Summary
Clustered protocadherin proteins (α-, β- and γ-Pcdhs) provide a high level of cell surface diversity to individual vertebrate neurons, engaging in highly specific homophilic interactions to mediate important roles in mammalian neural circuit development. How Pcdhs bind homophilically through their extracellular cadherin (EC) domains among dozens of highly similar isoforms has not been determined. Here we report crystal structures for extracellular regions from four mouse Pcdh isoforms (α4, α7, β6 and β8), revealing a canonical head-to-tail interaction mode for homophilic trans dimers comprising primary intermolecular EC1:EC4 and EC2:EC3 interactions. A subset of trans interface residues exhibit isoform-specific conservation, suggesting roles in recognition specificity. Mutation of these residues, along with trans-interacting partner residues, altered the specificities of Pcdh interactions. Together, these data show how sequence variation among Pcdh isoforms encodes their diverse strict homophilic recognition specificities, which are required for their key roles in neural circuit assembly.
Introduction
In functional neural circuits, axons and dendrites originating from the same neuron do not stably contact one another or their own cell body; however, they are free to interact with the processes of other neurons (Kramer and Kuwada, 1983; Kramer and Stent, 1985; Zipursky and Sanes, 2010; Zipursky and Grueber, 2013). This property of “self-avoidance”, with permissive interactions between non-self neurons, is mediated by mechanisms that generate high cell surface diversity such that each neuron acquires a distinct stochastically determined cellular identity (Zipursky and Grueber, 2013; Zipursky and Sanes, 2010; Hattori et al., 2008; Matthews et al., 2007; Chen and Maniatis, 2013; Kostadinov and Sanes, 2015). Pioneering work in Drosophila has shown that the Dscam1 gene, through stochastic alternative RNA splicing, encodes cell-surface protein isoforms with up to 19,008 distinct extracellular regions, each capable of highly specific homophilic recognition (Miura et al., 2013; Schmucker et al., 2000; Wojtowicz et al., 2007). Each neuron expresses a small but distinct stochastic repertoire of Dscam1 isoforms (Miura et al., 2013; Neves et al., 2004; Zhan et al., 2004), which confer sufficiently unique cell surface identities to mediate robust self-repulsion with permissive non-self engagement in the Drosophila nervous system (Hattori et al., 2007; Hattori et al., 2009). This mechanism appears to be common to insects and other arthropod invertebrates (Zipursky and Grueber, 2013).
By contrast, neuronal self-recognition and non-self discrimination in vertebrates is mediated, at least in part, by the clustered protocadherins (Pcdhs) (Zipursky and Sanes, 2010; Lefebvre et al., 2012; Chen and Maniatis, 2013; Zipursky and Grueber, 2013; Kostadinov and Sanes, 2015), a specialized family of cell-surface transmembrane cadherins (Suzuki, 1996). To date, functional studies have been reported for the Pcdhα and Pcdhγ gene clusters, but not for the Pcdhβ gene cluster. Pcdhα loss of function mutants display a subtle abnormality in the formation of glomeruli in the mouse olfactory bulb (Hasegawa et al., 2008; Hasegawa et al., 2012), and are also required for normal serotonergic projections in the mouse (Katori et al., 2009). Functional studies of the Pcdhγ gene cluster have been complicated by the observation that its loss leads to a cell death phenotype of many, though not all, neuronal cell types (Wang et al., 2002; Weiner et al., 2005; Lefebvre et al., 2008; Prasad and Weiner, 2011; Chen et al., 2012). The initial efforts to understand the in vivo function of the Pcdhγ cluster in mice also revealed defects in dendritic arborization in Pcdhγ mutants (Garrett et al., 2012). However, the key functional connection between the invertebrate Dscam1 and the vertebrate clustered Pcdhs was made by the observation that the Pcdhγ gene cluster is required for dendritic self-avoidance in mouse retinal starburst amacrine cells (SACs) and Purkinje cells (Lefebvre et al., 2012). In addition, the loss of self-avoidance in SACs results in the formation of self-synapses (autapses) (Kostadinov and Sanes, 2015). It is important to note, however, that these dendritic phenotypes are highly selective, as the wiring of most other neurons in the nervous system appears to be unaffected by the loss of Pcdhγ genes (Lefebvre et al., 2008). Thus, a general understanding of nervous system-wide function of the individual Pcdh gene clusters has yet to be determined.
With respect to protein function, like Dscam1 proteins, Pcdhs are a family of similar but distinct protein isoforms that mediate strictly homophilic cell-cell recognition and are stochastically expressed to provide diverse single-neuron identities (Yagi, 2013; Schreiner and Weiner, 2010; Thu et al., 2014; Rubinstein et al., 2015). In human and mouse, 53 and 58 Pcdhs respectively, are encoded by the tandemly arranged Pcdhα, β, and γ gene clusters (Wu and Maniatis, 1999; Wu et al., 2001). The mouse Pcdh gene cluster encodes 14 α, 22 β, and 22 γ isoforms. Each isoform is comprised of six extracellular cadherin domains (EC1–6), a transmembrane region, and, for α- and γ-Pcdhs, a short cytoplasmic extension (Wu and Maniatis, 1999). In contrast to Dscam1, single-cell specific expression of Pcdh isoforms is achieved through stochastic promoter choice rather than alternative splicing (Tasic et al., 2002; Wang et al., 2002; Esumi et al., 2005; Hirano et al., 2012; Kaneko et al., 2006). Each neuron is thought to express a small subset of Pcdh isoforms comprising a random repertoire of ~10 α, β, and γ isoforms, which are monoallelically expressed, along with constitutive biallelic expression of all 5 “C-type” isoforms, αC1–αC2 and γC3–γC5, which are divergent in sequence from other Pcdh variable isoforms (Esumi et al., 2005; Hirano et al., 2012; Kaneko et al., 2006). The function of Pcdh C-type isoforms is not well understood, although unlike the γ alternate isoforms, deletion of the C-type isoforms results in cell death (Chen et al., 2012).
Structure/function experiments from our laboratories have shown that α-, β- and γ-Pcdhs emanate from the membrane surface as EC6-mediated cis (same cell) dimers that appear to form promiscuously between Pcdh isoforms (Schreiner and Weiner, 2010; Thu et al., 2014; Rubinstein et al., 2015). In contrast, adhesive trans binding has been shown to be strongly homophilic (Schreiner and Weiner, 2010; Thu et al., 2014). Two recent papers reported crystal structures of γ-Pcdh (Nicoludis et al, 2015) and α-, β- and γ-Pcdh (Rubinstein et al., 2015) fragments comprising EC1–3. However, these constructs are monomeric in solution (Rubinstein et al., 2015) and thus neither study revealed the detailed molecular basis of Pcdh trans binding nor how the strict homophilic binding specificities of Pcdh isoforms are encoded by the sequence variations observed between isoforms. However, both studies suggested that, in contrast to classical cadherins, adhesive binding involves an EC1–4 trans interface.
Here we report the first crystal structures of α- and β-Pcdh extracellular fragments engaged in cognate homophilic recognition, revealing the atomic-level basis of highly specific trans recognition between Pcdhs from apposed cell surfaces. The dimer conformations are highly similar, each binding in head-to-tail orientation as predicted for α-, β- and γ-Pcdhs (Rubinstein et al., 2015; Nicoludis et al., 2015), and comprise EC1:EC4, EC2:EC3, and small α-specific EC3:EC3 and distinct β-specific EC3:EC3 and EC2:EC2 interactions. The intermolecular interfaces for these Pcdh trans dimers are extensive, burying between 3900 and 5200 Å2, revealing interaction surfaces populated by residues that vary among alternate α- and β-Pcdh isoforms, alongside some that are constant among alternate α- and β-Pcdh isoforms. We further show that mutation of interacting variable interfacial residues generates new trans binding specificities, demonstrating that these variable residues encode specificity within each Pcdh subfamily. Considering the conservation of the clustered Pcdhs from cephalopod invertebrates to man (Albertin et al., 2015), and the importance of Pcdh function to neural circuit assembly (Zipursky and Grueber, 2013; Chen and Maniatis, 2013; Kostadinov and Sanes, 2015), the atomic-level basis of isoform-specific Pcdh homophilic recognition revealed here represents a fundamental molecular mechanism required for normal neural circuit assembly.
