Abstract
Costimulatory and inhibitory receptors play a key role in regulating immune responses to infections. Recent translation of knowledge about inhibitory receptors such as CTLA-4 and PD-1 into the cancer clinic highlights the opportunities to manipulate these pathways to treat human disease. Studies in infectious disease have provided key insights into the specific roles of these pathways and the effects of their manipulation. Here, recent studies are discussed that have addressed how major inhibitory and costimulatory pathways play a role in regulating immune responses during acute and chronic infections. Mechanistic insights from studies of infectious disease provide opportunities to further expand our toolkit to treat cancer and chronic infections in the clinic.
Introduction
The original two signal model for lymphocyte activation (Bretscher and Cohn, 1970; Cunningham and Lafferty, 1977; Lafferty and Cunningham, 1975) posited that lymphocytes required not only antigen receptor signaling, but also a second or costimulatory signal to provide contextual information. This second signal ensures that lymphocytes receive proper activation only in the setting of antigen presentation by activated, “professional” antigen presenting cells (APCs). Since this original hypothesis and the early experimental data supporting this concept were published (Greenwald et al., 2005; Lenschow et al., 1996; Linsey and Ledbetter, 1993), several decades of work have revealed a tremendous diversity in not only positive costimulatory pathways that can augment lymphocyte activation, but also negative costimulatory, or inhibitory receptors that counterbalance these activation signals (Odorizzi and Wherry, 2012). Among the most important families of molecules involved in costimulation and co-inhibition of lymphocytes are those in the immunoglobulin (Ig)-superfamily, including molecules structurally related to CD28 and receptors and ligands in the tumor necrosis factor (TNF) receptor superfamily (Greenwald et al., 2005; Watts, 2005) (see reviews in this issue by Bluestone, Sharpe and Ware). Much of the current excitement about co-inhibitory molecules stems from the clinical success of blocking antibodies targeting CTLA-4 and PD-1, two inhibitory receptors expressed by lymphocytes. These blocking antibodies, including Ipilimumab, Pembrolizumab and Nivolumab, disrupt the negative regulatory signals mediated by these two receptors and have led to remarkable clinical regression of melanoma and other cancers (see Wolchok et al., 2016, this issue). In addition to regulating immune responses to tumors, co-inhibitory receptors have a central role in immune responses to infections, especially for pathogens that persist and continue to actively replicate. Indeed, considerable insights have been gained from interrogating costimulatory and inhibitory receptor pathways during infections. This review will focus on these pathways in acute and chronic infection.
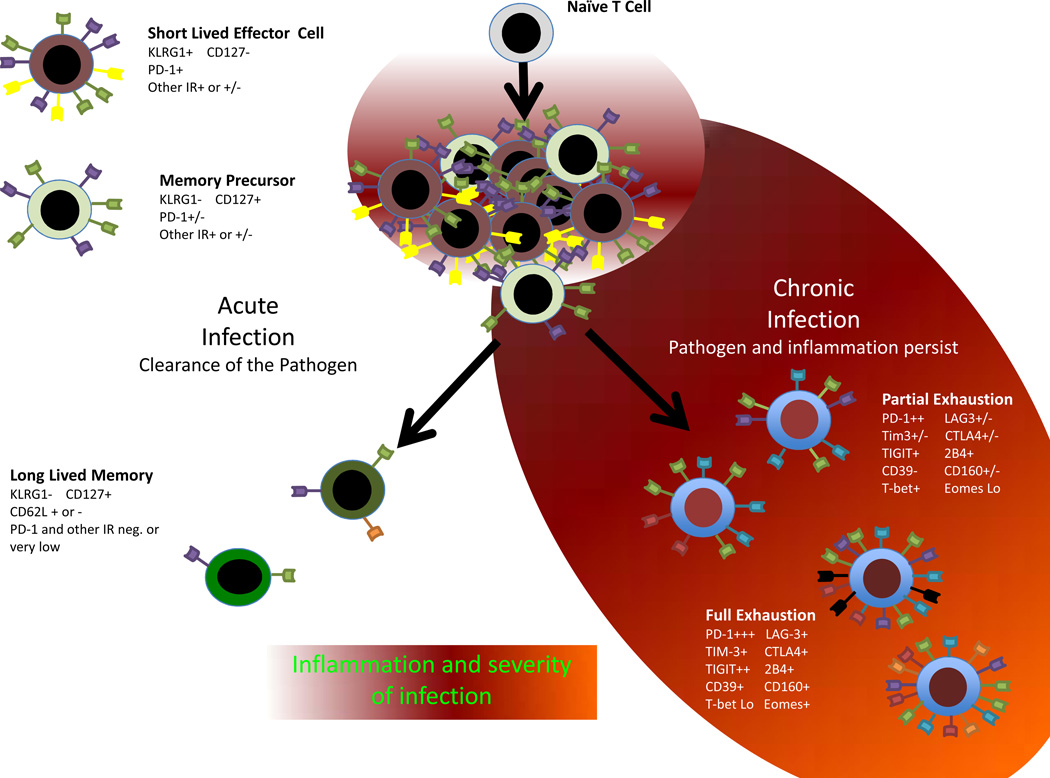
Exhaustion and inhibitory receptor expression
A prominent role for inhibitory receptors during infections has emerged largely from the study of persisting infections where immune function becomes suppressed, facilitating pathogen persistence. Exhaustion of T cells was first described in chronic LCMV infection where T cells are persistently stimulated and develop a series of defects, particularly in the ability to mediate effector functions, proliferate and acquire memory T cell properties (Fuller and Zajac, 2003; Moskophidis et al., 1993; Wherry et al., 2003; Zajac et al., 1998). Transcriptional profiling of exhausted (Tex) versus effector (Teff) or memory T (Tmem) cells revealed upregulation of a large number of cell surface inhibitory receptors (Wherry et al., 2007). Some of these inhibitory receptors expressed by Tex cells had been previously defined to negatively regulate lymphocyte function and/or contained inhibitory signaling motifs such as immunotyrosine inhibitory or switch motifs (ITIMs or ITSMs). These receptors included PD-1, CTLA4, LAG-3, 2B4, GP49B and others. While many inhibitory receptors are upregulated transiently during activation, high and sustained expression of multiple inhibitory receptors is now a canonical feature of Tex cells (Figure 1). These observations suggested a connection between inhibitory receptor expression and T cell exhaustion-- a connection confirmed initially by blocking these pathways in vivo (see below and (Barber et al., 2006)).
Figure 1.
Inhibitory Receptor Pattern of Expression in Infection
Several families of inhibitory receptors have been found to be important in negatively regulating responses of T cells and other leukocytes during persisting infection. These include the Ig superfamily with molecules often related to CD28 (Greenwald et al., 2005) and the C-Type lectin receptor family that includes molecules such as NKG2D and KLRG1 (Lanier, 1998). Molecules that do not fit neatly into either category exist, including TIM-3, which contains both an Ig domain and a mucin domain. Many of these receptors contain intracellular signaling domains capable of transmitting a negative signal, often through phosphatase activity or other adaptor molecules. ITIMs and ITSMs are common, but not essential for inhibition. Indeed, some inhibitory receptors have been suggested to operate by competition for ligand binding (Krummel and Allison, 1995; Yokosuka et al., 2010) or potentially mediate inhibitory function without a cytoplasmic tail (e.g. CD160; (Cai et al., 2008)). One of the first ITIM containing receptors studied was the FcγRIIb (Ravetch and Lanier, 2000), an inhibitory Fc receptor that may limit IFN production during viral infections (Flores et al., 2015) and have other negative regulatory roles. Since the role of ITIMs in this receptor were first studied, a large number of other ITIM containing and ITIM independent inhibitor receptors have been discovered (Odorizzi and Wherry, 2012). Some with prominent roles in chronic infection are discussed here.
PD-1
PD-1, a member of the CD28 subfamily of Ig receptors, is perhaps the most well studied of these inhibitory receptors in infections due to its role in T cell exhaustion (see review by Sharpe and colleagues, this issue). PD-1 binds two ligands. PD-L1 is expressed by many hematopoietic cells including most APC as well as non-hematapoietic cells such as stromal cells, epithelial cells and many tumor cells (Keir et al., 2008; Liang et al., 2003). PD-L1 expression is regulated by IFN-I and IFN-γ (Keir et al., 2008; Liang et al., 2003). PD-L2 expression is more restricted to dendritic cells (DCs) and germinal center B cells (Keir et al., 2008; Liang et al., 2003). Expression of PD-1 is upregulated on CD4+ and CD8+ T cells, as well as B cells and perhaps other leukocytes following acute and chronic infection (See Table 1) and PD-1 may also be expressed by a variety of hematopoietic and possibly non-hematopoietic cells in other settings (Keir et al., 2008; Liang et al., 2003). During acutely resolved infections, PD-1 expression is transient, returning in most cases to low levels after resolution of infection (Barber et al., 2006). In mice, PD-1 expression on memory CD8+ T cells is low, while memory CD4+ T cells and T follicular helper (Tfh) cells may retain higher expression. In humans, higher PD-1 expression may be maintained in memory CD8+ T cells (Duraiswamy et al., 2011). Whether this observation represents technical differences in detection, distinct antigenic history or true species differences in regulation is unclear.
Table 1.
