Abstract
Over-activation of brain renin-angiotensin system (RAS) has been implicated in the etiology of anxiety disorders. Angiotensin converting enzyme (ACE2) inhibits RAS activity by converting angiotensin II, the effector peptide of RAS, to angiotensin-(1-7), which activates Mas receptors (MasR). Whether increasing brain ACE2 activity reduces anxiety by stimulating central MasR is unknown. To test the hypothesis that increasing brain ACE2 activity reduces anxiety-like behavior via central MasR stimulation, we generated male mice overexpressing ACE2 (ACE2 KI mice) and wild type littermate controls (WT). ACE2 KI mice explored the open arms of the elevated plus maze (EPM) significantly more than WT, suggesting increasing ACE2 activity is anxiolytic. Central delivery of diminazene aceturate, an ACE2 activator, to C57BL/6 mice also reduced anxiety-like behavior in the EPM, but centrally administering ACE2 KI mice A-779, a MasR antagonist, abolished their anxiolytic phenotype, suggesting that ACE2 reduces anxiety-like behavior by activating central MasR. To identify the brain circuits mediating these effects, we measured Fos, a marker of neuronal activation, subsequent to EPM exposure and found that ACE2 KI mice had decreased Fos in the bed nucleus of stria terminalis but had increased Fos in the basolateral amygdala (BLA). Within the BLA, we determined that ~62% of GABAergic neurons contained MasR mRNA and expression of MasR mRNA was upregulated by ACE2 overexpression, suggesting that ACE2 may influence GABA neurotransmission within the BLA via MasR activation. Indeed, ACE2 overexpression was associated with increased frequency of spontaneous inhibitory postsynaptic currents (indicative of presynaptic release of GABA) onto BLA pyramidal neurons and central infusion of A-779 eliminated this effect. Collectively, these results suggest that ACE2 may reduce anxiety-like behavior by activating central MasR that facilitate GABA release onto pyramidal neurons within the BLA.
1. Introduction
Excessive uncontrollable anxiety is the most prevalent mental illness with ≈18% of adults in the U.S. being afflicted with some sort of anxiety disorder (Kessler et al., 2005). Despite therapeutic advancements, it is estimated that 40% of patients with anxiety disorders are resistant to treatment (Bystritsky, 2006). While medications such as benzodiazepines are effective at relieving anxiety, they are also associated with severe side effects such as sedation, memory impairments, tolerance and dependence (reviewed by (Uzun et al., 2010)). Accordingly, it is imperative to identify new medications that effectively relieve anxiety without deleterious side effects.
Patients with anxiety have increased risk for developing cardiovascular disease (Johannessen et al., 2006; Thurston et al., 2013) and a recent epidemiological study found that symptoms of posttraumatic stress disorder (PTSD), a type of anxiety disorder, are less severe in patients taking anti-hypertensive medications that target the renin-angiotensin-system (RAS) (Khoury et al., 2012). The effector peptide of the RAS is synthesized through a series of proteolytic cleavage events whereby renin converts angiotensinogen into angiotensin-I, which is cleaved by angiotensin converting enzyme (ACE) into angiotensin-II (Ang-II), which in turn, activates the angiotensin type 1a receptor (AT1aR). Several preclinical studies have found that stressful and anxiogenic stimilu promote Ang-II induced activation of AT1aRs but inhibiting this receptor or the synthesis of Ang-II dampens stress responsiveness and relieves fear and anxiety-like behavior in laboratory rats and mice (Castren and Saavedra, 1988; Krause et al., 2011b; Marvar et al., 2014; Saavedra et al., 2005). Taken together, these results suggest that over-activation of the ACE/Ang-II/AT1aR axis contributes to the etiology of anxiety disorders and therapies that limit its activation may be anxiolytic.
The relatively recent discovery of angiotensin converting enzyme 2 (ACE2) revealed a counter-regulatory limb of the RAS that opposes many of the deleterious consequences of AT1aR activation (reviewed by (Xu et al., 2011)). Angiotensin converting enzyme 2 metabolizes Ang-II into angiotensin 1-7 (Ang-(1-7)) which promotes cardio-protection by activating the Mas receptor (MasR) to cause vasodilation and decreased sympathetic nervous system activity (reviewed by (Xia and Lazartigues, 2010)). Emerging evidence suggests that this counter-regulatory limb of the RAS also influences physiological and behavioral responses to psychological stress. Genetic deletion of the MasR increases anxiety-like behavior in mice (Walther et al., 1998) but central administration of Ang-(1-7) blunts cardiovascular reactivity in response to psychogenic stress (Martins Lima et al., 2013) and attenuates anxiety-like behavior in rats (Bild and Ciobica, 2013; Kangussu et al., 2013). The relatively short half-life (≈ 10s) of Ang-(1-7) delivered in vivo in rats (Yamada et al., 1998) limits its therapeutic utility; however, ACE2 activity can be sustained pharmacologically in rats (Qi et al., 2013) and has the advantage of lowering Ang-II levels while also providing ligand for the MasR. Anti-hypertensive therapies have targeted the RAS for decades (Gavras et al., 1978), and consequently, it is established that interventions that suppress RAS activity are not associated with the deleterious side effects (e.g. sedation, dependence, memory impairment) that occur with some of the current anti-anxiety medications (Townsend, 2015). Therefore, ACE2 may be well-suited to serve as a therapeutic target to relieve anxiety; however, preclinical studies evaluating the effects of ACE2 on anxiety-like behavior have not been conducted.
The present study used genetic and pharmacological approaches in mice to test the hypothesis that increasing ACE2 in the brain reduces anxiety-like behavior by activating central MasRs. To identify brain circuits mediating the anxiolytic effects of ACE2, we assessed Fos induction within limbic regions subsequent to an anxiogenic stimulus. Experiments utilizing in situ hybridization and in vitro brain slice electrophysiology were conducted to reveal the neuronal phenotypes affected by MasR stimulation. Together, these studies suggest increasing brain ACE2 activity may be a novel therapuetic strategy for alleviating anxiety disorders.
2. Materials and Methods
2.1. Animals
All mice were male, 8–12 weeks-old at the initiation of the studies, individually-housed and given ad libitum access to pelleted rodent chow and water. All procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
2.1.1. ACE2 overexpressing mice
Using Cre-Lox technology, ROSA26Ace2/+ mice (created by Dr. Oh, University of Florida) with the FLAG-tagged mouse ACE2 gene expressed under the control of the endogenous ROSA26 promoter on one allele were generated. Briefly, a gene cassette containing mouse ACE2-Flag proceeded by a floxed Neo-STOP cassette was inserted after the ROSA26 locus to generate ROSA26Floxed Neo-STOP,Ace2/+ mice. Subsequently, ROSA26Floxed Neo-STOP,Ace2/+ mice were crossed with germline Cre deleter mice (β-actin-Cre) to remove the floxed Neo-STOP cassette and thus generate ROSA26Ace2/+;β-actin-Cre/+ mice. β-actin-Cre was then removed through a series of breeding steps to produce ROSA26Ace2/+ mice. ROSA26Ace2/+ mice were then inbred to generate ROSA26Ace2/Ace2 (ACE2 KI) and wild-type (WT) littermate control mice that were maintained on a 129/B6 mixed background. For all studies utilizing ACE2 KI mice, comparisons were made to their corresponding wild-type littermate controls with the exception of in vitro electrophysiological experiments which made comparisons between ACE2 KI mice and wild-type mice derived from similar parental lines.
