Abstract
Depression may be accompanied by increased oxidative stress and decreased circulating anti-oxidants. This study examines the association between depressive symptoms, F2-isoprostanes and carotenoids in a US community sample. The study includes 3009 participants (mean age 40.3, 54.2% female) from CARDIA (Coronary Artery Risk Development in Young Adults). Cross-sectional analyses were performed on data from the year 15 examination (2000–2001) including subjects whose depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) and had measurements of plasma F2-isoprostanes (gas chromatography/mass spectrometry) or serum carotenoids (high-performance liquid chromatography). Carotenoids zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, β-carotene were standardized and summed. Longitudinal analyses were conducted using the data from other examinations at 5-year intervals. Cross-lagged analyses investigated whether CES-D predicted F2-isoprostanes or carotenoids at the following exam, and vice versa. Regression analyses were controlled for sociodemographics, health and lifestyle factors. F2-isoprostanes were higher in subjects with depressive symptoms (CES-D⩾16) after adjustment for sociodemographics (55.7 vs 52.0 pg ml−1; Cohen's d=0.14, P<0.001). There was no difference in F2-isoprostanes after further adjustment for health and lifestyle factors. Carotenoids were lower in those with CES-D scores ⩾16, even after adjustment for health and lifestyle factors (standardized sum 238.7 vs 244.0, Cohen's d=−0.16, P<0.001). Longitudinal analyses confirmed that depression predicts subsequent F2-isoprostane and carotenoid levels. Neither F2-isoprostanes nor carotenoids predicted subsequent depression. In conclusion, depressive symptoms were cross-sectionally and longitudinally associated with increased F2-isoprostanes and decreased carotenoids. The association with F2-isoprostanes can largely be explained by lifestyle factors, but lower carotenoids were independently associated with depressive symptoms.
Introduction
Depression, besides being a leading cause of poor functioning and disability,1 is an independent predictor of the onset of somatic disease.2 Relative to individuals without, individuals with depression have a higher risk of developing cardiovascular disease,3 obesity,4 diabetes,5 cancer6 and cognitive impairment7 and have increased mortality rates.8 These associations have been found not only in individuals with major depressive disorder, but also in those with depressive symptoms.2 Increased oxidative stress and decreased antioxidant levels may be key mechanisms in this association. Oxidative stress is a complex and dynamic biological process that refers to the damaging effects of reactive oxygen species (ROS). ROS are normal products of aerobic metabolism and the body has a range of antioxidant defenses to protect against their potentially harmful effects. However, when there is either an increase in exposure to (or production of) ROS, or a decrease in anti-oxidants levels, damage may occur to lipids, proteins and DNA resulting in cellular dysfunction and disease, or ultimately cell death.9
A recent meta-analysis10 demonstrated that oxidative stress levels measured by F2-isoprostanes (reflecting oxidative lipid damage) are increased in persons with major depression and/or depressive symptoms, but the number of studies and their sample sizes were limited and many studies did not account for important potential confounding factors. Oxidative stress is associated with a range of sociodemographic, health and lifestyle factors, for example, socioeconomic status and smoking,9, 11, 12 that are also known to be associated with depression.4, 13, 14, 15 If taken into account, these factors could considerably influence the estimate of the association.
There is also evidence suggesting that anti-oxidants are decreased in depression, illustrated by lower antioxidant levels,16 including carotenoids,17, 18 and antioxidant enzymes in persons with depressive symptomatology.19 Measuring oxidative stress in vivo is challenging; levels of ROS are not easily determined owing to their short half-life and highly reactive nature. Alternative approaches include measurements of oxidative damage to protein, lipids or DNA or of levels of antioxidants.
F2-isoprostanes are currently considered to be the marker of choice for oxidative lipid damage.20, 21, 22, 23 F2-isoprostanes are formed solely through oxidative processes; this quality and the chemical stability of these compounds make them more reliable markers than other widely used measures of oxidative lipid damage, such as malondialdehyde or thiobarbituric reactive substances, that have limited validity due to the likelihood of artefactual formation.
Carotenoids have antioxidant capacity. This property may contribute to observational studies finding higher carotenoid levels to be associated with reduced risks of metabolic syndrome,24, 25 diabetes mellitus,26, 27, 28 cardiovascular disease29, 30 and cancer.31 In humans, the most important carotenoids include β-carotene, lycopene, zeaxanthin/lutein and β-cryptoxanthin. They owe their potent antioxidant action to their ability to quench ROS.32 As highly lipophilic molecules, they are located in the lipid bilayer of the cell membrane where they protect against lipid peroxidation.33 Studying carotenoids in unison with the F2-isoprostanes provides insight into two important and interdependent aspects of redox homeostasis.
This study describes the cross-sectional associations of depressive symptoms with F2-isosprostanes and carotenoids in large community samples, taking into account a wide range of important sociodemographic, health and lifestyle factors. In particular, this study includes data on dietary patterns, which is not available for the majority of previous research. Dietary patterns may be an important potential confounding factor in the association with depression; dietary intake is the sole source of carotenoids in humans and F2-isoprostanes have previously been demonstrated to be associated with dietary pattern independently of other health and lifestyle factors.34 In addition, this study examines the relationships between F2-isosprostanes, carotenoids and depressive symptoms over multiple time points to gain insight into the temporal directionality of the association as it is unclear whether oxidative stress leads to depressive symptoms or vice versa. It is possible that depressive symptoms lead to unhealthy behaviors that in turn increase exposure to oxidative stress. Alternatively, increased oxidative stress, to which the brain is particularly vulnerable, may cause oxidative damage, making an individual susceptible to developing depressive symptoms.35
This report comprises, to our knowledge, the largest sample in which the association of depressive symptoms, F2-isosprostanes and carotenoids have been examined together.
Materials and methods
Study sample
Data are from the CARDIA (Coronary Artery Risk Development in Young Adults) study and the ancillary YALTA (Young Adult Longitudinal Trends in Antioxidants) study. CARDIA is a longitudinal, multicenter epidemiological study on the development of risk factors for cardiovascular disease. The study design and recruitment of participants have been described elsewhere.36 In brief, from 1985 to 1986, subjects aged 18–30 years were recruited from four sites (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) in the United States. The sampling strategy resulted in a cohort balanced by race (52% black), sex (55% female) and education (40% with <12 years of education) comprising 5115 individuals at baseline. Follow-up assessments were conducted after 2, 5, 7, 10, 15, 20 years from the baseline assessment with retentions rates of 91%, 86%, 81%, 79%, 74%, 72% of the surviving cohort, respectively. YALTA measured serum carotenoids at years 0, 7 and 15 and plasma F2-isoprostanes at years 15 and 20. Institutional review committee approval was obtained from each site and written informed consent was obtained from the participants for all assessments.