Results
Structure Determination
Using a HEK-293 suspension-cell protein expression system, we produced a series of Pcdh ectodomains, each encompassing the EC1–4 trans interaction region. We produced both EC1–4 (Pcdhs α4, β6, β8) and EC1–5 (α7) proteins, and confirmed that these fragments formed dimers in solution by sedimentation-equilibrium analytical ultracentrifugation. The dimer dissociation constants (KDs) for α4EC1–4 and α7EC1–5 were 37 ± 8.2 nM (Table S1 and Figure S1) and 2.91 ± 0.55 μM respectively (Rubinstein et al, 2015). However, the much stronger binding observed for α4EC1–4 is beyond the normal limit of detection of ~0.5μM. Therefore, while it is clear that α4EC1–4 has a stronger dimerization affinity than α7EC1–5, it is unclear precisely how much stronger. The β-Pcdhs showed weaker binding, with respective KDs for β6EC1–4 and β8EC1–4 of 16.3 ± 2.08 μM and 24.0 ± 0.43 μM (Table S1 and Figure S1), similar to the previously published trans-binding affinities of γ (29–30 μM) and C-type (6–100 μM) Pcdh isoforms (Rubinstein et al., 2015). The functional significance of the differences in trans binding affinities among distinct Pcdh isoforms is unknown. However the Pcdh KDs are similar to those for trans homophilic binding between Dscam1 isoforms (1–2 μM; Wu et al., 2012), as well as classical cadherins (8–130 μM; Harrison et al., 2010; Vendome et al., 2014).
We determined crystal structures for these Pcdh ectodomain fragments—α4EC1–4, α7EC1–5, β6EC1–4 and β8EC1–4—each of which adopted dimeric conformations geometrically consistent with their functions in trans cell-cell recognition (Figure 1). We first determined the crystal structure of α7EC1–5 at 3.6 Å resolution, for which phases were determined by single-isomorphous replacement with anomalous scattering (SIRAS) with a platinum-derivative crystal (Table S2). Crystals of α4EC1–4, β6EC1–4 and β8EC1–4 all diffracted anisotropically with strong diffraction along the c*-axis (Figure S2), which was approximately parallel to the long-axis of the Pcdh molecule in each case. Each of these structures was determined by molecular replacement, using ellipsoidally truncated data with anisotropic diffraction limits of 3.1/3.4/2.4 Å, 3.6/4.3/2.9 Å, and 3.6/3.8/2.9 Å for α4EC1–4, β6EC1–4 and β8EC1–4, respectively (Figure S2, Table S2).
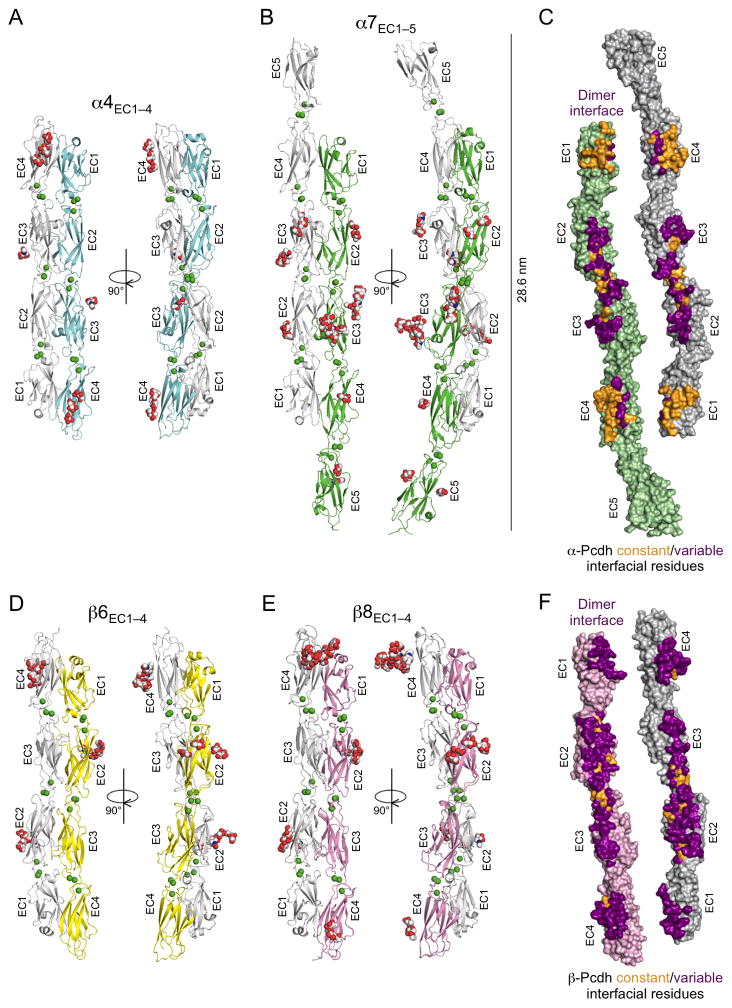
Figure 1. Crystal structures of the α- and β-Pcdh cell-cell recognition dimers.
A. Crystal structure of the α4EC1–4 dimer. The two EC1–4 protomers (colored cyan and grey) bind in a symmetrical anti-parallel configuration with EC1 interacting with EC4 and EC2 interacting with EC3. Bound calcium ions are shown as green spheres and glycans are shown as red, blue and white spheres.
B. The α7EC1–5 structure, protomers colored green and grey, shows a near identical EC1–4 mediated head-to-tail dimer to α4 (RMSD = 1.9 Å). The EC5 domains extend laterally, and are therefore not involved in the dimer interaction.
C. Surface representation of the two α7EC1–5 protomers, opened out to expose the dimer interface. Interfacial residues that are constant among α-Pcdhs are colored orange and those that vary among α-Pcdhs are colored purple. The interfacial residues are shown in detail in Figures 2 and 3.
D. The β6EC1–4 dimer, protomers colored yellow and grey, showing a similar EC1–4 mediated head-to-tail dimer to the α-Pcdh structures (RMSD to α4EC1–4 dimer = 4.7 Å).
E. The β8EC1–4 dimer (chains A and B from the crystal structure are shown), protomers colored pink and grey, is near identical to the β6EC1–4 dimer (RMSD = 1.6 Å)
F. Surface representation of the two β8EC1–4 protomers, opened out to expose the dimer interface. Interfacial residues that are constant among β-Pcdhs are colored orange and those that vary between β-Pcdhs are colored purple. The interfacial residues are shown in detail in Figures 2 and 4.
See also Figures S1–S3 and Tables S1–S4.
Overall structural features of Pcdh ectodomains
The four Pcdh ectodomain structures reported here are remarkably straight compared to classical cadherins (Figure S3A) and highly similar overall, with pairwise root mean square deviations for aligned Cα atoms (RMSDs) between individual protomers of 1.0–3.2 Å (Table S3). As expected, each comprises either four or five β-sandwich EC domains, arranged in tandem and connected by inter-domain linker regions (Figures 1 and S3), all of which bind three Ca2+ ions via the canonical cadherin Ca2+-binding motif as described previously (Rubinstein et al., 2015; Nicoludis et al., 2015). The individual protomers in the crystal structures are similar to the published monomeric EC1–3 structures of αC2, β1, γA8, γC3, and γC5, with RMSDs of 1.0–3.3 Å (Figures S3B–C; Rubinstein et al., 2015; Nicoludis et al., 2015) suggesting no significant conformational rearrangements on complex formation.
All four structures are decorated with N- and O-linked glycans on surface regions away from the trans dimer interfaces (Figure 1). Single O-mannose residues—an uncommon glycan that often modifies cadherin superfamily proteins (Vester-Christensen et al., 2013; Lommel et al., 2013)—were observed at positions 194 and 196 (α7 numbering) of EC2 in the α7, β6 and β8 structures. These glycans were previously identified by mass spectrometry in Pcdhs αC2, β1, γA8 and γC5, and S/T residues are conserved at these positions in most Pcdhs (Vester-Christensen et al., 2013; Rubinstein et al., 2015). The β8 structure also shows an additional O-mannose on the subsequent solvent exposed residue T199. Additionally α4 EC4 contains three O-mannoses on adjacent solvent exposed residues—T409, S411 and S413—and α7 is O-mannosylated on EC4 T409 and EC5 S449. The function of these O-mannose residues is currently unknown, yet it is remarkable that they appear to be spatially clustered on Pcdh ectodomain surfaces.
All structures show head-to-tail Pcdh trans recognition dimers
The structures of the trans dimers from the four different Pcdh isoforms are similar overall. They each reveal head-to-tail dimers, with partner molecules positioned as if emanating from adjacent cell surfaces (Figure 1). However, there are notable differences between the α- and β-EC1–4 Pcdh dimers, sufficient to provide family-wide recognition specificity between α- and β-Pcdhs. The RMSD between the α4EC1–4 and α7EC1–5 dimers is 1.9 Å, and the RMSDs between the β6EC1–4 and β8EC1–4 dimer structures range from 1.6–2.6 Å. In contrast, RMSDs between α- and β-Pcdh dimers are significantly larger, ranging from 3.7–4.7 Å (Figure S3D–J). This is due primarily to a difference in the relative rotations of the dimer-mate protomers in the α- and β-Pcdh trans dimers. Superimposing one protomer from each of the dimers reveals a family-specific ~30° difference in position of the dimer-partner molecule between the α- and β-Pcdh dimers (Figure S3K).
