Abstract
Substance P (SP) was recently reported to be expressed in human KNDy neurons and to enhance KNDy neuron excitability in the mouse hypothalamus.
We therefore examined 1) interactions of SP and kisspeptin in the mediobasal hypothalamus of adult male rhesus monkeys using immunofluorescence, and 2) the ability of SP to induce LH release in GnRH primed, agonadal juvenile male monkeys.
SP cell bodies were observed only occasionally in the arcuate nucleus (Arc), but more frequently dorsal to the Arc in the region of the pre-mammilary nucleus. Castration resulted in an increase in the number of SP cell bodies in the Arc but not in the other nuclei. SP fibers innervated the Arc where they were found in close apposition with kisspeptin perikarya in the periphery of this nucleus. Beaded SP axons projected to the median eminence where they terminated in the external layer and intermingled with beaded kisspeptin axons. Colocalization of the two peptides, however, was not observed. Although close apposition between SP fibers and kisspeptin neurons suggest a role for SP in modulating GnRH pulse generator activity, iv injections of SP failed to elicit release of GnRH (as reflected by LH) in the juvenile monkey.
Although the finding of structural interactions between SP and kisspeptin neurons are consistent with the notion that this tachykinin may be involved in regulating pulsatile GnRH release, the apparent absence of expression of SP in KNDy neurons suggests that this peptide is unlikely to be a fundamental component of the primate GnRH pulse generator.
Keywords: rhesus monkey, GnRH pulse generation, kisspeptin, substance P
Introduction
Substance P was the first tachykinin isolated, and this neuropeptide has been implicated in the control of LH for many years[1–3]. In women, circulating substance P levels increase at the time of the preovulatory gonadotropin surge [4], and systemic infusion of substance P in normal men induces a robust discharge of LH in the absence of a change in FSH secretion [5]. In addition, substance P levels in plasma, anterior pituitary and hypothalamus have been reported to fluctuate in phase with the menstrual cycle or with circulating estradiol levels in the monkey and human [4,6,7].
In addition to substance P, the tachykinin family also includes neurokinin B (NKB), neurokinin A (NKA), the N-terminally extended NKA peptides (neuropeptide K [NPK] and neuropeptide γ [NPγ]) and the endokinins [8]. The primate tachykinins are encoded by three different genes, TAC1, TAC3 and TAC4. While TAC3 encodes for only NKB, differential RNA splicing and posttranslational processing of preprotachykinins encoded by TAC1 gives rise to substance P, NKA, NPK and NPγ [8]. To date, three tachykinin receptors have been identified, NK1, NK2 and NK3; and each receptor shows preferential affinity for one of the tachykinins (NK1 for substance P; NK2 for NKA; NK3 for NKB). All tachykinins share the same hydrophobic C-terminal region, which is central to the activation of all three receptors, and substance P, NKA and NKB can activate any one of them, suggesting that there may be redundancy in tachykinin signaling [8–10].
The role of tachykinins in the hypothalamic regulation of gonadotropin secretion was rekindled by the discovery in man that loss of function mutations of either NKB or NK3, were associated with hypogonadotropism and delayed or absent puberty [11]. Interest was further stimulated when it was recognized that NKB had been earlier reported to be co-expressed in the arcuate nucleus of the ewe with kisspeptin [12], a neuropeptide currently considered to be the most critical stimulatory input to the GnRH neuronal network [13]. The kisspeptin/NKB coexpressing neurons in the arcuate nucleus have also been shown to co-synthesize dynorphin in a species dependent manner, and have been termed KNDy neurons [14].
The notion that the arcuate nucleus represents the site of the hypothalamic GnRH pulse generator originated from the early observation of Knobil’s laboratory that in the ovarariectomized rhesus monkey gonadotropin secretion was abolished by selective lesioning of this nucleus [15], and was reinforced by findings that multi-unit electrical activity in the vicinity of this nucleus was tightly coupled with pulsatile discharges of LH, reflecting corresponding release of GnRH into the pituitary portal circulation [16–19]. More recently, specific ablation of NKB receptor-expressing neurons in the arcuate nucleus of the rat has been shown to impair the post castration rise in gonadotropin secretion [20], providing evidence for a critical role of NKB signaling, and by inference KNDy neurons, in GnRH pulse generation. For these and other reasons, KNDy neurons are now viewed as a major component of most contemporary models of GnRH pulse generation. These models posit that pulsatile activity within the network of arcuate KNDy neurons is generated by a reciprocal interplay of stimulatory and inhibitory inputs provided by NKB and dynorphin signaling, respectively. The output of the pulse generator is provided by the pulsatile release of kisspeptin in the median eminence, which in turn induces corresponding intermittent GnRH discharges at this site [21–23]. The recent observation that substance P is co-localized in a subset of kisspeptin/NKB neurons in the human infundibular (aka arcuate nucleus) region [24], raises the possibility that substance P is a potential fourth player in the GnRH pulse generator.
The purpose of this study was twofold. First, to examine the morphological relationship between substance P and kisspeptin immunoactivity and potential interactions between these hypothalamic peptides in the region of the arcuate nucleus and median eminence of intact and castrate male rhesus monkeys. The castrate situation was evaluated because previous studies by our laboratory have shown that kisspeptin neurons are readily identifiable in the arcuate nucleus of the agonadal male [25]. Second, we accessed the effects of intravenous administration of substance P on basal LH secretion. For this purpose, the GnRH primed juvenile male rhesus monkey, in which endogenous GnRH secretion is minimal, was used to track induced GnRH release as described on several previous occasions [26–29].
Material and Methods
Tissue and Animals
The morphological relationship between immunopositive substance P and kisspeptin in the mediobasal hypothalamus (MBH) of the adult male rhesus monkey (Macaca mulatta) was examined using 4% paraformaldehyde perfusion fixed coronal hypothalamic hemi-sections obtained earlier from 4 intact (5.4–13.5 years old, 10.2–12.1 kg bw) and 4 castrated (4.1–12.1 years old; 5.6–14.7 kg bw) adult male rhesus monkeys. The castrates had been orchidectomized for 5 to 19 weeks before perfusion. Serial 25-µm sections throughout the entire hypothalamus had been cut on a freezing microtome and placed sequentially into a train of 10 wells so that each well contained a sequence of sections collected at 250-µm intervals (see reference [25] for details). The sections had been stored in cryoprotectant [30] at −20C in the laboratory’s tissue bank.
In order to examine the ability of substance P to induce GnRH release, four juvenile male rhesus monkeys (14.2–15.1 months of age; 2.8–3.2 kg body weight) obtained from the California National Primate Research Center (Davis, CA, USA) were used. These animals had been implanted with two indwelling venous catheters and bilaterally castrated for an unrelated University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approved physiological study, and were maintained under controlled photoperiod (lights on between 07.00– 19.00 h) and at approximately 21C as previously described in detail [26]. One iv line was dedicated to infusion of peptides (or vehicle) and the other to the withdrawal of blood to monitor LH discharges as an indirect measure of GnRH release. Animals were fed daily at approximately 0900h with monkey chow (Purina Monkey Chow 5045Z3N, St. Louis, MO, USA) and fruit and/or treats were provided in the mornings and afternoons. Water was available ad libitum. By the end of the present experiment, the agonadal monkeys ranged in age from 15.3–16.1 months: ie more than 12 months before the anticipated time of the pubertal rise in LH secretion in such animals [26,31]. The study was approved by the University of Pittsburgh IACUC.
Experimental Design
Experiment 1: Localization of immunopositive substance P in the MBH and median eminence of intact and castrate adult male monkeys and the relationship to kisspeptin in the arcuate nucleus and median eminence
A series of coronal hemi-sections taken every 500 µm, starting at the first retrochiasmatic section and continuing to the rostral aspect of the mammillary bodies, from each of the castrated and intact adult male monkeys were stained for substance P and kisspeptin as described below on one occasion. Substance P and kisspeptin-containing neurons were enumerated in each series of hemi-sections and each hemi-section was examined for double labeled somata and fibers. The distribution of immunopositive substance P and kisspeptin neurons in each group was aligned to the section containing the highest number of kisspeptin neurons, and designated section 0.
Experiment 2: Effect of iv bolus administration of substance P on LH release in the GnRH primed-agonadal juvenile male rhesus monkey
Before initiating this experiment, the responsiveness of the gonadotrophs to GnRH stimulation was first enhanced by a chronic intermittent iv infusion of GnRH (0.6µg GnRH/pulse once every h) as described previously [26]. LH secretion by the GnRH sensitized in situ pituitary was used as a bioassay for endogenous GnRH release as described previously on many occasions [26,29,32].
