Abstract
Streptococcus pneumoniae is the most common cause of pneumonia, which claims the lives of people over the age of 65 seven times more frequently than those aged 5–49. B-1a cells provide immediate and essential protection from S. pneumoniae through production of natural immunoglobulin, which has minimal insertion of N-region additions added by the enzyme TdT. In experiments with SCID mice infected with S. pneumoniae, we found passive transfer of IgG-depleted serum from aged (18–24-month old) mice had no effect whereas IgG-depleted serum from young (3-month old) mice was protective. This suggests protective natural IgM changes with age. Using single cell PCR we found N-region addition, which is initially low in fetal-derived B-1a cell IgM developing in the absence of TdT, increased in 7–24-month old mice as compared to 3-month old mice. To determine the mechanism responsible for the age related change in B-1a cell IgM, we established a mixed chimera system in which mice were reconstituted with allotype-marked mature peritoneal B-1a cells and adult bone marrow (BM) cells. We demonstrated even in the presence of mature peritoneal B-1a cells, adult BM contributed to the mature B-1a cell pool. More importantly, using this system we found over a 10-month period peritoneal B-1a cell IgM changed, showing the number of cells lacking N-region additions at both junctions fell from 49% to 29% of sequences. These results strongly suggest selection-induced skewing alters B-1a cell derived natural antibody, which may in turn be responsible for the loss of natural IgM-mediated protection against pneumococcal infection.
Introduction
Murine B-1a cells phenotypically characterized by the cell surface markers: CD5+, IgMhigh, IgDlow, B220low, MAC-1+, CD23−, and CD43+ (1, 2), are the primary source of natural IgM, which provides the first line of defense against infection (3). Natural antibodies are non-immune, polyreactive, low affinity immunoglobulin found in both humans and mice (4–6). In mice, 80% of natural IgM is produced by B-1a cells (7). Natural IgM structure is germline-like due to minimal insertion of non-template-encoded nucleotides (N-region addition), which are added to the V-D and D-J junctions by the enzyme terminal deoxytransferase (TdT) (8, 9). This B-1 cell derived germline-like natural IgM has been shown to be essential in 1) immediate defense against bacterial and viral pathogens (10–13); and, 2) elimination of autoantigens through removal of apoptotic cell debris and noxious molecular species (5, 14–16).
N-addition plays a key role in antigen receptor diversification and antibody effectiveness or lack thereof for antigen specificities predominately recognized by B-1a cells. Such specificities include phosphorylcholine (PC) (17). PC is a principal antigen found on the cell wall of S. pneumoniae. The prototypical B-1a anti-PC/anti-pneumococcal antibody, T15, has no N-addition, and is particularly effective (18, 19). Notably, vaccination of TdT transgenic mice with heat-killed S. pneumoniae elicited anti-PC Abs; however, these antibodies were not protective (20) due to forced N-addition in these anti-PC antibodies. Importantly, in the absence of B-1a cells, animals were unable to survive infection with S. pneumoniae due to the lack of natural IgM, in particular anti-PC and anti-pneumococcal capsular polysaccharide-3 (PPS-3) (21). These studies demonstrate the importance of structure in protection provided by germline-like natural antibody, and highlight the importance of natural IgM produced by B-1a cells in the immediate response to and therefore survival of infection (3).
Of the 1.2 million cases of pneumonia in the U.S. each year, one out of every 2.4 is caused by S. pneumoniae, making it the most common cause of pneumonia (22, 23). The incidence and mortality rate of pneumococcal infection increase dramatically in people over the age of 65, moving from an average incidence / mortality rate of 10.2 / 0.92 in ages 5–49 to 39.7 / 6.7 in people over the age of 65 (24). Clearly these numbers reveal an alarming disparity between the two age groups despite the availability of a vaccine, PPSV23, approved for adults 65 years of age and older since 1983 (23). These data clearly demonstrate pneumococcal infection still poses a great challenge in prevention and treatment in the elderly population, which immunization and B-2 cell adaptive immunity have not been able to overcome to date.
It has been shown mature B cell responses to antigen and the ability of B cells to produce effective antibodies are defective in the aged immune system in both mice and humans. After immunization of old mice the amount of antibody produced is not always changed but the affinity to the specific antigen is severely decreased as compared to antibody produced by immunization of young mice (25, 26). Antibodies against PC in older mice show usage of a variety of VH genes other than the restricted VH1 utilized by the T15 idiotype of younger mice (27). Significantly, these anti-PC antibodies found in older mice have been shown to be less protective than the T15 idiotype found in young mice (28, 29). In humans, post-vaccination IgG antibody titers against PPS antigens are the same in young adults (under the age of 45) and elderly adults (over the age of 65); however, the elderly population contains antibodies less effective at clearing bacteria (30–34). Furthermore, it was demonstrated that removing IgM from the serum of post-pneumococcal vaccinated samples eliminated the functional difference of bacterial killing between young adults and elderly individuals, which indicates the functional antibody deficiency observed in the elderly population lies within the IgM pool (35). Together these studies suggest immunization in the aged does not elicit generation of protective antibodies. Consequently, the aged individual is dependent upon natural IgM, which may be dysfunctional, for protection against infection.
The human equivalent of mouse B-1a cells was recently identified and shown to decrease in frequency with increasing age (36). Furthermore, it has been shown that natural IgM PPS specificities decrease in the aged (≥65 years) (37). While B-1a cell-derived natural antibody is crucial for survival of infected animals, the maintenance of B-1a cells and/or natural antibody in the aged immune system is poorly understood. Herein, we utilized the well-characterized murine system to uncover how B-1a cells and the protective natural IgM they produce are affected by age.
We have previously reported adult bone marrow-derived (BMD) B-1a cells are functionally and phenotypically similar to native (fetal-derived) B-1a cells from young animals in most respects. However, BMD B-1a cell antibody moves away from germline and lacks the same level of repertoire skewing as native B-1a cell antibody (38). Importantly, B-1a cells from middle-aged mice (6–10 months age) express antibody that contains more N-region additions than antibody produced by B-1a cells from young adult mice (38, 39). Such antibody might be considered less germline-like, less repertoire-skewed, more diverse, and ultimately less effective. We hypothesized that the level of natural IgM protection changes with age as a result of alterations to the B-1a cell pool over time. Here we demonstrate that: 1) the protection afforded by natural serum IgM against pneumococcal infection in vivo diminishes with advancing age; 2) the structure of B-1a cell-derived natural IgM changes by 6 months of age and this change is maintained into old age (18–24 months); 3) changes in structure and function of natural IgM with age are a consequence of selection pressures acting on the B-1a cell pool, which is comprised of both fetal and adult derived B-1a cells. The results presented here demonstrate how selection plays a significant role in the maintenance of the available natural antibody repertoire with age.
Materials and Methods
Mice
Male BALB/cByJ mice obtained from The Jackson Laboratory at 6–8 weeks of age were aged in our facility at 5 mice per cage for the time indicated. CB17-SCID or CB17 mice of 6–8 weeks of age were obtained from Taconic. Mice were cared for and handled in accordance with National Institutes of Health and institutional guidelines.
Cell Purification and Flow Cytometry
Peritoneal washouts and splenocytes were stained with immunofluorescent antibodies, and then analyzed on a LSRII flow cytometer or Influx cell sorter (BD Biosciences) with gating on live cells by forward scatter and side scatter. Images were constructed with FlowJo 6.0 software (Tree Star, San Carios, CA). The following antibodies were obtained from BD Pharmingen: Biotin-conjugated rat anti-mouse IgMb (clone AF6–78); FITC-conjugated rat anti-mouse IgMa (clone DS-1); PE-conjugated rat anti-mouse CD19 (clone ID3), CD43 (clone S7), Mac-1 (clone M1/70), B220/CD45 (clone RA3-6B2), CD23 (clone B3B4), PDL2 (clone TY25), CD86 (clone GL1) CD80 (clone 16-10A1), CD25 (clone PC61); PE-Cy5-conjugated rat anti-mouse CD5 (clone 53-7.3); Alexa647-conjugated rat anti-mouse CD5 (clone 53-7.3); and PerCP-Cy5.5-conjugated rat anti-mouse B220/CD45 (clone RA3-6B2). The following antibodies were obtained from Biolegend: PE-Cy7-conjugated rat anti-mouse CD23 (clone B3B4) and Pacific Blue-conjugated rat anti-mouse IgD (clone 11–26c.2a). The following Biotin-conjugated rat anti-mouse antibodies were used to discriminate lineage positive cells: Mac-1 (clone M1/70); TER-119; CD8a (clone 53-6.7); Ly-6C / Ly-6G (clone RB6-8C5); CD5 (clone 53-7.3); and IgM (Southern Biotech). For PC staining the following was used: PE-Cy7 labeled PC-BSA and FITC labeled BSA used at 10ug/ml (PC+BSA− B-1a cells were used for sorting). For all flow cytometry, live cells were gated first using FSC/SSC, which were then subsequently gated as indicated.
