Abstract
Background
Fluoxetine improves social interactions in children with autism, social anxiety and social phobia. It is not known whether this effect is mediated directly or indirectly by correcting the underlying pathology. Genetics may also influence the drug effect. Polymorphisms of the MAOA (monoamine oxidase A) gene interact with fluoxetine to influence metabolic profiles in juvenile monkeys. Juvenile nonhuman primates provide an appropriate model for studying fluoxetine effects and drug*gene interactions in children.
Methods
Male rhesus monkeys 1–3 years of age living in permanent social pairs were treated daily with a therapeutic dose of fluoxetine or vehicle (n=16/group). Both members of each social pair were assigned to the same treatment group. They were observed for social interactions with their familiar cagemate over a 2-year dosing period. Subjects were genotyped for MAOA variable number of tandem repeats (VNTR) polymorphisms categorized for high or low transcription rates (hi-MAOA, low-MAOA).
Results
Fluoxetine-treated animals spent 30% more time in social interaction than vehicle controls. Fluoxetine significantly increased the duration of quiet interactions, the most common type of interaction, and also of immature sexual behavior typical of rhesus in this age group. Specific behaviors affected depended on MAOA genotype of the animal and its social partner. When given fluoxetine, hi-MOAO monkeys had more social invitations and initiation behaviors and low-MAOA subjects with low-MAOA partners had more grooming and an increased frequency of some facial and vocal expressive behaviors.
Conclusions
Fluoxetine may facilitate social interaction in children independent of remediation of psychopathology. Common genetic variants may modify this effect.
1. Introduction
Social functioning is a major endpoint for pharmacotherapy of depression (1, 2). The selective serotonin reuptake inhibitor (SSRI) fluoxetine is approved for treatment of depression in children and is also used to treat social anxiety and autism in children (3–6), but there is no information on whether fluoxetine specifically improves social function during childhood. In adult depressed patients, SSRIs, including fluoxetine, improve social function (7–9). In normal adults, SSRIs (citalopram, reboxetine, escitalopram) have been demonstrated to facilitate social interactions although fluoxetine has not been studied in this regard (10–14). Similar studies of SSRI promotion of normal social interactions in children or juvenile animals were not identified in the literature. Based on findings with other SSRIs in normal adult humans, fluoxetine would be expected to facilitate social interactions in children.
In this study we measured peer social interactions in healthy young male rhesus monkeys. Ethical considerations preclude experiments in normal children with psychoactive drugs. A unique potential “side effect” of giving psychopharmacological agents to children is irreversible change in the trajectory of brain development that could lead to permanent impairment of brain function (15).
Nonhuman primates, particularly macaque monkeys, have become a valuable model for studies of psychoactive agents that translate to children (16–24). Macaque monkeys undergo a long period of brain development between infancy and puberty when higher cortical functions are being established in conjunction with synaptic pruning (25, 26, 27) and prefrontal cortex specialization and inclusion in brain circuits (28). Nonhuman primates are also a valuable model for studying gene*environment interactions (29).
In addition to drug effects, drug*genotype interactions were studied. Individuals within the two Treatment groups (fluoxetine, vehicle) were balanced for common variable number of tandem repeats (VNTR) polymorphisms of the serotonin metabolizing enzyme monoamine oxidase A (MAOA, LPR polymorphism). Each social dyad consisted of two partners in the same Treatment group, but with either the same or different MAOA genotypes (high or low transcription polymorphism). Thus MAOA genotype was also balanced within Treatment group, and individual, rather than dyad, was the unit of analysis. This design allowed evaluation of both variables with efficient use of animals. Monoamine oxidase metabolizes monoamine neurotransmitters including serotonin via oxidative deamination. The MAOA isoform has high selectivity for serotonin (30) and is localized primarily in brain (31). VNTR polymorphisms of MAOA occur in both macaques and humans and can be classified as producing greater (high-MAOA) or less (low-MAOA) transcription of the MAOA gene (32, 33). As an inhibitor of the serotonin reuptake, fluoxetine has potential to interact with the serotonin metabolism pathway. Interestingly, fluoxetine can also directly inhibit MAOA activity (34). In metabolomics studies of juvenile rhesus we found that fluoxetine administration interacted with MAOA polymorphism genotype in influencing metabolites in plasma and cerebrospinal fluid (35) indicating a biological basis for an interaction between fluoxetine and MAOA.
MAOA polymorphisms influence risk for, symptom patterns of, and severity of childhood behavior disorders including autism and ADHD (36–43). There is minimal research on MAOA polymorphism effects on behavior of normal infants and children (44, 45). We previously found that infant and juvenile monkeys with low-MAOA polymorphisms used more expressive vocalizations and facial expressions in social challenge situations (46). Hi-MAOA immature monkeys initiated more grooming episodes and displacements than their low-MAOA peers in round-robin testing (46) and also solved more cognitive puzzles (47). MAOA polymorphisms also interacted with developmental iron deficiency in macaques to influence social behavior (46). Based on this background, we hypothesized that MAOA polymorphism genotype would be a modifier of the behavioral response to fluoxetine.
2. Materials and Methods
2.1 Assurance of compliance with animal codes
All animal procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council and were approved by the UC Davis Institutional Animal Care and Use Committee.
2.2 Animal selection
Thirty-two male rhesus monkey infants were selected from the outdoor group- caged colony at the California National Primate Research Center (CNPRC) at 10 months of age and adapted to long-term indoor pair-housing as described previously (35). Males were selected for study because of the feasibility of obtaining MAOA genotype groups with high and low-expressing genotypes of the X-linked MAOA gene. The population of females homozygous for high and low-expressing alleles available for the study was limited due to the predominance of mixed allele heterozygotes.
