Abstract
Antimicrobial peptides (AMPs) are a key component of the host's innate immune system, targeting invasive and colonizing bacteria. For successful survival and colonization of the host, bacteria have a series of mechanisms to interfere with AMP activity, and AMP resistance is intimately connected with the virulence potential of bacterial pathogens. In particular, because AMPs are considered as potential novel antimicrobial drugs, it is vital to understand bacterial AMP resistance mechanisms. This review gives a comparative overview of Gram-positive and Gram-negative bacterial strategies of resistance to various AMPs, such as repulsion or sequestration by bacterial surface structures, alteration of membrane charge or fluidity, degradation and removal by efflux pumps.
This article is part of the themed issue ‘Evolutionary ecology of arthropod antimicrobial peptides’.
Keywords: bacterial resistance, antimicrobial peptides, Staphylococcus
1. Introduction
Antimicrobial peptides (AMPs) represent an important part of innate immune defences in many organisms [1]. They contribute to immune defences on epithelial surfaces and form part of the variety of antimicrobial agents by which leucocytes kill microorganisms after ingestion [2]. Many AMPs have activity against a wide range of pathogens. In addition to their antimicrobial activity, AMPs also have a signalling function. For example, they can activate components of the human acquired immune system, such as T cells and dendritic cells [3].
AMPs are synthesized as proforms before processing to the active, mature peptides occurs. Most AMPs are cationic (cationic AMPs, CAMPs) and show pronounced amphipathy. Although for many AMPs the mode of action is incompletely understood, these features contribute to binding to the anionic bacterial surface and integration into the cytoplasmic membrane, where many AMPs form pores to kill the target microorganism [4].
Most AMPs in humans belong to the beta-defensin family. Human beta-defensins 1–4 are produced by keratinocytes on the skin [5]. The only member of the cathelicidin family produced in humans is the LL-37 peptide [6]. While these peptides are cationic, humans also produce an anionic AMP, dermcidin, which recently has been shown to also work by pore formation in target membranes [7,8].
AMPs have often been suggested as potential novel antimicrobial compounds [9]. Notably, their commonly bactericidal mode of action makes them potential candidates for difficult-to-treat infections by slow-growing bacteria, such as biofilm infections. As the development of resistance to antimicrobial compounds represents one of the most serious problems for healthcare systems, a detailed understanding of resistance mechanism to AMPs is of vital importance.
AMP resistance mechanisms include proteolytic degradation or sequestration by secreted proteins, impedance by exopolymers and biofilm matrix molecules, circumvention of attraction by cell surface/membrane alteration, and export by efflux pumps. This review gives an overview of each mechanism in Gram-positive and Gram-negative bacteria and finishes by a presentation of mechanisms bacteria have to sense the presence of AMPs (see table 1 and figure 1 for a summary list and graphical presentation, respectively).
Table 1.
Overview of bacterial resistance mechanisms against antimicrobial peptides.
| mechanisms | Gram-positive bacteria | Gram-negative bacteria |
|---|---|---|
| extracellular proteins | proteolytic degradation sequestration |
proteolytic degradation |
| exopolymers | PIA, PGA | alginate, polysialic acid |
| surface modification | repulsion by d-alanylation of TA steric hindrance by l-rhamnosylation of WTA lipid II modification |
repulsion by lipid A phosphate modification increased OM rigidity by lipid A acylation O-antigen of LPS |
| cytoplasmic membrane alteration | charge repulsion by PG amino-acylation | increased IM rigidity by PG acylation |
| efflux pumps | export by ABC transporters | export by RND family efflux pumps |
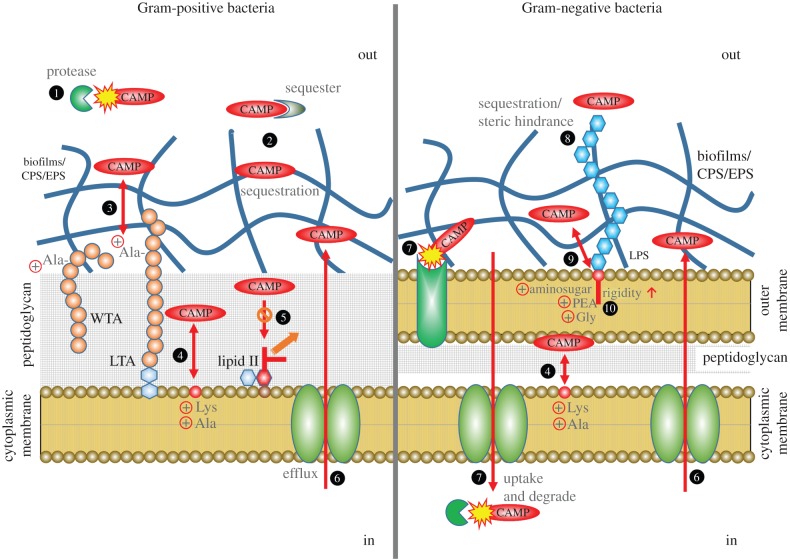
Figure 1.
Schematic diagram of bacterial AMP resistance mechanisms. Mechanisms in Gram-positive bacteria are shown on the left, and in Gram-negative bacteria on the right. Gram-positive bacteria retain the Gram stain owing to their thick peptidoglycan cell wall, while Gram-negative bacteria only have a thin peptidoglycan (PG) layer but possess an additional so-called ‘outer membrane’. 1, Proteolytic degradation by extracellular proteases; 2, sequestration by extracellular proteins or extracellular matrix; 3, electrostatic repulsion by alanylated teichoic acids (TA); 4, electrostatic repulsion by aminoacylated PG; 5, evasion of lipid II-binding AMPs by pentapeptide alteration; 6, export of AMPs by efflux pumps; 7, proteolytic cleavage by outer membrane protease or cytosolic protease after uptake by transporters; 8, sequestration or steric hindrance by O-antigen of lipopolysaccharide (LPS); 9, electrostatic repulsion by amine compound-added lipid A; 10, enhanced rigidity by lipid A acylation.
2. Extracellular proteins
Secreted bacterial proteins, such as proteases, are the first bacterial defence mechanisms that AMPs encounter when interacting with bacteria. Proteolytic degradation of AMPs by extracellular enzymes represents a simple, yet effective way of providing AMP resistance to microorganisms. Commensal bacteria that live on mammalian epithelial surfaces, such as staphylococci, secrete various proteases. In staphylococci, these include metalloproteases such as aureolysin and SepA, and serine endopeptidases such as the V8 protease, which are known to degrade linear CAMPs such as the human cathelicidin LL-37 [10,11]. Group A Streptococcus produces a protease called SpeB. This cysteine protease has been shown to fragmentize many host AMPs, including LL-37 and beta-defensins [12–15]. Interestingly, exploiting host proteins can intensify the proteolytic activity of SpeB. Namely, interaction of SpeB with cell wall-anchored G-related alpha2M-binding (GRAB) protein leads to a surface-bound complex of SpeB and a host proteinase inhibitor, alpha2-macroglobulin, which shows increased activity towards LL-37 [16,17]. In addition, as a secondary effect of SpeB proteolytic activity, degraded host proteoglycans release dermatan sulfate, which completely neutralizes human alpha-defensin, HNP-1 [18]. Finally, proteases from another Gram-positive pathogen, Enterococcus faecalis, and the Gram-negative Pseudomonas aeruginosa and Proteus mirabilis have also been reported to degrade LL-37 [12].
One of the most intensively studied group of proteases in Gram-negative bacteria is the omptin family, a group of aspartate proteases found in the enterobacterial outer membrane (OM) [19,20]. Some representative members of that protease, such as OmpT in Escherichia coli, PgtE in Salmonella enterica serotype Typhimurium (S. typhimurium) and Pla in Yersinia pestis, have been demonstrated to cleave AMPs including LL-37, the homologous murine cathelicidin-related antimicrobial peptide (CRAMP) and protamine [21–23]. Along with the opmtin family of proteases, metalloproteases play an important role in the defence of Gram-negative bacteria towards AMPs. For example, the metalloprotease ZapA of P. mirabilis neutralizes multiple targets such as LL-37, human beta-defensin HBD-1, and the porcine AMP protegrin-1 [24]. Burkholderia cenocepacia produces two metalloproteases, ZmpA and ZmpB, which can degrade various CAMPs [25]. Interestingly, ZmpA cleaves linear LL-37, whereas ZmpB targets the nonlinear HBD-1 [25]. Some Gram-negative bacteria also degrade AMPs in the intracellular environment after import by specific transport proteins. S. typhimurium and Haemophilus influenzae produce an ABC transporter encoded by the sapABCDFZ operon, which was reported to increase bacterial resistance to CAMPs [26–28]. Binding of CAMPs by SapA leads to increased expression of the genes in the sap operon, whose four-component ABC transporter product transfers CAMPs into the cytosol for intracellular proteolytic degradation [28–30]. H. influenzae SapA has been shown to bind to diverse AMPs such as LL-37, HNP-1, HBD-2, HBD-3 and melittin [31].
