Abstract
Rationale: Diesel exhaust inhalation, which is the model traffic-related air pollutant exposure, is associated with vascular dysfunction.
Objectives: To determine whether healthy subjects exposed to diesel exhaust exhibit acute vasoconstriction and whether this effect could be modified by the use of antioxidants or by common variants in the angiotensin II type 1 receptor (AGTR1) and other candidate genes.
Methods: In a genotype-stratified, double-blind, four-way crossover study, 21 healthy adult subjects were exposed at rest in a randomized, balanced order to diesel exhaust (200 μg/m3 particulate matter with an aerodynamic diameter ≤ 2.5 μm [PM2.5]) and filtered air, and to pretreatment with antioxidants (N-acetylcysteine and ascorbate) and placebo. Before and after each exposure, brachial artery diameter (BAd) was assessed using ultrasound. Changes in BAd were compared across pretreatment and exposure sessions. Gene–exposure interactions were evaluated in the AGTR1 A1166C polymorphism, on which recruitment was stratified, and other candidate genes, including TRPV1 and GSTM1.
Measurements and Main Results: Compared with filtered air, exposure to diesel exhaust resulted in a significant reduction in BAd (mean, −0.09 mm, 95% confidence interval [CI], −0.01 to −0.17; P = 0.03). Pretreatment with antioxidants augmented diesel exhaust–related vasoconstriction with a mean change in BAd of −0.18 mm (95% CI, −0.28 to −0.07 mm; P = 0.001). Diesel exhaust–related vasoconstriction was primarily observed in the variant alleles of AGTR1 and TRPV1. No association was found between diesel exhaust inhalation and flow-mediated dilation.
Conclusions: We confirmed that short-term exposure to diesel exhaust in healthy subjects is associated with acute vasoconstriction in a conductance artery and found suggestive evidence of involvement of nociception and renin–angiotensin systems in this effect. Pretreatment with an antioxidant regimen increased vasoconstriction.
Keywords: air pollution, environmental exposures, antioxidants, cardiovascular physiological processes/drug effects
At a Glance Commentary
Scientific Knowledge on the Subject
Traffic-related air pollution is a major risk factor for cardiovascular morbidity and mortality worldwide. The biologic mechanism is unknown, but studies suggest that nociceptive pathways may initiate the response to pollutants, and oxidative stress and the renin–angiotensin system may be downstream effector mechanisms.
What Does this Study Add to the Field
Our study confirms that short-term exposure to diesel exhaust in healthy participants is associated with acute vasoconstriction, a well-recognized intermediary outcome in cardiovascular disease. Our data on gene–pollutant interactions support the importance of nociceptive pathways and the renin–angiotensin system in causing this effect. Antioxidant supplementation increased the vasomotor dysfunction seen after diesel exhaust exposure.
Traffic-related air pollution is associated with significant cardiovascular mortality and morbidity. Acute exposure is linked to hospital admissions for angina, myocardial infarction, and heart failure, whereas long-term exposure increases the risk of death from coronary artery disease and sudden cardiac death (1–3). Because of the scale of exposure and severity of health outcomes, it is crucial to understand the biological pathways that lead to disease and to identify populations that may be most susceptible.
Oxidative stress is one of the proposed mechanisms of injury induced by air pollution. Inhalation of pollutant nanoparticles and associated gaseous copollutants may initiate nociception and lead to pulmonary and systemic inflammation, affecting downstream autonomic balance, increased endothelin release, prothrombotic changes, and vascular dysfunction potentially mediated by the renin–angiotensin system. Altered vasoreactivity can cause acute ischemia through plaque rupture and atherothrombosis, as well as chronic sequelae of accelerated atherosclerosis (1, 4).
Baseline brachial artery diameter (BAd) and flow-mediated dilation (FMD; the difference in BAd in response to vessel shear stress by cuff inflation) are noninvasive measurements of endothelial vascular function that predict the risks of cardiovascular events (5, 6). In a previous experiment, we observed BAd reduction, but did not observe a change in FMD with exposure to diesel exhaust (DE) (7). It is valuable to determine whether these effects are magnified or prevented by genetic variants in suspected physiological pathways or by pharmacologic agents that interfere in suspected molecular mechanisms.
In this study, we used diesel exhaust as a model for traffic-related air pollution. We examined whether healthy subjects exposed to DE exhibited acute vasoconstriction and whether this effect was blunted by antioxidant prophylaxis. In addition, we examined whether this effect was modified by variation within the renin–angiotensin system, oxidative stress, or nociception pathways. Some of the results of this study have been previously reported in the form of an abstract (8). Cosselman and colleagues previously published that DE exposure was associated with a rise in systemic blood pressure using data from this experiment combined with two additional studies using our exposure chamber (9).
Methods
Overview
Twenty-one subjects participated in a double-blind, placebo-controlled and genotype-stratified, four-arm crossover study to (1) confirm the vasoconstrictive effect of DE exposure in a conduit artery, (2) identify any modification of this effect by antioxidant pretreatment, and (3) explore possible modification of this effect by several genetic polymorphisms with known cardiovascular disease associations.
