011; 13(4):344-52; http://dx.doi.org/ 10.1111/j.1399-3062.2011.00602.x [DOI] [PubMed] [Google Scholar]
- 51.El Amari EB, Hadaya K, Buhler L, Berney T, Rohner P, Martin PY, Mentha G, van Delden C. Outcome of treated and untreated asymptomatic bacteriuria in renal transplant recipients. Nephrol, Dialysis, Transplant 2011; 26(12):4109-14; http://dx.doi.org/ 10.1093/ndt/gfr198 [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Bloodstream infection after living donor liver transplantation. Scandinavian J Infect Dis 2008; 40(6-7):509-16; http://dx.doi.org/ 10.1080/00365540701824116 [DOI] [PubMed] [Google Scholar]
- 53.Nieto-Rodriguez JA, Kusne S, Manez R, Irish W, Linden P, Magnone M, Wing EJ, Fung JJ, Starzl TE. Factors associated with the development of candidemia and candidemia-related death among liver transplant recipients. Ann Surgery 1996; 223(1):70-6; http://dx.doi.org/ 10.1097/00000658-199601000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bert F, Larroque B, Paugam-Burtz C, Janny S, Durand F, Dondero F, Valla DC, Belghiti J, Moreau R, Nicolas-Chanoine MH. Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transplant 2010; 16(3):393-401. [DOI] [PubMed] [Google Scholar]
- 55.Shi SH, Kong HS, Xu J, Zhang WJ, Jia CK, Wang WL, Shen Y, Zhang M, Zheng SS. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transplant Infect Dis 2009; 11(5):405-12; http://dx.doi.org/ 10.1111/j.1399-3062.2009.00421.x [DOI] [PubMed] [Google Scholar]
- 56.Singh N, Gayowski T, Wagener MM, Marino IR. Bloodstream infections in liver transplant recipients receiving tacrolimus. Clin Transplant 1997; 11(4):275-81. [PubMed] [Google Scholar]
- 57.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, et al.. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010; 50(8):1101-11; http://dx.doi.org/ 10.1086/651262 [DOI] [PubMed] [Google Scholar]
- 58.Husain S, Tollemar J, Dominguez EA, Baumgarten K, Humar A, Paterson DL, Wagener MM, Kusne S, Singh N. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation 2003; 75(12):2023-9; http://dx.doi.org/ 10.1097/01.TP.0000065178.93741.72 [DOI] [PubMed] [Google Scholar]
- 59.Giannella M, Bartoletti M, Morelli MC, Tedeschi S, Cristini F, Tumietto F, Pasqualini E, Danese I, Campoli C, Lauria ND, et al.. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015; 15(6):1708-15; http://dx.doi.org/ 10.1111/ajt.13136 [DOI] [PubMed] [Google Scholar]
- 60.Cervera C, van Delden C, Gavalda J, Welte T, Akova M, Carratala J, ESCMID Study Group for Infections in Compromised Hosts . Multidrug-resistant bacteria in solid organ transplant recipients. Clin Microbiol Infect. 2014; 20 Suppl 7:49-73; http://dx.doi.org/ 10.1111/1469-0691.12687 [DOI] [PubMed] [Google Scholar]
- 61.Russell DL, Flood A, Zaroda TE, Acosta C, Riley MM, Busuttil RW, Pegues DA. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant. 2008; 8(8):1737-43; http://dx.doi.org/ 10.1111/j.1600-6143.2008.02304.x [DOI] [PubMed] [Google Scholar]
- 62.Palmer SM, Alexander BD, Sanders LL, Edwards LJ, Reller LB, Davis RD, Tapson VF. Significance of blood stream infection after lung transplantation: analysis in 176 consecutive patients. Transplantation 2000; 69(11):2360-6; http://dx.doi.org/ 10.1097/00007890-200006150-00025 [DOI] [PubMed] [Google Scholar]