2.1.2. GABA-reporter mice
Mice that have Cre-recombinase directed to GABA synthesizing cells [vGAT-Cre; The Jackson Laboratory, stock # 016962] were bred to mice with a mutation of the Gt(ROSA)26 Sor locus with a loxP-flanked STOP cassette preventing transcription of a CAG promoter-driven sequence coding for the red fluorescent protein variant, tdTomato (Jackson Laboratory Stock # 007914). Mice co-expressing vGAT-Cre and loxP-flanked STOP-tdTomato have the loxP-flanked STOP cassette deleted specifically in vGAT-producing (i.e. GABAergic) cells, resulting in tdTomato expression specifically in GABAergic cells. To confirm that tdTomato expressing cells were indeed GABAergic we performed RNAscope in situ hybridization (Advanced Cell Diagnostics, Hayward, CA) to visualize GAD1 mRNA, a marker of GABA synthesis, in tdTomato expressing cells (Please refer to section 2.8).
2.1.3. C57BL/6 mice
Additional studies utilizing C57BL/6 mice obtained from Harlan Laboratories were conducted to evaluate the effect of diminazene aceturate ( DIZE; ACE2 activator) or A-779 (MasR antagonist) on anxiety-like behavior.
2.2. Assessment of hydromineral balance
Tail-vein blood samples (40 μl) were collected from ACE2 KI mice (n=12) and WT littermates (n=9). Subsequently, plasma sodium, hematocrit, and protein levels were measured as previously described (Frazier et al., 2013; Krause et al., 2011a; Smith et al., 2014).
2.3. Tissue dissection and semiquantitative real-time polymerase chain reaction (PCR)
Brains, pituitary glands, and adrenal glands were extracted from another group of mice (ACE2 KI mice n=9; WT littermates n=11) and flash frozen in dry ice cooled 2-methylbutane. To dissect the hypothalamus and amygdala, the brain was placed in a cooled brain block and a brain slice (4 mm) containing the hypothalamus and amygdala was isolated using razor blades. The amygdala was removed from the brain slice using a tissue punch (1.50 mm; Stoelting, Wood Dale, IL). The hypothalamus was isolated by removing tissue dorsal and lateral to the hypothalamus with a razor blade. RNA extraction, cDNA synthesis, and real-time PCR were conducted as previously described (de Kloet et al., 2013). Probes for real-time PCR were purchased from life technology (Grand Island, NY) and specific genes of interest included ACE2 (Mm01159003), AT1aR, (Mm01166161), angiotensin type-2 receptor (AT2R; Mm01341373), and MAS1 oncogene (MasR; Mm00434823_s1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Mm99999915) was used as the house-keeping gene and relative expression was quantified using the 2ΔΔCt method. Results were calculated as the percentage of the WT littermate controls.
2.4. ACE and ACE2 activity assay
Brains, pituitary glands, and adrenal glands were extracted from another group of mice (ACE2 KI mice n=4; WT littermates n=6) and proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer. Hypothalamus and amygdala tissues were dissected as described above. The activities of ACE and ACE2 were quantified by their ability to convert their fluorogenic substrates to fluorescent products. All samples were assayed in duplicate in 96-well plates. In the ACE activity assay, each well contains 2 μl sample, 1 μl C-16 (ACE2 antagonist, (Qi et al., 2013; Shenoy et al., 2013)), 10 μl fluorogenic peptide V (fluorogenic substrate for ACE, catalog. ID: ES005, R&D Systems, Minneapolis, MN), and 87 μl buffer ([1M NaCl, 75mM Tris-HCl, and 0.5μM ZnCl2, at pH 7.4). In the ACE2 activity assay, each well contains 2 μl sample, 1 μl captopril (ACE antagonist), 10 μl fluorogenic peptide VI (fluorogenic substrate for ACE2, catalog No: ES007, R&D Systems, Minneapolis, MN), and 87 μl buffer. The increase in fluorescence was measured using a fluorescence plate reader (Synergy HT, Biotek, USA). Details for the ACE2 assay were described previously (Qi et al., 2013). ACE and ACE2 activities were displayed as increase of relative fluorescence units (RFU)/min.
2.5. Testing anxiety-like behavior using the elevated plus maze
The elevated plus maze (EPM) consists of two opposing open (31 cm × 6 cm) and two opposing closed arms (31 cm × 6 cm) that were elevated 41 cm off the floor. Assessment of anxiety-like behavior was conducted in ACE2 KI mice (n=9) and WT littermates (n=12) during the light phase between 0800-1200 h. Mice were placed in the center of the EPM and then allowed to explore the maze for 5 min. Each testing session was recorded by a ceiling-mounted camera connected to a PC running TopScan software (CleverSys, Reston, VA).
2.6. Intracerebroventricular infusion of DIZE or A-779
C57BL/6 mice were centrally administered DIZE for 1 week to evaluate whether the anxiolytic effect associated with ACE2 overexpression can be reproduced by pharmacological activation of ACE2 in the brain. Micro-osmotic pumps (Model 1004, flow rate: 0.11μL/h, ALZET) were filled with normal saline (n=12) or DIZE (n=12/group; low dose group: 0.11 μg/h, medium dose group: 1.1 μg/h, high dose group: 1.1 μg/h), connected to brain infusion cannula (Brain Infusion Kit 3, ALZET), and then incubated at 37 °C in sterile saline for 48 h prior to implantation. Under isoflurane anesthesia, mice were placed in a stereotaxic apparatus and implanted with brain infusion cannulae terminating in the lateral ventricle using the following coordinates from Bregma: anterior −0.2 mm, lateral (right) 1.0 mm, ventral −2.5 mm. Brain infusion cannulae were connected to micro-osmotic pumps that were implanted subcutaneously. Mice recovered for 1 week during which time saline or DIZE was chronically delivered into the lateral ventricles. One-week after surgery, mice were assessed for anxiety-like behavior as described.
ACE2 KI mice (n=12) and WT littermates (n=9) were administered the MasR antagonist, A-779, into the lateral ventricles for 1 week (0.22 μg/h, 2 μg/μL delivered at a flow rate of 0.11 μL/h, BACHEM, Torrance, CA). Subsequently, anxiety-like behavior was assessed in the EPM to evaluate whether activation of MasRs in the brain contributed to the anxiolytic effect of increased ACE2 activity. To evaluate whether A-779 elicits anxiogenic and sedative effects, C57BL/6 mice were delivered saline vehicle (n=9) or A-779 (n=8) into the lateral ventricles as described above and 1 week later were tested in the EPM.
