Abstract
Aquaculture contributes more than one-third of the animal protein from marine sources worldwide. A significant proportion of aquaculture products are derived from marine protostomes that are commonly referred to as ‘marine invertebrates’. Among them, penaeid shrimp (Ecdysozosoa, Arthropoda) and bivalve molluscs (Lophotrochozoa, Mollusca) are economically important. Mass rearing of arthropods and molluscs causes problems with pathogens in aquatic ecosystems that are exploited by humans. Remarkably, species of corals (Cnidaria) living in non-exploited ecosystems also suffer from devastating infectious diseases that display intriguing similarities with those affecting farmed animals. Infectious diseases affecting wild and farmed animals that are present in marine environments are predicted to increase in the future. This paper summarizes the role of the main pathogens and their interaction with host immunity, with a specific focus on antimicrobial peptides (AMPs) and pathogen resistance against AMPs. We provide a detailed review of penaeid shrimp AMPs and their role at the interface between the host and its resident/pathogenic microbiota. We also briefly describe the relevance of marine invertebrate AMPs in an applied context.
This article is part of the themed issue ‘Evolutionary ecology of arthropod antimicrobial peptides’.
Keywords: invertebrate immunity, polymicrobial disease, vibrio, immune suppression, resistance, abiotic stress
1. Economic relevance of marine invertebrates
Oceans and seas cover two-thirds of our planet; provide ecosystem services, such as fishing, aquaculture, carbon sequestration, regulation of water quality and nutrient storage; and support numerous recreational activities, all of which significantly contribute to employment and economic activity [1]. In 2012, aquaculture provided an unprecedented total of 66.6 million tons of seafood, including 175 species of ‘marine invertebrates’, mainly crustaceans (Ecdysozosoa, Arthropoda) and molluscs (Lophotrochozoa, Mollusca) [2]. Farmed crustaceans accounted for 9.7% (6.4 million tons) of the seafood production by volume, but 22.4% (30.9 billion USD) by value. Shrimp is currently the largest single commodity in terms of value, with the main cultivated species being Litopenaeus vannamei and Penaeus monodon. In 2012, molluscs, such as oysters, mussels, scallops and clams, accounted for 20.5% (13.7 million tons) of seafood production. Molluscs are essentially produced for food, but they also contributed to 22 400 tons of non-food products, such as pearls and seashells for ornamental and decorative uses. However, disease outbreaks have impacted heavily on this intensifying production over the past few years, and emerging infectious diseases are predicted to increase in the future in both wild and farmed animals as a result of climate change [3].
2. Infectious diseases affecting marine invertebrates
The health status of marine invertebrates is intimately related to the microbial communities that are present in the aquatic environment, which include both commensals and opportunistic pathogens. While microorganisms hosted by invertebrates help maintain homeostasis, under stressful conditions some can become highly virulent and severely damage their host [4]. In coastal environments and lagoons, marine invertebrates are exposed to multiple abiotic stresses, which can be of anthropogenic origin. Thermal stress, high density [5] and nutrient-rich environments [6] are factors favouring infectious diseases in the wild as well as in intensive farming. Remarkably, while marine invertebrates are incredibly diverse in terms of phylogeny and ecological niche, disease patterns are repeatedly found across species. These diseases include temperature-dependent vibrioses [7,8] and polymicrobial diseases [9,10]. Differences are observed in the susceptibility of the animals, from larvae to juveniles and adults, and in the diversity of the pathogens (vibrios, viruses, etc.) that affect each developmental stage. Relevant examples of infectious diseases are presented below within three different phyla of marine invertebrates (Arthropoda, Mollusca and Cnidaria) that are directly/indirectly and intensively/extensively exploited by humans in a diversity of ecosystems.
Arthropods. Diseases in marine arthropods are dominated by those described in penaeid shrimp aquaculture, which is characterized by intensive cultural practices favouring disease development. Twenty viruses are known to infect penaeid shrimp. Two types of viruses cause major viral diseases, namely, DNA viruses, such as the monodon baculovirus [11], the white-spot syndrome virus (WSSV) [12], the hepatopancreatic parvovirus and the infectious hypodermal and haematopoietic virus [13], and RNA viruses, such as the yellow-head virus, the Taura syndrome virus [14] and the infectious myonecrosis virus [15]. WSSV is the most severe threat for farmed adult shrimp worldwide and is one of the best-studied crustacean viruses [16]. Bacterial infections, particularly vibrioses, are a major concern for the production of shrimp larvae and juveniles. Vibrio harveyi and V. vulnificus are associated with larvae mortality [17], whereas V. damsela, V. alginolyticus, V. parahaemolyticus, V. penaeicida and V. nichripulchritudo cause disease outbreaks in shrimp nurseries or grow-out ponds [17]. In 2010, a new shrimp disease that affects postlarvae has emerged from Asia [18]. This acute hepatopancreatic necrosis disease is caused by a highly virulent strain of V. parahaemolyticus, which has acquired a virulent plasmid encoding a pore-forming bacterial toxin that is as toxic as the insecticidal Bacillus Cry toxin [18].
Molluscs from the shellfish industry are affected by a variety of infectious diseases whose importance largely depends on the degree of exploitation and ecosystem health. The most significant epizootic events are caused by bacteria from the Vibrio genus [19,20], viruses from the Malacoherpesviridae family [21], and protozoans from the Perkinsus, Marteilia, Bonamia and Haplosporidium genera [17]. Some of these microorganisms can affect a broad range of mollusc species at all life stages around the world, while others are highly species-specific. The epizootic events that they cause are frequently devastating. Over the past few decades, abnormal mortalities of juvenile C. gigas oysters have affected the USA, Japan, Australia and Western Europe. Those mortalities of complex aetiology are due to a temperature-dependent polymicrobial disease that involves pathogenic Vibrio strains of the Splendidus clade and the ostreid herpes virus OsHV-1 µvar [9,17,22]. Vibrios also cause diseases at other oyster developmental stages. Vibrio aestuarianus is responsible for mortality of adult oysters [23], whereas V. tubiashi causes a necrotic disease in hatcheries [24].
Cnidarians, which live in coastal marine systems and lagoons, also suffer from infectious diseases. Among the known pathogens, all of the classical agents (eubacteria, cyanobacteria, fungi and viruses) have been described [25,26]. Most diseases were observed in corals. One of the most famous coral diseases is Type I White Band Disease (WBD). Described for the first time in the 1970s, WBD caused the loss of up to 95% of the acroporids found throughout the great Caribbean area [27]. However, as with many coral diseases, the causative agents of Type I WBD are unknown and do not fully satisfy the Koch postulate or correspond to microbial consortia [25]. Another well-known example is Black Band Disease, which is caused by cyanobacteria assemblages and other unidentified heterotrophic microbes [10,28]. Many of the known coral pathogens belong to the Vibrio genus. While V. harveyi/charcariae is the causative agent of Type II WBD and other ‘White Syndromes’ [29,30], V. shiloi and V. coralliilyticus are responsible for bacteria-induced bleaching in corals and tissue lysis in several other cnidarians [31–34].
3. Antimicrobial peptides in marine invertebrate immunity, a focus on penaeid shrimp
The economic consequences of infectious diseases affecting farmed bivalve molluscs and arthropods (crustaceans) have motivated a substantial research effort, which has considerably enriched our knowledge of the immune system of protostomes. Similarly, coral diseases are a threat for society and have inspired studies on the interaction of the immune system of cnidarians with its resident microbiota and pathogens. To date, antimicrobial peptides (AMPs) are among the best-described immune effectors of marine invertebrates.
(a). Diversity and specificity of AMPs in the immune response of marine invertebrates
Similar to AMPs from other phyla [35], most of the AMPs characterized in marine invertebrates including arthropods, molluscs and cnidarians are cationic and hydrophobic, and target essential components of microbial cell walls and membranes, which determines their spectrum of activity [36]. A high diversity of mechanisms of action has been reported for AMPs from marine invertebrates and is described in detail for some families. For example, mollusc defensins, which are essentially active against Gram-positive bacteria, bind to lipid II, the precursor of peptidoglycan [37]. Arthropod anti-lipopolysaccharide factors (ALFs) and mollusc bactericidal/permeability-increasing protein (BPI), which are essentially active against Gram-negative bacteria, bind to lipopolysaccharide (LPS) [38–40]. Finally, crustacean PvHCt, which is strictly antifungal, permeabilizes the fungal plasma membrane [41].