Three doses (0.5, 5 and 50 µg) of substance P were studied. The rational for the selection of this dose range was based on the earlier finding that an iv bolus of 2 µg kisspeptin elicits a robust discharge of LH in the GnRH primed juvenile male monkey (33), and the assumption that substance P would likely be markedly less potent than kisspeptin in this experimental paradigm. The 3 substance P doses and vehicle (see Peptides) were studied in 4 animals over a 4 day period in such a manner that, on any one day, 3 of the 4 animals received a different dose of substance P and the 4th animal received vehicle. Each animal received each of the 3 doses of substance P and vehicle. Each dose was administered as an iv bolus over approximately 1 min at time 0. Immediately after the substance P injection, the animals were checked for peripheral effects of the peptide such as facial flushing and lethargy, which has been reported in response to the vasodilatory and hypotensive actions of substance P [1]. A pre-injection blood sample was collected and post-injection samples were collected at 10, 20, 30, 60 and 110 min. Two hours following substance P injection or vehicle (time 120 min), a bolus iv injection of kisspeptin-10 (2 µg in 1 ml sterile saline) was given as a positive control for GnRH release. This dose of kisspeptin has been shown to elicit a discharge of LH comparable to that observed spontaneously in adult castrates [33].
Additional plasma samples were collected at time 130 and 140 min, ie 10 and 20 min after kisspeptin administration. After collection of the last blood sample on each of the first 3 days of the experiment, GnRH priming was briefly restored for at least 5 h, to maintain heightened sensitivity of the pituitary gonadotrophs.
Primary and secondary antibodies and specificity controls
Two substance P antisera, previously characterized by others, were screened for use in the present study. The first was a rabbit polyclonal antiserum generated against synthetic substance P by Eskay et al [34] that had previously been validated by Ronnekleiv et al for the use in the monkey hypothalamus [35]. The second was a monoclonal rat anti-substance P (AbD Serotec #8450-0505; Bio-Rad company, NC, USA) that had been used in the recent study of substance P localization in the human infundibular region [24]. The substance P antibodies were screened in a pilot study using both immunofluorescence and the ABC Nickel-intensified diaminobenzidine (Ni-DAB) methods [36]. Although both substance P antisera revealed a very similar pattern of labeling in the monkey MBH (data not shown), we chose to employ the rabbit antibody for double immunofluorescence labeling and quantification because it had been previously used by Ronnekliev et al [35] for examining substance P in the monkey hypothalamus. Immunofluorescence for double labeling was possible because this procedure and the more sensitive Ni-DAB technique revealed similar patterns of substance P immunopositivity.
For the NiDAB method, a goat anti-rabbit or a goat anti-rat secondary antibodies (cat# BA 1000 and BA 9400, respectively; Vector laboratories, Inc; Burlingame, CA) were used as appropriate.
For immunofluorescence, Cy3 donkey anti-rabbit (Jackson Immunoresearch Laboratories, West Grove, PA, USA) was used as the secondary antibody to detect the rabbit substance P antibody, and specificity was confirmed by pre-adsorption with synthetic substance P (AbD Serotec # 8450–1004) at a concentration of 5µg/ml. The kisspeptin antibody (GQ2) was raised in sheep against synthetic human kisspeptin-54 and was kindly provided by Dr. Stephen Bloom (Imperial College London, Hammersmith Hospital, London, UK). Alexa Fluor 488 donkey anti-sheep IgG (Invitrogen Corporation, Carlsbad, CA, USA) was used as the secondary antibody to detect GQ2, as previously described [25]. The specificity of GQ2 has been previously documented for use in radioimmunoassay (RIA) [37] and validated by this laboratory for immunohistochemical studies of kisspeptin in the monkey hypothalamus [25].
Double-labeling immunohistochemistry
Fluorescence immunocytochemistry for kisspeptin and substance P was performed on free-floating hemi-hypothalamic sections (500 µm apart) representing the anterior to posterior extent of the MBH. Sections were rinsed at room temperature in 50 mM KPBS (pH 7.3, 8 × 5 min), incubated for 30 min in 3% hydrogen peroxide, and rinsed again in 50 mM KPBS (5 × 10 min). The sections were then incubated for 60 min at room temperature in KPBS buffer containing 5% normal horse serum (Vector Laboratories, Inc., Burlingame, CA), 0.5% Triton X-100 and 0.1% BSA (Sigma Chemical Co., St. Louis, MO) to block nonspecific binding. For dual label immunofluorescence staining, the sections were incubated for 60 min at room temperature followed by 48h at 4C on a shaker in a cocktail of the primary antibodies (GQ2 at 1:120,000 and rabbit substance P at 1:15,000) prepared in the KPBS-horse serum buffer, and then washed in 50 mM KPBS (5 × 5 min) at room temperature. For immunofluorescence detection of kisspeptin and substance P, the sections were incubated for 1 h in the dark at room temperature in a cocktail of secondary antibodies; Alexa Fluor 488 donkey anti-sheep IgG and Cy3 donkey anti-rabbit were prepared in KPBS-horse serum buffer, both at a dilution of 1:200. The sections were mounted on slides (Fisherbrand Superfrost Plus; Fisher Scientific, Pittsburgh, PA) treated with subbing solution (0.1% gelatin and 0.01% chromium potassium sulfate), allowed to dry in the dark, coverslipped using GEL/MOUNT aqueous mounting medium with anti-fading agents (Biomeda Corp., Foster City, CA), and stored at 4C until analyses.
Ni-DAB immunohistochemistry
Hemi-hypothalamic sections of the MBH were first rinsed at room temperature in 50 mM KPBS (pH 7.3, 8 × 5 min), incubated for 30 min in 3% hydrogen peroxide, and rinsed again in 50 mM KPBS (5 × 10 min). The sections were incubated for 60 min at room temperature in KPBS buffer containing 5% normal goat serum (Vector Laboratories, Inc., Burlingame, CA), 0.5% Triton X-100 and 0.1% BSA (Sigma Chemical Co., St. Louis, MO) to block nonspecific binding and incubated in primary antisera (rabbit anti-substance P at 1:150,000 or rat anti-substance P 1:250) for 60 min at room temperature followed by 48h at 4C. The sections were then incubated with biotinylated secondary antibody; goat anti-rabbit or goat anti-rat at 1:600 for 60 min at room temperature. The signal was amplified using ABC Elite reagent kit (Vector; 1:200) for 60 min and visualized with Ni-DAB chromagen [36]. The sections were mounted on slides (Fisherbrand Superfrost Plus; Fisher Scientific, Pittsburgh, PA) treated with subbing solution (0.1% gelatin and 0.01% chromium potassium sulfate) and allowed to dry. The slides were dehydrated through graded alcohol, cleared in histoclear (National Diagnostics, Charlotte, NC, USA) and coverslipped using Permount (Fisher Scientific).
Confocal microscopy
Imaging of fluorescence labeling for kisspeptin and substance P was performed using a Nikon A1r confocal microscope (Nikon Instruments, Melville, NY). The excitation and emission wavelengths for Alexa Flour 488 (green) and Cy3 (red) were 488 / 520 nm and 543 / 570 nm, respectively. For low (×10) and high magnification (×40) profiles, 25–30 optical sections along the z-axis were collected at 1-µm intervals and reconstructed using Image J software (http://rsbweb.nih.gov/ij/). Composite digital images were then converted to tagged image file format and imported into Adobe Photoshop (Adobe Photoshop Lightroom®, version 5.3; Adobe Systems, Inc., San Jose, CA), in which color balance was generally adjusted.
Quantification of cell body number and statistical analyses
For enumerating the number of kisspeptin and substance P immunopositive perikarya in the MBH, a series of hemi-coronal sections at 500 µm intervals throughout the MBH was selected. The first anterior hemi-section in the series was 250 µm or less caudal to the optic chiasm. Kisspeptin and substance P cell bodies were counted using a fluorescence microscope (LEITZ DMRB; Leica, Wetzlar, Germany). The composite distribution of both neurons throughout the rostral-caudal extent of the MBH was compared by aligning cell counts/hemi-section to the section containing the highest number of kisspeptin neurons (designated section 0).
The significance of differences in the mean number of kisspeptin and substance P neurons/hemi-section in intacts and castrates was determined using the Student's t test (SigmaPlot 12.5; Systat Software Inc, San Jose, CA, USA). Significance was accepted at P < 0.05.