Single Cell Sequencing and Analysis
Peritoneal washout cells and splenocytes were obtained from BALB/c-ByJ mice at the ages indicated and stained with fluorescence labeled antibodies to B220, CD5, and CD23. B cell populations including native peritoneal CD5+ B1 cells (B220lo/CD5+) were then purified using an Influx cell sorter (BD Biosciences). Post-sort re-analysis of B cell populations showed them to be ≥98% pure. Peritoneal CD5+ B-1 cells were sorted onto a 48-well AmpliGrid (Advalytix). Single cell PCR was then carried out as previously described (40). Sequencing data was deposited into NCBI’s Genbank (http://www.ncbi.nlm.nih.gov/genbank/) and the accession numbers for the sequences are listed in Supplemental Figure 7.
Total Ig and antigen specific ELISA
Serum was collected from individual BALB/c-ByJ naïve mice at the time of euthanasia at the ages indicated. The serum was analyzed for total IgM or IgG by ELISA according to the manufacturer’s instructions (Bethyl Laboratories). IgM specific PC and PPS3 serum levels were measured by coating 96-well plates with PC-BSA (Biosearch Technologies) or PPS3 (American Type Culture Collection) at 5 µg/ml in 1× PBS. All plates were washed with TBS Tween (0.05%) and blocked with 1% BSA for 1 hour at room temperature. Bound IgM was measured using HRP-conjugated goat anti-mouse IgM (Bethyl Labs). IgM standards were included on each plate and PC / PPS3 specific antibody levels were interpreted as volume equivalent of IgM, as described in (41).
Allotype ELISA
Serum was collected from individual naïve chimera mice at the times indicated post transplantation, and from 3-month old BALB/c-ByJ mice, and 3-month old CB17 mice at the time of euthanasia. The serum was analyzed for total IgMa and IgMb by ELISA. Plates were coated with either anti-mouse IgMa (BD Pharmingen, clone DS-1) or anti-mouse IgMb (BD Pharmingen, clone AF6–78) at a final concentration of 10 µg/ml in 1× PBS. Plates were then washed 3× and blocked with 5% milk in 1× PBS with 0.05% Tween. Bound IgM was measured using HRP-conjugated goat anti-mouse IgM (Southern Biotech #1020-05) at 1:2000. IgMa and IgMb standards were included on each plate.
Adoptive Transfer
Lineage negative (Lin−) bone marrow obtained from 2-month old BALB/c-ByJ (IgMa) was sort purified using the Influx cell sorter (BD Biosciences), washed twice in 1× PBS, resuspended in 1× PBS, and then injected (i.v.) into recipient CB17-SCID mice at 0.2×106 cells per mouse in 0.2 ml. Recipient mice were not irradiated prior to transfer. At the same time of Lin− bone marrow injection, total peritoneal washout cells obtained from 2-month old CB17 (IgMb) mice were washed twice in 1× PBS, resuspended in 1× PBS, and then injected (i.p.) into the same recipient CB17-SCID mice at 3×106 cells per mouse in 0.2 ml. Serum, spleens, and peritoneal washout cells were collected from euthanized CB17-SCID recipients 1, 3, 6, and 10-months post transfer.
Pneumococcal Infection
Serum was obtained from naïve 3, 18, 23, and 24-month old BALB/c-ByJ mice at the time of euthanasia. The serum samples obtained from these mice were depleted of IgG using protein G (Santa Cruz Biotechnology). Each serum sample from each mouse was kept separate and not pooled. After depletion of IgG, the amount of IgM present in each sample was assessed by ELISA. Each sample to be injected was adjusted to 70 ug of total IgM in 400 µl. CB17-SCID mice were first injected i.p. with IgG-depleted serum samples containing 70 ug of IgM. Four hours after receiving the serum IgM, mice were injected i.p. with 60 CFU of Streptococcus pneumoniae strain WU2. The mice were then monitored for survival over the next 13 days. CB17-SCID mice receiving serum included: 16 CB17-SCID mice were injected with IgG-depleted serum samples containing 70 ug of IgM from 3 month old BALB/c-ByJ mice, 5 CB17-SCID mice were injected with IgG-depleted serum samples containing 70 ug of IgM from 18 month old BALB/c-ByJ mice, and 10 mice were injected with IgG-depleted serum samples containing 70 ug of IgM from 23–24 month old BALB/c-ByJ mice.
The infection experiment was performed with the whole bacteria WU2, which is a type 3 strain of strain of Streptococcus pneumoniae. The WU2 was kindly provided by Dr. David E. Briles (University of Alabama at Birmingham). This strain was grown in Todd-Hewitt broth supplemented with 0.5% yeast extract and once it reached mid-log phase of growth was frozen as glycerol stocks at −70°C. Frozen stocks were kept at −70°C for 2 weeks before determining CFUs by enumerating colony growth on blood agar plates (BAP). At the time of infection, bacteria were diluted in sterile PBS and the number of CFUs was reconfirmed.
Statistics
Comparisons were conducted as indicated using Graphpad Prism 6.0. Chi-squared analysis was performed using 2×4 and 2×2 comparisons as indicated. Student’s t-test was performed on data having a normal distribution. The Mann-Whitney test was performed on data without a normal distribution. Statistical analysis was performed on Kaplan-Meier survival curves using the log-rank test.
Results
Natural IgM from aged mice is not protective against pneumococcal infection
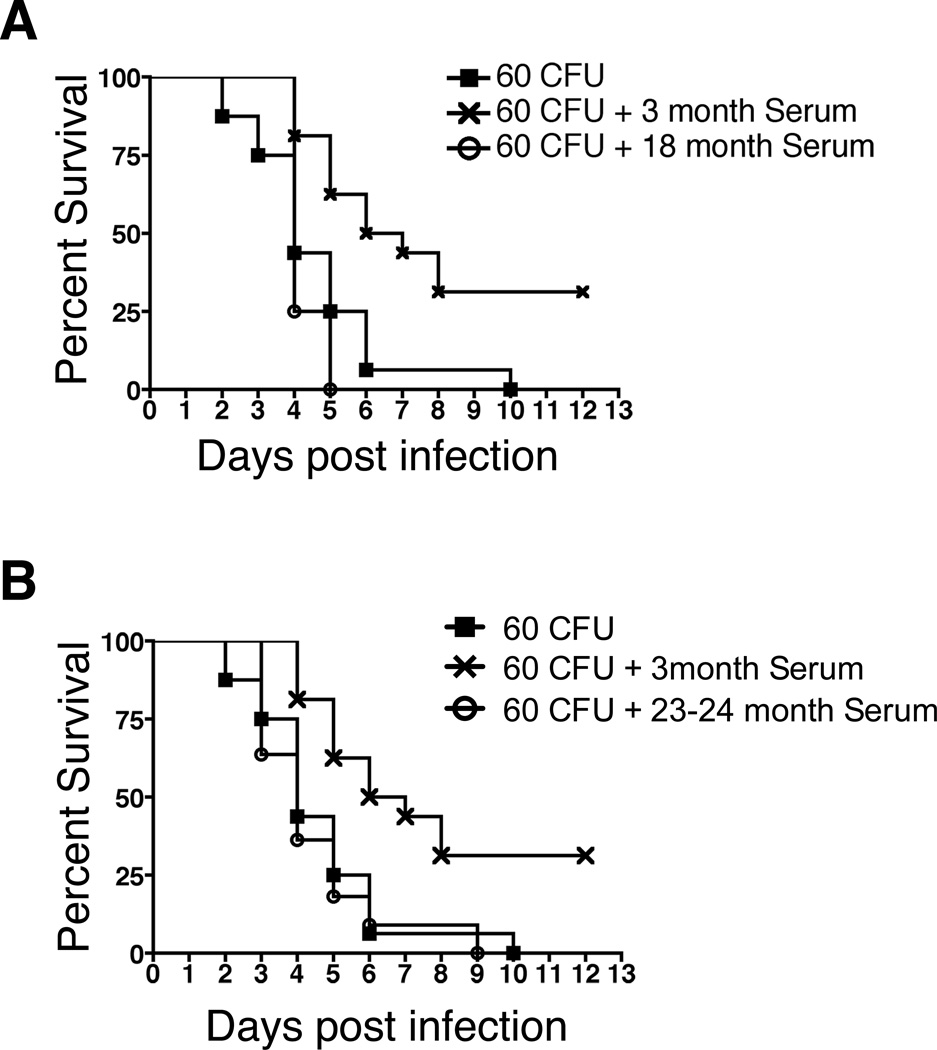
Previous studies have suggested that aging alters the protective capacity of anti-pneumococcal antibody (28, 29). However, this previous work examined adaptive antibodies elicited by immunization, whereas age-related changes in natural antibody, and in particular natural IgM antibody, have not been elucidated. To address the anti-microbial function of natural IgM antibody, we examined individual serum samples from 3-month (n=16), 18-month (n=5), and 23–24-month (n=11) old mice. Serum samples were depleted of IgG using protein G, after which they were assessed for total IgM and IgG. IgG was completely removed in all but three 3-month old serum samples and all but seven of the aged serum samples; however, the survival time of the samples did not differ from those in which IgG was completely removed (Supplemental Figure 1). CB17-SCID mice were then injected with PBS or 70 µg of serum IgM from either young or aged mice 4 hours prior to infection with 60 CFU of Streptococcus pneumoniae (WU2 strain), after which the fate of infected mice was monitored. We found that the survival of SCID mice receiving 3-month old mouse serum IgM was significantly longer than survival of SCID mice given PBS, as shown by Kaplan-Meier curves (p=0.002). In contrast, survival of SCID animals receiving serum IgM from either 18-month (Figure 1A) or 23–24-month (Figure 1B) old mice was significantly less than SCID mice receiving serum IgM from 3-month old mice (p=0.008 and p=0.003 respectively), and was not significantly different than survival of SCID mice receiving PBS. Thus, in contrast to the beneficial action of natural IgM from young mice, natural IgM from old mice provides no protection against pneumococcal infection, indicating an age-associated loss of natural antibody-mediated anti-microbial activity.
