Abstract
The aim of the study was to investigate the effect of 2-oxoglutaric acid (2-Ox) supplementation (a precursor of glutamine and hydroxyproline, the most abundant amino acid of collagen) on cartilage and bone in pigs after fundectomy. Pigs at the age of forty days were subjected to fundectomy and divided into two groups depending on 2-Ox supplementation (at the daily dosage of 0.4 g/kg of body weight). Other pigs were sham operated. Pigs were euthanized at the age of eight months. An analysis of the morphometry of trabeculae, growth plate and articular cartilage in fundectomy-induced osteopenic bone was performed. Moreover, the levels of expression of osteocalcin, osteopontin and osteoprotegerin in trabecular bone and osteocalcin in articular cartilage were evaluated. Articular cartilage was thinnest in fundectomized pigs and thickest in 2-Ox-supplemented animals after fundectomy. Moreover, 2-Ox supplementation after fundectomy enhanced the total thickness of the growth plate and trabeculae in fundectomized pigs. The most evident signal for osteocalcin and osteoprotegerin in trabecular bone was in sham-operated and 2-Ox-supplemented pigs; a low reaction was observed in the fundectomized group. Additionally, as a long-term postoperative consequence, a change was observed in the expression of osteocalcin in articular cartilage. It seems that 2-Ox is suitable for use in preventing the negative effects of fundectomy on cancellous bone and cartilage.
Keywords: 2-oxoglutaric acid, cartilage, fundectomy, swine
Fundectomy is the surgical removal of the fundus and is performed as a treatment for cancer or ulcer of the stomach in humans and animals. Moreover, such operations (bariatric) are increasingly being used in the treatment of obesity [8, 20, 32, 34]. Surgical removal of the stomach can have different short- or long-term postoperative consequences. After fundectomy, the daily intake of many macro- and microelements in the diet, vitamins and nutritive factors decreases to below the needed amount. Moreover, fundectomy induces complex hormonal and metabolic side effects that lead to the loss of bone mass in adults (about 25% of gastric surgery patients) or disturbance of longitudinal bone growth in children [6, 20, 35, 36]. An adult can lose bone minerals after fundectomy, while growing individuals cannot gain enough bone density. Fundectomy leads to bone metabolic instability without symptoms until an increased risk of fractures develops a few years after surgery [6, 17,18,19, 29, 35]. Side effects of such surgeries are studied in animal models, i.e., rats or pigs. Fundectomy in pigs induces osteopenic changes in bone similar to those observed in humans after gastrectomy or fundectomy [18, 19, 35, 41].
The positive impact of specific compounds in food is evident. For example, functional foods improve the state health and can reduce the risk of various diseases. Among these specific compounds is 2-oxoglutaric acid (2-Ox), a main source of energy for cells, one of the metabolites of the Krebs cycle and a precursor of glutamine, proline and hydroxyproline (main amino acids of collagen) as well as arginine and asparagine [8]. In many studies, 2-Ox has shown beneficial effects improving body weight gain. It decreases protein catabolism and increases protein synthesis in the skeletal muscles [7, 15, 47]. 2-Ox also shows a protective effect against the negative action of dexamethasone on bone and cartilage in animals with intrauterine growth restriction or treatemet with omeprazole [3, 4, 21, 35,36,37,38, 42, 45]. Moreover, long-term oral administration of 2-Ox improved bone mineralization and the mechanical endurance of long bones in fundectomized or gastrectomized animals compared with those that were not supplemented [4, 35, 36, 42].
Although, the histological structures of articular cartilage and the growth plate are well documented, little is known about the protective effects of dietary 2-Ox supplementation in growing fundectomized pigs on cancellous bone and cartilage. The influence of surgical removal of the fundus and postoperative diet on the growth plate and articular cartilage in fundectomy-induced osteopenic bone, however, has not been thoroughly studied with the morphometry and expression of the most abundant non-collagenous protein in cancellous bone and cartilage in pigs with experimentally performed fundectomy.
About 10% of different non-collagenous proteins are secreted by osteoblasts, osteocytes and chondrocytes in connective tissue. The cells of this tissue differ in morphology and metabolism from each other, but a type of connective tissue is best characterized by the extracellular matrix composition, which determines its division and classification [16]. The extracellular matrix is a dynamic environment that directly affects cells by limiting diffusion of substrates. Thus, the process of bone metabolism can be determined by the assessment of some non-collagenous proteins like osteocalcin (OCN), osteoprotegerin (OPG) or osteopontin (OPN). OPN can stimulate the activity of osteoclasts and is involved in bone resorption [2]. OPG is the most important inhibitor of osteoclast differentiation. When OPG expression is decreased, bone loss is observed [13, 26, 30]. OCN, a typical non-collagenous bone protein, has been accepted as a bone-specific product classified as the factor that most reflects bone formation. It is considered to be a more sensitive marker than serum bone alkaline phosphatase activity [16]. Monitoring of the OCN concentration may be useful in determination of the response to treatment or prediction of bone loss. OCN synthesis is enhanced during mineral deposition in the extracellular matrix. Moreover, it is a marker of the mature state of the osteoblasts in a mineralizing matrix. The expression of OCN is influenced by various growth factors or nutrition [9, 21, 24, 31].
The aim of the study was to investigate a dietary supplement that could attenuate, reduce or prevent the side effects of fundectomy. To evaluate the long-term effect of oral administration of 2-Ox on connective tissue in pigs subjected to fundectomy, histomorphometry of articular cartilage and the growth plate of the femur was performed. Moreover, the levels of expression of OCN, OPN and OPG in trabecular bone and OCN in articular cartilage were evaluated.
MATERIALS AND METHODS
The experimental procedures used throughout this study were approved (No. 43/04) by the Local Ethics Committee on Animal Experimentation of the University of Life Sciences of Lublin, Poland.
Animals, surgical procedure and animal diet: The study was performed on eighteen castrated male pigs of the Pulawska breed about 12 days after their weaning from sows. Pigs were placed under standard rearing conditions with constant access to fresh water. Castrated male pigs were randomly assigned into two weight-matched groups at the age of 40 days of life and then subjected to sham operation as a control (the control group, SHO; n=6) or experimental fundectomy (Fx; n=12). The mean initial body weights of pigs did not differ between the groups. The surgical procedures performed on 40-day-old pigs were similar to those described previously [36]. Fundectomy was carried out by resection of the fundus and glandular part of the stomach, and then the remaining part of the stomach was connected with the intestine in a manner that allowed for undisturbed passage of food to the duodenum. As a result of fundectomy, approximately 50% of the stomach was removed.