Finally, it needs to be stressed that the inactivation of AMPs by proteases is highly dependent on the structure of the target peptide [1]. For example, a linear AMP such as LL-37 is more prone to degradation than AMPs with nonlinear structures that contain disulfide bonds. The introduction of disulfide bonds, or the even more sophisticated post-translational modification found in some bacterial AMPs (bacteriocins) such as lantibiotics represent resistance mechanisms of AMPs towards proteolytic degradation by the host or competing microorganisms, exemplifying the multi-faceted evolutionary interplay of AMP producers and resistant microorganisms [1,32].
Along with proteolysis, sequestration has an important role in extracellular protein-mediated resistance to AMPs. Staphylokinase is one of the most prominent extracellular AMP-sequestering molecules. It is encoded by the sak gene in Staphylococcus aureus and sequesters alpha-defensins (HNP-1 and 2) [33,34]. Furthermore, binding to LL-37 increases staphylokinase-dependent plasminogen activation, which represents another crucial role of staphylokinase in pathogenesis [35]. Intriguingly, the activation of plasminogen by streptokinase (Ska) of group A streptococci, in addition to the original activities of plasmin such as fibrin clot degradation [36], leads to the destruction of LL-37 [37]. Another well-known streptococcal sequester, streptococcal inhibitor of complement (SIC), protects Streptococcus pyogenes from defensins and LL-37 [38] as well as the membrane attack complex [39]. Finally, some streptococcal cell surface-attached proteins such as the M1 protein of S. pyogenes and the PilB pilin subunit of Streptococcus agalactiae can bind cathelicidins (LL-37 and CRAMP), contributing to streptococcal AMP resistance [40,41].
3. Biofilms and exopolymers
Bacterial biofilm is a consortium of surface-attached bacterial cells that are embedded in a matrix composed mainly of extracellular proteins, extracellular DNA and exopolysaccharides (EPS) [42,43]. Bacteria in biofilms exhibit higher resistance (up to 1000-fold) to antibiotics and AMPs than planktonic bacteria [44,45]. The increased resistance is thought to be partly owing to the decreased penetration of AMPs through the matrix [46]. EPS and capsular polysaccharides (CPS) impede AMPs by capturing or repelling them. Polysaccharide intercellular adhesin (PIA, also known as poly-N-acetyl glucosamine), which is produced by S. aureus, Staphylococcus epidermidis, and a variety of other bacteria including other staphylococci and E. coli, is responsible for the resistance to cationic HBD-3 and LL-37 as well as anionic dermcidin [47,48]. IcaB-mediated deacetylation of PIA increases the positive net charge in PIA. This results in increased repulsion to CAMPs, but perhaps also increased sequestration of dermcidin, in addition to forming a mechanical barrier for both types of AMPs [49]. Additionally, PIA is crucial for in vitro and in vivo biofilm formation, thus contributing to biofilm formation as a general AMP resistance mechanism [49–51]. On the other hand, impedance of CAMPs such as polymyxin B, HNP-1, HBD-1, lactoferrin and protamine by the anionic CPS of Klebsiella pneumoniae, Streptococcus pneumoniae or P. aeruginosa is presumably the result of structural hindrance as well as electrostatic trapping [52,53]. Capsules in other streptococci also have an important role in sequestering AMPs. Hyaluronic acid capsule as well as M protein of group A streptococci mediate resistance to LL-37 [54]. Capsule synthesized by a phosphoglucomutase homologue in Streptococcus iniae has a role in resistance to the fish AMP, moronecidin [55]. Furthermore, as briefly mentioned in §2, P. aeruginosa, E. faecalis and S. pyogenes can exploit host polysaccharide to sequester AMPs after the degradation of the host proteoglycan matrix by secreted bacterial proteases [18].
In Pseudomonas species, alginate is a major component of the biofilm matrix. It is an acylated polysaccharide comprising anionic sugars such as guluronic acid and mannuronic acid [56,57], whose role in AMP resistance in biofilms was shown by genetic overexpression [58]. Mechanisms of resistance to LL-37 in P. aeruginosa and other lung pathogens are largely dependent on sequestration by EPS [59,60]. Finally, the Gram-negative bacterium, Neisseria meningitidis, has a capsular polysialic acid that prevents surface binding of defensins, cathelicidins, protegrins and polymyxin B [61,62].
Poly-gamma-glutamic acid (PGA), which is a homopolymer of glutamic acid linked by gamma-amide bonds, forms an extracellular capsule protecting bacteria from phagocytosis [63]. PGA is found only in Gram-positive bacteria, mainly in Bacillus species and coagulase-negative staphylococci including S. epidermidis [64,65]. In addition to its role in resisting ingestion by leucocytes, PGA provides protection from LL-37, HBD-3 and dermcidin, similar to PIA. However, in contrast to PIA, PGA does not directly facilitate biofilm formation [64,66], although the PGA biosynthesis cap operon is upregulated in biofilms of S. epidermidis [67,68].
Finally, it is important to note that AMPs have gained much attention as alternatives to traditional antibiotics in the treatment of biofilm-associated infection. This is mainly because the mode of action of AMPs is usually bactericidal, whereas many traditional antibiotics are commonly bacteriostatic and target fast-growing bacteria, thus lacking efficiency against biofilms [68,69]. However, despite numerous efforts of biofilm control with natural or synthetic AMPs, biofilm-intrinsic AMP resistance complicates the use of AMPs for the treatment of biofilm infections [70,71].
4. Surface modification
The bacterial cell envelope represents a major impediment for AMP activity. Because Gram-positive and Gram-negative bacteria have distinct cell envelope structures, they apply different strategies to modify their cell surface to resist AMPs. The key molecules for this purpose are anionic polymers attached to the outermost cell surface, teichoic acids (TA) in the Gram-positive cell wall and lipopolysaccharide (LPS) in the Gram-negative OM.
TA is the most abundant component in the Gram-positive cell wall, representing over 60% of its total mass [72]. It is composed of disaccharide anchors and phosphodiester-linked polyglycerol phosphate or polyribitol phosphate, which is responsible for the negative net charge of TA [73,74]. According to the anchoring location of TA to the cell surface, one distinguishes wall teichoic acid (WTA) and lipoteichoic acid. d-Alanylation on free hydroxyls of the repeating sugars, driven by the gene products of the dltABCD locus, adds a positive charge to TA. This generally lowers attraction of CAMPs and was shown in S. aureus for HNP-1,2,3, protegrins, magainin II, gallidermin and nisin [75]. In a more recent study on group B Streptococcus, it was shown that d-alanylation also increases cell wall density, which suggests a dual role of DltABCD, leading to electrostatic repulsion and a reduction of surface permeability [76]. d-Alanylation of TA as an AMP resistance strategy is widely used by various Gram-positive bacteria, including Staphylococcus, Streptococcus and Bacillus [75–83]. Interestingly, the Gram-negative bacterium, Bordetella pertussis, has a Dlt homologue, Dra, which leads to d-alanylation on the OM, resulting in a decreased negative charge that increases resistance to AMPs such as LL-37, HNP-1, HNP- 2 and polymyxin B [84]. Although the target component in the OM is yet to be determined, this finding suggests that Dlt-homologous proteins may have an even more widespread role in AMP resistance than previously believed. Recently, it was reported that l-rhamnosylation of WTA in L. monocytogenes protects from AMPs such as cathelicidins and gallidermin by increasing steric hindrance in the cell wall, proposing yet another TA decoration-related resistance mechanism [85].