Those AMP families have been generated through different patterns of diversification (gene duplication, gene copy number variation, recombination and allelic polymorphisms) [36] due to multiple evolutionary drivers and have given rise to functional divergence, as also observed for insect AMPs [42]. Some families appear to have evolved within specific phyla or species [31], whereas others are found in a diversity of phyla [32]. Most AMP families from molluscs, including defensins, big defensins and BPI (for recent review, see [33]), are also found in other protostomes (Ecdysozoa) and/or in deuterostomes (Mammalia). By contrast, in cnidarians, taxonomically restricted AMPs have been described, such as arminins, which are among the most highly expressed genes in Hydra [34], or damicornin in the coral Pocillopora damicornis [43]. In marine arthropods, the pioneering studies in Chelicerata (horseshoe crabs) have identified both taxonomically restricted (tachyplesins, ALFs) and more widely distributed AMPs (big defensins) [39,44,45]. Finally, in decapod crustaceans, highly diverse AMPs were characterized that are specific to penaeid shrimp (penaeidins, stylicins) or more widespread among arthropods (crustins, ALFs) [42,46,47]. The best-known AMPs from crustaceans were characterized in penaeid shrimp, and many of them are composed of structural domains that have distinct biological functions [46,48]. A description of the major AMP families in shrimp is provided below. Unlike in insects, there is still little knowledge about the molecular regulation of AMPs in marine invertebrates. In shrimp, which is one of the best-described organisms, some AMP families are controlled by NF-κB signalling pathways. Alternatively, mature AMPs can be stored in immune cells and are released upon challenge (see below).
(b). Gene-encoded AMPs from penaeid shrimp
(i). Penaeidins
Penaeidins were the first AMPs characterized in shrimp [49]. Those peptides, which are restricted to species of penaeid shrimp, are abundant in the circulating immune cells (the haemocytes) of L. vannamei [49]. They are cationic peptides (4.7–7.2 kDa; pI approx. 9) composed of an unconstrained N-terminal proline/arginine-rich domain followed by a C-terminal domain that contains an amphipathic helix and two coils stabilized by three disulfide bonds (table 1) [49,50]. Penaeidins can carry post-translational modifications, such as an N-terminal pyroglutamic acid and an amidated C-terminus. AMPs from this diverse family fall into four subgroups (PEN1/2, PEN3, PEN4 and PEN5; table 1), whose specific sequence signature and biochemical features have been used to standardize their nomenclature after the name of the shrimp species and the penaeidin subgroup [51]. Each subgroup is encoded by distinct genomic sequences [51,52]. While the PEN3 gene is widely distributed among species of penaeid shrimp, PEN1/2, PEN4 and PEN5 genes are restricted to a given species of shrimp [53]. PEN genes are highly and constitutively expressed in haemocytes of healthy individuals [54,55]. Penaeidins, which are stored in haemocyte granules, are released in response to microbial challenge [55].
Table 1.
Principal families of gene-encoded AMPs in penaeid shrimp.
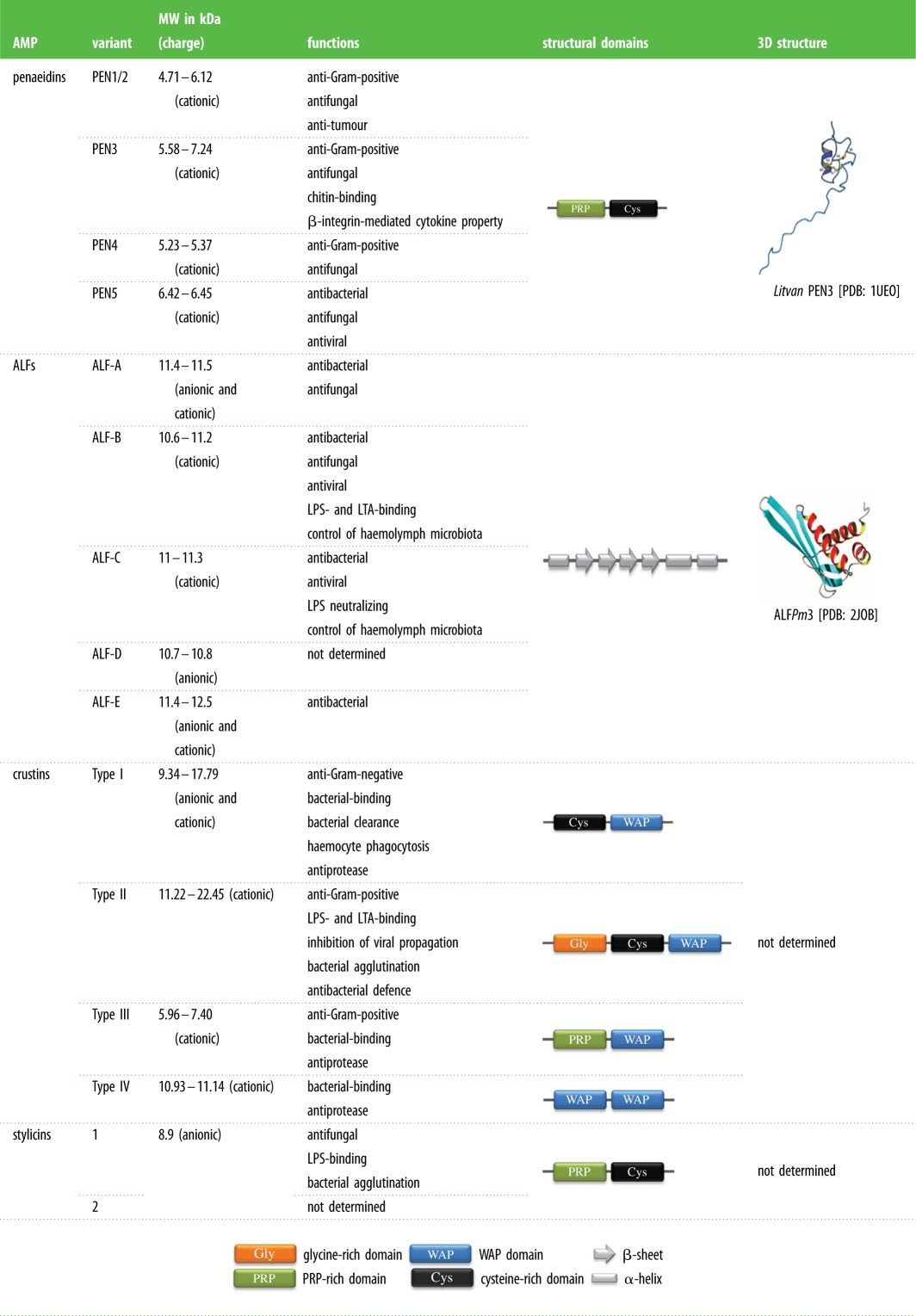 |
Penaeidin sequence diversity translates into diverse biological activities [56]. Most penaeidins from subgroups PEN1/2, PEN3 and PEN4 are active against Gram-positive bacteria and filamentous fungi, but not Gram-negative bacteria. By contrast, a PEN5 member (Fenchi PEN5) from Fenneropenaeus chinensis is active against Gram-negative bacteria [52]. Penmon PEN5 from P. monodon also participates in the shrimp antiviral defence against WSSV [57]. Although little is known about the mechanism of action of penaeidins, the function of the proline/arginine-rich domain was investigated in different penaeidins. This domain was devoid of antimicrobial activity in PEN3 [55], but possessed both antifungal and antibacterial activity in PEN4 [58]. Interestingly, this domain was also reported to behave as a cytokine by attracting haemocytes towards sites of injury [59]. In addition, the cysteine-rich domain of PEN3 was proposed to mediate penaeidin antifungal activity by binding to chitin [55]. The ability of penaeidins to bind to the chitin exoskeleton suggests that penaeidins could play a role in wound healing and/or moulting in shrimp [48].
(ii). Anti-lipopolysaccharide factors
ALFs form a diverse family of AMPs that are composed of both cationic and anionic polypeptides. First identified in horseshoe crabs and later in penaeid shrimp [60] and other crustaceans [53], ALFs were named ‘anti-LPS factors' for their immunomodulatory function. ALFs are able to inhibit the LPS-mediated activation of the limulid coagulation system [39,40]. ALFs contain a hydrophobic N-terminal region with two conserved cysteine residues. The disulfide bond delimits a β-hairpin structure that is referred to as the LPS-binding domain [61]. Most ALFs bind to Lipid A from Gram-negative bacteria, but they can also interact with lipoteichoic acid (LTA) from Gram-positive bacteria [62] and β-glucan from fungi [63]. The known three-dimensional structures of ALFs consist of three α-helices (one at the N-terminus and two at the C-terminus) packed against a four-stranded β-sheet (table 1) [61,64].