Each trans recognition dimer is composed of distinct sub-interfaces: large EC1:EC4 and EC2:EC3 interfaces are observed for both subfamilies; small EC3:EC3 interfaces distinct in α- and β-Pcdhs; and a small EC2:EC2 interface found only in β-Pcdhs. Altogether, large areas of molecular surface are buried in the trans dimer for each Pcdh structure: 4995 Å2 for α4EC1–4, 3904 Å2 for α7EC1–5, 4678 Å2 for β6EC1–4 and 5073–5228 Å2 for β8EC1–4.
The Pcdh head-to-tail trans interactions presented here are distinct from the classical cadherin trans interactions, which are mediated either by EC1 through a strand-swap mechanism or through the formation of a usually transient EC1–2-mediated X-dimer interface (Ciatto et al., 2010; Harrison et al., 2010) (Figure S3L). A crystal structure of a complex between non-clustered protocadherin proteins, cadherin-23 and protocadherin-15, revealed a heterophilic trans interface with an antiparallel orientation (Sotomayor et al., 2012), but the interfaces comprise only EC1 and EC2, and appear unrelated to the clustered Pcdhs (Figure S3L).
Conserved isoform-specific interface residues are likely specificity determinants
To help identify molecular regions important to establish specificities among α- and β-Pcdh isoforms, we produced sequence logos from multiple-sequence alignments of α- and β-Pcdh isoform-orthologs from numerous mammalian species (Tables S5 and S6). In the sequence logos, the height of each amino acid code indicates the relative frequency of that amino acid at that position in the multiple-sequence alignment, and the height of each letter stack represents the conservation level at that position. To focus on regions relevant to specificity, we extracted sub-logos depicting only interfacial residues (Figures 2D, 2G, 3E, and 4E). These show that some interfacial residues are conserved for all α or β isoforms; some residues vary among isoforms, but fail to show consensus among species; and some are both isoform-specific and also conserved among species. For example, interfacial residues 36 and 38 are QD in α8, but RA in other α-Pcdhs for >90% of vertebrate species (Figure 2D). Such isoform-specific species-conserved interfacial residues are highly likely to play a role in binding specificity, although those that are isoform-specific but not conserved among species could also play specificity roles in individual species.
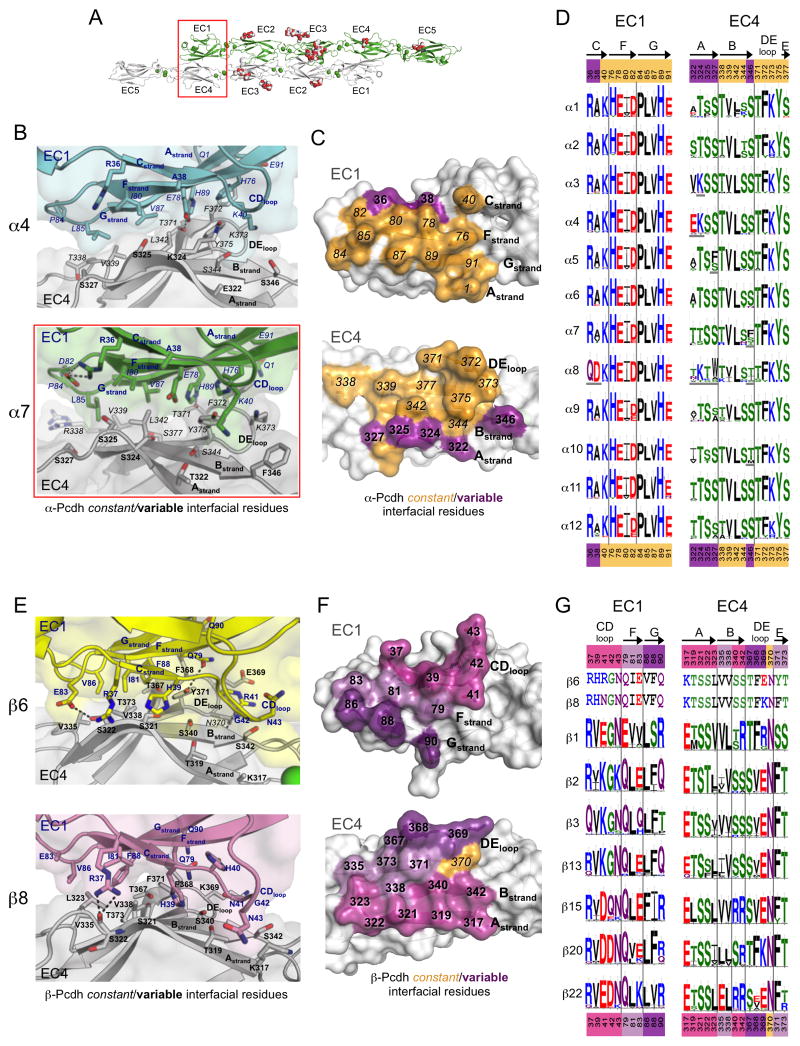
Figure 2. The EC1:EC4 interface is largely conserved amongst α-Pcdhs and highly diverse amongst β-Pcdhs.
A. The α7EC1–5 dimer structure with the EC1:EC4 interface highlighted (red box).
B. Close up of the EC1:EC4 interface in the α4EC1–4 (top, EC1 from one protomer is colored cyan the EC4 from the other protomer grey) and α7EC1–5 (bottom, EC1 green, EC4 grey) structures. Side chains are shown for all residues where the side chain contributes to the dimer interface.
C. Surface representation of α7 EC1 and EC4 interfacial regions from an opened out dimer, highlighting the putative specificity-determining residues. Interfacial residues are colored orange if they are conserved amongst all α-Pcdhs and purple if they show conserved differences in one or more of the 12 α-Pcdhs (see D).
D. α-Pcdh sequence logos of EC1:EC4 interface residues for each of the 12 mouse α-Pcdh isoforms, generated from sequence alignments of isoform orthologs (species used are listed in Table S5). Conserved isoform-specific residues are underlined. Secondary structure is indicated, and colors of residue numbers above and below correspond to part (C).
E. Close up of the EC1:EC4 interface in the β6EC1–4 (top, EC1 yellow, EC4 grey) and β8EC1–4 (bottom, EC1 pink, EC4 grey) structures.
F. Surface representation of β8 EC1 and EC4 interfacial regions from an opened out dimer, highlighting the putative specificity-determining residues. Interfacial residues are colored orange if they are conserved amongst all β-Pcdhs and shades of purple if they differ in one or more of the 22 mouse β-Pcdhs. Residues in EC1 and EC4 are colored matching shades of purple to show their predominant interaction.
G. β-Pcdh sequence logos of EC1:EC4 interface residues are shown for a subset of mouse β-Pcdh isoforms (species used are listed in Table S6). The mouse β6 and β8 interface residues are shown above the logos. Secondary structure is indicated on top, and colors of residue numbers correspond to part (F).
See also Figure S6 and Tables S4–S6.
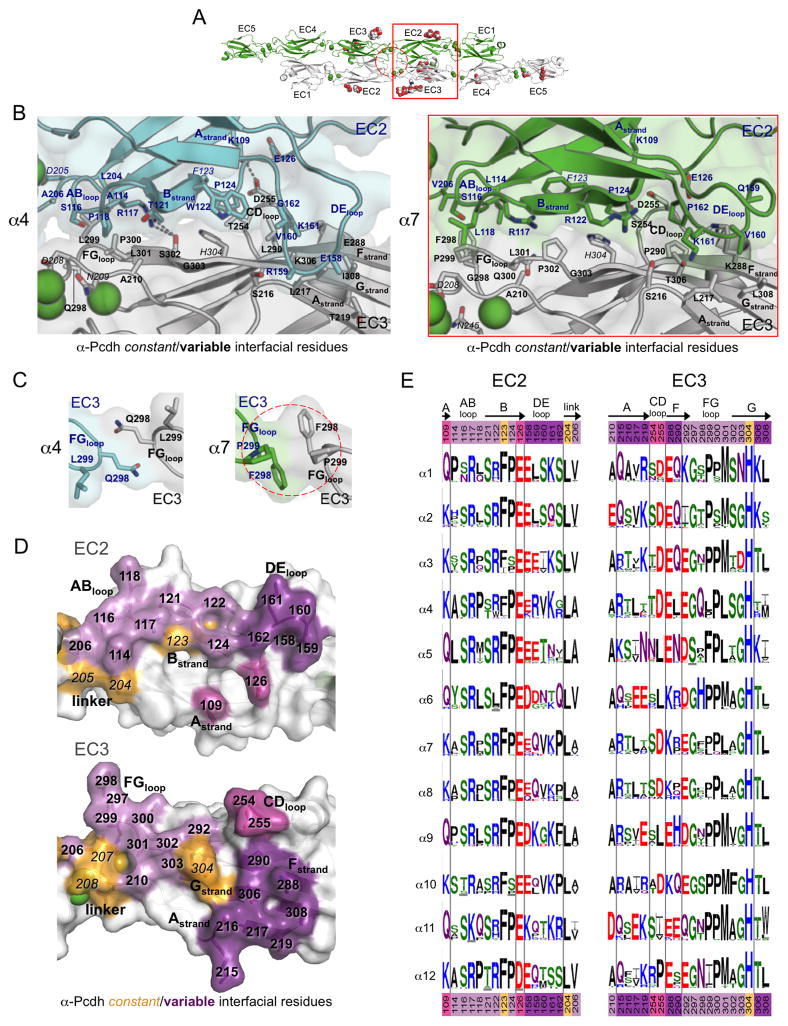
Figure 3. Diversity in the EC2:EC3 and EC3:EC3 interfaces of α-Pcdhs.