Fundectomized animals were divided into two groups depending on oral supplementation with 2-Ox: the 2-Ox group (n=6) received a solution of 2-Ox at the daily dosage of 0.4 g/kg of body weight, and the Fx group was not supplemented (n=6). The solution was mixed with diet and administered starting on day 41 of life up to the end of the experiment. This supplementation was performed regardless of feed procedure after recovery. Powdered 2-Ox (2-Ox; Protista International AB, Bjuv Sweden) of 99% purity was mixed with distilled water to obtain a solution. The pH of the stock solution was buffered by the addition of NaOH to a final pH of 7.3. The dosage of 2-Ox used in this study was the same as previously used in studies involving pigs and turkeys [14, 37,38,39,40, 44,45,46].
Throughout the entire period of the study, pigs from all the groups were housed indoors in three boxes (one box for each group, temperature 22°C and humidity 60–70%) equipped with feeders enabling food consumption for all animals within the group at the same time. Throughout the study, pigs were fed a commercial diet (twice a day) supplied in accordance with the stage of the production cycle and age as recommended [35]. Animals were weighed every week (to calculate the dose of 2-Ox) and sacrificed at the age of eight months.
Bone, articular cartilage and growth plate histomorphometry: Left femora were isolated immediately after euthanasia. Samples were taken from proximal part of femur after removal of soft tissues. The joint was opened, and specimens of full-thickness cartilage along with bone were excised. Cylindrical 20-mm-thick samples (cartilage and bone) were taken from the same anatomical position in the joint from the apical region of the head of the femur, except for the fovea of the femoral head [5]. Horizontal plane sections were used. Tissue samples were subjected to histology as described previously [4, 43]. Sections (4 µm thick) were stained with two methods: Goldner’s trichrome staining (GT) to assess the morphology of the growth plate and articular cartilage and picrosirius red staining (PSR) to evaluate the distribution of thick and thin collagen fibers of articular cartilage [9, 11, 12, 27, 33, 48]. Additionally, cartilage proteoglycans were stained with Safranine O [1]. Microscopic images (magnification ×100, ×200) were collected using an Axiovert 200M confocal microscope (Carl Zeiss, Jena, Germany) equipped with an AxioCam HRc camera (Carl Zeiss), halogen lamp and fluorescent lamp (excitation wavelength 450–490 nm for the evaluation of autofluorescence images). Sections stained with PSR were analyzed using an Olympus BX63 automated microscope (Olympus, Tokyo, Japan) equipped with filters to provide circularity polarized illumination. Images were collected with a digital color camera (UC50 Olympus). The analysis of collected images was performed with the graphical analysis software Olympus cellSens Version 1.5 (Olympus). The thicknesses of reserve cartilage, proliferative, hypertrophic and ossification zones were measured at four sites along the growth plate cartilage, and the average was taken. The four sites were selected a priori, and the zones were defined as described previously [5, 48]. Similarly, the thicknesses of three main zones of articular cartilage, such as horizontal (superficial surface), transitional and radial zones were measured. Individual layers of articular cartilage were defined as was described by Pearle et al. [25].
Among the parameters recommended by the American Society for Bone and Mineral Research, we assessed the volumes of bone trabeculae and the thicknesses of bone trabeculae [22, 23]. The bone and tissue volumes were measured in photographs of bone tissue sections using the pixel count, and relative bone volume (BV/TV%) was assessed. Moreover, the following parameters were examined for trabecular bone (epiphysis and metaphysis): mean and maximal trabeculae thickness (Tb.Th) and mean and maximal trabecular space (Tb.Sp), which was defined as the distance between the edges of adjacent trabeculae (measured directly).
Immunohistochemistry: Analysis of the expression of OCN, OPN and OPG in paraffin-embedded sections using immunohistochemistry was performed according to protocol described previously [44], except that antigen retrieval was achieved by enzymatic retrieval for 10 min with 1 mg/ml proteinase K (Sigma, Poznań, Poland) in TBS (ph 7.4) at 37°C. Mouse monoclonal to OCN anibodies and rabbit polyclonal OPN and OPG antibodies (Abcam, Cambridge, U.K., dilution 1:100) were used as primary antibodies. Biotinylated anti-mouse immunoglobulin (DakoCytomation, Glostrup, Denmark, dilution 1:200) and biotinylated anti-rabbit immunoglobulin (Abcam; dilution 1:200) were used as secondary antibodies, respectively. Negative control sections received identical preparations for immunohistochemical staining, except that the primary antibody was omitted. Microscopic observations of immunohistochemical reactions were further analyzed. The levels of expression of OCN, OPN and OPG were described as the intensity (a low- or high-power positive reaction) in trabecular osteocytes. Additionally, the intensity of OPN and OPG in osteocytes was measured by comparison of the pixel brightness value in the 8-bit grayscale images. The higher the pixel brightness value, the lower the intensity of immunoreactions. Moreover, the expression of OCN was described in articular cartilage as a positive (brown nuclei) or negative (blue nuclei) reaction.
Statistical analysis: All results are expressed as means ± SD (standard deviation). Differences between means were tested with the one-way ANOVA with the post hoc Tukey’s test as the correction for multiple comparisons. The normality of the distribution of data was examined using the Shapiro-Wilk test, and equality of variance was tested by the Brown-Forsythe test. If the distribution was not normal and/or the variances of data were unequal, Kruskal-Wallis ANOVA was used. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were carried out by means of the STATISTICA 10 software (StatSoft Inc., Tulsa, OK, U.S.A.).
RESULTS
Body weight, bone weight and length: The final body weight was lower in the Fx group (37.5 ± 9.5 kg) than in the SHO (108 ± 10 kg) or 2-Ox (65 ± 5 kg) group. Bones from the Fx group (16.5 ± 0.7 cm) were shorter than those in the SHO (20.6 ± 0.3 cm) and 2-Ox (19.3 ± 0.6 cm) groups. Bones from the Fx group (170.4 ± 17.5 g) were lighter than those in the SHO (280.4 ± 12.2 g) and 2-Ox (240.5 ± 3.1 g) groups.