In shrimp, ALFs form a large and diverse family of five groups, namely, ALF-A (anionic and cationic polypeptides of 11.4–11.5 kDa), ALF-B (highly cationic polypeptides of 10.6–11.2 kDa), ALF-C (cationic polypeptides of 11–11.3 kDa), ALF-D (highly anionic polypeptides of 10.7–10.8 kDa), and ALF-E (anionic and cationic polypeptides of 11.4–12.5 kDa; table 1) [65,66]. ALFs are encoded by separate genes and are transcribed at basal levels in healthy individuals [65]. In L. vannamei, ALF genes are differentially expressed in response to a fungal infection. Although ALF-A gene expression remains stable, the other ALF genes are inducible [65]. While ALFs can be detected in diverse shrimp tissues, results for ALFPm3 strongly suggest that the expression of ALF-B is restricted to haemocytes, which infiltrate shrimp tissues [62]. ALFs are potent and broad-spectrum AMPs. For example, cationic Group B ALFs are active against Gram-positive, Gram-negative bacteria, yeast, filamentous fungi and some enveloped viruses [46]. By contrast, anionic Group D ALFs have impaired LPS-binding properties and display very low antimicrobial activity in vitro [65].
(iii). Crustins
Crustins are antimicrobial polypeptides (6–22 kDa; pI 4–8) containing a whey acidic protein (WAP) domain [67]. This WAP domain, which is also found in some mammalian proteins, supports different biological functions, including antiprotease activities [68]. Crustins are widely distributed across crustaceans but some sequences have also been discovered in insect genomes [42]. Crustins from crustaceans are composed of four members (Types I–IV), which differ by their N-terminal sequence [67]. While the sequence of Type I crustins begins with a cysteine-rich domain, Type II crustins (subtypes IIa and IIb) harbour a glycine-rich hydrophobic domain at the N-terminal position ahead of the cysteine-rich domain that is also found in Type I crustins (table 1). Comparatively, Type III crustins can contain (or not) a short proline/arginine-domain at the N-terminus, whereas Type IV crustins are composed of two WAP domains, but do not harbour any specific N-terminal sequence (table 1) [67]. Data on crustinPm1 strongly suggest that crustins are mainly expressed by haemocytes [69].
The diversity of crustin sequences supports diverse biological functions. While Type I and II crustins are mainly antimicrobial, their spectrum of activity varies with the crustin type/subtype. For instance, in P. monodon, a Type IIa crustin is essentially active against Gram-positive bacteria, while Type IIb crustins are active against both Gram-positive and Gram-negative bacteria [69,70]. Interestingly, Type III and IV crustins show antimicrobial and/or antiprotease activity [67], whereas Type III crustins lacking the N-terminal proline/arginine-domain and Type IV crustins from shrimp [71,72] only have antiprotease activity [67]. Type IV crustins from crabs exhibit both antimicrobial and antiprotease activities [72,73].
(iv). Stylicins
Stylicins are anionic (pI 5) multi-domain peptides of 8.9 kDa found in penaeid shrimp. They are composed of an N-terminal proline/arginine-rich domain followed by a C-terminal domain containing 13 cysteine residues (table 1) [74]. The recombinant Ls-Stylicin-1 from Litopenaeus stylirostris is active against the filamentous fungus Fusarium oxysporum, but is not antibacterial. As observed for Group B ALFs, Ls-Stylicin-1 shows potent LPS-binding activity and was also able to agglutinate Gram-negative bacteria in vitro [74].
(c). Shrimp AMPs encrypted in multifunctional proteins
(i). Haemocyanin-derived fragments
In addition to gene-encoded AMPs, diverse AMPs from crustaceans are encrypted in large proteins carrying non-immune functions. Haemocyanins are respiratory proteins found in arthropods. Interestingly, crustacean haemocyanins release histidine-rich AMPs in response to microbial challenge [75,76]. In penaeid shrimp, strictly antifungal peptides are released from the C-terminus of haemocyanins [75]. Recently, the three-dimensional structure of the antifungal peptide PvHCt from L. vannamei was determined, and its mechanism of action was investigated. This histidine-rich AMP was shown to selectively bind to the fungal cell wall and permeabilize fungal membranes by adopting an amphipathic α-helical structure. Insertion of PvHCt into the plasma membrane disrupts its integrity as a permeability barrier, leading to a disruption of internal homeostasis and the death of the fungal pathogen [41].
(ii). Histones and derived fragments
Histones are essential protein components of the chromatin architecture. The antimicrobial activity of histones was first described in deuterostomes (mammalians) [77]. Histones and derived fragments are active against Gram-negative and Gram-positive bacteria, fungi and viruses with various modes of action, including the permeabilization of bacterial cell membrane and binding to bacterial DNA and/or RNA [78]. The role of histones in shrimp defence was first determined in L. vannamei [79]. The extracellular release of histones is associated with a defence reaction named ETosis, in which phagocytes release histones associated with extracellular traps (ETs) of DNA that entangle and eventually kill microbes [80]. ETs have now been described in deuterostomes [81] and protostomes, including species of Ecdysozoa (insects [82], crustaceans [83]) and Lophotrochozoa (molluscs [84]). This process is triggered by infection and/or tissue damage. ROS production is a signal that triggers ET formation in mammals [85] and lophotrochozoans [84]. In shrimp, haemocytes also release ETs in response to ROS inducers [83]. It will be of great interest to identify the haemocyte types that are involved in ETosis and determine whether the AMPs that are stored in haemocytes, such as penaeidins, contribute to the antimicrobial activity of shrimp ETs.
4. AMPs in marine invertebrate immune–microbiota interactions
Marine invertebrates host a broad diversity of microorganisms in their tissues and haemolymph, including vibrios [4], which have the potential to become pathogenic and cause severe disease outbreaks (see §2). Some adopt intracellular stages and are able to survive inside phagocytes [86,87]. As illustrated in §3, haemolymph, phagocytes and epithelial tissues are rich in AMPs. We are therefore facing a puzzling paradox, that is, microorganisms have evolved the ability to colonize immune cells/tissues that produce high local concentrations of AMPs. Although still incompletely understood, the recent literature sheds some light on the role of AMPs in the control of microbiota (including pathogens) and the mechanisms by which microorganisms avoid the complex chemical defences of their hosts.
(a). Essential role of AMPs in the control of the microbiota
The application of gene silencing to non-model organisms has opened the way for in vivo functional studies that provide a more exhaustive view on the role of AMPs at the interface between hosts and microorganisms (table 1). RNA interference confirmed the essential role of AMPs in controlling infections, as demonstrated for Type I crustins from Marsupenaeus japonicus, which participate in bacterial clearance in shrimp haemolymph [88,89]. It also confirmed the functional divergence of AMP variants. Indeed, in vivo, LvALF1 (Group A ALF) protected L. vannamei against V. penaeicida and F. oxysporum, but not WSSV [90], whereas ALFPm6 (Group C ALF) in P. monodon protected against both V. harveyi and WSSV [91]. However, gene silencing also showed a more complex role of AMPs in the immune response of shrimp. Indeed, Type II crustins, which are not active against Gram-negative bacteria in vitro [46], participate in the resistance of L. vannamei and M. japonicus to Gram-negative V. penaeicida in vivo [92,93].
In addition, through the use of gene silencing, AMPs were shown to orchestrate a key interface with the resident microbiota in marine invertebrates. In arthropods (shrimp), ALFs controlled shrimp-associated microbial communities [91,94]. Indeed, silencing of ALFPm3 (Group B ALF) in P. monodon caused a rapid propagation of bacteria, resulting in the death of the animals [91]. In freshwater cnidarians (Hydra), species-specific AMPs called arminins were found to define host species-specific bacterial associations [95]. With those findings, host-specific AMPs were proposed to have evolved in early branching metazoans because of the need to control the resident beneficial microbes and not because of invasive pathogens [96].
Novel functions (non-antimicrobial) of AMPs were also discovered through gene silencing. Thus, the silencing of PEN3 from the shrimp P. monodon resulted in a decrease in the β-integrin-dependent adhesion properties of shrimp haemocytes, revealing a previously unknown immunomodulatory function for penaeidins [97]. Gene silencing techniques applied to anionic AMPs, such as Group D ALFs [65] and stylicins [74], which show poor antimicrobial activity in vitro, should enable the characterization of their role in shrimp defence.
(b). Responses of pathogens to AMPs
(i). Immune suppression of AMPs by pathogens
Among strategies to escape the immune response, the suppression of AMP expression was shown in different phyla of marine invertebrates, including arthropods and cnidarians. In the shrimp L. vannamei, an infection by the fungal pathogen Fusarium solani dramatically repressed the expression of penaeidins, stylicins and Type II crustin [98]. Similarly, immune suppression of AMPs was reported in the coral P. damicornis, in which V. coralliilyticus exhibits temperature-dependent virulence, resulting in coral bleaching above 24°C and tissue lysis above 25°C [7]. In its non-virulent state (below 24°C), V. coralliilyticus induces the transcription of several coral immune genes [99] and the release of damicornin in coral mucus [43]. By contrast, in its virulent state, vibrios penetrating into coral tissues [100] induce a strong transient expression followed by a dramatic repression of damicornin transcription [43]. To date, the molecular mechanisms underlying the immune suppression of AMPs remains to be discovered.