Summary of Inhibitory Receptor Expression in Acute and Chronic Infection
| Infection | Inhibitory Receptors Expressed |
Cell Types Expressing Inhibitory Receptors |
Representative References |
|---|---|---|---|
| Acute LCMV Armstrong |
PD-1, Ly49 | CD4+, CD8+ | (Barber et al., 2006; McMahon et al., 2002; Wherry et al., 2007) |
| Influenza Virus | PD-1, TIM-3, LAG-3 | CD4+, CD8+ | (Erickson et al., 2012; Rutigliano et al., 2014; Strutt et al., 2012; Talay et al., 2009) |
|
Listeria monocytogenes |
PD-1, Ly49 | Dendritic Cells. CD4+ | (McMahon et al., 2002; Xu et al., 2013; Yao et al., 2009) |
| Ebola | PD-1 | CD8+ | (McElroy et al., 2015) |
| Chronic LCMV clone 13 |
PD-1, LAG-3, TIM-3, CD160, 2B4, TIGIT, GP49, |
CD4+, CD8+ | (Barber et al., 2006; Blackburn et al., 2009; Jin et al., 2010; Johnston et al., 2014; Richter et al., 2010; Wherry et al., 2007) |
| HBV | PD-1, CTLA-4, 2B4, LAG-3, TIM-3 |
CD4+, CD8+ | (Boettler et al., 2006; Boni et al., 2007; Kennedy et al., 2012; Raziorrouh et al., 2010; Schurich et al., 2011; Zhang et al., 2009; Zhang et al., 2008) |
| HCV | PD-1, 2B4, CD160, TIM-3, CTLA-4, TIGIT |
CD4+, CD8+ | (Bengsch et al., 2010; Gardiner et al., 2013; Golden-Mason et al., 2007; Golden-Mason et al., 2009; Kroy et al., 2014; McMahan et al., 2010; Nakamoto et al., 2009; Penna et al., 2007; Radziewicz et al., 2007; Urbani et al., 2006; Zhang et al., 2011) |
| HIV | PD-1, 2B4, CD160, TIM-3, CTLA-4,, TIGIT |
CD4+, CD8+ | (Buggert et al., 2014; Chew et al., 2016; Day et al., 2006; Jones et al., 2008; Kaufmann et al., 2007; Petrovas et al., 2006; Pombo et al., 2015; Trautmann et al., 2006) |
| SIV | PD-1 | CD4+, CD8+ | (Finnefrock et al., 2009; Petrovas et al., 2007; Velu et al., 2007; Velu et al., 2009) |
| Friend Virus | PD-1, CTLA-4, TIM-3 | CD8+ | (Takamura et al., 2010) |
| Toxoplasmosis | PD-1 | CD8+ | (Bhadra et al., 2011) |
| Malaria | PD-1, LAG-3, CTLA-4 | CD4+ CD8+ | (Butler et al., 2012; Hafalla et al., 2012) |
| Visceral Leishmania | PD-1 | CD4+CD8+ | (Esch et al., 2013) |
| Tuberculosis | PD-1 | CD4+, CD8+ | (Barber et al., 2011; Lazar-Molnar et al., 2010) |
| Pneumocystis | PD-1 | Macrophages | (Lei et al., 2015) |
| Histoplasma | PD-1 | (Lazar-Molnar et al., 2008) | |
| Sepsis | PD-1 | CD4+, CD8+, Macrophages |
(Brahmamdam et al., 2010; Zhang et al., 2010) |
During chronic infections, in contrast, expression of PD-1 by antigen-specific T cells is sustained and, indeed, the amount of PD-1 expressed in these settings often exceeds what is expressed during acute activation, a distinction that likely has important functional implications (Blackburn et al., 2008; Paley et al., 2012; Wei et al., 2013). Initial studies in chronic LCMV infection demonstrated high and sustained expression of PD-1 on exhausted CD8+ T cells in viremic mice (Barber et al., 2006). In vivo blockade of PD-1:PD-L1 interactions during chronic infection re-invigorated these exhausted CD8+ T cells and improved viral control (Barber et al., 2006). This work was rapidly extended to humans where a role for the PD-1 pathway was also found in chronic HIV (Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006). Considerable work by many groups has now confirmed the importance of PD-1 in settings of persisting viral, bacterial and parasitic infections including prominent roles in HCV, HBV, mycobacterium tuberculosis, malaria and many others (see Table 1).
CTLA-4
Early studies demonstrated an important role for CTLA-4 as a checkpoint in T cell activation (Krummel and Allison, 1995; Walunas et al., 1994). CTLA-4 is a CD28 homologue that binds CD80 and CD86 with a higher affinity than CD28 (see review by Sharpe and colleagues, this issue). CTLA-4 may inhibit T cell functions by directly competing for positive signals via CD28 by sequestering CD80 and/or CD86, but CTLA-4 has also been shown to transmit signals that disrupt proper AKT function (Parry et al., 2005). While some studies found relatively little impact of CTLA-4 deficiency on T cell responses to persisting infections in different chimera settings (Bachmann et al., 1998; Homann et al., 2006) or using CTLA-4 blockade (Barber et al., 2006), in other experimental models infections, such as murine retroviral infections, a role for CTLA-4 was observed (Iwashiro et al., 2001). In humans, a clear role for CTLA-4 during chronic infections has been found in HBV, HCV, HIV and other infections (Kaufmann et al., 2007; Nakamoto et al., 2009; Schurich et al., 2011). Blockade of CTLA-4 in vitro can often augment the function of pathogen-specific T cells from humans (Kaufmann et al., 2007; Nakamoto et al., 2009) and this role for CTLA-4 may involve functions on both Foxp3+ CD4+ regulatory T (Treg) cells and conventional T cells.
LAG-3
LAG-3 is a homologue of CD4 likely arising from a gene duplication and the two molecules share considerable structural similarity (Triebel, 2003). Like CD4, LAG-3 is capable of binding to MHC class II (Huard et al., 1997; Triebel et al., 1990). An inhibitory role for LAG-3 was originally suggested based on in vitro blockade studies using T cell clones (Huard et al., 1994). The role of LAG-3 as an immune negative regulator in vivo was evaluated by Workman, et. al. using a variety of in vivo approaches including acute Sendai virus infection and chronic infection with murine gammaherpesvirus where Lag3−/− T cells had greater expansion and/or IFN-γ production during acute (Sendai) or chronic (gammaherpesvirus) infection respectively, supporting an inhibitory role for this receptor (Workman et al., 2004). LAG-3 expression is also elevated and sustained on Tex cells in chronic LCMV infection (Blackburn et al., 2009; Wherry et al., 2007). While some studies suggest that manipulating LAG-3 alone in chronic LCMV has little impact on Tex cells (Richter et al., 2010), co-blockade of LAG-3 together with PD-1 led to remarkable synergy ((Blackburn et al., 2009) and see below) suggesting perhaps a subordinate role of LAG-3 when other strong inhibitory receptors are present. Based, in part, on these latter results, and other data on the role of LAG-3 in cancer (Grosso et al., 2007) and on Tregs (Huang et al., 2004), clinical trials targeting LAG-3 in cancer are underway (clinicaltrials.gov). Additional studies (see Table 1) have been extended to HBV, HCV, and HIV where LAG-3 expression often is found on TEX, though detection of LAG-3 in peripheral blood, compared to the site of infection, in humans is challenging in some settings (Kennedy et al., 2012; Kroy et al., 2014)
TIGIT
TIGIT is an immunomodulatory receptor expressed on T and NK cells that contains an immunoglobulin variable domain, a transmembrane domain, and an ITIM (Yu et al., 2009) (see review by Kuchroo and colleagues, this issue). TIGIT can bind CD155 or CD112 as ligands and deliver a negative signal (Joller et al., 2011), but competes with CD226, a positive costimulator, for these interactions. CD226-CD155 interactions promoted T cell activation, whereas binding of CD155 by TIGIT can negatively regulate T cell responses during chronic LCMV infection (Johnston et al., 2014). In addition, TIGIT-CD155 interactions induce production of IL-10 and decrease production of IL-12 by mature dendritic cells (Yu et al., 2009). TIGIT also has a role in viral hepatitis via inhibition of NK cells where TIGIT limits NK cell function through the ITIM domain (Stanietsky et al., 2009). In humans, cure of chronic HCV infection results in downregulation of TIGIT expression, suggesting a role for this molecule in T cell dysfunction during untreated chronic infection (Burchill et al., 2015). In HIV and SIV infection, TIGIT is coexpressed with PD-1 on Tex cells and may serve as a marker of the severity of infection (Chew et al., 2016). In this setting, blockade of TIGIT in vitro restored CD8 T cell function (Chew et al., 2016). Thus, TIGIT is an attractive inhibitory receptor to potentially consider targeting in vivo during chronic infections.
TIM-3
TIM-3 is a large transmembrane inhibitory receptor that contains an N-terminal Ig domain as well as a mucin domain that is highly glycosylated (Kuchroo et al., 2008) (see review by Kuchroo and colleagues, this issue). TIM-3 has been reported to bind to Galectin 9, likely through the TIM-3 mucin domain (Cao et al., 2007). TIM-3 also binds in cis to carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1) and this molecule facilitates the suppressive function of TIM-3 (Huang et al., 2015). The TIM-3 intracellular tail has several tyrosine motifs. BAT3 is a signaling adaptor molecule that inhibits signals from TIM-3 and may impair TIM-3 mediated T cell exhaustion (Rangachari et al., 2012). TIM-3 was described as a Th1 specific negative checkpoint regulator found on terminally differentiated cells (Hafler and Kuchroo, 2008). A broader role for TIM-3 in infection, originally described for HIV (Jones et al., 2008), is now appreciated on Tex cells from a variety of chronic infections including chronic LCMV and other infections in mice and HCV, HBV and HIV in humans (see Table 1). In vitro blocking experiments targeting TIM-3 often rescue cytokine production for human T cells and in vivo blockade augments responses in mouse models of chronic infection (Jin et al., 2010). Indeed, TIM-3 has emerged as an attractive target for immunotherapy due to the potency of re-invigoration of function in these settings.