Bone, articular and growth plate cartilages morphology: The total thicknesses of articular cartilage in the fundectomized group (819 ± 37 µm) and in sham operated group (849 ± 123 µm) were smaller than that in the group supplemented with 2-Ox after surgery (966 ± 104 µm; P<0.05). Long-term administration of 2-Ox after fundus removal resulted in 38% and 46% increases in the thickness of the transitional zone compared with sham-operated and fundectomized animals, respectively. Moreover, the superficial zone was thickest in the sham-operated group compared with the other groups, and there was a difference between the groups supplemented and not supplemented with 2-Ox after fundectomy (Table 1).
Table 1. The effect of oral administration of 2-oxoglutarate on the thicknesses of articular cartilage and the growth plate of the femur in fundectomized boars.
| Group | SHO group (n=6) | Fx group (n=6) | 2-Ox group (n=6) |
|---|---|---|---|
| Zones of growth plate | |||
| Reserve (µm) | 179.7 ± 53.2a) | 154.5 ± 40.2b) | 222.3 ± 57.9b) |
| Proliferative (µm) | 78.2 ± 22.8a) | 69.9 ± 20.4a) | 179.5 ± 33.1b) |
| Hypertrophic (µm) | 77.1 ± 18.1a) | 84.1 ± 22.2a) | 139.7 ± 28.3b) |
| Ossification (µm) | 298.8 ± 98.3a) | 318.2 ± 67.4a) | 248.5 ± 77.3b) |
| Zones of articular cartilage | |||
| Superficial (µm) | 99.8 ± 28.4a) | 46.8 ± 16.6b) | 55.5 ± 13.3c) |
| Transitional (µm) | 294.9 ± 100.9a) | 278.9 ± 34.5a) | 406.9 ± 39.1b) |
| Radial (µm) | 467.3 ± 108.5a) | 485.1 ± 43.7a) | 504.9 ± 120.8a) |
Data are shown as the mean ± SD. Values with different superscripts are significantly different (P<0.05). Fx group, the fundectomized group; 2-Ox group, supplemented with 2-Ox after fundectomy; SHO group, the sham operated group.
The growth plate was thinnest in the fundectomized (641 ± 79 µm) and sham-operated (622 ± 88 µm) animals and differed significantly from the growth plate in the group supplemented with 2-Ox after fundectomy (777 ± 83 µm; P<0.05). The thicknesses of the reserve, proliferative and hypertrophy zones increased by 24% and 44%, 129% and 160% and 80% and 65% compared with those of the sham-operated and fundectomized animals, respectively. But, the thickness of the ossification zone decreased by 22% and 16% in the 2-Ox group compared with those of other groups.
Histomorphometric analysis revealed significantly lower values for the relative bone volume of the femur in fundectomized pigs (Table 2). Moreover, the supplementation with 2-Ox did not influence bone volume after fundectomy. Even though there was no differences in the relative bone volume, postnatal 2-Ox supplementation enhanced the mean thickness of epiphyseal and metaphyseal trabeculae by 30.4% and 30.1%, when compared with the fundectomized group (Table 2). The maximal trabecular thickness of epiphyseal trabeculae decreased by 36% and 28.3% when compared with the control and 2-Ox-supplemented groups, respectively. The maximal trabecular thickness of metaphyseal trabeculae decreased by 31.5% and 21.6% when compared with the control and 2-Ox-supplemented groups, respectively. The lack of supplementation with 2-Ox resulted in 40% and 27% increases in mean trabecular space in the epiphysis and 93% and 44% increases in the metaphysis, when compared with the control and 2-Ox-supplemented groups, respectively. Moreover, the surgery enhanced the maximal trabecular space by 40% in the epiphysis and 74% in the metaphysis when compared with the control group. Supplementation with 2-Ox reduced this effect by 51% (Table 2).
Table 2. The effect of oral administration of 2-oxoglutarate on trabecular histomorphometry of the femur in fundectomized boars.
| Group | SHO group (n=6) | Fx group (n=6) | 2-Ox group (n=6) | |
|---|---|---|---|---|
| Epiphysis | ||||
| BV/TV% | 25.5 ± 2.3a) | 18.8 ± 3.1b) | 18.8 ± 2.4b) | |
| Tb.Th mean (µm) | 43.5 ± 1.6a) | 27.9 ± 2.5b) | 36.4 ± 4.9a) | |
| Tb.Th max (µm) | 129.7 ± 6.6a) | 82.6 ± 10.8b) | 115.2 ± 20.9a) | |
| Tb.Sp mean (µm) | 285.4 ± 45.8a) | 399.7 ± 63.7b) | 314.5 ± 91.9a) | |
| Tb.Sp max (µm) | 503.1 ± 88.2a) | 705.4 ± 126.8b) | 517.9 ± 39.5a) | |
| Metaphysis | ||||
| BV/TV% | 28.9 ± 1.9a) | 18.9 ± 2.1b) | 20.4 ± 3.2b) | |
| Tb.Th mean (µm) | 38.2 ± 2.9a) | 28.2 ± 2.3b) | 36.7 ± 4.7a) | |
| Tb.Th max (µm) | 127.2 ± 16.4a) | 87.1 ± 7.4b) | 111.2 ± 12.9a) | |
| Tb.Sp mean (µm) | 234.3 ± 33.2a) | 451.8 ± 57.6b) | 313.8 ± 90.3a) | |
| Tb.Sp max (µm) | 434.4 ± 40.6a) | 757.2 ± 57.6b) | 534.6 ± 79.9c) | |
Data are shown as the mean ± SD. Values with different superscripts are significantly different (P<0.05). BV/TV%, the relative bone volume; Tb.Th, trabeculae thickness; Tb.Sp, trabecular space; Fx group, the fundectomized group; 2-Ox group, supplemented with 2-Ox after fundectomy; SHO group, the sham operated group.
Osteocalcin, osteopontin and osteoprotegerin expression in trabeculae: High-intensity staining for OPG and moderate-intensity staining for OPN were observed in osteocytes of trabeculae from the sham-operated group (Fig. 1). The intensity of OPG and OPN expression in the 8-bit grayscale images reached values of 158 ± 14 and 102 ± 9, respectively. While significantly low staining for OPG in trabecular osteocytes was observed in the fundectomized group, the OPG expression in the 8-bit grayscale images reached a value of 79 ± 11. Moreover, the OPN reaction was moderate or intense in this group, and the expression in the 8-bit grayscale images reached a value 118 ± 10 and differed significantly from the other groups in intensity. The 2-Ox supplementation after fundectomy resulted in a value of 79 ± 15 for OPG in the 8-bit grayscale images and moderate staining for OPN (105 ± 9). The results of OCN staining are not shown, because the intensity of the reaction was very similar to that in OPG staining.