(ii). Resistance to AMPs in vibrios
The membranes of microorganisms are an important interface with host AMPs (see §3). Consequently, many of the most important mechanisms of AMP resistance rely on outer membrane remodelling [101]. Another important mechanism of resistance is the active efflux of AMPs from the bacterial cell [101]. Those mechanisms were recently reviewed in detail in vibrios [102]. The most significant are those in which the minimum inhibitory concentrations (MICs) increase by several folds and are summarized below.
Membrane charge alteration. As electrostatic interactions often initiate the binding of cationic AMPs to bacterial membranes, bacteria colonizing metazoan hosts have evolved strategies to lower the net negative charge of cell surface molecules. In Gram-negative bacteria, such modifications are often observed on LPS, the major constituent of their outer membrane [103,104]. Vibrios, including human pathogens, live in close association with marine invertebrates [105], which in turn may have selected AMP-resistant phenotypes among vibrios. A structural modification of LPS was recently found to be responsible for the different AMP-resistant phenotypes observed in V. cholerae. Indeed, the Lipid A structure (the anionic membrane anchor of LPS) of V. cholerae O1 and O139 [106] contains a hydroxylated secondary acyl chain that is also found in the squid symbiont V. fischeri [96]. This structure plays an important role in the resistance to AMPs [106,107], as it can be substituted by di-Glycine residues that lower the negative charge of the whole molecule [108].
Release of outer membrane vesicles. Upon outer membrane stress, such as that created by AMPs, bacteria can use the alternate σE factor to promote the expression of factors that help preserve and/or restore cell envelope integrity. The release of outer membrane vesicles (OMVs) is a σE-dependent mechanism [109], whose role in AMP resistance has been recently shown in vibrios. Vibrios, including the oyster pathogen V. tasmaniensis LGP32, were shown to release OMV protection against membrane-active AMPs [110,111]. Interestingly, OMV release was triggered by oyster plasma, suggesting, as shown in E. coli [112], that the membrane-active agents that are present in oyster plasma can trigger OMV release in vibrios. While the major protective effect of OMVs against AMPs has now been shown in two vibrio species and in E. coli [113], it is still unknown whether this effect results from a membranous shield-like effect in which OMVs surround vibrios and trap membrane-active AMPs, thereby preventing their interaction with the membranes of the bacterial cell, or a membrane renewal mechanism eliminating AMP-damaged membranes to maintain membrane integrity. The σE-dependent induction of OMV release tends to support the second hypothesis.
Active efflux of AMPs. Once AMPs have breached the membrane barriers of bacteria and reached the cytoplasmic space, they can be expelled into the extracellular milieu by diverse efflux pumps. Pumps of the resistance-nodulation-cell division superfamily (RND) contribute to AMP-resistance in vibrios. However, among the six RND efflux pumps of V. cholerae [114], only VexAB-TolC is required for AMP resistance in vitro [115,116]. The VexAB-TolC pump is structurally and functionally similar to the E. coli and Salmonella enterica AcrAB-TolC pump [117,118] and the Pseudomonas aeruginosa MexAB-OprM systems [119]. VexAB is also the main efflux pump that is involved in the resistance to bile acids, detergents, antibiotics and PmB [116,120]. To date, among vibrios, V. cholerae remains the only species in which AMP-resistance was mediated by an RND efflux system.
5. Marine invertebrate AMPs in an applied context
(a). AMPs as therapeutic drugs
Since their discovery in the 1980s, AMPs have been considered to be promising candidates for therapeutic uses in humans, animals and plant health. However, only a few AMPs have reached phases of clinical and preclinical pipelines [121,122]. AMPs with potential interest for biopharmaceutical companies have been isolated from marine invertebrates. Some AMPs, such as mollusc defensins, have very low MICs in the nanomolar range against Gram-positive bacteria [37]. Their fungal homologue, plectasin, is considered to be a major candidate for therapeutic use [123]. Importantly, AMPs are currently being used for drug development due to their activity as immune modulators, which give them clinical potential beyond the treatment of antibiotic-resistant strains [124]. Among AMPs from marine invertebrates, ALF-derived peptides have been shown to modulate the inflammatory response in murine macrophage cell lines [125] and display anti-tumour activity against HeLa cells through the alteration of the cell membrane [126]. Those novel activities may open the way to future drug developments.
(b). AMPs in aquaculture
Disease prevention in marine invertebrate aquaculture has been traditionally based on the control of pathogens or the selection of animals that are resistant to diseases. AMPs of marine invertebrates have been considered for both applications.
(i). AMPs in the control of pathogens in aquaculture
In the context of intensifying aquaculture, antibiotics have been used for disease prevention and management, which has resulted in increased bacterial resistance in the environment, and favoured the emergence of resistant strains of major human pathogens [127]. If AMPs are proposed as an alternative to the use of antibiotics, which should be reduced or avoided in aquaculture (FAO/OIE/WHO), there is a risk of promoting the emergence of AMP-resistant strains (see §4), and this resistance can readily be achieved experimentally [128]. However, AMPs present some important advantages over antibiotics, including the following: (i) they are much less stable in the environment; (ii) they often combine multiple mechanisms of action, e.g. they can both disrupt bacterial membranes and inhibit metabolic pathways; and (iii) they do not increase bacterial mutation rates [129]. However, most of all, it is now recognized that the antimicrobial activity of AMPs largely relies on their immune regulatory properties, including the recruitment and differentiation of immune cells [130], against which microorganisms cannot evolve simple mechanisms of resistance. The finding that AMPs are not simple antimicrobials but are complex orchestrators of host defences has opened new perspectives to combat bacterial infections. AMPs from marine invertebrates have appeared to be attractive candidates to reduce the impact of diseases in closed aquaculture systems that have a low impact on the environment [17]. However, only rare applications have been reported. AMPs have been recently applied in the Polynesian pearl industry [131]. For example, tachyplesin was combined with exopolysaccharides as filming agents instead of the antibiotics that are traditionally used in the grafting process to reduce oyster post-operative mortality and increase pearl quality. The result of this alternative process was similar to that of commercial nuclei that were treated with antibiotics [132]. To date, the use of AMPs as immune modulators has not been reported in aquaculture.
(ii). AMPs as markers for survival capacity in shrimp
AMPs control homeostasis in marine invertebrates and maintain individuals in a healthy state (see §4). Inspired by studies in humans, which have investigated the associations between the copy number of AMP-encoding genes and susceptibility to diseases [133,134], signatures of AMP expression have been studied in marine invertebrates that are susceptible or resistant to infectious diseases. Thus, in the shrimp L. stylirostris, the basal expression of AMP-encoding genes (PEN1/2 and PEN3, ALF-D, Type II crustin and lysozyme) has been correlated with the capacity of the shrimp to circumvent V. penaeicida infections. As a consequence, signatures of AMP expression have been proposed as original molecular markers for selection programmes that are dedicated to shrimp resistance to bacterial infections [135].
6. Conclusion
Based on our current knowledge, there is probably not a general scheme but a diversity of roles for AMPs in the homeostasis of marine invertebrates. Some AMP families are highly abundant in host cells and tissues, whereas others are expressed at levels below inhibitory concentrations. Although the antimicrobial activity of AMPs has been the main focus of research, the immune regulatory properties of AMPs in the defence of marine protostomes and cnidarians now deserve a much greater research effort. In addition, some AMPs appear to have evolved within specific phyla or species, whereas others evolved from a common ancestor and are widespread in the tree of life. The inspiring example of freshwater cnidarians prompts us to investigate how specific AMP repertoires may have shaped the host-specific microbiota formation of stable holobionts and compare those examples with other metazoan species with less specific AMP repertoires and more versatile microbiota. This research effort is now needed to obtain a more integrated view of the role of AMPs in symbiotic interactions that range from mutualistic to pathogenic in order to develop a better understanding of the role of AMPs in protostome and cnidarian health and disease.
Authors' contributions
The authors wrote and approved the final version of this manuscript.
Competing interests
We have no competing interests.
Funding
The authors received financial support from the ANR (Vibriogen project ANR-11-BSV7-0023 and Adacni project ANR-12-ADAP-0016), CAPES (CIMAR 1974/2014), CNPq (MEC/MCTI/CAPES/CNPq/FAPs PVE 401191/2014-1) and FONDECYT 11150009-2015. C.B. was supported by an MSc scholarship provided by CNPq-Brazil.