Peptides
A stock solution of substance P (Sigma-Aldrich Co., Cat # S6883; St. Louis, MO., USA). was prepared at 1 mg/ml in 0.1 M of acetic acid and stored in aliquots at −20C. 0.1 M acetic acid was used as the vehicle and aliquots were also stored at −20C. On days when substance P or vehicle challenges were administered, working solutions were freshly prepared in sterile saline immediately before iv injection. Human kisspeptin-10 was synthesized at the Peptide/Protein Core Facility of the Massachusetts General Hospital Endocrine/Reproductive Endocrinology Unit. A stock solution (1 mg/ml) in 5% dimethylsulfoxide in sterile saline was prepared and stored at –20C. GnRH was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). A stock solution (1 mg/ml) was prepared in sterile saline and stored at −20C. A working GnRH dilution (0.6 µg/ml) was also prepared in sterile saline as previously described [29].
LH assay
Plasma LH concentrations were estimated using a homologous RIA system as described previously [38]. This RIA consisted of recombinant cynomolgus (rc) LH (AFP6936A) as the standard and radioiodinated trace, and a polyclonal rabbit antiserum (AFP34294) raised against rcLH. The sensitivity of the LH RIA ranged between 0.1–0.2 ng/ml. The intra- and inter-assay coefficients of variation were ≤6% and ≤13%, respectively. LH concentrations below detection were assigned a value equivalent to the sensitivity of the assay. Significance of differences in plasma hormone concentrations was determined by Two-way ANOVA followed by Sidak's multiple comparisons test. Data are presented as mean ± SEM. Significance was accepted at P < 0.05.
Results
Experiment 1: Localization of immunopositive substance P in the MBH and median eminence of intact and castrate adult male monkeys and the relationship to kisspeptin in the arcuate nucleus and median eminence
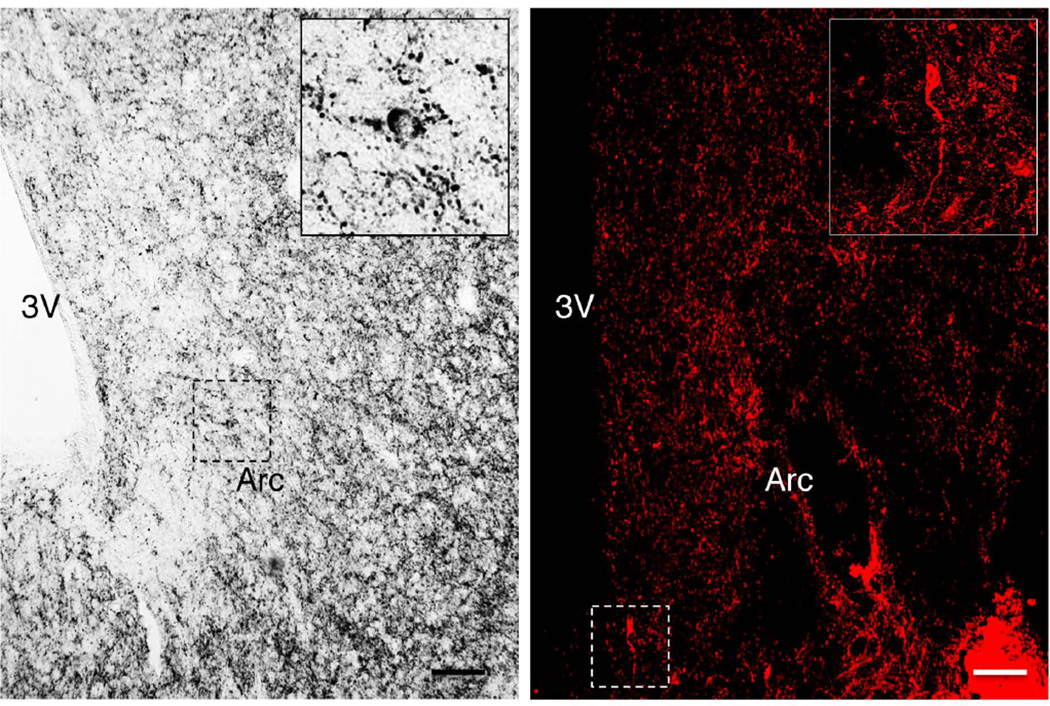
Only occasional immunopositive substance P perikarya were identified in the MBH, regardless of whether Ni-DAB or immunofluorescence was used for visualization. The few substance P neurons that were observed in the arcuate nucleus of both intact and castrate adult male monkeys were homogenously immunopositive and generally found in close proximity to the third ventricle (Fig. 1). On the other hand, fibers immunopositive for substance P were observed to innervate the entire length of the arcuate nucleus and the median eminence: innervation of the arcuate nucleus was particularly marked at the periphery of this structure (Fig. 2). In addition, intense immunopositivity was observed in the pars tuberalis, particularly in the anterior aspects of this structure (Fig 2, left panel). Pre-adsorption of the substance P antibody with authentic peptide eliminated all staining for the tackykinin in both median eminence and arcuate nucleus (Fig. 3).
Figure 1.
Hemi-hypothalamic coronal sections through the arcuate nucleus (Arc) of castrate adult male monkey showing immunopositive substance P neurons (insets) revealed with the Eskay antibody using Ni-DAB (left panel) and immunofluorescence (right panel; confocal projection 40×; 1 µm optical sections). Insets show at higher magnification corresponding neurons demarcated by dotted boxes. 3V, third ventricle; scale bar, 100µm.
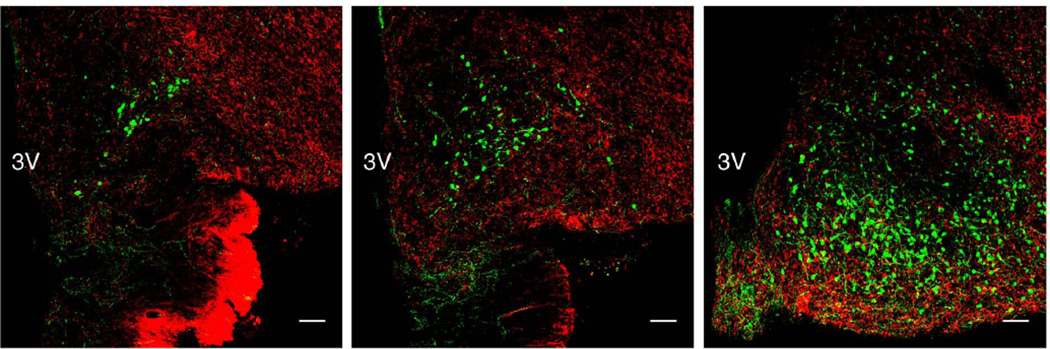
Figure 2.
Confocal immunofluorescence projections (10×; 1 µm optical sections) demonstrating the distribution of substance P (red) fibers in relation to kisspeptin neurons (green) in representative coronal hemi-sections of the anterior (left panel), mid (center panel) and posterior (right panel) regions of the arcuate nucleus in an agonadal male monkey. 3V, third ventricle; scale bar, 100µm. See also Fig. 7.