Fig. 1.
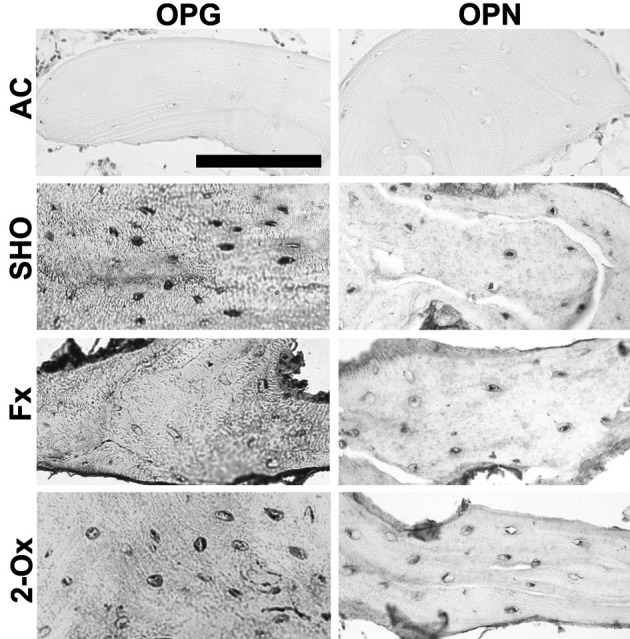
Representative pictures of the immunohistochemical analysis of the expression of osteopontin (OPN) and osteoprotegerin (OPG) carried out on formaldehyde-fixed sections from femoral trabeculae of pigs from the Fx group, the fundectomized group; the 2-Ox group, supplemented with 2-Ox after fundectomy; and the SHO group, the sham-operated group. AC, antibody control. Scale bar for all panels: 200 µm.
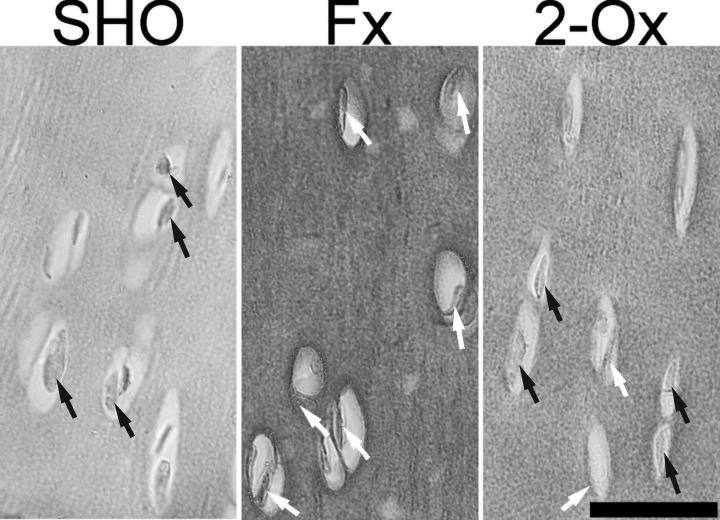
Osteocalcin expression in articular cartilage: In chondrocytes of normal cartilage (from the SHO group), the protein staining was negative, and chondrocytes had blue nuclei (Fig. 2). The power signal for OCN in many cells in the deep cartilage layer was various in the articular cartilage in fundectomized animals. The 2-Ox supplementation resulted in very low-power positive staining for OCN in a few cells of articular cartilage (Fig. 2).
Fig. 2.
Representative pictures of the immunohistochemical analysis of the expression of osteocalcin (OCN) carried out on formaldehyde-fixed sections from femoral articular cartilage of pigs from the Fx group, the fundectomized group; the 2-Ox group, supplemented with 2-Ox after fundectomy; and the SHO group, the sham-operated group. In normal chondrocyte from the SHO group, the nucleus is stained blue (the negative OCN signal) and indicated by black arrows. The positive OCN signal is indicated in cells from the Fx group by white arrows. Scale bar: 200 µm.
The distribution of thick and thin collagen fibers and proteoglycans in articular cartilage: The structural information obtained from analysis of fibrous components in PSR-stained section revealed a difference between the large (red-orange) and thin collagen fibers including reticular fibers (green). Supplementation of fundectomized animals with 2-Ox enhanced thin fibers (green) and decreased thick (red) fibers in articular cartilage, resulting in greenish dark and red radial and transitional fibers (Fig. 3). The opposite proportion was observed in fundectomized pigs that were not supplemented, with primarily dark and bright red thick fibers being present in the radial zone compared with other groups. Fundectomy resulted also in the lowest amount of thin (green) fibers in the transitional zone. Moreover, control cartilage from sham-operated animals contained both type of fibers with the predominance of thick fibers (bright red) in the radial zone. Many visible bright green fibers were around chondrocytes.
Fig. 3.
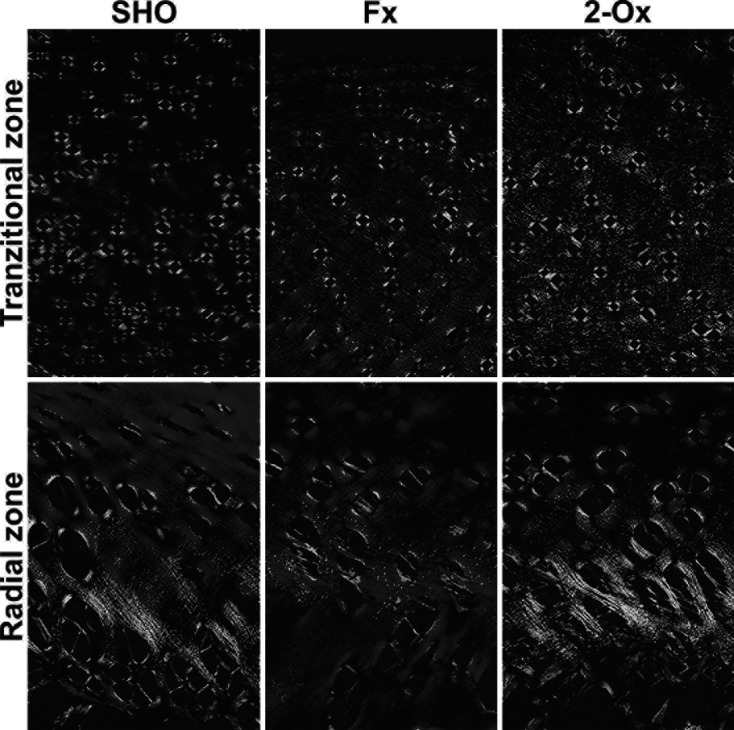
Representative images of PSR staining carried out on formaldehyde-fixed sections from femoral articular cartilage of pigs. Vertical section of transitional and radial zones of femoral articular cartilage from the Fx group, the fundectomized group; the SHO group, the sham-operated group; and the 2-Ox group, supplemented with 2-Ox after fundectomy. Magnification ×100.