2.3 Drug treatment
Drug treatment began at one year of age (367±0.4 days of age, mean±sem). Half of the group was administered an oral dose of fluoxetine (Webster Veterinary Supply, Devens, MA). Monkeys were trained to receive the daily dose diluted in a flavorful vehicle by oral syringe between 1 and 2 pm. Vehicle controls received only the flavorful vehicle. A dose of 2.0 mg/kg was known to be therapeutic in adult rhesus (48–52) and determined to be in the therapeutic range for juvenile rhesus (53). During the two years of treatment (from 1 to 3 years of age) the dose was initiated at 1.6 mg/kg for the first 11 months and increased to 2.4 mg/kg for the remainder of the study. In a pilot study, serum levels of fluoxetine and its active metabolite norfluoxetine averaged 336±40 ng/mL (mean±sem) 2–10 h after a single dose of 2.0 mg/kg in rhesus juveniles (53). Fluoxetine and norfluoxetine averaged 273±31 ng/mL when measured 20 h after dosing with 2.4 mg/kg at the end of the current study. Comparable values in children are 363 ng/mL measured 8–12 h after dosing in a pharmacokinetic study of pediatric patients treated with fluoxetine at therapeutic doses (54) and 213 ng/mL from a therapeutic monitoring study in pediatric patients (55).
2.4 Study Design
The design of the study is shown in Table 1. Most rhesus infants at CNPRC are genotyped for common VNTR polymorphisms of the serotonin transporter gene (SERT, 5HTTLPR polymorphism, LL, SL and SS variant groups) and the serotonin metabolizing enzyme monoamine oxidase (MAOA, hi-MAOA and low-MAOA variant groups) allowing us to select subjects to balance these variables within treatment groups. SERT polymorphisms were not found to influence social behavior. An extensive battery of behavioral evaluations was conducted during this time (Table S1), including 3, 30-min sessions of social interaction observation conducted 6 months, 12 months and 20 months after initiation of dosing (18, 24 and 32 months of age).
Table 1.
Design of the drug*genotype study. Each subject was paired with a partner in the same treatment group.
| Drug Treatment | MAOA transcription category |
Partner MAOA transcription category |
|---|---|---|
| Vehicle (n=16) | Hi-MAOA (n=8) | Same (n=4) |
| Different (n=4) | ||
| Low-MAOA (n=8) | Same (n=4) | |
| Different (n=4) | ||
| Fluoxetine (n=16) | Hi-MAOA (n=8) | Same (n=4) |
| Different (n=4) | ||
| Low-MAOA (n=8) | Same (n=4) | |
| Different (n=4) |
Social observations were conducted in the home housing situation. All animals in the study were housed in an indoor caging room in a suite of two cages (each 60 by 65 by 79 cm) connected by a door. The two cagemates assigned for compatibility by animal management staff remained together throughout the study with the door between the two cages open except during dosing and behavioral testing. Both cagemates were assigned to the same treatment (fluoxetine or vehicle). The sessions were conducted in the afternoon (1500 h) by the same observer seated with a laptop computer in a neutral location that differed for each dyad. Observations using a social ethogram (Table S2) were recorded with Observer software (Noldus Observer XT 11.0, Noldus Information Technology, Netherlands) after a 3 min adaptation period. No more than two dyads were observed on one afternoon.
The ethogram contained 30 social interactions and social expressive behaviors (see behavior definitions in Table S2). All other behavior was recorded as “nonsocial” with the exception of stereotypic behaviors which were recorded separately. The major categories (Table 2) of social interactions were passive contact, quiet interaction, active play and immature sexual behavior. Social expressive behaviors (primarily facial expressions and vocalizations) were scored for frequency of occurrence. Expressive behaviors were not necessarily part of an interaction with the partner.
Table 2.
Summary variables constructed from individual ethogram behaviors. The categories were based on nonhuman primate behavior literature and examination of covariance matrices from the study. Summary variables were analyzed first in the tiered statistical analysis approach.
| Social Interaction categories: coded for both duration and frequency | |
| Passive contact | Bodies are in contact but there is no active interaction |
| Quiet affiliation | Cling, groom |
| Play | Rough and tumble, contact play, chase |
| Immature sexual | Mount, genital inspection, rump present |
| Social invitation/initiation: coded for frequency | |
| Social Invitation | Approach, groom present, play face |
| Social Interaction initiation | Partner responsible |
| Expressive Behavior clusters: coded only for frequency | |
| PC1 | Scratch, coo, fear grimace |
| PC2 | Grunt, threat, lipsmack, yawn, sniff |
After the first observation session, refinements were made for the second and third sessions: the cagemate initiating and terminating each social interaction was recorded and invitations to social behavior (approach, play face, groom present) were added to the ethogram. Thus these data were only available for the last two sessions.
2.5 Statistical analysis
Statistical analysis used a tiered approach, first examining apical variables such as the total amount of social interaction, and then following up with examination of subcategories and individual behaviors. Prior to statistical analysis, all endpoints were screened for potential covariates including age, size, cohort, cage position, and 5HTTLPR genotype. These potential covariates, including 5HTTLPR genotype, did not significantly influence behavioral endpoints. However, individual MAOA genotype and the partner’s MAOA genotype category (analyzed as same or different) had strong and extensive influence on social interactions and expressive endpoints. The treatment groups had been balanced a priori for these MAOA genotype variables, so that they could be included as independent variables in the analyses. Thus, summary social interaction durations and expressive behavior frequencies (Table 2) were analyzed by three-way ANOVA (fluoxetine, MAOA genotype and partner’s MAOA genotype) including the interactions. Social invitation behavior frequencies (approach, play face, groom present) and frequencies of initiation of social interaction categories were analyzed by two-way ANOVA (individual MAOA genotype, fluoxetine) including the interaction. When significant drug*genotype interactions were identified, two post hoc planned contrasts were conducted to determine whether treatment effects occurred within each of the two MAOA individual groups or partner (same, different) groups. Significance was identified at p<0.05 for ANOVA main effects and interactions.