Each subject underwent four exposure sessions, randomized to order, and conducted according to the four possible combinations of antioxidant pretreatment (AO) or placebo (PL) and DE or filtered air (FA): DE/AO, DE/PL, FA/AO, and FA/PL. Brachial artery dimensions were assessed before and after each exposure session.
The study was designed to stratify for genotypic variation of a specific common variant of AGTR1 (A1166C). Study subjects, staff in contact with subjects, and staff conducting analyses were blinded to both exposure and treatment status.
Subjects fasted for at least 10 hours before each exposure session. Exposure days for a given subject were separated by at least 3 weeks to allow adequate washout of possible DE or AO effects. Exposures were conducted only during the first half of the menstrual cycle in female subjects.
Subject Selection
Subjects were recruited if they were in overall good health and between the ages of 18 and 49 years. Exclusion criteria included current smoking (by self-report and urine cotinine) or pregnancy, history of hypertension, asthma, diabetes, hypercholesterolemia, or other chronic conditions that required ongoing medical care. Subjects were screened and excluded if they had body mass index (BMI) greater than 27 kg/m2, elevated fasting blood sugar, bradycardia, hypotension, evidence of a medical condition requiring treatment, or if we determined that brachial artery images would be suboptimal for analysis. Recruitment screening ensured that equal numbers of subjects would carry the AA, AC, and CC alleles at A1166C on the AGTR1 gene.
All study subjects gave written, informed consent. The consent form and study protocol were approved by the University of Washington’s Human Subjects Division.
Antioxidant Treatment Protocol
The AO regimen and matching PL capsules were prepared by the Investigational Drug Service at the University of Washington Medical Center. One week before each exposure session, all subjects were required to refrain from taking nutritional supplements and foods highly fortified in vitamin C. The AO regimen included 500-mg vitamin C (ascorbate [AA]) capsules taken twice daily for 7 days until the exposure day and two 600-mg N-acetylcysteine (NAC) capsules taken twice on the day before the exposure session. On the morning of the exposure session, subjects were administered 1000 mg AA and 600 mg NAC. Ascorbate levels were measured before each exposure session to document compliance with and effect of supplementation.
Exposure Session Protocol
Subjects were exposed at rest to either FA or DE continuously for 120 minutes, beginning at approximately 8:30 a.m.. We assessed subjects’ perceptions of the exposure during each session. Subject blinding to exposure in our system has previously been shown to be successful (10).
Exposure System
A complete description of the exposure system is available elsewhere (11). A 2002 model turbocharged, direct-injection 5.9-L Cummins B-series engine (6BT5.9G6; Cummins, Inc., Columbus, IN) in a 100-kW generator set was used to generate the DE to which subjects were exposed. The system was run at steady state before exposure, and 75% of the rated capacity was maintained using a load-adjusting load bank (Simplex, Springfield, IL). No. 2 undyed on-highway low sulfur diesel fuel and Valvoline 15W-40 crankcase oil (Valvoline, Lexington, KY) were used. Emissions were diluted in two phases to maintain the final concentration of particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5) of 200 μg/m3 in the breathing zone. Real-time assessments of PM2.5 concentrations were performed using a tapered element oscillating microbalance (1400a PM2.5; Rupprecht and Patashnick Co., Albany, NY), and continuous adjustments were performed using a nephelometry-based feedback control system. The mass median diameter of the DE particles was 0.080 μm, and particle counts per cubed centimeters for FA and DE exposures were 1.7 × 104 and 7.1 × 105, respectively. The volume of the exposure room was 116 m3, and its temperature and humidity were maintained throughout exposure sessions at 20°C to 21°C and 50%, respectively. FA was produced by passing ambient air through HEPA and carbon matrix filters. This FA was used both for the FA exposures and for dilution of the DE for the DE exposures.
Genotyping
Blood drawn for genotyping was collected at the initial subject screening in sodium citrate. DNA was isolated using the DNeasy kit (Qiagen, Valencia, CA), and was genotyped for the regions of interest using the TaqMan SNP genotyping with specific fluorogenic probes and primers from Applied Biosystems (ThermoFisher Scientific, Waltham, MA). Probes were 3′-labeled with TAMRA quencher dye; wild-type and variant probes were 5′-labeled with 6-FAM and VIC reporter dyes, respectively. Sequencing reactions and analysis were performed using the Applied Biosystems 7,900 Fast Real-Time PCR System. In addition to genotyping for the AGTR1 variant used in study selection (rs5186), we also genotyped subjects for AGTR1 rs4488792, TRPV1 (rs8065080 and rs222747), and the GSTM1 deletion (OMIM 138,350).
Brachial Artery Assessment
BAd was measured on the subjects’ extended right arm after 10 minutes at rest in a supine position. Brachial artery measurements were performed using a HDI 5000 Ultrasound Instrument (Philips Medical Systems, Bothell, WA) with a 5- to 12-MHz linear array transducer by one experienced sonographer, 30 minutes before initiation of exposure and 30 minutes after completion of exposure. The brachial artery was scanned above the antecubital fossa in a longitudinal orientation. The location of the ultrasound probe during the subjects’ first test was recorded and used for later tests. Measurements were taken in a dark, quiet room, with an ambient air temperature between 20 and 26°C.