Figure 1. Serum IgM from aged mice is less protective against pneumococcal infection.
Serum samples were obtained from 3, 18, or 23–24-month old male BALB/c-ByJ mice at time of euthanasia. The samples were depleted of IgG by protein G clearance. An equal quantity of serum IgM (70µg) was injected in a total volume of 400 µl (i.p.) into CB17-SCID mice from the 3-month (n=16), 18-month (n=5), 23–24-month (n=11) old serum samples, or PBS only (n=16). Four hours post injection the CB17-SCID mice were injected (i.p.) with 60 CFU of Streptococcus pneumoniae WU2 strain. Statistical analysis was performed using the log rank test: 3-month vs. PBS, p=0.002; 3-month vs 18-month, p=0.008; 3-month vs 24-month, p=0.003.
Serum anti-PC and anti-PPS3 levels do not explain the age-associated loss of anti-microbial activity
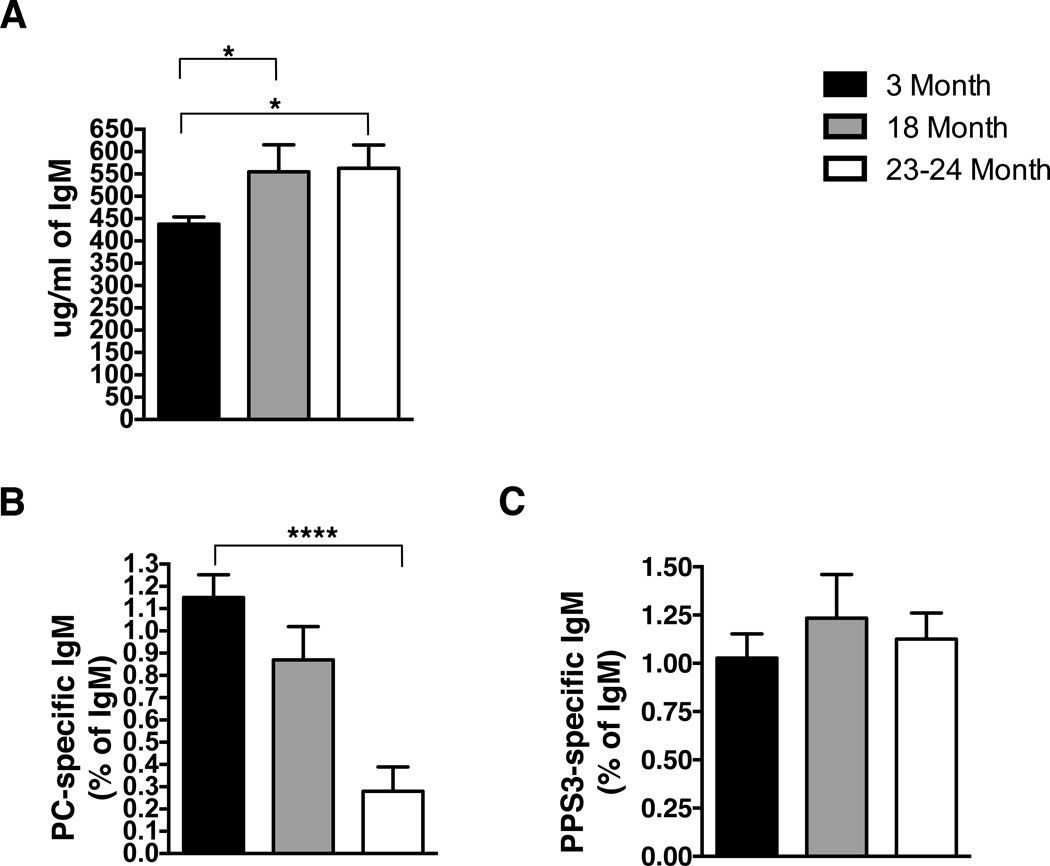
The loss of antibody anti-microbial function could be due to a quantitative decline or a qualitative change. To understand the mechanism of diminished anti-pneumococcal activity in natural IgM from old mice, we first examined serum samples for PC- and PPS-3-specific IgM, which have been shown to be required for protection against S. pneumoniae infection (21). Initially, sera from young adult (3-month) and aged adult (18–23-month) mice were assessed for total IgM levels by ELISA. We found the total amount of serum IgM (Figure 2A) was significantly higher in 18-month and 23-month old mice as compared to 3-month old mice; regardless, all infected mice (Figure 1) received the same amount of total IgM. Next, the same serum samples were assessed for PC− (Figure 2B) and PPS-3-specific IgM (Figure 2C). The amount of PC-specific IgM was not significantly different in 18-month old mice as compared to 3-month old mice. However, a significant decrease in PC-specific IgM was observed in 23-month old mice (p<0.0001). In contrast, the level of PPS-3 specific IgM was not significantly different in 18 or 23-month old mice as compared to 3-month old mice. As shown in Supplemental Figure 2, the total amount of anti-PC or anti-PPS-3-specific IgM mimics the amount of each relative to the total amount of IgM, which is presented in Figure 2. Note that serum IgM from 18-month old mice failed to protect against infection (Figure 1A) despite the fact that the levels of anti-PC and anti-PPS-3 specific IgM at 18-months did not differ from 3-month old mice (Figure 2A,B and Supplemental Figure 2A,B). We used protein G to deplete the serum of IgG; however, protein G would not remove any IgA present. Therefore, we assessed the levels of total and PC-specific IgA in 3-month old and 15-month old mice. Interestingly, these demonstrate the level of anti-PC-specific IgA in 3-month old mice (0.04 ug/ml) is more than 100-fold less than the level of anti-PC-specific IgM (5 ug/ml) (Supplementary Figures 3 and 2, respectively); yet, there is still significant protection against S. pneumoniae infection from 3-month old serum (Figure 1). Furthermore, serum from aged mice 15-month) showed significantly more anti-PC-specific IgA (2.6 ug/ml) over the level seen in the 3-month old mice (0.04 ug/ml); yet, the aged serum was still less protective than young serum despite this increase in the level of anti-PC-specific IgA.
Figure 2. Serum Ig from aged mice is significantly different from young serum.
Serum was collected from 3 (n=9), 18 (n=5), 23-month (n=6) old BALB/c-ByJ mice at time of euthanasia. Serum samples were analyzed for (A) total IgM, (B) PC-specific IgM, and (C) PPS3-specific IgM. The total amount of PC and PPS3 specific IgM was normalized as a percent of total IgM in each sample. Black bars represent 3-month old mice, grey bars represent 18-month old mice, and white bars represent 23-month old mice. Values are displayed as the mean (±SEM) of individual mouse serum samples. Statistics were performed using unpaired, two-tailed student’s t-test. Serum IgM: 3-month vs. 18-month, p=0.03; 3-month vs. 23-month, p=0.01. PC-specific IgM: 3-month vs. 23-month, p<0.0001.
Together, these results demonstrate a significant loss of the protective capacity of natural serum IgM in aged mice that is not accounted for by a change in the level of PC- or PPS3-specific IgM or PC-specific IgA, and raise the possibility that the anti-microbial activity of serum natural antibody could be affected by an aged-related change in IgM sequence.