2.7. Fos induction subsequent to EPM exposure
To determine whether the anxiolytic effects of ACE2 overexpression were associated with altered patterns of neuronal activation, another cohort of ACE2 KI mice (n=10) and WT littermates (n=12) were placed in the EPM for 5 min and recovered in their home cages. Ninety-minutes after the onset of EPM exposure, mice were anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 4% paraformaldehyde (PFA). Brains were extracted and post-fixed in 4% PFA for 4 h and then cyroprotected in 30% sucrose at 4°C. Coronal serial sections (30 μm) were taken on a Leica CM3050 S cryostat, and subsequently, tissue was stored in cryoprotective solution at −20°C until processing for immunohistochemistry (IHC). All solutions for IHC were made in 50 mM K-PBS and each incubation was followed by 5 rinses (5 min each rinse) in 50 mM K-PBS. Brain sections were incubated in 3% hydrogen peroxide for 15 min, and then blocked for 1 h in 0.2% Triton X-100 (Sigma-Aldrich), 2% bovine serum albumin (BSA, Sigma-Aldrich) containing solution. Brain sections were then incubated in primary antibody (1:2500 in blocking solution, sc-52 rabbit anti c-Fos, Santa Cruz Biotechnology) at 4 °C overnight. The following day, brain sections were incubated for 45 min in secondary antibody (1:500 in blocking solution, biotinylated goat anti rabbit, Vector Laboratories) at room temperature and subsequently incubated for 1 h in avidin-biotin complex (1:500 in 50 mM K-PBS solution, Vector Laboratories). Brain sections were then developed for 10 min in a solution containing 3, 3 diaminobenzidine, hydrogen peroxide, and nickel (Vector Laboratories). Finally, brain sections were mounted onto microscope slides, rinsed in graded ethanol (50%, 75%, 85%, 95%, and 100%) and xylene solutions, and cover-slipped using DPX mountant for microscopy (VWR). Image capturing and counting of Fos positive nuclei were performed by researchers blind to the experimental conditions. Briefly, regions of interest (ROI) were identified according to the stereotaxic coordinates of (Franklin and Paxinos, 2008). To obtain the ROI with the highest resolution, images were captured under the highest magnification that allowed the ROI within the visual field. Rostrocaudal sections of the ROI were matched using anatomical landmarks and each section was counted for Fos positive nuclei. Total counts were calculated for each ROI and then averaged per experimental group (WT littermates vs. ACE2 KI mice) to allow between group comparisons.
2.8. Validation of GABA reporter mice using RNAscope in situ hybridization
We used RNAscope in situ hybridization (ISH; Advanced Cell Diagnostics, Hayward, CA) to evaluate the accuracy of tdTomato reporting for GABA in the BLA. Tissue preparation and RNAscope in situ hybridization was performed as previously described (de Kloet et al., 2014b; Smith et al., 2014). Briefly, mice (n=3) were euthanized with an overdose of sodium pentobarbital and then were transcardioally perfused with 4% PFA. Brains were extracted, postfixed in 4% PFA for 4 h and then cyroprotected in 30% sucrose at 4 °C. Brains were coronally sectioned at 20 μm using a Leica CM3050S cryostat, rinsed twice, and then mounted onto Superfrost Plus Gold slides. Afer air-dried for ~20 min, slides were stored at −80 °C. To run RNAscope ISH, slides were allowed to reach room temperature for 30 min, incubated with pretreat 4 (Advanced Cell Diagnostics, Hayward, CA) for 20 min, and then underwent the hybridization procedures as described in the RNAscope® Multiplex Fluorescent Kit User Manual PART 2 (Advanced Cell Diagnostics, Hayward, CA). Specific hybridization probes used in this experiment were: (1) DapB, negative control, (2) Ubc, positive control, (3) GAD1 (indicative of GABA synthesis). After hybridization procedures, slides were cover-slipped using ProLong® Gold antifade reagent (Life technologies).
2.9. Dual RNAscope in situ hybridization and immunohistochemistry
GABA reporter mice (n=3) were used for experiments determining whether the MasR is expressed on GABAergic neurons in the BLA. Tissue preparation and RNAscope in situ hybridization was performed as described in section 2.8. To quantify the frequency with which the MasR mRNA and GABA co-localize in the BLA, we used the following probes: (1) DapB, negative control, (2) Ubc, positive control, (3) Mas1, probe for MasR. To amplify the signal for tdTomato, tissue sections underwent IHC. Briefly, sections were rinsed 5 times in 50 mM potassium PBS (KPBS) and placed in a blocking solution (50 mM KPBS with 2% normal goat serum and 0.2% Triton X-100) for 1 h at room temperature. Subsequently, sections were incubated in rabbit anti-red fluorescent protein (1:200; Abcam, Cambridge, MA) in the blocking solution for 48 h at 4°C. Sections were then brought to room temperature, rinsed, and incubated for 1 h in biotinylated goat anti-rabbit (1:500; Vector Laboratories, Burlingame, CA) made in a blocking solution. Sections were then rinsed and incubated for 1 h in avidin-biotin complex (Vectastain ABC Elite, 1:500; Vector Laboratories) and, after another series of rinses, were incubated in DyLight 549 streptavidin (1:500; Vector Laboratories) for 1 h and then rinsed and cover-slipped using ProLong® Gold antifade reagent (Life technologies).
2.10. Capture and analysis of fluorescent images
Fluorescent images were captured using an AxioImager M.2 fluorescent Apotome microscope (Carl Zeiss, Thornwood, New York). Z-stack images were captured at 20X, 40X, and 60X magnifications throughout the BLA. Z-steps were set at 1 μm, with an average of 16 optical sections per image. The optimal exposure time and optimal image processing procedures were determined using sections hybridized with the Ubc (positive control). Using these parameters, fluorescence was undetectable in sections hybridized with DapB (negative control). All images were captured using this same exposure time and were processed using the same parameters. Number of colocalizations between mRNA molecules and tdTomato-positive cells were counted manually under 20X. It was considered as a colocalization when at least one mRNA molecule localized within the tdTomato-positive cell.