In each examination, preexposure recording of vessel images followed baseline Doppler assessment of blood flow velocity. After exposure, vessel images were again recorded, followed by inflation of a cuff on the forearm to 50 mm Hg above systolic pressure for 5 minutes. The blood pressure cuff was placed on the forearm distal to the location of the ultrasound probe. Blood flow velocity was then obtained after cuff release, and the brachial artery was imaged and recorded for 5 minutes. Images were digitized from the video output of the ultrasound machine using a frame grabber under control of custom software on a personal computer. Image acquisition was gated with an electrocardiography signal to capture end-diastole in each cardiac cycle.
BAd was assessed by a single operator using a validated and automated, beat-by-beat image processing software package (Vascular Tools 4.6; Medical Imaging Applications, Coralville, IA) (12). The operator defined a vascular region of interest, which was then applied automatically to identify the media-to-media diameters in each frame over time. Frames with a detected vessel border of <70% of the width of the region of interest were excluded from analysis. Custom software developed in the MATLAB programming environment (MathWorks, Natick, MA) was used to extract measurements from the time series of diameter measurements. We previously reported a small day-to-day variability of baseline BAd for the described methods with minimal modifications (coefficient of variation, 3.4 ± 1.3%) (7, 13).
Change in BAd was defined as the difference between mean postexposure measurement and mean preexposure measurement, assessed before cuff inflation. FMD was calculated as a percent increase to maximal BAd after cuff deflation from mean BAd before cuff inflation.
Statistical Analysis
Statistical analyses were performed using Stata 13 (Stata Corp, College Station, TX). Statistical significance was defined by a two-tailed P value with α = 0.05. Our primary analysis of vascular outcomes was conducted in a multilevel mixed effects linear regression model, with main effects for exposure and antioxidant treatment adjusted for covariates of interest (including age, sex, BMI, subject perception of exposure) and random effects for subjects. This analysis permitted us to use all available information, including subjects who were missing data from one or more exposure situations, and to draw statistical power across sessions with similar characteristics. Similar analyses were also performed to test for effect modification based on pretreatment and genotypes with the inclusion of an interaction term for exposure–antioxidant and exposure–genotype, respectively. We also tested for period and carryover effects.
Results
The baseline characteristics of the 21 study subjects are shown in Table 1. One subject (not included in analyses or results) was excluded because of a positive urine cotinine level. Two additional subjects had a remote history of tobacco use with no current active or passive smoke exposure. Genetic polymorphisms studied are presented in Table 2. Preexposure AA levels confirmed adherence to the prescribed AO regimen. Mean ± SE AA level was 0.89 ± 0.04 mg/dl when subjects took PL and 1.44 ± 0.06 mg/dl when subjects took the AO regimen. All subjects’ AA levels were higher when assigned to AO pretreatment, with individual increases ranging from 0.11 to 1.30 mg/dl.
Table 1.
Baseline Characteristics
| Characteristic | |
|---|---|
| Age, yr, mean (range) | 28.6 (19–47) |
| Sex, female:male | 8:13 |
| White, n (%) | 18 (86) |
| Body mass index, kg/m2 | 23 ± 0.48 |
| Blood pressure, mm Hg | |
| Systolic | 115 ± 2.8 |
| Diastolic | 71 ± 2.0 |
| Total cholesterol, mg/dl | 162.9 ± 6.2 |
| Triglycerides, mg/dl | 76 ± 10 |
| Glucose, mg/dl | 89.6 ± 1.0 |
Values are mean ± SE, unless otherwise noted. N = 21.
Table 2.
Genotype of Study Participants
| Gene/Locus | Reference | Mutation | −/− | +/− | +/+ |
|---|---|---|---|---|---|
|
AGTR1* |
rs5186 |
A→C |
7 |
7 |
7 |
|
AGTR1 |
rs4488792 |
C→T |
10 |
11 |
0 |
| TRPV-1 | rs8065080 | C→T | 2 | 10 | 9 |
| Gene/Locus | Reference | Mutation | Wild Type | Null | |
|---|---|---|---|---|---|
| GSTM1 | OMIM 138350 | Deletion | 11 | 10 |
rs5186 was the focus of subject recruitment and stratification.
Vascular Caliber Measurements
Data were not available for BAd measurements in three subjects in 1 or 2 exposure sessions because of problems with exposure generation or ultrasound equipment. In an additional subject, one session was excluded from analysis because measurements were taken from the incorrect artery segment. Fifteen subjects had complete data from all four exposure and/or treatment conditions, five subjects had three complete sessions, and one subject had one complete session.
The average BAd measures for the different pretreatment and exposure groups are displayed in Table 3. The average preexposure BAd were not statistically different among the pretreatment or exposure groups: PL/FA 3.55 mm; PL/DE 3.55 mm; AO/FA 3.58 mm; and AO/DE 3.55 mm (P = 0.97). Overall, exposure to DE was associated with vasoconstriction with a decrease in BAd of 0.09 mm (95% confidence interval [CI], 0.01–0.17 mm; P = 0.03). Age, sex, BMI, and perception of exposure did not modify the vasoconstrictive effect of DE.
Table 3.
