Abstract
Serine/threonine kinase PAK1 is activated by estrogen and plays an important role in breast cancer. However, the integration of PAK1 into the estrogen response is not fully understood. In this study, we investigated the mechanisms underlying hormone-induced activation of estrogen receptor (ERα, ESR1). We show that estrogen activated PAK1 through both the ERα and GPER1 membrane receptors. Estrogen-dependent activation of PAK1 required the phosphorylation of tyrosine residues by Etk/Bmx and protein kinase A (PKA) within an assembled signaling complex comprising pTyr-PAK1, Etk/Bmx, the heterotrimer G-protein subunits Gβ1, Gγ2 and/or Gγ5, PAK-associated guanine nucleotide exchange factor (βPIX, ARHGEF7), and PKA. Moreover, the PKA RIIβ subunit is a direct target of PAK1, and thus in response to estrogen, the activated pTyr-PAK1 complex reciprocally potentiated PKA activity, suggesting a positive feedback mechanism. We also demonstrate that PKA phosphorylated Ser305-ERα in response to estrogen, but pTyr-PAK1 phosphorylated Ser305-ERα in response to prolactin (PRL), implying that maximal ERα phosphorylation is achieved when cells are exposed to both PRL and estrogen. Furthermore, S305-ERα activation led to enhanced phosphorylation of Ser118-ERα and promoted cell proliferation and tumor growth. Together, these data strongly support a critical interplay between PRL and estrogen via PAK1, and suggest that ligand-independent activation of ERα through PRL/PAK1 may impart resistance to anti-estrogen therapies.
Keywords: PAK1, estrogen, prolactin, PKA, estrogen receptor
Introduction
Estrogens play a crucial role in regulating the growth and differentiation of breast cancers, with about two thirds of all breast tumors expressing the estrogen receptor alpha (ERα) [1]. Although the involvement of estrogen in breast cancer tumorigenicity has been known for years, the exact mechanisms are unclear. Estrogen action is mediated by “classical” (initiated in nucleus) and “alternative” (initiated at the plasma membrane) pathways. The “alternative” pathway promotes a fast response to estrogen and is mediated by membrane-localized ERα (mERα) that activates Gα and Gβγ proteins, leading to calcium and cyclic AMP (cAMP) generation [2]. Estrogen also signals through G-protein-coupled receptor 30 (GPR30, or G-protein-coupled estrogen receptor 1 (GPER1)) [3]. GPER1 activation results in stimulation of both Gαs and Gαi/o, through which adenylyl cyclase (AC) is either positively or negatively regulated, leading to changes in cAMP level and protein kinase A (PKA) activity. Upon activation of both mER and GPER1, Gα dissociates from Gβγ, and both trigger activation of downstream effectors [3].
The role of prolactin (PRL) in the pathogenesis of breast cancer is increasingly appreciated (reviewed in [4,5,6]). PRL receptor (PRLR) is expressed in many breast cancers, including both ERα+ and ERα− tumors [7], and PRLR mRNA is elevated in tumors [8]. Augmented circulating PRL was linked to a 60% increased risk of ER+ tumors [4] and elevated circulating PRL 10 years prior to diagnosis was associated with the risk for metastatic ER+ disease [9]. Circulating PRL and PRL secreted in hyperprolactinemia have been also associated with metastatic breast cancer [10,11].
Additionally, breast cancer tumorigenicity is amplified by PRL produced locally by the breast tumor [12]. In response to PRL, PRLR activates non-receptor tyrosine kinase Janus-kinase 2 (JAK2) which activates several signaling cascades, including signal transducers and activators of transcription (STATs) [13].
Although PRL and estrogen exert independent effects on breast cancer cells, there is crosstalk between the two hormones that leads to enhanced breast cancer cell proliferation [14]. This cross-talk occurs at multiple levels and is bidirectional in that modulation of the ER pathway influences PRL pathways and vice versa. PRL increases expression and phosphorylation of ERα, and enhances alternative estrogen signaling, while estrogen induces transcription of both PRL and PRLR and potentiates PRL-dependent STAT5 activity (reviewed in [15]). Defining the mechanisms of cross-talk between estrogen and PRL has important implication for treatment of tumor resistance and for identifying new therapeutic targets in breast cancer. Here we provide evidence that the serine-threonine kinase PAK1 is a common node in PRL and estrogen cross-talk.
The misregulation of PAK1 activity has been demonstrated in many cancers and has been extensively studied in breast cancer [16]. PAK1 directly phosphorylates ERα at Ser305 to promote the transactivation of the receptor leading to upregulation of cyclin D1. This transactivation may contribute to the development of estrogen-independent growth of breast cancer cells and tamoxifen resistance [17]. However, the role of PAK1 in the respective signaling pathways affected is not defined.
We have previously discovered that PAK1 is a target for PRL-activated JAK2 and that JAK2 phosphorylates PAK1 on tyrosines 153, 201, and 285 [18]. Tyrosyl phosphorylation of PAK1 (pTyr-PAK1) enhances PAK1 kinase activity and its ability to form protein/protein interactions. Both PAK1 activities are important for PRL-dependent breast cancer cell adhesion, motility, and invasion [19,20,21]. pTyr-PAK1 also regulates PRL-induced cyclin D1 promoter activity [22]. Similarly to PRL treatment, irradiation of lung cancer cells also leads to phosphorylation of tyrosines 153, 201 and 285 by JAK2 resulting in increases in PAK1 stability, epithelial-mesenchymal transition and radioresistance [23]. In addition to JAK2, the nonreceptor tyrosine kinase Etk/Bmx phosphorylates and activates PAK1 leading to increased proliferation of MCF-7 cells [24].
In the present study, we sought to elucidate the mechanism by which estrogen and PRL co-activate ERα and a role of pTyr-PAK1 in this process. We demonstrated that estrogen-activated Etk directly phosphorylated PAK1 on Tyr153. Activated Gβγ bound to PAK1 in a complex with Etk and PAK1-associated βPIX to potentiate PKA activation. PKA-Cα directly phosphorylated PAK1 while PKA-RIIβ was a direct target of PAK1. We also demonstrated that, in response to estrogen, PKA phosphorylated Ser305-ERα whereas PRL caused phosphorylation of the same site by PAK1. Our results identified reciprocal relationships between PAK1 and PKA, wherein each activated the other in response to estrogen. Our findings established PAK1 as a new target in both estrogen and prolactin signaling and provide a possible explanation of how these two hormones synergistically regulate breast cancer cell proliferation.
Materials and Methods
Plasmids, antibodies, siRNA, reagents and inhibitors are described in Supplementary Material and Methods and in Supplemental Table 1S.
Cells
MCF-7 and T47D cell lines were purchased from the ATCC. The following cell lines were kindly donated: TMX2-28 (Dr. Eisenmann, University of Toledo, OH); SKBR3 (Dr. Chen, Clemson University, SC), HC-11 (Dr. Carter-Su, University of Michigan, MI), MDA-MB-134 and BT474 (Dr. Rae, University of Michigan, MI). All cell lines were authenticated using Promega GenePrint10 System and were grown up for fewer than 6 months following resuscitation. They were routinely verified to be mycoplasma-free using the Mycoprobe Mycoplasma Detection Kit (R&D Systems). All cell lines were tested and authenticated in November 2015. MCF-7 clones, SKBR3, HC-11, MDA-MB-134, and BT474 cells were maintained in DMEM (Corning-Cellgro) supplemented with 10% FBS (Sigma-Aldrich). T47D and TMX2-28 clones were maintained as described in [19,20].
Immunoprecipitation, PAK1 in vitro kinase assay and gene silencing were performed as described in [21]. Briefly, IP’d PAK1 was subjected to an in vitro kinase assay in the presence of 10 μCi of [γ-32P]ATP and histone H4 (substrate of PAK1). For siRNA transfection, 100 nM of specific siRNAs (PAK1 and βPIX (Santa-Cruz), Etk/Bmx, GPER1, ERα (Cell Signaling)) were transiently transfected using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s instructions.