Owing to its pivotal role in cell proliferation, the bacterial peptidoglycan precursor, lipid II, represents a further prominent AMP target. Lipid II is a cell wall building block consisting of undecaprenyl pyrophosphate and pentapeptide connected to the cell wall disaccharide of N-acetyl muramic acid (MurNAc) and N-acetyl glucosamine (GlcNAc) [86]. Many bacteria modify lipid II to evade AMP killing. Of note, unlike AMP-induced envelope alteration, modification of lipid II is a consistent change. The best-known lipid II modification is replacement of the terminal d-alanine to evade the activity of the glycopeptide antibiotic, vancomycin. Vancomycin binds to the d-Ala–d-Ala dipeptide found in the cell wall-cross-linking pentapeptide bridge and blocks the transpeptidation reaction that is required for cell wall synthesis [87]. Vancomycin-resistant strains contain lipid II with d-lactate or d-serine instead of the terminal d-alanine and show 1000-fold increased resistance [88]. Another well-known group of AMPs targeting lipid II include the bacterial lantibiotic-type bacteriocins, such as nisin or epidermin, which also use lipid II as docking molecule for subsequent pore formation [89,90]. In addition to vancomycin and lantibiotics, the human AMPs HNP-1 and HBD-3 target lipid II to block cell wall biosynthesis [91,92]. Finally, there are reports of resistance to lysozyme that is conferred by acetylation of MurNAc [93] or deacetylation of GlcNAc [94].
Similar to Gram-positive bacteria, Gram-negative bacteria accomplish resistance to AMPs by alteration of net charge and permeability of the cell surface. The counterpart of TA in Gram-negative bacteria is LPS, which forms the main component of the outer leaflet of the OM, covering 75% of the cell surface [95] and causing a net negative surface charge. Prevention of electrostatic binding of AMPs to the Gram-negative cell surface is achieved by amine-containing molecules (amino sugars, phosphoethanolamine (PEA) or glycine), which increase the positive charge of the anionic LPS component lipid A. P. aeruginosa and S. typhimurium attach aminoarabinose to a phosphate group in lipid A [96,97]. Acinetobacter baumannii, Francisella novicida and Bordetella species modify lipid A phosphate with galactosamine or glucosamine [98–101]. Furthermore, Gram-negative bacteria use PEA to decrease the anionic properties of LPS. S. typhimurium, Neisseria gonorrhoeae and A. baumannii attach PEA onto phosphates in lipid A [101–103]. Finally, Vibrio cholerae imports glycines into lipid A acyl chains to increase the positive charge and diminish AMP attraction [104]. Especially Burkholderia species and N. meningitidis constitutively express aminoarabinose- and PEA-attached lipid A, respectively; thus, inherently they have higher AMP resistance than other bacteria [105,106]. In addition to adding positively charged molecules to LPS, Gram-negative bacteria can obtain the same result by removing anionic phosphate groups from lipid A. In F. novicida, the LpxE and LpxF proteins have phosphatase activities, catalysing the removal of phosphate from the 1 and 4′ position of lipid A, respectively [107,108]. In contrast, LpxT is responsible for additional phosphorylation on lipid A [109,110]. Activation of LpxE/LpxF and inhibition of LpxT may contribute to AMP resistance in Gram-negative bacteria by decreasing the net negative charge [111–113]. Finally, it has recently been discovered that LPS dephosphorylation by LpxF is critical for survival of the human gut commensal Bacteroidetes, by protecting this bacterium from inflammatory perturbation originating from AMPs [114].
Another strategy of increasing AMP resistance in Gram-negative bacteria is to enhance the rigidity of the OM by adding extra acyl chains into lipid A [115,116]. Lipid A acylation is often mediated by the PagP enzyme, which was shown to promote resistance to various AMPs such as C18G, protegrin, polymyxin B, LL-37 and magainin II in S. typhimurium, E. coli, Yersinia enterocolitica and V. cholerae [115–117]. Furthermore, in a recent study on A. baumannii, which does not have PagP, homologues of LpxL and LpxM were shown to promote lipid A acylation to prevent the activities of C18G and polymyxin [118].
In addition to changing surface charge and permeability of the OM, the outermost long polysaccharide chain in LPS, called O-antigen, confers an extra AMP barrier to Gram-negative bacteria [72]. Both LPS core and the O-antigen have been proven essential for AMP resistance in B. cenocepacia and Brucella abortus using mutants that lack the respective sugar structures in LPS [119,120].
Finally, during symbiotic interaction with insects, changes in the bacterial surface structure are observed that decrease AMP resistance and increase susceptibility to host innate immune defences. Namely, Burkholderia species were shown to lose the LPS O-antigen during symbiotic colonization of the gut of the bean bug, Riptortus pedestris, resulting in increased susceptibility to purified host AMPs [121]. For a detailed review on insect–symbiont interaction, the reader is referred to the article by Masson et al. [122].
5. Alteration of the cytoplasmic membrane structure
After passing through all the outer barriers on the bacterial surface, AMPs finally confront the cytoplasmic membrane. Because this is the major target of AMPs, bacteria frequently have strategies to modify the membrane in a fashion that decreases AMP attraction and insertion. The most abundant phospholipids in bacterial membranes are phosphatidylglycerol (PG) and diphosphatidylglycerol (DPG, also called cardiolipin), which have anionic head groups that attract CAMPs. This attraction may be perturbed, for example, by amino-acylation of the PG head group, which masks anionic phosphates with cationic primary amines. Originally described in S. aureus, the bifunctional, integral membrane protein, multipeptide resistance factor (MprF) is responsible for amino-acylation of PG with lysine, which results in AMP resistance by electrostatic repulsion [123]. The C-terminal synthase domain of MprF uses PG and aminoacyl–tRNA as substrates. The synthesized aminoacyl-PG is translocated to the outer leaflet of the cytoplasmic membrane by an N-terminal flippase domain in MprF [124]. In a recent study performed in S. aureus, further detailed structural information on MprF, the only known bacterial phospholipid flippase, was revealed, including multi-domain interaction and oligomerization [125]. MprF proteins of different bacteria use different amino acid and phospholipid substrates, an adaptation that may explain why MprF is widely spread among bacteria except for enterobacteria [126]. While the majority of bacteria including Staphylococcus and Bacillus species only generate Lys-PG from PG and lysyl-tRNA, some other bacteria use a different combination of substrates: Lys-PG and Lys-CL in Listeria monocytogenes [127], Ala-PG in P. aeruginosa [128], Lys-PG and Ala-PG in Clostridium perfringens [129]. Compared with other bacteria that are devoid of MprF such as group A or B streptococci [76,130], staphylococci have an especially thick cationic surface barrier, possibly explaining why staphylococci are particularly resistant to CAMPs. Although PG lysylation has been reported mostly in Gram-positive bacteria, there are some studies that report the presence of Lys-PG in Gram-negative bacteria such as Caulobacter crescentus [131], Rhizobium tropici [132] and P. aeruginosa.
In addition to lipid A, the PagP protein also palmitoylates PG on the OM [133]. As PagP is involved in the homeostasis of membrane hydrophobicity, it is certainly imaginable that the protein may also repair AMP-damaged membranes, which would constitute another AMP resistance mechanism [134]. In general, higher membrane rigidity can be achieved by an increase of saturated acyl chains in the membrane, which has been reported to confer elevated resistance to pediocin and nisin in E. faecalis and L. monocytogenes, respectively [135,136]. In S. aureus, enhanced membrane rigidity can be achieved by increased incorporation of the carotenoid staphyloxanthin, which stabilizes acyl chains in the membrane [137]. In contrast, unsaturated fatty acyl chains increase membrane fluidity, which underlies resistance to thrombin-induced platelet microbicidal proteins (tPMP) in S. aureus [138]. Generally, the roles of membrane rigidity and fluidity in AMP resistance are still ambiguous and will require enhanced research efforts.
6. Efflux pumps
Even when AMPs have already attached to and inserted in the cytoplasmic membrane, bacteria can still remove them using dedicated efflux pumps, which constitute another important AMP resistance component. The resistance/nodulation/cell division (RND) family transporters, AMP efflux pumps that are present in many Gram-negative bacteria, have been studied for some time [139,140]. A typical member of this family is composed of three components: an inner membrane proton/AMP antiporter, an OM transporter and a periplasmic accessory protein that completes and stabilizes the entire efflux complex [141,142]. For example, MtrCDE of N. gonorrhoeae and N. meningitides is responsible for resistance to LL-37 and protegrin-1 [106,143]. AcrAB-TolC in K. pneumoniae is active on polymyxin B as well as human defensins (HNP-1, HBD-1, 2) [144]. Finally, V. cholerae VexAB-TolC protects from polymyxin B [145]. Importantly, some RND family efflux pumps from other bacteria have no function in AMP resistance. In those cases (e.g. AcrAB-TolC of E. coli, MexAB-OprM of P. aeruginosa), no significant differences in the susceptibilities towards cathelicidin, and various human defensins could be observed using overexpression and isogenic efflux pump gene deletion strains [146]. Finally, in another Gram-negative pathogen, Y. enterocolitica, RosAB, a major facilitator superfamily (MFS) efflux pump was reported to confer resistance to polymyxin B [147].