Brachial Artery Diameter at Baseline and after Exposure and Percentage Change after Flow-mediated Dilation
| BAd (mm) | Placebo | Antioxidant | All |
|---|---|---|---|
| FA | |||
| Preexposure | 3.55 ± 0.14 | 3.58 ± 0.15 | 3.56 ± 0.10 |
| Postexposure | 3.50 ± 0.14 | 3.60 ± 0.15 | 3.54 ± 0.10 |
| Postexposure − preexposure | −0.05 ± 0.03 | 0.07 ± 0.05 | 0 ± 0.03 |
| 200 μg/m3 DE | |||
| Preexposure | 3.55 ± 0.15 | 3.55 ± 0.15 | 3.55 ± 0.11 |
| Postexposure | 3.62 ± 0.15 | 3.45 ± 0.14 | 3.53 ± 0.10 |
| Postexposure − preexposure | −0.06 ± 0.02 | −0.10 ± 0.04 | −0.08 ± 0.02 |
| FMD (%) | |||
| FA | 10.8 ± 1.5 | 8.3 ± 1.4 | 9.7 ± 1.0 |
| 200 μg/m3 DE | 11.2 ± 1.4 | 11 ± 1.3 | 11 ± 0.9 |
Definition of abbreviations: BAd = brachial artery diameter; DE = diesel exhaust; FA = filtered air; FMD = flow-mediated dilation.
Values are mean ± SE.
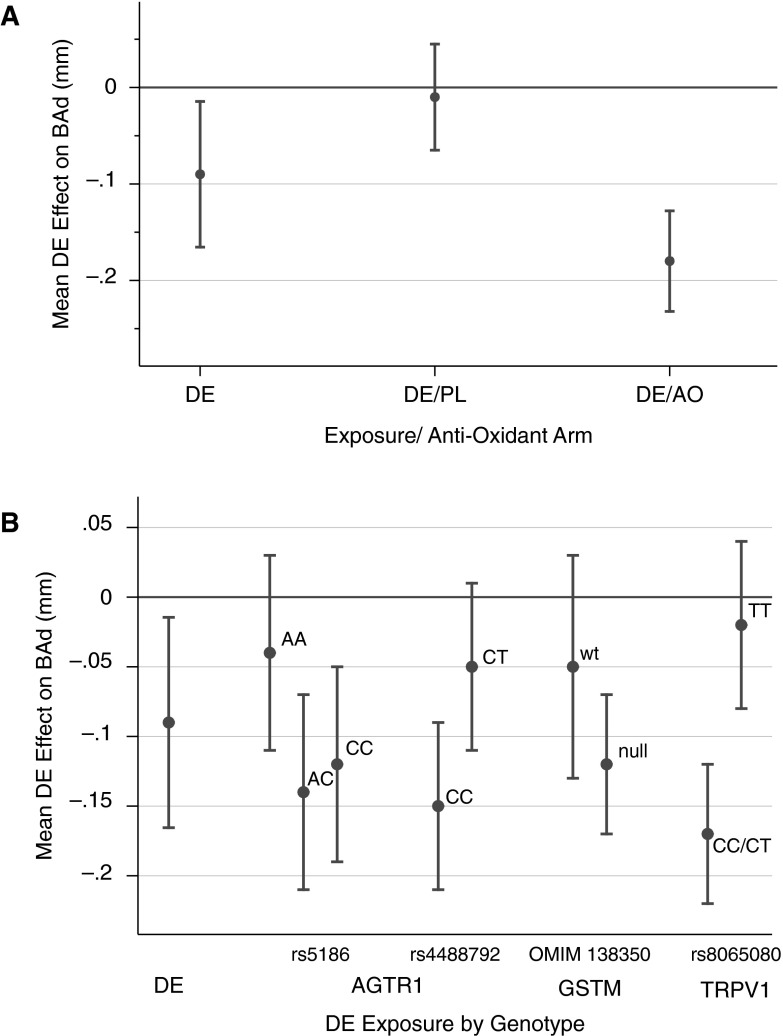
Figure 1A shows the interaction between the pretreatment and exposure regimens in mixed models. Pretreatment with AO had no effect on baseline BAd (P = 0.92) or on changes in BAd (P = 0.4) as a main effect in the multivariable model. However, in our mixed effects model, pretreatment with AOs modified the effect of DE on BAd. Contrary to our hypothesis, exposure to DE elicited greater vasoconstriction in subjects who were pretreated with AOs compared with those who received PL, with a DE effect of −0.18 mm (95% CI, −0.28 to −0.07 mm; P = 0.01) compared with −0.01 (95% CI, −0.11 to 0.9 mm; P = 0.81), respectively.
Figure 1.
Effect of diesel exhaust (DE) exposure on brachial artery caliber: postexposure − preexposure session difference in adjusted mean brachial artery diameter (BAd) comparing DE exposure to filtered air exposure. (A) Antioxidant (AO)–DE effect with mixed models demonstrating the overall DE effect, the impact of the placebo (DE/PL), and the impact of the antioxidant regimen (DE/vitamin C + N-acetylcysteine). (B) Gene–pollutant effect with mixed models demonstrating the overall DE effect and the DE effect in preselected genotypes of AGTR1, GSTM1, and TRPV. wt = wild type.