PKA kinase assay
Cells were lysed by 5 freeze/thaw cycles. Equal amount of protein was subjected to an in vitro kinase assay in the presence of 5 μCi [γ-32P] ATP, and kemptide (synthetic peptide substrate). 20μl of the reaction volume was spotted on P81 phosphocellulose paper (EMD Millipore) and subjected to liquid scintillation counting [25,26].
Cell proliferation assay
The cells were deprived for 24h in phenol red-free DMEM supplemented with 1% charcoal-stripped serum, treated with 500 ng/ml PRL or 1 nM E2. Cell proliferation was assessed in 7 days by MTT cell proliferation assay (Life Technologies) according to manufacturer’s protocol [25,26].
Treatment with inhibitors
Serum-deprived cells were treated with E2 (1nM, 30min) in the presence or absence of inhibitors: LY294002 (10μM, 1h); UO126 (10μM, 1h); SB203580 (10μM, 6h); SP600125 (30μM, 1h); AG879 (increasing concentrations, 1h); PP1 or PP2, (increasing concentrations, 2h ); GF109203X (5μM, 2h); IKK inhibitor VII (5μM, 16h); imatinib mesylate (STI 571; increased concentrations, 24h); or canertinib (CNT, increased concentrations, 4h). To inhibit PKA, H89 (10μM, 1h); myristolated-PKI amide (14–22) (myr; 20μM, 1h), Rp-cAMP (100μM, 2h), and 4-cyano-3 methylisoquinoline (4C3MQ; 2μM, 4h) were used. Whole cell lysates were probed with indicated Abs. For ETK and Src inhibition, ETK and Src were IP’d with the αETK and αSrc respectively and subjected to in vitro kinase assay.
Etk in vitro kinase assay
HA-PAK1 WT/pCMV6 or PAK1 mutants were translated in vitro using TNT Coupled Reticulocyte Lysate System T7 (Promega) with S35-methionine according to the manufacturer’s instruction. The mixture of recombinant active Etk (50 ng) and in vitro translated or IP’d PAK1 was incubated in the presence of 10 μCi of [γ-32P]ATP or 20 μM non-radioactive ATP.
Xenograft model
MCF-7 clones stably overexpressing vector, PAK1 WT or PAK1 Y3F were inoculated directly into mammary fat pad of NSG (NOD/SCID/ IL2Rgamma) female mice. The mice were supplied with Dox (2mg/ml) in drinking water and implanted with slow-release estradiol pellets. hPRL (20 μg/100 μl) was injected subcutaneously every other day until the end of experiment. Tumors were measured and volume calculated as V = (4/3)π R12 R2. Mouse experimental procedures were performed in the animal research core of Lerner Research Institute, Cleveland Clinic (Dr. Lindner), and were approved by the Institutional Animal Care and Use Committee, Cleveland Clinic.
Statistical Analysis
Data from at least 3 separate experiments were pooled and analyzed using 1-way analysis of variance plus Tukey’s honest significant difference test. Differences were considered to be statistically significant at a P < 0.05. Results are expressed as the mean ± standard error (S.E.).
Results and Discussion
Estrogen activates PAK1 in a tyrosyl phosphorylation-dependent manner
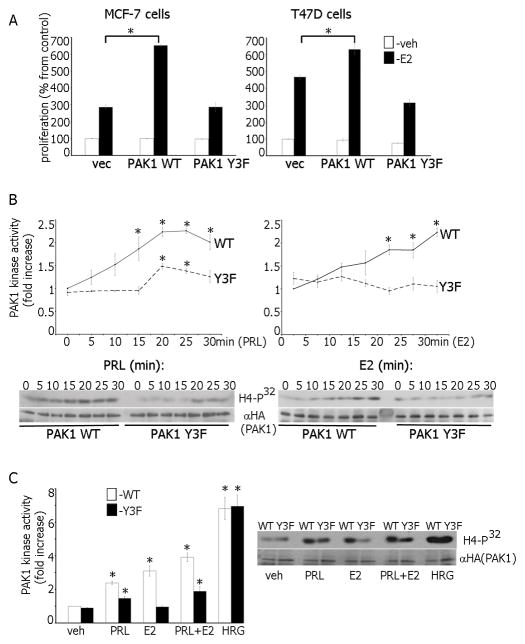
17β-estradiol (E2) is responsible for proliferation of normal and neoplastic breast tissue. Because PAK1 also stimulates cell proliferation [16], we tested whether PAK1 overexpression enhances the E2-dependent proliferation of MCF-7 and T47D breast cancer cells. As demonstrated previously, E2 induced proliferation of T47D and MCF-7 cells (Fig. 1A, vec). PAK1 WT overexpression did not affect basal cell proliferation, and E2 increased cell growth in both lines (Fig. 1A, PAK1 WT cells). In contrast, overexpression of PAK1 Y3F (3 JAK2 phosphorylation sites mutated) strongly decreased E2-dependent cell proliferation relative to PAK1 WT overexpression (Fig. 1A, PAK1 Y3F cells). Similar results were obtained in BT474, MDA-MB-134 and HC-11 cell lines (Fig. S1A) indicating that this finding was not restricted to MCF-7 and T47D cells. To confirm the role of PAK1 in cell proliferation, we treated control MCF-7 and T47D cells with PAK1 inhibitor IPA-3 (Fig. S1B; [27]). IPA-3 did not inhibit proliferation of vehicle-treated cells and was not toxic but it did inhibit E2-dependent cell proliferation in a concentration-dependent manner (Fig. S1C) confirming that PAK1 participates in E2-dependent cell proliferation.
Figure 1.
Estrogen activates PAK1. (A) MCF-7 and T47D cells overexpressing PAK1 WT or PAK1 Y3F were incubated with/without E2. Changes in cell numbers at day 7 are shown as percentages of the veh-treated cell number. *, p <0.05 compared with control cells in the same condition, n=3. (B) PAK1 WT or Y3F MCF-7 clones were treated with PRL (left), or E2 (right). PAK1 was IP’d and subjected to an in vitro kinase assay with H4 histone as a substrate. PAK1 kinase activity was normalized by IP’d PAK1 for each lane and plotted. (C) PAK1 WT or Y3F clones were treated with veh, PRL, E2, PRL+E2 or HRG and assessed as in B. *, p<0.05, n=3.
To uncover the mechanism by which pTyr-PAK1 regulates E2-dependent cell proliferation, we assessed PAK1 kinase activity in E2-treated cells, because E2 activates PAK1 [28]. As demonstrated previously, PRL activated PAK1 WT significantly stronger than PAK1 Y3F confirming that tyrosines 153, 201 and 285 are required for full activation (Fig. 1B, left; [19,20]). PAK1 WT activation by E2 or PRL were similar. Surprisingly, E2 failed to activate PAK1 Y3F (Fig. 1B, right). Additionally, E2-induced phosphorylation of Thr423-PAK1 (a marker of PAK1 activation) was detected in PAK1 WT but not in PAK1 Y3F cells (Fig. S1D) demonstrating that PAK1 tyrosyl phosphorylation is important for E2-dependent PAK1 activation. To confirm that PAK1 Y3F retains its kinase activity, we assessed effect of the heregulin (HRG), a known activator of PAK1 [29], on PAK1 activation. HRG-induced PAK1 kinase activity was similar in cells expressing PAK1 WT and PAK1 Y3F (Fig. 1C) confirming that PAK1 Y3F is functionally active.