2.11. In vitro whole-cell recording
Mice were anesthetized with a single intraperitoneal injection of ketamine (~100 mg kg−1) and rapidly decapitated. The brains were quickly removed and immersed in ice cold artificial cerebral spinal fluid (ACSF). Coronal brain slices (300 μm thick) through the BLA were obtained using a vibrating tissue slicer, and transferred to an incubator that contained ACSF preheated to 30–35 °C. After a 30 min incubation period slices were allowed to equilibrate to room temperature for a minimum of 30 additional minutes before use. For electrophysiological studies, slices were transferred to a recording chamber and were superfused at a constant rate of 2 ml min−1 with ACSF saturated with 95% O2 and 5% CO2. ACSF for sectioning and incubation contained the following (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.5 MgSO4, 10 D-glucose, 1 CaCl2, and 25.9 NaHCO3, saturated with 95% O2 and 5% CO2. ACSF for recording contained (in mM): 124 NaCl, 5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4, 25 D-glucose and was maintained at 28–30°C. Electrode tip resistance was 5–7 MΩ when filled with an internal solution that contained (in mM): 100 K-gluconate, 40 KCl, 10 NaCl, 2 MgCl2, 1 EGTA, 10 Hepes, 2 Na2-ATP, 0.3 Na-GTP, 10 HEPES, pH to 7.3 using KOH and volume adjusted to 290–300 mosmol l−1. The pyramidal neurons of the BLA were identified by their large somata, moderate to high input resistance, and varying degrees of spike frequency adaptation (Duvarci and Pare, 2007; Rainnie et al., 1993). After initial characterization, neurons were voltage-clamped at −70 mV and the basal frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) was observed for 5 min in the presence of the ionotropic glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3-Dione (DNQX; 20 μM) and DL-2-amino-5-phosphonovaleric acid (APV; 40 μM). In a subset of experiments the GABAergic nature of these events was confirmed by blocking them with bath application of the GABAA receptor antagonist picrotoxin (100 μM). After recording, sIPSCs were detected using parameter based event detection software written in OriginC (OriginLab Corp., Northampton MA, USA) by CJF. Mice (n=3/group) used in this experiment included WT mice (9 neurons recorded), ACE2 KI mice (8 neurons recorded), WT mice infused (ivc) with A-779 for 1 week (17 neurons recorded), and ACE2 KI mice infused (icv) with A-779 for 1 week (15 neurons recorded).
2.12. Statistical analysis
All data were analyzed and graphed using Prism 5 (GraphPad Software, La Jolla, CA) or OriginPro 9 (OriginLab Corp., Northampton, MA) and presented as mean and SEM. EPM results after DIZE infusion were analyzed using one-way ANOVA. All other results were analyzed using two-tailed student t-test.
3. Results
3.1. ACE2 KI mice robustly express ACE2 mRNA and have increased MasR mRNA in the amygdala
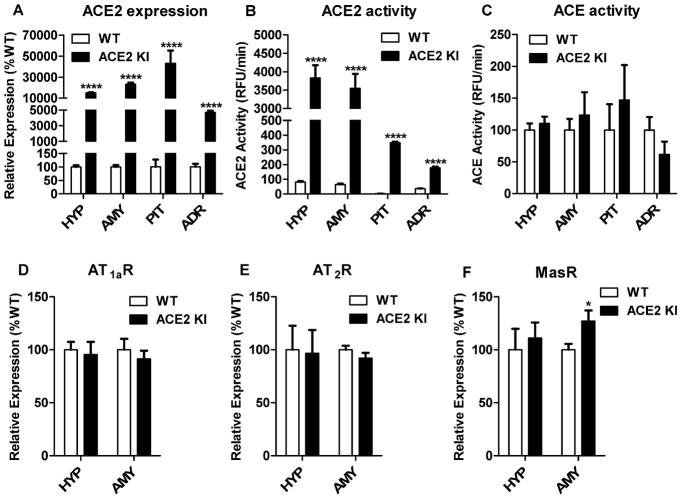
The hypothalami, amygdala, pituitaries and adrenals of ACE2 KI mice had significantly increased levels (P<0.0001) of ACE2 mRNA (Figure 1A) and significantly elevated (P<0.0001) ACE2 activity (Figure 1B) relative to WT littermates but ACE activity was similar between the groups (Figure 1C). We measured mRNA levels for the AT1aR, AT2R, and MasR to determine whether increasing ACE2 influenced the expression of receptors for angiotensin-related peptides. Increasing ACE2 did not affect AT1aR and AT2R mRNA in the hypothalamus and amygdala (Figure 1D, 1E); however, mRNA for the MasR was significantly increased in the amygdala of ACE2 KI mice relative to WT littermates (P<0.05, Figure 1F).
Figure 1. ACE2 KI mice robustly express ACE2 and have increased MasR mRNA in the amygdala.
(A) Levels of ACE2 mRNA expression and (B) ACE2 activity in central and peripheral tissues. (C) ACE activity in central and peripheral tissues. Levels of mRNA expression for (D) AT1aR, (E) AT2R, and (F) MasR in the hypothalamus and amygdala. HYP, hypothalamus; AMY, amygdala; PIT, pituitary gland; ADR, adrenal gland. Bars represent mean and SEM. * = P < 0.05; **** = P < 0.0001.
3.2. ACE2 overexpression decreases hematocrit but does not affect body weight or other indices of hydromineral balance
We measured body weight, plasma sodium, plasma proteins and hematocrit to determine whether ACE2 overexpression affected these indices of metabolism and hydromineral balance. Overexpression of ACE2 had no effect on body weight (WT littermates; 21.3 g ± 1.46 g vs. ACE2 KI; 23.7 g ± 1.06 g), plasma sodium (WT littermates; 131 mM ± 3.40 mM vs. ACE2 KI; 136 mM ± 3.83 mM) or plasma protein (WT littermates; 6.72 g/dl ± 0.11 g/dl vs. ACE2 KI; 6.37 g/dl ± 0.17 g/dl); however, hematocrit was significantly decreased (WT littermates; 51.7% ± 0.94% vs. ACE2 KI; 46.9 % ± 1.10%; P<0.01) relative to that of WT littermates.