3. Results
3.1 Social interaction: Fluoxetine increased time spent in social interaction
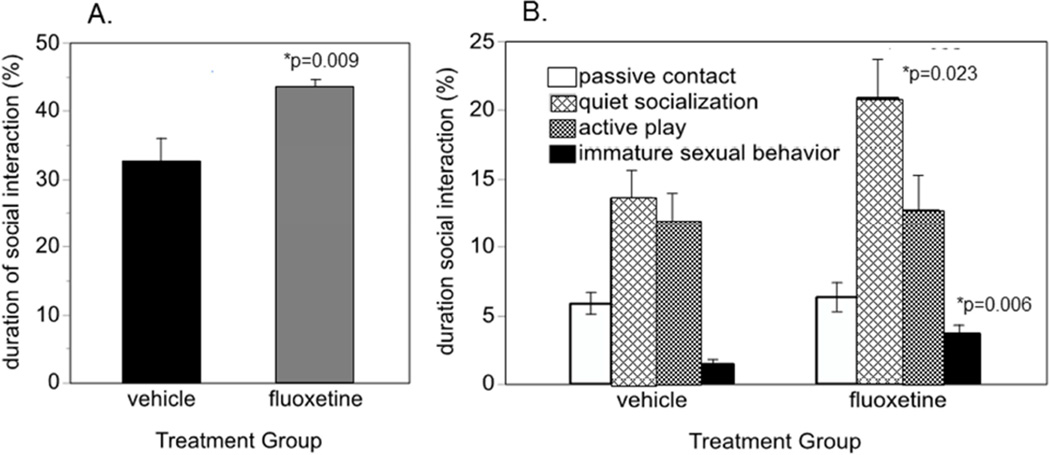
The average amount of time spent in social interactions was 38% of the 90 min observation period. The duration of social interactions was 30% greater in the fluoxetine-treated animals than in controls (F1,26=8.27, p=0.008) (Figure 1A). Looking at the individual summary categories of social interaction (Figure 1B), this effect was seen for quiet interactions and immature sexual behavior, but not for play or for passive contact. As described below, partner genotype also influenced time spent in various social interaction categories. In addition, some behaviors showed trends across the three individual sessions that were evaluated statistically. These analyses are detailed below.
Figure 1.
Time spent in social interaction during 3 30-min sessions over a 2-year period of dosing. A. Time spent in all social interactions. B. Time spent in individual categories of social interaction. Mean ± s.e.m. are shown. N=16/group. * significantly different from corresponding vehicle mean.
The time spent in passive contact was 6% of the 90 min observation time. No fluoxetine or MAOA genotype effect or interaction was seen.
The time spent in quiet social interaction averaged 17%. Fluoxetine increased quiet socialization duration, summed across all sessions, by 61% compared to vehicle controls (F1,26=4.92, p=0.035). Partner genotype differentially affected the two components of quiet social interaction, groom and cling. Grooming duration (14% of observation time) was greater in monkeys whose partners had the same genotype (F1,26=6.71, p=0.0155) while clinging (3% of observation time) was greater, though not significantly (p=0.13), in monkeys whose partners had different genotypes. There were also interactions between fluoxetine and partner genotype. Fluoxetine increased time spent grooming for the monkeys whose partners had the same genotype (F1,26=6.55, p=0.017) but increased time spent clinging for the monkeys whose partner had a different MAOA genotype (F1,26=6.52, p=0.017). Thus fluoxetine increased the quiet social behavior that was more common in the pair.
The grooming analysis also revealed a 3-way interaction between fluoxetine, individual MAOA genotype, and partner’s (same/different) genotype (F1,26=7.47, p=0.011). Specifically, monkeys with the low-MAOA genotype, whose partners also had the same genotype, had greater durations of grooming when treated with fluoxetine than their vehicle treated counterparts (p=0.0003). In fact, this subgroup had the highest grooming durations indicating a greater sensitivity to fluoxetine.
Time spent in play averaged 12% with no fluoxetine or MAOA genotype effects. Similarly, there was no effect of fluoxetine on duration of play in the subcategories of play: contact play, rough and tumble play, and chase play. Play was consistent in duration across sessions in controls but increased in the fluoxetine treated group (p=0.018, paired t-test) from a level initially lower than controls (first session, p=0.033).
Immature sexual behavior averaged 2.57% of the observation time and was 60% higher in the fluoxetine treated group (F1,26=8.57, p=0.007). Immature sexual behavior is a normal part of the play and stress-relief behavioral repertoire of juvenile macaques (56). No influences of fluoxetine or MAOA genotype were seen.
Notably, in these young familiar dyad partners, there was no incidence of aggression. Displacement behaviors, which are often used as an index of dominance in nonhuman primates, occurred at a low frequency (median=1/90 min) and were not influenced by fluoxetine or MAOA genotype.