Similar to the S. aureus MFS family efflux protein NorA [146], most staphylococcal multidrug-resistance transporters are not active on human AMPs. However, the S. aureus MFS efflux pump, QacA, has been shown to confer resistance to tPMP [148]. Including in S. aureus, a large number of AMP-exporting efflux pumps in Gram-positive bacteria are ATP-binding cassette (ABC) transporters that are designated for the secretion of, or producer immunity towards, newly synthesized AMPs [149]. For example, NisT of Lactococcus lactis exports newly synthesized nisin [150] and EpiFEG of S. epidermidis protects from epidermin and the structurally similar gallidermin [151]. Notably, these proteins have a limited role in general AMP resistance because of their narrow substrate specificity. However, the other group of two-component ABC transporters, called BceAB type, are active on a wider range of AMPs, including lantibiotics, cyclic AMPs, glycopeptides, cathelicidin and defensins [149]. While most BceAB-type transporters accept as substrates only one or two classes of AMPs (mostly lantibiotics and bacitracin), the S. aureus VraFG ABC transporter confers resistance to a variety of AMPs, including nisin, bacitracin, vancomycin, indolicidin, LL-37 and HBD-3 [149,152,153]. In addition, S. aureus has two more BceAB-type ABC transporters, BraDE and VraDE, but their substrates are limited to nisin and bacitracin [154–156]. BceAB-type ABC transporters have been also found in B. subtilis, S. pneumoniae, L. monocytogenes and many other Gram-positive bacteria [149,157–160]. Of note, this type of transporter often appears to be involved with AMP sensing (see §7). Finally, a unique S. pneumoniae dual efflux pump of both the MFS and ABC families, MefE/Mel, is inducible by cathelicidin, and confers resistance to LL-37 as well as macrolides [161,162].
7. Antimicrobial peptide sensing systems
Although some bacterial AMP resistance mechanisms, such as the modification of lipid II, represent constitutive changes, most are strictly regulated by bacterial sensor/regulators. This is likely to minimize a fluctuation in anionic homeostasis and expression of energy-consuming resistance mechanisms when they are not needed.
The first Gram-positive AMP sensing system was identified in S. epidermidis and named antimicrobial peptide sensor (Aps), also known as GraRS or GraRSX [163]. It is unusual in comprising three components: a membrane-bound AMP sensing histidine kinase (ApsS), a DNA-binding response regulator (ApsR) and a third component (ApsX) of yet unknown function, all of which are essential for AMP-dependent gene regulation. The Aps system regulates the expression of genes involved in major AMP resistance mechanisms, such as the dlt operon for TA alanylation, the mprF gene for PG lysinylation and the vraFG ABC transporter. The latter was recently reported to also be involved in AMP-dependent signal transduction [153]. Interestingly, a very short extracellular loop in ApsS interacts with AMPs and determines substrate specificity [152]. Aps-homologous systems are found in multiple Gram-positive pathogens including S. pneumoniae, L. monocytogenes, Bacillus anthracis, Clostridium difficile and Staphylococcus haemolyticus [163]. Other Gram-positive two-component systems that sense AMPs and regulate resistance mechanisms include BceSR of B. subtilis [157], BraSR, VraSR in S. aureus [154] and LiaFSR in streptococci [164,165]. Often, they cooperate with corresponding ABC transporters, such as VraFG in the case of Aps [153].
A central regulator for AMP resistance in Gram-negative bacteria is the PhoPQ two-component system [166,167]. PhoQ is a histidine kinase sensor located in the cytoplasmic membrane and PhoP is the corresponding DNA-binding response regulator. PhoPQ and a further PhoPQ-regulated two-component system, PmrAB, work together to regulate most lipid A modifications involved with AMP resistance, which include PagP-mediated palmitoylation, amine-compound addition to phosphates and alteration of the degree of phosphorylation in lipid A [97,102,107,108,113,115,168]. PhoPQ-homologous systems for AMP resistance are found in various Gram-negative bacteria, including S. typhimurium, Y. pestis and Shigella flexneri [169–172]. Sodalis glossinidius, an endosymbiont of the tsetse fly, also has the PhoP/PhoQ system, but by loss of PhoP's sensory capacity constitutively expresses genes facilitating resistance to host AMPs during colonization [173].
8. Conclusion
Mechanisms of resistance to AMPs are widespread in bacteria. Many of those are based on changes in the physico-chemical properties of surface molecules and the cytoplasmic membrane. These changes commonly confer moderate levels of resistance and are relatively non-specific. Possibly, they are not exclusively involved with AMP resistance. For example, the primary task of many surface molecules is the formation of a biofilm matrix and that of many secreted proteases the acquisition of nutrients, and most AMP efflux pumps also accept other antimicrobial molecules as substrates. For drug development efforts in the field of AMPs, it is important that AMP resistance, which may develop due to selective pressure [174], is thus not based on dedicated resistance genes that are conferred, such as in the case of many antibiotic resistance mechanisms, by horizontal gene transfer [175]. Rather, these observations mean that for any AMP-based drug, a certain increase of resistance after exposure to the drug is to be expected (‘MIC creep’), such as observed for the AMP-related antibiotic, daptomycin [126,176].
Even if many details about bacterial resistance to AMPs have been discovered, several topics await more in-depth investigation, such as membrane repair mechanisms, the role of membrane fluidity/rigidity, the involvement of host components and resistance mechanisms of the Gram-negative inner membrane.
Competing interests
We have no competing interests.