Etk kinase directly phosphorylates and activates PAK1 in response to estrogen
To understand how E2 mediated PAK1 tyrosyl phosphorylation and activation, we tested whether E2 activates JAK2. As expected, PRL, but not E2, strongly activated JAK2 (Fig. S2A). We hypothesized that kinase(s) other than JAK2 tyrosyl phosphorylated PAK1 in response to E2, and tested Etk/Bmx, Src, c-Abl and ErbB because they have been previously implicated in tyrosyl phosphorylation of either PAK1 or PAK2 [24,30,31,32]. Inhibition of c-Abl by STI571 and ErbB by canertinib (CNT) did not affect E2-dependent PAK1 tyrosyl phosphorylation, although Erk 1/2 activation was eliminated by both inhibitors (Fig. S2B). Similarly, Src inhibitors PP1 and PP2 did not affect E2-dependnet PAK1 tyrosyl phosphorylation, but blocked Src activation (Fig. S2C).
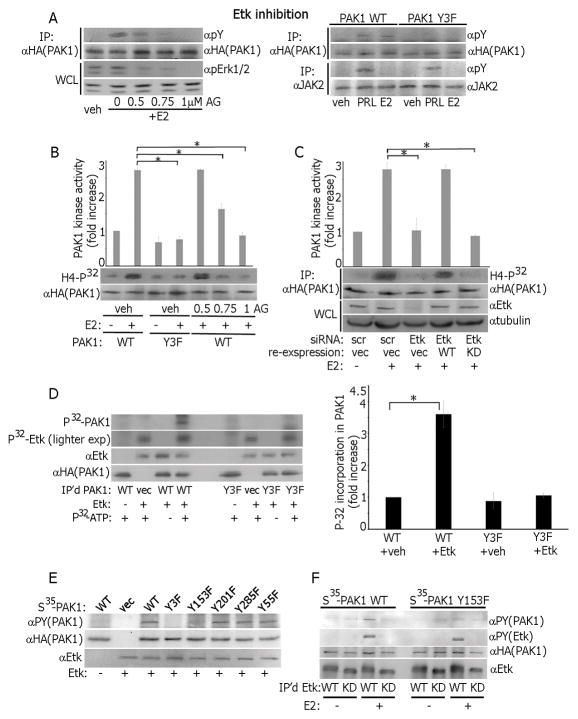
AG879 inhibition of Etk resulted in a concentration-dependent decrease in pTyr-PAK1 (Fig. 2A, left) suggesting that Etk is involved in E2-dependent PAK1 phosphorylation. Tyrosines 153, 201 and 285 are implicated in this E2-dependent phosphorylation because Y3F was not phosphorylated in response to neither PRL nor E2 (Fig. 2A, right). More importantly, Etk inhibition attenuated E2-dependent PAK1 kinase activity (Fig. 2B). To support this finding, we knocked-down Etk by siRNA and assessed E2-dependent PAK1 activity. Indeed, Etk silencing decreased E2-dependent PAK1 activity (Fig. 2C). We recovered E2-dependent PAK1 activation by re-introducing WT, but not kinase-dead Etk [33], in the Etk-depleted cells (Fig. 2C). These data strongly suggest that Etk tyrosyl phosphorylates and activates PAK1 in response to E2. To mechanistically link Etk and PAK1, we IP’d PAK1 WT or Y3F, performed the in vitro kinase assay with active recombinant Etk and demonstrated that Etk phosphorylates PAK1 WT but not Y3F (Fig. 2D). To map PAK1 phosphorylation site(s), we performed in vitro kinase assays using recombinant Etk and in vitro-translated PAK1 WT, Y3F, Y153F, Y201F, Y285F or Y55F PAK1 mutants. Etk failed to phosphorylates both PAK1 Y3F and Y153F (Fig. 2E). To show that Etk phosphorylates Tyr-153 of PAK1 in response to E2, we IP’d overexpressed Etk WT or kinase-dead Etk from MCF-7 cells treated with/without E2 and assessed kinase activity in vitro with in vitro-translated PAK1 WT and Y153F. As expected, E2-activated Etk directly phosphorylated PAK1 WT but not Y153F (Fig. 2F). Our data strongly suggest that E2-activated Etk directly phosphorylates PAK1 WT on Tyr 153 causing PAK1 activation.
Figure 2.
Etk directly phosphorylates and activates PAK1 in response to estrogen. (A) PAK1 WT cells were treated with Etk inhibitor AG879 before E2 treatment and IP’d PAK1 or whole cell lysate (WCL) were probed with Abs (left panel). PAK1 or JAK2 were IP’d from PAK1 WT and Y3F clones treated with E2 or PRL and immunoblotted (right panel). (B) Cells were treated with AG879. PAK1 was IP’d and subjected to an in vitro kinase assay as in Fig. 1. (C) Cells were transfected with control (scr) or Etk siRNA. In siRNA rescue experiments, 24h after Etk siRNA transfection, cells were transfected with either Etk WT or Etk kinase-dead mutant (KD). PAK1 was IP’d and subjected to in vitro kinase assay as in Fig. 1. (D) PAK1 WT and Y3F were IP’d and assessed in the in vitro kinase assay in the presence of recombinant active Etk. The levels of P32 incorporation in PAK1 were plotted. (E) PAK1 WT and indicated tyrosine-deficient PAK1 mutants were translated in vitro and subjected to the in vitro kinase assay in the presence of recombinant Etk and non-radioactive ATP. (F) WT or kinase-dead (KD) Etk were IP‘d from the cells treated with/without E2 and assessed in the in vitro kinase assay in the presence of either PAK1 WT or Y153F as in E. Each figure represents the same blot reblotted with the indicated antibodies. All blots are representative of at least 3 experiments.*, p<0.05, n=3.
Although the mechanisms by which estrogen receptors regulate Etk kinase need to be further investigated, we speculate that Etk may be an effector of Gβγ subunits in response to E2 (see below).
E2 promotes PAK1 activation through both ERα and GPER1 plasma membrane receptors
The rapid activation of PAK1 by E2 (Fig. 1B) indicates involvement of either membrane-localized estrogen receptor (mER) and/or GPER1. These two estrogen-responsive receptors are structurally different and can trigger different signaling pathways in the same cell including MCF-7 cells [34,35,36].
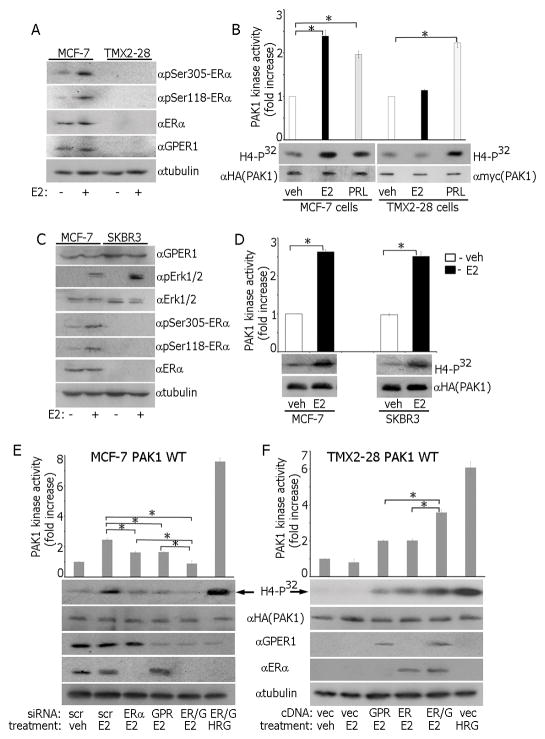
To test whether mERα is required for E2-triggered PAK1 activation, we used TMX2-28 cells, an ER-negative variant of MCF-7 cells [37]. As expected, E2 caused phosphorylation of Ser118-ERα and Ser305-ERα (markers of ER activation) in MCF-7 cells but not in TMX2-28 cells (Fig. 3A). Furthermore, TMX2-28 cells are also GPER1-deficient (Fig. 3A). E2 activated PAK1 in MCF-7 cells but not in TMX2-28 cells while PRL activated PAK1 similarly in both lines (Fig. 3B) suggesting that either mERα or GPER1 are required for E2-dependent PAK1 activation.
Figure 3.