- 63.Danziger-Isakov LA, Sweet S, Delamorena M, Huddleston CB, Mendeloff E, Debaun MR. Epidemiology of bloodstream infections in the first year after pediatric lung transplantation. Pediatric Infect Dis J 2005; 24(4):324-30; http://dx.doi.org/ 10.1097/01.inf.0000157089.42020.41 [DOI] [PubMed] [Google Scholar]
- 64.Aris RM, Routh JC, LiPuma JJ, Heath DG, Gilligan PH. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am J Respir Crit Care Med 2001; 164(11):2102-6; http://dx.doi.org/ 10.1164/ajrccm.164.11.2107022 [DOI] [PubMed] [Google Scholar]
- 65.Fauroux B, Hart N, Belfar S, Boule M, Tillous-Borde I, Bonnet D, Bingen E, Clément A. Burkholderia cepacia is associated with pulmonary hypertension and increased mortality among cystic fibrosis patients. J Clin Microbiol 2004; 42(12):5537-41; http://dx.doi.org/ 10.1128/JCM.42.12.5537-5541.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Soyza A, Meachery G, Hester KL, Nicholson A, Parry G, Tocewicz K, Pillay T, Clark S, Lordan JL, Schueler S, et al.. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. J Heart Lung Transplant. 2010; 29(12):1395-404; http://dx.doi.org/ 10.1016/j.healun.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 67.Alexander BD, Petzold EW, Reller LB, Palmer SM, Davis RD, Woods CW, Lipuma JJ. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant. 2008; 8(5):1025-30; http://dx.doi.org/ 10.1111/j.1600-6143.2008.02186.x [DOI] [PubMed] [Google Scholar]
- 68.Chaparro C, Maurer J, Gutierrez C, Krajden M, Chan C, Winton T, Keshavjee S, Scavuzzo M, Tullis E, Hutcheon M, et al.. Infection with Burkholderia cepacia in cystic fibrosis: outcome following lung transplantation. Am J Respir Crit Care Med. 2001; 163(1):43-8; http://dx.doi.org/ 10.1164/ajrccm.163.1.9811076 [DOI] [PubMed] [Google Scholar]
- 69.Mahenthiralingam E, Vandamme P, Campbell ME, Henry DA, Gravelle AM, Wong LT, Davidson AG, Wilcox PG, Nakielna B, Speert DP. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin Infect Dis. 2001; 33(9):1469-75; http://dx.doi.org/ 10.1086/322684 [DOI] [PubMed] [Google Scholar]
- 70.Zhou J, Chen Y, Tabibi S, Alba L, Garber E, Saiman L. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrobial Agents Chemotherapy 2007; 51(3):1085-8; http://dx.doi.org/ 10.1128/AAC.00954-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nash EF, Coonar A, Kremer R, Tullis E, Hutcheon M, Singer LG, Keshavjee S, Chaparro C. Survival of Burkholderia cepacia sepsis following lung transplantation in recipients with cystic fibrosis. Transplant Infect Dis 2010; 12(6):551-4; http://dx.doi.org/ 10.1111/j.1399-3062.2010.00525.x [DOI] [PubMed] [Google Scholar]
- 72.Poston RS, Husain S, Sorce D, Stanford E, Kusne S, Wagener M, Griffith BP, Kormos RL. LVAD bloodstream infections: therapeutic rationale for transplantation after LVAD infection. J Heart Lung Transplant. 2003; 22(8):914-21; http://dx.doi.org/ 10.1016/S1053-2498(02)00645-9 [DOI] [PubMed] [Google Scholar]
- 73.Healy AH, Baird BC, Drakos SG, Stehlik J, Selzman CH. Impact of ventricular assist device complications on posttransplant survival: an analysis of the United network of organ sharing database. Ann Thoracic Surgery 2013; 95(3):870-5; http://dx.doi.org/ 10.1016/j.athoracsur.2012.10.080 [DOI] [PubMed] [Google Scholar]