References
- 1.Millennium, Assessment & Ecosystem. 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press. [Google Scholar]
- 2.FAO. 2014. The state of world fisheries and aquaculture. Rome: http://www.fao.org/3/a-i3720e.pdf. [Google Scholar]
- 3.Burge C, et al. 2014. Climate change influences on marine infectious diseases: implications for management and society. Ann. Rev. Mar. Sci. 6, 249–277. ( 10.1146/annurev-marine-010213-135029) [DOI] [PubMed] [Google Scholar]
- 4.Schmitt P, Rosa RD, Duperthuy M, de Lorgeril J, Bachère E, Destoumieux-Garzón D. 2012. The antimicrobial defense of the Pacific Oyster, Crassostrea gigas. How diversity may compensate for scarcity in the regulation of resident/pathogenic microflora. Front. Microbiol. 3, 160 ( 10.3389/fmicb.2012.00160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno J, Selig E, Casey K, Page C, Willis B, Harvell C, Sweatman H, Melendy A. 2007. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 5, 1220–1227. ( 10.1371/journal.pbio.0050124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega Thurber R, Burkepile D, Fuchs C, Shantz A, McMinds R, Zaneveld J. 2014. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 20, 544–554. ( 10.1111/gcb.12450) [DOI] [PubMed] [Google Scholar]
- 7.Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J, Rosenberg E. 2003. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53, 309–315. ( 10.1099/ijs.0.02402-0) [DOI] [PubMed] [Google Scholar]
- 8.Fukui Y, Saitoh S-I, Sawabe T. 2010. Environmental determinants correlated to Vibrio harveyi-mediated death of marine gastropods. Environ. Microbiol. 12, 124–133. ( 10.1111/j.1462-2920.2009.02052.x) [DOI] [PubMed] [Google Scholar]
- 9.Petton B, Bruto M, James A, Labreuche Y, Alunno Bruscia M, Le Roux F. 2015. Crassostrea gigas mortality in France: the usual suspect, a herpes virus, may not be the killer in this polymicrobial opportunistic disease. Front. Microbiol. 6, 1–10. ( 10.3389/fmicb.2015.00686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barneah O, Ben-Dov E, Kramarsky-Winter E, Kushmaro A. 2007. Characterization of black band disease in Red Sea stony corals. Environ. Microbiol. 9, 1995–2006. ( 10.1111/j.1462-2920.2007.01315.x) [DOI] [PubMed] [Google Scholar]
- 11.Lightner D, Redman R. 1981. A baculovirus-caused disease of penaeid shrimp, Penaeus monodon. J. Invert. Pathol. 38, 299–302. ( 10.1016/0022-2011(81)90137-3) [DOI] [Google Scholar]
- 12.Lo C, et al. 1996. White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis. Aquat. Organ. 27, 215–225. ( 10.3354/dao027215) [DOI] [Google Scholar]
- 13.Lightner D. 1996. Epizootiology, distribution and the impact on international trade of two penaeid shrimp viruses in the Americas. Rev. Sci. Tech. 15, 579–601. [DOI] [PubMed] [Google Scholar]
- 14.Dhar A, Cowley J, Hasson K, Walker P. 2004. Genomic organization, biology, and diagnosis of Taura syndrome virus and yellowhead virus of penaeid shrimp. Adv. Virus Res. 63, 353–421. ( 10.1016/S0065-3527(04)63006-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulos B, Tang K, Pantoja C, Bonami J, Lightner D. 2006. Purification and characterization of infectious myonecrosis virus of penaeid shrimp. J. Gen. Virol. 87, 987–996. ( 10.1099/vir.0.81127-0) [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Paz A. 2010. White spot syndrome virus: an overview on an emergent concern. Vet. Res. 41, 43 ( 10.1051/vetres/2010015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachère E. 2003. Anti-infectious immune effectors in marine invertebrates: potential tools for disease control in larviculture. Aquaculture 227, 427–438. ( 10.1016/S0044-8486(03)00521-0) [DOI] [Google Scholar]
- 18.Lee C-T, et al. 2015. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl Acad. Sci. USA 112, E5445 ( 10.1073/pnas.1517100112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romalde JL, Diéguez AL, Lasa A, Balboa S. 2014. New Vibrio species associated to molluscan microbiota: a review. Front. Microbiol. 4, 413 ( 10.3389/fmicb.2013.00413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travers M-A, Miller KB, Roque A, Friedman CS. 2015. Bacterial diseases in marine bivalves. J. Invertebr. Pathol. 131, 11–31. ( 10.1016/j.jip.2015.07.010) [DOI] [PubMed] [Google Scholar]
- 21.Segarra A, Pépin JF, Arzul I, Morga B, Faury N, Renault T. 2010. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oyster, Crassostrea gigas, in France in 2008. Virus Res. 153, 92–99. ( 10.1016/j.virusres.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 22.Lemire A, Goudenège D, Versigny T, Petton B, Calteau A, Labreuche Y, Le Roux F. 2015. Populations, not clones, are the unit of vibrio pathogenesis in naturally infected oysters. ISME J. 9, 1523–1531. ( 10.1038/ismej.2014.233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudenège D, et al. 2015. A single regulatory gene is sufficient to alter Vibrio aestuarianus pathogenicity in oysters. Environ. Microbiol. 17, 4189–4199. ( 10.1111/1462-2920.12699) [DOI] [PubMed] [Google Scholar]
- 24.Travers M-A, et al. 2014. First description of French V. tubiashii strains pathogenic to mollusk: I. Characterization of isolates and detection during mortality events. J. Invertebr. Pathol. 123, 38–48. ( 10.1016/j.jip.2014.04.009) [DOI] [PubMed] [Google Scholar]
- 25.Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD. 2009. Microbial disease and the coral holobiont. Trends Microbiol. 17, 554–562. ( 10.1016/j.tim.2009.09.004) [DOI] [PubMed] [Google Scholar]
- 26.Soffer N, Brandt ME, Correa AMS, Smith TB, Thurber RV. 2014. Potential role of viruses in white plague coral disease. ISME J. 8, 271–283. ( 10.1038/ismej.2013.137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronson R, Precht W. 2001. White-band disease and the changing face of Caribbean coral reefs. In The ecology and etiology of newly emerging marine diseases (ed. Porter J.), pp. 25–38. Berlin, Germany: Springer. [Google Scholar]
- 28.Sekar R, Mills DK, Remily ER, Voss JD, Richardson LL. 2006. Microbial communities in the surface mucopolysaccharide layer and the black band microbial mat of black band-diseased Siderastrea siderea. Appl. Environ. Microbiol. 72, 5963–5973. ( 10.1128/AEM.00843-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luna G, Biavasco F, Danovaro R. 2007. Bacteria associated with the rapid tissue necrosis of stony corals. Environ. Microbiol. 9, 1851–1857. ( 10.1111/j.1462-2920.2007.01287.x) [DOI] [PubMed] [Google Scholar]
- 30.Luna G, Bongiorni L, Gili C, Biavasco F, Danovaro R. 2010. Vibrio harveyi as a causative agent of the White Syndrome in tropical stony corals. Environ. Microbiol. Rep. 2, 120–127. ( 10.1111/j.1758-2229.2009.00114.x) [DOI] [PubMed] [Google Scholar]
- 31.König E, Bininda-Emonds ORP, Shaw C. 2015. The diversity and evolution of anuran skin peptides. Peptides 63, 96–117. ( 10.1016/j.peptides.2014.11.003) [DOI] [PubMed] [Google Scholar]
- 32.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720. ( 10.1038/nri1180) [DOI] [PubMed] [Google Scholar]
- 33.Bachère E, Rosa RD, Schmitt P, Poirier AC, Merou N, Charrière GM, Destoumieux-Garzón D. 2015. The new insights into the oyster antimicrobial defense: cellular, molecular and genetic view. Fish Shellfish Immunol. 46, 50–64. ( 10.1016/j.fsi.2015.02.040) [DOI] [PubMed] [Google Scholar]
- 34.Augustin R, Anton-Erxleben F, Jungnickel S, Hemmrich G, Spudy B, Podschun R, Bosch TCG. 2009. Activity of the novel peptide arminin against multiresistant human pathogens shows the considerable potential of phylogenetically ancient organisms as drug sources. Antimicrob. Agents Chemother. 53, 5245–5250. ( 10.1128/AAC.00826-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395. ( 10.1038/415389a) [DOI] [PubMed] [Google Scholar]
- 36.Schmitt P, Rosa RD, Destoumieux-Garzón D. 2015. An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes. BBA Biomembr. 1858, 958–970. ( 10.1016/j.bbamem.2015.10.011) [DOI] [PubMed] [Google Scholar]