Both GPER1 and mERα are involved in estrogen-dependent PAK1 activation. MCF-7 and TMX2-28 cells were treated with E2 and either WCL were probed with indicated Abs (A) or in vitro kinase assay was performed (B). (C–D) MCF-7 and SKBR3 cells were treated with E2 and either WCL were probed with the indicated Abs (C) or in vitro kinase assay was performed (D). (E) MCF-7 PAK1 WT cells were transfected with control (scr), ERα, GPER1 (GPR) or ERα+GPER1 (ER/G) siRNAs. HA-PAK1 was IP’d and subjected to in vitro kinase assay. (F) TMX2-28 PAK1 WT cells were transfected cDNAs encoding GPER1 (GPR), ERα, or ERα+GPER1 (ER/G). HA-PAK1 was IP’d and subjected to the in vitro kinase assay.
To determine whether E2 activates PAK1 via GPER1, we employed ER-negative/GPER1-positive SKBR3 cells. E2 activated GPER1 in both MCF-7 and SKBR3 cells as demonstrated by Erk1/2 phosphorylation while E2 activated ERα only in MCF-7 cells (Fig. 3C). E2 activated PAK1 in both lines suggesting that GPER1 participates in PAK1 activation (Fig. 3D). To confirm this observation, we performed siRNA knock-down of either GPER1, ERα or both receptors in MCF-7 cells. Silencing of either ERα or GPER1 significantly decreased E2-triggered PAK1 activation, however, only downregulation of the ERα and GPER1 together completely abolished E2-dependent PAK1 activation (Fig. 3E). We also overexpressed GPER1, ERα or both receptors in TMX2-28 cells and showed that E2 caused maximal PAK1 activation when both GPER1 and ERα were present (Fig. 3F). These data suggest that E2 promotes PAK1 activation through both mERα and GPER1 plasma membrane receptors.
Gβγ subunits of G protein activate PAK1 in response to estrogen
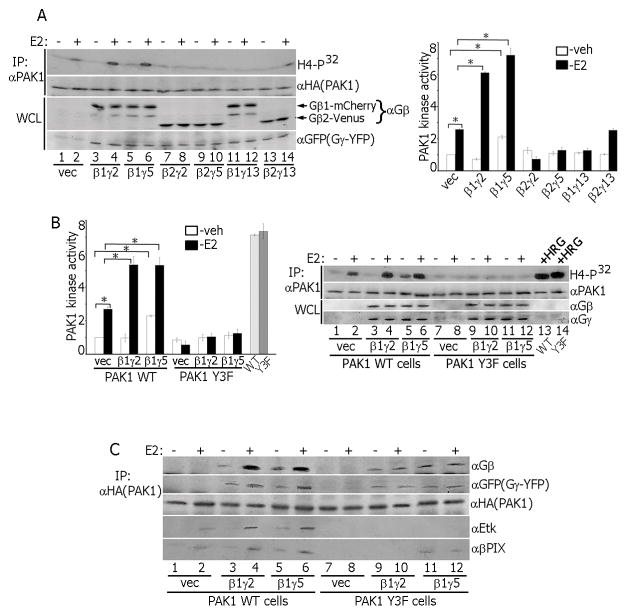
mERα and GPER1 activate heterotrimeric G-proteins [3]. The Gβγ dimer directly binds to both PAK1 [38] and E2-activated mERα [39]. To determine which combination of β and γ subunits participates in E2-dependent PAK1 activation, we overexpressed β1 or β2 together with γ2, γ5 or γ13 in different combinations and assessed E2-dependent PAK1 activity (Fig. 4A). As expected, E2 activated PAK1 (lane 2 vs. lane 1). β1γ2 and β1γ5 overexpression strongly enhanced E2-dependent PAK1 activity (lanes 4 and 6). Interestingly, β2γ2 (lane 8), β2γ5 (lane 10) or β1γ13 (lane 12) overexpression inhibited E2-dependent PAK1 activity while β2γ13 (lane 14) overexpression had no effect compared to control (lane 2). To test whether PAK1 tyrosines play a role in activation, we overexpressed β1γ2 and β1γ5 subunits in PAK1 WT and Y3F clones and assessed E2-dependent PAK1 activation. β1γ5 overexpression increased both basal and E2-stimulated PAK1 WT activity (Fig. 4B, lanes 5–6) while β1γ2 overexpression enhanced PAK1 WT activity only in response to E2 (lanes 3–4). β1γ2 and β1γ5 overexpression did not activate PAK1 Y3F (lanes 9–12) whereas HRG strongly activated PAK1 WT and Y3F (lanes 13–14). To assess PAK1 binding to β1γ2 and β1γ5, PAK1 WT or Y3F were IP’d and assessed for β1γ2, β1γ5 (Fig. 4C) and β1γ13 (Fig. S2D). PAK1 WT bound to E2-activated β1γ2 and to β1γ5 independent of E2 (Figs. 4C and S2D, lanes 3–6). We considered PAK1 WT binding to β1γ2 without E2 (Figs. 4C and S2D, lane 3), to β1γ13 (Fig. S2D, lanes 1–2) and Y3F to β1γ13, β1γ2 and β1γ5 (Fig. 4C and S2D) as negligible. Endogenous Etk was associated with E2-activated PAK1 WT (αEtk blot, lanes 2, 4 and 6 in Figs. 4C and S2D) while PAK1 Y3F failed to bind to Etk (lanes 7–12). These data suggest that E2 treatment promotes formation of Gβ1γ2,5/PAK1/Etk complex.
Figure 4.
Gβγ subunits activate PAK1 in response to estrogen. (A, B) Combinations of β1, β2, γ2, γ5 and γ13 subunits were overexpressed. E2-dependent PAK1 activation was assessed in the in vitro kinase assay. Both gray bars in B represent the cells treated with HRG. (C) HA-PAK1 was IP’d from PAK1 WT or Y3F clones overexpressing β1γ2 or β1γ5 subunits and immunoblotted.
PAK1 can be activated by β1γ2 or β1γ5 subunits via the PI3-kinase and Akt pathways independently of Rac1 or Cdc42 [40]. In that study EGF, PMA, LPA and carbachol did not augment PAK1 activity beyond that induced by β1γ2 or β1γ5 [40]. Our data demonstrated additive effect of E2 underlying a high degree of specificity for this pathway.
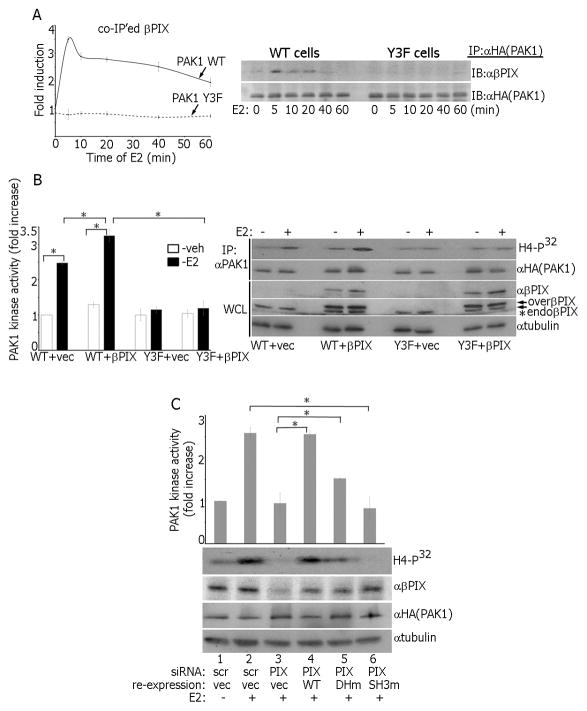
βPIX facilitates E2-dependent PAK1 activation
In response to chemoattractants, Gβγ binds directly to PAK1, which simultaneously associates with αPIX (PAK-associated guanine nucleotide exchange factor) to form a Gβγ/PAK1/αPIX/Cdc42 complex leading to PAK1 activation [38]. Because we have previously shown that PRL increases association of βPIX with PAK1 WT but not Y3F and Y285F mutants [21], we reasoned that formation of the Gβγ/PAK1/βPIX complex plays a role in E2-dependent PAK1 activation. We IP’d PAK1 from E2-treated PAK1 WT and Y3F cells and probed for endogenous βPIX. E2 increased βPIX association with PAK1 WT but not with Y3F (Fig. 5A), demonstrating that tyrosyl phosphorylation of PAK1 facilitates E2-dependent binding with βPIX. We next overexpressed βPIX in WT and Y3F clones and assess E2-dependent PAK1 kinase activity. βPIX significantly activated PAK1 WT vs. Y3F in response to E2 (Fig. 5B). βPIX silencing abolished E2-dependent PAK1 activation (Fig. 5C, lane 3) while re-expression of βPIX WT rescued PAK1 activation (lane 4). Re-expression of βPIX DHm(L238R/L239S), lacking GEF activity, partially rescued PAK1 kinase activity (lane 5) and re-expression of βPIX SH3m(W43K), which lacks PAK1 binding activity, did not (lane 6). These data suggest that βPIX binding to PAK1 is required for its activation and that βPIX GEF activity is sufficient for E2-dependent PAK1 activation. Furthermore, PAK1 tyrosyl phosphorylation is also required for E2-dependent βPIX binding.