A. The α7EC1–5 dimer structure, with the EC2:EC3 interface (red box) and the EC3:EC3 interface (dashed red circle) highlighted.
B. Close-up view of the EC2:EC3 interface in α4EC1–4 (left, EC2 cyan, and EC3 grey) and α7EC1–5 structures (right, EC2 in green, EC3 in grey). Side chains are shown for all residues where the side chain contributes to the dimer interface. Bound calcium ions are shown as green spheres.
C. Close-up views of the EC3:EC3 interface in the α-Pcdh dimer structures. EC3 FG-loop residue 298 makes a symmetrical contact with itself in both the α4 (left, cyan and grey protomers) and α7 (right, green and grey protomers) dimers.
D. Surface representation of α7 EC2 and EC3 interfacial regions from an opened out dimer, highlighting the putative specificity-determining residues. Interfacial residues are colored orange if they are conserved amongst all α-Pcdh isoforms and shades of purple if they differ in one or more of the 12 α-Pcdhs. Matching shades of purple denote EC2 and EC3 interacting residues.
E. α-Pcdh sequence logos of EC2 and EC3 interface residues (species used are listed in Table S5). Secondary structure is indicated, and colors of residue numbers at top and bottom correspond to part (D).
See also Tables S4–S5.
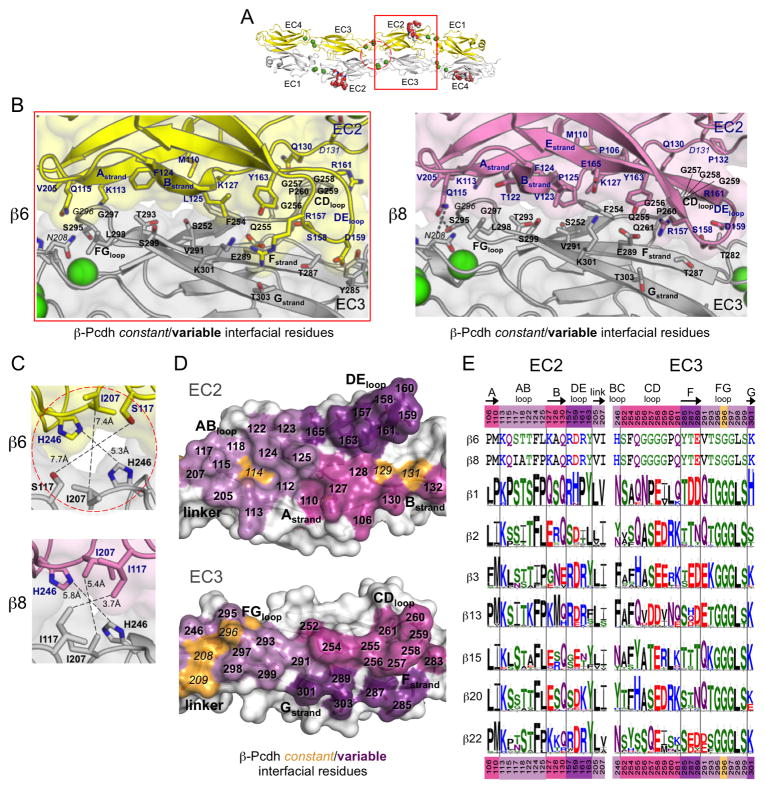
Figure 4. Diversity in the EC2:EC3 and center of symmetry interfaces of β-Pcdhs.
A. The β6EC1–4 dimer structure, with the EC2:EC3 interface (red box) and the center of symmetry interfaces (dashed red circle) highlighted.
B. Close-up view of the EC2:EC3 interface in β6EC1–4 (left, EC2 yellow, EC3 grey) and β8EC1–4 structures (right, EC2 pink, EC3 grey). Side chains are shown for all residues where the side chain contributes to the dimer interface. Bound calcium ions are shown as green spheres.
C. Close-up views of the center of symmetry interfacial regions in the β6 (top, protomers yellow and grey) and β8 (bottom, protomers pink and grey) dimer structures.
D. Surface representation of β8 EC2 and EC3 interfacial regions from an opened out dimer, highlighting the putative specificity-determining residues. Interfacial residues are colored orange if they are conserved amongst all β-Pcdh isoforms and shades of purple if they differ in one or more of the 22 β-Pcdhs.
E. β-Pcdh sequence logos of key EC2 and EC3 interface residues are shown for a subset of the mouse β-Pcdh isoforms (species used are listed in Table S6). The mouse β6 and β8 interface residues are shown above the logos. Secondary structure is indicated, and colors of residue numbers at top and bottom correspond to part (D).
See also Tables S4 and S6.
The “opened-up” depictions of the α7EC1–5 and β8EC1–4 homodimer interfaces (Figures 1C and F), reveal that variable residues—likely specificity residues—generally make intermolecular contacts with other variable residues on the partner molecule, while constant residues generally contact other constant residues. Constant residues contribute ~40–50% of the buried surface area (BSA) for α-Pcdhs, but only ~5% of the BSA in the β-Pcdh dimers (Table S4).
α-Pcdh EC1:EC4 interface
In the α-Pcdh structures, the EC1:EC4 interface (Figures 2A–D) buries ~975 Å2, thereby contributing ~1950 Å2 BSA overall via the two copies of EC1:EC4 present in each of the dimers (Table S4). The EC1:EC4 interface runs over about half the length of each EC domain, involving the N-terminal end of the EC1 C, F, and G-strands and the C-terminal end of the A, D, E, and B-strands of EC4. Constant interactions predominate, accounting for ~74–88% of the EC1:EC4 interface BSA (Table S4). These include a cluster of conserved aliphatic interface residues from the EC1 FG region (I80, P84, L85, and V87) and the B and D-strands of EC4 (T338, V339, L342, and T371) (Figure 2B–C, left side), as well as conserved interactions between constant polar and charged residues from the EC1 C, F, and G-strands (K40, H76, E78, H89 and E91) and the EC4 DE-loop (F372, K373, and Y375) (Figures 2B–C, right side).
Variable elements of the EC1:EC4 interface are localized to the edge of the interface, where variable residues from the C-strand of EC1 pack near variable residues of the EC4 A-strand. Additionally the α4 structure reveals an isoform-specific salt bridge between constant residue E78 and variable residue K324 (Figures 2B and 2D).
β-Pcdh EC1:EC4 interface
The β-Pcdh EC1:EC4 interface involves similar residues and structural elements to the α-Pcdh EC1:EC4 interface, although there is a greater contribution to the interface from the EC1 C-strand and CD-loop, and no contribution from the EC1 A-strand (Figure 2E). The β-Pcdhs have a slightly larger EC1:EC4 interface than the α-Pcdhs, contributing 2202 and 2102–2287 Å2 BSA respectively to the β6 and β8 trans dimers. In contrast to α-Pcdhs, almost all of the interface residues vary among mouse β isoforms (Figure S4). All of these variable interface residues show isoform-specific conservation, underscoring their likely function in recognition specificity (Figures 2F and 2G).
β6 and β8 are the most closely related β-Pcdhs with 92% sequence identity in their EC1–4 regions. Consequently their EC1:EC4 interfaces (Figure 2E) are nearly identical, with only three differences in the identity of the interfacial residues, two of which interact—R/N41:E/K369—explaining the previously demonstrated role in β6/β8 specificity for residue 41 (Rubinstein et al., 2015).
α-Pcdh EC2:EC3 interface
The EC2:EC3 interface is highly variable for all three Pcdh families and appears to represent the primary specificity region for α-Pcdhs, with variable residues accounting for 88–92% of the EC2:EC3 BSA (Table S4). Taken as a whole, the symmetric contiguous EC2–3:EC2–3 interface region buries 2983 Å2 in the α4 dimer and 1975 Å2 in the α7 dimer, involving contacts between the ABED-face of EC2 and the AGFC-face of EC3 (Figure 3B).
The majority of EC2:EC3 interfacial area in both α-Pcdh structures is provided by interaction of the EC2 AB-loop, the EC3 FG-loop and the EC2–3 linker region (colored pale purple and orange in Figure 3D). Aside from the conserved calcium-coordinating linker residues (D205, D208 and N209), over half of the surface residues in these contacting regions differ between α4 and α7 (Figure 3B and 3E). The EC2-AB-loop:EC3-FG-loop interaction is predominantly hydrophobic, except for EC2 AB-loop residue R117, which hydrogen bonds with EC3 FG-loop residue S302 in α4 and with Q300 in α7. This region contains one notable α-Pcdh constant interaction between H304 (EC3 G-strand) and F123 (EC2 B-strand) (Figure 3D–E).