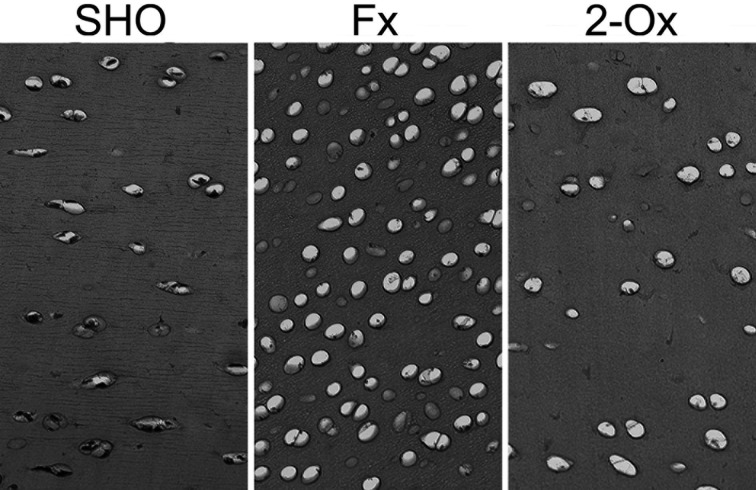
Cartilage proteoglycan staining with Safranine O showed that there was no difference in proteoglycan content (the color intensity) in articular cartilage belonging to boars supplemented with 2-Ox and boars not supplemented with 2-Ox after fundectomy as well as articular cartilage belonging to sham-operated boars (Fig. 4). Even though the chondrocyte number per square mm of articular cartilage was not calculated, an increase in chondrocyte number in fundectomized animals was seen. Moreover, a lack of enhancement of the chondrocyte number in articular cartilage was observed in 2-Ox-supplemented animals after fundectomy.
Fig. 4.
Representative images of Safranin O staining carried out on formaldehyde-fixed sections from femoral articular cartilage of pigs from the Fx group, the fundectomized group; the 2-Ox group, supplemented with 2-Ox after fundectomy; and the SHO group, the sham-operated group. Highly cellular articular cartilage in fundectomized animals with chondrocytes is separated by thin septa of matrix in the Fx group compared with the SHO and 2-Ox groups. Magnification ×200.
DISCUSSION
The influence of fundectomy on bone metabolism and development in growing pigs has been proven in a previous study, and it has been shown that fundectomy in growing pigs leads to decreased body mass and bone loss followed by malnutrition [35, 36]. Another study showed that gastrectomy greatly affects articular cartilage and the growth plate in rats, and that dietary 2-Ox administration is capable of counteracting the gastrectomy-evoked impairment of this crucial tissue [5]. However, there have been no reports about the effect of fundectomy on hyaline cartilage or opportunities to minimize its side effects. Regarding this in particular, the mechanism of bone loss after bariatric surgeries is known; that is, it is known that negative effects on the skeletal system are associated with decreased activity of the gastric-hypothalamic-pituitary axis. Fundectomy lowers the growth hormone, leptin and insulin-like growth hormone (IGF-1) concentrations in blood serum [35]. However, little is known about the role of food containing precursors of glutamine in the expression of non-collagenous protein in fundectomy-induced osteopenic bone. Non-collagenous protein expression in trabeculae after fundectomy has not been reported as well. It is important for matrix transformation to occur not only in the growth plate but also in the osteoid. However, the cells, collagen and other proteins in these tissues are different.
Our study indicated that fundectomy led to lowered expression of OPG and OCN in cancellous bone. This was probably an additional mechanism (after decreased activity of the gastric-hypothalamic-pituitary axis) underlying insufficient bone mineralization after fundectomy linked with malnutrition. Thus, the lack of 2-Ox treatment resulted in a decrease in relative bone volume and thickness of trabeculae compared with the animals receiving the sham operation and 2-Ox supplementation. An increase of trabecular space also was observed. Therefore, the resulting reduction in bone mass after fundectomy was characterized by thinning of trabeculae with relative preservation of the trabecular architecture. Moreover, the growth plate in fundectomized animals was reduced. It should be emphasized that the longitudinal growth occurs as a result of an increase in cell number resulting from the divisions in the zone of proliferation. Disturbances of bone growth also influence their width and thickness, not only the architecture of the trabeculae.
This result is in agreement with another study that showed that gastrectomy in rats decreases the relative bone volume in tibial trabeculae [11]. The present study also showed that gastrectomy reduces BMD and bone strength at skeletal sites rich in cancellous bone compared with sites rich in cortical bone and that the gastectomy-induced decrease in bone mass is greater in cancellous bone than in cortical bone. Our results support other studies that show little reduction of cortical bone in the femur or calvaria [3, 17].
Cell physiology and metabolism in the growth plate depend on the blood supply and the growth and energy factors present in the blood. The results of the present study showed that treatment with 2-Ox improved the growth plate morphology and histomorphometric parameters of trabeculae. The expression of OPG and OCN, non-collagenous proteins closely related with bone metabolism, in the fundectomy-induced osteopenic bone was enhanced after 2-Ox supplementation, which probably provided additional energy for cells, in addition to its function as a precursor of glutamine for the synthesis of collagen. This alteration evoked by 2-Ox supplementation could have the potential to affect the degree of mineralization, which depends on the size and number of cells in the hypertrophic zone of the growth plate, and the amount of calcium released from the mitochondrion. However, results could be interpreted as indicating that 2-Ox supplementation might reduce excessive bone loss and the risk of the fracture in fundectomized animals.