Funding
The authors are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
References
- 1.Peschel A, Sahl HG. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4, 529–536. ( 10.1038/nrmicro1441) [DOI] [PubMed] [Google Scholar]
- 2.Otto M. 2010. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev. Dermatol. 5, 183–195. ( 10.1586/edm.10.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, et al. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528. ( 10.1126/science.286.5439.525) [DOI] [PubMed] [Google Scholar]
- 4.Brown KL, Hancock RE. 2006. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18, 24–30. ( 10.1016/j.coi.2005.11.004) [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720. ( 10.1038/nri1180) [DOI] [PubMed] [Google Scholar]
- 6.Durr UH, Sudheendra US, Ramamoorthy A. 2006. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425. ( 10.1016/j.bbamem.2006.03.030) [DOI] [PubMed] [Google Scholar]
- 7.Schittek B, et al. 2001. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2, 1133–1137. ( 10.1038/ni732) [DOI] [PubMed] [Google Scholar]
- 8.Song C, et al. 2013. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc. Natl Acad. Sci. USA 110, 4586–4591. ( 10.1073/pnas.1214739110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51. ( 10.1038/nrd3591) [DOI] [PubMed] [Google Scholar]
- 10.Sieprawska-Lupa M, et al. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 48, 4673–4679. ( 10.1128/AAC.48.12.4673-4679.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teufel P, Gotz F. 1993. Characterization of an extracellular metalloprotease with elastase activity from Staphylococcus epidermidis. J. Bacteriol. 175, 4218–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46, 157–168. ( 10.1046/j.1365-2958.2002.03146.x) [DOI] [PubMed] [Google Scholar]
- 13.Baranska-Rybak W, Sonesson A, Nowicki R, Schmidtchen A. 2006. Glycosaminoglycans inhibit the antibacterial activity of LL-37 in biological fluids. J. Antimicrob. Chemother. 57, 260–265. ( 10.1093/jac/dki460) [DOI] [PubMed] [Google Scholar]
- 14.Nelson DC, Garbe J, Collin M. 2011. Cysteine proteinase SpeB from Streptococcus pyogenes – a potent modifier of immunologically important host and bacterial proteins. Biol. Chem. 392, 1077–1088. ( 10.1515/BC.2011.208) [DOI] [PubMed] [Google Scholar]
- 15.Frick IM, Nordin SL, Baumgarten M, Morgelin M, Sorensen OE, Olin AI, Egesten A. 2011. Constitutive and inflammation-dependent antimicrobial peptides produced by epithelium are differentially processed and inactivated by the commensal Finegoldia magna and the pathogen Streptococcus pyogenes. J. Immunol. 187, 4300–4309. ( 10.4049/jimmunol.1004179) [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen M, Muller HP, Bjorck L. 1999. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding alpha2-macroglobulin. J. Biol. Chem. 274, 15 336–15 344. ( 10.1074/jbc.274.22.15336) [DOI] [PubMed] [Google Scholar]
- 17.Nyberg P, Rasmussen M, Bjorck L. 2004. Alpha2-macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J. Biol. Chem. 279, 52 820–52 823. ( 10.1074/jbc.C400485200) [DOI] [PubMed] [Google Scholar]
- 18.Schmidtchen A, Frick IM, Bjorck L. 2001. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol. Microbiol. 39, 708–713. ( 10.1046/j.1365-2958.2001.02251.x) [DOI] [PubMed] [Google Scholar]
- 19.Haiko J, Suomalainen M, Ojala T, Lahteenmaki K, Korhonen TK. 2009. Invited review: Breaking barriers--attack on innate immune defences by omptin surface proteases of enterobacterial pathogens. Innate Immun. 15, 67–80. ( 10.1177/1753425909102559) [DOI] [PubMed] [Google Scholar]
- 20.Kukkonen M, Korhonen TK. 2004. The omptin family of enterobacterial surface proteases/adhesins: from housekeeping in Escherichia coli to systemic spread of Yersinia pestis. Int. J. Med. Microbiol. 294, 7–14. ( 10.1016/j.ijmm.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 21.Stumpe S, Schmid R, Stephens DL, Georgiou G, Bakker EP. 1998. Identification of OmpT as the protease that hydrolyzes the antimicrobial peptide protamine before it enters growing cells of Escherichia coli. J. Bacteriol. 180, 4002–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guina T, Yi EC, Wang H, Hackett M, Miller SI. 2000. A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to alpha-helical antimicrobial peptides. J. Bacteriol. 182, 4077–4086. ( 10.1128/JB.182.14.4077-4086.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvan EM, Lasaro MA, Schifferli DM. 2008. Capsular antigen fraction 1 and Pla modulate the susceptibility of Yersinia pestis to pulmonary antimicrobial peptides such as cathelicidin. Infect. Immun. 76, 1456–1464. ( 10.1128/IAI.01197-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belas R, Manos J, Suvanasuthi R. 2004. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect. Immun. 72, 5159–5167. ( 10.1128/Iai.72.9.5159-5167.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooi C, Sokol PA. 2009. Burkholderia cenocepacia zinc metalloproteases influence resistance to antimicrobial peptides. Microbiology 155, 2818–2825. ( 10.1099/mic.0.028969-0) [DOI] [PubMed] [Google Scholar]
- 26.Groisman EA, Parra-Lopez C, Salcedo M, Lipps CJ, Heffron F. 1992. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc. Natl Acad. Sci. USA 89, 11 939–11 943. ( 10.1073/pnas.89.24.11939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra-Lopez C, Baer MT, Groisman EA. 1993. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 12, 4053–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason KM, Munson RS Jr, Bakaletz LO. 2005. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect. Immun. 73, 599–608. ( 10.1128/IAI.73.1.599-608.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason KM, Bruggeman ME, Munson RS, Bakaletz LO. 2006. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 62, 1357–1372. ( 10.1111/j.1365-2958.2006.05460.x) [DOI] [PubMed] [Google Scholar]
- 30.Shelton CL, Raffel FK, Beatty WL, Johnson SM, Mason KM. 2011. Sap transporter mediated import and subsequent degradation of antimicrobial peptides in Haemophilus. PLoS Pathog. 7, e1002360 ( 10.1371/journal.ppat.1002360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason KM, Raffel FK, Ray WC, Bakaletz LO. 2011. Heme utilization by nontypeable Haemophilus influenzae is essential and dependent on Sap transporter function. J. Bacteriol. 193, 2527–2535. ( 10.1128/JB.01313-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bierbaum G, Sahl HG. 2009. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 10, 2–18. ( 10.2174/138920109787048616) [DOI] [PubMed] [Google Scholar]
- 33.Bokarewa M, Tarkowski A. 2004. Human alpha-defensins neutralize fibrinolytic activity exerted by staphylokinase. Thromb. Haemost. 91, 991–999. ( 10.1267/THRO04050991) [DOI] [PubMed] [Google Scholar]
- 34.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. 2004. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J. Immunol. 172, 1169–1176. ( 10.4049/jimmunol.172.2.1169) [DOI] [PubMed] [Google Scholar]
- 35.Braff MH, Jones AL, Skerrett SJ, Rubens CE. 2007. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis. J. Infect. Dis. 195, 1365–1372. ( 10.1086/513277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun H, et al. 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305, 1283–1286. ( 10.1126/science.1101245) [DOI] [PubMed] [Google Scholar]
- 37.Hollands A, Gonzalez D, Leire E, Donald C, Gallo RL, Sanderson-Smith M, Dorrestein PC, Nizet V. 2012. A bacterial pathogen co-opts host plasmin to resist killing by cathelicidin antimicrobial peptides. J. Biol. Chem. 287, 40 891–40 897. ( 10.1074/jbc.M112.404582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frick IM, Akesson P, Rasmussen M, Schmidtchen A, Bjorck L. 2003. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 278, 16 561–16 566. ( 10.1074/jbc.M301995200) [DOI] [PubMed] [Google Scholar]
- 39.Akesson P, Sjoholm AG, Bjorck L. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271, 1081–1088. ( 10.1074/jbc.271.2.1081) [DOI] [PubMed] [Google Scholar]
- 40.Lauth X, von Kockritz-Blickwede M, McNamara CW, Myskowski S, Zinkernagel AS, Beall B, Ghosh P, Gallo RL, Nizet V. 2009. M1 protein allows group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J. Innate Immun. 1, 202–214. ( 10.1159/000203645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maisey HC, Quach D, Hensler ME, Liu GY, Gallo RL, Nizet V, Doran KS. 2008. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 22, 1715–1724. ( 10.1096/fj.07-093963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. ( 10.1126/science.284.5418.1318) [DOI] [PubMed] [Google Scholar]
- 43.Otto M. 2006. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr. Top. Microbiol. Immunol. 306, 251–258. ( 10.1007/3-540-29916-5_10) [DOI] [PubMed] [Google Scholar]
- 44.Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39. ( 10.1016/S0966-842X(00)01913-2) [DOI] [PubMed] [Google Scholar]
- 45.Nickel JC, Ruseska I, Wright JB, Costerton JW. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27, 619–624. ( 10.1128/AAC.27.4.619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otto M. 2012. Molecular basis of Staphylococcus epidermidis infections. Semin. Immunopathol. 34, 201–214. ( 10.1007/s00281-011-0296-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Preston JF III, Romeo T. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186, 2724–2734. ( 10.1128/JB.186.9.2724-2734.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 6, 269–275. ( 10.1046/j.1462-5822.2004.00367.x) [DOI] [PubMed] [Google Scholar]