There are two additional interaction regions that make up the EC2:EC3 interface. First, there is a small EC2 A-strand:EC3 CD-loop contact, which, in both α4 and α7, is a salt bridge formed between K109 and D255. Second, there is an EC2 DE-loop:EC3 AGF-sheet interaction, which is the most divergent region of the EC2:EC3 interface since, as well as being highly variable in sequence (Figure 3E), the EC2 DE-loop also adopts different conformations in the α4 and α7 structures, resulting in differing interactions with the EC3 AGF-sheet surface (Figure 3B). Poor electron density for the DE-loop regions in both structures suggest that the EC2 DE-loop is more mobile than other α-Pcdh interfacial elements. Interestingly, a number of the contacts in this interface appear to be main-chain mediated. The role this region could play in α-Pcdh specificity is therefore unclear.
β-Pcdh EC2:EC3 interface
The β-Pcdh EC2:EC3 interface, between the EC2 ABED-face and EC3 GFC-face, involves both residues in common with the α-interface as well as residues that are distinct (Figure 4B). It is highly diversified amongst β isoforms, with 92–93% of the BSA contributed by variable residues (Figure 4D–E, Figure S4 and Table S4). However the β6 and β8 isoforms differ only in the identity of one EC2:EC3 interface residue, L/P125 in EC2. The EC2:EC3 interface buries 2477 Å2 in the β6 dimer and 2759–3027 Å2 in the β8 dimers.
The β-Pcdh EC2 AB-loop and EC3 FG-loop form interactions similar to those observed in the α structures; although the contacts are not as extensive in β-Pcdhs because the EC3 FG-loop is two residues shorter and the apex of the loop consists of two glycine residues in β6 and β8 (three in most other β isoforms). Additionally, unlike α-Pcdhs, the EC3 CD-loop makes extensive contacts in the β6 and β8 structures (Figure 4B). However, the EC3 CD-loop contains four glycine residues in β6 and β8, but not in any other β-Pcdh (Figure 4E), and therefore may not be able to insert so readily between the EC2 B-strand and DE-loop in other β isoforms. The EC2 DE-loop also engages EC3 F and G-strand residues, like in the α’s, although the DE-loop is not so closely packed to the EC3 surface resulting in more side chain:side chain interactions.
Small EC2:EC2 and EC3:EC3 interfaces at the trans-dimer 2-fold
For the α-Pcdhs, a small region of EC3:EC3 intermolecular interaction is formed by symmetry-related contacts between the side chains of FG-loop residue 298 from each protomer of the trans dimer (Figure 3C). In α7 P299 also contributes to this interaction alongside the hydrophobic F298:F298 interaction. Notably the EC3 FG-loop is two residues shorter in β-Pcdhs, too short to make contact at the 2-fold axis.
The β-Pcdh ectodomain structures indicate that small interaction regions at the trans dimer two-fold axis—involving the self-interactions of the EC3 BC-loop residue 246 and the EC2 AB-loop residue 117—will occur when these residues are large, as is the case for some but not all β-Pcdhs (Figure 4C). Residue H246 does not make self-contacts in the β6 and β8 dimers. However, residue 246 is a phenylalanine or tyrosine in 12 of the 22 mouse β-Pcdhs, which are both large enough to self-interact in the trans dimer, and therefore contribute to the binding energy and potentially to specificity. Additionally residue I117 self-interacts in the β8 crystal structure, but the smaller S117 in the β6 structure does not. Residue 117 is also an isoleucine in β13, and an asparagine, which could also self-interact, in 10 other mouse β-Pcdhs.
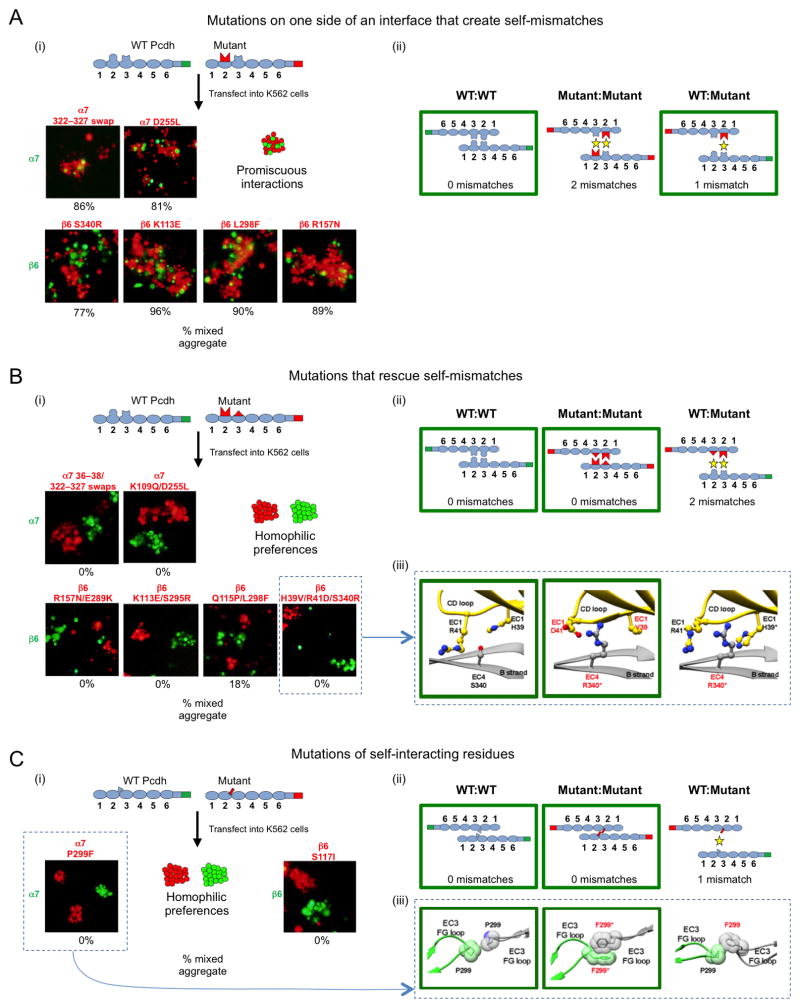
Generating new homophilic Pcdh specificities via mutation of interface residues
In order to identify residues important for recognition specificity, we performed mutagenesis experiments both in cells and in silico. We used sequence logo analysis (Figures 2–4) to identify interface residues that are conserved across species in individual Pcdh isoforms, and then evaluated residue interactions across the trans interface. We chose pairs (or in two cases, clusters) of interacting residues that had significant differences in chemical character in different isoforms and asked whether we could change binding preferences by replacing residues of one isoform with those of another. Binding preferences were determined using the K562 cell aggregation assay (Schreiner and Weiner, 2010; Thu et al., 2014). Cells were transfected with either mCherry labeled mutants or mVenus labeled WT Pcdhs, mixed, and allowed to aggregate with mild shaking. We then assessed whether the mutant and WT cell populations formed separate aggregates—which would indicate that the mutant Pcdh bound to itself more strongly than to the WT protein—or alternatively formed mixed aggregates, indicating that the mutant specificity was functionally indistinguishable from the parental WT Pcdh.
We first tested the effect of mutating only one side of an interacting pair/cluster. As shown in Figure 5Aii, when only one side of an interface is mutated, there are three distinct complexes that could form in the mixed cell assays involving WT and mutant protein: two corresponding to homophilic Pcdh preferences (WT:WT and mutant:mutant) and one representing the heterophilic preference (WT:mutant). In all cases tested (Figure 5Ai), mixed aggregates were observed, showing that no preference can be distinguished for binding between cells expressing mutant and WT protein. This is unsurprising because WT:mutant interfaces have fewer expected mismatches than mutant:mutant interfaces so that the cells expressing mutant protein prefer to adhere to cells expressing the WT protein (Figure 5Aii).
Figure 5. Pcdh homophilic recognition depends on trans-interacting variable residues.
A. Single-sided α7 or β6 interface mutants were assayed for binding specificity in a mixed cell aggregation assay with wild-type (WT) α7 or β6. (i) Schematic of WT mVenus-tagged Pcdh and mutant mCherry-tagged Pcdh (top). Representative images of mixed cell aggregation assays are shown for each mutant (bottom). In all cases mixed aggregates were observed. The percentage of mixed (red/green) aggregates, from three independent experiments, is given below each image. (ii) Schematic illustrations of the three possible Pcdh trans dimers that can form between the interacting cells. In each case, the number of expected mismatched residues is indicated. The green boxes highlight the interactions that mediate the observed aggregates in panel (i).
B. Complementary (two-sided) mutations of trans-interacting residues were introduced in α7 or β6, and binding specificity was assessed in the mixed cell aggregation assay. (i) Separate aggregates of WT and mutant Pcdh expressing cells were formed in all cases. (ii) The schematic shows that the putative mutant:WT interaction would be expected to contain two mismatched residue interactions, explaining why mixed aggregates were not observed. (iii) Structural models for the two homophilic (WT:WT and mutant:mutant) interactions that mediate the observed cell aggregates, and the putative heterophilic (WT:mutant) interface, which does not form, are shown for the β6 WT with β6 H39V/R41D/S340R mutant case.