- 37.Schmitt P, Wilmes M, Pugnière M, Aumelas A, Bachère E, Sahl H-G, Schneider T, Destoumieux-Garzón D. 2010. Insight into invertebrate defensin mechanism of action: oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J. Biol. Chem. 285, 29 208–29 216. ( 10.1074/jbc.M110.143388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González M, et al. 2007. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gigas Cg-BPI. Proc. Natl Acad. Sci. USA 104, 17 759–17 764. ( 10.1073/pnas.0702281104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka S, Nakamura T, Morita T, Iwanaga S. 1982. Limulus anti-LPS factor: an anticoagulant which inhibits the endotoxin mediated activation of Limulus coagulation system. Biochem. Biophys. Res. Commun. 105, 717–723. ( 10.1016/0006-291X(82)91493-0) [DOI] [PubMed] [Google Scholar]
- 40.Muta T, Miyata T, Tokunaga F, Nakamura T, Iwanaga S. 1987. Primary structure of anti-lipopolysaccharide factor from American horseshoe crab, Limulus polyphemus. J. Biochem. 101, 1321–1330. [DOI] [PubMed] [Google Scholar]
- 41.Petit VW, et al. 2015. A hemocyanin-derived antimicrobial peptide from the penaeid shrimp adopts an alpha-helical structure that specifically permeabilizes fungal membranes. BBA Gen. Subj. 1860, 557–568. ( 10.1016/j.bbagen.2015.12.010) [DOI] [PubMed] [Google Scholar]
- 42.Mylonakis E, Podsiadlowski L, Muhammed M, Vilcinskas A. 2016. Diversity, evolution and medical applications of insect antimicrobial peptides. Phil. Trans. R. Soc. B 371, 20150290 ( 10.1098/rstb.2015.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal-Dupiol J, et al. 2011. Innate immune responses of a scleractinian coral to vibriosis. J. Biol. Chem. 286, 22 688–22 698. ( 10.1074/jbc.M110.216358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito T, Kawabata S, Shigenaga T, Takayenoki Y, Cho J, Nakajima H, Hirata M, Iwanaga S. 1995. A novel big defensin identified in horseshoe crab hemocytes: isolation, amino acid sequence, and antibacterial activity. J. Biochem. 117, 1131–1137. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T, Furunaka H, Miyata T, Tokunagas F, Mutas T, Iwanagall S, Niwa M, Takao T, Shimonishi Y. 1988. Tachyplesin, a Class of Antimicrobial Peptide from the Hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 263, 16 709–16 713. [PubMed] [Google Scholar]
- 46.Rosa RD, Barracco MA. 2010. Antimicrobial peptides in crustaceans. Invertebr. Surviv. J. 7, 262–284. [Google Scholar]
- 47.Smith VJ, Dyrynda EA. 2015. Antimicrobial proteins: from old proteins, new tricks. Mol. Immunol. 68, 383–398. ( 10.1016/j.molimm.2015.08.009) [DOI] [PubMed] [Google Scholar]
- 48.Bachère E, Gueguen Y, Gonzalez M, De Lorgeril J, Garnier J, Romestand B. 2004. Insights into the anti-microbial defense of marine invertebrates: the penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 198, 149–168. ( 10.1111/j.0105-2896.2004.00115.x) [DOI] [PubMed] [Google Scholar]
- 49.Destoumieux D, Bulet P, Loew D, Van Dorsselaer A, Rodriguez J, Bachère E. 1997. Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). J. Biol. Chem. 272, 28 398–28 406. ( 10.1074/jbc.272.45.28398) [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Poncet J, Garnier J, Zatylny C, Bachère E, Aumelas A. 2003. Solution structure of the recombinant penaeidin-3, a shrimp antimicrobial peptide. J. Biol. Chem. 278, 36 859–36 867. ( 10.1074/jbc.M305450200) [DOI] [PubMed] [Google Scholar]
- 51.Gueguen Y, et al. 2006. PenBase, the shrimp antimicrobial peptide penaeidin database: sequence-based classification and recommended nomenclature. Dev. Comp. Immunol. 30, 283–288. ( 10.1016/j.dci.2005.04.003) [DOI] [PubMed] [Google Scholar]
- 52.Kang C-J, Xue J-F, Liu N, Zhao X-F, Wang J-X. 2007. Characterization and expression of a new subfamily member of penaeidin antimicrobial peptides (penaeidin 5) from Fenneropenaeus chinensis. Mol. Immunol. 44, 1535–1543. ( 10.1016/j.molimm.2006.08.025) [DOI] [PubMed] [Google Scholar]
- 53.Tassanakajon A, Amparyup P, Somboonwiwat K, Supungul P. 2011. Cationic antimicrobial peptides in penaeid shrimp. Mar. Biotechnol. 13, 639–657. ( 10.1007/s10126-011-9381-8) [DOI] [PubMed] [Google Scholar]
- 54.Cuthbertson BJ, Shepard EF, Chapman RW, Gross PS. 2002. Diversity of the penaeidin antimicrobial peptides in two shrimp species. Immunogenetics 54, 442–445. ( 10.1007/s00251-002-0487-z) [DOI] [PubMed] [Google Scholar]
- 55.Destoumieux D, Muñoz M, Cosseau C, Rodriguez J, Bulet P, Comps M, Bachère E. 2000. Penaeidins, antimicrobial peptides with chitin-binding activity, are produced and stored in shrimp granulocytes and released after microbial challenge. J. Cell Sci. 113, 461–469. [DOI] [PubMed] [Google Scholar]
- 56.Cuthbertson BJ, Deterding LJ, Williams JG, Tomer KB, Etienne K, Blackshear PJ, Büllesbach EE, Gross PS. 2008. Diversity in penaeidin antimicrobial peptide form and function. Dev. Comp. Immunol. 32, 167–181. ( 10.1016/j.dci.2007.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woramongkolchai N, Supungul P, Tassanakajon A. 2011. The possible role of penaeidin5 from the black tiger shrimp, Penaeus monodon, in protection against viral infection. Dev. Comp. Immunol. 35, 530–536. ( 10.1016/j.dci.2010.12.016) [DOI] [PubMed] [Google Scholar]
- 58.Cuthbertson BJ, Büllesbach EE, Fievet J, Bachère E, Gross PS. 2004. A new class (penaeidin class 4) of antimicrobial peptides from the Atlantic white shrimp (Litopenaeus setiferus) exhibits target specificity and an independent proline-rich-domain function. Biochem. J. 381, 79–86. ( 10.1042/BJ20040330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Song Y. 2010. Proline-rich domain of penaeidin molecule exhibits autocrine feature by attracting penaeidin-positive granulocytes toward the wound-induced inflammatory site. Fish Shellfish Immunol. 29, 1044–1052. ( 10.1016/j.fsi.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 60.Gross P, Bartlett T, Browdy C, Chapman R, Warr G. 2001. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp L. setiferus. Dev. Comp. Immunol. 25, 565–577. ( 10.1016/S0145-305X(01)00018-0) [DOI] [PubMed] [Google Scholar]
- 61.Hoess A, Watson S, Siber GR, Liddington R. 1993. Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 A resolution. EMBO J. 12, 3351–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somboonwiwat K, Bachère E, Rimphanitchayakit V, Tassanakajon A. 2008. Localization of anti-lipopolysaccharide factor (ALF Pm 3) in tissues of the black tiger shrimp, Penaeus monodon, and characterization of its binding properties. Dev. Comp. Immunol. 32, 1170–1176. ( 10.1016/j.dci.2008.03.008) [DOI] [PubMed] [Google Scholar]
- 63.Sun C, Xu W, Zhang H, Dong L. 2011. An anti-lipopolysaccharide factor from red swamp cray fish, Procambarus clarkii, exhibited antimicrobial activities in vitro and in vivo. Fish Shellfish Immunol. 30, 295–303. ( 10.1016/j.fsi.2010.10.022) [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, et al. 2009. NMR structure of rALF-Pm3, an anti-lipopolysaccharide factor from shrimp: model of the possible lipid A-binding site. Biopolymers 91, 207–220. ( 10.1002/bip.21119) [DOI] [PubMed] [Google Scholar]
- 65.Rosa RD, et al. 2013. Functional divergence in shrimp anti-lipopolysaccharide factors (ALFs): from recognition of cell wall components to antimicrobial activity. PLoS ONE 8, 17–19. ( 10.1371/journal.pone.0067937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang H, Zhang Q, Zhao Y, Jia W, Zhao X, Wang J. 2015. A new group of anti-lipopolysaccharide factors from Marsupenaeus japonicus functions in antibacterial response. Dev. Comp. Immunol. 48, 33–42. ( 10.1016/j.dci.2014.09.001) [DOI] [PubMed] [Google Scholar]
- 67.Tassanakajon A, Somboonwiwat K, Amparyup P. 2015. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev. Comp. Immunol. 48, 324–341. ( 10.1016/j.dci.2014.05.020) [DOI] [PubMed] [Google Scholar]