Our results regarding genetic variations are shown in Table 4 and Figure 1B. In our primary genotype-stratified analysis of AGTR1 A1166C (rs5186), subjects with the AC/CC alleles showed greater vasoconstriction after DE exposure than subjects with the common AA allele, with a −0.13 mm (95% CI, −0.23 to −0.04 mm; P = 0.007) versus a −0.04 mm (95% CI, −0.18 to 0.10 mm; P = 0.60) change in BAd. At the AGTR1 intron locus assessed (rs4488792), subjects with the CC allele exhibited more vasoconstriction than subjects with the heterozygous CT allele, with a −0.15 mm (95% CI, −0.20 to −0.04 mm; P = 0.01) versus a −0.05 mm (95% CI, −0.16 to 0.06 mm; P = 0.4) change in BAd. At the TRPV allele (rs8065080), subjects with the CC/CT allele exhibited more vasoconstriction than subjects with the TT allele, with a −0.17 mm (95% CI, −0.27 to −0.06 mm; P = 0.002) versus a −0.02 mm (95% CI, −0.13 to 0.10 mm; P = 0.62) change in BAd. Formal testing for effect modification by genetic variants did not demonstrate statistical significance.
Table 4.
Gene–Pollutant Interactions: Mixed Model of Diesel Exhaust Effect on Brachial Artery Caliber in Preselected Genotypes
| DE Effect | SE | 95% CI | P Value | |
|---|---|---|---|---|
|
AGTR1 (AC1166) |
||||
| AA | −0.04 | 0.07 | −0.18 to 0.11 | 0.60 |
| AC | −0.14 | 0.07 | −0.28 to −0.01 | 0.03 |
| CC | −0.12 | 0.07 | −0.26 to 0.02 | 0.09 |
| AGTR1 (intron) | ||||
| CC | −0.15 | 0.06 | −0.2 to −0.04 | 0.01 |
| CT | −0.05 | 0.06 | −0.16 to 0.06 | 0.40 |
| GSTM1 | ||||
| Wild type | −0.05 | 0.08 | −0.21 to 0.11 | 0.53 |
| Null | −0.12 | 0.05 | −0.23 to −0.02 | 0.02 |
| TRPV1 | ||||
| CC/CT | −0.17 | 0.05 | −0.27 to 0.06 | 0.002 |
| TT | −0.02 | 0.06 | −0.13 to 0.10 | 0.62 |
Definition of abbreviations: CI = confidence interval; DE = diesel exhaust.
FMD
FMD for subjects based on pretreatment and exposure status is shown in Table 3 (full results of the analysis can be found in the online supplement). Neither AO pretreatment nor DE exposure elicited a statistically significant change in FMD.
Discussion
Our study confirms that DE induces acute vasoconstriction of a medium-sized conductance vessel in healthy subjects. Assessed as an overall effect, this change in brachial artery caliber is similar to that observed in a previous study by our group (7), which found an average 0.11-mm decrease in BAd 30 minutes after exposure to DE at 200 μg/m3. This finding adds to the growing body of literature confirming that acute exposure to traffic-related air pollutants has a consistent and measurable effect on vasocontractility (7, 14–18) associated with a simultaneous rise in systemic blood pressure (9, 19). Interestingly, in this study, we found evidence that both genetic variations and AO supplementation may alter individual susceptibility to the DE exposure.
Although the implication of vasomotor dysfunction is not completely understood, we postulate that it provides a mediating mode of action for the adverse cardiovascular findings reported in epidemiologic studies of air pollution exposure. The brachial constriction that has now been repeatedly demonstrated in human-controlled experiments most likely parallels processes occurring within epicardial vessels and the cardiac microvasculature, and may presage plaque destabilization, vasospasm, and the resulting risk of myocardial infarction or arrhythmia. In individuals with preexisting coronary artery disease, the consequences can be a clinically significant increase in the ischemic burden (20).
Antioxidant Pretreatment
Pretreatment with an AO regimen of AA and NAC resulted in greater DE-induced vasoconstriction, with a 0.18-mm decrease in BAd when subjects received the AO regimen compared with a 0.01-mm decrease in BAd when subjects received PL. Although AO pretreatment significantly modified the effect of DE inhalation on BAd, the drug therapy was not otherwise associated with a change in vasoreactivity.
In this analysis of effect modification by AO pretreatment, the vasoconstrictive impact of DE exhaust was not apparent in the placebo group. We considered two explanations for this apparent null response: (1) this could be due to chance observation; and (2) although somewhat unlikely, the subjects taking the PL regimen had a relative depletion in AOs, which could reduce the susceptibility to the vasoactive effect of DE.
We selected a regimen of AA and NAC to target vascular tone mediated by locally produced vasodilators and vasoconstrictors. In particular, the vasodilator nitric oxide (NO) is important in maintaining basal vascular tone and regulating the response to short-term increases in shear stress (21). Previous animal and human studies have demonstrated that acute PM exposure reduces the bioavailability of NO by direct scavenging of NO through reactive oxygen species (ROS) and through impaired endothelial NO synthetase (eNOS), resulting in vasoconstriction (22, 23).