Sequence Analysis of Natural IgM from PC binding B-1a cells demonstrates changes with age
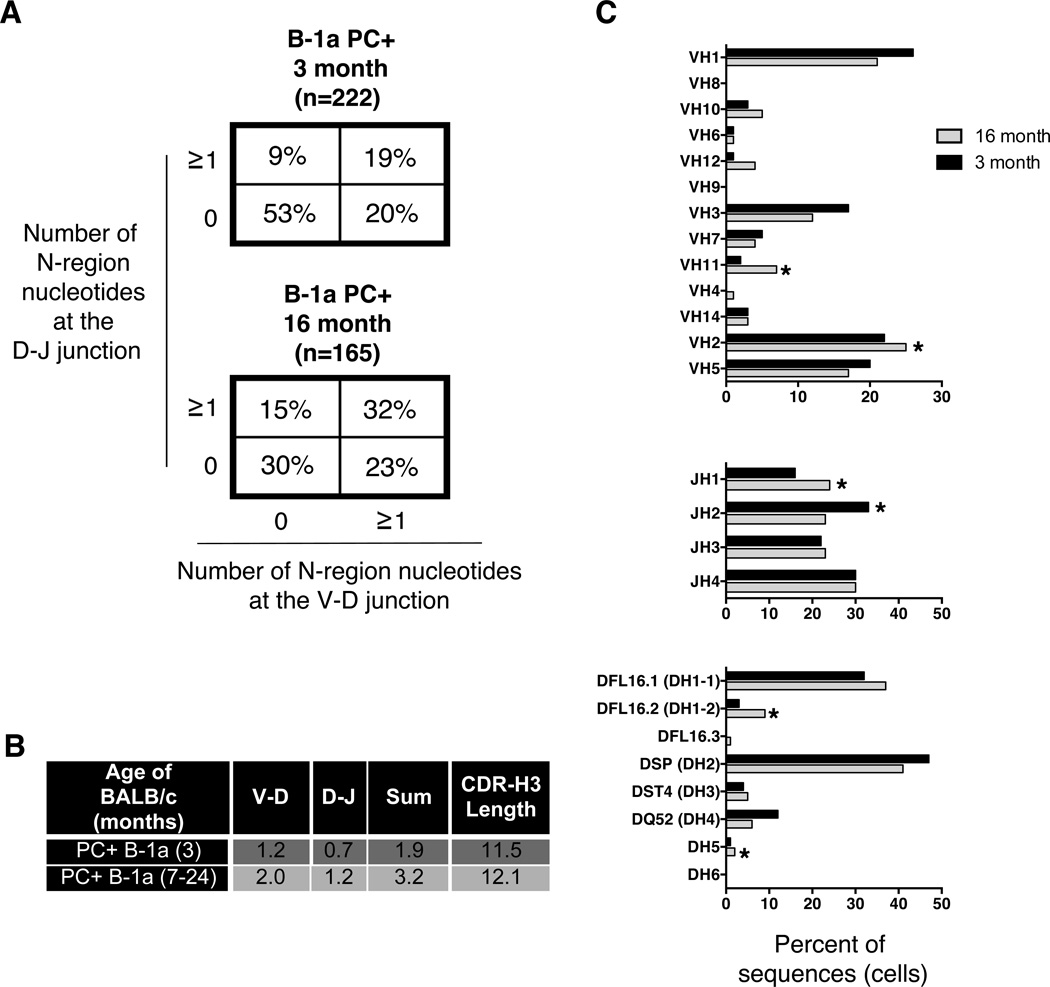
We assessed the nucleotide sequence of IgM from PC binding B-1a cells in young adult and aged mice. As shown in Figure 3A, peritoneal PC+ B-1a cells from young mice produce IgM with few N-additions (53% of sequences have zero N-additions at both junctions). Peritoneal PC+ B-1a cells from aged mice produce IgM with significantly more N-additions (30% of sequences have zero N-additions at both junctions). Taking into account both junctions (analysis of the junctions: 0 at both, 0 at V-D, ≥1 at both, and 0 at D-J) × 2 populations = 4×2 Chi-square analysis), peritoneal PC+ B-1a cell IgM from aged mice is significantly different than peritoneal PC+ B-1a cell IgM from young mice (p<0.0001). Furthermore, we found N-addition lengths are significantly longer in PC+ B-1a cell IgM from aged mice as compared to PC+ B-1a cell IgM from young mice at the V-D junction (p=0.0002), the D-J junction (p<0.0001), and for the sum of the two junctions (p<0.0001). CDR-H3 analysis also demonstrated an increase in length in PC+ B-1a cell IgM from aged (12.1± 0.194) as compared to young (11.5± 0.155) mice (p=0.01). These results are summarized in Figure 3B.
Figure 3. Sequence analysis of natural IgM from peritoneal PC binding B-1a cells in aged and young adult mice.
PC+ peritoneal B-1a cells were single cell sorted from 3 and 16-month old (as indicated) male BALB/c-ByJ mice. The VH region was amplified and sequenced as detailed in the Materials and Methods section. (A) The percent of sequences with zero N-additions at both junctions, one or more N-additions at both junctions, zero N-additions at V-D and 1 or more at D-J junctions, or zero N-additions at D-J and 1 or more at V-D junctions is shown. (B) Average number of N-additions at the V-D, D-J, or sum of the two junctions is shown along with CDR3 length. (C) VH, DH, and JH gene segment usage in B-1a cells from 3-month (black bars) or 16-month (grey bars) old B-1a cells is displayed. Results are based on 2 independent experiments with sequences combined from each independent experiment.
Analysis of VH, DH, and JH usage revealed a few significant differences. Peritoneal PC+ B-1a cells from aged mice display an increase in VH2 (p=0.014), VH11 (p=0.03), JH1 (p=0.04), DFL16.2 (p=0.01), and DH5 (p=0.04) usage, but a decrease in JH2 (p=0.04) usage (Figure 3C). Examination of the CDR-H3 loop region revealed a decrease in charge in aged mice (−0.125± 0.024) as compared to young old mice (−0.182± 0.022) (p=0.04) (Supplemental Figure 4). Somatic hypermutation (SHM) analysis revealed PC+ B-1a cell IgM from aged mice display a higher rate of SHM (7.4± 1.4) than PC+ B-1a cell IgM from young mice (5.9± 0.8) (Supplemental Figure 6A), but this difference did not reach the level of significance.
These results demonstrate a shift away from germline in the antibody of PC+ B-1a cells from aged mice. These results together with previous studies showing less protection from S. pneumoniae infection in TdT transgenic mice, which have forced N-addition in all PC antibodies (20), demonstrate a change in antibody nucleotide sequence created by increased N-addition produces less protective natural IgM.
Sequence Analysis of Natural IgM from all B-1a cells demonstrates changes with age
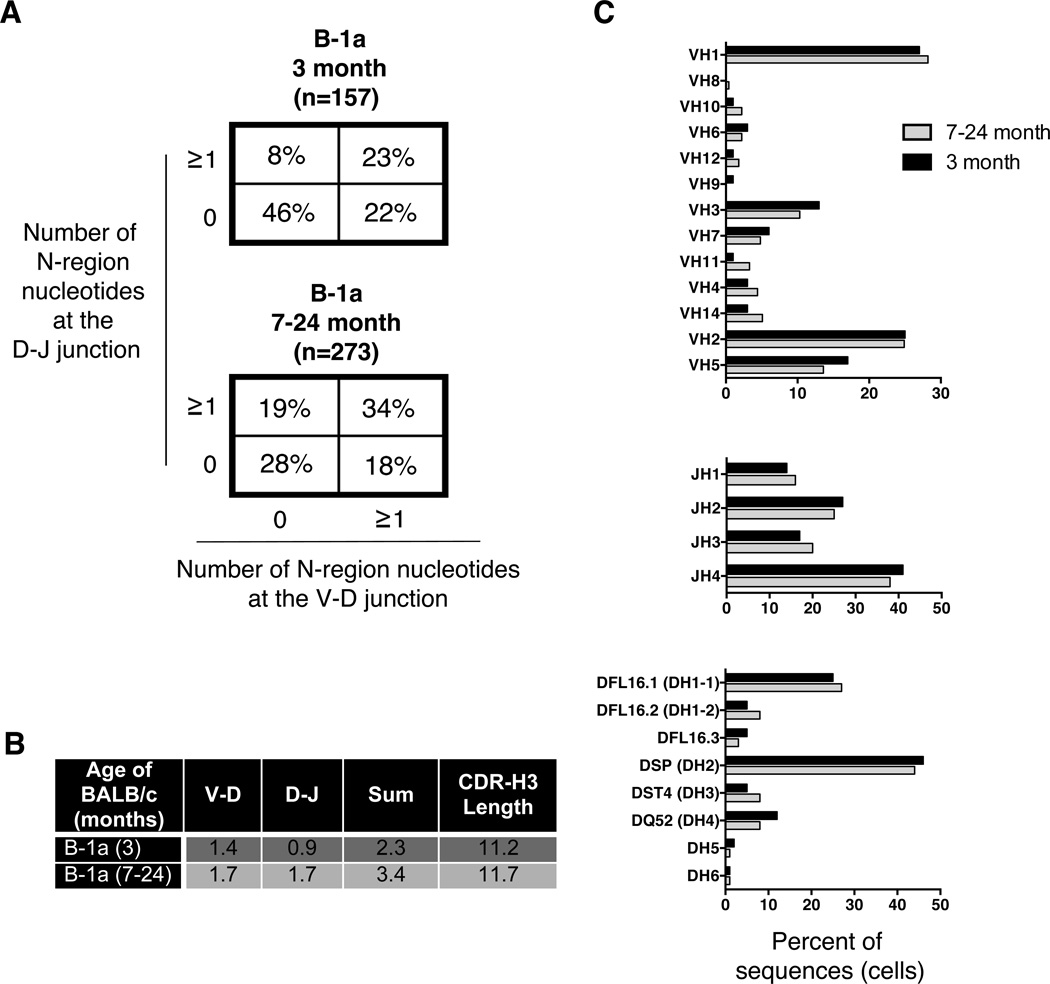
Prior results suggest that the change in N-addition found in B-1a PC-binding immunoglobulin from aged mice may be generalizable to the entire population of B-1a cells (38, 39), although earlier work did not look beyond 10 months of age. Therefore, we used single cell PCR to evaluate natural IgM from the entire peritoneal B-1a cell population obtained from male BALB/c-ByJ mice at various ages from 3 to 24 months (Supplemental Figure 5). We found a marked difference in the number of N-additions in B-1a cell antibodies from young versus aged mice; however, this difference was fully developed by 7 months of age. There is no statistically significant difference in the increased level of N-additions within the various age groups beyond 7-months of age (7–12-month vs. 18-month old mice; 7–12-month vs. 23–24-month old mice; or 18-month vs. 23–24-month) by Chi-square analysis (Supplemental Figure 5). Therefore, for repertoire analysis of the total B-1a cell population, we compared two groups, young adult mice (3 months; n=157) versus older adult mice (7–24 months; n=273). The percent of sequences lacking N-addition at both junctions in 3-month old mice (46%) was similar to our previous report (38) and quite different from the percent of sequences lacking N-addition at both junctions in 7–24-month old mice (28%) (Figure 4A). When both junctions are considered at the same time, (analysis of the junctions (0 at both, 0 at V-D, ≥1 at both, and 0 at D-J) × 2 populations = 4×2 Chi-square analysis) there is a significant increase in N-region additions with age comparing 3-month old mice to 7–24-month old mice (p<0.0001) (Figure 4A). Furthermore, we found N-addition lengths were significantly longer in B-1a cell IgM from aged mice as compared to B-1a cell IgM from young mice at the D-J junction (p<0.0001) and for the sum of the two junctions (p=0.0003). Beyond N-addition, we analyzed CDR-H3 length. We found a significant increase in antibody CDR-H3 length from aged mouse B-1a cells (11.7± 0.151) as compared to young mouse B-1a cells (11.2± 0.238) (p=0.03). These results are summarized in Figure 4B. Thus, both N-addition and CDR3 length differ in aged versus young mice.