- 68.Ranganathan S, Simpson KJ, Shaw DC, Nicholas KR. 1999. The whey acidic protein family: a new signature motif and three-dimensional structure by comparative modeling. J. Mol. Graph. Model. 17, 106–113. ( 10.1016/S1093-3263(99)00023-6) [DOI] [PubMed] [Google Scholar]
- 69.Supungul P, Tang S, Maneeruttanarungroj C, Rimphanitchayakit V, Hirono I, Aoki T, Tassanakajon A. 2008. Cloning, expression and antimicrobial activity of crustinPm1, a major isoform of crustin, from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 32, 61–70. ( 10.1016/j.dci.2007.04.004) [DOI] [PubMed] [Google Scholar]
- 70.Amparyup P, Kondo H, Hirono I, Aoki T, Tassanakajon A. 2008. Molecular cloning, genomic organization and recombinant expression of a crustin-like antimicrobial peptide from black tiger shrimp Penaeus monodon. Mol. Immunol. 45, 1085–1093. ( 10.1016/j.molimm.2007.07.031) [DOI] [PubMed] [Google Scholar]
- 71.Du Z-Q, Ren Q, Zhao X-F, Wang J-X. 2009. A double WAP domain (DWD)-containing protein with proteinase inhibitory activity in Chinese white shrimp, Fenneropenaeus chinensis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 154, 203–210. ( 10.1016/j.cbpb.2009.06.004) [DOI] [PubMed] [Google Scholar]
- 72.Li S, Jin X-K, Guo X-N, Yu A-Q, Wu M-H, Tan S-J, Zhu Y-T, Li W-W, Wang Q. 2013. A double WAP domain-containing protein Es-DWD1 from Eriocheir sinensis exhibits antimicrobial and proteinase inhibitory activities. PLoS ONE 8, e73563 ( 10.1371/journal.pone.0073563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du Z-Q, Yuan J-J, Ren D-M. 2015. A novel single WAP domain-containing protein isoform with antibacterial relevance in Litopenaeus vannamei. Fish Shellfish Immunol. 44, 478–484. ( 10.1016/j.fsi.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 74.Rolland JL, Abdelouahab M, Dupont J, Lefevre F, Bachère E, Romestand B. 2010. Stylicins, a new family of antimicrobial peptides from the Pacific blue shrimp Litopenaeus stylirostris. Mol. Immunol. 47, 1269–1277. ( 10.1016/j.molimm.2009.12.007) [DOI] [PubMed] [Google Scholar]
- 75.Destoumieux-Garzón D, Saulnier D, Garnier J, Jouffrey C, Bulet P, Bachère E. 2001. Crustacean Immunity: antifungal peptides are generated from the c terminus of shrimp hemocyanin in response to microbial challenge. J. Biol. Chem. 276, 47 070–47 077. ( 10.1074/jbc.M103817200) [DOI] [PubMed] [Google Scholar]
- 76.Lee SY, Lee BL, Söderhäll K. 2003. Processing of an antibacterial peptide from hemocyanin of the freshwater crayfish Pacifastacus leniusculus. J. Biol. Chem. 278, 7927–7933. ( 10.1074/jbc.M209239200) [DOI] [PubMed] [Google Scholar]
- 77.Miller B, Abrams R, Dorfman A, Klein M. 1942. Antibacterial properties of protamine and histone. Science 96, 428–430. ( 10.1126/science.96.2497.428) [DOI] [PubMed] [Google Scholar]
- 78.Kawasaki H, Iwamuro S. 2008. Potential roles of histones in host defense as antimicrobial agents. Infect. Disord. Drug Targets 8, 195–205. ( 10.2174/1871526510808030195) [DOI] [PubMed] [Google Scholar]
- 79.Patat SA, Carnegie RB, Kingsbury C, Gross PS, Chapman R, Schey KL. 2004. Antimicrobial activity of histones from hemocytes of the Pacific white shrimp. Eur. J. Biochem. 271, 4825–4833. ( 10.1111/j.1432-1033.2004.04448.x) [DOI] [PubMed] [Google Scholar]
- 80.Guimarães-Costa AB, Nascimento MTC, Wardini AB, Pinto-da-Silva LH, Saraiva EM. 2012. ETosis: a microbicidal mechanism beyond cell death. J. Parasitol. Res. 2012, 1–11. ( 10.1155/2012/929743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. ( 10.1126/science.1092385) [DOI] [PubMed] [Google Scholar]
- 82.Altincicek B, Stötzel S, Wygrecka M, Preissner KT, Vilcinskas A, Sto S. 2008. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J. Immunol. Methods 181, 2705–2712. ( 10.4049/jimmunol.181.4.2705) [DOI] [PubMed] [Google Scholar]
- 83.Ng TH, Wu M-H, Chang S-H, Aoki T, Wang H-C. 2015. The DNA fibers of shrimp hemocyte extracellular traps are essential for the clearance of Escherichia coli. Dev. Comp. Immunol. 48, 229–233. ( 10.1016/j.dci.2014.10.011) [DOI] [PubMed] [Google Scholar]
- 84.Poirier AC, Schmitt P, Rosa RD, Vanhove AS, Kieffer-Jaquinod S, Rubio TP, Charrière GM, Destoumieux-Garzón D. 2014. Antimicrobial histones and DNA traps in invertebrate immunity: evidences in Crassostrea gigas. J. Biol. Chem. 289, 24 821–24 831. ( 10.1074/jbc.M114.576546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keshari RS, Verma A, Barthwal MK, Dikshit M. 2013. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 114, 532–540. ( 10.1002/jcb.24391) [DOI] [PubMed] [Google Scholar]
- 86.Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. 2009. Translocation of a Vibrio cholerae Type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5, 234–243. ( 10.1016/j.chom.2009.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duperthuy M, et al. 2011. Use of OmpU porins for attachment and invasion of Crassostrea gigas immune cells by the oyster pathogen Vibrio splendidus. Proc. Natl Acad. Sci. USA 108, 2993–2998. ( 10.1073/pnas.1015326108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang H-T, Yang M-C, Sun J-J, Guo F, Lan J-F, Wang X-W, Zhao X-F, Wang J-X. 2015. Catalase eliminates reactive oxygen species and influences the intestinal microbiota of shrimp. Fish Shellfish Immunol. 47, 63–73. ( 10.1016/j.fsi.2015.08.021) [DOI] [PubMed] [Google Scholar]
- 89.Liu N, Lan J-F, Sun J-J, Jia W-M, Zhao X-F, Wang J-X. 2015. A novel crustin from Marsupenaeus japonicus promotes hemocyte phagocytosis. Dev. Comp. Immunol. 49, 313–322. ( 10.1016/j.dci.2014.11.021) [DOI] [PubMed] [Google Scholar]
- 90.De la Vega E, O'Leary N, Shockey JE, Robalino J, Payne C, Browdy CL, Warr GW, Gross PS. 2008. Anti-lipopolysaccharide factor in Litopenaeus vannamei (Lv ALF): a broad spectrum antimicrobial peptide essential for shrimp immunity against bacterial and fungal infection. Mol. Immunol. 45, 1916–1925. ( 10.1016/j.molimm.2007.10.039) [DOI] [PubMed] [Google Scholar]
- 91.Ponprateep S, Tharntada S, Somboonwiwat K, Tassanakajon A. 2012. Gene silencing reveals a crucial role for anti-lipopolysaccharide factors from Penaeus monodon in the protection against microbial infections. Fish Shellfish Immunol. 32, 26–34. ( 10.1016/j.fsi.2011.10.010) [DOI] [PubMed] [Google Scholar]
- 92.Shockey JE, O'Leary NA, de la Vega E, Browdy CL, Baatz JE, Gross PS. 2009. The role of crustins in Litopenaeus vannamei in response to infection with shrimp pathogens: An in vivo approach. Dev. Comp. Immunol. 33, 668–673. ( 10.1016/j.dci.2008.11.010) [DOI] [PubMed] [Google Scholar]
- 93.Hipolito SG, Shitara A, Kondo H, Hirono I. 2014. Role of Marsupenaeus japonicus crustin-like peptide against Vibrio penaeicida and white spot syndrome virus infection. Dev. Comp. Immunol. 46, 461–469. ( 10.1016/j.dci.2014.06.001) [DOI] [PubMed] [Google Scholar]
- 94.Wang XW, Xu JD, Zhao XF, Vasta GR, Wang JX. 2014. A shrimp C-type lectin inhibits proliferation of the hemolymph microbiota by maintaining the expression of antimicrobial peptides. J. Biol. Chem. 289, 11 779–11 790. ( 10.1074/jbc.M114.552307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franzenburg S, Walter J, Künzel S, Wang J, Baines JF, Bosch TCG, Fraune S. 2013. Distinct antimicrobial peptide expression determines host species-specific bacterial associations. Proc. Natl Acad. Sci. USA 110, E3730–E3738. ( 10.1073/pnas.1304960110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bosch T. 2013. Cnidarian-microbe interactions and the origin of innate immunity in metazoans. Annu. Rev. Microbiol. 67, 499–518. ( 10.1146/annurev-micro-092412-155626) [DOI] [PubMed] [Google Scholar]
- 97.Li CY, Yan HY, Song YL. 2010. Tiger shrimp (Penaeus monodon) penaeidin possesses cytokine features to promote integrin-mediated granulocyte and semi-granulocyte adhesion. Fish Shellfish Immunol. 28, 1–9. ( 10.1016/j.fsi.2009.09.003) [DOI] [PubMed] [Google Scholar]