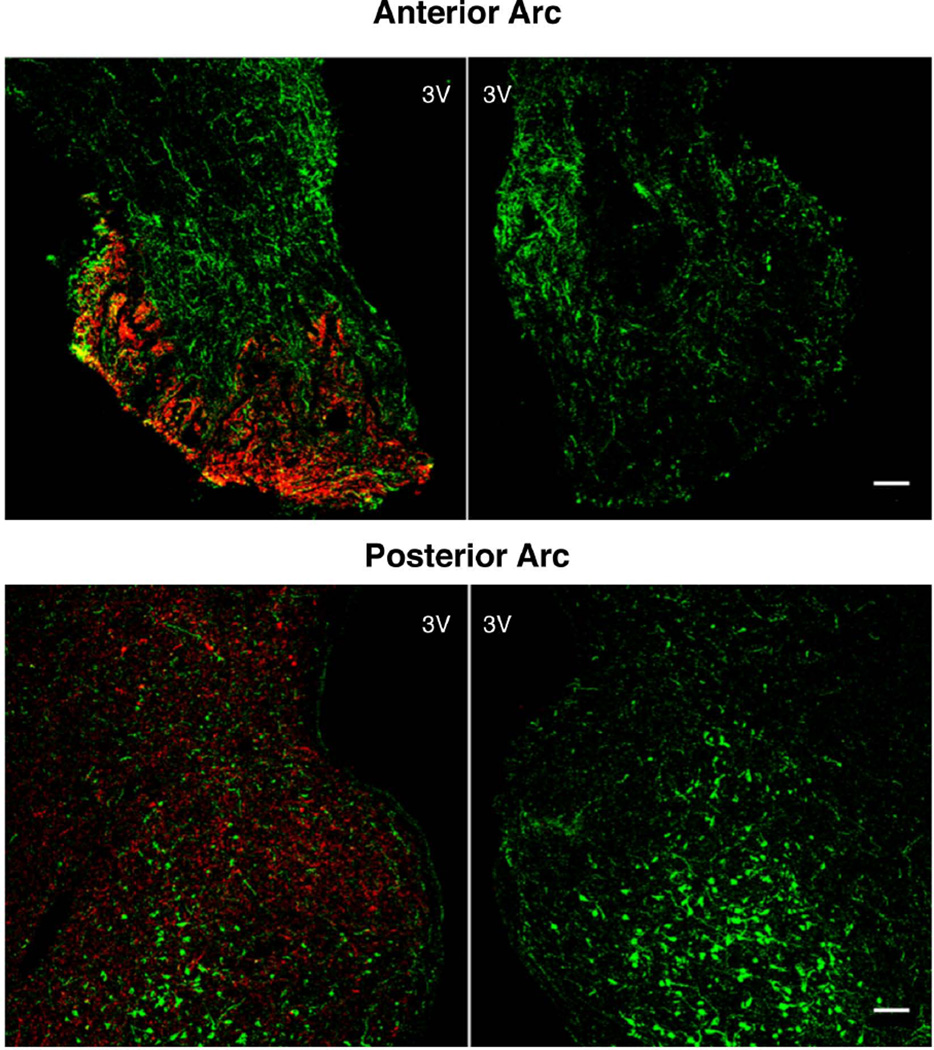
Figure 3.
Confocal projections (10×; 1 µm optical sections) of double immunofluorescence images for substance P (red) and kisspeptin (green) of a hypothalamic section from the anterior (upper panels) and posterior (lower panels) arcuate nucleus of a castrate adult male monkey. The sections shown in the right hand panels were stained after the substance P antibody was pre-adsorbed with synthetic peptide at a concentration of 5 µg/ml. 3V, third ventricle; scale bar, 100µm.
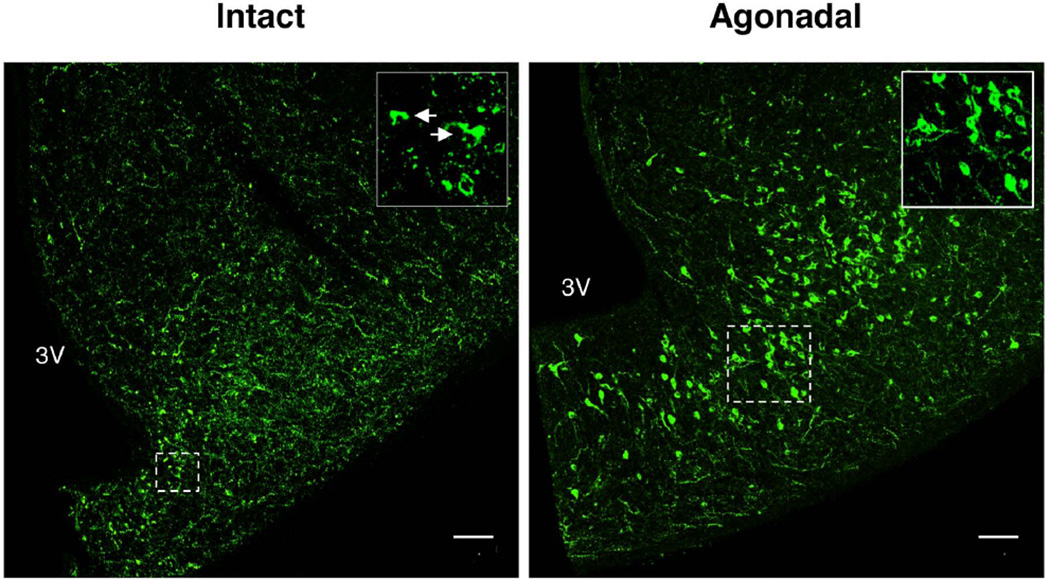
As expected, kisspeptin immunopositive perikarya and fibers were also found in the arcuate nucleus of both intact and castrated adult monkeys (Fig. 4). Many heavily and homogeneously immunopositive kisspeptin perikarya were found in this hypothalamic nucleus of agonadal adult monkeys (Fig 4). In intact animals, however, the intensity of kisspeptin staining in the soma was much less than that in the agonadal situation, and, in contrast to castrates, the distribution of immunopositive peptide within the cytoplasm of the cell body was not homogeneous, but rather appeared as cytoplasmic granules or as a cytoplasmic “cap” (Fig. 4). On the other hand, the intensity of kisspeptin fiber staining tended to be greater in the MBH of intact compared to castrated monkeys (Fig. 4).
Figure 4.
Confocal projections (10×; 1 µm optical sections) of single immunofluorescence images of kisspeptin in a hypothalamic hemi-section from posterior arcuate nucleus of an intact (left hand panel) and agonadal (right hand panel) adult male monkey. Insets show at higher magnification corresponding neurons demarcated by dotted boxes. White arrows show kisspeptin neurons with cytoplasmic “caps” seen in intact animals. 3V, third ventricle; scale bar, 100µm.
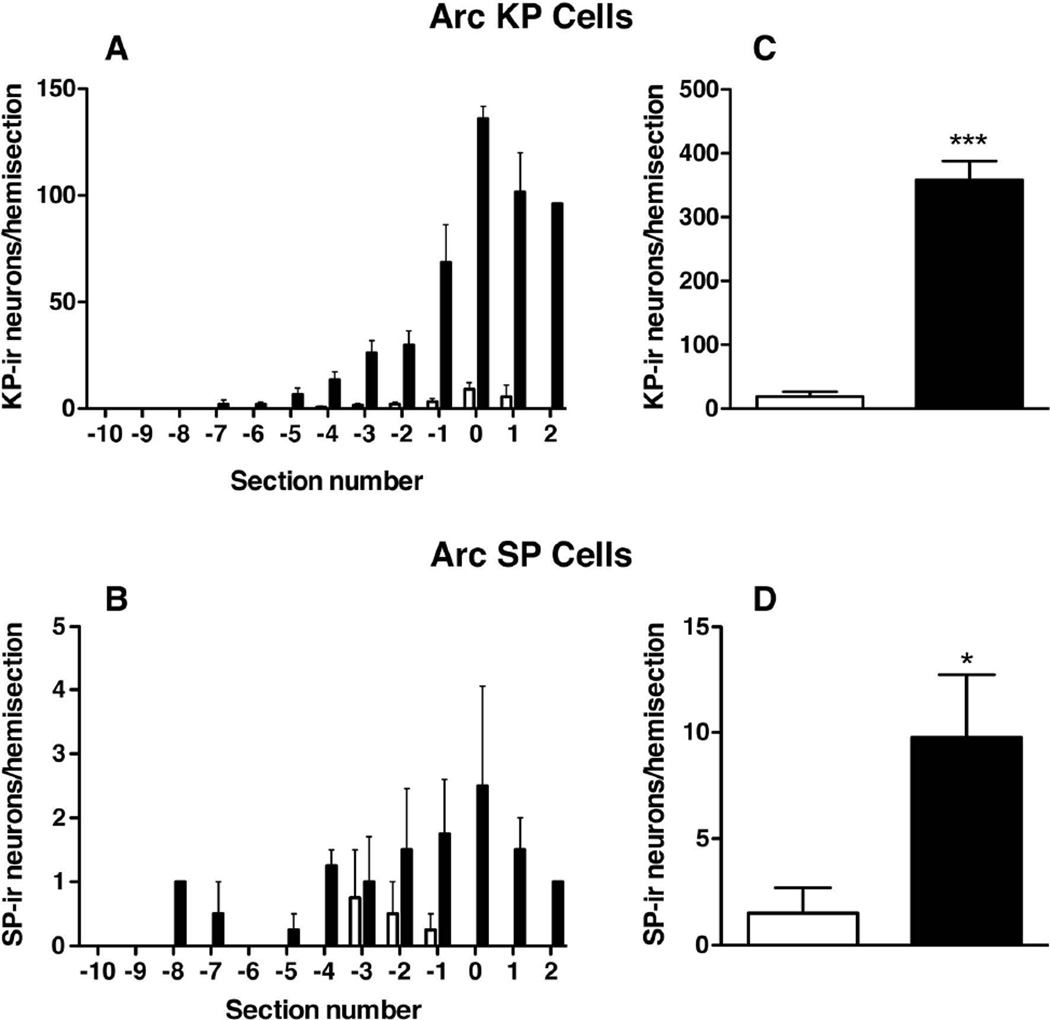
The number of kisspeptin neurons per hemi-section increased progressively in the anterior to posterior direction in the arcuate nucleus (Fig. 2 and 5A). In castrates, the first identified kisspeptin perikarya were seen, on average, in sections taken approximately 1,000 µm posterior from the optic chiasm. However, in intact animals it was rather variable. In the three intact monkeys in which kisspeptin perikarya were detected, the first identified kisspeptin neurons tended to be located more posteriorly, approx 4,000 µm posterior to the optic chiasm (Fig. 5A). Substance P immunopositive perikarya in the arcuate nucleus were located in the mid/posterior region of the nucleus, with occasional neurons found in the anterior region (Fig. 5B). The number of kisspeptin and substance P neurons in the hemi-sections analyzed were greater in the castrated monkeys compared to intact animals (358.0 ± 29.9 vs 19.00 ± 7.3; P<0.0001 and 9.8 ± 3.0 vs 1.5 ± 1.2; P<0.05, respectively) (Fig. 5 C, D).