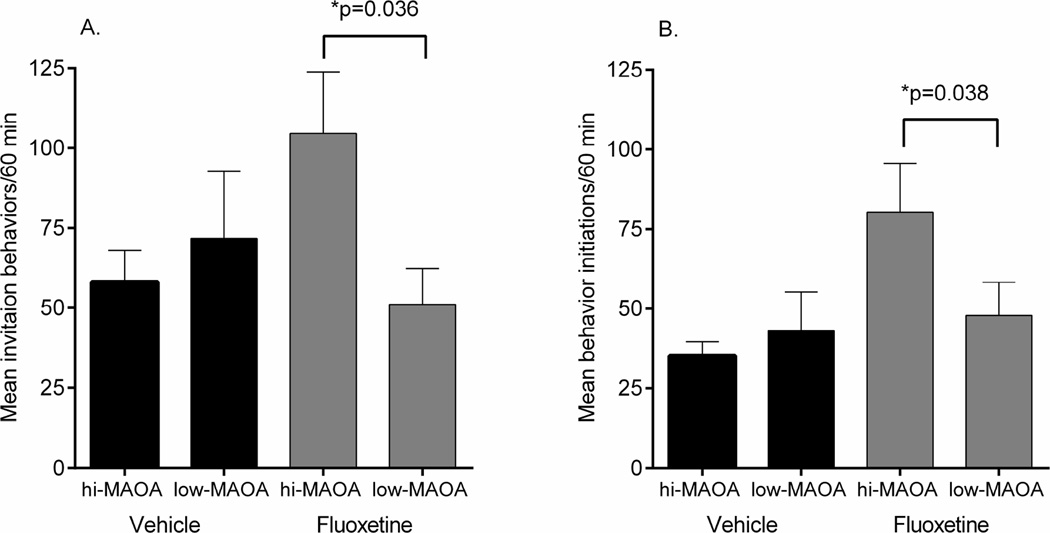
3.2 Fluoxetine increased the invitation to social interaction and the initiation of social interaction in hi-MAOA individuals
In addition to duration of different social interactions, the frequency of invitation to social interactions (approach, play face, groom present) was examined in sessions 2 and 3. A fluoxetine effect emerged in the form of an interaction with individual MAOA genotype (F1,26=4.47, p=0.044). In the fluoxetine treated animals, the hi-MAOA subgroup had an increased number of invitation behaviors (approach, play face, and groom present) compared to the low-MAOA subgroup (p=0.036) (Figure 2A).
Figure 2.
Invitation to social interactions and initiation of social interaction during 2nd and 3rd 30-min observation sessions. A. Invitation behaviors (approach, play face, groom present). B. Initiation of social interactions. Mean ± s.e.m. are shown. N=16/group.
Data on which individuals initiated and terminated each social interaction were also recorded in sessions 2 and 3. As was the case with behavior invitations, a fluoxetine effect emerged in the form of an interaction with individual MAOA genotype (F1,26=4.85, p=0.036). In the fluoxetine treated animals, the hi-MAOA subgroup had an increased number of behavior initiations compared to the low-MAOA subgroup (p=0.038) (Figure 2B). Fluoxetine and MAOA genotype were not found to influence the number of behavior terminations.
In general, fluoxetine could be seen to increase social interactions by increasing the invitation to social interaction and the initiation of social interaction by hi-MAOA individuals and by extending the duration of grooming in low-MAOA individuals whose partners had the same genotype.
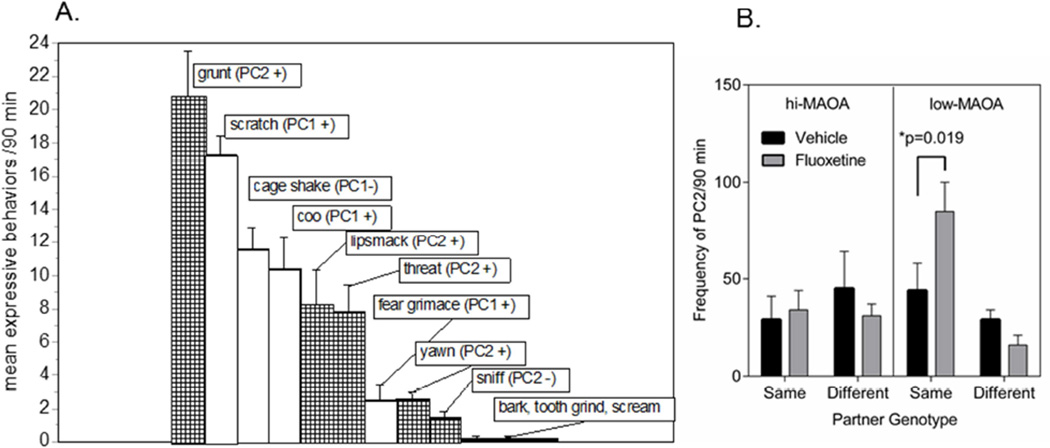
3.3 Fluoxetine increased occurrence of some expressive behaviors in low-MAOA animals whose partners had the same genotype
The amount of expressive behavior (sum of all occurrences) was not influenced by fluoxetine but showed a trend toward effects of partner genotype (p=0.081) and toward an interaction between MAOA genotype and partner genotype (p=0.081). Low-MAOA individuals whose partner had the same genotype had the highest frequency of all expressive behaviors. This suggested possible effects on a subgroup of the expressive behaviors. Because the incidence of most individual expressive behaviors was low (Figure 3A), clusters of these behaviors were needed to conduct statistical analysis. A preliminary Principal Component Analysis (PCA) yielded two clusters (Figure 3A). The first expressive cluster included coo, fear grimace, and scratch. When the summed incidence of these behaviors was analyzed, no fluoxetine, MAOA genotype, partner genotype effects or interactions were shown. The second cluster included grunt, threat, lipsmack, sniff and yawn and showed a trend toward a partner genotype effect (p=0.069), and a marginal fluoxetine interaction with partner genotype (p=0.053). Further analysis showed that the fluoxetine by partner genotype interaction occurred only in monkeys with the low-MAOA genotype (F1,12=5.58, p=0.036) and reflected a much higher frequency of these expressive behaviors in fluoxetine treated monkeys whose partners also had the low-MAOA genotype (Figure 3B). Some of these behaviors (threat) are interpreted as “aggressive” in adult social interactions (57, 58) but it should be noted that these expressive behaviors are not well integrated at these early ages and often appear out of the contexts in which they occur in adults (59). Further, it is important to note that clustering is based on the incidence of occurrence in individuals across the observation period, not on temporal association of behaviors as the animals interact.