NAC stimulates glutathione synthesis, which functions as a free radical scavenger to counteract oxidative stress (24). AA potentiates the synthesis of eNOS via stabilization of tetrahydrobiopterin, reduces oxygen free radical production, and decreases NO consumption. In diseased subjects who smoke, have diabetes, hypertension, hyperlipidemia, and coronary artery disease, AA supplementation has been shown to improve FMD (25–27). Most of these human exposure studies used high-dose intra-arterial AA and measured vascular outcomes shortly after administration.
The epidemiology research using AO supplementation to prevent adverse cardiovascular events has been disappointing, with multiple studies showing no improvement in outcomes (28–32). The results in human particulate air pollution exposure studies have been more mixed. Two studies in an older cohort in Mexico with metabolic syndrome showed attenuation of the acute PM effect on heart rate variability after long-term supplementation with fish oil (33, 34). In a controlled exposure experiment to ozone, Samet and colleagues (35) showed a smaller decrement in lung function after supplementation with a cocktail of AA, α-tocopherol, and vegetables. Of note, subjects adhered to a strict diet leading to vitamin C depletion before initiation of the supplement regimen. In contrast, in a controlled human exposure study by Brook and colleagues (16) a single preexposure dose (2000 mg) of vitamin C did not mitigate the effects of ambient PM exposure on blood pressure changes.
The vasoconstrictive response that we observed adds to these disparate findings with implications regarding the use of AOs as a preventive measure. There is evidence that AA supplementation in healthy individuals without vitamin C deficiency or other states that induce a functional depletion may exert a pro-oxidant effect (36–38). Vitamin-derived radicals can accumulate within vessel walls, causing detrimental effects on the cardiovascular system through an imbalance of the endogenous redox state. One study of long-term AA supplementation in pigs resulted in impaired myocardial perfusion and coronary endothelial function (39). Other studies have shown that AA can inhibit flow and agonist-mediated vasodilation by preventing the release of endothelial-derived hyperpolarizing factor, resulting in vasoconstriction (40–42). Alternatively, because the adverse effects of air pollution are likely mediated by multiple pathways, it is possible that the vasomotor response is less dependent on oxidative stress than on activation of the autonomic nervous system.
Because some have considered AO supplementation to prevent pollution-related adverse outcomes, our work indicates more research is needed before recommendations can be made.
Genetic Variation and Mechanisms of DE Effects
Our efforts regarding gene–environment interactions were intended to examine potential mechanistic pathways underlying the vasoconstrictive effects of DE. We hypothesized that (1) nociceptive pathways may initiate the response to DE, and (2) oxidative stress and the renin–angiotensin system may be downstream effector mechanisms. We selected previously characterized specific human variants for each of these mechanistic elements.
We selected the AGTR1 gene based on its physiologic role in the renin–angiotensin system, with activation of the receptor leading to increased vascular tone, the generation of ROS, and cellular proliferation with accelerated atherosclerosis (43–45). Previous animal and in vitro studies report that exposure to PM increases angiotensin II in rats and activates the AGTR1 receptor, with resulting vasoconstriction in human pulmonary artery endothelial cells (45, 46).
To our knowledge, this is one of very few randomized clinical trials that has recruited and stratified subjects based on genotype (47). In particular, we stratified on the common A1166C polymorphism (rs5186) in the 3′-untranslated region of the AGTR1 gene, based on its impact on mRNA stability and subsequent transcription of the AGTR1 receptor. When the CC or AC polymorphism is present, there is an increase in AGTR1 levels, which correlates with elevated blood pressures (44, 48). Similarly, we found that subjects with the AC/CC allele exhibited greater vasoconstriction after DE exposure compared with the common AA allele. Although we did not measure AGTR1 levels, this suggests that the renin–angiotensin system is important in modifying the effects of air pollution.
We also examined polymorphisms in the TRPV1 and GSTM genes to target nociceptive receptors in the lungs and the endogenous ability to clear ROS, respectively. Previous in vivo and in vitro studies have shown that DE activates pulmonary sensory TRPV1 receptors. Receptor antagonists that block TRPV1 have been shown to decrease the systemic rise in blood pressure and incidence of arrhythmias in rats exposed to DE (49). Some epidemiologic studies report that people with a deletion in GSTM1, and thus with less ability to process ROS, show increased adverse effects from air pollution exposure (44). In this study, at the TRPV1 functional single nucleotide polymorphism, DE exposure elicited greater vasoconstriction in the CC and CT alleles compared with the TT allele. This supports the hypothesis that nociceptive pathways contribute to the initiation of these processes.
Study Limitations
There were several aspects of our study that may limit the generalization of our findings. In contrast to real-life exposure to traffic-related air pollution, which is long-term and repetitive, our study focused on physiologic responses after brief exposure to high levels of DE. Although we could control pollution exposure during sessions, we could not control individual exposures between sessions. We tried to minimize this variability by requiring subjects to use the same form of transportation to get to the study center for each session. We waited at least 3 weeks between sessions to allow an adequate washout period, although it is possible that there were some lingering effects from previous exposures to DE in the same subject. Finally, we studied vascular reactivity in a small group of healthy subjects, and their responses may not mimic the physiologic changes seen in a population of many with additional cardiovascular comorbidities.