- 74.Aslam S, Hernandez M, Thornby J, Zeluff B, Darouiche RO. Risk factors and outcomes of fungal ventricular-assist device infections. Clin Infect Dis. 2010; 50(5):664-71; http://dx.doi.org/ 10.1086/650454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh RP, Farney AC, Rogers J, Ashcraft E, Hart L, Doares W, Hartmann EL, Reeves-Daniel A, Adams PL, Stratta RJ. Analysis of bacteremia after pancreatic transplantation with enteric drainage. Transplant Proc 2008; 40(2):506-9; http://dx.doi.org/ 10.1016/j.transproceed.2008.02.015 [DOI] [PubMed] [Google Scholar]
- 76.Kawecki D, Kwiatkowski A, Michalak G, Sawicka-Grzelak A, Mlynarczyk A, Sokol-Leszczynska B, Kot K, Czerwinski J, Lisik W, Bieniasz M, et al.. Etiologic agents of bacteremia in the early period after simultaneous pancreas-kidney transplantation. Transplant Proc 2009; 41(8):3151-3; http://dx.doi.org/ 10.1016/j.transproceed.2009.07.064 [DOI] [PubMed] [Google Scholar]
- 77.Sigurdsson L, Reyes J, Kocoshis SA, Mazariegos G, Abu-Elmagd K, Green M. Bacteremia after intestinal transplantation in children correlates temporally with rejection or gastrointestinal lymphoproliferative disease. Transplantation 2000; 70(2):302-5; http://dx.doi.org/ 10.1097/00007890-200007270-00011 [DOI] [PubMed] [Google Scholar]
- 78.Akhter K, Timpone J, Matsumoto C, Fishbein T, Kaufman S, Kumar P. Six-month incidence of bloodstream infections in intestinal transplant patients. Transplant Infect Dis 2012; 14(3):242-7. [DOI] [PubMed] [Google Scholar]
- 79.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, et al.. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011; 39(4 Suppl 1):S1-34; http://dx.doi.org/ 10.1016/j.ajic.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 80.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, et al.. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010; 50(5):625-63; http://dx.doi.org/ 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 81.American Thoracic S, Infectious Diseases Society of A . Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171(4):388-416; http://dx.doi.org/ 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 82.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, et al.. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011; 52(9):e162-93; http://dx.doi.org/ 10.1093/cid/cir257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouza E, Burillo A, Guembe M. Managing intravascular catheter-related infections in heart transplant patients: how far can we apply IDSA guidelines for immunocompromised patients? Current opinion in infectious diseases. 2011; 24(4):302-8. [DOI] [PubMed] [Google Scholar]
- 84.Choi SU, Lee JH, Oh CK, Shin GT, Kim H, Kim SJ, Kim SI. Clinical significance of prophylactic antibiotics in renal transplantation. Transplant Proc 2013; 45(4):1392-5; http://dx.doi.org/ 10.1016/j.transproceed.2012.10.059 [DOI] [PubMed] [Google Scholar]
- 85.Singh N, Squier C, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Impact of an aggressive infection control strategy on endemic Staphylococcus aureus infection in liver transplant recipients. Infect Control Hospital Epidemiol. 2006; 27(2):122-6; http://dx.doi.org/ 10.1086/500651 [DOI] [PubMed] [Google Scholar]
- 86.Raakow R, Steffen R, Lefebre B, Bechstein WO, Blumhardt G, Neuhaus P. Selective bowel decontamination effectively prevents gram-negative bacterial infections after liver transplantation. Transplant Proc 1990; 22(4):1556-7. [PubMed] [Google Scholar]