Figure 3.
Expressive behaviors. A. Frequency of occurrence of different expressive behaviors during 60 min of observation (2nd and 3rd observation sessions). Mean ± s.e.m. N=32. Tags indicate which behaviors were significant members of the first and second principal components (PC) in a preliminary principle components analysis. Low incidence behaviors (bark, tooth grind, scream) did not contribute to the PC1 or PC2. B. Effect of fluoxetine on PC2 expressive behaviors in monkeys with high or low-MAOA genotypes and partners with the same or opposite genotype. * Significant fluoxetine effect from planned comparison. Frequency of occurrence of PC1 variables was not affected by fluoxetine.
3.4 Stereotypy was infrequent and not influenced by fluoxetine
Stereotypy, either self-directed or motor, is also thought to reflect emotional state. Stereotypy averaged 0.7% of the observation period and about a third (10/32) of the animals never displayed stereotypy during the 90 min observation period. Neither the number of animals displaying stereotypy nor total time spent in stereotypy differed by treatment group, individual genotype or partner genotype.
4. Discussion
In this study chronic fluoxetine treatment at therapeutic doses increased the amount of time spent in social interactions by juvenile monkeys interacting with a familiar peer. The normal time budget of social interaction was not disrupted and increases in abnormal or aggressive behavior did not occur. Instead fluoxetine enhanced the behaviors most commonly seen in the familiar dyads.
Basic research on the effects of other SSRIs on normal social interactions in adults is relevant to our study. Tse and colleagues have studied effects of SSRIs (citalopram and reboxetine) on social behavior in normal volunteers participating in detailed experiments using structured social interaction paradigms. These studies found promotion of affiliative behavior and social bonding by SSRIs (10, 12) and suggested greater noradrenergic than serotonergic influences through the use of SSRIs more selective for norepinephrine (11, 13). Most recently, experiments from these investigators supported the hypotheses that SSRIs enhance reward sensitivity of social stimuli (14). In nonhuman primates, the SSRI sertraline was found to increase affiliative social behavior and decrease dominance-related behaviors in long-term social groups of female cynomolgus macaques (60).
In addition to main effects on apical variables, fluoxetine was found to interact with MAOA genotype of the subjects and their partners in influencing specific behaviors. Specifically, fluoxetine increased duration of grooming and frequency of expressive vocalizations the most in low-MAOA individuals interacting with low-MAOA partners. There was a main effect of MAOA genotype on initiation of social interactions. Hi-MAOA individuals initiated more social interactions, and this genotype effect was significant in the fluoxetine-treated individuals. A similar fluoxetine*MAOA interaction was seen for invitation to social interaction. These patterns are probably specific to the age, sex, housing and observation conditions. However, understanding that the pattern of change in specific behaviors may depend on genotype of the children may help personalize the use of psychoactive drugs to promote social interaction. Promoting positive social interaction is an important therapeutic target for disorders like autism, depression and social anxiety in children. Childhood and adolescence are periods of intense peer interaction that form a basis for success later in life.
Our study suggests that fluoxetine can facilitate positive social behavior in familiar situations. Most research on fluoxetine and social behavior focuses on correcting pathology, counteracting aggression or modifying response to strangers. In patient populations, fluoxetine has been demonstrated to increase social interactions in depressed patients (1, 2, 7, 8) and is used as a treatment for social phobia (5, 61–63). In animal models, fluoxetine effects on social interaction have been studied in two mouse models of autism, the BTBR mouse strain (64) and the MAOA knockout or hypomorphic mouse (65). Adult sexual, aggressive, or social approach behaviors have been studied in rodent models in brief structured stranger interaction paradigms during fluoxetine dosing (66–68). In nonhuman primates fluoxetine has been studied in connection with aggressive response to a stranger in adult vervet monkeys (69), with response to social separation in juvenile rhesus monkeys (49) and with behavior during a brief encounter with strangers in juvenile rhesus (23) and adult marmosets (Kinnally 2006). However, other studies of extended interaction of juveniles with familiar peers in children or experimental animals were not located in the literature.
In addition to social interactions, we studied expressive behaviors often seen in a social context. Vocal and facial expressions can serve signaling or social communication purposes but also are seen as manifestations of emotional state (57). In the observation situation used here, expressive behaviors could be directed at other animals in the room, and at the observer, as well as at the proximal social partner (cagemate) and were not necessarily imbedded in an interaction sequence. These considerations limit interpretation. In adult and infant macaques, multivariate analysis of these expressive behaviors has shown relationships as factors that are often interpreted as “aggressive” but differential use of these behaviors is gradually acquired during juvenile development (59). In the current study, fluoxetine did not generally change frequency of expressive behaviors, but in dyads with two low-MAOA partners receiving fluoxetine treatment, the frequency of this specific cluster of expressive behaviors was increased.