- 49.Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, Otto M. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 279, 54 881–54 886. ( 10.1074/jbc.M411374200) [DOI] [PubMed] [Google Scholar]
- 50.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20, 1083–1091. ( 10.1111/j.1365-2958.1996.tb02548.x) [DOI] [PubMed] [Google Scholar]
- 51.Rupp ME, Ulphani JS, Fey PD, Bartscht K, Mack D. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67, 2627–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 72, 7107–7114. ( 10.1128/Iai.72.12.7107-7114.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llobet E, Tomas JM, Bengoechea JA. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 154, 3877–3886. ( 10.1099/mic.0.2008/022301-0) [DOI] [PubMed] [Google Scholar]
- 54.Cole JN, Pence MA, von Kockritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, Nizet V. 2010. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. MBio 1, e00191. ( 10.1128/mBio.00191-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchanan JT, Stannard JA, Lauth X, Ostland VE, Powell HC, Westerman ME, Nizet V. 2005. Streptococcus iniae phosphoglucomutase is a virulence factor and a target for vaccine development. Infect. Immun. 73, 6935–6944. ( 10.1128/IAI.73.10.6935-6944.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans LR, Linker A. 1973. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 116, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoiby N. 1974. Pseudomonas aeruginosa infection in cystic fibrosis. Relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 82, 551–558.4213330 [Google Scholar]
- 58.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183, 5395–5401. ( 10.1128/JB.183.18.5395-5401.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foschiatti M, Cescutti P, Tossi A, Rizzo R. 2009. Inhibition of cathelicidin activity by bacterial exopolysaccharides. Mol. Microbiol. 72, 1137–1146. ( 10.1111/j.1365-2958.2009.06707.x) [DOI] [PubMed] [Google Scholar]
- 60.Herasimenka Y, Benincasa M, Mattiuzzo M, Cescutti P, Gennaro R, Rizzo R. 2005. Interaction of antimicrobial peptides with bacterial polysaccharides from lung pathogens. Peptides 26, 1127–1132. ( 10.1016/j.peptides.2005.01.020) [DOI] [PubMed] [Google Scholar]
- 61.Spinosa MR, Progida C, Tala A, Cogli L, Alifano P, Bucci C. 2007. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect. Immun. 75, 3594–3603. ( 10.1128/IAI.01945-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones A, Georg M, Maudsdotter L, Jonsson AB. 2009. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J. Bacteriol. 191, 3861–3868. ( 10.1128/JB.01313-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogunleye A, Bhat A, Irorere VU, Hill D, Williams C, Radecka I. 2015. Poly-gamma-glutamic acid: production, properties and applications. Microbiology 161, 1–17. ( 10.1099/mic.0.081448-0) [DOI] [PubMed] [Google Scholar]
- 64.Kocianova S, Vuong C, Yao Y, Voyich JM, Fischer ER, DeLeo FR, Otto M. 2005. Key role of poly-gamma-dl-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Invest. 115, 688–694. ( 10.1172/JCI23523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candela T, Fouet A. 2006. Poly-gamma-glutamate in bacteria. Mol. Microbiol. 60, 1091–1098. ( 10.1111/j.1365-2958.2006.05179.x) [DOI] [PubMed] [Google Scholar]
- 66.Otto M. 2008. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322, 207–228. ( 10.1007/978-3-540-75418-3_10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao Y, Sturdevant DE, Otto M. 2005. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J. Infect. Dis. 191, 289–298. ( 10.1086/426945) [DOI] [PubMed] [Google Scholar]
- 68.Batoni G, Maisetta G, Brancatisano FL, Esin S, Campa M. 2011. Use of antimicrobial peptides against microbial biofilms: advantages and limits. Curr. Med. Chem. 18, 256–279. ( 10.2174/092986711794088399) [DOI] [PubMed] [Google Scholar]
- 69.Strempel N, Strehmel J, Overhage J. 2015. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr. Pharm. Des. 21, 67–84. ( 10.2174/1381612820666140905124312) [DOI] [PubMed] [Google Scholar]
- 70.Joo HS, Otto M. 2012. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 19, 1503–1513. ( 10.1016/j.chembiol.2012.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Luca M, Maccari G, Nifosi R. 2014. Treatment of microbial biofilms in the post-antibiotic era: prophylactic and therapeutic use of antimicrobial peptides and their design by bioinformatics tools. Pathog. Dis. 70, 257–270. ( 10.1111/2049-632X.12151) [DOI] [PubMed] [Google Scholar]
- 72.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414 ( 10.1101/cshperspect.a000414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, Gotz F. 2007. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J. Bacteriol. 189, 280–283. ( 10.1128/JB.01221-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kojima N, Araki Y, Ito E. 1985. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J. Bacteriol. 161, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410. ( 10.1074/jbc.274.13.8405) [DOI] [PubMed] [Google Scholar]
- 76.Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, Shai Y. 2012. d-Alanylation of lipoteichoic acids confers resistance to cationic peptides in Group B Streptococcus by increasing the cell wall density. PLoS Pathog. 8, e1002891 ( 10.1371/journal.ppat.1002891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, Trieu-Cuot P. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43, 1–14. ( 10.1046/j.1365-2958.2002.02723.x) [DOI] [PubMed] [Google Scholar]
- 78.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. 2005. d-Alanylation of teichoic acids promotes Group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187, 6719–6725. ( 10.1128/JB.187.19.6719-6725.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fabretti F, Theilacker C, Baldassarri L, Kaczynski Z, Kropec A, Holst O, Huebner J. 2006. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect. Immun. 74, 4164–4171. ( 10.1128/IAI.00111-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kovacs M, Halfmann A, Fedtke I, Heintz M, Peschel A, Vollmer W, Hakenbeck R, Bruckner R. 2006. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in Gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 188, 5797–5805. ( 10.1128/JB.00336-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan KG, Mayer M, Davis EM, Halperin SA, Lin TJ, Lee SF. 2007. Role of d-alanylation of Streptococcus gordonii lipoteichoic acid in innate and adaptive immunity. Infect. Immun. 75, 3033–3042. ( 10.1128/IAI.01549-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abi Khattar Z, et al. 2009. The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. J. Bacteriol. 191, 7063–7073. ( 10.1128/JB.00892-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Revilla-Guarinos A, Gebhard S, Alcantara C, Staron A, Mascher T, Zuniga M. 2013. Characterization of a regulatory network of peptide antibiotic detoxification modules in Lactobacillus casei BL23. Appl. Environ. Microbiol. 79, 3160–3170. ( 10.1128/AEM.00178-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taneja NK, Ganguly T, Bakaletz LO, Nelson KJ, Dubey P, Poole LB, Deora R. 2013. d-Alanine modification of a protease-susceptible outer membrane component by the Bordetella pertussis dra locus promotes resistance to antimicrobial peptides and polymorphonuclear leukocyte-mediated killing. J. Bacteriol. 195, 5102–5111. ( 10.1128/JB.00510-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carvalho F, Atilano ML, Pombinho R, Covas G, Gallo RL, Filipe SR, Sousa S, Cabanes D. 2015. l-Rhamnosylation of Listeria monocytogenes wall teichoic acids promotes resistance to antimicrobial peptides by delaying interaction with the membrane. PLoS Pathog. 11, e1004919 ( 10.1371/journal.ppat.1004919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Heijenoort J. 2007. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 71, 620–635. ( 10.1128/MMBR.00016-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kahne D, Leimkuhler C, Lu W, Walsh C. 2005. Glycopeptide and lipoglycopeptide antibiotics. Chem. Rev. 105, 425–448. ( 10.1021/cr030103a) [DOI] [PubMed] [Google Scholar]
- 88.Bugg TD, Wright GD, Dutka-Malen S, Arthur M, Courvalin P, Walsh CT. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30, 10 408–10 415. ( 10.1021/bi00107a007) [DOI] [PubMed] [Google Scholar]
- 89.Brotz H, Josten M, Wiedemann I, Schneider U, Gotz F, Bierbaum G, Sahl HG. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30, 317–327. ( 10.1046/j.1365-2958.1998.01065.x) [DOI] [PubMed] [Google Scholar]
- 90.Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl HG, de Kruijff B. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286, 2361–2364. ( 10.1126/science.286.5448.2361) [DOI] [PubMed] [Google Scholar]