Figure 4. Sequence analysis of natural IgM from peritoneal B-1a cells obtained from aged and young adult mice.
Peritoneal B-1a cells were single cell sorted from 3, 7, 9, 10, 12, 18, 23, and 24-month old BALB/c-ByJ mice. The VH region was amplified and sequenced as detailed in the Materials and Methods section. (A) The percent of sequences with zero N-additions at both junctions, one or more N-additions at both junctions, zero N-additions at V-D and 1 or more at D-J junctions, or zero N-additions at D-J and 1 or more at V-D junctions is shown. (B) Average number of N-additions at the V-D, D-J, or sum of the two junctions is shown along with CDR3 length. (C) VH, DH, and JH gene segment usage in B-1a cells from 3-month (black bars) or 7–24-month (grey bars) old B-1a cells is displayed. Results are based on 8 independent experiments with sequences combined from each independent experiment.
Further analysis of VH, DH, and JH usage did not reveal any statistically significant differences in 3-month old versus 7–24 month old mice (Figure 4C). Examination of the CDR-H3 loop region revealed an increase in charge in 7–24 month old mice (−0.099± 0.024) as compared to 3-month old mice (−0.021± 0.026) (p<0.0001) (Supplemental Figure 4). The increase in CDR-H3 charge in the aged population might be predicted since autoreactive antibodies are more charged in CDR-H3 (42), increase with age, and are typically B-1a cell-derived (43). Somatic hypermutation (SHM) analysis revealed B-1a cell IgM from aged mice display a significantly higher rate of SHM (11.21± 1.25) than B-1a cell IgM from young mice (8.78± 1.50) (p=0.01) (Supplemental Figure 6A).
Together, these results demonstrate B-1a cell antibody structure drifts away from germline with age as evidenced by an increase in N-region additions and CDR-H3 length. Interestingly, this change in structure occurs by 6 months of age and is not altered thereafter for the life of the animal as measured up to 24 months ((38) and Supplemental Figure 5).
Both Bone Marrow and Fetal Derived B-1a cells contribute to the B-1a cell pool with age
It has been previously shown that bone marrow derived (BMD) B-1a cells produce Ig with significantly more N-region addition than fetal liver derived B-1a cells (38, 40, 44). Therefore, we hypothesized the age-related change in natural antibody sequence and decreased protective capacity of natural IgM could be due to an increase in diversity produced by emigration of BMD B-1a cells into the adult B-1a cell pool. In previous experiments bone marrow cells were adoptively transferred into immunodeficient “empty vessel” mice with no native B cells; however, this approach could have yielded nonphysiological results inasmuch as B-1a cells are known to exert feedback inhibition on B-1a development (45).
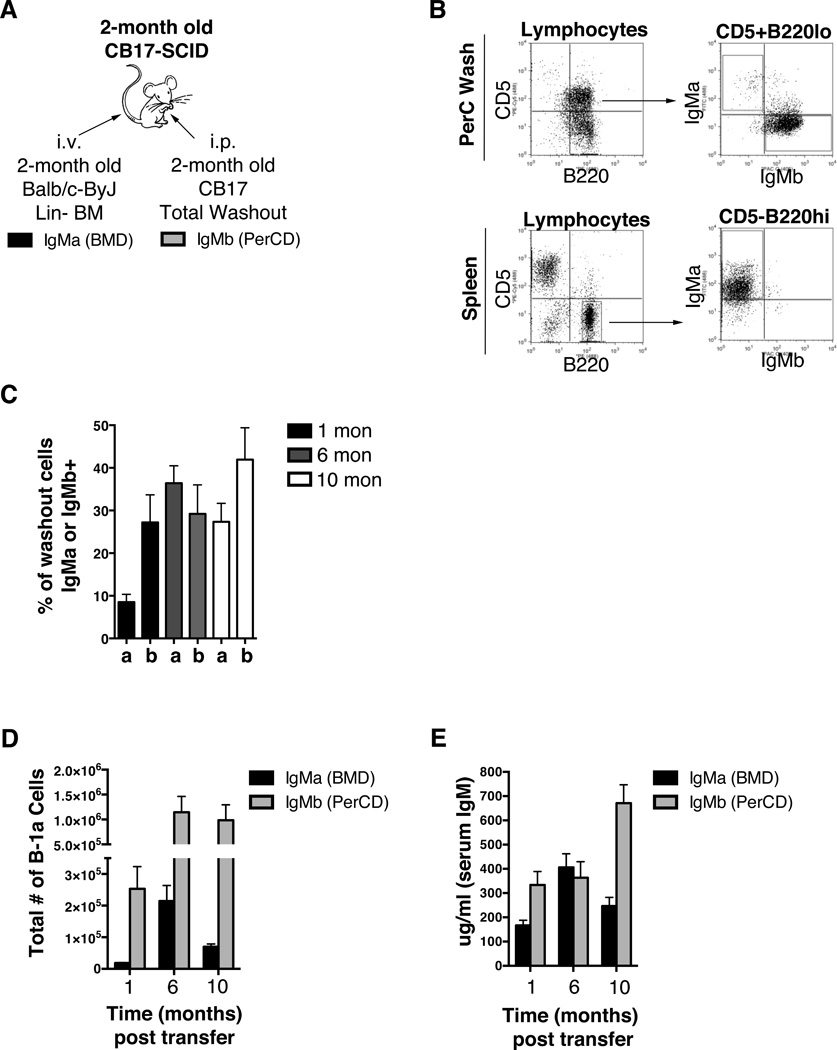
To directly test the extent to which BMD B-1a cells contribute to the B-1a cell pool in presence of competing B-1a cells, we set up a mixed chimera system. Figure 5A illustrates the experimental design that involved simultaneous injection of lineage negative bone marrow (BM) from 2-month old BALB/c-ByJ mice and total peritoneal cavity cells (PerC) from 2-month old CB17 mice into 2-month old CB17-SCID recipients. Allotypic differences between BALB/c-ByJ (IgMa) and CB17 (IgMb) mice were used to assess the individual contribution of peritoneal cavity and bone marrow derived B-1a cells to the overall B-1a cell pool with increasing age. We found the peritoneal and splenic compartments were reconstitued at 1, 6, and 10 months after transfer of BM and PerC cells (Figure 5B displays representative dot plots at 10 months post transfer). On average the percent of live splenocytes that consisted of reconstituted B-2 cells at 1, 6, and 10 months post-transfer were 20%, 36%, and 38%, respectively. We then examined the total number of peritoneal B-1a cells derived from each source (Figure 5D). At all time points the number of PerC-derived (PerCD) B-1a cells was significantly higher than the number of BMD B-1a cells. Interestingly, the number of BMD B-1a cells increased from 1 to 6-months post transfer (p=0.004) but decreased from 6 to 10-months post transfer (p=0.02). However, the total number of BMD peritoneal B-1a cells was still significantly higher at 10 months compared to 1 month post transfer (p=0.0004). The total number of B-1a cells derived from peritoneal cavity cells increased from 1 month to 6 months (p=0.03) post transfer and then remained steady from 6 to 10 months post transfer. Together these results clearly show BMD B-1a cells contribute to the B-1a cell pool with age, although this appears to be time limited. Moreover, despite a peak at 6 months post transfer, the contribution of BMD B-1a cells to the B-1a cell pool was significantly lower than that of PerCD B-1a cells.
Figure 5. Contribution of native and BMD B-1a cells with age.