Figure 5.
βPIX facilitates E2-dependent PAK1 activation. (A) IP’d PAK1 from WT and Y3F clones treated with E2 was assessed for endogenous βPIX. (B) βPIX was overexpressed in WT and Y3F clones and PAK1 kinase activity was assessed. * indicates longer exposure with αβPIX revealing endogenous βPIX (lower double-arrow). Overexpressed βPIX is indicated by the upper double-arrow. (C) The cells were transfected with control (scr; lanes 1–2) or βPIX siRNA (lanes 3–4). In siRNA rescue experiments, βPIX DHm(L238R/L239S) (lane 5) or SH3m(W43K) (lane 6) mutants were re-expressed and PAK1 activation was assessed in the in vitro kinase.
To test whether βPIX is a member of Gβ1γ2,5/PAK1/Etk complex, we IP’d either WT or Y3F from cells treated with/without E2 and blotted for βPIX. βPIX was associated with E2-activated PAK1 WT (Figs. 4C and S2D, αβPIX blot, lanes 2, 4, 6). PAK1 Y3F complexed with β1γ5 only, and independently of E2 (lanes 11–12). E2 also promotes formation of this Etk/PAK1/βPIX complex in T47D, BT474, MDA-MB-134 and HC-11 cells (Fig. S3A). These data suggest that E2 induces a multiprotein signaling complex containing pTyr-PAK1, Etk, β1/γ2 or β1/γ5 subunits and βPIX. It appears that three tyrosines of PAK1 are required for the formation of this complex which facilitates PAK1 activation.
E2-dependent PKA activity is reciprocally regulated by activated pTyr-PAK1
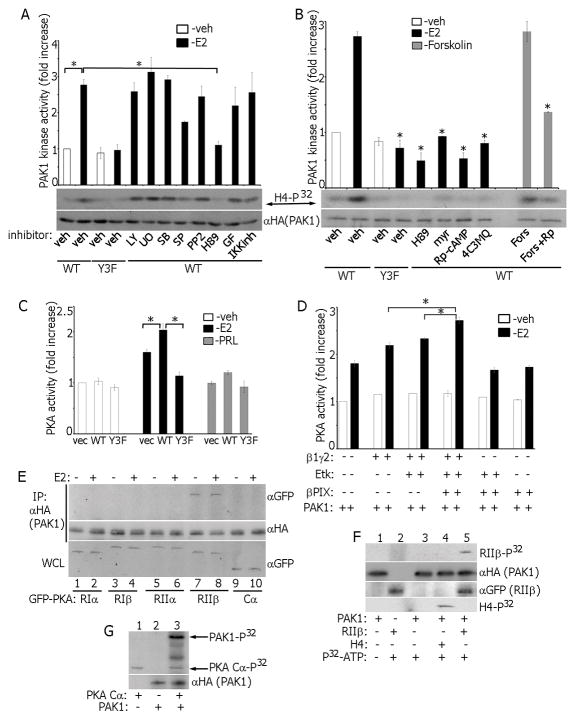
E2 rapidly activates multiple signaling pathways, including MAPKs, PI3K, PKC, PKA, NFkB and Src [2]. To determine which of these pathways are implicated in E2-triggered PAK1 activation, we treated MCF-7 cells with E2 and selective inhibitors. Inhibitor specificity was confirmed (Fig. S4A) and all inhibitors had no effect on PAK1 activity in vehicle-treated cells, demonstrating that they did not independently effect PAK1 activity (Fig. S3B). Only PKA inhibition by H89 inhibited E2-dependent PAK1 kinase activity (Fig. 6A). To confirm that the effect of H89 was specific to estrogen, we treated PAK1 WT cells with PRL+H89 and showed that PAK1 kinase activity was unaffected (Fig. S3C). In addition to H89, other selective PKA inhibitors, myristolated-PKI amide (14–22) (myr), Rp-cAMP and 4-cyano-3 methylisoquinoline (4C3MQ) (Fig. S4B) also inhibited E2-activated PAK1 (Fig. 6B). Furthermore, PAK1 activity was enhanced by Forskolin, which triggers general cAMP elevation and PKA activation, and abolished by Rp-cAMP (Fig. 6B).
Figure 6.
Reciprocal regulation of PAK1 and PKA in E2 signaling. (A) Cells were treated with inhibitors: UO126, SB203580, SP600125, LY294002, IKK inhibitor, GF109203X and H89, then with E2 and PAK1 activation was assessed in the in vitro kinase assay. (B) PAK1 activation was assessed as in A in cells treated with indicated different PKA inhibitors. *, P < 0.05 compared with cells expressing PAK1 WT and treated with veh+E2. (C) Cell lysates were assessed for PKA activity in the in vitro kinase assay. (D) Combinations of β1γ2 subunits, Etk and βPIX were overexpressed in MCF-7 PAK1 WT cells. E2-dependent PKA activation was assessed in the in vitro kinase assay. (E) mEGFP-tagged cDNAs encoding different PKA subunits (RIα, RIβ, RIIα, RIIβ and Cα) were overexpressed in MCF-7 PAK1 WT clone and treated with/without E2. HA-PAK1 was IP’s with αHA and immunoblotted. (F) In vitro translated PAK1 was incubated with IP’d PKA RIIβ in the in vitro kinase assay. H4 histone was used as a PAK1 substrate (lane 4). (G) In vitro translated PAK1 was incubated with recombinant active PKA Cα (catalytic subunit) in the in vitro kinase assay. Incorporation of P32 in PAK1 (upper arrow) and in PKA Cα (low arrows) is indicated.
In response to E2 both mERα and GPER1 activate Gαs leading to stimulation of AC, cAMP and subsequent activation of PKA [2,3]. PKA is a PAK1 regulator in anchorage-dependent signal transduction [41]. PKA phosphorylates βPIX leading to activation of PAK1 effector, Cdc42 [42]. Furthermore, PKA phosphorylates PAK1 and the PKA catalytic subunit phosphorylates and complexes with PAK1/4 [41,43,44]. Hence, we hypothesized that activated PAK1 may in turn modulate PKA activity. PKA in vitro kinase activity was significantly elevated in E2-treated, but not in RPL-treated, PAK1 WT cells (Fig. 6C). Interestingly, PAK1 Y3F inhibited E2-triggered PKA activation (Fig. 6C). To study whether formation of Gβ1γ2/Etk/PAK1/βPIX complex facilitates E2-dependent PKA activation, we overexpressed these proteins in PAK1 WT cells and assessed endogenous PKA in vitro kinase activity. PKA activity was maximal when β1γ2, Etk, PAK1, and βPIX were present and E2 was added (Fig. 6D). To determine which PKA subunit complexed with PAK1, different PKA subunits were overexpressed in PAK1 WT cells, treated with/without E2. PKA-RIIβ subunit interacted with PAK1 WT in E2-independent manner (Fig. 6E). To demonstrate that PKA RIIβ is a direct substrate of PAK1, we assessed in vitro translated HA-PAK1 with IP’d PKA-RIIβ in the in vitro kinase assay. Translated PAK1 was active as judged by the in vitro phosphorylation of H4 histone (PAK1 substrate, Fig. 6F, lane 4) and PAK1 directly phosphorylated RIIβ (Fig. 6F, lane 5). In agreement with published data [41], in vitro translated PAK1 was directly phosphorylated by recombinant active PKA-Cα (catalytic subunit) in the in vitro kinase assay (Fig. 6G). These data suggest that PKA-Cα directly phosphorylates PAK1 while PKA-RIIβ is the direct target of PAK1. In response to E2, activated pTyr-PAK1 complexes with β1γ2/Etk/βPIX to potentiate PKA activation. At the same time, PKA is required for full E2-dependent PAK1 activation, demonstrating positive feedback between these two kinases.