3.3. Increasing brain ACE2 activity attenuates anxiety-like behavior by activating central Mas receptors
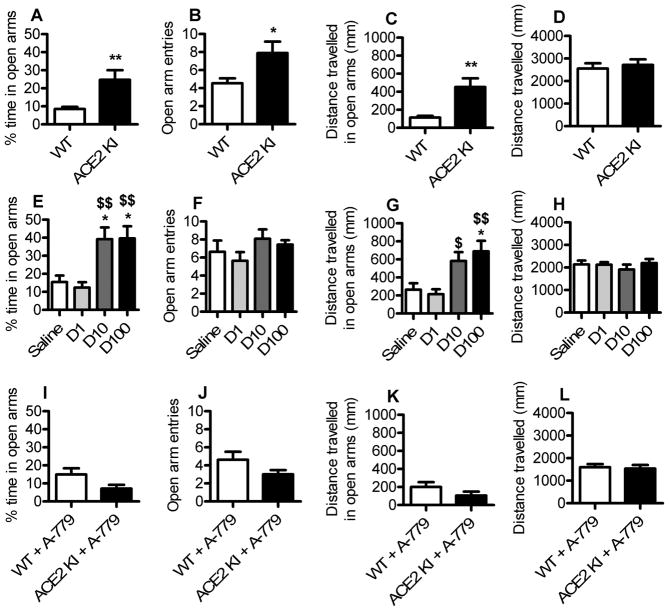
ACE2 KI mice and WT littermates were assessed in the EPM to test the hypothesis that ACE2 overexpression is anxiolytic. ACE2 KI mice spent significantly more time (P<0.01) in the open arms, had significantly more (P<0.05) open arm entries, and traveled more distance in the open arms (P<0.01) compared to WT littermates (Figure 2A–C). Despite the increased distance traveled in the open arms, ACE2 KI mice did not exhibit an overall increase in total distance traveled compared to WT littermates (Figure 2D). Interestingly, pharmacologically augmenting brain ACE2 activity in C57BL/6 mice with chronic central delivery of DIZE recapitulated the anxiolytic effects of genetic ACE2 overexpression. Specifically, relative to saline infused controls, chronic icv infusion of DIZE increased open arm time (Figure 2E) as well as the distance traveled in the open arms of the EPM (Figure 2G) without affecting total locomotor activity (Figure 2H). Anxiety-like behavior was assessed subsequent to central administration of A-779 to test the hypothesis that the anxiolytic effects of ACE2 overexpression were the result of activation of MasRs within the brain. Consistent with this hypothesis, central infusion of A-779 eliminated the anxiolytic effects of ACE2 overexpression (Figure 2I–L). C57BL/6 mice administered saline or A-779 had similar performance in the EPM: open arm time (saline; 8.43 % ± 2.65 vs. A-779; 9.1 % ± 2.19), open arm entries (saline; 4 ± 0.82 vs. A-779; 3.86 ± 0.63), and distance traveled in open arms (saline; 121 mm ± 46.9 vs. A-779; 114 mm ± 34.2). These results suggest that augmented ACE2 activity is necessary for the anxiolysis induced by increased MasR activation. Importantly, the locomotor activity of mice given A-779 and those given saline was similar (saline; 1821 mm ± 168.5 vs. A-779; 1669 mm ± 197.4), demonstrating that the increased anxiety-like behavior that accompanied A-779 administration to ACE2 KI mice was not the result of motor impairments.
Figure 2. ACE2 overexpression attenuates anxiety-like behavior, an effect that is reproduced by pharmacological activation of brain ACE2 and is eliminated by blockade of central MasR.
(A–D) Anxiety-like behavior of WT littermates and ACE2 KI mice tested in the elevated plus maze (EPM). (E–H) Anxiety-like behavior of C57BL/6 mice tested in the EPM after icv infusion (0.11 μl/h) of normal saline or DIZE (D1, 1 μg/μl; D10, 10 μg/μl; D100, 100 μg/μl). (I–L), Anxiety-like behavior of WT littermates and ACE2 KI mice tested in the EPM after icv infusion (0.11 μl/h) of A-779 (2 μg/μl). Bars represent mean and SEM. * = P < 0.05 vs. WT or Saline; ** = P < 0.01; $ = P < 0.05 vs. D1; $$ = P < 0.01 vs. D1.
3.4. ACE2 overexpression alters neuronal activation after EPM exposure
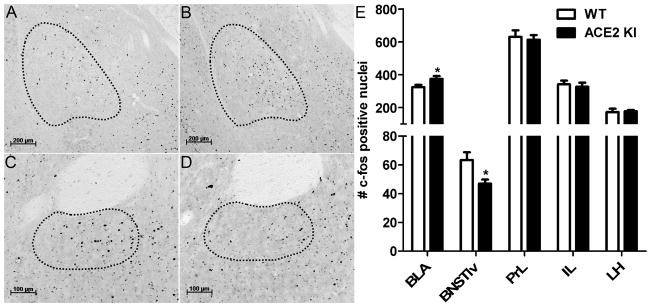
We evaluated Fos induction after exposure to the EPM to investigate whether ACE2 induced anxiolysis is associated with altered neuronal activation within specific brain nuclei. Relative to WT littermates, ACE2 KI mice had significantly more Fos positive nuclei (P<0.05) in the posterior division of the anterior BLA (Figure 3 A–C) but had significantly fewer Fos positive nuclei (P<0.05) in BNSTlv (Figure 3D–F). Subsequent to EPM exposure, overexpression of ACE2 had no effect on the Fos in the prelimbic cortex (PrL), infralimbic cortex (IL), and lateral hypothalamus (LH).
Figure 3. ACE2 overexpression alters neuronal activation after EPM exposure.
Representative immunohistochemistry images for Fos, depicted as black dots, within outlined regions of interest. (A) The BLA of a WT littermate and (B) an ACE2 KI mouse. (C) BNSTlv of a WT littermate and (D) an ACE2 KI mouse. (E) Relative to WT littermates, ACE2 KI mice have more Fos positive nuclei in the posterior division of the anterior basolateral amygdala (BLA) but have less Fos positive nuclei in the lateroventral division of the bed nucleus of stria terminalis (BNSTlv). Number of Fos positive nuclei in prelimbic cortex (PrL), infralimbic cortex (IL), and lateral hypothalamus (LH) are not different between ACE2 KI mice and WT littermates. Bars represent mean and SEM. * = P < 0.05.
3.5. MasR mRNA is expressed by GABAergic neurons in the BLA
To confirm that tdTomato-expressing cells in GABA reporter mice were indeed GABAerigc, we performed RNAscope in situ hybridization to visualize GAD1 mRNA, a marker of GABA synthesis, in tdTomato expressing cells. We found that 93% of tdTomato-positive cells in the BLA of GABA-reporter mouse also express GAD1 mRNA (depicted as punctate magenta dots Figure 4C), demonstrating the GABA-reporter mouse reliably reports GABAergic neurons.
Figure 4. MasRs are expressed on GABAergic neurons in the BLA.
(A) Atlas section corresponding to the level of sections used for in situ hybridization. The red highlighted area indicates the BLA. (B) 5X image of a coronal section through the BLA (dotted outline) of a GABA reporter mouse (Green, tdTomato signal representing vGAT). (C) 20X image and (C inset) 40X image of GAD1 mRNA (depicted as punctate magenta dots) frequently co-localizing with tdTomato signal representing vGAT (green), indicating that the reporter gene accurately tracks GABA synthesizing neurons. (D) 20X images and (D inset) 63X image of tdTomato signal representing vGAT (green) with DapB mRNA (negative control, undetectable). (E) 20X images and (E inset) 63X image of tdTomato signal representing vGAT (green) with Ubc mRNA (positive control, depicted as punctate red dots). (F) 20X image and (F inset) 63X image of mRNA for the MasR (depicted as punctate red dots) frequently co-localizing with tdTomato signal representing vGAT (green), demonstrating MasR mRNA is expressed in GABAergic neurons in the BLA. Signals of tdTomato were colored as green for better display of the collocalizations.
To investigate whether MasRs are expressed on GABAergic neurons in the BLA, we conducted RNAscope in situ hybridization to visualize mRNA for MasR on brain sections obtained from GABA-reporter mice. As shown in Figure 4F, mRNA for the MasR, depicted as punctate red dots, was frequently co-expressed with tdTomato, indicating that MasRs are expressed on GABAergic neurons in the BLA. Quantitatively, we examinated 3715 GABAergic neurons in the BLA and found that 2301 or 61.9% of these neurons expressed at least one molecule of MasR mRNA.