(A) Allotype mixed chimeras were set-up by injecting linage negative (Lin−) bone marrow (i.v.) obtained from BALB/c-ByJ mice (IgMa) and total peritoneal washout cells (i.p.) obtained from CB17 mice (IgMb) into CB17-SCID recipients at the same time. The CB17-SCID recipients were 2-months of age upon transfer. The Lin− bone marrow and total peritoneal washout cells were obtained from 2-month old BALB/c-ByJ and CB17 mice respectively. (B) Representative FACS plots of lymphocytes from peritoneal washout cells (PerC Wash) and splenocytes 10 months post-transfer. Gated peritoneal B-1a (CD5+B220lo) or splenic B-2 (CD5-B220hi) cells are shown and then analyzed for IgMa or IgMb. (C, D, E) At 1, 6, and 10-months post transfer of Lin− bone marrow and total peritoneal washout cells into SCID recipients, serum and peritoneal washout cells were obtained from the recipients at each time point. (C) The percent of live lymphocytes positive for IgMa (a) or IgMb (b) in the collected washout cells was assessed. (D) The number of peritoneal B-1a cells derived from Lin− bone marrow (IgMa, black bars) or PWC B-1a cells from transferred washout cells (IgMb, grey bars). (E) Serum from the mixed chimeras was assessed for IgMa (black bars) and IgMb (grey bars).
Next, we evaluated the separate contributions of BMD and PerCD B cells to total resting serum IgM by measuring the amount of serum IgMa and IgMb from the mixed chimera mice (Figure 5E). The amount of serum IgM derived from BMD B cells increased significantly from 1 month to 6 months (p=0.004) post transfer. Interestingly, the amount of serum IgM derived from BMD B cells then decreased from 6 months to 10 months (p=0.04) post transfer. These results correlate with the increase in BMD B-1a cell number from 1 to 6 months post transfer and the decrease of BMD B-1a cell number from 6 to 10 months post transfer (Figure 5D). Resting serum IgM derived from PerCD B cells stayed at a fairly constant level from 1 to 6 months with an increase at 10 months post transfer. Overall, these results demonstrate the ability of both BMD and PerCD B cells to contribute to resting serum IgM with age.
Change in natural IgM sequence with age is the result of selection
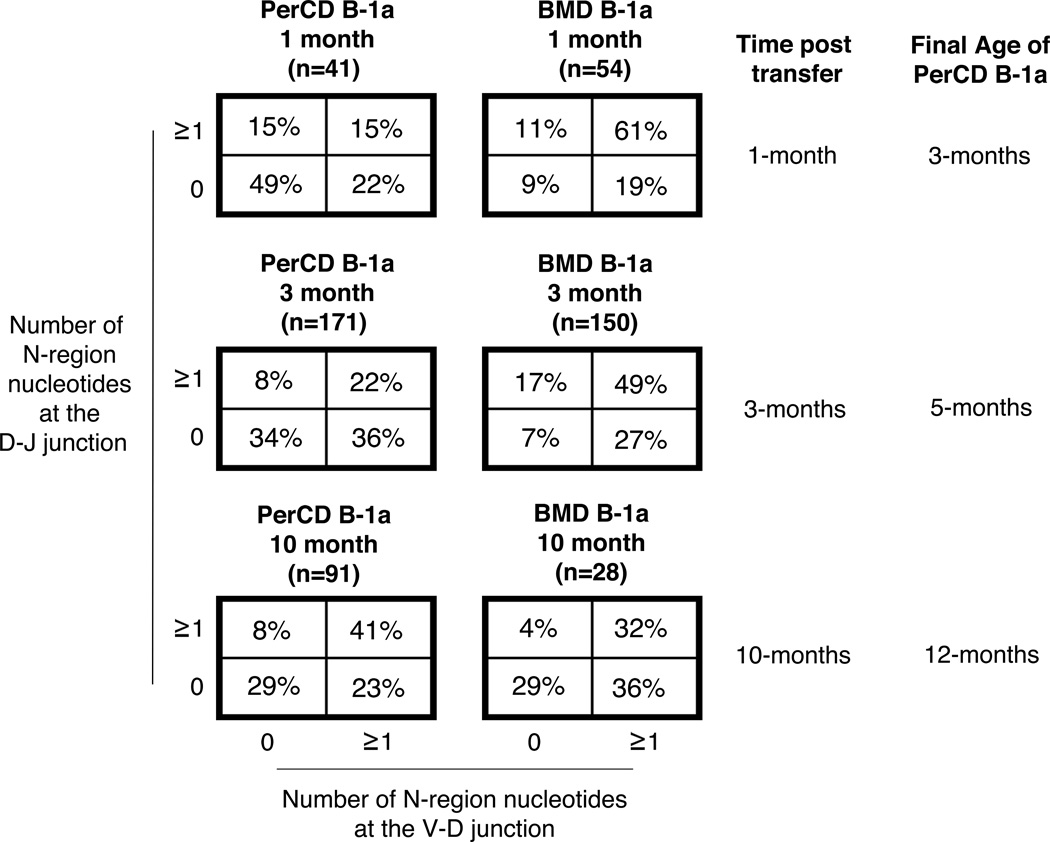
We next asked how natural IgM antibodies produced by either PerCD or BMD B-1a cells change with age. Using PerCD B-1a cells and BMD B-1a cells identified by allotype from the mixed chimeras set-up in Figure 5, we performed single cell PCR to analyze the sequence of Ig from these separately sourced B-1a cells developing in the same environment. Results are displayed in Figure 6. IgM from PerCD B-1a cells 1 month post-transfer had few N-additions with 49% of sequences completely lacking nontemplated nucleotides at both junctions. Note that these PerCD B-1a cells are essentially 3 months old because they were harvested from 2 month old mice; thus it is not unexpected that their low level of N-addition would parallel that of native 3 month old B-1a cells. By 3 months post transfer PerCD B-1a cell IgM showed more N-addition (34% of sequences lack N-additions at both junctions), and then by 10 months post transfer PerCD B-1a cell IgM looked identical to IgM of native B-1a cells from 18–23 month old BALB/c-ByJ mice (29% of sequences lacking N-additions at both junctions). On the other hand, IgM from BMD B-1a cells contained a large number of N-additions at both 1 month and 3 months post transfer with 9% and 7% of sequences lacking N-additions at both junctions, respectively. These results are consistent with our previously published data demonstrating BMD B-1a cells are derived from progenitors with high levels of TdT and produce IgM with abundant N-additions (40, 44). Interestingly, at 10 months post transfer IgM from BMD B-1a cells displayed a decrease in the number of N-additions at both junctions. From 3 months post-transfer to 10 months post transfer the percent of sequences lacking N-additions at both junctions increased from 7% to 29%. By testing allotype-marked PerCD B-1a cells and BMD B-1a cells independently in this system (Figure 5), we assessed changes in antibody structure for each population separately without any potential emigration of BMD B-1a cells into the peritoneal B-1a cell pool (Figure 6). Our results indicate clearly that, with BMD B-1a cell emigration excluded, the peritoneal pool of natural antibody producing B-1a cells changes with age. This indicates a selective process is engaged. Surprisingly, the same is also true for BMD B-1a cells, except selection for N-addition occurs in the opposite direction. As a result, regardless of the source of B-1a cells, the number of N-additions present in IgM from aged B-1a cells is similar with around 29% of sequences lacking N-additions at both junctions.
Figure 6. N-region addition in BMD and native B-1a cells with age.
Allotype mixed chimeras (Figure 5) were generated by injecting lineage negative (Lin−) bone marrow (i.v.) obtained from BALB/c-ByJ mice (IgMa) and total peritoneal washout cells (i.p.) obtained from CB17 mice (IgMb) into CB17-SCID recipients at the same time. The Lin− bone marrow and total peritoneal washout cells were obtained from 2-month old BALB/c-ByJ and CB17 mice respectively. At 1, 3, and 10-months post transfer of Lin− bone marrow and total peritoneal washout cells into SCID recipients B-1a cells derived from bone marrow (IgMa+) or peritoneal washout cells (IgMb+) were obtained from the peritoneal cavities of recipients at each time point. The B-1a cells from each source were single cell sorted and the VH region was amplified and sequenced. The percent of sequences with zero N-additions at both junctions, one or more N-additions at both junctions, zero N-additions at V-D and 1 or more at D-J junctions, or zero N-additions at D-J and 1 or more at V-D junctions is displayed in chart form for each group of B-1a cells.