Increased social behavior could be interpreted as part of a more general activation of behavior. “Activation” of behavior in children by fluoxetine and other SSRIs was recognized early in its therapeutic use in children (70) and is seen as having predominately negative implications (71). The current study does not suggest that the increased socialization is associated with “activation”. Similarly, no association with disinhibition (69) was indicated, but rather facilitation of normal interaction patterns in a familiar social relationship as reported previously in adult macaques (60).
5. Conclusion
Social behavior was facilitated by chronic fluoxetine treatment in juvenile rhesus monkeys interacting with a familiar peer. The type of social behavior affected depended on the MAOA genotype of the monkey and its partner. Translation of these findings to use of fluoxetine therapy in children would require extension of research to larger, more complex and more challenging social situations including different age groups, maturational stages that include aggressive behaviors, and environments with more behavioral options.
Supplementary Material
Highlights.
Fluoxetine increased social interaction in familiar pairs of young monkeys
Behaviors affected depended on MAOA polymorphism genotypes of the partners
Fluoxetine therapy may facilitate social interaction of pediatric patients
Acknowledgments
The authors would like to thank the Research Services staff at CNPRC for providing dosing support. This work was supported by NIH grants HD065826 (to MSG), OD010962 (to John Capitanio) and OD011107 (to Harris Lewin). The study sponsors had no role in the design, execution, or interpretation of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material is available at the Neuropharmacology website.
Financial Disclosures
The authors report no biomedical financial interests or conflicts of interest.
Contributor Information
Mari S. Golub, Department of Environmental Toxicology, University of California Davis, Davis, CA 95616
Casey E. Hogrefe, California National Primate Research Center, University of California Davis, Davis, CA 95616
Alicia M. Bulleri, California National Primate Research Center, University of California Davis, Davis, CA 95616
References
- 1.Bech P. Social functioning: should it become an endpoint in trials of antidepressants? CNS Drugs. 2005;19(4):313–324. doi: 10.2165/00023210-200519040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Healy D, McMonagle T. The enhancement of social functioning as a therapeutic principle in the management of depression. J Psychopharmacol. 1997;11(4 Suppl):S25–S31. [PubMed] [Google Scholar]
- 3.Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD004677.pub3. Cd004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar B, Prakash A, Sewal RK, Medhi B, Modi M. Drug therapy in autism: a present and future perspective. Pharmacol Rep. 2012;64(6):1291–1304. doi: 10.1016/s1734-1140(12)70927-1. [DOI] [PubMed] [Google Scholar]
- 5.Scharfstein LA, Beidel DC, Finnell LR, Distler A, Carter NT. Do pharmacological and behavioral interventions differentially affect treatment outcome for children with social phobia? Behav Modif. 2011;35(5):451–467. doi: 10.1177/0145445511408590. [DOI] [PubMed] [Google Scholar]
- 6.Stevanovic D, Tadic I, Knez R. Are antidepressants effective in quality of life improvement among children and adolescents? A systematic review. CNS Spectr. 2014;19(2):134–141. doi: 10.1017/S1092852913000576. [DOI] [PubMed] [Google Scholar]
- 7.Briley M, Moret C. Improvement of social adaptation in depression with serotonin and norepinephrine reuptake inhibitors. Neuropsychiatr Dis Treat. 2010;6:647–655. doi: 10.2147/NDT.S13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubini A, Bosc M, Polin V. Noradrenaline-selective versus serotonin-selective antidepressant therapy: differential effects on social functioning. J Psychopharmacol. 1997;11(4 Suppl):S17–S23. [PubMed] [Google Scholar]
- 9.Sghendo L, Mifsud J. Understanding the molecular pharmacology of the serotonergic system: using fluoxetine as a model. J Pharm Pharmacol. 2012;64(3):317–325. doi: 10.1111/j.2042-7158.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 10.Tse WS, Bond AJ. Serotonergic intervention affects both social dominance and affiliative behaviour. Psychopharmacology (Berl) 2002;161(3):324–330. doi: 10.1007/s00213-002-1049-7. [DOI] [PubMed] [Google Scholar]
- 11.Tse WS, Bond AJ. Difference in serotonergic and noradrenergic regulation of human social behaviours. Psychopharmacology (Berl) 2002;159(2):216–221. doi: 10.1007/s00213-001-0926-9. [DOI] [PubMed] [Google Scholar]
- 12.Tse WS, Bond AJ. Reboxetine promotes social bonding in healthy volunteers. J Psychopharmacol. 2003;17(2):189–195. doi: 10.1177/0269881103017002007. [DOI] [PubMed] [Google Scholar]
- 13.Tse WS, Bond AJ. Noradrenaline might enhance assertive human social behaviours: an investigation in a flatmate relationship. Pharmacopsychiatry. 2006;39(5):175–179. doi: 10.1055/s-2006-948328. [DOI] [PubMed] [Google Scholar]
- 14.Tse WS, Chow H, Wing YK, Bond AJ. Using a partner’s facial emotion to elucidate social dominance motivation induced by an SSRI. Eur Neuropsychopharmacol. 2014;24(10):1641–1649. doi: 10.1016/j.euroneuro.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Christian RB, Gaynes BN, Saavedra LM, Sheitman B, Wines R, Jonas DE, et al. Use of antipsychotic medications in pediatric and young adult populations: future research needs. J Psychiatr Pract. 2015;21(1):26–36. doi: 10.1097/01.pra.0000460619.10429.4c. [DOI] [PubMed] [Google Scholar]