Figure 5.
Distribution of kisspeptin (KP, A,C) and substance P (SP, B,D) immunopositive neurons in the arcuate nucleus (Arc) of intact (open bar, n=4) and castrate (closed bar, n=4) adult male monkeys. KP and SP neurons were enumerated in a series of hemi-sections taken at 500 µm intervals throughout the medial basal hypothalamus starting at the first retrochiasmatic section. In A and B, the distribution of both peptides in each group of animals was aligned to the section containing the highest number of KP neurons (indicated as 0 on the abscissa). C and D, comparison of the sum (mean±SEM) of KP (C) and SP (D) neurons counted in the hemi-sections analysed. *** P<0.001 and *P <0.05.
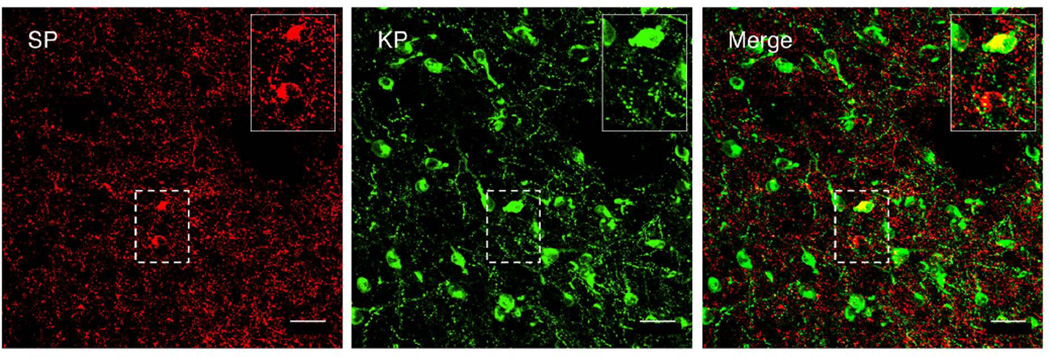
Evidence of co-localization of kisspeptin and substance P in cell bodies was observed on only one occasion and this was in a castrate animal: one perikaryon out of 1,432 neurons immunopositive kisspeptin neurons analyzed in this study exhibited evidence of double labeling (Fig 6). Similarly, unequivocal double-labeled fibers were not observed in the arcuate nucleus, median eminence or elsewhere in the MBH (Fig. 7 and Supplementary Video).
Figure 6.
Confocal projection (40×; 1 µm optical sections) showing the relationship between substance P (SP, left panel, red) and kisspeptin (KP, center panel, green) neurons in a coronal hemi-section of the arcuate nucleus in a castrate adult male monkey. Merged projection indicating co-localization of both peptides in one out of the two substance P neurons present in the Arc (right panel). Insets show at higher magnification corresponding neurons demarcated by dotted boxes. Scale bar, 50µm.
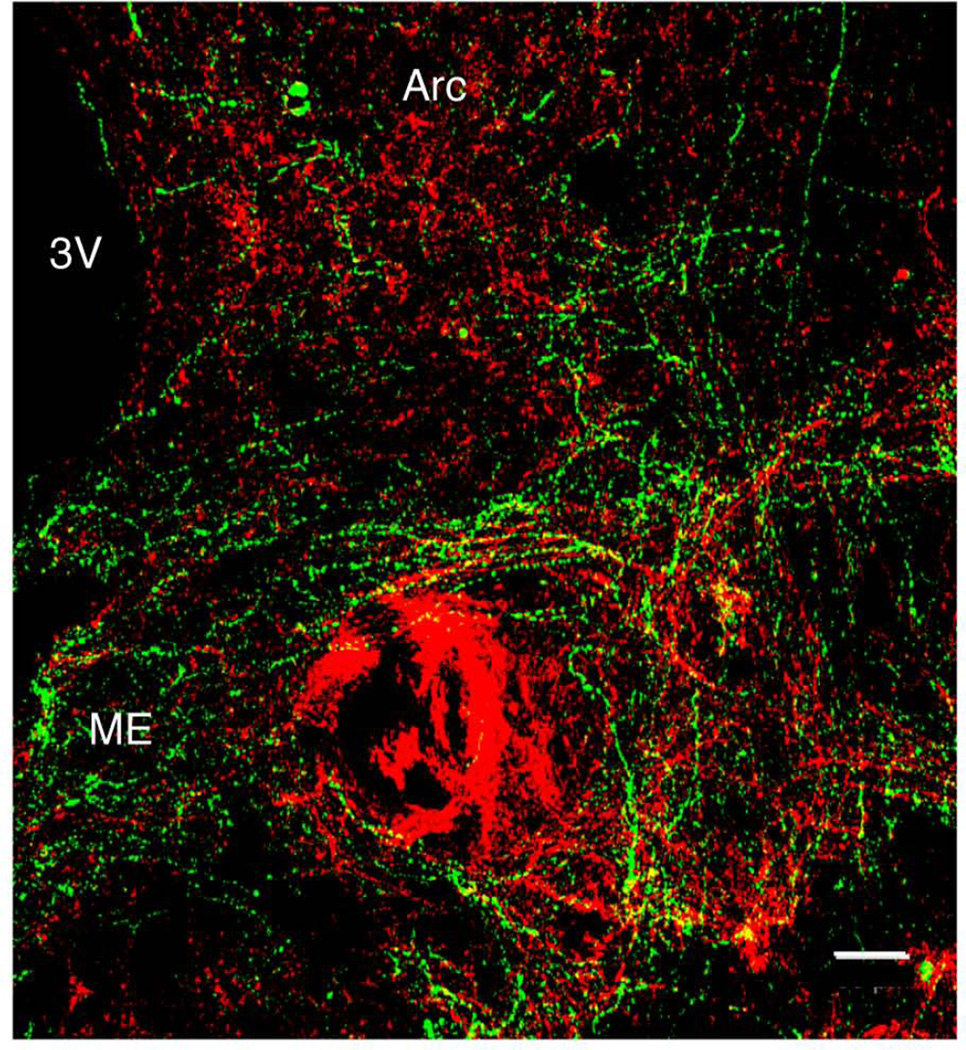
Figure 7.
Confocal projection (10×; 1 µm optical sections) illustrating the relationship between immunopositive substance P (red) and kisspeptin (green) fiber projections to the median eminence (ME) in a coronal hemi-hypothalamic section at a mid-tuberal level of a castrate adult male monkey. Substance P fibers project to the ME, and at this level clusters of substance P and kisspeptin beaded axons were observed running in close association in a near horizontal plane. Double labeled fibers were not observed. 3V, third ventricle. Arc, arcuate nucleus. Scale bar, 50µm. See also Supplementary Video to examine individual optical sections.
On the other hand, intimate axo-axonal interactions between these two peptides were seen at the level of the median eminence, where kisspeptin beaded-axons appeared to run horizontally along the arcuate-median eminence boundary intimately intermingling with substance P fibers (Fig. 7). Many of the substance P projections appeared to converge in the external zone of the median eminence forming dense plexuses appearing to surround the capillaries of the hypophysial portal system (Fig. 7).