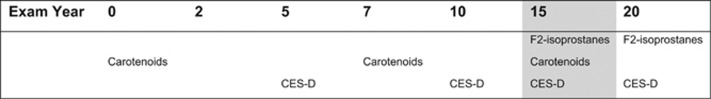
The cross-sectional analyses in this study were conducted with data from the year 15 CARDIA assessment, as this is the only time point that allowed analysis of both F2-isoprostanes and carotenoids (see Figure 1) with depressive symptoms. The sample comprised 3009 participants for whom data were available on depressive symptoms measured by the Center for Epidemiologic Studies Depression Scale (CES-D) and data on either F2-isoprostanes (n=2974) or carotenoids (n=2889). For the longitudinal analyses, data were used on F2-isoprostanes from years 15 and 20, carotenoids from baseline and years 7 and 15, and CES-D from years 5, 10, 15 and 20.
Figure 1.
Available data on F2-isoprostanes, carotenoids and CES-D in CARDIA exam years 0–20. CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale.
Depressive symptoms
Depressive symptoms were assessed using the 20-item CES-D.37 Subjects indicate on a four-point scale how often they experienced a symptom in the past week. Four items relating to positive affect are reverse scored and the overall score is a sum of the responses across the 20 items (possible range 0–60). A cutoff score of ⩾16 indicates a clinically significant depressed mood. The CES-D has been found to have good internal consistency and adequate test–retest reliability. Construct validity of the scale is supported by correlations with other self-report measures, clinical ratings of depression, and clinical interviews.38
CES-D scores were analyzed in four different ways. First, total CES-D scores were analyzed as a continuous measure. Second, the CES-D was used as a categorical measure using the cutoff score ⩾16. Third, a categorical measure based on CES-D scores and antidepressant use comparing subjects with CES-D⩾16 and/or current antidepressant to subjects with CES-D<16 and no antidepressant use was created. (Data on antidepressant use was obtained through a structured interview assessed at the same examination point.) Finally, to study the impact of exposure to chronic depressive symptoms, a count variable was created representing the number of times the CES-D was ⩾16 based on the assessments at years 5, 10 and 15. Subjects were compared with those who never had a CES-D score ⩾16.
Oxidative stress and anti-oxidants
All the participants were instructed to adhere to an overnight fast and were asked to avoid smoking and heavy physical activity for at least 2 h before blood collection at each examination. After serum and plasma separation from whole blood, aliquots were stored at −70 °C until they were shipped on dry ice to a central laboratory.
Plasma F2-isoprostanes
YALTA used plasma obtained at CARDIA years 15 and 20 to measure F2-isoprostanes with a gas chromatography–mass spectrometry-based method39 by the Molecular Epidemiology and Biomarker Research Laboratory at the University of Minnesota (Minneapolis, MN, USA), as previously described.40 All the samples were analyzed within 1 year of collection. Substudies demonstrated stability of F2-isoprostanes (no ex vivo loss or formation) during blood collection and processing procedures. Analytical variation of the method was 10% for each of three control pools assembled in 2000 and assayed repeatedly between October 2000 and August 2007; the values of these control pools were stable over time, implying that both assay and stored samples were stable.34 Thus, year 15 and 20 plasma F2-isoprostane concentrations are directly comparable.
Serum carotenoids
YALTA used sera obtained at CARDIA years 0, 7 and 15 to assay the carotenoids, α- and β-carotene, lycopene, zeaxanthin/lutein and β-cryptoxanthin (Molecular Epidemiology and Biomarker Research Laboratory, University of Minnesota), with an HPLC-based assay modified from the method of Bieri et al.41 to optimize detection of carotenoids with calibration as described by Craft et al.42 and sample handling as described by Gross et al.43 Calibration was performed with pure compounds (Hoffmann-La Roche, Basel, Switserland; Sigma Chemical (now Sigma-Aldrich, St Louis, MO, USA)). Quality-control procedures included routine analysis of plasma and serum control pools containing high and low concentrations of each analyte. In addition, the laboratory routinely analyzed NIST reference sera and was a participant in the NIST Fat-Soluble Vitamin Quality Assurance Group. The coefficients of variance were <10% for all analytes and control pools. The intra-class correlation coefficients (between-person variance/between-person plus within-person variance) were 0.93 for α-carotene, 0.98 for β-carotene, 0.73 for zeaxanthin/lutein, 0.97 for β-cryptoxanthin and 0.73 for lycopene.44
From a dietary and physiological perspective, the five carotenoids (zeaxanthin/lutein, β-cryptoxanthin, lycopene, α- and β-carotene) are closely interconnected and were therefore analyzed as total carotenoids (sum of five carotenoids) and as well as individually, similar to previous reports on carotenoids in this sample45 and previous studies on carotenoids and depressive symptoms.46
Covariates
Demographic variables
Participants' age, sex and race were assessed by self-report. Education was assessed by asking participants to report their highest level of education.
Number of somatic diseases
Participants reported whether they had ever been diagnosed with any of twenty-seven major or chronic health conditions, and whether they had had these conditions in the past year (see footnote 'd' in Table 1). The total number of self-reported diagnoses of diseases served as the covariate.
Table 1. Sample characteristics by depressive symptoms (CES-D< or ⩾16).