C. Mutations of residues that interact symmetrically between the two protomers. (i) Separate aggregates of WT and mutant cells were formed in all cases indicating that a new homophilic Pcdh has been generated. (ii) As in (B), homophilic preferences of WT and mutant proteins can be explained by the presence of mismatched residue interactions in the WT:mutant complex. (iii) Structural models are shown for the potential α7 WT and α7 P299F mutant complexes.
See also Figure S5.
Figure 5B summarizes the results observed when complementary mutations are introduced on both sides of the recognition interface. Comparison of the number of expected mismatches in each of the three possible complexes (Figure 5Bii) shows that homophilic mutant:mutant interactions contain no mismatches whereas WT:mutant interactions generate two mismatches. Consequently, two homophilic aggregates are formed (Figure 5Bi). These experiments demonstrate that replacing just a single interacting pair is sufficient to abrogate binding to the WT protein, and to generate a new Pcdh that binds homophilically. In order to assess the likely structural impact of the new complementary residues that were introduced, we used the side chain modeling program SCAP (Xiang and Honig, 2001) to generate structural models of heterophilic (WT:mutant) and homophilic (mutant:mutant) trans dimers for each mutant (see Experimental Procedures for details). Generally, models of the heterophilic WT:mutant interactions showed mismatches and clashes while models of the homophilic mutant:mutant interactions were compatible between interacting residues (Figures 5B–C and S6A–F). In several cases models of WT:mutant interactions showed electrostatic incompatibility, while models of mutant:mutant interactions showed electrostatic complementarity between the interacting residues. This can be seen in the β6 EC1:EC4 interface mutant H39V/R41D/S340R (Figure 5Bii), the α7 EC2:EC3 interface mutant K109Q/D255L (Figure S5B), as well as two β6 EC2:EC3 interface mutants K113E/S295R and R157N/E289K (Figure S5C–D). Whereas for the β6 Q115P/ FL298F mutant, modeling suggested that the homophilic cell aggregation specificity observed between WT and mutant resulted from Van der Waals clashes between the mutant and WT residues, making heterophilic interaction unfavorable (Figure S5E).
Finally, constructs involving single mutations of self-interacting residues preferred to aggregate homophilically (Figure 5Ci). Similar to the results shown in Figure 5B, no mismatches are expected for the homophilic mutant:mutant interaction, whereas the WT:mutant interaction is inhibited by mismatches (Figure 5Cii). For the α7 mutant, a structural model of the WT:mutant complex suggested that mismatches arise due to Van der Waals clashes between the interacting P299:F299 residues, while F299:F299 interactions in the mutant:mutant complex showed Van der Waals complementarity (Figure 5Cii).
Overall, these results demonstrate that residues in the crystallographically determined EC1–4 interfaces, which are isoform-specific as determined by the logo analysis, function as specificity-determining residues for α- and β-Pcdh isoforms. Not only do these residues interact favorably across the homophilic interface, but they also ensure that heterophilic binding will not occur, primarily through steric and electrostatic repulsion in putative heterophilic interfaces.
Discussion
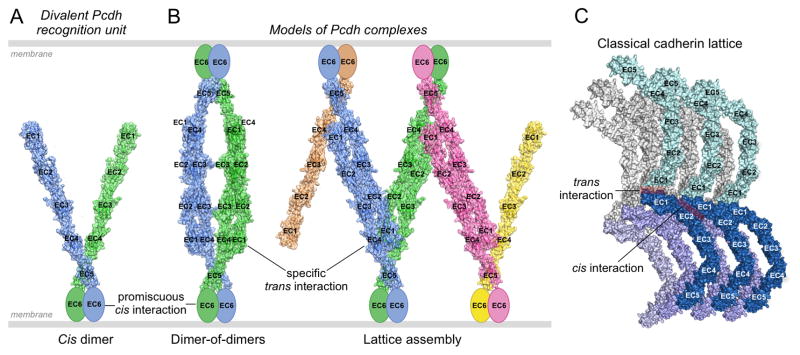
There is strong evidence suggesting that Pcdhs form EC6 domain-mediated cis dimers on cell surfaces (Rubinstein et al., 2015). Cis associations between isoforms appear to be promiscuous (Schreiner and Weiner, 2010; Thu et al., 2014), enabling the formation of a highly diverse repertoire of recognition units comprising any pair of Pcdh isoforms expressed by a neuron (except α/α pairs). It is this repertoire of bivalent recognition units, each containing two highly specific homophilic trans binding regions, that underlies recognition specificity between vertebrate neurons. A model of a bivalent cis dimer is shown in Figure 6A highlighting the two ectodomain arms, which need not be identical, and mediate trans homophilic recognition through their EC1–4 domain regions. Each structure presented in this work represents the bound form of one arm of the bivalent recognition unit; it is the homophilic interface formed by each arm that is responsible for the trans binding specificity of each recognition unit. Below we discuss the molecular basis and the evolution of the homophilic properties of individual Pcdh isoforms, compare the molecular logic of Pcdh and Dscam1 mediated recognition, and consider the implications of Pcdh architecture in self-recognition and non-self discrimination by neurons.
Figure 6. Pcdh-mediated recognition units and possible binding modes between opposed cell surfaces.
A. Model of a cis-dimeric Pcdh recognition unit. The dimerization interface is located on EC6 which is depicted as an ellipse since no structure for this domain is available. Each of the two arms is taken from the 5-domain structure of α7EC1–5. The angle between them is arbitrary. The two arms are depicted in different colors since they may correspond to different isoforms.
B. Models of possible Pcdh recognition complexes. The left panel depicts a dimer of recognition dimers yielding a tetrameric complex. Although the two arms are depicted here as different, the only tetramer detected so far (in solution, Rubinstein et al., 2015) has both arms identical. The right panel depicts the initiation of a linear zipper formed from different recognition units on each membrane that contain one common isoform. Mismatched isoforms, expressed by one cell but not the other, are represented in pink and yellow, and in principle would terminate growth of the intercellular Pcdh zipper.
C. Classical cadherin intercellular assembly, corresponding to the extracellular structure of adherens junctions, mediated by cis and trans interactions.
Determinants of binding specificity
We have described crystal structures of Pcdhs α4EC1–4, α7EC1–5, β6EC1–4, and β8EC1–4, each depicting cognate homophilic trans dimer complexes. These structures reveal canonical modes of trans interaction that are similar but distinct for α- and β-Pcdhs. They each bind in a head-to-tail orientation, with interfaces comprising EC1:EC4, EC2:EC3, and small α-specific EC3:EC3 and distinct β-specific EC3:EC3 and EC2:EC2 interactions. These structures explain the results of prior mutagenesis studies (Schreiner and Weiner, 2010; Thu et al., 2014; Rubinstein et al., 2015), and also provide new insights into disease-associated Pcdh variants in humans (Figure S6) (Iossifov et al., 2014). Moreover, new structure-guided mutants reported here show that trans interface residues that vary among isoforms, but are conserved in a given Pcdh isoform across multiple species, play important roles in homophilic specificity. Together with our previous mutagenesis results (Rubinstein et al., 2015), these data show that the combined specificity of the EC1:EC4 and EC2:EC3 interfaces, along with the smaller EC3:EC3 interface in α-Pcdhs, and the EC2:EC2, and potentially the EC3:EC3 interface(s) in β-Pcdhs, underlie the strict homophilic recognition specificities of Pcdh isoforms. Moreover similar effects of domain deletions, swaps between isoforms, and point mutations, are suggestive of a related mechanism for γ- and C-type Pcdhs (Rubinstein et al., 2015). Indeed the structure of a monomeric EC1–3 fragment of Pcdh γA1 showed an EC2:EC3 interaction in the crystal lattice (Nicoludis et al., 2015), which is similar overall to the EC2:EC3 interfaces described here for α- and β-Pcdhs (Figure S3D–J).
We used the observation that numerous Pcdh interface residues show isoform-specific conservation as the basis of functional studies in which we mutated these conserved residues—alone or along with their trans-interacting partner residues—to determine if mutation of the interacting pairs were sufficient to change Pcdh binding specificities. Most single mutants (Figure 5A), introducing one or more mutations to one side of the interface to create residue/residue mismatches in the interface, yielded WT and mutant transfected cell populations that formed mixed aggregates—indicating they are insufficient to change binding preferences. In contrast, most double mutants, which introduced new complementary intermolecular interactions—led to the formation of separate cell aggregates, indicating that a new Pcdh had been generated that binds homophilically, but not to the parent WT protein from which it was derived (Figure 5B). Thus, interface residues identified by their isoform-specific conservation play roles both in homophilic specificity, and in the ability to distinguish between self (e.g., a double mutant) and non-self (the parental WT Pcdh).