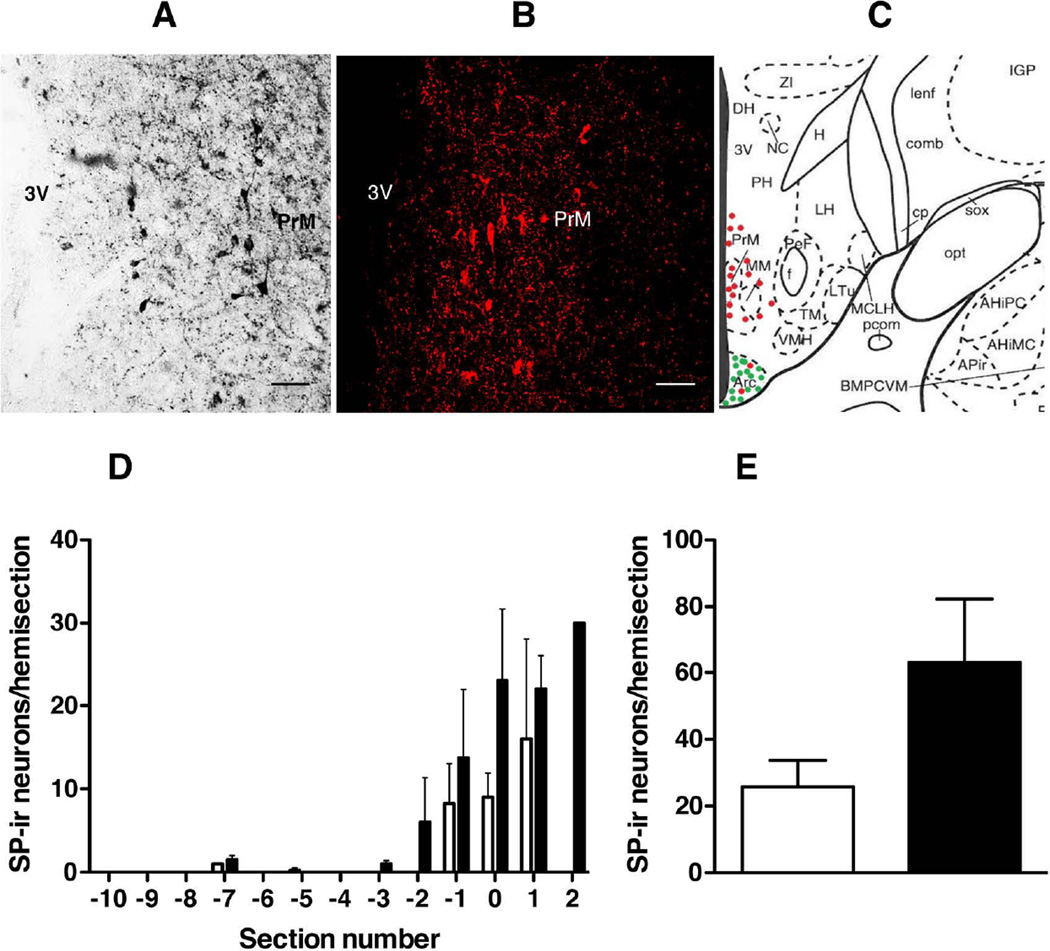
A well-defined population of periventricular substance P neurons was seen dorsal to the arcuate nucleus in the posterior hypothalamus at the level of the pre-mammilary nucleus with both immunofluorescence and Ni-DAB (Fig. 8 A, B and C). Unlike substance P neurons in the arcuate nucleus, the number of non-arcuate substance P neurons was not significantly influenced by castration (Figure 8 D and E; castrates: 63.0 ± 19.3 vs intacts: 25.8 ±8.0; P=0.124).
Figure 8.
(A, B) Hemi-hypothalamic coronal sections through the region of the pre-mammilary nucleus (PrM) and posterior arcuate nucleus (Arc) of castrate adult male monkeys showing of a cluster of periventricular substance P (SP) neurons dorsal to the Arc in the region of the PrM revealed with the Eskay antibody using Ni-DAB (A) and immunofluorescence (B; confocal projection 10×; 1 µm optical sections). (C) Schematic diagram of a coronal hemi-section of the posterior medial basal hypothalamus (MBH) of the rhesus monkey [54, permission pending] depicting the distribution of Arc and non- Arc SP cells (red dots) and their relationship to kisspeptin neurons (green dots). (D) Distribution of non-Arc SP neurons in intact (open bar, n=4) and castrate (closed bar, n=4) adult male monkeys. Immunopositive SP neurons were enumerated in a series of hemi-sections at 500 µm intervals throughout the MBH. As in Fig. 5, the distributions of SP neurons are aligned to the section with the greatest number of Arc kisspeptin neurons (indicated as 0 on the abscissa). (E), Non significant effect of castration on number (mean±SEM) of immunopositive non-Arc SP neurons (open bar, intacts; closed bar, castrates). Scale bar, 50µm.
Experiment 2: Effect of iv bolus administration of substance P on LH release in the GnRH primed-agonadal juvenile male rhesus monkey
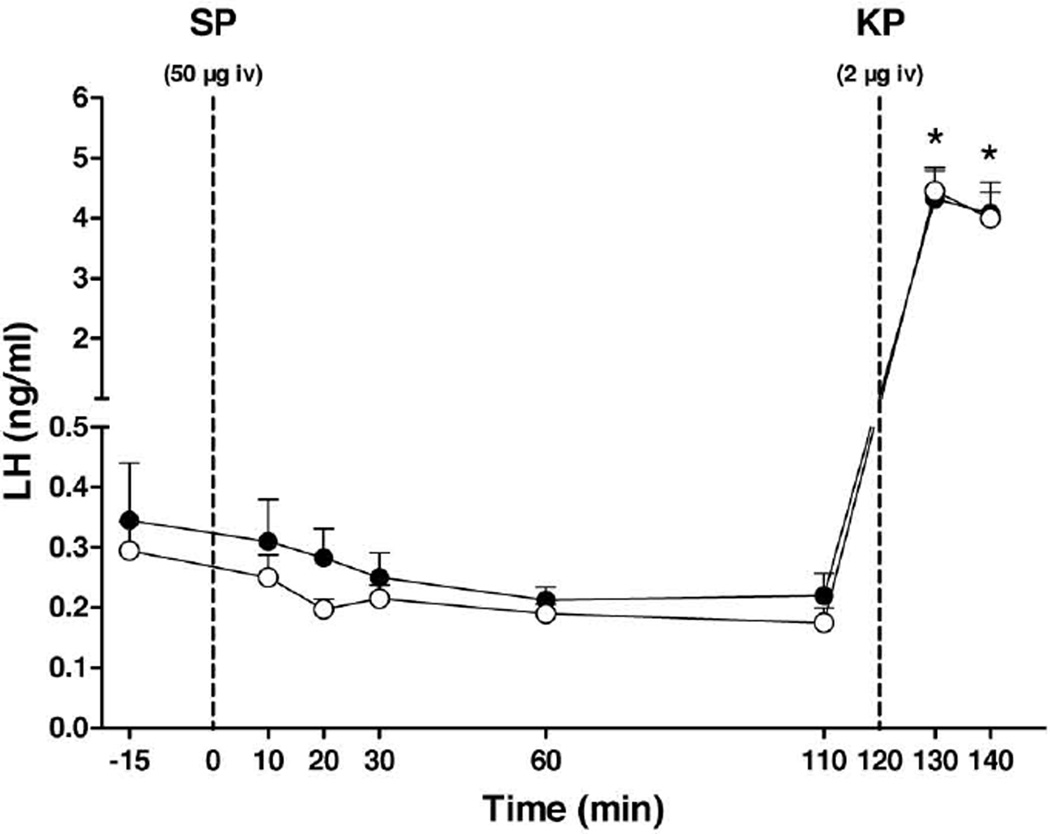
Circulating concentrations of LH following iv administration of substance P at all doses examined (0.5, 5 or 50 µg/monkey) were indistinguishable from those that followed injection of vehicle. In sharp contrast, a similar mode of kisspeptin administration 2 h after the substance P injection resulted, as anticipated, in an immediate, consistent and dramatic increase in plasma LH concentrations. Figure 9 shows the plasma LH response to the highest dose of substance P (50µg) and to the kisspeptin injection. Flushing of the face immediately after injection of substance P was observed in 75% of the monkeys at each of the 3 doses, although the identity of the 3 monkeys exhibiting this response were different for each dose. Flushing when observed typically lasted for 2 – 5 min. Repetitive injections of the peptide did not result in sensitization of the facial response.
Figure 9.
Effect of an iv bolus of substance P (SP; 50 µg/monkey, closed data point) or vehicle (open data point) followed 2 h later by kisspeptin (KP; 2µg/monkey) on circulating LH concentrations in GnRH-primed agonadal juvenile male rhesus monkeys (n=4). Data points show mean±SEM. *P<0.001, time points 130 and 140 min vs all the other time points in both groups (Two-way ANOVA followed by Sidak's multiple comparisons test). Dotted vertical lines indicate time of the iv injections of SP and KP.