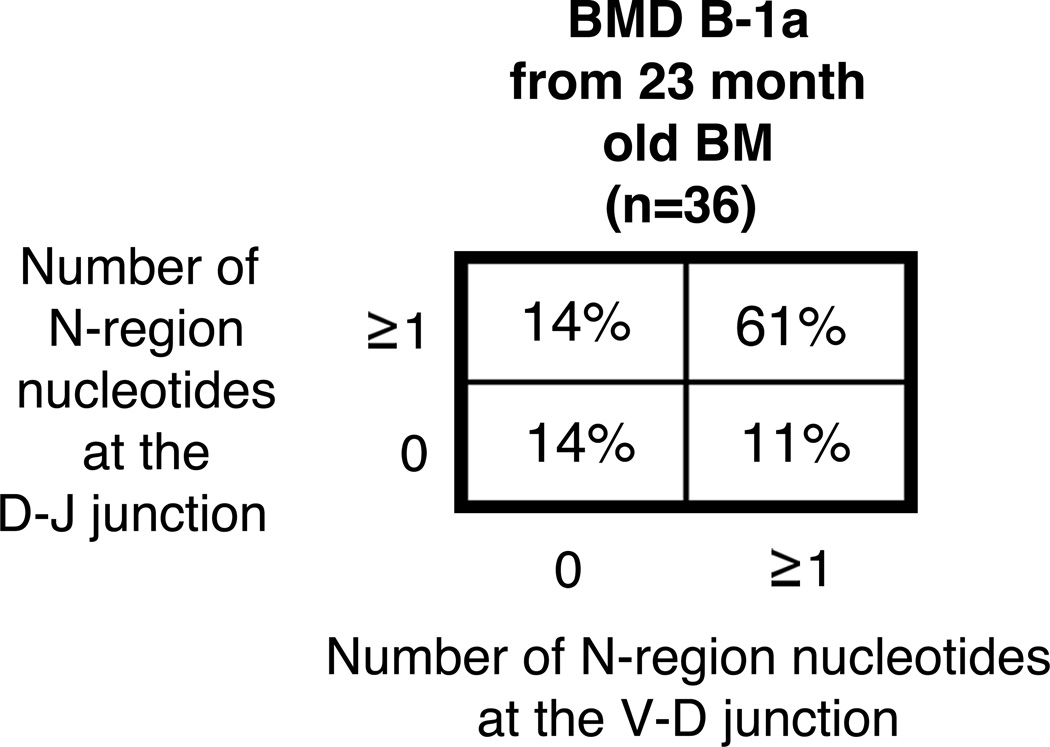
An alternative explanation for these results might be if bone marrow (BM) from aged mice produce B-1a cells expressing IgM with low levels of N-addition, which if true, would contrast with previous work demonstrating BMD B-1a cells from young mice produce IgM with abundant N-additions (40). Thus, we considered the possibility that changes observed in N-addition from BMD B-1a cell IgM 10 months after adoptive transfer of bone marrow (Figure 6) could be due to an age-related change in the function of BM progenitors. To address this, we adoptively transferred total lineage negative BM from 22–23 month old mice and evaluated B-1a cell antibodies one month post transfer. We found no statistical difference in the level of IgM N-addition from B-1a cells derived from young BM versus old BM (Figure 7). These results rule out aged BM dysfunction as an explanation for the age-associated change in BMD B-1a IgM N-addition, and reinforce our conclusion that selection causes the shift away from protective germline IgM in the aged.
Figure 7. N-region addition analysis of B-1a cells derived from aged BM.
Total linage negative (Lin−) bone marrow was obtained from 22–23-month old BALB/c-ByJ mice and injected (i.v.) into CB17-SCID recipients. Four weeks post transplantation the mice were euthanized and evaluated for reconstitution of B-1a cells in the peritoneal cavity. The BMD B-1a cells were single cell sorted and the VH region was amplified and sequenced as detailed in the Materials and Methods section. The percent of sequences with zero N-additions at both junctions, one or more N-additions at both junctions, zero N-additions at V-D and 1 or more at D-J junctions, or zero N-additions at D-J and 1 or more at V-D junctions is shown.
Discussion
B-1a cell-derived natural IgM provides broadly reactive antibody, which controls infection while the adaptive immune system mounts a specific response. However, pneumococcal infections still pose a great challenge in prevention and treatment in the elderly population (46). In the case of adaptive antibodies to PC formed after immunization, older mice produce less protective anti-PC antibodies than those mounted in young mice (28, 29). As a result, natural anti-pneumococcal antibodies are increasingly important; yet, there has been a gap in our knowledge about whether aging affects the protective function of B-1a cell derived natural IgM, and how the available natural repertoire is affected by increasing age.
Herein, our study demonstrates the protective capacity of serum natural IgM produced by B-1a cells decreases substantially with age. Study of PC positive peritoneal B-1a cell natural IgM showed that antibody with this anti-pneumococcal specificity moves away from germline as evidenced by increased N-addition. In addition, we show natural IgM from the total B-1a cell pool changes with age away from the germline. Importantly, we further investigated the mechanism by which these age related changes occur and find that selection plays a key role in the determining the repertoire of B-1a cell natural IgM over time.
Recently, it was demonstrated B-1a cells develop prior to emergence of hematopoietic stem cells in the yolk sac (YS) and para-aortic splanchnopleura (PSp) endothelium (47, 48). These embryonic and fetal derived B-1 cells are maintained in the adult by self-renewal. Moreover, once the B-1a cell pool is formed there is feedback inhibition preventing new input (45). Taken together, there would seem to be little room for any alteration in the B-1a cell natural IgM repertoire during adulthood. Nevertheless, within the last few years several groups have demonstrated the presence of B-1a cell progenitors in adult bone marrow and that these progenitors are capable of producing mature B-1a cells (38, 44, 49, 50). Of particular interest, bone marrow derived B-1a cells differ from fetal-derived B-1a cells in that their natural IgM has significantly more N-region additions (38, 44). Such findings raise questions regarding the stability and source of the B-1 cell pool in adult and aged animals, and have given rise to the two-pathway model of B-1 cell development as proposed in a recent review (7). In this model, the B-1a cell pool formed prior to birth is maintained by self-renewal throughout the life of the animal with some input from the bone marrow in adulthood.
The two-pathway model was built on adoptive transfer experiments in which recipients initially lacked lymphocytes (38, 44, 49, 50). This has led to uncertainty over whether adult bone marrow generates B-1a cells in the presence of other lymphocytes, especially other B-1a cells that can feedback inhibit B-1a cell development. Herein, we addressed this issue by specifically testing whether input from BMD B-1a cells into the peritoneal B-1a cell pool occurs when mature peritoneal B-1a cells are present. To do this we set-up a mixed chimera system (Figure 5A). We found BMD B-1a cells could enter the adult B-1a cell pool in a limited manner and correspondingly contribute to serum IgM even in the presence of mature peritoneal B-1a cells (Figure 5). These results suggest the change in B-1a cell-derived IgM structure with age is the result of input from BMD B-1a cells into the pool of self-renewing fetal derived B-1a cells with age. However, 10 months post transfer the contribution of BMD B-1a cells and the amount of serum IgM derived from BMD B-1a cells decreased. This limited contribution of BMD B-1a cells to the adult B-1a cell pool fits with previous studies demonstrating limited ability of B-1a cell progenitors found in adult bone marrow to produce B-1a cells (51).
These mixed chimera results raised the question of whether something else is responsible, at least in part, for the altered N-addition in aged B-1a cell antibody. To further test this we used allotype markers to separately analyze the structure of peritoneal-derived and bone marrow-derived B-1a cell antibodies. In particular, this experimental design provided the opportunity to evaluate the age-related fate of peritoneal B-1a cells in the absence of any contribution from adult bone marrow. We found in the absence of any BM contribution that peritoneal (fetal-derived) B-1a cell IgM experiences an increase in the number of N-additions with age. Furthermore, despite the source of B-1a cells, fetal- or bone marrow-derived, the number of N-additions in IgM by 10-months of age was similar (Figure 6). Not only was the number of N-additions similar between the two sources of B-1a cells at 10-months post transfer, but it was also much like the number of N-additions found in B-1a cell derived Ig from normal aged BALB/c-ByJ mice (Figure 4). In other words, peritoneal B-1a cell N-addition increased with age; whereas, BMD B-1a cell N-additions decreased with age. Since there was no intermingling of allotype separable pools, these results indicate selection is operating on each population leading to a convergence in N-addition level. In particular, this demonstrates that the B-1a cell repertoire undergoes selection with age regardless of the source (fetal or bone marrow derived), and this adds a new layer to B-1a cell physiology in terms of the time-dependent dynamics of repertoire display.
Thus, two processes, diversification and selection, are at work during B-1a cell aging. On the one hand, there is a mechanism in adulthood allowing B-1a cell input from bone marrow progenitors (diversification). On the other hand, the aging process also applies pressure on the renewal/expansion/viability of the B-1a cell pool during adulthood (selection). Therefore, both bone marrow input and more importantly selection determine the overall structure of natural IgM with increasing age.