Additionally, the obtained results proved that 2-Ox given over a long period of time caused significant increase of the articular cartilage thickness in fundectomized male pigs. Further, widening of the transitional zone can influence the transfer and distribution of loads through the joint with functional consequences. This might prevent the degradation of articular cartilage, particularly with increasing age. On the other hands, articular cartilage in fundectomized animals became highly cellular, and chondrocytes were separated by thin septa of matrix. Moreover, the lack of 2-Ox supplementation after fundectomy led to decreased thickness of articular cartilage. This was due to lack of the precursor of glutamine as a primary source of energy, which could influence chondrocyte metabolism. The articular cartilage is characterized by a high volume ratio of matrix to cells with low metabolic activity. The physical properties of articular cartilage are determined by the diversity in the components of the matrix and the difference in the quantitative mutual relations of collagen fibers.
In the mature adult articular cartilage, chondrocytes have a stable phenotype. They synthesize physiological matrix components, such as type II and VI collagen [28]. Type II collagen interacts with proteoglycans and glycoproteins to form the cartilage matrix. This composition ensures sustainability and a low friction surface, and contributes to flexibility and plasticizing of hyaline cartilage. However, they begin to express bone-related proteins, such as OCN, alkaline phosphatase and type X collagen under some conditions. A change in the activity of chondrocytes alters the composition of the matrix including the collagen network and the microenvironment of chondrocytes, which can lead to degeneration and osteoarthritis [26, 28]. This process may have an impact on the physiological changes that occur in the cartilage with age, as proteoglycans bind more water in young animals; in elderly animals, there is less water and the stiffness of cartilage increases.
The expression of OCN in articular cartilage in fundectomized pigs has not been reported so far. The current analysis showed that fundectomy resulted in well-marked cytoplasmic signal for OCN in many chondrocytes of articular cartilage. This alteration of chondrocyte activity can result in microdegeneration in articular cartilage, which can accumulate with age and predispose articular cartilage to damage.
It is noteworthy that in normal articular cartilage, there is a lack of OCN expression, but in progressive degeneration, OCN is present in the synovial fluid [28]. Moreover, serum OCN has been proposed as a possible biomarker for early detection and monitoring of arthritic degeneration, and its concentration reflects processes that are directly implicated in the metabolism of articular cartilage [10]. The diagnosis of any disease requires the presence of clinical signs. It might be that OCN is an early presymptomatic biomarker of degradation of cartilage, which, if detected, could allow for earlier diagnosis in a pre-radiographic phase or a later radiographic phase with visible structural joint changes (articular matrix breakdown) [10]. Thus, OCN has opened novel possibilities for earlier diagnosis and monitoring of the degradation of articular cartilage. Whether OCN is useful in this process is still unclear, but Pullig et al. demonstrated that in osteoarthritic human cartilage, chondrocyte hypertrophy is accompanied by the expression of OCN [28].
One more effect of fundectomy was observed (Figs. 2 and 4); that is, articular chondrocytes in the articular cartilage of pigs subjected to surgery become more circular in shape, despite the loss of body weight and lowered load of the joint. A similar effect was observed in our earlier study in which we performed gastrectomy in rats, which showed a decrease in chondrocyte perimeter in tibial articular cartilage [5]. It might be that both effects (OCN expression and alteration of the shape as a sign of hypertrophy) are interconnected. The affected chondrocytes of fundectomized pigs might express a type of collagen other than that expected in normal cartilage. Therefore, abnormal production of collagen could affect the structural integrity of articular cartilage. This needs further investigation. Finally, the involvement of the increase of chondrocytes activity in articular cartilage fibers content must be considered. Based on the structural information obtained from analysis of fibers components in PSR-stained sections, it can be concluded that when fundectomized animals were not administered 2-Ox, thin fibers (green) did not appear. This may indicate that the molecular signals were too low and could not potentially guide the structure of collagen in growing fundectomized animals. This collagen network was subsequently remodeled after treatment with 2-Ox, which served as the main source of proline and hydroxyproline used in collagen synthesis.
This is consistent with our other study, which showed that gastrectomy greatly affects hyaline cartilage and that dietary 2-Ox administration is capable of counteracting the gastrectomy-evoked impairment of this crucial tissue [5].
This study observed the influence of 2-Ox supplementation on the growth of pigs under the condition of fundectomy and revealed that surgical removal of part of the stomach significantly reduces the thickness of articular cartilage. Additionally, as a long-term postoperative consequence, a change in the expression of non-collagenous proteins was observed. Further, based on histomorphometry of trabeculae, this study showed that osteopenia was observed in cancellous bone. Further studies are needed that include dynamic bone morphometric analysis with tetracycline labeling in animals with fundectomy-induced osteopenia.
Gastric surgery patients have significantly decreased energy intake leading to weight loss. Individuals must be monitored for complications. Moreover, measures for prevention of bone loss should be carried. Fundectomized individuals need to be treated carefully by a functional or special balanced diet or a cocktail of supplements.
It seems that 2-Ox is suitable for use in minimizing some side effects of fundectomy. It can be concluded that 2-Ox supplementation can mainly improve the collagen network in articular cartilage after fundectomy.