A recent study showed that the bacterial ParD-ParE toxin-antitoxin system evolved specific protein-protein interactions through promiscuous intermediate proteins, rather than through non-interacting intermediate proteins that were rescued by compensatory mutations (Aakre et al., 2015). Interestingly, the mutagenesis results presented here, as well as results from domain-shuffling mutagenesis in our previous study (Rubinstein et al., 2015), show that mutating only one side of the Pcdh interface, e.g. EC1 without its trans-interacting partner residues in EC4, typically resulted in a functional isoform with promiscuous interactions, able to recognize both self and parent WT isoforms. This may at least partly mirror the process by which Pcdhs diversified in evolution, where initial mutations could result in promiscuous interactions, and a second interacting mutation could then generate a novel highly specific homophilic interaction.
Comparing the molecular logic of recognition for Pcdhs and Dscam1
While the structures of Pcdhs are different from those of the Dscam1 proteins, there appear to be common features in the character of Pcdh and Dscam1 domain:domain interactions. Recognition between Dscam1 isoforms is mediated by three self-to-self Ig domain interface regions, Ig2:Ig2, Ig3:Ig3 and Ig7:Ig7, and homophilic binding requires identical matches for all three domains (Zipursky and Grueber, 2013). A truncated Dscam1 molecule containing only the N-terminal horseshoe structure that includes the Ig2:Ig2 and Ig3:Ig3 interfaces was monomeric (Meijers et al., 2007). Additionally, high-throughput binding experiments with Dscam1 protein isoforms showed that matches in all three specificity domains were required for binding in the vast majority of cases (Wojtowicz et al., 2007). For Pcdhs, EC1–3 fragments do not dimerize in solution despite the fact that they contain the regions necessary for the EC2:EC3 recognition interface to form (Rubinstein et al, 2015; Nicoludis et al., 2015). In addition, domain-shuffling experiments with Pcdh chimeras showed that matches in both EC1:EC4 and EC2:EC3 interfaces are required to mediate aggregation in K562 cell aggregation assays (Rubinstein et al., 2015). Thus for Pcdhs, as for Dscam1 isoforms, homophilic specificity derives from the combination of all variable interface regions. This feature is essential if heterophilic trans dimerization is to be avoided. Specifically, if sub-regions of an interface could dimerize on their own then binding could occur independent of the nature of the remainder of the interface; this would, in principle, allow for heterophilic binding.
A major difference between Dscam1 and Pcdh isoforms lies in the fact that in the former, the Ig domains of individual Dscam1 isoforms are combined from alternative exons within the Dscam1 gene so that the product of 12 distinct Ig2 domains, 48 distinct Ig3 domains and 33 distinct Ig7 domains yields 19,008 distinct isoforms. In contrast, the interacting EC domains in Pcdhs are encoded within a single large exon chosen by alternative promoter choice; thus there is no combinatorial effect to generate the dramatic diversity characteristic of Dscam1 proteins. For this reason, Pcdhs have evolved a different logic of neuronal recognition that is not dependent on the existence of a large number of different isoforms.
Relating Pcdh structure to neuronal recognition
We previously described a phenomenon we termed “interference” whereby even a single mismatched isoform between two cells precludes Pcdh-mediated cell recognition, even when the other expressed isoforms are identical (Thu et al., 2014). For example, a test cell population expressing 5 different Pcdhs can efficiently co-aggregate with a second cell population expressing the same set of 5. However, if the second cell population expresses only 4 of the 5 isoforms co-aggregation is not observed (Thu et al. 2014). As we discussed, interference makes it possible for the smaller number of total Pcdh isoforms in vertebrates to mediate neuronal self-avoidance as effectively as the much larger number of Dscam isoforms in arthropod invertebrates (Thu et al., 2014).
We proposed a molecular mechanism for interference based on dimeric Pcdh recognition units shown in Figure 6A (Rubinstein et al., 2015). Figure 6B shows two ways that these recognition units can interact. In the left panel they form a dimer of dimers, which yields the tetramers observed in solution biophysics studies (Rubinstein et al., 2015). The right panel illustrates a model whereby a bivalent recognition unit on one cell surface binds to two units on the opposing surface, leading to a zipper-like assembly whose growth is terminated by incorporation of a Pcdh isoform not shared between the interacting cell surfaces (Rubinstein et al., 2015). Monte Carlo simulations of the effects of such Pcdh mismatches reveal a profound effect on the size of the assembly, even for a single isoform mismatch (Rubinstein et al., 2015). The model posits both that formation of a large assembly generates a signal that activates downstream processes, and that this signal can only be generated in the presence of an apposed neuronal surface with an identical Pcdh isoform repertoire—the natural representation of “self”.
Although the mode of trans interaction differs between Pcdhs and classical cadherins, both molecular families are characterized by the formation of cis and trans self-interactions. The notion of a signal generated by an ordered lattice is consistent with the well-characterized self-assembly of type I classical cadherin ectodomains, which form the ordered extracellular superstructure of adherens junctions through combined cis and trans interactions—but only in the presence of an apposed cell expressing the cognate cadherin (Harrison et al., 2011; Figure 6C). The type I classical cadherin lattice is formed through a cooperative transition involving adhesive trans interactions and much weaker cis interactions involving domains EC2 and EC3 that only become significant upon cell-cell contact (Wu et al., 2012). In contrast, the cis interactions in Pcdhs are mediated primarily by EC6 and are strong enough to be observed in solution, even in the absence of trans interactions. We note that the formations of a zipper or 2D array through combined non-overlapping cis and trans interactions has been suggested as a signaling mechanism for numerous immunoglobulin superfamily cell adhesion molecules such as NCAM, nectin-like proteins, and receptor protein tyrosine phosphatase μ (for review see Aricescu and Jones, 2007).
While the zipper model for Pcdh signaling has not been experimentally validated, it arises naturally from the crystal structures and from the compelling evidence for the formation of promiscuous cis dimers mediated by EC6 (Rubinstein et al., 2015). Future structures, biophysical measurements and assays of cluster size in a cellular context should determine whether the model provides a molecular basis for understanding the ability of neurons to distinguish self from non-self.
Experimental Procedures
Protein production and crystallography
Pcdh protein fragments were expressed in suspension HEK293 cells (Invitrogen). The α7EC1–5 crystal structure was solved using the SIRAS technique with a platinum-derivative crystal, and all other structures were solved by molecular replacement.
Sedimentation equilibrium analytical ultracentrifugation
Proteins were diluted to an absorbance at 10 mm and 280 nm of 0.65, 0.43 and 0.23 absorbance units. All samples were run at four speeds, 9000, 11000, 13000 and 15000 rpm, at 25°C. Measurements were taken at 25°C, and detection was by UV at 280 nm. HeteroAnalysis, University of Connecticut (www.biotech.uconn.edu/auf) was used calculate the dimeric KD.
Co-aggregation assay to test trans binding specificity
Cell aggregation assays were performed as previously described in Thu et al., 2014. mCherry- or mVenus-tagged wild type or mutant Pcdh expression constructs were transfected into K562 cells (human leukemia cell line, ATCC CCL243). Transfected cells were mixed after 24 h by shaking for 1–3 h. Cell aggregates were then imaged.
Structural modeling
Models of homophilic complexes formed by both the WT and mutant isoforms, as well the heterophilic dimer between mutant and WT isoforms, were generated using the crystal structures of α7, α4 and β8 as structural templates. Side chains of mutated residues were first modified to alanine and then modeled using SCAP with default parameters (Xiang and Honig, 2001). Importantly, the three models were generated using the same procedure and therefore, there was no bias towards homophilic or heterophilic models.
Supplementary Material
Acknowledgments
We thank Igor Kourinov and Surajit Banerjee for help with synchrotron data collection at the APS NE-CAT 24-ID-C/E beamlines, supported by NIH P41 GM103403. We thank Chaim Schramm (Columbia) for help with preparing the sequence logo analysis. We thank Wayne Hendrickson (Columbia) and Michael Sawaya (UCLA) for helpful discussions on handling X-ray diffraction anisotropy. This work was supported by a National Science Foundation grant to B.H. (MCB- 1412472), an NIH grant to L.S. (R01GM062270), and a joint NIH grant to T.M. and L.S. (R01GM107571).
Footnotes
Accession numbers
Atomic coordinates and structure factors have been deposited to the protein data bank with accession codes 5DZW, 5DZV, 5DZX and 5DZY for α4EC1–4, α7EC1–5, β6EC1–4 and β8EC1–4 respectively.