- 91.de Leeuw E, Li C, Zeng P, Li C, Diepeveen-de Buin M, Lu WY, Breukink E, Lu W. 2010. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 584, 1543–1548. ( 10.1016/j.febslet.2010.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sass V, Schneider T, Wilmes M, Korner C, Tossi A, Novikova N, Shamova O, Sahl HG. 2010. Human beta-defensin 3 inhibits cell wall biosynthesis in staphylococci. Infect. Immun. 78, 2793–2800. ( 10.1128/IAI.00688-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bera A, Biswas R, Herbert S, Gotz F. 2006. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect. Immun. 74, 4598–4604. ( 10.1128/IAI.00301-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vollmer W, Tomasz A. 2000. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J. Biol. Chem. 275, 20 496–20 501. ( 10.1074/jbc.M910189199) [DOI] [PubMed] [Google Scholar]
- 95.Whitfield C, Trent MS. 2014. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 83, 99–128. ( 10.1146/annurev-biochem-060713-035600) [DOI] [PubMed] [Google Scholar]
- 96.Moskowitz SM, Ernst RK, Miller SI. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186, 575–579. ( 10.1128/JB.186.2.575-579.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M, Miller SI. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27, 1171–1182. ( 10.1046/j.1365-2958.1998.00757.x) [DOI] [PubMed] [Google Scholar]
- 98.Llewellyn AC, et al. 2012. NaxD is a deacetylase required for lipid A modification and Francisella pathogenesis. Mol. Microbiol. 86, 611–627. ( 10.1111/mmi.12004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanistanon D, et al. 2008. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 4, e24 ( 10.1371/journal.ppat.0040024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shah NR, Hancock RE, Fernandez RC. 2014. Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation. Antimicrob. Agents Chemother. 58, 4931–4934. ( 10.1128/AAC.02590-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK. 2013. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 57, 4831–4840. ( 10.1128/AAC.00865-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee H, Hsu FF, Turk J, Groisman EA. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 186, 4124–4133. ( 10.1128/JB.186.13.4124-4133.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lewis LA, Shafer WM, Dutta Ray T, Ram S, Rice PA. 2013. Phosphoethanolamine residues on the lipid A moiety of Neisseria gonorrhoeae lipooligosaccharide modulate binding of complement inhibitors and resistance to complement killing. Infect. Immun. 81, 33–42. ( 10.1128/IAI.00751-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. 2012. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc. Natl Acad. Sci. USA 109, 8722–8727. ( 10.1073/pnas.1201313109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loutet SA, Valvano MA. 2011. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front. Microbiol. 2, 159 ( 10.3389/fmicb.2011.00159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tzeng YL, Ambrose KD, Zughaier S, Zhou X, Miller YK, Shafer WM, Stephens DS. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 187, 5387–5396. ( 10.1128/JB.187.15.5387-5396.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CR. 2004. MsbA transporter-dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of Francisella novicida LpxE expressed in Escherichia coli. J. Biol. Chem. 279, 49 470–49 478. ( 10.1074/jbc.M409078200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang X, McGrath SC, Cotter RJ, Raetz CR. 2006. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J. Biol. Chem. 281, 9321–9330. ( 10.1074/jbc.M600435200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones JW, Shaffer SA, Ernst RK, Goodlett DR, Turecek F. 2008. Determination of pyrophosphorylated forms of lipid A in Gram-negative bacteria using a multivaried mass spectrometric approach. Proc. Natl Acad. Sci. USA 105, 12 742–12 747. ( 10.1073/pnas.0800445105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Touze T, Tran AX, Hankins JV, Mengin-Lecreulx D, Trent MS. 2008. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol. Microbiol. 67, 264–277. ( 10.1111/j.1365-2958.2007.06044.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karbarz MJ, Kalb SR, Cotter RJ, Raetz CR. 2003. Expression cloning and biochemical characterization of a Rhizobium leguminosarum lipid A 1-phosphatase. J. Biol. Chem. 278, 39 269–39 279. ( 10.1074/jbc.M305830200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trent MS. 2004. Biosynthesis, transport, and modification of lipid A. Biochem. Cell Biol. 82, 71–86. ( 10.1139/o03-070) [DOI] [PubMed] [Google Scholar]
- 113.Herrera CM, Hankins JV, Trent MS. 2010. Activation of PmrA inhibits LpxT-dependent phosphorylation of lipid A promoting resistance to antimicrobial peptides. Mol. Microbiol. 76, 1444–1460. ( 10.1111/j.1365-2958.2010.07150.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cullen TW, et al. 2015. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347, 170–175. ( 10.1126/science.1260580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, Hackett M, Miller SI. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95, 189–198. ( 10.1016/S0092-8674(00)81750-X) [DOI] [PubMed] [Google Scholar]
- 116.Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CR. 2000. Transfer of palmitate from phospholipids to lipid A in outer membranes of Gram-negative bacteria. EMBO J. 19, 5071–5080. ( 10.1093/emboj/19.19.5071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matson JS, Yoo HJ, Hakansson K, Dirita VJ. 2010. Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by MsbB. J. Bacteriol. 192, 2044–2052. ( 10.1128/JB.00023-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boll JM, Tucker AT, Klein DR, Beltran AM, Brodbelt JS, Davies BW, Trent MS. 2015. Reinforcing lipid A acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. MBio 6, e00478. ( 10.1128/mBio.00478-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loutet SA, Flannagan RS, Kooi C, Sokol PA, Valvano MA. 2006. A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to antimicrobial peptides and bacterial survival in vivo. J. Bacteriol. 188, 2073–2080. ( 10.1128/JB.188.6.2073-2080.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allen CA, Adams LG, Ficht TA. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66, 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim JK, et al. 2015. Insect gut symbiont susceptibility to host antimicrobial peptides caused by alteration of the bacterial cell envelope. J. Biol. Chem. 290, 21 042–21 053. ( 10.1074/jbc.M115.651158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Masson F, Zaidman-Rémy A, Heddi A. 2016. Antimicrobial peptides and cell processes tracking endosymbiont dynamics. Phil. Trans. R. Soc. B 371, 20150298 ( 10.1098/rstb.2015.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peschel A, et al. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193, 1067–1076. ( 10.1084/jem.193.9.1067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ernst CM, Staubitz P, Mishra NN, Yang SJ, Hornig G, Kalbacher H, Bayer AS, Kraus D, Peschel A. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 5, e1000660 ( 10.1371/journal.ppat.1000660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ernst CM, Kuhn S, Slavetinsky CJ, Krismer B, Heilbronner S, Gekeler C, Kraus D, Wagner S, Peschel A. 2015. The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits. MBio 6, e02340. ( 10.1128/mBio.02340-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ernst CM, Peschel A. 2011. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 80, 290–299. ( 10.1111/j.1365-2958.2011.07576.x) [DOI] [PubMed] [Google Scholar]
- 127.Thedieck K, Hain T, Mohamed W, Tindall BJ, Nimtz M, Chakraborty T, Wehland J, Jansch L. 2006. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 62, 1325–1339. ( 10.1111/j.1365-2958.2006.05452.x) [DOI] [PubMed] [Google Scholar]
- 128.Klein S, et al. 2009. Adaptation of Pseudomonas aeruginosa to various conditions includes tRNA-dependent formation of alanyl-phosphatidylglycerol. Mol. Microbiol. 71, 551–565. ( 10.1111/j.1365-2958.2008.06562.x) [DOI] [PubMed] [Google Scholar]
- 129.Johnston NC, Baker JK, Goldfine H. 2004. Phospholipids of Clostridium perfringens: a reexamination. FEMS Microbiol. Lett. 233, 65–68. ( 10.1016/j.femsle.2004.01.048) [DOI] [PubMed] [Google Scholar]
- 130.LaRock CN, Nizet V. 2015. Cationic antimicrobial peptide resistance mechanisms of streptococcal pathogens. Biochim. Biophys. Acta 1848, 3047–3054. ( 10.1016/j.bbamem.2015.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones DE, Smith JD. 1979. Phospholipids of the differentiating bacterium Caulobacter crescentus. Can. J. Biochem. 57, 424–428. ( 10.1139/o79-054) [DOI] [PubMed] [Google Scholar]
- 132.Sohlenkamp C, Galindo-Lagunas KA, Guan Z, Vinuesa P, Robinson S, Thomas-Oates J, Raetz CR, Geiger O. 2007. The lipid lysyl-phosphatidylglycerol is present in membranes of Rhizobium tropici CIAT899 and confers increased resistance to polymyxin B under acidic growth conditions. Mol. Plant Microbe Interact. 20, 1421–1430. ( 10.1094/MPMI-20-11-1421) [DOI] [PubMed] [Google Scholar]