Conclusions
These results confirm that short-term exposure to DE in healthy subjects is associated with acute vasoconstriction in a conductance artery; the impact was augmented after pretreatment with an AO regimen. Our observations regarding genetic variation suggest the role of nociceptive receptors in the initiation of the vasoconstrictive response, and of the renin–angiotensin system in effecting this response. Further research is needed to determine the role of oxidative stress in mediating vascular health related to exposure to traffic-related air pollution.
Acknowledgments
Acknowledgment
The authors thank the study subjects and the faculty and staff of the Controlled Exposure Laboratory at the University of Washington, in particular, Jim Stewart, Tim Larson, Tim Gould, and Mary Aulet. In addition, they extend special thanks to the technical expertise of Marla Paun, who died shortly after this study was complete.
Footnotes
Supported by National Institute of Environmental Health Science grants K24ES013195, P30ES07033, and P50ES015915 and NHLBI grant T32HL007287.
Author Contributions: Conception and design: J.D.K. and A.P. Acquisition of data: K.L.J., P.L.S., J.A., C.A.T., and A.P. Analysis and interpretation: C.S.S., C.O., J.D.K., and K.L.J. Drafting of the initial manuscript: C.S.S. All authors provided important intellectual content, critical review and revisions, and approved the final manuscript to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201506-1247OC on November 24, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Hart JE, Chiuve SE, Laden F, Albert CM. Roadway proximity and risk of sudden cardiac death in women. Circulation. 2014;130:1474–1482. doi: 10.1161/CIRCULATIONAHA.114.011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, et al. ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation; ESC Heart Failure Association. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson K, Duffin R, Langrish JP, Miller MR, Mills NL, Poland CA, Raftis J, Shah A, Shaw CA, Newby DE. Nanoparticles and the cardiovascular system: a critical review. Nanomedicine (Lond) 2013;8:403–423. doi: 10.2217/nnm.13.16. [DOI] [PubMed] [Google Scholar]
- 5.Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J. 2005;26:363–368. doi: 10.1093/eurheartj/ehi017. [DOI] [PubMed] [Google Scholar]
- 6.Faulx MD, Wright AT, Hoit BD. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J. 2003;145:943–951. doi: 10.1016/S0002-8703(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 7.Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA, Kaufman JD. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–942. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sack C, Jansen K, Peretz A, Olives C, Kaufman J. Diesel exhaust inhalation causes acute arterial vasoconstriction not affected by antioxidant treatment [abstract] Am J Respir Crit Care Med. 2015;191:A5251. doi: 10.1164/rccm.201506-1247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosselman KE, Krishnan RM, Oron AP, Jansen K, Peretz A, Sullivan JH, Larson TV, Kaufman JD. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension. 2012;59:943–948. doi: 10.1161/HYPERTENSIONAHA.111.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsten C, Oron AP, Curtiss H, Jarvis S, Daniell W, Kaufman JD. Symptoms in response to controlled diesel exhaust more closely reflect exposure perception than true exposure. PLoS One. 2013;8:e83573. doi: 10.1371/journal.pone.0083573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould T, Larson T, Stewart J, Kaufman JD, Slater D, McEwen N. A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhal Toxicol. 2008;20:49–52. doi: 10.1080/08958370701758478. [DOI] [PubMed] [Google Scholar]
- 12.Sonka M, Liang W, Lauer RM. Automated analysis of brachial ultrasound image sequences: early detection of cardiovascular disease via surrogates of endothelial function. IEEE Trans Med Imaging. 2002;21:1271–1279. doi: 10.1109/TMI.2002.806288. [DOI] [PubMed] [Google Scholar]
- 13.Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, Paun M, Gill EA, Kaufman JD. Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC Cardiovasc Disord. 2007;7:11. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dales R, Liu L, Szyszkowicz M, Dalipaj M, Willey J, Kulka R, Ruddy TD. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health. 2007;81:159–164. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 15.Wilker EH, Ljungman PL, Rice MB, Kloog I, Schwartz J, Gold DR, Koutrakis P, Vita JA, Mitchell GF, Vasan RS, et al. Relation of long-term exposure to air pollution to brachial artery flow-mediated dilation and reactive hyperemia. Am J Cardiol. 2014;113:2057–2063. doi: 10.1016/j.amjcard.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanobetti A, Luttmann-Gibson H, Horton ES, Cohen A, Coull BA, Hoffmann B, Schwartz JD, Mittleman MA, Li Y, Stone PH, et al. Brachial artery responses to ambient pollution, temperature, and humidity in people with type 2 diabetes: a repeated-measures study. Environ Health Perspect. 2014;122:242–248. doi: 10.1289/ehp.1206136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong H, Rappold AG, Caughey M, Hinderliter AL, Graff DW, Berntsen JH, Cascio WE, Devlin RB, Samet JM. Cardiovascular effects caused by increasing concentrations of diesel exhaust in middle-aged healthy GSTM1 null human volunteers. Inhal Toxicol. 2014;26:319–326. doi: 10.3109/08958378.2014.889257. [DOI] [PubMed] [Google Scholar]