PKA phosphorylates Ser305-ERα in response to E2 and PAK1 phosphorylates Ser305-ERα in response to PRL
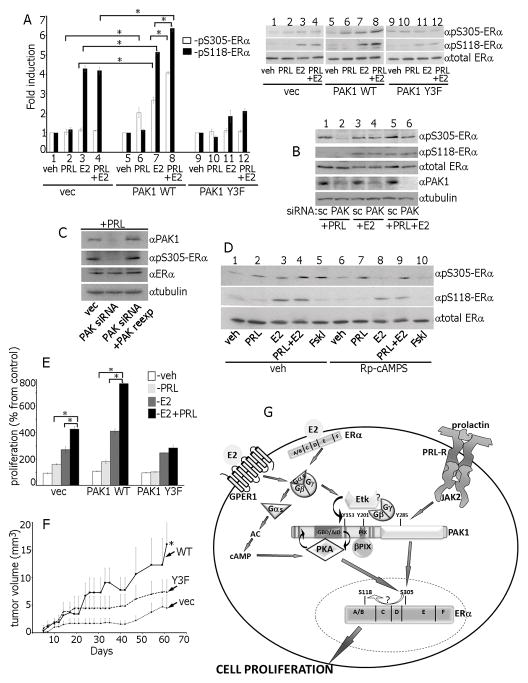
Ser305-ERα can be phosphorylated by PAK1 and PKA [45,46]. Constitutively active PAK1 phosphorylates Ser305-ERα resulting in subsequent phosphorylation of Ser118-ERα, ligand-independent ER activation and tamoxifen resistance [45,47]. PAK1 overexpression increased phosphorylation of both Ser305-ERα and Ser118-ERα upon E2 treatment (Fig. 7A, lanes 3 vs. 7, white and black bars) while Y3F overexpression inhibited pSer118-ERα (Fig. 7A, lanes 11 vs. 3). PRL treatment induced Ser305-ERα, but not Ser118-ERα phosphorylation in WT PAK1 cells, but had no effect in Y3F cells (Fig. 7A, lanes 2, 6 and 10).
Figure 7.
Ser305-ERα is phosphorylated by PKA in response to E2 and by PAK1 in response to PRL. (A) Control, PAK1 WT or PAK1 Y3F clones of MCF-7 cells were treated with veh, E2, PRL or PRL+E2 and WCL were immunoblotted. (B) Cells were transfected with control (scr) or PAK1 siRNA, treated with PRL, E2 or PRL+E2 and WCL were immunoblotted. (C) In siRNA rescue experiments, 24h after PAK1 siRNA transfection, cells were transfected with control or PAK1 WT, treated with PRL and WCL were immunoblotted. (D) Cells were incubated with either veh or Rp-cAMP, treated with veh, PRL, E2, PRL+E2 or Forskolin. (E) MCF-7 overexpressing vec, PAK1 WT or PAK1 Y3F were incubated with veh, PRL, E2 or PRL+E2 and assessed for cell proliferation as in Fig.1. (F) pTyr-PAK1 accelerates tumor growth in vivo. MCF-7 clones were injected into the mammary pad of female NSG mice. Mice were treated with PRL+E2. *, p<0.05 compared with control (vec) mice, n=8. (G) Proposed mechanisms for the role of PAK1 in synergetic effects of PRL and E2 on ERα activation. E2 activates membrane-localized ERα and GPER1 leading to G-protein and Etk kinase activation. E2-activated Etk binds to and directly phosphorylates PAK1 on Tyr153. Gβγ subunits also bind to pTyr-PAK1, facilitating recruitment and activation of βPIX, which in turn binds and further activates PAK1 and establishes a self-amplifying cascade. Gαs subunit activates PKA, which binds to PAK1 via PKA-RIIβ while PKA-Cα directly phosphorylates PAK1. Gβγ/Etk/pTyr-PAK1/βPIX complex reciprocally activates PKA in response to E2 and activated PKA phosphorylates Ser305-ERα leading to enhanced S118-ERα phosphorylation. PRL-activated JAK2 phosphorylates and activates PAK1 which also activates ERα by phosphorylation of S305-ERα. Thus, in response to E2+PRL, pS305-ERα is phosphorylated by both PAK1 and PKA that leads to enhanced cell proliferation.
Next we aimed to determine whether PAK1 or PKA participate in E2- and PRL- triggered ERα phosphorylation. PAK1 siRNA silencing abolished PRL-dependent Ser305-ERα phosphorylation, but not E2-dependent Ser305-ERα phosphorylation (Fig. 7B, lanes 2 vs. 4). To confirm that PAK1 phosphorylates Ser305-ERα in response to PRL, we re-introduced PAK1 into PAK1-silenced cells and recovered PRL-dependent Ser305-ERα phosphorylation (Fig. 7C). To test whether PKA phosphorylates Ser305-ERα in response to E2, we inhibited PKA by Rp-cAMPs, and observed that pSer305-ERα signal was strongly decreased in the cells treated with E2 or Foskolin (Fig. 7D, lanes 3 vs. 8 and 5 vs. 10) but not in the cells treated with PRL or PRL+E2 (Fig. 7D, lanes 2 vs. 7 and 4 vs. 9).
These data suggest that PKA phosphorylates Ser305-ERα in response to E2 while PAK1 phosphorylates Ser305-ERα in response to PRL. PAK1 augments PKA-dependent Ser305-ERα phosphorylation, as PAK1 overexpression significantly increased E2-dependent Ser305-ERα phosphorylation (Fig. 7A, lines 7 vs. 3). Furthermore, PAK1 tyrosines 153, 201 and 285 participate in both signaling pathways because Y3F overexpression inhibited Ser305-ERα phosphorylation after both PRL and E2 treatments (Fig. 7A, lanes 10–12). Since PAK1 WT, but not Y3F, significantly enhanced E2-dependent PKA activation (Fig. 6D), our data suggest that although PAK1 does not phosphorylate Ser305-ERα upon E2 treatment, it augments PKA activation and subsequent Ser305-ERα phosphorylation, indirectly increasing estrogen responsiveness.