3.6. ACE2 overexpression increases sIPSC frequency in BLA pyramidal neurons by activating MasR
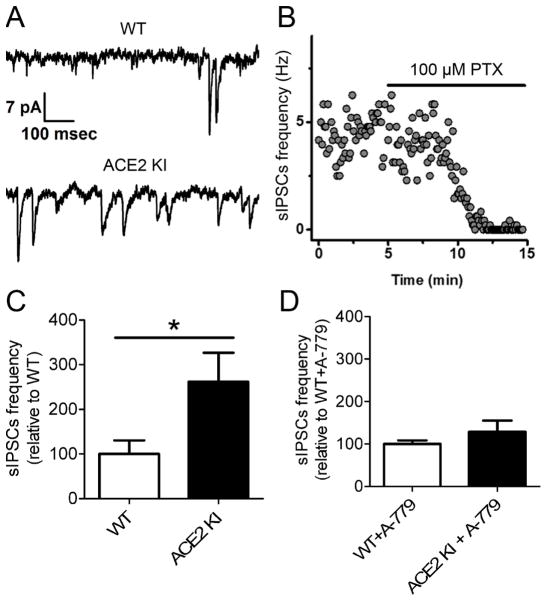
To test the hypothesis that increased ACE2 activity promotes the synaptic release of GABA onto BLA pyramidal neurons, we performed whole-cell patch clamp recordings in an acute BLA slice preparation. Relative to WT controls, the frequency of sIPSCs recorded in BLA pyramidal neurons was significantly higher (P<0.05) in ACE2 KI mice (Figure 5A, 5C), yet there was no significant difference in sIPSC amplitude (12.0 ± 2.45 pA WT, 15.6 ± 2.19 pA ACE2 KI, P=0.30). The increased sIPSCs in BLA pyramidal neurons of ACE2 KI mice relative to WT controls were eliminated by chronic central delivery of A-779 (Figure 5D). Additionally, holding current (at −70 mV) and input resistance were not different in neurons from WT vs. ACE2 KI animals (iHold: −62±13.8 pA WT, −72±40.5 pA ACE2 KI, p=0.80, Rm: 105 ± 10.3 MΩ WT, 100 ± 12.6 MΩ ACE2 KI, P=0.74)
Figure 5. ACE2 overexpression increases basal sIPSCs frequency in BLA pyramidal neurons by acting on MasRs.
(A) Representative recording of sIPSCs from pyramidal neurons in BLA of a WT control (top) and ACE2 KI mouse (bottom). (B) Representative recording of sIPSCs from principal neurons in the BLA showing sensitivity to picrotoxin (PTX), a GABAA receptor antagonist. (C) Summary data for sIPSCs frequency recorded from animals without A-779 infusion. (D) Summary data for sIPSCs frequency recorded from animals that received chronic brain infusion of A-779 (2μg/μl) for 7 days. Bars represent mean and SEM. * = P < 0.05
4. Discussion
The goal of this study was to evaluate the effect of increased ACE2 on anxiety-like behavior in male mice. To this end, we determined that ubiquitous overexpression of ACE2 significantly reduced anxiety-like behavior assessed in the EPM. Increasing brain ACE2 activity with central administration of DIZE recapitulated the anxiolytic effects of ubiquitous ACE2 overexpression, demonstrating that pharmacologically up-regulating ACE2 activity in the brain is anxiolytic. Central administration of A-779 abrogated the decreased anxiety-like behavior associated with ubiquitous ACE2 overexpression, suggesting that increasing ACE2 activity promotes anxiolysis by stimulating MasRs within the brain. Functional anatomical studies were conducted to gain insight towards the neuronal circuits underlying the anxiolytic effects of ACE2. ACE2 overexpression altered the pattern of Fos induction that occurred in the BLA and BNSTlv subsequent to an anxiogenic stimulus. The BLA and BNSTlv comprise a neuronal circuit implicated in the control of fear and anxiety-like behavior and we determined that over 60% of GABAergic neurons in the BLA express mRNA for the MasR. Intriguingly, in vitro electrophysiological recordings revealed that ACE2 overexpression enhances GABAergic neurotransmission within the BLA and this effect is mediated by activation of the MasR. Taken together, our results demonstrate that increasing ACE2 activity in the brain is anxiolytic and this effect may be the result of increased MasR stimulation that enhances GABAergic neurotransmission within brain circuits controlling fear and anxiety-like behavior.
A major component of the stress response is increased cardiac reactivity derived from augmented sympathetic outflow and systemic delivery of Ang-(1-7) or an ACE2 activator decreases cardiac reactivity in response to emotional stress (Martins Lima et al., 2013). Our study did not measure cardiovascular reactivity and it is possible that the anxiolysis that accompanies augmented ACE2 can be attributed to decreased sympathetic outflow and lowered blood pressure. Conversely, mood disorders are predictive of elevated sympathetic outflow and hypertension (Kayano et al., 2012; Yeragani et al., 2002) and recent research has found that increased activation of the amygdala, a brain nuclei known to mediate cardiovascular responses to psychological stress, underlies the elevated blood pressure that is observed in the BPH/2J mouse model of hypertension (Davern et al., 2009; Jackson et al., 2014). Therefore, it is also possible that the sympathoinhibition and reduced cardiovascular reactivity that accompanies increased ACE2 manifests from altered neuronal activity within brain nuclei controlling responses to psychological stress.
Angiotensin II is known to influence body weight and hydromineral balance through activation of AT1aR and AT2R (de Kloet et al., 2013; de Kloet et al., 2014a; Grobe et al., 2011; Krause et al., 2008). Overexpression of ACE2 likely disrupts the actions of Ang-II, which in turn, may affect anxiety-like behavior by altering body weight or hydromineral balance. In this regard, WT littermates and ACE2 KI mice had similar body weight, plasma sodium and protein concentrations as well as mRNA levels for the AT1a and AT2 receptors. Hematocrit was slightly but significantly lower in ACE2 KI mice relative to controls. Elevated hematocrit is a risk factor for cardiovascular disease (Paul et al., 2012; Sorlie et al., 1981) and the decreased hematocrit that is observed in ACE2 KI mice is consistent with the cardio-protective effects of the ACE2 (reviewed by (Ferreira et al., 2008)]. Interestingly, psychological stress increases hematocrit (Kitahara et al., 1988) and the lower hematocrit of ACE2 KI mice is also consistent with their anxiolytic phenotype. Collectively, the results suggest that the decreased anxiety-like behavior that is observed in ACE2 KI mice is not secondary to metabolic alterations or hydromineral imbalance.