- 19.Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, Fokkens PH, Boon NA, Sandstrom T, Blomberg A, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32:2660–2671. doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills NL, Törnqvist H, Gonzalez MC, Vink E, Robinson SD, Söderberg S, Boon NA, Donaldson K, Sandström T, Blomberg A, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 21.Stoner L, Erickson ML, Young JM, Fryer S, Sabatier MJ, Faulkner J, Lambrick DM, McCully KK. There’s more to flow-mediated dilation than nitric oxide. J Atheroscler Thromb. 2012;19:589–600. doi: 10.5551/jat.11973. [DOI] [PubMed] [Google Scholar]
- 22.Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Moseley AM, Cumpston JL, Goodwill AG, Frisbee SJ, Perrotta PL, Brock RW, et al. HEI Health Review Committee. Pulmonary particulate matter and systemic microvascular dysfunction. Res Rep Health Eff Inst. 2011;164:3–48. [PubMed] [Google Scholar]
- 23.Wauters A, Dreyfuss C, Pochet S, Hendrick P, Berkenboom G, van de Borne P, Argacha JF. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension. 2013;62:352–358. doi: 10.1161/HYPERTENSIONAHA.111.00991. [DOI] [PubMed] [Google Scholar]
- 24.Lu Q, Björkhem I, Xiu RJ, Henriksson P, Freyschuss A. N-acetylcysteine improves microcirculatory flow during smoking: new effects of an old drug with possible benefits for smokers. Clin Cardiol. 2001;24:511–515. doi: 10.1002/clc.4960240719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 26.Heitzer T, Just H, Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 27.Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 28.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 30.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e56803. doi: 10.1371/journal.pone.0056803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Sao Paulo Med J. 2015;133:164–165. doi: 10.1590/1516-3180.20151332T1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romieu I, Téllez-Rojo MM, Lazo M, Manzano-Patiño A, Cortez-Lugo M, Julien P, Bélanger MC, Hernandez-Avila M, Holguin F. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005;172:1534–1540. doi: 10.1164/rccm.200503-372OC. [DOI] [PubMed] [Google Scholar]
- 35.Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, Levine M, McDonnell WF, Devlin RB. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med. 2001;164:819–825. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- 36.Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. Biomed Res Int. 2014;2014:801896. doi: 10.1155/2014/801896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 38.Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39:671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 39.Versari D, Daghini E, Rodriguez-Porcel M, Sattler K, Galili O, Pilarczyk K, Napoli C, Lerman LO, Lerman A. Chronic antioxidant supplementation impairs coronary endothelial function and myocardial perfusion in normal pigs. Hypertension. 2006;47:475–481. doi: 10.1161/01.HYP.0000201445.77125.26. [DOI] [PubMed] [Google Scholar]
- 40.May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013;19:2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stirrat A, Nelli S, Dowell FJ, Martin W. Flow-induced enhancement of vasoconstriction and blockade of endothelium-derived hyperpolarizing factor (EDHF) by ascorbate in the rat mesentery. Br J Pharmacol. 2008;153:1162–1168. doi: 10.1038/sj.bjp.0707499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeish AJ, Nelli S, Wilson WS, Dowell FJ, Martin W. Differential effects of ascorbate on endothelium-derived hyperpolarizing factor (EDHF)-mediated vasodilatation in the bovine ciliary vascular bed and coronary artery. Br J Pharmacol. 2003;138:1172–1180. doi: 10.1038/sj.bjp.0705143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mottl AK, Shoham DA, North KE. Angiotensin II type 1 receptor polymorphisms and susceptibility to hypertension: a HuGE review. Genet Med. 2008;10:560–574. doi: 10.1097/gim.0b013e3181809613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanobetti A, Baccarelli A, Schwartz J. Gene-air pollution interaction and cardiovascular disease: a review. Prog Cardiovasc Dis. 2011;53:344–352. doi: 10.1016/j.pcad.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghelfi E, Wellenius GA, Lawrence J, Millet E, Gonzalez-Flecha B. Cardiac oxidative stress and dysfunction by fine concentrated ambient particles (CAPs) are mediated by angiotensin-II. Inhal Toxicol. 2010;22:963–972. doi: 10.3109/08958378.2010.503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Carter JD, Dailey LA, Huang YC. Pollutant particles produce vasoconstriction and enhance MAPK signaling via angiotensin type I receptor. Environ Health Perspect. 2005;113:1009–1014. doi: 10.1289/ehp.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frampton MW, Pietropaoli A, Dentler M, Chalupa D, Little EL, Stewart J, Frasier L, Oakes D, Wiltshire J, Vora R, et al. Cardiovascular effects of ozone in healthy subjects with and without deletion of glutathione-S-transferase M1. Inhal Toxicol. 2015;27:113–119. doi: 10.3109/08958378.2014.996272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung MM, Rao F, Poddar S, Mahata M, Khandrika S, Mahata SK, O’Connor DT. Early inflammatory and metabolic changes in association with AGTR1 polymorphisms in prehypertensive subjects. Am J Hypertens. 2011;24:225–233. doi: 10.1038/ajh.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robertson S, Thomson AL, Carter R, Stott HR, Shaw CA, Hadoke PW, Newby DE, Miller MR, Gray GA. Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV1 and β1 adrenoreceptors. Part Fibre Toxicol. 2014;11:12. doi: 10.1186/1743-8977-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]