Synergistic effects of PRL and E2 on ERα activation, cell proliferation ant tumor growth
If both PKA and PAK1 phosphorylate the same site on ERα, we hypothesized that PRL+E2 treatment would have additive effects on Ser305-ERα phosphorylation and subsequent Ser118-ERα phosphorylation. Indeed, we observed amplified pSer305-ERα and pSer118-ERα when PAK1 WT cells were treated with PRL+E2 vs. either with PRL or E2 alone (Fig. 7A, lanes 8 vs. 7 and 6). S305-ERα phosphorylation by PAK1 promotes ERα transactivation, leading to up-regulation of cyclin D1 and hormone-independence [48]. To determine whether enhanced ERα activation impacted breast cancer cells, we evaluated the effect of PRL+E2 on proliferation. In agreement with published studies demonstrating that PRL enhances E2-induced proliferation [14,49,50,51,52], PRL+E2 induced proliferation of control MCF-7 cells (Fig. 7E, vec). PAK1 overexpression further enhanced cell proliferation in response to E2 +PRL as compared to cells treated with either PRL or E2 (Fig. 7E, PAK1 WT cells). In contrast, Y3F inhibited E2+PRL- dependent proliferation (PAK1 Y3F cells) implicating these tyrosines in PAK1 regulation. To demonstrate that the role of PAK1 in synergistic PRL+E2 -mediated signaling is not limited to MCF-7 cells, we assessed proliferation of different human breast cancer cell lines endogenously expressing PRLR and ER (T47D, BT474 and MDA-MB-134) as well as mouse mammary HC-11 cells. In all lines PAK1 overexpression enhanced cell proliferation in response to E2 +PRL, compared to the cells treated with either PRL or E2 (Fig. S5). Finally, PRL+E2 -activated pTyr-PAK1 enhances breast tumor growth in a xenograft mouse model (Fig. 7F).
We have demonstrated here that tyrosines 153, 201 and 285 play a role in PAK1 activation by E2 (but not HRG), ability of PAK1 to complex with Gβ1γ2 and 5, Etk and βPIX, activate PKA, phosphorylate S305-ERα and, finally, enhance PRL+E2-dependent proliferation and tumor growth. Kim and colleagues have proposed that tyrosyl phosphorylation of these sites may disrupt the inhibitory conformation of PAK1 and/or maintain PAK1 active conformation because Tyr153 resides nearby Ser144 and Ser149, Tyr201 locates near Ser199 and Ser204 (autophosphorylation sites) and Tyr285 lies in the kinase domain and may stabilize the interaction between K299 and ATP molecules [23]. Although it should be confirmed by structural studies, this proposition is very attractive. Another possibility is that pTyr-PAK1 may create additional docking sites to recruit SH2 domain-containing proteins to facilitate PAK1 functions. Either way, these tyrosine phosphorylation events are critical for PAK1 functions.
Based on our finding and previous studies, we propose a model for PAK1 in estrogen- and PRL-triggered intracellular signaling leading to a synergistic effect of PRL and E2 on Ser305-ERα phosphorylation, cell proliferation and tumor growth (Fig. 7G). E2 activates membrane-localized ERα and GPER1 leading to G-protein activation that dissociates into α and βγ subunits both of which activate additional effectors. Gαs activates PKA, which can phosphorylates many substrates, including PAK1 and Ser305-ERα. In addition, β1γ2 and β1γ5 are released and bind to PAK1 which is directly phosphorylated by Etk on Tyr153. pTyr-PAK1 recruits and binds to βPIX, an association which further activates PAK1. Although PKA is the main kinase phosphorylating Ser305-ERα in response to E2, PKA is reciprocally regulated by activated PAK1 as a component of β1γ2/Etk/βPIX/pTyr-PAK1 complex. If cells are exposed to both PRL and E2, Ser305-ERα is phosphorylated by both PKA and pTyr-PAK1 resulting in maximal signal. Furthermore, S305-ERα activation enhances Ser118-ER phosphorylation, presumably due to an intramolecular conformational change of ERα [46,47], and finally induces cell proliferation. E2-dependent Etk and PRL-dependent JAK2 PAK1 tyrosyl phosphorylation are important, as PAK1 Y3F fails to establish this self-amplifying cascade and promote cell proliferation and tumor growth.
Another notable finding is that Ser305-ERα is phosphorylated by PAK1 in response of PRL in the absence of estrogen. Ser305-ERα phosphorylation was implicated in tamoxifen resistance and a correlation between high level of PAK1 and tamoxifen sensitivity in breast cancer patients described [47,53]. Our data predict that patients with augmented levels of PRL will likely develop tamoxifen resistance due to Ser305-ERα phosphorylation by PRL-activated PAK1. Furthermore, the synergistic effects of estrogen and PRL signaling described here is physiologically and therapeutically relevant since breast cells are constantly exposed to both hormones, but anti-estrogen therapy targets only the estrogen component. We introduce PAK1 as a common node for estrogen- and PRL-dependent pathways making it an attractive target for anti-cancer therapy.
Supplementary Material
Acknowledgments
This work was supported by a Grant from the National Institutes of Health (R01 DK88127 to MD)
We thank Drs. Sorokin (Medical College of Wisconsin, WI) and Chen (Institute of Biological Chemistry, Taiwan) for providing cDNAs encoding βPIX WT, βPIX mutants, Etk WT and kinase-dead Etk mutant, respectively. We thank Drs. Eisenmann (University of Toledo, OH), Chen (Clemson University, SC), Carter-Su (University of Michigan, MI) and Rae (University of Michigan, MI) for providing cells.
List of Abbreviation
- c-Abl
Abelson murine leukemia viral oncogene homolog 1
- AC
adenylate cyclase
- cAMP
cyclic adenosine monophosphate
- Dox
doxycycline
- E2
17β-estradiol
- ER
estrogen receptor
- Erk
extracellular signal-regulated kinases
- FBS
fetal bovine serum
- GPER1
G protein-coupled estrogen receptor 1
- HA
hemagglutinin
- HRG
heregulin
- IP
immunoprecipitation
- IP’d
immunoprecipitated
- JAK2
Janus-kinase 2
- MAPK
mitogen-activated protein kinases
- mER
membrane-localized estrogen receptor
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAK1
p21-activated serine-threonine kinase
- PI3K
phosphatidylinositol 3-kinase
- βPIX
PAK1-interacting guanine-nucleotide exchange factor β
- PKA
protein kinase A
- PKC
protein kinase C
- PRL
prolactin
- pTyr
tyrosyl phosphorylated
- STAT
signal transducers and activators of transcription
- vec
vector
- veh
vehicle
Footnotes
The authors have nothing to disclose.