| CARDIA exam year 15 | CES-D<16 | CES-D⩾16 | P | Total | ||
|---|---|---|---|---|---|---|
| (N=2538) | (N=471) | (N=3009)a | ||||
| Oxidative stress | ||||||
| F2-isoprostanes pg ml−1 | Median (IQR) | 58.2 (38.2–68.3) | 55.4 (41.9–75.0) | <0.001 | 50.9 (38.5–69.4) | |
| Carotenoids | ||||||
| Sum of five carotenoids (t-scores)b | Median (IQR) | 252.0 (228.6–268.5) | 234.4 (221.3–252.4) | <0.001 | 243.4 (227.2–266.50) | |
| Zeaxanthin/lutein μg dl−1 | Median (IQR) | 25.9 (17.5–31.3) | 20.2 (15.6–27.6) | <0.001 | 23.1 (17.1–30.7) | |
| β-cryptoxanthin μg dl−1 | Median (IQR) | 12.1 (6.5–15.2) | 8.4 (5.8–12.9) | <0.001 | 9.6 (6.4–14.7) | |
| Lycopene μg dl−1 | Median (IQR) | 40.2 (27.3–50.4) | 35.0 (24.4–46.0) | <0.001 | 37.7 (26.9–49.7) | |
| α-carotene μg dl−1 | Median (IQR) | 5.9 (2.0–7.3) | 2.6 (1.3.1–4.9) | <0.001 | 3.7 (1.9–6.9) | |
| β-carotene μg dl−1 | Median (IQR) | 22.5 (9.5–16.4) | 12.1 (7.1–20.1) | <0.001 | 15.6 (9.1–26.4) | |
| Depressive symptoms | ||||||
| CES-D | Mean (s.d.) | 6.3 (4.2) | 22.8 (7.0) | 8.9 (7.7) | ||
| CES-D⩾16 | — | — | 15.7% | |||
| CES-D⩾16 and/or antidepressant use | — | — | 20.0% | |||
| Antidepressant users | 5.1% | 19.1% | <0.001 | 7.3% | ||
| N of times CES-D⩾16 | 0 | 75.4% | — | <0.001 | 63.9% | |
| over years 0, 10 and 15c | 1 | 19.3% | 29.0% | 20.8% | ||
| 2 | 5.3% | 35.0% | 9.8% | |||
| 3 | — | 36.0% | 5.5% | |||
| Sociodemographics | ||||||
| Age | Mean (s.d.) | 40.3 (3.6) | 40.1 (3.7) | 0.15 | 40.3 (3.6) | |
| Female | 52.4% | 63.9% | <0.001 | 54.2% | ||
| White | 58.3% | 37.2% | <0.001 | 55.0% | ||
| Education | <0.001 | |||||
| ⩽High school | 44.4% | 55.9% | 46.2% | |||
| (some) College | 43.6% | 33.3% | 42.0% | |||
| ⩾Master's degree | 11.9% | 10.9% | 11.8% | |||
| Health and lifestyle | ||||||
| N somatic diseasesd | Mean (s.d.) | 0.9 (1.1) | 1.4 (1.5) | <0.001 | 1.0 (1.2) | |
| Supplement userse | 61.4% | 62.2% | 0.74 | 61.5% | ||
| Diet quality scoref | Mean (s.d.) | 67 (11) | 63 (11) | <0.001 | 67 (11) | |
| Smoking | Non (never and former) | 81.8% | 65.7% | <0.001 | 79.3% | |
| Current | 18.2% | 34.3% | 20.7% | |||
| Alcohol | <0.001 | |||||
| ♂/♀ 0 units per week | 46.5% | 50.1% | 47.1% | |||
| ♂⩽14/♀⩽7 units per week | 42.0% | 33.3% | 40.7% | |||
| ♂>14/♀>7 units per week | 11.5% | 16.6% | 12.3% | |||
| BMI kg/m2 | Mean (s.d.) | 28.3 (6.0) | 29.7 (7.3) | <0.001 | 28.5 (6.2) | |
| Physical activity (exercise units) | Mean (s.d.) | 367 (290) | 281 (252) | <0.001 | 353 (286) | |
Abbreviations: BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale; IQR, interquartile range; N, number.
Year 15 N=3009 with valid CES-D score and valid F2-isoprostane (N=2974), at least one carotenoid value (N=2889), all five carotenoid values (N=2865).
Sum of standardized values (t-scores) of zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, β-carotene.
Number of times CES-D⩾16 over assessments at CARDIA exam years 5, 10, 15.
Number or somatic diseases included in count: high blood pressure, high cholesterol, heart problem, diabetes, hepatitis in the past year, kidney failure/dialysis/transplant in the past year, nephritis in the past year, other kidney disease in the past year, liver cirrhosis, other liver disease in the past year, gallstones in the past year, migraine in the past year, peripheral vascular disease, cancer (ever), thyroid disease (ever), ulcer in the past year, other digestive disease in the past year, gout in the past year, asthma in the past year, epilepsy with seizures in the past year, tuberculosis in the past year, emphysema in the past year, multiple sclerosis in the past year, stroke in the past year, chronic bronchitis in the past year, HIV (ever), blood clot (past year), other major disease, polycystic ovarian syndrome.
Use of a multivitamin, vitamin A, C, E, beta-carotene or an antioxidant combination.
Average score over CARDIA exam years 0, 7 and 20.
Supplement use
Participants were asked whether they had used any vitamin or mineral supplements in the past year as part of the CARDIA Diet Practices, Behaviors and Attitudes Questionnaire. Those using a multivitamin, vitamin A, C, E, beta-carotene or an antioxidant combination were defined as supplement users.
Diet quality
Diet was assessed at years 0, 7 and 20 through an interviewer-administered validated diet history in which participants were asked open-ended questions about their dietary pattern in the last month. Food groups were classified as beneficial (n=20), adverse (n=13) or neutral (n=13) in terms of hypothesized health effects. This score (A Priori Diet Quality Score) has been previously described in more detail.34 The theoretical range of score is 0–132 with higher scores indicating higher hypothesized diet quality. This diet quality score is characterized by long-term stability. Correlation coefficients for dietary scores at years 0 and 7, and years 7 and 20 were 0.65 and 0.64, respectively. As dietary data are not available for the year 15 assessment, the mean of the year 0, 7 and 20 diet scores was calculated based on as many of the three data points as were available.
Smoking status
Self-reported smoking was classified as nonsmoker (never and former) or current smoker.
Alcohol consumption
Participants were categorized as non-drinker, moderate drinker (⩽14 units per week for males and ⩽7 units per week for females) or heavy drinker (>14 units per week for males and >7 units per week for females).
Physical activity
A validated interview-administered questionnaire (CARDIA Physical Activity History, a simplified version of the Minnesota Leisure Time Physical Activity Questionnaire47) was used to assess physical activity in the past year, from which exercise units were calculated.
Body mass index
Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Height and weight were recorded to the nearest 0.5 cm and 0.2 kg.
All scales used to assess health behaviors are accessible on http://www.cardia.dopm.uab.edu/.
Statistical analyses
Statistical analyses were performed using SPSS version 20.0 (IBM, Armonk, NY, USA). Independent t-tests were used to compare means of continuous variables and chi-square tests to compare categorical variables between persons with and without depressive symptoms. To analyze total carotenoids, a sum score of the standardized values (t-scores) of the five carotenoids was created. F2-isoprostanes and the sum of five carotenoids were log-transformed for the analysis.
Linear regression examining the cross-sectional association between F2-isoprostanes or carotenoids (dependent variables) and CES-D scores (main predictor) were conducted with three models. Model 1 was adjusted for sociodemographics (age, sex, race and education); model 2 was additionally adjusted for supplement use and the number of somatic diseases; model 3 was additionally adjusted for health and lifestyle factors, including diet, BMI, smoking, alcohol consumption and physical activity. All the models were adjusted for research center (Birmingham, AL; Chicago, IL; Minneapolis, MN or Oakland, CA). Analysis of covariance was used to calculate the mean levels of F2-isoprostanes and carotenoids in those with and without depressive symptoms (CES-D cutoff ⩾16). Reported levels were back-transformed to geometric means. Effect's sizes Cohen's d48 were calculated on the basis of the means, standard deviations and number of subjects.