- 98.Goncalves P, Guertler C, Bachère E, de Souza CRB, Rosa RD, Perazzolo LM. 2014. Molecular signatures at imminent death: hemocyte gene expression profiling of shrimp succumbing to viral and fungal infections. Dev. Comp. Immunol. 42, 294–301. ( 10.1016/j.dci.2013.09.017) [DOI] [PubMed] [Google Scholar]
- 99.Vidal-Dupiol J, Dheilly NM, Rondon R, Grunau C, Cosseau C, Smith KM, Freitag M, Adjeroud M, Mitta G. 2014. Thermal stress triggers broad Pocillopora damicornis transcriptomic remodeling, while Vibrio coralliilyticus infection induces a more targeted immuno-suppression response. PLoS ONE 9, e107672 ( 10.1371/journal.pone.0107672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vidal-Dupiol J, Ladriere O, Meistertzheim A-L, Foure L, Adjeroud M, Mitta G. 2011. Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus. J. Exp. Biol. 214, 1533–1545. ( 10.1242/jeb.053165) [DOI] [PubMed] [Google Scholar]
- 101.Joo H-S, Fu C-l, Otto M. 2016. Bacterial Strategies of resistance to antimicrobial peptides. Phil. Trans. R. Soc. B 371, 20150292 ( 10.1098/rstb.2015.0292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Destoumieux-Garzón D, Duperthuy M, Vanhove AS, Schmitt P, Wai SN. 2014. Resistance to antimicrobial peptides in vibrios. Antibiotics 3, 540–563. ( 10.3390/antibiotics3040540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cullen TW, et al. 2015. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Nature 347, 170–175. ( 10.1126/science.1260580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo L, Lim K, Gunn J, Bainbridge B, Darveau R, Hackett M, Miller S. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276, 250–253. ( 10.1126/science.276.5310.250) [DOI] [PubMed] [Google Scholar]
- 105.Austin B. 2010. Vibrios as causal agents of zoonoses. Vet. Microbiol. 140, 310–317. ( 10.1016/j.vetmic.2009.03.015) [DOI] [PubMed] [Google Scholar]
- 106.Hankins JV, Madsen JA, Giles DK, Childers BM, Klose KE, Brodbelt JS, Trent MS. 2011. Elucidation of a novel Vibrio cholerae lipid A secondary hydroxy-acyltransferase and its role in innate immune recognition. Mol. Microbiol. 81, 1313–1329. ( 10.1111/j.1365-2958.2011.07765.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matson JS, Yoo HJ, Hakansson K, Dirita VJ. 2010. Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by MsbB. J. Bacteriol. 192, 2044–2052. ( 10.1128/JB.00023-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. 2012. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc. Natl Acad. Sci. USA 109, 8722–8727. ( 10.1073/pnas.1201313109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song T, et al. 2008. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 70, 100–111. ( 10.1111/j.1365-2958.2008.06392.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duperthuy M, Sjöström AE, Sabharwal D, Damghani F, Uhlin BE, Wai SN. 2013. Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 9, 1–12. ( 10.1371/journal.ppat.1003620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vanhove A, et al. 2015. Outer membrane vesicles are vehicles for the delivery of Vibrio tasmaniensis virulence factors to oyster immune cells. Environ. Microbiol. 17, 1152–1165. ( 10.1111/1462-2920.12535) [DOI] [PubMed] [Google Scholar]
- 112.Manning A, Kuehn M. 2013. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J. Mol. Microbiol. Biotechnol. 23, 131–141. ( 10.1159/000346548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Manning AJ, Kuehn MJ. 2011. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 11, 258 ( 10.1186/1471-2180-11-258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitaoka M, Miyata ST, Unterweger D, Pukatzki S. 2011. Antibiotic resistance mechanisms of Vibrio cholerae. J. Med. Microbiol. 60, 397–407. ( 10.1099/jmm.0.023051-0) [DOI] [PubMed] [Google Scholar]
- 115.Bina JE, Mekalanos JJ. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 69, 4681–4685. ( 10.1128/IAI.69.7.4681-4685.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bina XR, Provenzano D, Nguyen N, Bina JE. 2008. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect. Immun. 76, 3595–3605. ( 10.1128/IAI.01620-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nikaido H, Zgurskaya HI. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3, 215–218. [PubMed] [Google Scholar]
- 118.Buckley A, Webber M, Cooles S, Randall L, La Ragione R, Woodward M, Piddock L. 2006. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell. Microbiol. 8, 847–856. ( 10.1111/j.1462-5822.2005.00671.x) [DOI] [PubMed] [Google Scholar]
- 119.Poole K. 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3, 255–264. [PubMed] [Google Scholar]
- 120.Bina JE, Provenzano D, Wang C, Bina XR, Mekalanos JJ. 2006. Characterization of the Vibrio cholerae vexAB and vexCD efflux systems. Arch. Microbiol. 186, 171–181. ( 10.1007/s00203-006-0133-5) [DOI] [PubMed] [Google Scholar]
- 121.Kang S, Park S, Mishig-Ochir T, Lee B. 2014. Antimicrobial peptides: therapeutic potentials. Expert Rev. Anti. Infect. Ther. 12, 1477–1486. ( 10.1586/14787210.2014.976613) [DOI] [PubMed] [Google Scholar]
- 122.Cheung RCF, Ng TB, Wong JH. 2015. Marine peptides: bioactivities and applications. Mar. Drugs 13, 4006–4043. ( 10.3390/md13074006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mygind PH, et al. 2005. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437, 975–980. ( 10.1038/nature04051) [DOI] [PubMed] [Google Scholar]
- 124.Hilchie AL, Wuerth K, Hancock REW. 2013. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 9, 761–768. ( 10.1038/nchembio.1393) [DOI] [PubMed] [Google Scholar]
- 125.Lin M-C, Lin S-B, Lee S-C, Lin C-C, Hui C-F, Chen J-Y. 2010. Antimicrobial peptide of an anti-lipopolysaccharide factor modulates of the inflammatory response in RAW264.7 cells. Peptides 31, 1262–1272. ( 10.1016/j.peptides.2010.04.004) [DOI] [PubMed] [Google Scholar]
- 126.Lin M-C, Lin S-B, Chen J-C, Hui C-F, Chen J-Y. 2010. Shrimp anti-lipopolysaccharide factor peptide enhances the antitumor activity of cisplatin in vitro and inhibits HeLa cells growth in nude mice. Peptides 31, 1019–1025. ( 10.1016/j.peptides.2010.02.023) [DOI] [PubMed] [Google Scholar]
- 127.Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 15, 1917–1942. ( 10.1111/1462-2920.12134) [DOI] [PubMed] [Google Scholar]
- 128.Perron GG, Zasloff M, Bell G. 2006. Experimental evolution of resistance to an antimicrobial peptide. Proc. R. Soc. B 273, 251–256. ( 10.1098/rspb.2005.3301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodríguez-Rojas A, Makarova O, Rolff J. 2014. Antimicrobials, stress and mutagenesis. PLoS Pathog. 10, e1004445 ( 10.1371/journal.ppat.1004445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hancock REW, Nijnik A, Philpott DJ. 2012. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254. ( 10.1038/nrmicro2745) [DOI] [PubMed] [Google Scholar]
- 131.Guezennec J, Gueguen Y, Bachere E, Kouzayha A, Simon-Colin C. 2013. Nucleus coated with a film-forming coating having antibacterial and cicatrizing properties, and method for obtaining same. US20130152865 A1. [DOI] [PMC free article] [PubMed]
- 132.Simon-Colin C, Gueguen Y, Bachere E, Kouzayha A, Saulnier D, Gayet N, Guezennec J. 2015. Use of natural antimicrobial peptides and bacterial biopolymers for cultured pearl production. Mar. Drugs 13, 3732–3744. ( 10.3390/md13063732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cantsilieris S, White SJ. 2013. Correlating multiallelic copy number polymorphisms with disease susceptibility. Hum. Mutat. 34, 1–13. ( 10.1002/humu.22172) [DOI] [PubMed] [Google Scholar]
- 134.Hollox EJ, Hoh B-P. 2014. Human gene copy number variation and infectious disease. Hum. Genet. 133, 1217–1233. ( 10.1007/s00439-014-1457-x) [DOI] [PubMed] [Google Scholar]
- 135.de Lorgeril J, Gueguen Y, Goarant C, Goyard E, Mugnier C, Fievet J, Piquemal D, Bachère E. 2008. A relationship between antimicrobial peptide gene expression and capacity of a selected shrimp line to survive a Vibrio infection. Mol. Immunol. 45, 3438–3445. ( 10.1016/j.molimm.2008.04.002) [DOI] [PubMed] [Google Scholar]