In the current study, ubiquitous overexpression of ACE2 greatly elevated ACE2 mRNA and ACE2 activity in the brain; however, a recent study from our group determined ACE2 KI mice also have elevated ACE2 mRNA and ACE2 activity in peripheral tissues and plasma, respectively (Qi et al., 2015). Therefore, our subsequent experiments evaluated whether the suppressed anxiety-like behavior observed with augmented ACE2 was centrally mediated. In this regard, delivery of DIZE, an ACE2 activator, selectively into the brain decreased anxiety-like behavior and central adminstration of the MasR antagonist, A-779, abolished the anxiolytic effects of ubiquitous ACE2 overexpression. Abrogation of the anxiolytic effects of ACE2 with A-779 cannot be attributed to sedation because mice given A-779 had locomotor activity similar to those given saline. Moreover, impaired motor function resulting from surgical manipulation is unlikely to underlie the effects of A-779 because mice delivered DIZE underwent indentical surgical procedures and an anxiolytic phenotype was still observed. To the best of our knowledge, these results are the first to demonstrate that increasing ACE2 activity in the brain is anxiolytic, which is consistent with previous reports indicating that activating central MasRs with icv administration of Ang-(1-7) decreases anxiety-like behavor in the EPM (Bild and Ciobica, 2013; Kangussu et al., 2013). Taken together, these results suggest that increasing ACE2 activity reduces anxiety by activating central MasRs.
Both ACE2 and MasRs are endogenously expressed in brain nuclei controlling behavioral and physiological responses to anxiety and stress, such as the amygdala, paraventricular hypothalamic nucleus, nucleus of the solitary tract, and ventrolateral medulla (Becker et al., 2007; Doobay et al., 2007; Freund et al., 2012). Overexpression of ACE2 elevated MasR mRNA in the amygdala, a brain region that controls the behavioral manifestations of fear and anxiety (Davis, 1992). Therefore, we examined Fos induction within amygdala-related circuits to investigate neuronal mechanisms underlying the anxiolytic effects of ACE2. In this regard, overexpression of ACE2 significanly increased Fos in the BLA but decreased Fos induction in the BNSTlv in response to an anxiogenic stimulus. The ACE2 overpression did not affect Fos induction in the PRL, IL or LH, suggesting that increasing brain ACE2 activity selectively influences anxiety evoked neuronal activation within the BLA and BNSTlv. The BLA has direct excitatory projections to the BNST that regulate the expression of fear and anxiety-like behaviors (for review see (Davis et al., 2010)). The BLA is comprised of pyramidal neurons that send glutamatergic projections to brain nuclei regulating stress responsiveness or GABAergic interneurons that influence the excitability of these pyramidal neurons. Activation of BLA pyramidal neurons promotes anxiety (Felix-Ortiz et al., 2013; Tye et al., 2011) and it is unlikely that the increased Fos observed in the BLA of ACE2 KI mice can be attributed to activation of pyramidal neurons because the anxiolytic effects of ACE2 are not congruent with such activation. Increasing GABA tone within the BLA inhibits the activation of BLA pyramidal neurons and diminishes anxiety (Tian et al., 2013). Taking this into account, an alternative interpretation is that the increased Fos that occurred within the BLA of ACE2 KI mice was due to enhanced activation of GABAergic interneurons that inhibit pyramidal neurons, which in turn, decreased Fos expression in the BNSTlv.
We generated GABA reporter mice and used RNAscope in situ hybridization to visualize MasR mRNA within GABAergic neurons in the BLA to evaluate the influence of ACE2 on intra-amygdala circuits. Interestingly, > 60% of GABAergic neurons within the BLA expressed mRNA for the MasR, suggesting that MasRs are well positioned to influence GABA release onto pyramidal neurons. To test the hypothesis that increasing ACE2 activity in the brain promotes GABAergic inhibition of pyramidal neurons in the BLA, we took electrophysiological recordings from brain slices obtained from ACE2 KI mice and observed increased frequency of sIPSCs within pyramidal neurons of the BLA. These results indicate that ACE2 overexpression promotes the presynaptic release of GABA onto BLA pyramidal neurons. The increase of sIPSCs frequency observed in ACE2 KI mice was abolished after chronic central infusion of the MasR antagonist, suggesting activation of MasRs, which are expressed by GABA neurons within the BLA, promotes the release of inhibitory transmitter onto BLA pyramidal neurons. In other words, ACE2 may inhibit amygdala neurons that potentiate stress and anxiety by enhancing activation of MasRs expressed on GABAergic interneurons within the BLA.
The present study demonstrates that augmenting ACE2 activity in the brain reduces anxiety-like behavior by stimulating central MasRs, an effect that is associated with enhanced GABAergic inhibitory transmission within the amygdala. As mentioned, the substrate for ACE2 is Ang-II, a peptide that promotes fear and anxiety by activating AT1aR (Krause et al., 2011b; Marvar et al., 2014; Saavedra and Benicky, 2007). The anxiogenic effects of Ang-II induced activation of AT1aRs have led several groups to propose the use of angiotensin receptor blockers as therapeutics for anxiety disorders (Khoury et al., 2012; Morrison and Ressler, 2014; Saavedra et al., 2005; Saavedra et al., 2011). Interestingly, patients taking angiotensin receptor blockers had increased ACE2 level (Furuhashi et al., 2015) and activation of ACE2/Ang-(1-7)/MasR axis contributed to the therapeutic effect of ACE inhibition (Luque et al., 1996). These studies, in conjunction with our results, suggest that central augmentation of the ACE2/Ang-(1-7)/MasR axis may complement therapuetic strategies that limit Ang-II induced activation of AT1aR to alleviate stress-related diseases, like anxiety disorders.
Overexpression of Angiotensin Converting Enzyme (ACE2) is anxiolytic.
Activation of ACE2 in the brain using diminazene aceturate is anxiolytic.
Central antagonism of Mas Receptor (MasR) abolishes the anxiolytic effect of ACE2.
MasRs are highly expressed on GABAergic neurons in the basolateral amygdala (BLA).
ACE2 overexpression promotes GABAergic transmission in the BLA by acting on MasR.