To demonstrate which health and lifestyle factors had the greatest effects on the association between F2-isoprostanes/carotenoids and CES-D scores, change of estimate analyses were conducted. Models were created for each health and lifestyle factor by adding them to the model already adjusted for sociodemographics (model 1). The percentage of change in standardized regression coefficient of the CES-D score after the addition of a health and lifestyle factor was calculated for each covariate.
To determine whether depressive symptoms predict oxidative stress levels over time (or vice versa), cross-lagged linear regression analyses were conducted. F2-isoprostane levels at year 20 (dependent variable) and CES-D at year 15 (main predictor) were adjusted for covariates at year 15 and F2-isoprostanes at year 15. Similarly, analyses were conducted with CES-D at year 20 (dependent variable) and F2-isoprostanes at year 15 (main predictor), adjusted for covariates at year 15 and CES-D at year 15. For the sum of the five carotenoids at year 15, cross-lagged analyses were conducted with CES-D at year 10 as the main predictor, with covariates at year 10, additionally adjusted for carotenoids at year 7 (no data were available on carotenoids at year 10). Finally, analyses were conducted with CES-D at year 20 (dependent variable) and the carotenoids at year 15 (main predictor), adjusted for covariates at year 15 and CES-D at year 15.
Results
Sample characteristics
The sample was 54% female, 55% white and the mean age was 40.3 years (s.d. 3.6). The mean CES-D score was 8.9 (s.d. 7.7) with 15.7% scoring ⩾16 and 20.0% scoring ⩾16 and/or currently using antidepressants. Those with a CES-D score ⩾16 were more likely to be female, black and have less than high school education. They differed significantly from those with CES-D score lower than 16 on all health and lifestyle factors, except supplement use. Those with depressive symptoms were more likely to be smokers, heavy drinkers, have a higher BMI, have somatic diseases, have a lower diet quality score and to be less physically active (Table 1). F2-isoprostanes and the summed carotenoids were significantly inversely correlated (Pearson's rho: −0.27, P<0.001). Table 2 shows the associations of these markers with the sociodemographic, health and lifestyle covariates. (See Supplementary Table 1 for the results of the individual carotenoids.)
Table 2. Cross-sectional univariate associations of F2-isoprostanes and carotenoids with covariatesa.
| CARDIA exam year 15 |
F2-isoprostanes |
Sum of five carotenoidsb |
||||
|---|---|---|---|---|---|---|
| β | P | β | P | |||
| Depressive symptoms | ||||||
| CES-D | 0.07 | <0.001 | −0.16 | <0.001 | ||
| CES-D⩾16 | 0.08 | <0.001 | −0.14 | <0.001 | ||
| CES-D⩾16 and/or antidepressant use | 0.09 | <0.001 | −0.13 | <0.001 | ||
| Antidepressant users | 0.07 | <0.001 | −0.06 | <0.001 | ||
| N of times CES-D⩾16 | 0 | Ref. | Ref. | |||
| over years 0, 10 and 15c | 1 | 0.06 | 0.006 | −0.05 | 0.007 | |
| 2 | 0.07 | <0.001 | −0.09 | <0.001 | ||
| 3 | 0.06 | 0.003 | −0.09 | <0.001 | ||
| Sociodemographics | ||||||
| Age | −0.02 | 0.180 | 0.04 | 0.023 | ||
| Sex | (male reference) | 0.27 | <0.001 | 0.00 | 0.984 | |
| Race | (white reference) | 0.00 | 0.820 | −0.13 | <0.001 | |
| Education | ⩽High school | Ref. | Ref. | |||
| (some) College | −0.09 | <0.001 | 0.23 | <0.001 | ||
| ⩾Master's degree | −0.03 | 0.137 | 0.08 | <0.001 | ||
| Health and lifestyle | ||||||
| N somatic diseasesd | 0.07 | <0.001 | −0.07 | <0.001 | ||
| Supplement userse | −0.13 | <0.001 | 0.12 | <0.001 | ||
| Diet quality scoref | −0.11 | <0.001 | 0.33 | <0.001 | ||
| Smoker | Non (never and former) | Ref. | Ref. | |||
| Current | 0.08 | <0.001 | −0.23 | <0.001 | ||
| Alcohol | ♂/♀ 0 Units per week | Ref. | Ref. | |||
| ♂⩽14 /♀⩽7 Units per week | −0.10 | <0.001 | 0.10 | <0.001 | ||
| ♂>14/♀>7 Units per week | 0.07 | <0.001 | −0.03 | 0.078 | ||
| BMI kg/m2 | 0.34 | <0.001 | −0.28 | <0.001 | ||
| Physical activity | Exercise units (EU) | −0.16 | <0.001 | 0.13 | <0.001 | |
Abbreviations: BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale; N, number; Ref., reference.
F2-isoprostanes and carotenoids log-transformed for linear regression analysis. Results are reported as standardized regression coefficients. All the results are adjusted for CENTER at baseline.
Sum of standardized values (t-scores) of zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, β-carotene
Comparison of 1, 2 or 3 times CES-D⩾16 with CES-D score 0 times ⩾16 (over CARDIA exam years 5, 10, 15)
Number of self-reported somatic diseases included in count: high blood pressure, high cholesterol, heart problem, diabetes, hepatitis in the past year, kidney failure/dialysis/transplant in the past year, nephritis in the past year, other kidney disease in the past year, liver cirrhosis, other liver disease in the past year, gallstones in the past year, migraine in the past year, peripheral vascular disease, cancer (ever), thyroid disease (ever), ulcer in the past year, other digestive disease in the past year, gout in the past year, asthma in the past year, epilepsy with seizures in the past year, tuberculosis in the past year, emphysema in the past year, multiple sclerosis in the past year, stroke in the past year, chronic bronchitis in the past year, HIV (ever), blood clot (past year), other major disease, polycystic ovarian syndrome.
Use of a multivitamin, vitamin A, C, E, beta-carotene or an antioxidant combination.
Average score over CARDIA exam years 0, 7 and 20.