Discussion
The distribution of immunopositive substance P in the MBH of the adult male rhesus monkey observed in the present study was qualitatively similar to that previously reported for this macaque and other highly evolved primates including man [24,35,39,40]. Dense networks of substance P fibers were found in the arcuate nucleus and the external zone of the median eminence, where they were observed surrounding capillary blood vessels. In addition, high concentrations of substance P-containing granules were found in the pars tuberalis.
The overall topography and morphology of substance P neurons that we observed in the MBH of the rhesus monkey was in agreement with that previously reported for this macaque by Ronnekleiv and colleagues [35]. Substance P perikarya were typically located in the arcuate and ventromedial nuclei, with those in the arcuate nucleus usually found in the mid to posterior region of this structure. However, the number of substance P neurons found in the arcuate nucleus in the present study was notably less than that previously reported for male and female rhesus monkeys [35]. Here it may be noted that in a study of the African green monkey substance P neurons were not found in the arcuate nucleus [39].
In the two studies of the rhesus monkey, differences in immunohistochemical methods employed are unlikely to explain the quantitative discrepancy. The same primary substance P antiserum was used in both studies, and in our hands visualization with peroxidase, used by Ronnekleiv et al, [35], and fluorescence, employed by us, yielded similar results in terms of numbers of immunopositive substance P neurons revealed. The quantitative difference cannot be attributed to gonadal status since the present study and that of Ronnekleiv et al [35] used castrate adult animals. The possibility of a male-female difference, however, cannot be excluded because, although both male and female castrates were studied previously, in contrast to the present investigation that only examined males, the number of arcuate substance P neurons for each sex was not reported in the earlier study. In this regard, Hrabovsky et al [24] recently reported that the number of substance P somata in the infundibular nucleus of women was 5 times greater than that in men.
In the rat, populations of substance P cells were found throughout the MBH, especially in the ventromedial nucleus [41,42], and in the mouse robust expression of Tac1 has been demonstrated in the arcuate and ventromedial nuclei by in situ hybridization [43].
In man, approximately 65% of substance P positive neurons in the arcuate nucleus have been shown to co-express kisspeptin [24]. In contrast, neurons of the arcuate nucleus of rhesus monkeys do not appear to co-express these two neuropeptides; a situation very different from the relationship between kisspeptin and neurokinin B, which are colocalized in approximately 60% of kisspeptin positive neurons in this nucleus [44]. The mouse appears to be similar to the monkey in this regard since Tac1-expressing neurons in the arcuate nucleus of the female although closely associated with Kiss-1 positive neurons, were not observed to co-express the latter gene [43]. The absence of robust substance P expression in arcuate kisspeptin neurons in the rhesus monkey and mouse suggests that there is no pressing need to incorporate this tachykinin into the KNDy model of mammalian GnRH pulse generation, proposed by several groups and recently reviewed by Goodman and Inskeep [45].
Substance P, however, may act as a neuromodulator of the GnRH pulse generator. In support of this view, Navarro et al [43] reported that nearly 50% of Kiss-1 neurons in the arcuate nucleus of female mice express Tacr1 mRNA, the transcript encoding NK1 - the substance P receptor, and de Croft et al [46] demonstrated that substance P was able to depolarize KNDy neurons. Moreover, icv infusion of a substance P agonist (GR 73632) induced GnRH release in adult male and ovariectomized, estradiol treated mice by a kisspeptin-dependent mechanism as reflected by the finding that this action of substance P was not observed in Kiss1r−/− mice [43]. It should be noted that Tacr1 is also expressed in a subset of GnRH neurons in the female mouse [43] and therefore substance P may modulate GnRH release directly in this species.
Whether NK1 is expressed by KNDy and/or GnRH neurons in the monkey and other highly evolved primates is unknown. However, the close association between substance P fibers and kisspeptin neurons in parts of the arcuate nucleus and the intimate intermingling of substance P and kisspeptin (and GnRH by inference) axonal projections in the ME observed in the present study and an earlier investigation of postmenopausal women [40] is consistent with the notion of substance P signaling on KNDy soma and terminals and GnRH terminals.
The present finding that the number of immunopositive substance P neurons in the MBH of the male monkey was 6.5-fold greater in castrates than that in intact animals is consistent with the earlier observation of Rance and Young [47] in the infundibular nucleus of pre- and post-menopausal women. The results of both studies suggest that down regulation of substance P in hypothalamic neurons may mediate, at least in part, the negative feedback action of gonadal steroids on gonadotropin secretion. This view is consistent with the finding in men that systemic infusion of substance P induced a robust discharge of LH [5]. In the ovariectomized estradiol replaced rat iv injection of substance P (10 and 50 µg) also stimulated LH release [48] likely by an action primarily at the hypothalamic level [48–50].
In the present study, iv administration of increasing doses of substance P consistently failed to induce LH secretion in castrated juvenile male monkeys. Since all animals displayed facial flushing, a vasomotor reaction in response to substance P infusion [1], the lack of an endocrine effect of substance P cannot be attributed to loss of biological activity. Similarly, insufficient responsiveness of the pituitary gland to GnRH was not responsible for the failure of substance P to induce LH release because the control kisspeptin challenge consistently induced a robust discharge of LH. There are several obvious possibilities to explain the difference in the LH response to substance P administration in juvenile monkeys (present study) and men [5]. First, in the juvenile monkey, the GnRH pulse generator is restrained by a developmental control system [51], while in the men studied by Coiro et al [5] GnRH pulse generation was presumably robust. Second, in the present study substance P was administered as an iv bolus but in men the peptide was iv infused for one hour. Third, the monkeys studied by us were castrated while the men were intact, and there are several examples in the literature of gonadal steroids profoundly modulating the neuroendocrine response of neuropeptides and neurotransmitters [52]. We consider that differences in dose and mode of administration of substance P employed in the present study (126 – 12,600 pmol/kg/min for 1 min) and the earlier study of men (1.5 pmol/kg/min for 60 min) are unlikely to explain the failure of substance P to elicit LH release in the agonadal juvenile monkey. With regard to the rate of infusion of substance P (0.5 – 1.5 pmol/kg/min) employed in the study of men [5], it is of considerable interest to note that it was less than that used by Dhillo et al [37] to initially demonstrate the ability of kisspeptin (4 pmol/kg/min) to elicit LH release in the human male.
Although the present finding that the number of kisspeptin neurons in the arcuate nucleus of castrate adult males was nearly 20-fold greater than that in intact animals was anticipated because KISS1 in this hypothalamic nucleus of the castrate adult male monkey is dramatically down regulated by testosterone replacement [53], this effect of the primate testis to suppress expression of the peptide has not been quantified before. A similar action of testicular steroids on kisspeptin neurons in the arcuate nucleus has been reported previously in mice and sheep [54,55] an effect that appears to be mediated by both estrogen and androgen receptor signaling [52]. This biology is consistent with a role of kisspeptin in mediating, at least in part, the negative feedback actions of gonadal steroids on GnRH secretion [13].
In summary, the present study provides for the adult male rhesus monkey a detailed description of the distribution of immunopositve substance P perikarya and fibers in the MBH and median eminence and of their relationship to kisspeptin neurons in these hypothalamic regions of both intact and castrate adult animals. Evidence for co-localization of substance P and kisspeptin in either cell bodies or fibers was not observed, although immunopositive fibers for these two peptides were intimately intermingled in the median eminence. Administration of substance P by iv injection to GnRH primed, agonadal juvenile monkeys failed to elicit GnRH release as reflected by LH. The foregoing findings suggest that this tachykinin is unlikely to be a fundamental component of the GnRH pulse generator in the monkey, but does not exclude the possibility that substance P may modulate the pulsatile release of the decapeptide.