- 16.Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, et al. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37(12):2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandell DJ, Unis A, Sackett GP. Post-drug consequences of chronic atypical antipsychotic drug administration on the ability to adjust behavior based on feedback in young monkeys. Psychopharmacology (Berl) 2011;215(2):345–352. doi: 10.1007/s00213-010-2147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattison DR, Plant TM, Lin HM, Chen HC, Chen JJ, Twaddle NC, et al. Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. Proc Natl Acad Sci U S A. 2011;108(39):16301–16306. doi: 10.1073/pnas.1102187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson TA, Li M, Hotchkiss CE, Mauz A, Eddie M, Greischel A, et al. Toxicity assessment of pramipexole in juvenile rhesus monkeys. Toxicology. 2010;276(3):164–171. doi: 10.1016/j.tox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, et al. Chronic marijuana smoke exposure in the rhesus monkey. II: Effects on progressive ratio and conditioned position responding. J Pharmacol Exp Ther. 1992;260(1):210–222. [PubMed] [Google Scholar]
- 21.Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive-behavioral development in nonhuman primates I. Neurotoxicol Teratol. 2001;23(4):319–332. doi: 10.1016/s0892-0362(01)00156-8. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, et al. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol Teratol. 2010;32(2):142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, et al. Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry. 2014;171(3):323–331. doi: 10.1176/appi.ajp.2013.13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soto PL, Wilcox KM, Zhou Y, Kumar A, Ator NA, Riddle MA, et al. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmac ol ogy. 2012;37(12):2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elston GN, Oga T, Okamoto T, Fujita I. Spinogenesis and pruning from early visual onset to adulthood: an intracellular injection study of layer III pyramidal cells in the ventral visual cortical pathway of the macaque monkey. Cereb Cortex. 2010;20(6):1398–1408. doi: 10.1093/cercor/bhp203. [DOI] [PubMed] [Google Scholar]
- 26.Elston GN, Okamoto T, Oga T, Dornan D, Fujita I. Spinogenesis and pruning in the primary auditory cortex of the macaque monkey (Macaca fascicularis): an intracellular injection study of layer III pyramidal cells. Brain Res. 2010;1316:35–42. doi: 10.1016/j.brainres.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 27.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 28.Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, et al. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2(6):336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 30.Murphy DL, Redmond DE, Jr, Garrick N, Baulu J. Brain region differences and some characteristics of monoamine oxidase type A and B activities in the vervet monkey. Neurochem Res. 1979;4(1):53–62. doi: 10.1007/BF00963831. [DOI] [PubMed] [Google Scholar]
- 31.Westlund KN, Krakower TJ, Kwan SW, Abell CW. Intracellular distribution of monoamine oxidase A in selected regions of rat and monkey brain and spinal cord. Brain Res. 1993;612(1–2):221–230. doi: 10.1016/0006-8993(93)91664-e. [DOI] [PubMed] [Google Scholar]
- 32.Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57(2):167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, et al. Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet. 2006;36(2):163–172. doi: 10.1007/s10519-005-9017-8. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee J, Yang ZY. Monoamine oxidase A inhibition by fluoxetine: an in vitro and in vivo study. Synapse. 1999;31(4):285–289. doi: 10.1002/(SICI)1098-2396(19990315)31:4<285::AID-SYN6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Hogrefe CE, Grapov D, Palazoglu M, Fiehn O, Turck CW, et al. Identifying individual differences of fluoxetine response in juvenile rhesus monkeys by metabolite profiling. Transl Psychiatry. 2014;4:e478. doi: 10.1038/tp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis LK, Hazlett HC, Librant AL, Nopoulos P, Sheffield VC, Piven J, et al. Cortical enlargement in autism is associated with a functional VNTR in the monoamine oxidase A gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1145–1151. doi: 10.1002/ajmg.b.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen IL, Liu X, Schutz C, White BN, Jenkins EC, Brown WT, et al. Association of autism severity with a monoamine oxidase A functional polymorphism. Clin Genet. 2003;64(3):190–197. doi: 10.1034/j.1399-0004.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 38.Jaiswal P, Guhathakurta S, Singh AS, Verma D, Pandey M, Varghese M, et al. SLC6A4 markers modulate platelet 5-HT level and specific behaviors of autism: a study from an Indian population. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:196–206. doi: 10.1016/j.pnpbp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Verma D, Chakraborti B, Karmakar A, Bandyopadhyay T, Singh AS, Sinha S, et al. Sexual dimorphic effect in the genetic association of monoamine oxidase A (MAOA) markers with autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:11–20. doi: 10.1016/j.pnpbp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Tassone F, Qi L, Zhang W, Hansen RL, Pessah IN, Hertz-Picciotto I. MAOA, DBH, and SLC6A4 variants in CHARGE: a case-control study of autism spectrum disorders. Autism Res. 2011;4(4):250–261. doi: 10.1002/aur.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCracken JT, Badashova KK, Posey DJ, Aman MG, Scahill L, Tierney E, et al. Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics J. 2014;14(3):295–302. doi: 10.1038/tpj.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen IL, Liu X, Lewis ME, Chudley A, Forster-Gibson C, Gonzalez M, et al. Autism severity is associated with child and maternal MAOA genotypes. Clin Genet. 2011;79(4):355–362. doi: 10.1111/j.1399-0004.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoo HJ, Lee SK, Park M, Cho IH, Hyun SH, Lee JC, et al. Family- and population-based association studies of monoamine oxidase A and autism spectrum disorders in Korean. Neurosci Res. 2009;63(3):172–176. doi: 10.1016/j.neures.