2B4
2B4 (CD244) is a member of the signaling lymphocyte activated molecule (SLAM) subfamily of Ig superfamily (IgSF) receptors and contains 4 cytoplasmic ITSM motifs. 2B4 is expressed by NK cells and activated CD8 T cells, and binds to CD48 on the surface of hematopoietic cells (McNerney et al., 2005). CD48 also interacts with CD2, an interaction that may have a role in lipid raft formation and costimulation (Muhammad et al., 2009). Thus, 2B4-CD48 interactions may deliver negative signals via 2B4 directly, but also by disrupting CD48-CD2 complexes. However, direct signaling by 2B4 can be either costimulatory or inhibitory depending on which ITSM is phosphorylated (Eissmann et al., 2005), as well as the expression level of the receptor itself and the downstream signaling adaptors SAP and EAT-2 (Chlewicki et al., 2008). Indeed, 2B4 expression has been strongly linked to positive regulation of cytotoxic activity of NK and CD8 T cells (Bloch-Queyrat et al., 2005). 2B4 expression is also upregulated on antigen specific CD8 T cells in chronic HBV, EBV, and HIV (Raziorrouh et al., 2010), as well as in mouse models of chronic infection (Lee et al., 2004; Mooney et al., 2004). Interestingly, the adaptor molecule, SAP, is a key adaptor involved in positive signaling by 2B4. SAP is more highly expressed in functional Teff cells compared to Tex cells during chronic LCMV infection, whereas the latter have much higher expression of 2B4 (Wherry et al., 2007). In such a setting, the ratio of the positive adaptor, SAP, to receptor is predicted to decrease leading to inhibitory 2B4 signaling, consistent with in vivo data in the LCMV system (West et al., 2011).
In addition to the receptors discussed above, a large number of other known or potentially inhibitory cell surface receptors can be expressed by lymphocytes and other hematopoietic cells during chronic infections. These other molecules include many other members of the IgSF, and C-type lectin families and are often expressed by T cell, B cells and NK cells. In fact, much of our conceptual understanding of inhibitory receptors on lymphocytes arose from the study of NK inhibitory receptor pathways such as the Ly49, KLR and KIR families (Carrington and Alter, 2012; Orr and Lanier, 2011) where concepts emerged, including the balance of positive and negative receptor signaling and recognition of cellular changes, such as stress or loss of MHC expression, which results in activation.
Co-expression of Inhibitory Receptors
Although lymphocytes are clearly impacted by expression of individual inhibitory receptors, it was rapidly appreciated that exhausted CD8 and CD4 T cells during chronic infections co-expressed multiple inhibitory receptors simultaneously and that the overall number and pattern of receptors co-expressed had important functional implications (Crawford and Wherry, 2009). The concept of inhibitory receptor co-expression has been extended to numerous mouse models of chronic infection and also to humans (Zehn and Wherry, 2015). It is possible that specific combinations of inhibitory receptor co-expression may impart specificity or unique negative regulatory circuits in some settings. Overall, however, the patterns of inhibitory receptor co-expression in different settings remains incompletely defined and how these distinct patterns may reflect the underlying biology are only just beginning to be understood.
The level of expression of individual receptors, and often the number of receptors co-expressed can reflect the severity of chronic infection (Figure 1; (Blackburn et al., 2009)). In many cases this expression pattern likely reflects the strength of antigenic stimulation (Blackburn et al., 2009), although there are examples of inflammatory signals (Gerner et al., 2013; Kinter et al., 2008; Zhu et al., 2015) or epigenetic changes (Youngblood et al., 2011) also playing a role. In the latter case, methylation patterns established at the Pdcd1 locus (encoding PD-1) during chronic infections can persist long-term even after resolution or control of infection and may influence persistence or re-expression of PD-1 (Youngblood et al., 2013; Youngblood et al., 2011). However, in most cases, the precise regulation of inhibitory receptor expression by TCR versus inflammatory signals remains incompletely defined.
Inhibitory Receptor Blockade Synergy in Chronic Viral Infection
A major breakthrough in our understanding of inhibitory receptor function during infections came from initial studies blocking the PD-1 pathway in chronic LCMV infection (Barber et al., 2006). In these studies, treating mice with blocking antibodies against PD-1 or PD-L1 reversed T cell exhaustion and improved viral control, demonstrating a central role for signals from the PD-1 pathway in restraining ongoing responses to chronic infections. These, and other early studies using inhibitory receptor blockade demonstrated several important concepts. First, they demonstrated that TEX, at least as a population, could be rejuvenated by simply blocking signals from a single receptor. Second, they highlighted the potential of therapeutically targeting inhibitory receptor pathways in chronic infections, a concept that was also emerging for cancer. What has become clear is that in settings of chronic infection, co-expression of multiple inhibitory receptors is common and, in addition to potentially reflecting information about the severity and possibly inflammatory milieu of chronic infections, co-expression of multiple inhibitory receptors also (Figure 1) has therapeutic implications. For example, in initial studies, the PD-1 pathway was blocked while simultaneously blocking LAG-3 (Blackburn et al., 2009). Combined blockade of PD-L1 and LAG-3 led to synergy and substantially more robust reversal of T cell exhaustion and control of viral load than blockade of either pathway alone (Blackburn et al., 2009). These findings have been confirmed and extended for other infectious and cancer models using co-targeting of PD-1 and LAG-3 (Butler et al., 2012; Okazaki et al., 2011; Woo et al., 2012). In addition, this concept has been extended to other combinations of inhibitory receptor cotargeting, including PD-1 and Tim-3, PD-1 and TIGIT, PD-1 and CTLA4 + others (see Table 2), demonstrating a broad application of the potential to quantitatively, but also possibly qualitatively tune the efficacy of checkpoint blockade. Recent work on exhausted B cells in HIV and malaria where expression of other inhibitory receptor is prominent (Moir et al., 2008; Weiss et al., 2009) suggests possible opportunities with inhibitory receptor targeting to improve humoral immunity. Moreover, it has been possible to extend this concept to the clinic where combined targeting of PD-1 and CTLA-4 has substantial clinical benefit in advanced melanoma and is now an approved therapeutic approach ((Wolchok et al., 2013) and see (see review by Wolchok and colleagues, this issue)). Numerous trials co-targeting multiple inhibitory receptor pathways or inhibitory receptor and costimulatory pathways are now in progress in cancer (clinicaltrials.gov).
Table 2.
Summary of Multiple Inhibitory Receptor Blockade or Inhibitory Receptor Blockade plus Costimulation agonist in vivo
| Target 1 | Target 2 | Infection | Major Cell Type affected |
Result | Improvement on Single Blockade? |
Reference |
|---|---|---|---|---|---|---|
| PD-L1 | LAG-3 | Chronic LCMV | CD8+ | Improved CTL Function, Lower Viremia |
Yes | (Blackburn et al., 2009) |
| PD-L1 | TIM-3 | Chronic LCMV | CD8+ | Improved CTL Function, Lower Viremia |
Yes | (Jin et al., 2010) |
| PD-L1 | TIGIT | Chronic LCMV | CD4+ CD8+ |
Improved CTL Function, Lower Viremia |
Yes | (Johnston et al., 2014) |
| PD-L1 | LAG3 | Malaria | CD4+,CD 8+, B cells |
Clearance of parasite |
Yes | (Butler et al., 2012) |
| PD-L1 | CTLA-4 | Cerebral Malaria |
CD4+, CD8+ |
Enhanced Disease |
No | (Hafalla et al., 2012) |
| PD-L1 | Ox40 | Malaria | CD4+ CD8+ B Cell |
Increased Parasitemia |
No | (Zander et al., 2015) |
| PD-L1 | 4-1BB | Chronic LCMV | CD8+ | CD8+ | Yes (depending on dose of 4-1BB) |
(Vezys et al., 2011) |
While the precise molecular mechanisms of inhibitory receptor co-blockade remain to be defined, these observations point to an important set of concepts. First, Tex cells are under multiple layers of negative regulation and (at least some) inhibitory receptors are non-redundant in their mechanism of action. Second, differences in cellular expression pattern, distinct structures of inhibitory receptors suggest both non-overlapping intracellular signaling pathways and extracellular ligand interactions that may impart specificity and/or tissue or cell type specific regulatory activity. Third, synergy upon co-blockade of inhibitory receptors also suggests that it may be possible to tailor the type of re-invigorated response desired. For example, reinvigoration of cytokine production versus cytotoxicity versus proliferation or even B cell help may be possible with a more complete understanding of how these pathways and their therapeutic targeting operates. Some evidence exists for selective re-invigoration of different effector functions of Tex cells in vitro (Blackburn et al., 2009), though considerably more work is required to fully interrogate this issue. Nevertheless, the potential to tune re-invigoration of responses during chronic infections by co-targeting multiple negative regulatory pathways has considerable appeal if appropriate targets can be defined.
Costimulatory pathways and combined blockades with inhibitory receptors
In addition to inhibitory receptors, activated T cells and Tex cells also upregulate numerous costimulatory molecules (Crawford et al., 2014). The upregulation of costimulatory molecules by Tex cells is somewhat counterintuitive since these cells are often poorly functional. However, costimulatory molecule expression on Tex cells may reflect an elevated activation state and/or recent TCR signaling. Many costimulatory pathways are important for regulating responses to persisting infections and genetic loss of CD28:B7, ICOS:ICOS-L, CD40:CD40L, OX40:O40L, 4-1BB:4-1BBL, and others result in impaired immune responses and failure to control persisting pathogen replication (Andreasen et al., 2000; Bertram et al., 2002b; Kopf et al., 2000; Kopf et al., 1999; Suresh et al., 2001; Tan et al., 1999; Whitmire et al., 1999). Whether these defects in pathogen control using germline knockout mice reflect defects in initial T cell priming or a role for these pathways in ongoing responses during chronic infection is not always clear. Counterintuitive results, however, have been obtained with CD27 blockade in chronic viral infection where loss of signaling in this pathway augments, rather than inhibits immunity due to decreased IFN-γ production and preservation of germinal centers (Matter et al., 2006). In general, however, costimulatory signals play positive roles in the response to infections. In addition to the synergy achieved by blocking multiple negative regulatory pathways, blocking a cell surface inhibitory receptor while simultaneously agonizing a costimulatory pathway has proven an effective strategy to re-invigorate T cell responses during chronic infections. For example, agonizing several costimulatory pathways including 4-1BB, OX40, and GITR has been demonstrated to synergize with PD-1 pathway blockade (See Table 2). Selected examples are discussed here.