F2-isoprostanes
Cross-sectional analyses
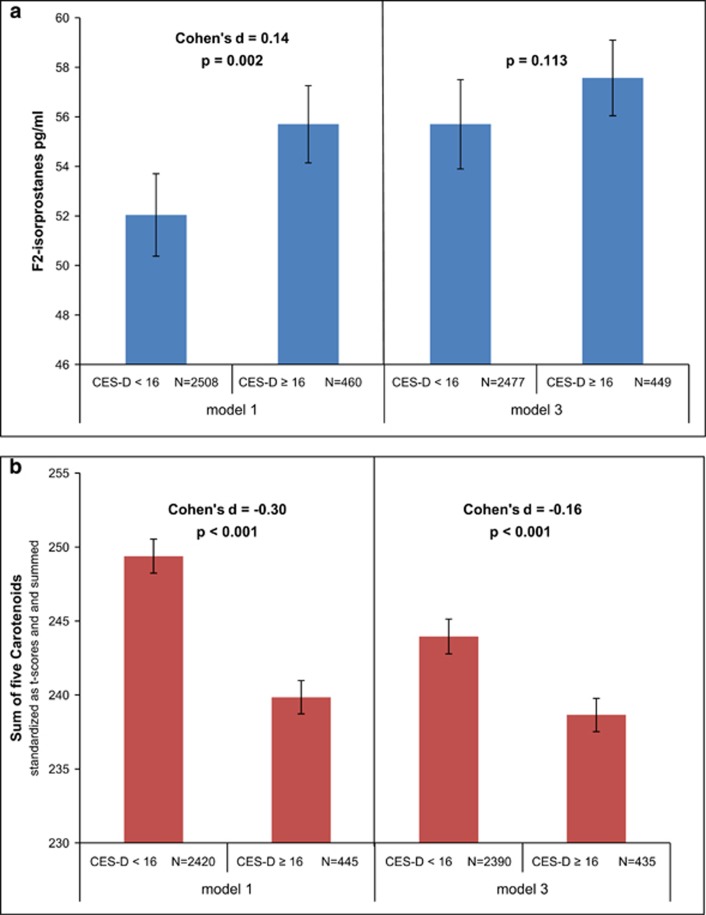
CES-D (continuous score) was positively associated with F2-isoprostanes after adjustment for sociodemographics (model 1: β=0.05, P=0.009) and supplement use and somatic disease (model 2: β=0.05, P=0.010; Table 3). After additional adjustment for lifestyle, there was no association between CES-D and F2-isoprostanes (model 3: β=0.01, P=0.731). In depressed subjects (CES-D⩾16), the mean F2-isoprostane level was 55.7 pg ml−1 (95% CI=53.6–58.0) vs 52.0 pg ml−1 (95% CI=51.0–53.1) in the non-depressed after adjustment for sociodemographics (model 1: Cohen's d= 0.14, P=0.001; Figure 2). There was no significant difference between the groups after adjustment for health and lifestyle factors: mean F2-isoprostane level was 57.5 pg ml−1 (95% CI=55.4–59.9) in depressed versus 55.7 pg ml−1 in non-depressed subjects (95% CI=54.4–57.0; P=0.113; Figure 2a). Change in estimate analyses revealed BMI, smoking and diet had the greatest effects on the association (See Supplementary Table 3).
Table 3. Cross-sectional multivariable associations of F2-isoprostanes and carotenoids with depressive symptoms (CES-D)a.
| CARDIA exam year 15 | Model 1 | Model 2 | Model 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age, sex, race, education |
Age, sex, race,
education,
somatic disease,
supplement use |
Age, sex, race,
education,
somatic disease,
supplement use,
diet, BMI,
smoking, alcohol,
physical activity |
||||||||||||||||
| N | β | P | N | β | P | N | β | P | ||||||||||
| F2-isoprostanes | ||||||||||||||||||
| CES-D score | 2968 | 0.05 | 0.009 | 2955 | 0.05 | 0.010 | 2926 | 0.01 | 0.731 | |||||||||
| CES-D⩾16 | 2968 | 0.06 | 0.002 | 2955 | 0.06 | 0.001 | 2926 | 0.03 | 0.113 | |||||||||
| CES-D⩾16 and/or AD use | 2968 | 0.06 | 0.001 | 2955 | 0.06 | 0.001 | 2926 | 0.03 | 0.047 | |||||||||
| N CES-D ⩾16b | 0 | 2592 | Ref. | 2582 | Ref. | 2558 | Ref. | |||||||||||
| 1 | 0.03 | 0.123 | 0.03 | 0.130 | 0.01 | 0.704 | ||||||||||||
| 2 | 0.05 | 0.009 | 0.05 | 0.008 | 0.03 | 0.111 | ||||||||||||
| 3 | 0.04 | 0.033 | 0.04 | 0.036 | 0.01 | 0.603 | ||||||||||||
| Sum of five carotenoidsc | ||||||||||||||||||
| CES-D score | 2865 | −0.13 | <0.001 | 2853 | −0.13 | <0.001 | 2825 | −0.07 | <0.001 | |||||||||
| CES-D ⩾16 | 2865 | −0.11 | <0.001 | 2853 | −0.11 | <0.001 | 2825 | −0.06 | <0.001 | |||||||||
| CES-D ⩾16 and/or AD use | 2865 | −0.11 | <0.001 | 2853 | −0.10 | <0.001 | 2825 | −0.06 | <0.001 | |||||||||
| N CES-D ⩾16b | 0 | 2508 | Ref. | 2499 | 2475 | Ref. | ||||||||||||
| 1 | −0.03 | 0.083 | −0.03 | 0.096 | 0.00 | 0.981 | ||||||||||||
| 2 | −0.07 | 0.001 | −0.06 | 0.001 | −0.02 | 0.206 | ||||||||||||
| 3 | −0.07 | <0.001 | −0.07 | <0.001 | −0.03 | 0.062 | ||||||||||||
Abbreviations: AD, antidepressant; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale; N, number; Ref., reference.
F2-isoprostanes and carotenoids are log-transformed for linear regression analysis. Results are reported as standardized regression coefficients. All the results are adjusted for CENTER at baseline.
Comparison of 1, 2 or 3 CES-D⩾16 with CES-D score 0⩾16 over years 5, 10, 15 as reference group.
Sum of standardized values (t-scores) of zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, β-carotene.
Figure 2.
Mean levels of F2-isoprostanes (a) and carotenoids (b) in non-depressed (CES-D<16) and in depressed subjects (CES-D⩾16) adjusted for sociodemographics only (model 1) and adjusted for both sociodemographics and health and lifestyle factors (model 3), CARDIA exam year 15. In a, mean levels of F2-isoprostanes in pg ml−1. In b, mean levels of the sum of five carotenoids, standardized as t-scores and summed. Model 1: center, age, sex, race, education. Model 3: center, age, sex, race, education, supplement use, somatic disease, diet, alcohol consumption, smoking, BMI, physical activity. F2-isoprostanes and carotenoids were log-transformed for analysis. Reported levels are back-transformed values. CES-D cutoff score ⩾16, increased depressive symptoms. BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale.
Depression defined as CES-D⩾16 and/or current antidepressant use was positively associated with F2-isoprostanes after adjustment for sociodemographics (model 1: β=0.06, P<0.001) and after health and lifestyle (model 3: β=0.03, P=0.047). The association of F2-isoprostanes and the number of times that CES-D scores were ⩾16 over the years 5, 10 and 15 was significant for those with increased scores two or three times (after adjustment for sociodemographics), illustrating the association was stronger when depressive symptoms had a more chronic course (model 1: CES-D⩾16 two times, β=0.05, P=0.009; CES-D⩾16 three times, β=0.04, P=0.033). The association was no longer significant after additional adjustment for health and lifestyle (model 3: CES-D⩾16 two times, β=0.03, P=0.111; CES-D⩾16 three times, β=0.01, P=0.603).