Conflict of Interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Camplejohn K. L., Allard S. A.1988. Limitations of safranin ‘O’ staining in proteoglycan-depleted cartilage demonstrated with monoclonal antibodies. Histochemistry 89: 185–188. doi: 10.1007/BF00489922 [DOI] [PubMed] [Google Scholar]
- 2.Dallas S. L., Prideaux M., Bonewald L. F.2013. The osteocyte: an endocrine cell ... and more. Endocr. Rev. 34: 658–690. doi: 10.1210/er.2012-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrowolski P. J., Piersiak T., Surve V. V., Kruszewska D., Gawron A., Pacuska P., Håkanson R., Pierzynowski S. G.2008. Dietary alpha-ketoglutarate reduces gastrectomy-evoked loss of calvaria and trabecular bone in female rats. Scand. J. Gastroenterol. 43: 551–558. doi: 10.1080/00365520701824951 [DOI] [PubMed] [Google Scholar]
- 4.Dobrowolski P., Tomaszewska E., Radzki R. P., Bienko M., Wydrych J., Zdybel A., Pierzynowski S. G.2013. Can 2-oxoglutarate prevent changes in bone evoked by omeprazole? Nutrition 29: 556–561. doi: 10.1016/j.nut.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 5.Dobrowolski P., Tomaszewska E., Kurlak P., Pierzynowski S. G.2015. Dietary 2-oxoglutarate mitigates gastrectomy-evoked structural changes in cartilage of female rats. Exp. Biol. Med. (Maywood) (in press) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipponi P., Gregorio F., Cristallini S., Mannarelli C., Blass A., Scarponi A. M., Vespasiani G.1990. Partial gastrectomy and mineral metabolism: effects on gastrin-calcitonin release. Bone Miner. 11: 199–208. doi: 10.1016/0169-6009(90)90059-O [DOI] [PubMed] [Google Scholar]
- 7.Hammarqvist F., Wernerman J., von der Decken A., Vinnars E.1991. Alpha-ketoglutarate preserves protein synthesis and free glutamine in skeletal muscle after surgery. Surgery 109: 28–36. [PubMed] [Google Scholar]
- 8.Heiskanen J. T., Kröger H., Pääkkönen M., Parviainen M. T., Lamberg-Allardt C., Alhava E.2001. Bone mineral metabolism after total gastrectomy. Bone 28: 123–127. doi: 10.1016/S8756-3282(00)00404-X [DOI] [PubMed] [Google Scholar]
- 9.Hoang Q. Q., Sicheri F., Howard A. J., Yang D. S. C.2003. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 425: 977–980. doi: 10.1038/nature02079 [DOI] [PubMed] [Google Scholar]
- 10.Huang C. C., Lee C. C., Wang C. J., Wang F. S., Huang H. Y., Ng S. H., Tseng C. Y., Ko S. F.2014. Effect of age-related cartilage turnover on serum C-telopeptide of collagen type II and osteocalcin levels in growing rabbits with and without surgically induced osteoarthritis. Biomed. Res. Int. 2014: 284784. doi: 10.1155/2014/284784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamoto J., Sato Y., Matsumoto H.2013. Influence of gastrectomy on cortical and cancellous bones in rats. Gastroenterol. Res. Pract. 2013: 381616.doi.org/10.1155/2013/381616. doi: 10.1155/2013/381616 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Junqueira L. C., Bignolas G., Brentani R. R.1979. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11: 447–455. doi: 10.1007/BF01002772 [DOI] [PubMed] [Google Scholar]
- 13.Kostenuik P. J.2005. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr. Opin. Pharmacol. 5: 618–625. doi: 10.1016/j.coph.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 14.Kowalik S., Filip R. S., Śliwa E., Tatara M. R.2005. Pierzynowski S. G., Studziński T.: Influence of alpha-ketoglutarate on bone mineral density of the femur in piglets. Bull. Vet. Inst. Pulawy 49: 343–348. [Google Scholar]
- 15.Kristensen N. B., Jungvid H., Fernández J. A., Pierzynowski S. G.2002. Absorption and metabolism of alpha-ketoglutarate in growing pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 86: 239–245. doi: 10.1046/j.1439-0396.2002.00380.x [DOI] [PubMed] [Google Scholar]
- 16.Lee A. J., Hodges S., Eastell R.2000. Measurement of osteocalcin. Ann. Clin. Biochem. 37: 432–446. doi: 10.1177/000456320003700402 [DOI] [PubMed] [Google Scholar]
- 17.Lehto-Axtelius D., Stenström M., Johnell O.1998. Osteopenia after gastrectomy, fundectomy or antrectomy: an experimental study in the rat. Regul. Pept. 78: 41–50. doi: 10.1016/S0167-0115(98)00101-3 [DOI] [PubMed] [Google Scholar]
- 18.Maier G. W., Kreis M. E., Zittel T. T., Becker H. D.1997. Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann. Surg. 225: 181–192. doi: 10.1097/00000658-199702000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellström D., Johansson C., Johnell O., Lindstedt G., Lundberg P. A., Obrant K., Schöön I. M., Toss G., Ytterberg B. O.1993. Osteoporosis, metabolic aberrations, and increased risk for vertebral fractures after partial gastrectomy. Calcif. Tissue Int. 53: 370–377. [PubMed] [Google Scholar]
- 20.Melton L. J., 3rd, Crowson C. S., Khosla S., O’Fallon W. M.1999. Fracture risk after surgery for peptic ulcer disease: a population-based cohort study. Bone 25: 61–67. doi: 10.1016/S8756-3282(99)00097-6 [DOI] [PubMed] [Google Scholar]
- 21.Meyer J. H.1994. Nutritional outcomes of gastric operations. Gastroenterol. Clin. North Am. 23: 227–260. [PubMed] [Google Scholar]
- 22.Mocetti P., Ballanti P., Zalzal S., Silvestrini G., Bonucci E., Nanci A.2000. A histomorphometric, structural, and immunocytochemical study of the effects of diet-induced hypocalcemia on bone in growing rats. J. Histochem. Cytochem. 48: 1059–1078. doi: 10.1177/002215540004800804 [DOI] [PubMed] [Google Scholar]
- 23.Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R.1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2: 595–610. doi: 10.1002/jbmr.5650020617 [DOI] [PubMed] [Google Scholar]
- 24.Patti A., Gennari L., Merlotti D., Dotta F., Nuti R.2013. Endocrine actions of osteocalcin. Int. J. Endocrinol. 2013: 846480. doi: 10.1155/2013/846480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearle A. D., Warren R. F., Rodeo S. A.2005. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 24: 1–12. doi: 10.1016/j.csm.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Peel N.2009. Bone remodeling and disorders of bone metabolism. Surgery 27: 70–74. [Google Scholar]