- 87.Arnow PM. Prevention of bacterial infection in the transplant recipient. The role of selective bowel decontamination. Infect Dis Clin North Am 1995; 9(4):849-62. [PubMed] [Google Scholar]
- 88.Badger IL CH, Kong KL, et al.. Is selective bowel decontamination of the digestive tract beneficial in liver transplant patients? Interim results of a prospective, randomized trial. Transplant Proc 1991. Feb; 23(1 Pt 2):1460-1. [PubMed] [Google Scholar]
- 89.Smith SD, Jackson RJ, Hannakan CJ, Wadowsky RM, Tzakis AG, Rowe MI. Selective decontamination in pediatric liver transplants. A randomized prospective study. Transplantation. 1993; 55(6):1306-9; http://dx.doi.org/ 10.1097/00007890-199306000-00018 [DOI] [PubMed] [Google Scholar]
- 90.Sanchez Garcia M, Cambronero Galache JA, Lopez Diaz J, Cerda Cerda E, Rubio Blasco J, Gomez Aguinaga MA, Núnez Reiz A, Rogero Marín S, Onoro Canaveral JJ, Sacristán del Castillo JA. Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled, multicenter trial. Am J Respir Crit Care Med. 1998; 158(3):908-16; http://dx.doi.org/ 10.1164/ajrccm.158.3.9712079 [DOI] [PubMed] [Google Scholar]
- 91.Aguilar-Guisado M, Givalda J, Ussetti P, Ramos A, Morales P, Blanes M, Bou G, de la Torre-Cisneros J, Román A, Borro JM, et al.. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007; 7(8):1989-96; PMID:17617864; http://dx.doi.org/ 10.1111/j.1600-6143.2007.01882.x [DOI] [PubMed] [Google Scholar]
- 92.Tolkoff-Rubin NE, Cosimi AB, Russell PS, Rubin RH. A controlled study of trimethoprim-sulfamethoxazole prophylaxis of urinary tract infection in renal transplant recipients. Rev Infect Dis 1982; 4(2):614-8; PMID:7051249; http://dx.doi.org/ 10.1093/clinids/4.2.614 [DOI] [PubMed] [Google Scholar]
- 93.Fox BC, Sollinger HW, Belzer FO, Maki DG. A prospective, randomized, double-blind study of trimethoprim-sulfamethoxazole for prophylaxis of infection in renal transplantation: clinical efficacy, absorption of trimethoprim-sulfamethoxazole, effects on the microflora, and the cost-benefit of prophylaxis. Am J Med 1990; 89(3):255-74; PMID:2118307; http://dx.doi.org/ 10.1016/0002-9343(90)90337-D [DOI] [PubMed] [Google Scholar]
- 94.Vidal E, Cervera C, Cordero E, Arminanzas C, Carratala J, Cisneros JM, et al.. Management of urinary tract infection in solid organ transplant recipients: Consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI). Enferm Infecc Microbiol Clin. 2015 Dec;33(10):680-7. [DOI] [PubMed] [Google Scholar]
- 95.Tissot F, Pascual M, Hullin R, Yerly P, Tozzi P, Meylan P, Manuel O. Impact of targeted antifungal prophylaxis in heart transplant recipients at high risk for early invasive fungal infection. Transplantation. 2014; 97(11):1192-7; PMID:24521774; http://dx.doi.org/ 10.1097/01.tp.0000441088.01723.ee [DOI] [PubMed] [Google Scholar]
- 96.Fortun J, Martin-Davila P, Montejo M, Munoz P, Cisneros JM, Ramos A, Aragón C, Blanes M, San Juan R, Gavaldá J, et al.. Prophylaxis with caspofungin for invasive fungal infections in high-risk liver transplant recipients. Transplantation 2009; 87(3):424-35; PMID:19202450; http://dx.doi.org/ 10.1097/TP.0b013e3181932e76 [DOI] [PubMed] [Google Scholar]
- 97.Silveira FP, Kusne S, Practice ASTIDCo . Candida infections in solid organ transplantation. Am J Transplant. 2013; 13 Suppl 4:220-7; PMID:23465015 [DOI] [PubMed] [Google Scholar]