- 133.Dalebroux ZD, Matamouros S, Whittington D, Bishop RE, Miller SI. 2014. PhoPQ regulates acidic glycerophospholipid content of the Salmonella typhimurium outer membrane. Proc. Natl Acad. Sci. USA 111, 1963–1968. ( 10.1073/pnas.1316901111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Band VI, Weiss DS. 2015. Mechanisms of antimicrobial peptide resistance in Gram-negative bacteria. Antibiotics (Basel) 4, 18–41. ( 10.3390/antibiotics4010018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumariya R, Sood SK, Rajput YS, Saini N, Garsa AK. 2015. Increased membrane surface positive charge and altered membrane fluidity leads to cationic antimicrobial peptide resistance in Enterococcus faecalis. Biochim. Biophys. Acta 1848, 1367–1375. ( 10.1016/j.bbamem.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 136.Mazzotta AS, Montville TJ. 1997. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10 degrees C and 30 degrees C. J. Appl. Microbiol. 82, 32–38. ( 10.1111/j.1365-2672.1997.tb03294.x) [DOI] [PubMed] [Google Scholar]
- 137.Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, McKinnell J, Bayer AS. 2011. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 55, 526–531. ( 10.1128/AAC.00680-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bayer AS, et al. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68, 3548–3553. ( 10.1128/IAI.68.6.3548-3553.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Piddock LJ. 2006. Multidrug-resistance efflux pumps - not just for resistance. Nat. Rev. Microbiol. 4, 629–636. ( 10.1038/nrmicro1464) [DOI] [PubMed] [Google Scholar]
- 140.Delmar JA, Su CC, Yu EW. 2014. Bacterial multidrug efflux transporters. Annu. Rev. Biophys. 43, 93–117. ( 10.1146/annurev-biophys-051013-022855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Piddock LJ. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19, 382–402. ( 10.1128/CMR.19.2.382-402.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruggerone P, Murakami S, Pos KM, Vargiu AV. 2013. RND efflux pumps: structural information translated into function and inhibition mechanisms. Curr. Top. Med. Chem. 13, 3079–3100. ( 10.2174/15680266113136660220) [DOI] [PubMed] [Google Scholar]
- 143.Shafer WM, Qu X, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl Acad. Sci. USA 95, 1829–1833. ( 10.1073/pnas.95.4.1829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Padilla E, Llobet E, Domenech-Sanchez A, Martinez-Martinez L, Bengoechea JA, Alberti S. 2010. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 54, 177–183. ( 10.1128/AAC.00715-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bina XR, Provenzano D, Nguyen N, Bina JE. 2008. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect. Immun. 76, 3595–3605. ( 10.1128/IAI.01620-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rieg S, Huth A, Kalbacher H, Kern WV. 2009. Resistance against antimicrobial peptides is independent of Escherichia coli AcrAB, Pseudomonas aeruginosa MexAB and Staphylococcus aureus NorA efflux pumps. Int. J. Antimicrob Agents 33, 174–176. ( 10.1016/j.ijantimicag.2008.07.032) [DOI] [PubMed] [Google Scholar]
- 147.Bengoechea JA, Skurnik M. 2000. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol. Microbiol. 37, 67–80. ( 10.1046/j.1365-2958.2000.01956.x) [DOI] [PubMed] [Google Scholar]
- 148.Kupferwasser LI, Skurray RA, Brown MH, Firth N, Yeaman MR, Bayer AS. 1999. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: role of the qacA locus. Antimicrob. Agents Chemother. 43, 2395–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gebhard S. 2012. ABC transporters of antimicrobial peptides in Firmicutes bacteria – phylogeny, function and regulation. Mol. Microbiol. 86, 1295–1317. ( 10.1111/mmi.12078) [DOI] [PubMed] [Google Scholar]
- 150.Qiao M, Saris PE. 1996. Evidence for a role of NisT in transport of the lantibiotic nisin produced by Lactococcus lactis N8. FEMS Microbiol. Lett. 144, 89–93. ( 10.1111/j.1574-6968.1996.tb08513.x) [DOI] [PubMed] [Google Scholar]
- 151.Otto M, Peschel A, Gotz F. 1998. Producer self-protection against the lantibiotic epidermin by the ABC transporter EpiFEG of Staphylococcus epidermidis Tu3298. FEMS Microbiol. Lett. 166, 203–211. [DOI] [PubMed] [Google Scholar]
- 152.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. 2007. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66, 1136–1147. ( 10.1111/j.1365-2958.2007.05986.x) [DOI] [PubMed] [Google Scholar]
- 153.Falord M, Karimova G, Hiron A, Msadek T. 2012. GraXSR proteins interact with the VraFG ABC transporter to form a five-component system required for cationic antimicrobial peptide sensing and resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 56, 1047–1058. ( 10.1128/AAC.05054-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hiron A, Falord M, Valle J, Debarbouille M, Msadek T. 2011. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 81, 602–622. ( 10.1111/j.1365-2958.2011.07735.x) [DOI] [PubMed] [Google Scholar]
- 155.Yoshida Y, Matsuo M, Oogai Y, Kato F, Nakamura N, Sugai M, Komatsuzawa H. 2011. Bacitracin sensing and resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 320, 33–39. ( 10.1111/j.1574-6968.2011.02291.x) [DOI] [PubMed] [Google Scholar]
- 156.Kolar SL, et al. 2011. NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 157, 2206–2219. ( 10.1099/mic.0.049692-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ohki R, Giyanto, Tateno K, Masuyama W, Moriya S, Kobayashi K, Ogasawara N. 2003. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 49, 1135–1144. ( 10.1046/j.1365-2958.2003.03653.x) [DOI] [PubMed] [Google Scholar]
- 158.Becker P, Hakenbeck R, Henrich B. 2009. An ABC transporter of Streptococcus pneumoniae involved in susceptibility to vancoresmycin and bacitracin. Antimicrob. Agents Chemother. 53, 2034–2041. ( 10.1128/AAC.01485-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Collins B, Curtis N, Cotter PD, Hill C, Ross RP. 2010. The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various beta-lactam antibiotics. Antimicrob. Agents Chemother. 54, 4416–4423. ( 10.1128/AAC.00503-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nawrocki KL, Crispell EK, McBride SM. 2014. Antimicrobial peptide resistance mechanisms of Gram-positive bacteria. Antibiotics (Basel) 3, 461–492. ( 10.3390/antibiotics3040461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ambrose KD, Nisbet R, Stephens DS. 2005. Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrob. Agents Chemother. 49, 4203–4209. ( 10.1128/AAC.49.10.4203-4209.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zahner D, Zhou X, Chancey ST, Pohl J, Shafer WM, Stephens DS. 2010. Human antimicrobial peptide LL-37 induces MefE/Mel-mediated macrolide resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 54, 3516–3519. ( 10.1128/AAC.01756-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. 2007. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl Acad. Sci. USA 104, 9469–9474. ( 10.1073/pnas.0702159104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eldholm V, Gutt B, Johnsborg O, Bruckner R, Maurer P, Hakenbeck R, Mascher T, Havarstein LS. 2010. The pneumococcal cell envelope stress-sensing system LiaFSR is activated by murein hydrolases and lipid II-interacting antibiotics. J. Bacteriol. 192, 1761–1773. ( 10.1128/JB.01489-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Suntharalingam P, Senadheera MD, Mair RW, Levesque CM, Cvitkovitch DG. 2009. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J. Bacteriol. 191, 2973–2984. ( 10.1128/JB.01563-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miller SI, Kukral AM, Mekalanos JJ. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl Acad. Sci. USA 86, 5054–5058. ( 10.1073/pnas.86.13.5054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Groisman EA, Chiao E, Lipps CJ, Heffron F. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl Acad. Sci. USA 86, 7077–7081. ( 10.1073/pnas.86.18.7077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gunn JS, Miller SI. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178, 6857–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122, 461–472. ( 10.1016/j.cell.2005.05.030) [DOI] [PubMed] [Google Scholar]
- 170.Oyston PC, Dorrell N, Williams K, Li SR, Green M, Titball RW, Wren BW. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68, 3419–3425. ( 10.1128/IAI.68.6.3419-3425.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rebeil R, Ernst RK, Gowen BB, Miller SI, Hinnebusch BJ. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 52, 1363–1373. ( 10.1111/j.1365-2958.2004.04059.x) [DOI] [PubMed] [Google Scholar]
- 172.Moss JE, Fisher PE, Vick B, Groisman EA, Zychlinsky A. 2000. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell Microbiol. 2, 443–452. ( 10.1046/j.1462-5822.2000.00065.x) [DOI] [PubMed] [Google Scholar]
- 173.Pontes MH, Smith KL, De Vooght L, Van Den Abbeele J, Dale C. 2011. Attenuation of the sensing capabilities of PhoQ in transition to obligate insect–bacterial association. PLoS Genet. 7, e1002349 ( 10.1371/journal.pgen.1002349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Perron GG, Zasloff M, Bell G. 2006. Experimental evolution of resistance to an antimicrobial peptide. Proc. R. Soc. B 273, 251–256. ( 10.1098/rspb.2005.3301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Juhas M. 2015. Horizontal gene transfer in human pathogens. Crit. Rev. Microbiol. 41, 101–108. ( 10.3109/1040841X.2013.804031) [DOI] [PubMed] [Google Scholar]
- 176.Baltz RH. 2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13, 144–151. ( 10.1016/j.cbpa.2009.02.031) [DOI] [PubMed] [Google Scholar]