Cross-lagged longitudinal analyses
Cross-lagged analyses of CES-D and F2-isoprostanes at years 15 and 20 revealed CES-D⩾16 (as well as CES-D⩾16 and/or current antidepressant) at year 15 were associated with higher F2-isoprostanes at year 20 (model 1: β=0.05, P=0.003; Table 4). These findings remained significant after adjustment for health and lifestyle factors (model 3: β=0.03, P=0.048). F2-isoprostanes at year 15 were associated with CES-D scores at year 20 after adjustment for sociodemographics (model 1: β=0.04, P=0.028), but not after further adjustment for health and lifestyle (model 3: β=0.03, P=0.095).
Table 4. Cross-lagged longitudinal multivariable analyses of F2-isoprostanes and carotenoids with depressive symptoms (CES-D)a.
|
CARDIA exam years 10, 15 and 20 |
Model 1 | Model 2 | Model 3 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variable | Dependent variable | Adjusted for |
Age, sex, race,
education |
Age, sex, race,
education,
somatic disease,
supplement use |
Age, sex, race,
education,
somatic disease,
supplement use,
diet, BMI,
smoking, alcohol,
physical activity |
|||||||||||||||
| N | β | P | N | β | P | N | β | P | ||||||||||||
| F2-isoprostanes | ||||||||||||||||||||
| CES-Db | F2-isoprostanes 5 years later | Previous F2-isoprostane levels | 2334 | 0.06 | 0.001 | 2321 | 0.05 | 0.002 | 2304 | 0.03 | 0.064 | |||||||||
| CES-D⩾16b | F2-isoprostanes 5 years later | Previous F2-isoprostane levels | 2334 | 0.05 | 0.003 | 2321 | 0.05 | 0.004 | 2304 | 0.03 | 0.048 | |||||||||
| CES-D⩾16 and/or ADb | F2-isoprostanes 5 years later | Previous F2-isoprostane levels | 2334 | 0.06 | <0.001 | 2321 | 0.06 | 0.001 | 2304 | 0.04 | 0.012 | |||||||||
| F2-isoprostanesc | CES-D 5 years later | Previous CES-D scores | 2589 | 0.04 | 0.028 | 2576 | 0.04 | 0.028 | 2506 | 0.03 | 0.095 | |||||||||
| Sum of five carotenoidsd | ||||||||||||||||||||
| CES-De | Sum of five carotenoids 5 years later | Previous carotenoid levels | 2356 | −0.07 | <0.001 | 2347 | −0.07 | <0.001 | 2306 | −0.05 | 0.004 | |||||||||
| CES-D⩾16e | Sum of five carotenoids 5 years later | Previous carotenoid levels | 2356 | −0.06 | <0.001 | 2347 | −0.06 | <0.001 | 2306 | −0.05 | 0.002 | |||||||||
| CES-D⩾16 and/or ADe | Sum of five carotenoids 5 years later | Previous carotenoid levels | 2356 | −0.06 | <0.001 | 2347 | −0.06 | <0.001 | 2306 | −0.05 | 0.004 | |||||||||
| Sum of five carotenoidsf | CES-D 5 years later | Previous CES-D scores | 2504 | −0.03 | 0.069 | 2492 | −0.03 | 0.086 | 2476 | −0.01 | 0.560 | |||||||||
Abbreviations: AD, antidepressant; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale; N, number.
F2-isoprostanes and carotenoids log-transformed for linear regression analysis. Results are reported as standardized regression coefficients. All the results are adjusted for CENTER at baseline.
CES-D at year 15, F2-isoprostanes at year 20, covariates at year 15; adjusted for F2-isoprostanes at year 15.
F2-isoprostanes at year 15, CES-D at year 20, covariates at year 15; adjusted for CES-D scores at year 15.
Sum of standardized (t-scores) zeaxanthin/lutein, β-cryptoxanthin, lycopene, α-carotene, β-carotene.
CES-D at year 10, sum of five carotenoids at year 15, covariates at year 10; adjusted for carotenoids at year 7 (as there are no data on carotenoids available in year 10 (see Figure 1)).
Sum of five carotenoids at year 15, CES-D at year 20, covariates at year 15; adjusted for CES-D scores at year 15.
Carotenoids
Cross-sectional analyses
CES-D (continuous score) was negatively associated with the sum of five carotenoids after adjustment for sociodemographics (model 1: β=−0.13, P<0.001), and after additional adjustment for supplement use and somatic disease (model 2: β=−0.13, P<0.001), and also after adding lifestyle factors (model 3: β=−0.07, P<0.001; Table 3). In those with depressive symptoms, the mean level of the sum of the five carotenoids was 239.8 (95% CI=237.2–242.7) vs 249.4 (95% CI=248.1–250.9; standardized sum) in those without depressive symptoms after adjustment for sociodemographics (model 1: Cohen's d=−0.30, P<0.001; Figure 2b) and 238.7 (95% CI=236.0–241.3) vs 244.0 (95% CI=242.5–245.7) after additional adjustment for health and lifestyle factors (model 3: Cohen's d=−0.16, P<0.001; Figure 2b). Change in estimate analyses revealed that smoking, diet and to a lesser extent BMI had the greatest effects on the association (See Supplementary Table 3). The number of previously measured CES-D⩾16 tended to be related only for those who scored ⩾16 at all three measurements (model 3: β=−0.03, P=0.062). The pattern of results was similar across the individual carotenoids (see Supplementary Table 2).
Cross-lagged longitudinal analyses
Cross-lagged analyses revealed all CES-D variables at year 10 predicted carotenoids at year 15 after adjustment for sociodemographics (CES-D⩾16 model 1: β=−0.07, P<0.001) as well as after adjustment for health and lifestyle factors (CES-D⩾16 model 3: β=−0.05, P=0.002). Carotenoids at year 15 were not associated with CES-D at year 20 (model 3: β=−0.01, P=0.560; Table 4).
Discussion
Higher F2-isoprostanes and lower carotenoid levels were cross-sectionally associated with increased depressive symptoms. For the F2-isoprostanes this association was largely explained by the lifestyle factors smoking, diet and BMI. For the carotenoids, this association is partially attenuated by these factors but remains present even after controlling for them. Longitudinal analyses show that depressive symptoms predict both the F2-isoprostane and carotenoids levels 5 years later; however, neither marker predicts future depressive symptoms. Overall, the effect sizes found in this study were small in size.48
A recent meta-analysis10 found increased F2-isoprostanes in persons with major depressive disorder and depressive symptoms. This is in contrast with the results of the present study and may be explained by the fact that several of the studies in this meta-analysis took into account some, but not all of the health and lifestyle factors included in these analyses. Previous large scale studies also reported decreased carotenoid levels in depressive symptoms.17, 18 A novel finding in this study is that the association remains present even after controlling for diet quality, which should be considered an important confounder as dietary intake is the sole source of carotenoids. The longitudinal associations, in which depressive symptoms predict future oxidative stress/antioxidant levels, are small in size but significant, despite the conservative method of estimation (correcting for previous levels of F2-isoprostanes or carotenoids).