References
- 1.Barone I, Brusco L, Fuqua SA. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2702–2708. doi: 10.1158/1078-0432.CCR-09-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin ER. Plasma membrane estrogen receptors. Trends in endocrinology and metabolism: TEM. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson BO, Olde B, Leeb-Lundberg LM. G protein-coupled oestrogen receptor 1 (GPER1)/GPR30: a new player in cardiovascular and metabolic oestrogenic signalling. British journal of pharmacology. 2011;163:1131–1139. doi: 10.1111/j.1476-5381.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tworoger SS, Hankinson SE. Prolactin and breast cancer etiology: an epidemiologic perspective. Journal of mammary gland biology and neoplasia. 2008;13:41–53. doi: 10.1007/s10911-008-9063-y. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez I, Touraine P, Goffin V. Prolactin and human tumourogenesis. Journal of neuroendocrinology. 2010;22:771–777. doi: 10.1111/j.1365-2826.2010.02011.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary KA, Shea MP, Schuler LA. Modeling prolactin actions in breast cancer in vivo: insights from the NRL-PRL mouse. Advances in experimental medicine and biology. 2015;846:201–220. doi: 10.1007/978-3-319-12114-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaminathan G, Varghese B, Fuchs SY. Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:81–91. doi: 10.1007/s10911-008-9068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas A, Trivin C, Postel-Vinay MC, Kuttenn F, Kelly PA. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. 1998;83:667–674. doi: 10.1210/jcem.83.2.4564. [DOI] [PubMed] [Google Scholar]
- 9.Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, Hankinson SE. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer research. 2013;73:4810–4819. doi: 10.1158/0008-5472.CAN-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mujagic Z, Mujagic H. Importance of serum prolactin determination in metastatic breast cancer patients. Croatian medical journal. 2004;45:176–180. [PubMed] [Google Scholar]
- 11.Mujagic Z, Mujagic H, Prnjavorac B. Circulating levels of prolactin in breast cancer patients. Medicinski arhiv. 2005;59:33–35. [PubMed] [Google Scholar]
- 12.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clevenger CV, Gadd SL, Zheng J. New mechanisms for PRLr action in breast cancer. Trends Endocrinol Metab. 2009;20:223–229. doi: 10.1016/j.tem.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Gutzman JH, Nikolai SE, Rugowski DE, Watters JJ, Schuler LA. Prolactin and estrogen enhance the activity of activating protein 1 in breast cancer cells: role of extracellularly regulated kinase 1/2-mediated signals to c-fos. Mol Endocrinol. 2005;19:1765–1778. doi: 10.1210/me.2004-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carver KC, Arendt LM, Schuler LA. Complex prolactin crosstalk in breast cancer: new therapeutic implications. Mol Cell Endocrinol. 2009;307:1–7. doi: 10.1016/j.mce.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eswaran J, Li DQ, Shah A, Kumar R. Molecular pathways: targeting p21-activated kinase 1 signaling in cancer--opportunities, challenges, and limitations. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3743–3749. doi: 10.1158/1078-0432.CCR-11-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayala SK, Kumar R. Sliding p21-activated kinase 1 to nucleus impacts tamoxifen sensitivity. Biomed Pharmacother. 2007 doi: 10.1016/j.biopha.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Rider L, Shatrova A, Feener EP, Webb L, Diakonova M. JAK2 tyrosine kinase phosphorylates PAK1 and regulates PAK1 activity and functions. J Biol Chem. 2007;282:30985–30996. doi: 10.1074/jbc.M701794200. [DOI] [PubMed] [Google Scholar]
- 19.Rider L, Oladimeji P, Diakonova M. PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV. Molecular endocrinology. 2013;27:1048–1064. doi: 10.1210/me.2012-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer A, Rider L, Oladimeji P, Cook L, Li Q, Mattingly RR, Diakonova M. Tyrosyl Phosphorylated PAK1 Regulates Breast Cancer Cell Motility in Response to Prolactin through Filamin A. Mol Endocrinol. 2013;27:455–465. doi: 10.1210/me.2012-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer A, Oladimeji P, De Las Casas LE, Diakonova M. Phosphorylation of tyrosine 285 of PAK1 facilitates betaPIX/GIT1 binding and adhesion turnover. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:943–959. doi: 10.1096/fj.14-259366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao J, Oladimeji P, Rider L, Diakonova M. PAK1-Nck Regulates Cyclin D1 Promoter Activity in Response to Prolactin. Mol Endocrinol. 2011;25:1565–1578. doi: 10.1210/me.2011-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim E, Youn H, Kwon T, Son B, Kang J, Yang HJ, Seong KM, Kim W, Youn B. PAK1 tyrosine phosphorylation is required to induce epithelial-mesenchymal transition and radioresistance in lung cancer cells. Cancer research. 2014;74:5520–5531. doi: 10.1158/0008-5472.CAN-14-0735. [DOI] [PubMed] [Google Scholar]
- 24.Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, Kumar R. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J Biol Chem. 2001;276:29403–29409. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 25.Corbin JD, Reimann EM. Assay of cyclic AMP-dependent protein kinases. Methods in enzymology. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- 26.Oladimeji P, Kubohara Y, Kikuchi H, Oshima Y, Rusch C, Skerl R, Diakonova M. A Derivative of Differentiation-Inducing Factor-3 Inhibits PAK1 Activity and Breast Cancer Cell Proliferation. International journal of cancer and clinical research. 2015;2:1–6. doi: 10.23937/2378-3419/2/4/1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chemistry & biology. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/s0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 29.Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 30.Renkema GH, Pulkkinen K, Saksela K. Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol Cell Biol. 2002;22:6719–6725. doi: 10.1128/MCB.22.19.6719-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roig J, Tuazon PT, Zipfel PA, Pendergast AM, Traugh JA. Functional interaction between c-Abl and the p21-activated protein kinase gamma-PAK. Proc Natl Acad Sci U S A. 2000;97:14346–14351. doi: 10.1073/pnas.97.26.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McManus MJ, Boerner JL, Danielsen AJ, Wang Z, Matsumura F, Maihle NJ. An oncogenic epidermal growth factor receptor signals via a p21-activated kinase-caldesmon-myosin phosphotyrosine complex. J Biol Chem. 2000;275:35328–35334. doi: 10.1074/jbc.M005399200. [DOI] [PubMed] [Google Scholar]
- 33.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, Black MA, Donato AL, Arterburn JB, Oprea TI, Prossnitz ER, Dun NJ, Jordan VC. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer research. 2010;70:1184–1194. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Molecular endocrinology. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 36.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 37.Fasco MJ, Amin A, Pentecost BT, Yang Y, Gierthy JF. Phenotypic changes in MCF-7 cells during prolonged exposure to tamoxifen. Mol Cell Endocrinol. 2003;206:33–47. doi: 10.1016/s0303-7207(03)00256-9. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 39.Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Molecular endocrinology. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 40.Menard RE, Mattingly RR. Gbetagamma subunits stimulate p21-activated kinase 1 (PAK1) through activation of PI3-kinase and Akt but act independently of Rac1/Cdc42. FEBS letters. 2004;556:187–192. doi: 10.1016/s0014-5793(03)01406-6. [DOI] [PubMed] [Google Scholar]
- 41.Howe AK, Juliano RL. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol. 2000;2:593–600. doi: 10.1038/35023536. [DOI] [PubMed] [Google Scholar]
- 42.Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. The Journal of biological chemistry. 2005;280:578–584. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- 43.Bachmann VA, Riml A, Huber RG, Baillie GS, Liedl KR, Valovka T, Stefan E. Reciprocal regulation of PKA and Rac signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8531–8536. doi: 10.1073/pnas.1215902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park MH, Lee HS, Lee CS, You ST, Kim DJ, Park BH, Kang MJ, Heo WD, Shin EY, Schwartz MA, Kim EG. p21-Activated kinase 4 promotes prostate cancer progression through CREB. Oncogene. 2013;32:2475–2482. doi: 10.1038/onc.2012.255. [DOI] [PubMed] [Google Scholar]
- 45.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. Embo J. 2002;21:5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, Floore A, Velds A, van’t Veer L, Neefjes J. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, Aldaz M, Khan S, Kumar R. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006;66:1694–1701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 48.Balasenthil S, Barnes CJ, Rayala SK, Kumar R. Estrogen receptor activation at serine 305 is sufficient to upregulate cyclin D1 in breast cancer cells. FEBS Lett. 2004;567:243–247. doi: 10.1016/j.febslet.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez L, Zambrano A, Lazaro-Trueba I, Lopez E, Gonzalez JJ, Martin-Perez J, Aranda A. Activation of the unliganded estrogen receptor by prolactin in breast cancer cells. Oncogene. 2009;28:1298–1308. doi: 10.1038/onc.2008.473. [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen LM, Frederiksen KS, Din N, Galsgaard E, Christensen L, Berchtold MW, Panina S. Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocrine-related cancer. 2010;17:809–822. doi: 10.1677/ERC-09-0326. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Huang K, Chen KE, Walker AM. Prolactin and estradiol utilize distinct mechanisms to increase serine-118 phosphorylation and decrease levels of estrogen receptor alpha in T47D breast cancer cells. Breast cancer research and treatment. 2010;120:369–377. doi: 10.1007/s10549-009-0400-7. [DOI] [PubMed] [Google Scholar]
- 52.Sato T, Tran TH, Peck AR, Liu C, Ertel A, Lin J, Neilson LM, Rui H. Global profiling of prolactin-modulated transcripts in breast cancer in vivo. Molecular cancer. 2013;12:59. doi: 10.1186/1476-4598-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holm C, Kok M, Michalides R, Fles R, Koornstra RH, Wesseling J, Hauptmann M, Neefjes J, Peterse JL, Stal O, Landberg G, Linn SC. Phosphorylation of the oestrogen receptor alpha at serine 305 and prediction of tamoxifen resistance in breast cancer. The Journal of pathology. 2009;217:372–379. doi: 10.1002/path.2455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.