Supplementary Material
Acknowledgments
We thank Dr. Oline Ronnekleiv (Oregon National Primate Research Center, Portland) for providing us with the Eskay substance P antibody, Dr. Stephen R Bloom (Imperial Collage, London) for the kisspeptin antibody, Dr. Stephanie B Seminara (Massachuseetts General Hospital, Boston) for kisspeptin-10, Dr. Philippe Ciofi (French Institute of Health and Medical Research, Paris) for help with selection of primary antibodies for substance P, and Dr. Gloria E Hoffman (Morgan State University, Baltimore) for valuable guidance in the immunohistochemical technique. We also thank Dr. Judith Yanowitz (Magee Womens Research Institute, Pittsburgh) for her guidance with confocal microscopy and Ms. Carolyn Phalin for conducting the radioimmunassays. This study was supported by São Paulo Research Foundation 2013/03596-1 grant awarded to BK and NIH Grants R01 HD13254 and U54 HD08610 awarded to TMP.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Von Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessop DS, Chowdrey HS, Larsen PJ, Lightman SL. Substance P: multifunctional peptide in the hypothalamo-pituitary system? J Endocrinol. 1992;132:331–337. doi: 10.1677/joe.0.1320331. [DOI] [PubMed] [Google Scholar]
- 3.Lasaga M, Debeljuk L. Tachykinins and the hypothalamo–pituitary–gonadal axis: An update. Peptides. 2011;32:1972–1978. doi: 10.1016/j.peptides.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Kerdelhué B, Lenoir V, Scholler R, Jones HW. Substance P plasma concentration during the LH preovulatory surge of the menstrual cycle in the human. [Internet] Neuro Endocrinol Lett. 2006;27:359–364. [PubMed] [Google Scholar]
- 5.Coiro V, Volpi R, Capretti L, Caiazza A, Marcato A, Bocchi R, et al. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism. 1992;41:689–691. doi: 10.1016/0026-0495(92)90305-t. [DOI] [PubMed] [Google Scholar]
- 6.Kerdelhué B, Williams RF, Lenoir V, Fardin V, Kolm P, Hodgen GD, et al. Variations in plasma levels of substance P and effects of a specific substance P antagonist of the NK(1) receptor on preovulatory LH and FSH surges and progesterone secretion in the cycling cynomolgus monkey. Neuroendocrinology. 2000;71:228–236. doi: 10.1159/000054540. [DOI] [PubMed] [Google Scholar]
- 7.Kerdelhué B, Jones GS, Gordon K, Seltman H, Lenoir V, Millar RP, et al. Hypothalamo-anterior pituitary gonadotropin-releasing hormone and substance-P systems during the 17 beta-estradiol-induced plasma luteinizing hormone surge in the ovariectomized female monkey. Endocrinology. 1993;132:1151–1157. doi: 10.1210/endo.132.3.7679971. [DOI] [PubMed] [Google Scholar]
- 8.Page NM. New challenges in the study of the mammalian tachykinins. Peptides. 2005;26:1356–1368. doi: 10.1016/j.peptides.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Harmar AJ, Armstrong A, Pascall JC, Chapman K, Rosie R, Curtis A, et al. cDNA sequence of human beta-preprotachykinin, the common precursor to substance P and neurokinin A. FEBS Lett. 1986;208:67–72. doi: 10.1016/0014-5793(86)81534-4. [DOI] [PubMed] [Google Scholar]
- 10.Debeljuk L, Lasaga M. Modulation of the hypothalamo-pituitary-gonadal axis and the pineal gland by neurokinin A, neuropeptide K and neuropeptide gamma. Peptides. 1999;20:285–299. doi: 10.1016/s0196-9781(98)00159-4. [DOI] [PubMed] [Google Scholar]
- 11.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 13.Herbison AE. Physiology of the adult gonadotropin – releasing hormone neuronal network. In: Plant T, Zelznik A, editors. Knobil and Neill’s Physiology of Reproduction. 4th. London: Elsevier; 2015. pp. 399–467. [Google Scholar]
- 14.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RC, Kesner JS, Kaufman J-M, Uemura T, Akema T, Knobil E. Central Electrophysiologic Correlates of Pulsatile Luteinizing Hormone Secretion in the Rhesus Monkey. Neuroendocrinology. 1984;39:256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 17.Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, et al. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology. 1991;53:392–395. doi: 10.1159/000125746. [DOI] [PubMed] [Google Scholar]
- 18.Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among Kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59:40–48. doi: 10.1262/jrd.2012-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami M, Uemura T, Hayashi R. Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology. 1982;35:63–67. doi: 10.1159/000123356. [DOI] [PubMed] [Google Scholar]
- 20.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. doi: 10.1007/978-1-4614-6199-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrabovszky E, Borsay BÁ, Rácz K, Herczeg L, Ciofi P, Bloom SR, et al. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2013;8:e72369. doi: 10.1371/journal.pone.0072369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta) Endocrinology. 1998;139:2774–2783. doi: 10.1210/endo.139.6.6055. [DOI] [PubMed] [Google Scholar]
- 27.Suter KJ, Pohl CR, Plant TM. Indirect assessment of pulsatile gonadotropin-releasing hormone release in agonadal prepubertal rhesus monkeys (Macaca mulatta) J Endocrinol. 1999;160:35–41. doi: 10.1677/joe.0.1600035. [DOI] [PubMed] [Google Scholar]
- 28.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 29.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 31.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- 32.Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol Cell Endocrinol. 2006;254–255:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 34.Eskay RL, Long RT, Iuvone PM. Evidence that TRH, somatostatin and substance P are present in neuro-secretory elements of the vertebrate retina. Brain Res. 1980;196:554–559. doi: 10.1016/0006-8993(80)90424-2. [DOI] [PubMed] [Google Scholar]
- 35.Rønnekleiv OK, Kelly MJ, Eskay RL. Distribution of immunoreactive substance P neurons in the hypothalamus and pituitary of the rhesus monkey. J Comp Neurol. 1984;224:51–59. doi: 10.1002/cne.902240105. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman GE, Le WW, Sita LV. The importance of titrating antibodies for immunocytochemical methods. Curr Protoc Neurosci. 2008;Chapter 2(Unit 2.12) doi: 10.1002/0471142301.ns0212s45. [DOI] [PubMed] [Google Scholar]
- 37.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 38.El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. Neuropeptide Y: A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci U S A. 2000;97:6179–6184. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hökfelt T, Pernow B, Nilsson G, Wetterberg L, Goldstein M, Jeffcoate SL. Dense plexus of substance P immunoreactive nerve terminals in eminentia medialis of the primate hypothalamus. Proc Natl Acad Sci U S A. 1978;75:1013–1015. doi: 10.1073/pnas.75.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borsay BÁ, Skrapits K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, et al. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance p immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100:141–152. doi: 10.1159/000368362. [DOI] [PubMed] [Google Scholar]
- 41.Ljungdahl A, Hökfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- 42.Palkovits M, Kakucska I, Makara GB. Substance P-like immunoreactive neurons in the arcuate nucleus project to the median eminence in rat. Brain Res. 1989;486:364–368. doi: 10.1016/0006-8993(89)90524-6. [DOI] [PubMed] [Google Scholar]
- 43.Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156:627–637. doi: 10.1210/en.2014-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman RL, Inskeep EK. Control of the Ovarian Cycle of the Sheep. In: Plant TM, Zeleznick AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th. Elsevier; 2015. pp. 1259–1296. [Google Scholar]
- 46.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 47.Rance NE, Young WS. Hypertrophy and Increased Gene Expression of Neurons Containing Neurokinin-B and Substance-P Messenger Ribonucleic Acids in the Hypothalami of Postmenopausal Women*. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 48.Arisawa M, De Palatis L, Ho R, Snyder GD, Yu WH, Pan G, et al. Stimulatory role of substance P on gonadotropin release in ovariectomized rats. Neuroendocrinology. 1990;51:523–529. doi: 10.1159/000125386. [DOI] [PubMed] [Google Scholar]
- 49.Vijayan E, McCann SM. In vivo and in vitro effects of substance P and neurotensin on gonadotropin and prolactin release. Endocrinology. 1979;105:64–68. doi: 10.1210/endo-105-1-64. [DOI] [PubMed] [Google Scholar]
- 50.Ohtsuka S, Miyake A, Nishizaki T, Tasaka K, Aono T, Tanizawa O. Substance P stimulates gonadotropin-releasing hormone release from rat hypothalamus in vitro with involvement of oestrogen. Acta Endocrinol (Copenh) 1987;115:247–252. doi: 10.1530/acta.0.1150247. [DOI] [PubMed] [Google Scholar]
- 51.Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73–88. doi: 10.1016/j.yfrne.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso-Solís R, Abreu P, López-Coviella I, Hernández G, Fajardo N, Hernández-Díaz F, et al. Gonadal steroid modulation of neuroendocrine transduction: A transynaptic view. Cell Mol Neurobiol. 1996;16:357–382. doi: 10.1007/BF02088101. [DOI] [PubMed] [Google Scholar]
- 53.Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19:432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 55.Nestor CC, Briscoe AMS, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153:2756–2765. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, USA: Academic Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.