4-1BB
The first studies targeting both costimulatory and inhibitory receptors simultaneously in vivo during chronic infection involved the costimulatory receptor 4-1BB (Vezys et al., 2011). 4-1BB is a TNFR superfamily member expressed by activated T cells including subsets of conventional CD4 T cells, Treg and Tex cells (see review by Ware and colleagues, this issue). The ligand, 4-1BBL is expressed mainly by activated APC (Watts, 2005). CD8 T cells are especially responsive to costimulation via 4-1BB during activation (Shuford et al., 1997) and, indeed, 4-1BB signaling domains have become an integral feature of engineered chimeric antigen receptors used in cellular therapy for cancer (Barrett et al., 2014). Expression of TRAF1, a key signaling adaptor downstream of 4-1BB is downregulated in T cells during chronic infection with LCMV or HIV (Wang et al., 2012) suggesting that high 4-1BB levels on T cells in these settings may be compensating for poor signaling. While mice that received treatment with a 4-1BB agonist antibody during the acute phase of LCMV had little change in the response to virus, combining 4-1BB agonist treatments with PD-1:PD-L1 pathway blockade increased the number, cytolytic activity, and cytokine production of virus specific CD8+ T cells-- changes that were substantially better than blockade of PD-1:PD-L1 alone (Vezys et al., 2011). Co-treatment resulted in a substantial reduction in viral replication (Vezys et al., 2011). Interestingly, during chronic HIV infection, 4-1BB is down-regulated on antigen specific CD4 T cells and reduced expression of 4-1BB correlates with a loss of IL-2 production (Kassu et al., 2009). CD4 T cells upregulate 4-1BB and IL-2 production after anti-retroviral therapy and control of HIV replication. Interestingly, treating chronic LCMV infected mice with an agonist 4-1BB antibody in combination with IL-7, a key cytokine involved in T cell survival and memory, showed beneficial effects on reversing exhaustion (Wang et al., 2012). Thus, 4-1BB:4-1BBL appears to be a critical costimulatory pathway for T cells during chronic viral infections, providing a rationale for targeting this pathway in combination with inhibitory receptor blockade or other signals such as cytokines.
OX40
OX40, another TNFR superfamily member expressed on Th1, Th2, Tfh, Treg and CD8 T cells has also been explored in co-targeting approaches during chronic infections. The OX40:OX40L axis is responsible for regulating several anti-apoptotic pathways, including BCL-2/BCL-xL through NF-kB and AKT signaling (Song et al., 2005; Song et al., 2008). OX40 signals also play a key role in T cell differentiation (Walker et al., 1999). Interestingly, OX40 expression strongly correlates with PD-1 expression in both acute and chronic LCMV infection, where the kinetics of surface expression suggest regulation driven by the presence of antigen (Boettler et al., 2012b). During chronic LCMV, mice lacking OX40 experience severe immunopathology, significantly lower numbers of anti-viral CD8 T cells, and a higher viral burden (Boettler et al., 2012b; Kopf et al., 1999). This outcome suggests a partially impaired T cell response that is unable to contain virus, yet still able to cause tissue damage due to greater viral dissemination and immunopathology. Further, these mice are unable to form germinal centers and thus have lower plasma cell numbers likely contributing to higher viral load (Boettler et al., 2012b; Kopf et al., 1999). The fact that OX40 influences multiple arms of adaptive immunity makes it an attractive immunotherapeutic target. OX40:OX40L interactions in vivo impact different populations of CD4 T cells. Treatment of mice with an agonistic OX40 antibody in the chronic stage of malaria infection results in expansion of Th1, Th2, and Treg populations. Further, this treatment augments germinal center formation, and subsequently the number of IgM+ plasmablasts (Zander et al., 2015). In experimental malaria infection, dual treatment with PD-1 pathway blockade and an OX40 agonist reverses the benefits of OX40 treatment alone (Zander et al., 2015). This effect was due to increased IFNγ production from CD8 T cells following PD-1 blockade that inhibited Tfh differentiation and germinal center formation (Zander et al., 2015). In contrast, in the setting of HIV infection, OX40 ligation on CD4 T cells enhances “help” to antigen specific CD8 T cells resulting in better proliferation and cytolytic activity (Yu et al., 2006). Together, these data suggest a role for OX40 during chronic infections, however the precise consequences of targeting and co-targeting this pathway with PD-1 therapeutically, may be context dependent. Nevertheless, promising results in these preclinical models suggest a deeper understanding of the OX40 pathway during chronic infections could lead to clinical therapeutic opportunities.
GITR
The TNFR family member GITR, has a similar transient expression pattern to OX40. GITR interacts with GITRL on APC. During chronic LCMV infection, mice genetically deficient in GITR experience more severe T cell exhaustion, fewer antigen specific CD8 T cells and higher viral loads (Clouthier et al., 2015). During chronic LCMV infection, the ligand for GITR is highly upregulated early in infection, but expression rapidly returns to low levels within a week, suggesting a potential role for this pathway normally early in infection rather than during the chronic phase (Clouthier et al., 2014). Mice constitutively expressing the ligand for GITR on APCs have significantly higher numbers of antigen specific CD8 T cells with better cytokine production by CD4 and CD8 T cells and lower viral load (Clouthier et al., 2014; Pascutti et al., 2015). Treatment with a single dose of a GITR agonist antibody during chronic infection produced a similar virological outcome without increased immunopathology (Clouthier et al., 2014). Relatively little information exists on the potential synergy between GITR and inhibitory receptor pathways in infectious settings. Given the high expression of GITR on Treg, it will be interesting to further interrogate the importance of GITR costimulatory signals on conventional T cells versus Treg in chronic infections.
It is interesting and potentially therapeutically important that co-stimulatory and inhibitory molecules can often compete for binding to the same ligands. CTLA4 and CD28 competition for CD80/CD86 binding is one example, already being targeted clinically. In an interesting example of cross-family interactions, the TNFR family ligand HVEM interacts not only with the TNFR family receptor LIGHT, but also with the IgSF members BTLA and CD160 (Cai et al., 2008). LIGHT, BTLA and CD160 can all be expressed by activated lymphocytes, but while LIGHT provides costimulatory signals, ligation of HVEM with either BTLA or CD160 inhibits cellular function (Cai et al., 2008). The herpesvirus glycoprotein gD, can bind to the BTLA/CD160 binding sight on HVEM blocking interactions of these inhibitory receptors, but not interactions with the costimulatory molecule LIGHT (Stiles et al., 2010). Lasaro et al were able exploit this property of gD to block the inhibitory effects of BTLA and/or CD160 interaction with HVEM and provide better antigen-specific anti-viral responses (Lasaro et al., 2008). It remains unclear whether gD binding to HVEM simply blocks negative BTLA or CD160 interactions or enhances LIGHT/HVEM signaling, however these results point to a potential therapeutic opportunity with this multi-interaction pathway. While this example provides proof of principle for therapeutic effects of interfering with these complex interactions, manipulating such pathways may be complex and dependent on the expression patterns of multiple ligands and receptors in addition to the specific molecule targeted. Indeed, such complexity might explain the different outcomes observed for targeting the same molecule in different infections. Such complexity may exist in multiple inhibitory receptor pathways. For example PD-L1 interacts with B7-1 and PD-L2 with RMGb, adding other receptors besides PD-1 to this pathway and interactions between CD2-CD48-2B4 discussed above can influence the functional consequences in interactions in that pathway.
It may also be important to note that while pathway complexity is one potential reason for distinct results of blockade in different settings, the antibodies used in these studies could also influence outcomes. Affinity/avidity of antibodies used may differ in different studies and the actual sites targeted on the costimulatory or inhibitory molecules may have different effects. One must also consider the other side of the antibody molecule and recent data has demonstrated important differences in targeting PD-1 versus PD-L1 in vivo that depend on Fc receptor binding of the latter antibody in a tumor model (Dahan et al., 2015). Moreover, Fc receptor mediated myeloid cell function can change dramatically during chronic infections, substantially affecting antibody-mediated depletion (Wieland et al., 2015; Yamada et al., 2015). Whether this latter effect influences the response to blocking or agonizing antibodies during chronic infections remains unclear.
Thus, existing data support an important role targeting costimulatory pathways in chronic infections. During established chronic infection when CD4 and CD8 T cells are exhausted, providing additional stimulation through these receptors appears to provide substantial benefit. The initial studies mentioned above that combined co-stimulatory agonists with checkpoint blockade suggest potentially attractive approaches that should be further explored experimentally and potentially clinically in infectious disease.