Our results suggest that health and lifestyle factors are important mechanisms in the association between depressive symptoms and oxidative stress, especially for F2-isoprostanes. The lower carotenoid levels found in depressive symptoms may be a consequence of relatively low intake of foods rich in carotenoids, such as fruits and vegetables; a dietary pattern that has previously been associated with depression.49, 50 In this study, however, the association between depressive symptoms and carotenoids was also present after adjusting analyses for a measure of diet quality. Other unhealthy lifestyle behaviors associated with depressive symptoms, such as smoking and alcohol consumption, increase the exposure to oxidative damage, as reflected by increased F2-isoprostane levels.51 One of the mechanisms by which smoking may decrease carotenoids levels is through causing an increase in metabolic rate, which in turn increases oxidative stress exposure, leading to a higher expenditure of antioxidant micronutrients such as the carotenoids.52 Exposure of human plasma to cigarette smoke has been shown to reduce carotenoids and other antioxidant levels.53
There is also evidence suggesting that common genetic factors underlie both low carotenoid levels and depressive symptoms: a recent study demonstrated a single-nucleotide polymorphism associated with low levels of β-cryptoxanthin was also associated with depressive symptoms.54
Besides increased exposure to ROS from exogenous sources owing to poor health and lifestyle behaviors, there is evidence to suggest that depression is also accompanied by increased endogenous production of ROS, possibly through mitochondrial dysfunction.55 The brain is particularly vulnerable to oxidative damage owing to its large oxygen consumption and relatively weak antioxidant defenses. Sustained oxidative brain damage during a depressive episode may make a sufferer prone to developing another depressive episode. Therefore, it has been hypothesized that exposure to oxidative stress could be an explanatory mechanism in the remitting and chronic course of depressive disorders.35
There is some evidence to suggest that antidepressants have antioxidant properties and may act through reducing pro-inflammatory cytokines and ROS production and improving levels of antioxidants such as superoxide dismutase.56 A number of studies have demonstrated that successful treatment with antidepressants decreases markers of oxidative stress and/or increases markers of antioxidant activity.57, 58, 59, 60, 61 However, these findings are limited and conflicting.19, 62, 63, 64, 65, 66, 67 To illustrate, Chung et al.64 found that serotonin re-uptake inhibitor treatment actually increased F2-isoprostane levels, despite reducing depressive symptoms.
Further understanding of the complex biological processes involved in oxidative stress will be necessary to successfully exploit (anti-)oxidative processes as an avenue for treatment. Even in diseases in which the role of oxidative stress is considered well established (such as cancer), the results of preventive treatment with antioxidants have been disappointing, or even harmful.68, 69, 70 The paradigm within which oxidative stress is perceived as damaging and antioxidants as protective is likely not an adequate model to this end. Although previously ROS were thought of purely as damaging to cells, the vital role they play in defense against pathogens and intracellular signaling is now recognized. Similarly antioxidants should not be thought of purely as benevolent factors. The finding that β-carotene supplementation in smokers lead to a significant increase in lung cancer incidence,71, 72 is a clinical example of this. Omega-3 fatty acids are among the most widely studied supplements for the treatment of depression and may reduce oxidative stress through their anti-inflammatory properties. A recent meta-analysis of randomized trials found that they indeed reduce depressive symptoms in patients with a diagnosis of major depressive disorder or increased depressive symptoms.73 The effect of carotenoid supplements on depression has (to our knowledge) not yet been investigated, but observational data suggest that dietary patterns are associated with depressive symptoms.74 The same can be said of smoking, alcohol use, obesity and physical inactivity, all of which occur more frequently in those with depressive symptoms,4, 13, 14, 15 and all of which are associated with oxidative stress.9, 11 This suggests that addressing these behaviors may be the most effective course of action in depression.
This study has some important limitations. Although the CES-D is a well-established and sensitive tool for assessing depressive symptoms, like most self-report questionnaires for depression it lacks the specificity of diagnosis by a clinician or through a (semi-)structured interview. This may have led to misclassification of some subjects in this study as being depressed. In addition, due to the observational design and the varying availability of oxidative stress, antioxidant and depression data at different time points, the possibilities for examination of the longitudinal associations were limited. The effect sizes are statistically significant but small; this should be considered when interpreting the potential clinical impact of the association. Individual markers of oxidative stress and/or antioxidants cannot and do not fully reflect the ongoing and complex biological process of redox homeostasis. This limitation applies to all human studies in the field of oxidative stress and should be considered in the interpretation of all study results. F2-isoprostanes reflect oxidative lipid damage, but are only one product of lipid peroxidation. Furthermore oxidative damage also affects DNA and proteins, but markers of these processes such as 8-OHdG and protein carbonyls, are not available in this study. Although carotenoids are important potent antioxidants, their levels do not reflect all aspects of the antioxidant defense system, that includes many more enzymatic (for example, superoxide dismutase, catalase) and non-enzymatic antioxidants (ascorbic acid). The main strengths of this study are the large sample size, the measurements of circulating F2-isoprostanes and carotenoids with gold-standard techniques, the ability to control for a wide range of important health and lifestyle confounders, and determination of the temporal associations between depression and F2-isoprostanes and carotenoids.
In conclusion, this study demonstrates that F2-isoprostanes and carotenoids are associated with depressive symptoms, both cross-sectionally and longitudinally, and that health and lifestyle factors are important mechanisms in this association, especially in F2-isoprostanes. Further large scale research on oxidative stress and antioxidants should investigate these associations in individuals meeting the diagnostic criteria for a mood disorder.