- 27.Puchtler H., Waldrop F. S., Valentine L. S.1973. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr. Pathol. 150: 174–187. doi: 10.1016/S0005-8165(73)80016-2 [DOI] [PubMed] [Google Scholar]
- 28.Pullig O., Weseloh G., Ronneberger D., Käkönen S., Swoboda B.2000. Chondrocyte differentiation in human osteoarthritis: expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif. Tissue Int. 67: 230–240. doi: 10.1007/s002230001108 [DOI] [PubMed] [Google Scholar]
- 29.Puzio I., Kapica M., Filip R., Bienko M., Radzki R. P.2005. Fundectomy evokes elevated gastrin and lowered ghrelin serum levels accompanied by decrease in geometrical and mechanical properties of femora in rats. Bull. Vet. Inst. Pulawy 49: 69–73. [Google Scholar]
- 30.Ralston S. T.2013. Bone structure and metabolism. Medicine 41: 581–585. doi: 10.1016/j.mpmed.2013.07.007 [DOI] [Google Scholar]
- 31.Rich L., Whittaker P.2005. Collagen and picrosirius red staining: a polazized light assessment of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 22: 97–104. [Google Scholar]
- 32.Schölmerich J.2004. Postgastrectomy syndromes—diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 18: 917–933. doi: 10.1016/S1521-6918(04)00122-2 [DOI] [PubMed] [Google Scholar]
- 33.Suvara S. K., Layton C., Bancroft J. D.2013. Theory and Practice of Histological Techniques. 7th ed., Churchill Livingstone Elsevier, Amsterdam. [Google Scholar]
- 34.Svedlund J., Sullivan M., Liedman B., Lundell L.1999. Long term consequences of gastrectomy for patient’s quality of life: the impact of reconstructive techniques. Am. J. Gastroenterol. 94: 438–445. doi: 10.1111/j.1572-0241.1999.874_c.x [DOI] [PubMed] [Google Scholar]
- 35.Śliwa E.2010. 2-Oxoglutaric acid administration diminishes fundectomy-induced osteopenia in pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 94: e86–e95. doi: 10.1111/j.1439-0396.2009.00985.x [DOI] [PubMed] [Google Scholar]
- 36.Śliwa E., Adaszek Ł., Tatara M., Dobrowolski P.2010. Short- and long-term consequences on biochemical markers after fundectomy in pigs supplemented with 3-hydroxy-3-methylbutyrate and alpha-ketoglutarate. Berl. Munch. Tierarztl. Wochenschr. 123: 397–405. [PubMed] [Google Scholar]
- 37.Śliwa E., Tatara M. R., Nowakowski H., Pierzynowski S. G., Studziński T.2006. Effect of maternal dexamethasone and alpha-ketoglutarate administration on skeletal development during the last three weeks of prenatal life in pigs. J. Matern. Fetal Neonatal Med. 19: 489–493. doi: 10.1080/14767050600850381 [DOI] [PubMed] [Google Scholar]
- 38.Śliwa E., Dobrowolski P., Tatara M. R., Piersiak T., Siwicki A., Rokita E., Pierzynowski S. G.2009. Alpha-ketoglutarate protects the liver of piglets exposed during prenatal life to chronic excess of dexamethasone from metabolic and structural changes. J. Anim. Physiol. Anim. Nutr. (Berl.) 93: 192–202. doi: 10.1111/j.1439-0396.2007.00805.x [DOI] [PubMed] [Google Scholar]
- 39.Śliwa E., Kowalik S., Tatara M. R., Krupski W., Majcher P., Łuszczewska-Sierakowska I., Pierzynowski S. G., Studziński T.2005. Effect of alpha-ketoglutarate (AKG) given to pregnant sows on development of humerus and femur in newborns. Bull. Vet. Inst. Pulawy 49: 117–120. [Google Scholar]
- 40.Tatara M. R., Brodzki A., Krupski W., Śliwa E., Silmanowicz P., Majcher P., Pierzynowski S. G., Studziński T.2005. Effects of alpha-ketoglutarate on bone homeostasis and plasma amino acids in turkeys. Poult. Sci. 84: 1604–1609. doi: 10.1093/ps/84.10.1604 [DOI] [PubMed] [Google Scholar]
- 41.Tatara M. R., Krupski W., Śliwa E., Maciejewski R., Dąbrowski A.2007. Fundectomy-evoked osteopenia in pigs is mediated by the gastric-hypothalamic-pituitary axis. Exp. Biol. Med. (Maywood) 232: 1449–1457. doi: 10.3181/0608-RM-196 [DOI] [PubMed] [Google Scholar]
- 42.Tomaszewska E., Dobrowolski P., Wydrych J.2012. Postnatal administration of 2-oxoglutaric acid improves articular and growth plate cartilages and bone tissue morphology in pigs prenatally treated with dexamethasone. J. Physiol. Pharmacol. 63: 547–554. [PubMed] [Google Scholar]
- 43.Tomaszewska E., Dobrowolski P., Puzio I.2013. Morphological changes of the cartilage and bone in newborn piglets evoked by experimentally induced glucocorticoid excess during pregnancy. J. Anim. Physiol. Anim. Nutr. (Berl.) 97: 785–796. doi: 10.1111/j.1439-0396.2012.01319.x [DOI] [PubMed] [Google Scholar]
- 44.Tomaszewska E., Dobrowolski P., Puzio I.2012. Postnatal administration of 2-oxoglutaric acid improves the intestinal barrier affected by the prenatal action of dexamethasone in pigs. Nutrition 28: 190–196. doi: 10.1016/j.nut.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 45.Tomaszewska E., Dobrowolski P., Bieńko M., Prost Ł., Szymańczyk S., Zdybel A.2015. Effects of 2-oxoglutaric acid on bone morphometry, densitometry, mechanics, and immunohistochemistry in 9-month-old boars with prenatal dexamethasone-induced osteopenia. Connect. Tissue Res. 56: 483–492. doi: 10.3109/03008207.2015.1069822 [DOI] [PubMed] [Google Scholar]
- 46.Tomaszewska E., Dobrowolski P., Kostro K., Jakubczak A., Taszkun I., Jaworska-Adamu J., Żmuda A., Rycerz K., Muszyński S.2015. The effect of HMB and 2-Ox administered during pregnancy on bone properties in primiparous and multiparous minks (Neivison vison). Bull. Vet. Inst. Pulawy 59: 563–568. doi: 10.1515/bvip-2015-0084 [DOI] [Google Scholar]
- 47.Wernerman J., Hammarqvist F., Vinnars E.1990. Alpha-ketoglutarate and postoperative muscle catabolism. Lancet 335: 701–703. doi: 10.1016/0140-6736(90)90811-I [DOI] [PubMed] [Google Scholar]
- 48.Ushiki T.2002. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 65: 109–126. doi: 10.1679/aohc.65.109 [DOI] [PubMed] [Google Scholar]