Extrinsic pathways and combined inhibitory receptor blockade
Blockade of inhibitory receptors during chronic infections can also complement other therapeutic approaches including targeting cytokines, Treg and use of vaccines. For example, reinvigoration of Tex cells during chronic viral infection is enhanced by co-blocking the IL-10 pathway (Brooks et al., 2008; Porichis et al., 2014), providing exogenous IL-2 (West et al., 2013), augmenting CD4 T cell help (Aubert et al., 2011), depletion of Tregs (Penaloza-MacMaster et al., 2014) or therapeutic vaccination (Ha et al., 2008). Each approach has attractive aspects that may be of interest in different contexts of infection. This latter effect of therapeutic vaccination is somewhat surprising given the notion that chronic antigen stimulation is thought to be a factor driving T cell exhaustion. Typically, therapeutic vaccines have been poorly effective (Autran et al., 2004), an outcome thought to be at least partially connected to the problem of simply providing more antigen to T cells that are overstimulated, though there is some reason for optimism at least in HCV (Barnes et al., 2012). However, the addition PD-1 pathway blockade appears to be able to recalibrate this antigen stimulation threshold allowing robust expansion and re-invigoration of exhausted T cells (Ha et al., 2008). Several other T cell extrinsic pathways may synergize with inhibitory receptor blockades such IL-27 (Hirahara et al., 2012), IL-35 (Collison et al., 2007), IFN-I (Teijaro et al., 2013; Wilson et al., 2013), Tgf-β (Garba et al., 2002; Tinoco et al., 2009) and others, though the role of this latter pathway in chronic viral infection remains controversial (Boettler et al., 2012a; Garidou et al., 2012), .
Transcriptional regulation of inhibitory receptor pathways during chronic infection
A recent area of investigation has been the transcriptional control of inhibitory receptor expression during chronic infections. While there has been in depth analysis of only a handful of inhibitory receptor genes, these data have been informative. For example, NFAT has been directly implicated in transcriptional regulation of Pdcd1 and PD-1. NFATc1 (NFAT2) binds to two sites within ∼1500bp of the transcriptional start site and induces transcriptional expression of Pdcd1 (Oestreich et al., 2008). These observations are interesting in light of studies that demonstrate that NFAT acts together with AP-1 family transcription factors to promote development of a functional Teff cell effector gene expression program whereas, NFAT binding without canonical AP-1 promotes expression of a gene expression program of exhaustion including PD-1 (Martinez et al., 2015). Not only do these studies highlight a key role of NFAT activity in PD-1 expression tying PD-1 to TCR and Ca++ signaling, but they also point to the importance of context specific transcription factor activity in T cells undergoing different programs of differentiation.
The NFAT connection may have additional relevance since PD-1 signaling can promote expression of BATF (Quigley et al., 2010), an AP-1 family transcription factor that positively regulates Th17 differentiation (Murphy et al., 2013) and early CD8 T cell activation (Kurachi et al., 2014). BATF is capable of binding Fos and displacing Jun from canonical AP-1 dimers. BATF lacks the transactivation domain found in Jun proteins and can act as a transcriptional repressor. BATF expression can repress effector genes such as Ifng and Il2 and knockdown of BATF in exhausted T cells from chronic HIV infection can restore function (Quigley et al., 2010). PD-1 signaling can induce BATF and may foster a feed-forward loop of additional “AP-1-less” NFAT signaling due to disruption of canonical fos/Jun AP-1 dimers. Further, the NFAT axis may be a central pivot integrating other signals to foster productive T cell activation and effector activity or elevated PD-1 expression and exhaustion, tolerance or other forms of T cell dysfunction. Partnerless NFAT activity has also been implicated in the regulation of other inhibitory receptors including LAG-3 and TIM-3 (Martinez et al., 2015).
The transcriptional repressor Blimp-1 also has a role in inhibitory receptor expression. Initial studies found a strong positive correlation between high Blimp-1 expression in Tex cells during chronic infection and high expression of PD-1, LAG-3, CD160 and 2B4 (Shin et al., 2009). Expression of these molecules was substantially reduced in the absence of Blimp-1 during chronic infection (Shin et al., 2009). During acute infection Blimp-1 fosters the development of terminally differentiated Teff cells that express high KLRG1 (Kallies et al., 2009; Rutishauser et al., 2009). In most settings, KLRG1 and PD-1 only partially overlap in expression; PD-1Hi CD8 T cells have low KLRG1, while KLRG1Hi terminally differentiated effector CD8 T cells are typically PD-1Int (Angelosanto et al., 2012; Blackburn et al., 2009). These observations suggest a complex role for Blimp-1 in PD-1 expression. In contrast to the role during chronic infection and T cell exhaustion, during acute T cell activation, Blimp-1 can repress transcription of Pdcd1 directly and indirectly (Lu et al., 2014). Blimp-1 also has a role in epigenetic remodeling of the Pdcd1 locus (Lu et al., 2014) and DNA methylation of this locus can have a dramatic influence on PD-1 expression and re-induction upon restimulation (Youngblood et al., 2011).
In contrast to the positive regulation by NFAT and Blimp-1 during chronic infection, the T-box transcription factor T-bet represses high expression of PD-1 by binding near NFAT sites just upstream of the transcriptional start site (Kao et al., 2011). This repression by T-bet does not fully ablate PD-1 expression, but acts to negatively regulate a distinct PD-1Hi expression state (Kao et al., 2011; Paley et al., 2012). High expression of T-bet represses from PD-1Hi to PD-1Int but does not eliminate PD-1 expression. Thus, while there is a moderate effect of T-bet on the level of PD-1 expression in acutely activated TEFF, the role of T-bet as a repressor of Pdcd1 is predominantly found in Tex cells during chronic infections (Kao et al., 2011; Paley et al., 2012). T-bet also regulates the expression of a suite of other inhibitory receptors where it represses expression of LAG-3, CD160, BTLA and CD244, but promotes expression of KLRG1 (Joshi et al., 2007; Kao et al., 2011). T-bet can also positively regulate TIM-3 during initial T cell activation (Anderson et al., 2010), but negatively regulate TIM-3 in Tex cells (Kao et al., 2011).
There appears to be a reciprocal relationship between T-bet and the related T-box transcription factor Eomesodermin (Eomes) in regulating inhibitory receptor expression. While T-bet represses expression, Eomes expression is associated with high expression of PD-1 and other inhibitory molecules during chronic, but not acutely resolved infections (Paley et al., 2012). Whether Eomes is directly involved in transcriptional regulation of inhibitory receptors in these settings is unclear. However, the potential for these transcription factors to bind similar DNA sequences, cooperate, operate redundantly or antagonize each other (Intlekofer et al., 2008; Pearce et al., 2003) suggests a key role for Eomes in inhibitory receptor expression. One surprising feature of T-box transcription factor control of inhibitory receptors (and other genes) is that the same transcription factor can have distinct, and often non-overlaping functions in Tex cells from chronic infection compared to functional Teff or Tmem cells from acute infections (Doering et al., 2012). Indeed, transcriptional network analysis has demonstrated that T-bet and Eomes have highly context specific activities in functional T cells versus Tex cells (Doering et al., 2012). For Eomes, this is interesting considering a functional role in quiescent and self-renewing central Tmem cells following acutely resolved infections (Banerjee et al., 2010; Paley et al., 2013; Zhou et al., 2010), but also in the terminally differentiated subset of Tex cells during chronic infection (Buggert et al., 2014; Paley et al., 2012). These examples of context specific control of inhibitory receptor expression by transcription factors may be due to cofactors such as the NFAT-AP-1 setting, expression level of the transcription factor or the epigenetic landscape in which these transcription factors function.
The transcription factor FoxO1 suppresses differentiation and proliferation of Teff, and survival of long lived Tmem cells (Hess Michelini et al., 2013; Kim et al., 2013). In the setting of chronic TCR stimulation FoxO1 accumulates in the nucleus where it controls expression of several genes important for CD8 T cell biology. In addition to containing binding sites for T-Bet/Eomes, Blimp-1, NFAT, and, Pdcd1 also contains a binding region for FoxO1. Overexpression of constitutively active FoxO1 leads to increased Pdcd1 promoter activity, suggesting a positive transcriptional regulation of Pdcd1 by FoxO1 (Staron et al., 2014). Indeed, genetic deficiency in FoxO1 causes a loss of PD-1 high exhausted CD8 T cells in chronic infection (Staron et al., 2014).
The transcription factor Nfil3 has been shown to regulate TIM-3 expression in a circuit including T-bet and STAT3 (Zhu et al., 2015). Using in vitro activated T cells, Zhu, et. al. found that IL-27 and IL-10 can cooperate to induce an Nfil3 and T-bet dependent set of epigenetic changes in the Havcr2 locus (encoding TIM-3) that control TIM-3 expression. Whether these epigenetic changes result in a change in requirement of Nfil3 and/or T-bet for control of TIM-3 expression in chronically activated T cells is unclear. However, these studies connect the immunoregulatory cytokines to inhibitory receptor expression by the T cells through Nfil3 and T-bet suggesting that targeting these cytokines could alter TIM-3 expression.
Given both direct physical, transcriptional and potentially metabolic interactions between NFAT, AP-1, Tbet, Eomes, Blimp-1, Nfil3 and FoxO1 the context specific transcriptional events regulating expression of PD-1 and other inhibitory receptors is only just beginning to emerge. Developing an integrated understanding of how these transcription factor pathways collaborate will be key to effectively manipulating immune regulators.