Acknowledgments
Support: HL096830 (EGK), HL122494 (EGK), HL125805 (ADdK), HL116074 (ADdK), HL033610 (MKR), HL102033 (MKR)
Footnotes
Funding and Disclosure
This study was supported by HL096830 (EGK), HL122494 (EGK), HL125805 (ADdK), HL116074 (ADdK), HL033610 (MKR), HL102033 (MKR and MJK), HL 05921 (MKR and MJK) and HL05921 (MKR and MJK).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. 2007;293:H1416–1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- Bild W, Ciobica A. Angiotensin-(1-7) central administration induces anxiolytic-like effects in elevated plus maze and decreased oxidative stress in the amygdala. J Affect Disord. 2013;145:165–171. doi: 10.1016/j.jad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry. 2006;11:805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- Castren E, Saavedra JM. Repeated stress increases the density of angiotensin II binding sites in rat paraventricular nucleus and subfornical organ. Endocrinology. 1988;122:370–372. doi: 10.1210/endo-122-1-370. [DOI] [PubMed] [Google Scholar]
- Davern PJ, Nguyen-Huu TP, La Greca L, Abdelkader A, Head GA. Role of the sympathetic nervous system in Schlager genetically hypertensive mice. Hypertension. 2009;54:852–859. doi: 10.1161/HYPERTENSIONAHA.109.136069. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33:4825–4833. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014a;136:31–38. doi: 10.1016/j.physbeh.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2014b doi: 10.1007/s00429-014-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AJ, Hernandez Prada JA, Ostrov DA, Raizada MK. Cardiovascular protection by angiotensin-converting enzyme 2: a new paradigm. Future Cardiol. 2008;4:175–182. doi: 10.2217/14796678.4.2.175. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates 2008 [Google Scholar]
- Frazier CJ, Pati D, Hiller H, Nguyen D, Wang L, Smith JA, MacFadyen K, de Kloet AD, Krause EG. Acute hypernatremia exerts an inhibitory oxytocinergic tone that is associated with anxiolytic mood in male rats. Endocrinology. 2013;154:2457–2467. doi: 10.1210/en.2013-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund M, Walther T, von Bohlen und Halbach O. Immunohistochemical localization of the angiotensin-(1-7) receptor Mas in the murine forebrain. Cell Tissue Res. 2012;348:29–35. doi: 10.1007/s00441-012-1354-3. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- Gavras H, Brunner HR, Turini GA, Kershaw GR, Tifft CP, Cuttelod S, Gavras I, Vukovich RA, McKinstry DN. Antihypertensive effect of the oral angiotensin converting-enzyme inhibitor SQ 14225 in man. N Engl J Med. 1978;298:991–995. doi: 10.1056/NEJM197805042981803. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension. 2011;57:600–607. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KL, Palma-Rigo K, Nguyen-Huu TP, Davern PJ, Head GA. Major contribution of the medial amygdala to hypertension in BPH/2J genetically hypertensive mice. Hypertension. 2014;63:811–818. doi: 10.1161/HYPERTENSIONAHA.113.02020. [DOI] [PubMed] [Google Scholar]
- Johannessen L, Strudsholm U, Foldager L, Munk-Jorgensen P. Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. J Affect Disord. 2006;95:13–17. doi: 10.1016/j.jad.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Kangussu LM, Almeida-Santos AF, Bader M, Alenina N, Fontes MA, Santos RA, Aguiar DC, Campagnole-Santos MJ. Angiotensin-(1-7) attenuates the anxiety and depression-like behaviors in transgenic rats with low brain angiotensinogen. Behav Brain Res. 2013;257:25–30. doi: 10.1016/j.bbr.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Kayano H, Koba S, Matsui T, Fukuoka H, Toshida T, Sakai T, Akutsu Y, Tanno K, Geshi E, Kobayashi Y. Anxiety disorder is associated with nocturnal and early morning hypertension with or without morning surge: ambulatory blood pressure monitoring. Circ J. 2012;76:1670–1677. doi: 10.1253/circj.cj-11-1085. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara Y, Imataka K, Nakaoka H, Ishibashi M, Yamaji T, Fujii J. Hematocrit increase by mental stress in hypertensive patients. Jpn Heart J. 1988;29:429–435. doi: 10.1536/ihj.29.429. [DOI] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Flak JN, Smeltzer MD, Solomon MB, Evanson NK, Woods SC, Sakai RR, Herman JP. Hydration state controls stress responsiveness and social behavior. J Neurosci. 2011a;31:5470–5476. doi: 10.1523/JNEUROSCI.6078-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci. 2011b;31:15009–15015. doi: 10.1523/JNEUROSCI.0892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–6424. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- Martins Lima A, Xavier CH, Ferreira AJ, Raizada MK, Wallukat G, Velloso EP, dos Santos RA, Fontes MA. Activation of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol. 2013;305:H1057–1067. doi: 10.1152/ajpheart.00433.2013. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, Ressler KJ. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry. 2014;75:864–872. doi: 10.1016/j.biopsych.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depress Anxiety. 2014;31:279–290. doi: 10.1002/da.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L, Jeemon P, Hewitt J, McCallum L, Higgins P, Walters M, McClure J, Dawson J, Meredith P, Jones GC, Muir S, Dominiczak AF, Lowe G, McInnes GT, Padmanabhan S. Hematocrit predicts long-term mortality in a nonlinear and sex-specific manner in hypertensive adults. Hypertension. 2012;60:631–638. doi: 10.1161/HYPERTENSIONAHA.112.191510. [DOI] [PubMed] [Google Scholar]
- Qi Y, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, Song C, Pepine CJ, Katovich MJ, Raizada MK. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62:746–752. doi: 10.1161/HYPERTENSIONAHA.113.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YF, Zhang J, Wang L, Shenoy V, Krause E, Oh SP, Pepine CJ, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. J Mol Med (Berl) 2015 doi: 10.1007/s00109-015-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1362. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept. 2005;128:227–238. doi: 10.1016/j.regpep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10:185–193. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy V, Gjymishka A, Jarajapu YP, Qi Y, Afzal A, Rigatto K, Ferreira AJ, Fraga-Silva RA, Kearns P, Douglas JY, Agarwal D, Mubarak KK, Bradford C, Kennedy WR, Jun JY, Rathinasabapathy A, Bruce E, Gupta D, Cardounel AJ, Mocco J, Patel JM, Francis J, Grant MB, Katovich MJ, Raizada MK. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187:648–657. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Wang L, Hiller H, Taylor CT, de Kloet AD, Krause EG. Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiol Behav. 2014;136:91–96. doi: 10.1016/j.physbeh.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie PD, Garcia-Palmieri MR, Costas R, Jr, Havlik RJ. Hematocrit and risk of coronary heart disease: the Puerto Rico Health Program. Am Heart J. 1981;101:456–461. doi: 10.1016/0002-8703(81)90136-8. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Rewak M, Kubzansky LD. An anxious heart: anxiety and the onset of cardiovascular diseases. Prog Cardiovasc Dis. 2013;55:524–537. doi: 10.1016/j.pcad.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Tian Z, Wang Y, Zhang N, Guo YY, Feng B, Liu SB, Zhao MG. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. 2013;38:2218–2233. doi: 10.1016/j.psyneuen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Townsend RR. In: Major side effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. Post TW, editor. UpToDate; Waltham, MA: 2015. [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzun S, Kozumplik O, Jakovljevic M, Sedic B. Side effects of treatment with benzodiazepines. Psychiatr Danub. 2010;22:90–93. [PubMed] [Google Scholar]
- Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C, Ganten D, Bader M. Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem. 1998;273:11867–11873. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM. Converting enzyme determines plasma clearance of angiotensin-(1-7) Hypertension. 1998;32:496–502. doi: 10.1161/01.hyp.32.3.496. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Rao KA, Smitha MR, Pohl RB, Balon R, Srinivasan K. Diminished chaos of heart rate time series in patients with major depression. Biol Psychiatry. 2002;51:733–744. doi: 10.1016/s0006-3223(01)01347-6. [DOI] [PubMed] [Google Scholar]