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C) and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content. The Young Adult Longitudinal Trends in Antioxidants Study (YALTA) is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (R01 HL 53560). CNB and BWJHP are supported through an NWO-VICI grant (number 91811602).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013; 11: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27: 2763–2774. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67: 220–229. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31: 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008; 5: 466–475. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry 2006; 63: 273–279. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord 2002; 72: 227–236. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84. [DOI] [PubMed] [Google Scholar]
- Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BWJH. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2014; 51C: 164–175. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem 2006; 52: 601–623. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Matthews KA, Gross MD, Jacobs DRJ. Socioeconomic status, antioxidant micronutrients, and correlates of oxidative damage: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med 2009; 71: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE et al. Smoking, smoking cessation, and major depression. JAMA 1990; 264: 1546–1549. [PubMed] [Google Scholar]
- Abu-Omar K, Rutten A, Lehtinen V. Mental health and physical activity in the European Union. Soz Praventivmed 2004; 49: 301–309. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA, O'Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med 2005; 118: 330–341. [DOI] [PubMed] [Google Scholar]
- Palta P, Samuel LJ, Miller ER 3rd, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med 2014; 76: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Boueiz A, Shroff MR, Zonderman AB. Antioxidant status and its association with elevated depressive symptoms among US adults: National Health and Nutrition Examination Surveys 2005-6. Br J Nutr 2013; 109: 1714–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Bandinelli S, Penninx BW, Corsi AM, Lauretani F, Vazzana R et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry 2012; 13: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol 2007; 22: 67–73. [DOI] [PubMed] [Google Scholar]
- Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta 2014; 1840: 809–817. [DOI] [PubMed] [Google Scholar]
- Milne GL, Dai Q, Roberts LJ. The isoprostanes-25 years later. Biochim Biophys Acta 2015; 1851: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 2005; 38: 698–710. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol 2007; 433: 113–126. [DOI] [PubMed] [Google Scholar]
- Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003; 52: 2346–2352. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang Y, Zonderman AB. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr 2011; 141: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S et al. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr 2005; 82: 685–693. [DOI] [PubMed] [Google Scholar]
- Ford ES, Will JC, Bowman BA, Narayan KM. Diabetes mellitus and serum carotenoids: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 1999; 149: 168–176. [DOI] [PubMed] [Google Scholar]
- Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee D-H. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 2006; 163: 929–937. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr 2004; 79: 47–53. [DOI] [PubMed] [Google Scholar]
- Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Manson JE, Willett WC. Dietary carotenoids and risk of coronary artery disease in women. Am J Clin Nutr 2003; 77: 1390–1399. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr 2015; 101: 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem 2013; 70: 102–110. [DOI] [PubMed] [Google Scholar]
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014; 6: 466–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KA, Sijtsma FPC, Nettleton JA, Steffen LM, Horn L, Van, Shikany JM et al. Dietary patterns are associated with plasma F2-isoprostanes in an observational cohort study of adults. Free Radic Biol Med 2014; 57: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 2013; 18: 595–606. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41: 1105–1116. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385–401. [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 2006; 62: 123–146. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ 2nd. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol 1999; 300: 3–12. [DOI] [PubMed] [Google Scholar]
- Gross M, Steffes M, Jacobs DR, Yu X, Lewis L, Lewis CE et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA study. Clin Chem 2005; 51: 125–131. [DOI] [PubMed] [Google Scholar]
- Bieri JG, Brown ED, Smith JC. Determination of individual carotenoids in human-plasma by high-performance liquid-chromatography. J Liq Chromatogr 1985; 8: 473–484. [Google Scholar]
- Craft NE, Brown ED, Smith JCJ. Effects of storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clin Chem 1988; 34: 44–48. [PubMed] [Google Scholar]
- Gross MD, Prouty CB, Jacobs DRJ. Stability of carotenoids and alpha-tocopherol during blood collection and processing procedures. Clin Chem 1995; 41: S943–S944. [PubMed] [Google Scholar]
- Iribarren C, Folsom AR, Jacobs DRJ, Gross MD, Belcher JD, Eckfeldt JH. Association of serum vitamin levels, LDL susceptibility to oxidation, and autoantibodies against MDA-LDL with carotid atherosclerosis. A case-control study. The ARIC Study Investigators. Atherosclerosis risk in communities. Arterioscler Thromb Vasc Biol 1997; 17: 1171–1177. [DOI] [PubMed] [Google Scholar]
- Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee D-H. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALT. Clin Chem 2007; 53: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Fanelli-Kuczmarski MT, Kitner-Triolo MH, Beydoun HA, Kaufman JS, Mason MA et al. Dietary antioxidant intake and its association with cognitive function in an ethnically diverse sample of US adults. Psychosom Med 2015; 77: 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DRJ P, Hahn LPM, Haskell WLP, Pirie PP, Sidney SM. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989; 9: 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- McMartin SE, Jacka FN, Colman I. The association between fruit and vegetable consumption and mental health disorders: evidence from five waves of a national survey of Canadians. Prev Med 2013; 56: 225–230. [DOI] [PubMed] [Google Scholar]
- Payne ME, Steck SE, George RR, Steffens DC. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet 2012; 112: 2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseervatham GSB, Sivasudha T, Jeyadevi R, Arul Ananth D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—an overview. Environ Sci Pollut Res Int 2013; 20: 4356–4369. [DOI] [PubMed] [Google Scholar]
- Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology 2002; 180: 121–137. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr 1996; 63: 559–565. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Nalls MA, Canas JA, Evans MK, Zonderman AB. Gene polymorphisms and gene scores linked to low serum carotenoid status and their associations with metabolic disturbance and depressive symptoms in African-American adults. Br J Nutr 2014; 112: 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hroudová J, Fišar Z. Connectivity between mitochondrial functions and psychiatric disorders. Psychiatry Clin Neurosci 2011; 65: 130–141. [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Lee S-J, Han C, Patkar AA, Masand PS, Pae C-U. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2013; 46: 224–235. [DOI] [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep 2003; 8: 365–370. [DOI] [PubMed] [Google Scholar]
- Galecki P, Szemraj J, Bienkiewicz M, Zboralski K, Galecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol 2009; 24: 277–286. [DOI] [PubMed] [Google Scholar]
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res 2007; 38: 247–252. [DOI] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci 2009; 63: 639–645. [DOI] [PubMed] [Google Scholar]
- Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1284–1290. [DOI] [PubMed] [Google Scholar]
- Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 2001; 64: 43–51. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Lower plasma Coenzyme Q10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuro Endocrinol Lett 2009; 30: 462–469. [PubMed] [Google Scholar]
- Chung CP, Schmidt D, Stein CM, Morrow JD, Salomon RM. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res 2012; 206: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawdin BS, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun 2012; 31: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JW, Wang J-F, Andreazza AC, Shao L, Yatham LN, Young LT. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int J Neuropsychopharmacol 2011; 14: 1069–1074. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang J-F, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 2011; 14: 123–130. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012; 3: CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst Rev 2008; CD004183. [DOI] [PubMed]
- Cortés-Jofré M, Rueda J-R, Corsini-Muñoz G, Fonseca-Cortés C, Caraballoso M, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev 2012; 10: CD002141. [DOI] [PubMed] [Google Scholar]
- Satia JA, Littman A, Slatore CG, Galanko JA, White E. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the VITamins And Lifestyle (VITAL) study. Am J Epidemiol 2009; 169: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanvetyanon T, Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer 2008; 113: 150–157. [DOI] [PubMed] [Google Scholar]
- Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One 2014; 9: e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr 2014; 99: 181–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.