Inhibitory receptors during acute viral infection and vaccination
PD-1 and other inhibitory receptors are rapidly induced upon activation and are likely involved in attenuating primary T cell responses. Indeed, in acutely activated T cells CTLA-4, PD-1 and other inhibitory receptors inhibit proximal TCR signaling and downstream events (Chemnitz et al., 2004; Parry et al., 2005; Patsoukis et al., 2012; Riley, 2009; Yokosuka et al., 2012). Multiple mechanisms have been implicated based on in vitro activation studies including inhibiting proximal TCR signaling, antagonizing CD28 mediated costimulation, inhibition of AKT and PI3K and regulation of cell cycle (Chemnitz et al., 2004; Parry et al., 2005; Yokosuka et al., 2012). In general, these effects may have been co-opted from evolutionarily selected mechanisms to regulate self-tolerance. During many infections, these pathways are likely to curb the later phase of T cell expansion and/or activation. Knocking out or blocking the PD-1 pathway in mice can augment responses and/or survival following infection with Histoplasma capsulatum or rabies virus (Lafon et al., 2008; Lazar-Molnar et al., 2008). In settings of virulent infections negative regulation of T cell responses by inhibitory receptors such as PD-1 can be critical to avoid lethal immunopathology. For example, in the absence of PD-1 signals mice infected with rapidly disseminating or “chronic” strains of LCMV die from lethal immunopathology within 1 week (Barber et al., 2006; Frebel et al., 2012). This pathology is associated with high levels of systemic cytokines and death resulting from pulmonary pathology due to endothelial cell killing (Frebel et al., 2012). In acute HBV infection in humans, severe liver pathology and liver failure resulting in death may be associated with failure to properly induce PD-1 expression on HBV-specific CD8 T cells (Zhang et al., 2008). Similarly, PD-1–deficient mice have an advantage in clearance of adenovirus from the liver, but also develop more severe hepatocellular injury, likely due to an overaggressive adaptive immune response (Isogawa et al., 2005). During respiratory viral infections with influenza virus and metapneumovirus, PD-1 signals may also attenuate T cell effector functions limiting damage to sensitive respiratory tissues (Erickson et al., 2012). Blockade of PD-1 signals can increase pathology, but also may augment viral control (Erickson et al., 2012; McNally et al., 2013). While examples of extreme pathology are uncommon during acute infection, a potential role of inhibitor receptors in helping to establish the balance between pathogen clearance and limited tissue damage is emerging and may be an important consideration for PD-1 maintenance therapy in cancer patients. Notably, while rare, pneumonitis has been reported during PD-1 blockade in humans (Topalian et al., 2012), though whether this is related to an infectious event or other immunological interactions is unclear.
There are also some reports of a positive role for signals from inhibitory receptors such as PD-1 during acute infections. For example, the absence or blockade of PD-L1 can decrease the CD8+ T cell response to acute Listeria monocytogenes infection (Rowe et al., 2008; Xu et al., 2013). Whether this effect is due to a true positive costimulatory signal generated via PD-1 or a result of protection from overstimulation and antigen-induced cell death is unclear. In vitro studies provide some evidence for the latter. Using cultured peritoneal macrophages and Th1 cells, Yamazaki, et. al. found that blockade of PD-1 signals resulted in better IFN-γ production that, in turn, provoked higher nitric oxide (NO) from the infected macrophages (Yamazaki et al., 2002). This NO suppressed the responses of activated Th1 cells (Yamazaki et al., 2002). Thus, in vivo, PD-1 pathway blockade during acute infection could conceivably enhance activation induced cell death, perhaps especially for pathogens that reside in myeloid cells capable of robust oxidative burst. A role for inhibitory receptors on DCs and/or macrophages may also influence the precise balance of positive versus negative signals from these pathways in vivo (Seo et al., 2008; Talay et al., 2009; Yamazaki et al., 2002). Nevertheless, consistent with the original concept of tempering acute T cell activation, a beneficial effect of inhibitory receptor pathways may exist in vivo during acute infections to prevent overstimulation of T cells and/or immunopathology.
Many other inhibitory receptors including CTLA-4 (Raue and Slifka, 2007), Lag-3 (Workman et al., 2004), CD200:CD200R (Snelgrove et al., 2008), and some Ly49 family members (McMahon et al., 2002) have been examined on T cells during acute infection, while the roles of many others remain to be explored. There has also been some progress in understanding how inhibitory receptor signals may influence the generation and function of Tmem cells. Genetic deletion of PD-1 skews the CD8 Tmem cell pool towards central memory and may also influence secondary CD8 T cell responses upon re-infection especially for CD8 T cells primed without CD4 T cell help (Allie et al., 2011). In addition, LAG-3 can negatively regulate the development of CD8 Tmem cells following Sendai virus infection (Workman et al., 2004) and may affect homeostatic proliferation, a key function of Tmem cells (Workman and Vignali, 2005). Recent work on the methylation of the Pdcd1 locus also found permanent demethylation following chronic infections such as LCMV or HIV, even if these infections are resolved (Youngblood et al., 2013; Youngblood et al., 2011). Thus, memory-like CD8 T cells that develop following antiretroviral treatment of HIV infection may harbor permanent epigenetic changes that, in the case of Pdcd1 result in more rapid re-expression of PD-1 upon reactivation (Youngblood et al., 2011) . Thus, the nature of primary acute stimulus may have a direct influence on the role of inhibitory receptors in Tmem cells. Indeed, stimulation with IL-12 versus IFN-I as a signal 3 during priming can also have an impact on PD-1 re-expression (Gerner et al., 2013). In a tumor model, IFN-α costimulated CD8 T cells upregulated PD-1 more easily and became exhausted, whereas IL-12 generated cells more effectively controlled the tumor (Gerner et al., 2013). Whether there is an epigenetic impact on the Pdcd1 locus in this setting is unknown, but these observations may have direct relevance to inhibitory receptors during infection given the bias of many infections towards IL-12 or IFN-I inflammation. Despite this work, our understanding of how these receptor pathways function during acute infections remains limited. Opportunities should arise due to cancer immunotherapy to ask many of these questions not only in animal models but also directly in humans.
Inhibitory receptor pathways and blockade during bacterial and parasitic infections
Much of the discussion above has focused on viral infections. Inhibitory receptors also play central roles in regulating immune responses to non-viral pathogens. For example, a role for PD-1 and/or other inhibitory receptors has been reported for persisting infection with Toxoplasma gondii, Mycobacterium tuberculosis (mTb), Leishmania major, malaria, trypanasoma cruzi and even during bacterial sepsis (See Table 1) and in a number of these settings elevated inhibitory receptor expression is associated with T cell dysfunction or exhaustion.
PD-1:PD-L interactions are important in the pathogenesis of mTb infection. PD-1 expression is increased and is sustained on CD4+ T cells during mTb infection in mice and in humans suggesting that PD-1 signals may suppress immunity to this persisting bacterial infection. Interestingly, however, mice deficient in PD-1 are highly susceptible, rather than resistant to mTb (Barber et al., 2011; Lazar-Molnar et al., 2010). Blocking PD-1 in vivo also drastically reduces survival (Lazar-Molnar et al., 2010). One interpretation of these observations is that control of mTb infection requires a carefully balanced immune response that contains the infection without causing pathology. In the absence of PD-1 signals, immune driven pathology allows greater dissemination leading to a cycle of bacterial spread and tissue damage. Additional studies are needed to further investigate these issues and perhaps identify other inhibitory receptors pathways that may allow tuning of responses to be more effective at control of mTb.
T-cell dysfunction or exhaustion has been described during infection with t. gondii, L. major and malaria; persistent infections caused by parasitic protozoans. In each of these settings there is some evidence for a role for PD-1 or other inhibitory pathways limiting the effectiveness of the immune response. During chronic t gondii infection, CD8 T cells have high expression of PD-1(Wilson et al., 2009). Blocking the PD-1:PDL-1 interactions in vivo improves T cell function and survival and this effect is at least partially CD8 T cell dependent (Bhadra et al., 2011). IL-27 has been implicated in driving PD-L1 expression in this setting (Hirahara et al., 2012). Additional studies suggest possible roles for other inhibitory receptors during t. gondii infection (Wu et al., 2013), though the precise role of these pathways remains poorly understood.
Leishmaniasis is a chronic, parasitic infection that can present with several different clinical manifestations. Typically, cutaneous leishmaniasis causes a local lesion where parasite replication is contained, but not eliminated (Scott and Hunter, 2002). In visceral leismaniasis, the most aggressive form of the disease, a highly immuno-suppressive environment is created. CD4 and CD8 T cells highly express PD-1 and produce profinflammatory cytokines poorly (Esch et al., 2013). Blocking PDL-1 in vivo has been shown to enhance the number and function of antigen specific T cell in visceral leishmaniasis suggesting T cell exhaustion/dysfunction (Esch et al., 2013). Increased IFNγ production by CD8 T cells following PD-L1 blockade leads to an increase in superoxide production by monocytes and a lower parasite burden. Thus, at least in this most severe form of this parasitic infection, evidence suggests a prominent role for the PD-1 pathway.
Generating an effective vaccine against malaria has proven to be challenging (Langhorne et al., 2008), though recent results with the RTS’S malaria vaccine show progress in this area (Miura, 2016). Nevertheless, better treatment options are needed for individuals already infected. Previous studies have shown that CD4 T cells are critical for immunity to plasmodium infection (Vinetz et al., 1990). During chronic malaria infection, however, these cells have increased expression of PD-1, (LAG-3 and other inhibitory receptors and produce less cytokines consistent with T cell exhaustion (Butler et al., 2012). Simultaneous blockade of PD-1 and LAG-3 in vivo significantly improved CD4 T cell function, parasite clearance and memory formation (Butler et al., 2012). In addition, this treatment improved the numbers of antibody secreting, long-lived plasmablasts independent of plasmodium strain, suggesting potential impact of such therapeutic interventions. Given the challenges with malaria vaccination and challenges for patients chronically infected, exploration of inhibitory receptor pathways as targets in malaria may warrant further attention. However, PD-1 and CTLA-4 signals have also been reported to also have independent and important roles negatively regulating T cell responses in a model of acute cerebral malaria. In this setting, blockade of either pathway induces immunopathology (Hafalla et al., 2012), suggesting caution should be exercised in the situations where immunity to malaria is augmented by inhibitory receptor blockade
The rapid course of disease in sepsis makes this an unlikely place to observe a key role for inhibitory receptors. Interestingly, however, PD-1 is upregulated by CD4 and CD8 T cells as early as 48h after the onset of sepsis (Brahmamdam et al., 2010; Guignant et al., 2011), an expression pattern that is associated with profound T cell dysfunction. Indeed mice deficient in PD-1 have improved survival during sepsis compared to their wild type counterparts suggesting a potential causal link between PD-1, immune dysfunction and pathological consequences during this highly acute inflammatory response (Huang et al., 2009). Administration of anti-PD-1:PD-L blocking antibody to septic mice dramatically increases long-term survival (Brahmamdam et al., 2010; Zhang et al., 2010). Indeed, several clinical trials are now addressing the potential role of the PD-1 pathway as a therapeutic target during human sepsis. It remains unclear, however, which cell types are the major targets of PD-1:PD-L regulation during sepsis and which immune pathways are critical for the beneficial effects of blocking PD-1 interactions during this acute hyperinflammatory, yet immunosuppressive disease.