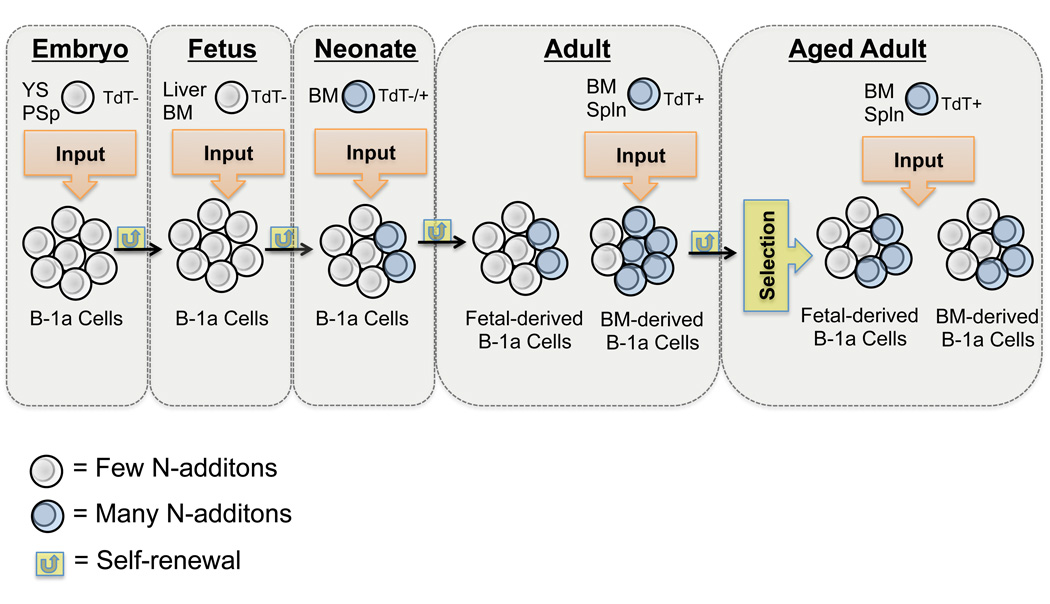
Selection has been discussed in terms of the early developmental history of B-1a cells. It has been shown B-1a cells are positively selected based on their ability to bind self-antigens such as but not limited to phosphorylcholine (PC) (17, 52), phosphatidylcholine (PtC) (52), and Thy-1 (CD90) (53). Furthermore, BCR signal strength plays a crucial role in positive selection of B-1a cells as evidenced by the relative increase or decrease of B-1a cells in mice lacking certain negative or positive signaling mediators, respectively (54, 55). More recently, it has been shown that B-1a cell selection occurs from neonatal life through young adulthood, which is dependent upon antigens not found in the microbiota (56). The results presented here are novel in terms of examining the selection of adult B-1a cells, from different developmental sources (fetal and bone marrow), with age. Clearly both repertoire selection operating on mature B-1a cells, and input of B-1a cells developing from adult progenitors, contribute to the shape of the B-1a cell pool with age, which results in less effective protection against a clinically relevant infectious organism. The way in which diversity is maintained over time in the B-1a cell pool and how selection pressures might work with this diversity is summarized in Figure 8.
Figure 8. Creation of the aged B-1a cell pool.
B-1 cell development begins in the embryo where B-1 cell progenitors are found in the yolk sac (YS) and para-aortic splanchnopleura (PSp) (47). B-1a cell development continues in the fetal liver and bone marrow (49). The mature B-1a cells developing during fetal life produce Ig lacking N-additions due to the absence of TdT expression during fetal life (8). As the animal moves into adult life TdT expression allows for some diversity in the B-1a cell pool. In particular, the adult B-1a progenitors express high levels of TdT, which give rise to B-1a cells producing Ig with many N-additions (40). In the work presented here we demonstrate bone marrow B-1a cell progenitors add to the adult B-1a cell pool and both the fetal and bone marrow derived B-1a cells are exposed to selection pressures with increasing age. Both diversification and selection of the B-1a cell pool over time leads to a decrease in protective capacity of natural IgM in the aged.
These results clarify the origins of age-related changes to the B-1a cell pool, which affects the protective capacity of the natural IgM B-1a cells produce. Future investigation needs to be performed on the cell intrinsic and extrinsic factors that affect the selection of these cells with age, and the role, if any, of circulating IgA natural antibodies. Understanding how natural antibodies change with age will have application beyond clearance of bacteria. This has been recently shown in a study demonstrating natural IgM is required to control the accumulation of autoantibodies via its ability to regulate B cell development and selection (57). Therefore, such future studies will aid in understanding the natural available repertoire that is present in older age, which could shed light on improving therapeutic strategies against S. pneumoniae in the elderly (58).
Supplementary Material
Acknowledgments
This work was supported by the Public Health Service grant AI029690 awarded by the National Institutes of Health.
We would like to thank Drs. Andre Vale and Harry Schroeder for their invaluable help in analysis of the CDR-3H region. In addition, we thank Drs. Ana Maria Hernandez, Tam Quach, and Nely Rodriquez Zhurbenko for their critical review and discussion of the manuscript.
References
- 1.Hardy RR, Hayakawa K. B cell development pathways. Annual review of immunology. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein TL. Cutting edge commentary: two B-1 or not to be one. J Immunol. 2002;168:4257–4261. doi: 10.4049/jimmunol.168.9.4257. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 4.Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunology today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 6.Casali P, Schettino EW. Structure and function of natural antibodies. Current topics in microbiology and immunology. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 7.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 8.Feeney AJ. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990;172:1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 10.Su SD, Ward MM, Apicella MA, Ward RE. The primary B cell response to the O/core region of bacterial lipopolysaccharide is restricted to the Ly-1 lineage. J Immunol. 1991;146:327–331. [PubMed] [Google Scholar]
- 11.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 12.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 15.Weksler ME, Pawelec G, Franceschi C. Immune therapy for age-related diseases. Trends Immunol. 2009;30:344–350. doi: 10.1016/j.it.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Horkko S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. Journal of lipid research. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 18.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict CL, Gilfillan S, Thai TH, Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. [PubMed] [Google Scholar]
- 20.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 21.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. :1–20. 24. [PubMed] [Google Scholar]
- 23.Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, provisional-2009. 2010 [Google Scholar]
- 25.Ben-Yehuda A, Szabo P, LeMaoult J, Manavalan JS, Weksler ME. Increased VH 11 and VH Q52 gene use by splenic B cells in old mice associated with oligoclonal expansions of CD5 + B cells. Mech Ageing Dev. 1998;103:111–121. doi: 10.1016/s0047-6374(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 26.Dobken J, Weksler ME, Siskind GW. Effect of age on ease of B-cell tolerance induction. Cell Immunol. 1980;55:66–73. doi: 10.1016/0008-8749(80)90137-9. [DOI] [PubMed] [Google Scholar]
- 27.Klinman NR, Kline GH. The B-cell biology of aging. Immunol Rev. 1997;160:103–114. doi: 10.1111/j.1600-065x.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 28.Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 29.Yang X, Stedra J, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. IV. Study of VH and VL gene utilization in splenic antibody foci by in situ hybridization. J Immunol. 1994;152:2214–2221. [PubMed] [Google Scholar]
- 30.Lee H, Nahm MH, Kim KH. The effect of age on the response to the pneumococcal polysaccharide vaccine. BMC Infect Dis. 2010;10:60. doi: 10.1186/1471-2334-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone J, Eurich DT, Minhas JK, Marrie TJ, Majumdar SR. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis. 51:15–22. doi: 10.1086/653114. [DOI] [PubMed] [Google Scholar]
- 33.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, Raga X, Gomez F, Valdivieso E, Fuentes C, Palacios L. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine. 2008;26:1955–1962. doi: 10.1016/j.vaccine.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Chidiac C, Ader F. Pneumococcal vaccine in the elderly: a useful but forgotten vaccine. Aging Clin Exp Res. 2009;21:222–228. doi: 10.1007/BF03324905. [DOI] [PubMed] [Google Scholar]
- 35.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infection and immunity. 2011;79:314–320. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simell B, Lahdenkari M, Reunanen A, Kayhty H, Vakevainen M. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clinical and vaccine immunology : CVI. 2008;15:1391–1397. doi: 10.1128/CVI.00110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39:2383–2394. doi: 10.1002/eji.200838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu H, Forster I, Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. The EMBO journal. 1990;9:2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holodick NE, Vizconde T, Rothstein TL. B-1a cell diversity: nontemplated addition in B-1a cell Ig is determined by progenitor population and developmental location. J Immunol. 2014;192:2432–2441. doi: 10.4049/jimmunol.1300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol. 185:525–531. doi: 10.4049/jimmunol.0902841. [DOI] [PubMed] [Google Scholar]
- 42.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis research & therapy. 2004;6:131–139. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E, Reth M, Buch T, Waisman A, Kretschmer K, Weiss S. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 45.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 46.Lang PO, Govind S, Bokum AT, Kenny N, Matas E, Pitts D, Aspinall R. Immune senescence and vaccination in the elderly. Current topics in medicinal chemistry. 2013;13:2541–2550. doi: 10.2174/15680266113136660181. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghosn EE, Waters J, Phillips M, Yamamoto R, Long BR, Yang Y, Gerstein R, Stoddart CA, Nakauchi H, Herzenberg LA. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem cell reports. 2015 doi: 10.1016/j.stemcr.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 50.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci U S A. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghosn EE, Yamamoto R, Hamanaka S, Yang Y, Herzenberg LA, Nakauchi H, Herzenberg LA. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci U S A. 2012;109:5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD, Herzenberg LA. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 54.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 55.Guo B, Rothstein TL. RasGRP1 Is an Essential Signaling Molecule for Development of B1a Cells with Autoantigen Receptors. J Immunol. 2016 doi: 10.4049/jimmunol.1502132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, Herzenberg LA. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. eLife. 2015;4 doi: 10.7554/eLife.09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen TT, Elsner RA, Baumgarth N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol. 2015;194:1489–1502. doi: 10.4049/jimmunol.1401880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vale AM, Kapoor P, Skibinski GA, Elgavish A, Mahmoud TI, Zemlin C, Zemlin M, Burrows PD, Nobrega A, Kearney JF, Briles DE, Schroeder HW., Jr The link between antibodies to OxLDL and natural protection against pneumococci depends on D(H) gene conservation. J Exp Med. 2013;210:875–890. doi: 10.1084/jem.20121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.