Author contributions
K.M.G., R.R., C.A.T., T.M., B.H. and L.S. designed experiments, analyzed data and wrote the paper. F.B., S.M. and K.M.G. cloned, expressed, purified and crystallized all proteins. K.M.G. determined the crystal structures. S.M. and F.B. performed the site-directed mutagenesis. R.R. conducted the sequence and structural analysis of specificity. C.A.T. and C.R. performed and analyzed the cell aggregation experiments. G.A. performed and analyzed the analytical ultracentrifugation experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aakre CD, Herrou J, Phung TN, Perchuk BS, Crosson S, Laub MT. Evolving New Protein-Protein Interaction Specificity through Promiscuous Intermediates. Cell. 2015;163:594–606. doi: 10.1016/j.cell.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JR, Edsinger-Gonzales E, Brenner S, Ragsdale CW, Rokhsar DS. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature. 2015;524:220–224. doi: 10.1038/nature14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricescu AR, Jones EY. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Curr Opin Cell Biol. 2007;19:543–50. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Chen WV, Alvarez FJ, Lefebvre JL, Friedman B, Nwakeze C, Geiman E, Smith C, Thu CA, Tapia JC, Tasic B, et al. Functional significance of isoform diversification in the protocadherin gamma gene cluster. Neuron. 2012;75:402–9. doi: 10.1016/j.neuron.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WV, Maniatis T. Clustered protocadherins. Development. 2013;140:3297–3302. doi: 10.1242/dev.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciatto C, Bahna F, Zampieri N, VanSteenhouse HC, Katsamba PS, Ahlsen G, Harrison OJ, Brasch J, Jin X, Posy S, et al. T-cadherin structures reveal a novel adhesive binding mechanism. Nat Struct Mol Biol. 2010;17:339–47. doi: 10.1038/nsmb.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Garrett AM, Schreiner D, Lobas MA, Weiner JA. γ-protocadherins control cortical dendrite arborization by regulating the activity of a FAK/PKC/MARCKS signaling pathway. Neuron. 2012;74:269–76. doi: 10.1016/j.neuron.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Lin C, Meng S, Wang X. Proteomics analysis reveals overlapping functions of clustered protocadherins. Mol Cell Proteomics. 2010;9:71–83. doi: 10.1074/mcp.M900343-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Hamada S, Kumode Y, Esumi S, Katori S, Fukuda E, Uchiyama Y, Hirabayashi T, Mombaerts P, Yagi T. The protocadherin-alpha family is involved in axonal coalescence of olfactory sensory neurons into glomeruli of the olfactory bulb in mouse. Mol Cell Neurosci. 2008;38:66–79. doi: 10.1016/j.mcn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Hirabayashi T, Kondo T, Inoue K, Esumi S, Okayama A, Hamada S, Yagi T. Constitutively expressed Protocadherin–α regulates the coalescence and elimination of homotypic olfactory axons through its cytoplasmic region. Front Mol Neurosci. 2012;5:97. doi: 10.3389/fnmol.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Chen Y, Matthews BJ, Salwinski L, Sabatti C, Grueber WB, Zipursky SL. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature. 2009;461:644–648. doi: 10.1038/nature08431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Kaneko R, Izawa T, Kawaguchi M, Kitsukawa T, Yagi T. Single-neuron diversity generated by Protocadherin-beta cluster in mouse central and peripheral nervous systems. Front Mol Neurosci. 2012;5:90. doi: 10.3389/fnmol.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem. 2006;281:30551–3560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci. 2009;29:9137–47. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinov D, Sanes JR. Protocadherin-dependent dendritic self-avoidance regulates neural connectivity and circuit function. Elife. 2015;4:e08964. doi: 10.7554/eLife.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AP, Kuwada JY. Formation of the receptive fields of leech mechanosensory neurons during embryonic development. J Neurosci. 1983;3:2474–2486. doi: 10.1523/JNEUROSCI.03-12-02474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AP, Stent GS. Developmental arborization of sensory neurons in the leech Haementeria ghilianii. II Experimentally induced variations in the branching pattern. J Neurosci. 1985;5:768–775. doi: 10.1523/JNEUROSCI.05-03-00768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivov GG, Shapovalov MV, Dunbrack RL., Jr Improved prediction of protein side-chain conformations with SCWRL4. Proteins. 2009;77:778–795. doi: 10.1002/prot.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. Gamma-protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–51. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel M, Winterhalter PR, Willer T, Dahlhoff M, Schneider MR, Bartels MF, Renner-Muller I, Ruppert T, Wolf E, Strahl S. Protein O-mannosylation is crucial for E- cadherin-mediated cell adhesion. Proc Natl Acad Sci USA. 2013;110:21024–21029. doi: 10.1073/pnas.1316753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Meijers R, Puettmann-Holgado R, Skiniotis G, Liu JH, Walz T, Wang JH, Schmucker D. Structural basis of Dscam isoform specificity. Nature. 2007;449:487–91. doi: 10.1038/nature06147. [DOI] [PubMed] [Google Scholar]
- Miura SK, Martins A, Zhang KX, Graveley BR, Zipursky SL. Probabilistic splicing of Dscam1 establishes identity at the level of single neurons. Cell. 2013;155:1166–1177. doi: 10.1016/j.cell.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Zucker J, Daly M, Chess A. Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nat Genet. 2004;36:240–246. doi: 10.1038/ng1299. [DOI] [PubMed] [Google Scholar]
- Nicoludis JM, Lau SY, Schärfe CPI, Marks DS, Weihofen WA, Gaudet R. Structure and Sequence Analyses of Clustered Protocadherins Reveal Antiparallel Interactions that Mediate Homophilic Specificity. Structure. 2015;23:2087–98. doi: 10.1016/j.str.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad T, Weiner JA. Direct and Indirect Regulation of Spinal Cord Ia Afferent Terminal Formation by the γ-Protocadherins. Front Mol Neurosci. 2011;4:54. doi: 10.3389/fnmol.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255–68. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Ribich S, Tasic B, Maniatis T. Identification of long-range regulatory elements in the protocadherin-alpha gene cluster. Proc Natl Acad Sci USA. 2006;103:19719–19724. doi: 10.1073/pnas.0609445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein R, Thu CA, Goodman KM, Wolcott HN, Bahna F, Mannepalli S, Ahlsén G, Chevee M, Halim A, Clausen H, Maniatis T, Shapiro L, Honig B. Molecular logic of neuronal self-recognition through protocadherin domain interactions. Cell. 2015;163:629–642. doi: 10.1016/j.cell.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya MR, Wojtowicz WM, Andre I, Qian B, Wu W, Baker D, Eisenberg D, Zipursky SL. A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell. 2008;19:1007–18. doi: 10.1016/j.cell.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker D, Chen B. Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009;23:147–156. doi: 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci USA. 2010;107:14893–14898. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature. 2012;492:128–132. doi: 10.1038/nature11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki ST. Protocadherins and diversity of the cadherin superfamily. J Cell Sci. 1996;109:2609–2611. doi: 10.1242/jcs.109.11.2609. [DOI] [PubMed] [Google Scholar]
- Tasic B, Nabholz CE, Baldwin KK, Kim Y, Rueckert EH, Ribich SA, Cramer P, Wu Q, Axel R, Maniatis T. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell. 2002;10:21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Thu CA, Chen WV, Rubinstein R, Chevee M, Wolcott HN, Felsovalyi KO, Tapia JC, Shapiro L, Honig B, Maniatis T. Single-cell identity generated by combinatorial homophilic interactions between alpha, beta, and gamma protocadherins. Cell. 2014;158:1045–1059. doi: 10.1016/j.cell.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendome J, Felsovalyi K, Song H, Yang Z, Jin X, Brasch J, Harrison OJ, Ahlsen G, Bahna F, Kaczynska A, Katsamba PS, Edmond D, Hubbell WL, Shapiro L, Honig B. Structural and energetic determinants of adhesive binding specificity in type I cadherins. Proc Natl Acad Sci USA. 2014;111:E4175–84. doi: 10.1073/pnas.1416737111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci USA. 2013;110:21018–21023. doi: 10.1073/pnas.1313446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su H, Bradley A. Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev. 2002;16:1890–1905. doi: 10.1101/gad.1004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang T, Cheng JF, Kim Y, Grimwood J, Schmutz J, Dickson M, Noonan JP, Zhang MQ, Myers RM, Maniatis T. Comparative DNA sequence analysis of mouse and human protocadherin gene clusters. Genome Res. 2001;11:389–404. doi: 10.1101/gr.167301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Ahlsen G, Baker D, Shapiro L, Zipursky SL. Complementary chimeric isoforms reveal Dscam1 binding specificity in vivo. Neuron. 2012;74:261–268. doi: 10.1016/j.neuron.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475:510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Honig B. Extending the accuracy limits of prediction for side-chain conformations. J Mol Biol. 2001;311:421–430. doi: 10.1006/jmbi.2001.4865. [DOI] [PubMed] [Google Scholar]
- Yagi T. Molecular codes for neuronal individuality and cell assembly in the brain. Front Mol Neurosci. 2012;5:45. doi: 10.3389/fnmol.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T. Genetic basis of neuronal individuality in the mammalian brain. J Neurogenet. 2013;27:97–105. doi: 10.3109/01677063.2013.801969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Grueber WB. The molecular basis of self-avoidance. Ann Rev Neurosci. 2013;36:547–568. doi: 10.1146/annurev-neuro-062111-150414. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.