Clinical success and Ongoing Trials
The overwhelming majority of the clinical trials involving immune checkpoint blockade in chronic disease are in cancer. In these settings, while adverse events have been observed, PD-1 blockade with Nivolumab or Pembrolizumab is generally well tolerated. Importantly, while nearly all humans likely harbor multiple persisting viruses (Virgin et al., 2009), no pathogen associated pathologies have been reported with PD-1 blockade. Despite this safety profile and thousands of patients treated with blockade of at least one inhibitory receptor in various cancers, some of whom have had unprecedented clinical results, information from human trials of inhibitory receptor blockade during infection is limited. BMS-936558, a monoclonal IgG4 anti-PD-1 antibody, was studied in patients with chronic HCV infection (Gardiner et al., 2013). Anti-PD-1 treatment was well tolerated in this setting. In this study, 5 patients in the anti-PD-1 treated group achieved a reduction in viral load versus only 1 in the control arm, though in only 1 subject was this a durable clearance of virus. These observations are perhaps more remarkable, however, when one considers that this was a dose escalation study with 15/35 treated patients receiving 0.3 mg/kg or less of the antibody far below the 3 or 10 mg/kg given to cancer patients. Moreover, immune dysfunction in HCV can be quite severe and patients infected for long periods of time, such as those on this trial, often have severe dysfunction and numerical reductions in the HCV-specific T cell responses making this a challenging patient population for reversal of immune exhaustion.
Limited immunology was performed in the human anti-PD-1 trial above. Three chimpanzees chronically infected with HCV have also been treated with blocking anti-PD-1 antibody (Fuller et al., 2013). One of the three animals had a significant virological response and controlled HCV replication until treatment was discontinued. This temporary viral control occurred without liver injury but was associated with restoration of intrahepatic CD4 and CD8 T cell responses to the virus. The animal that responded had a more vigorous pre-existing T cell response to HCV compared to the two non-responder animals suggesting that the level of pre-existing T cells available for re-invigoration may be an important variable in therapeutic efficacy of PD-1 pathway blockade in chronic infections and cancer.
While other trials in infectious disease are not yet available, data in SIV infection also suggests promise for blocking the PD-1 pathway in HIV. Blocking PD-1 in SIV infected rhesus macaques enhanced not only T cell responses but also B cells and antibody (Velu et al., 2009). Moreover, this treatment extended the lifespan of the treated animals (Velu et al., 2009) and may even reduce chronic inflammation (Dyavar Shetty et al., 2012). These observations have been extended to a humanized mouse model of HIV infection where blocking the PD-1 pathway led to significant reduction in viral load and augmented CD8 T cells (Seung et al., 2013). CTLA-4 blockade has also been tested in SIV infection. In one case some benefit was observed on T cell responses and viral load (Hryniewicz et al., 2006), in other studies CTLA-4 blockade drove immune activation, CD4 proliferation and higher viral load (Cecchinato et al., 2008). Safety is a paramount concern when considering inhibitory receptor blockade during infectious disease given previous experiences with antibodies targeting CD28 (Sharpe and Abbas, 2006). Nevertheless, with increasing experience in cancer, the risk benefit balance may become clearer for antibodies against PD-1 to be used in infectious disease. Indeed, there may be increasing opportunities to consider PD-1 pathway targeting in HIV cure approaches or as complementary strategies to antivirals in HBV infection and beyond.
Acknowledgments
We thank the members of Wherry lab for helpful discussions. EJW is supported by the National Institutes of Health (grants AI105343, AI112521, AI082630, AI115712, AI117950, AI108545). EJW has a patent licensing agreement on the PD-1 pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no additional conflicts of interest.
References
- Allie SR, Zhang W, Fuse S, Usherwood EJ. Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J Immunol. 2011;186:6280–6286. doi: 10.4049/jimmunol.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AC, Lord GM, Dardalhon V, Lee DH, Sabatos-Peyton CA, Glimcher LH, Kuchroo VK. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur J Immunol. 2010;40:859–866. doi: 10.1002/eji.200939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen SO, Christensen JE, Marker O, Thomsen AR. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J Immunol. 2000;164:3689–3697. doi: 10.4049/jimmunol.164.7.3689. [DOI] [PubMed] [Google Scholar]
- Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305:205–208. doi: 10.1126/science.1100600. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Waterhouse P, Speiser DE, McKall-Faienza K, Mak TW, Ohashi PS. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J Immunol. 1998;160:95–100. [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Science translational medicine. 2012;4:115ra111. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annual review of medicine. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram EM, Lau P, Watts TH, Guinn BA. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection 4-1BBL enhances anti-tumor responses in the presence or absence of CD28 but CD28 is required for protective immunity against parental tumors. Journal of Immunology. 2002a;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- Bertram EM, Tafuri A, Shahinian A, Chan VS, Hunziker L, Recher M, Ohashi PS, Mak TW, Watts TH. Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol. 2002b;32:3376–3385. doi: 10.1002/1521-4141(200212)32:12<3376::AID-IMMU3376>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch-Queyrat C, Fondaneche MC, Chen R, Yin L, Relouzat F, Veillette A, Fischer A, Latour S. Regulation of natural cytotoxicity by the adaptor SAP and the Src-related kinase Fyn. J Exp Med. 2005;202:181–192. doi: 10.1084/jem.20050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler T, Cheng Y, Ehrhardt K, von Herrath M. TGF-beta blockade does not improve control of an established persistent viral infection. Viral Immunol. 2012a;25:232–238. doi: 10.1089/vim.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, Croft M, von Herrath MG. OX40 facilitates control of a persistent virus infection. PLoS Pathog. 2012b;8:e1002913. doi: 10.1371/journal.ppat.1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggert M, Tauriainen J, Yamamoto T, Frederiksen J, Ivarsson MA, Michaelsson J, Lund O, Hejdeman B, Jansson M, Sonnerborg A, et al. T-bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection. PLoS Pathog. 2014;10:e1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat. 2015;22:983–991. doi: 10.1111/jvh.12465. [DOI] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4(+) T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008 doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Carrington M, Alter G. Innate immune control of HIV. Cold Spring Harbor perspectives in medicine. 2012;2:a007070. doi: 10.1101/cshperspect.a007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- Clouthier DL, Zhou AC, Watts TH. Anti-GITR agonist therapy intrinsically enhances CD8 T cell responses to chronic lymphocytic choriomeningitis virus (LCMV), thereby circumventing LCMV-induced downregulation of costimulatory GITR ligand on APC. J Immunol. 2014;193:5033–5043. doi: 10.4049/jimmunol.1401002. [DOI] [PubMed] [Google Scholar]
- Clouthier DL, Zhou AC, Wortzman ME, Luft O, Levy GA, Watts TH. GITR intrinsically sustains early type 1 and late follicular helper CD4 T cell accumulation to control a chronic viral infection. PLoS Pathog. 2015;11:e1004517. doi: 10.1371/journal.ppat.1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AJ, Lafferty KJ. A simple conservative explanation of the H-2 restriction of interactions between lymphocytes. Scandinavian journal of immunology. 1977;6:1–6. doi: 10.1111/j.1365-3083.1977.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell. 2015;28:285–295. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186:4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, Amara RR. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, Williams JV. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J Immunol. 2013;191:5542–5550. doi: 10.4049/jimmunol.1301810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M, Chew C, Tyan K, Huang WQ, Salem A, Clynes R. FcgammaRIIB prevents inflammatory type I IFN production from plasmacytoid dendritic cells during a viral memory response. J Immunol. 2015;194:4240–4250. doi: 10.4049/jimmunol.1401296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, Oxenius A. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209:2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A, et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc Natl Acad Sci U S A. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- Gardiner D, Lalezari J, Lawitz E, Dimicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, et al. A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients with Chronic Hepatitis C Virus Infection. PLoS ONE. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garidou L, Heydari S, Gossa S, McGavern DB. Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection. J Virol. 2012;86:7060–7071. doi: 10.1128/JVI.00164-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF. Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. J Immunol. 2013;191:1011–1015. doi: 10.4049/jimmunol.1300652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafalla JC, Claser C, Couper KN, Grau GE, Renia L, de Souza JB, Riley EM. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology. PLoS Pathog. 2012;8:e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, Kuchroo V. TIMs: central regulators of immune responses. J Exp Med. 2008;205:2699–2701. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210:1189–1200. doi: 10.1084/jem.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, Sciume G, Hall AO, Dupont CD, Francisco LM, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Dummer W, Wolfe T, Rodrigo E, Theofilopoulos AN, Oldstone MB, von Herrath MG. Lack of intrinsic CTLA-4 expression has minimal effect on regulation of antiviral T-cell immunity. J Virol. 2006;80:270–280. doi: 10.1128/JVI.80.1.270-280.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, Maigret B, Dreano M, Triebel F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A. 1997;94:5744–5749. doi: 10.1073/pnas.94.11.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol. 1994;24:3216–3221. doi: 10.1002/eji.1830241246. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci U S A. 2001;98:9226–9230. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs a gradient of T-bet expression that specifies memory precursor and short-lived effector CD8 T cell fates. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8(+) T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassu A, D’Souza M, O’Connor BP, Kelly-McKnight E, Akkina R, Fontenot AP, Palmer BE. Decreased 4-1BB expression on HIV-specific CD4+ T cells is associated with sustained viral replication and reduced IL-2 production. Clin Immunol. 2009;132:234–245. doi: 10.1016/j.clim.2009.03.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan A, Naik S, Foster G, Bertoletti A. Preserved T Cell Function in Children and Young Adults With Immune Tolerant Chronic Hepatitis B. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- Kroy DC, Ciuffreda D, Cooperrider JH, Tomlinson M, Hauck GD, Aneja J, Berger C, Wolski D, Carrington M, Wherry EJ, et al. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology. 2014;146:550–561. doi: 10.1053/j.gastro.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M, Allison J. CD28 and CTLA-4 Have Opposing Effects on the Response of T cells to Stimulation. JEM. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- Kurachi M, Barnitz RA, Yosef N, Odorizzi PM, DiIorio MA, Lemieux ME, Yates K, Godec J, Klatt MG, Regev A, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol. 2014;15:373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- Lafon M, Megret F, Meuth SG, Simon O, Velandia Romero ML, Lafage M, Chen L, Alexopoulou L, Flavell RA, Prehaud C, Wiendl H. Detrimental contribution of the immuno-inhibitor B7-H1 to rabies virus encephalitis. J Immunol. 2008;180:7506–7515. doi: 10.4049/jimmunol.180.11.7506. [DOI] [PubMed] [Google Scholar]
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Lasaro MO, Tatsis N, Hensley SE, Whitbeck JC, Lin SW, Rux JJ, Wherry EJ, Cohen GH, Eisenberg RJ, Ertl HC. Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses. Nat Med. 2008 doi: 10.1038/nm1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar-Molnar E, Gacser A, Freeman GJ, Almo SC, Nathenson SG, Nosanchuk JD. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc Natl Acad Sci U S A. 2008;105:2658–2663. doi: 10.1073/pnas.0711918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D, Walunas T, Bluestone J. CD28/B7 system of T cell Costimulation. Annu Rev Im. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- Linsey P, Ledbetter J. The Role of the CD28 Receptor During T cell Responses to Antigen. Annu Rev Im. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Lu P, Youngblood BA, Austin JW, Mohammed AU, Butler R, Ahmed R, Boss JM. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med. 2014;211:515–527. doi: 10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GJ, Pereira RM, Aijo T, Kim EY, Marangoni F, Pipkin ME, Togher S, Heissmeyer V, Zhang YC, Crotty S, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203:2145–2155. doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- McNally B, Ye F, Willette M, Flano E. Local blockade of epithelial PDL-1 in the airways enhances T cell function and viral clearance during influenza virus infection. J Virol. 2013;87:12916–12924. doi: 10.1128/JVI.02423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–494. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev Vaccines. 2016 doi: 10.1586/14760584.2016.1141680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney JM, Klem J, Wulfing C, Mijares LA, Schwartzberg PL, Bennett M, Schatzle JD. The murine NK receptor 2B4 (CD244) exhibits inhibitory function independent of signaling lymphocytic activation molecule-associated protein expression. J Immunol. 2004;173:3953–3961. doi: 10.4049/jimmunol.173.6.3953. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells [published erratum appears in Nature 1993 Jul 15;364(6434):262] [see comments] Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Schiller HB, Forster F, Eckerstorfer P, Geyeregger R, Leksa V, Zlabinger GJ, Sibilia M, Sonnleitner A, Paster W, Stockinger H. Sequential cooperation of CD2 and CD48 in the buildup of the early TCR signalosome. J Immunol. 2009;182:7672–7680. doi: 10.4049/jimmunol.0800691. [DOI] [PubMed] [Google Scholar]
- Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J Immunol. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MT, Lanier LL. Inhibitory Ly49 receptors on mouse natural killer cells. Curr Top Microbiol Immunol. 2011;350:67–87. doi: 10.1007/82_2010_85. [DOI] [PubMed] [Google Scholar]
- Paley MA, Gordon SM, Bikoff EK, Robertson EJ, Wherry EJ, Reiner SL. Technical Advance: Fluorescent reporter reveals insights into eomesodermin biology in cytotoxic lymphocytes. J Leukoc Biol. 2013;93:307–315. doi: 10.1189/jlb.0812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascutti MF, Geerman S, Slot E, van Gisbergen KP, Boon L, Arens R, van Lier RA, Wolkers MC, Nolte MA. Enhanced CD8 T cell responses through GITR-mediated costimulation resolve chronic viral infection. PLoS Pathog. 2015;11:e1004675. doi: 10.1371/journal.ppat.1004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Science signaling. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O’Mara L, Yang S, Konieczny BT, Sharpe AH, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, McMullen A, Brennan T, Ahmed R, Freeman GJ, Kavanagh DG, Kaufmann DE. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J Virol. 2014;88:2508–2518. doi: 10.1128/JVI.02034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue HP, Slifka MK. Pivotal advance: CTLA-4+ T cells exhibit normal antiviral functions during acute viral infection. J Leukoc Biol. 2007;81:1165–1175. doi: 10.1189/jlb.0806535. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Raziorrouh B, Schraut W, Gerlach T, Nowack D, Gruner NH, Ulsenheimer A, Zachoval R, Wachtler M, Spannagl M, Haas J, et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934–1947. doi: 10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22:13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Johanns TM, Ertelt JM, Way SS. PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J Immunol. 2008;180:7553–7557. doi: 10.4049/jimmunol.180.11.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, Maini MK. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- Scott P, Hunter CA. Dendritic cells and immunity to leishmaniasis and toxoplasmosis. Curr Opin Immunol. 2002;14:466–470. doi: 10.1016/s0952-7915(02)00353-9. [DOI] [PubMed] [Google Scholar]
- Seo SK, Jeong HY, Park SG, Lee SW, Choi IW, Chen L, Choi I. Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection. Immunology. 2008;123:90–99. doi: 10.1111/j.1365-2567.2007.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PLoS ONE. 2013;8:e77780. doi: 10.1371/journal.pone.0077780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A Role for the Transcriptional Repressor Blimp-1 in CD8(+) T Cell Exhaustion during Chronic Viral Infection. Immunity. 2009 doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, Cui G, Li MO, Kaech SM. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles KM, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C. Herpes simplex virus glycoprotein D interferes with binding of herpesvirus entry mediator to its ligands through downregulation and direct competition. J Virol. 2010;84:11646–11660. doi: 10.1128/JVI.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci U S A. 2009;106:2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619–622. doi: 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Penaloza-Macmaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB Signaling Synergizes with Programmed Death Ligand 1 Blockade To Augment CD8 T Cell Responses during Chronic Viral Infection. J Immunol. 2011 doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinetz JM, Kumar S, Good MF, Fowlkes BJ, Berzofsky JA, Miller LH. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. J Immunol. 1990;144:1069–1074. [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Wang C, McPherson AJ, Jones RB, Kawamura KS, Lin GH, Lang PA, Ambagala T, Pellegrini M, Calzascia T, Aidarus N, et al. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J Exp Med. 2012;209:77–91. doi: 10.1084/jem.20110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A. 2013;110:E2480–E2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, et al. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity. 2011;35:285–298. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Flavell RA, Grewal IS, Larsen CP, Pearson TC, Ahmed R. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- Wieland A, Shashidharamurthy R, Kamphorst AO, Han JH, Aubert RD, Choudhury BP, Stowell SR, Lee J, Punkosdy GA, Shlomchik MJ, et al. Antibody effector functions mediated by Fcgamma-receptors are compromised during persistent viral infection. Immunity. 2015;42:367–378. doi: 10.1016/j.immuni.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks AG. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- Wu B, Huang B, Chen Y, Li S, Yan J, Zheng H, Huang S, Shen J, Lun ZR, Wang Y, et al. Upregulated expression of Tim-3 involved in the process of toxoplasmic encephalitis in mouse model. Parasitol Res. 2013;112:2511–2521. doi: 10.1007/s00436-013-3416-1. [DOI] [PubMed] [Google Scholar]
- Xu D, Fu HH, Obar JJ, Park JJ, Tamada K, Yagita H, Lefrancois L. A potential new pathway for PD-L1 costimulation of the CD8-T cell response to Listeria monocytogenes infection. PLoS One. 2013;8:e56539. doi: 10.1371/journal.pone.0056539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada DH, Elsaesser H, Lux A, Timmerman JM, Morrison SL, de la Torre JC, Nimmerjahn F, Brooks DG. Suppression of Fcgamma-receptor-mediated antibody effector function during persistent viral infection. Immunity. 2015;42:379–390. doi: 10.1016/j.immuni.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Takamatsu M, Sakata-Sogawa K, Zeng H, Hashimoto-Tane A, Yagita H, Tokunaga M, Saito T. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Noto A, Porichis F, Akondy RS, Ndhlovu ZM, Austin JW, Bordi R, Procopio FA, Miura T, Allen TM, et al. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191:540–544. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486–2495. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function [see comments] J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe. 2015;17:628–641. doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Wherry EJ. Immune Memory and Exhaustion: Clinically Relevant Lessons from the LCMV Model. Adv Exp Med Biol. 2015;850:137–152. doi: 10.1007/978-3-319-15774-0_10. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–1949. doi: 10.1053/j.gastro.2008.03.037. 1949 e1931–1933. [DOI] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nature communications. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]