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Chen X, Deng H, Lu Z. Identifying the interaction of maternal sensitivity and two serotonin-related gene polymorphisms on infant self-regulation. Infant Behav Dev. 2014;37(4):606–614. doi: 10.1016/j.infbeh.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Chen X, Way N, Yoshikawa H, Deng H, Ke X, et al. The association between infants’ self-regulatory behavior and MAOA gene polymorphism. Dev Sci. 2011;14(5):1059–1065. doi: 10.1111/j.1467-7687.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 46.Golub MS, Hogrefe CE, Unger EL. Influence of prenatal iron deficiency and MAOA genotype on response to social challenge in rhesus monkey infants. Genes Brain Behav. 2012;11(3):278–290. doi: 10.1111/j.1601-183X.2012.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golub M, Hogrefe C. Prenatal iron deficiency and monoamine oxidase A (MAOA) polymorphisms: combined risk for later cognitive performance in rhesus monkeys. Genes Nutr. 2014;9(2):381. doi: 10.1007/s12263-013-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke AS, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry. 1999;46(2):221–228. doi: 10.1016/s0006-3223(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 49.Clarke AS, Kraemer GW, Kupfer DJ. Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Res. 1998;79(2):91–104. doi: 10.1016/s0165-1781(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 50.Fontenot MB, Musso MW, McFatter RM, Anderson GM. Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2009;48(2):176–184. [PMC free article] [PubMed] [Google Scholar]
- 51.Fontenot MB, Padgett EE, 3rd, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med. 2005;55(1):67–74. [PubMed] [Google Scholar]
- 52.Sawyer EK, Howell LL. Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology. 2011;88(1–2):44–49. doi: 10.1159/000329417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golub MS, Hogrefe CE. Fluoxetine: juvenile pharmacokinetics in a nonhuman primate model. Psychopharmacology (Berl) 2014;231(20):4041–4047. doi: 10.1007/s00213-014-3537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilens TE, Cohen L, Biederman J, Abrams A, Neft D, Faird N, et al. Fluoxetine pharmacokinetics in pediatric patients. J Clin Psychopharmacol. 2002;22(6):568–575. doi: 10.1097/00004714-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Koelch M, Pfalzer AK, Kliegl K, Rothenhofer S, Ludolph AG, Fegert JM, et al. Therapeutic drug monitoring of children and adolescents treated with fluoxetine. Pharmacopsychiatry. 2012;45(2):72–76. doi: 10.1055/s-0031-1291294. [DOI] [PubMed] [Google Scholar]
- 56.Gunst N, Leca JB, Vasey PL. Development of sexual and socio-sexual behaviors in free-ranging juvenile male Japanese macaques, Macaca fuscata. Behavi our. 2013;150:1225–1254. [Google Scholar]
- 57.Waller BM, Micheletta J. Facial expressions in nonhuman animals. Emotion Reviews. 2013;5(1):54–59. [Google Scholar]
- 58.Chevalier-Skolnikoff S. Facial expression of emotion in nonhuman primates. In: Ekman P, editor. Darwin and Facial Expression. New York: Academic Press; 1973. pp. 11–89. [Google Scholar]
- 59.Mason WA. Experiential influences on the development of expressive behaviors in rhesus monkeys. In: Zivin G, editor. The development of expressive behavior: Biology-environment interactions. New York: Academic Press; 1985. pp. 117–152. [Google Scholar]
- 60.Shively CA, Register TC, Higley JD, Willard SL. Sertraline effects on cerebrospinal fluid monoamines and species-typical socioemotional behavior of female cynomolgus monkeys. Psychopharmacology (Berl) 2014;231(7):1409–1416. doi: 10.1007/s00213-013-3329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622–1632. doi: 10.1097/chi.0b013e318154bb57. [DOI] [PubMed] [Google Scholar]
- 62.Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 63.Hedges DW, Brown BL, Shwalb DA, Godfrey K, Larcher AM. The efficacy of selective serotonin reuptake inhibitors in adult social anxiety disorder: a meta-analysis of double-blind, placebo-controlled trials. J Psychopharmacol. 2007;21(1):102–111. doi: 10.1177/0269881106065102. [DOI] [PubMed] [Google Scholar]
- 64.Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97(3):586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Godar SC, Bortolato M, Castelli MP, Casti A, Casu A, Chen K, et al. The aggression and behavioral abnormalities associated with monoamine oxidase A deficiency are rescued by acute inhibition of serotonin reuptake. J Psychiatr Res. 2014;56:1–9. doi: 10.1016/j.jpsychires.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villalba C, Boyle PA, Caliguri EJ, De Vries GJ. Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster) Horm Behav. 1997;32(3):184–191. doi: 10.1006/hbeh.1997.1420. [DOI] [PubMed] [Google Scholar]
- 67.Ricci LA, Melloni RH., Jr Repeated fluoxetine administration during adolescence stimulates aggressive behavior and alters serotonin and vasopressin neural development in hamsters. Behav Neurosci. 2012;126(5):640–653. doi: 10.1037/a0029761. [DOI] [PubMed] [Google Scholar]
- 68.Moy SS, Nonneman RJ, Shafer GO, Nikolova VD, Riddick NV, Agster KL, et al. Disruption of social approach by MK-801, amphetamine, and fluoxetine in adolescent C57BL/6J mice. Neurotoxicol Teratol. 2013;36:36–46. doi: 10.1016/j.ntt.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology. 2001;24(4):370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 70.Riddle M, King R, Hardin M, Scahill L, Ort S, Chappell P, et al. Behavioral side effects of fluoxetine in children and adolescents. J Child Adolesc Psychopharm. 1990/91;1(3):193–198. [Google Scholar]
- 71.Bussing R, Murphy TK, Storch EA, McNamara JP, Reid AM, Garvan CW, et al. Psychometric properties of the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) in youth with OCD. Psychiatry Res. 2013;205(3):253–261. doi: 10.1016/j.psychres.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.