Abstract
Background
Embryonic-stem-cell (ESC)-derived islets hold the promise of providing a renewable source of tissue for the treatment of insulin-dependent diabetes. Encapsulation may allow ESC-derived islets to be transplanted without immunosuppression, thus enabling wider application of this therapy.
Methods
In this study, we investigated the immunogenicity of mouse pancreatic progenitor cells and efficacy of a new macroencapsulation device in protecting these cells against alloimmune and autoimmune responses in mouse models.
Results
Mouse pancreatic progenitor cells activated the indirect but not the direct pathway of alloimmune response and were promptly rejected in immune competent hosts. The new macroencapsulation device abolished T cell activation induced by allogeneic splenocytes and protected allogeneic MIN6 β cells and pancreatic progenitors from rejection even in pre-sensitized recipients. In addition, the device was effective in protecting MIN6 cells in spontaneously diabetic non-obese diabetic recipients against both alloimmune and recurring autoimmune responses.
Conclusion
Our results demonstrate that macroencapsulation can effectively prevent immune sensing and rejection of allogeneic pancreatic progenitor cells in fully sensitized and autoimmune hosts.
Introduction
Islet transplantation is an effective therapy for type 1 diabetes (T1D).1,2 However, donor shortage and the toxicity of chronic immunosuppression limit its use to patients with brittle diabetes.3 The development of a renewable source of β-cells that can be transplanted without immunosuppression is needed for the wider application of this therapy. Human embryonic stem cells (hESC) can be differentiated in vitro into pancreatic endoderm cells that further develop into functional β-cells after transplantation in vivo.4–6 Combining hESC-derived pancreatic progenitor cells with an immune-protective encapsulation device is a potential solution to this unmet clinical need.
Encapsulation for cellular transplantation should support the function of encapsulated cells and prevent immune responses against the cells. T cells are the primary drivers of alloimmune responses. T cells can be activated “directly” by MHC expressed on the transplanted cells or “indirectly” by peptides from allogeneic donor cells which are acquired and presented by recipient antigen presenting cells.7 While the direct pathway requires contact between host immune cells and intact graft cells, the indirect pathway can be activated by antigenic fragments shed from the graft and is thus potentially more difficult to block by physical separation using encapsulation. In addition, recipients may be sensitized to alloantigens because of prior or concurrent transplants and have pre-formed effector T cells with lower threshold for activation. Moreover, for autoimmune diabetes recipients, recurrent autoimmunity against islet antigens can contribute to graft rejection.8 Thus an effective encapsulation device should prevent activation of direct and indirect T cell responses and also prevent rejection by pre-formed alloimmune and autoimmune effector cells.
In this study, we investigated the efficacy of a new macroencapsulation device in protecting allogeneic mouse pancreatic progenitor cells against alloimmune- and autoimmune-mediated rejection in mouse models.
Methods
Mice
C57BL/6 (B6), BALB/c, non-obese diabetic (NOD), NOD.Rag2−/−, B6.(Cg)-Tyrc−2J/J (albino B6) and B6.Tg(Ins1-luc)77Park (MIP-Luc) mice9 were purchased from Jackson Laboratories (Bar Harbor, ME). B6.CD11c-mCherry transgenic mice were obtained from Dr. Kamal Khanna.10 4C, TCR75, and 2C.Rag1−/− mice were as described previously 7. Briefly, transgenic (Tg) 4C T cells are derived from H-2b B6 background and directly recognize the MHC class II molecule I-Ad.11 Tg 2C T cells are derived from H-2b B6 background and directly recognize the class I MHC H-2Ld .12 Tg TCR75 T cells indirectly recognize a peptide derived from H-2Kd MHC class I molecule presented by MHC class II molecule I-Ab.13 NOD mice were monitored weekly for diabetes. Diabetic mice were maintained with insulin pellets (Linbit, Linshin, Toronto, Canada) until cell transplantation. All animals were housed and bred in a specific-pathogen-free facility at UCSF. All procedures were performed in accordance with the ethical guidelines of the Institutional Animal Care and Use Committee of UCSF.
Mouse pancreatic progenitor cell preparation
Mouse pancreatic progenitor cells were collected from pancreata of 11.5- to 16.5-day old embryos by micro-dissecting pancreatic buds, followed by trypsin digestion and mechanical removal of mesenchyme from the epithelia as described previously.14 The epithelium-enriched tissue was cultured overnight in DMEM H-16 and F/12 (1:1) augmented with penicillin and steptomycin, insulin, transferrin, selenium, and DNAse before transplantation the following day. MIN6 cells used in this study were at low passages (30–40) and were cultured as described previously.15 MIN6 cells were transduced with a lentiviral vector expressing GFP and firefly luciferase genes driven by a ferritin promoter.16 Transduced cells were isolated using fluorescence activated cell sorting based on GFP expression and expanded for imaging studies. Newborn pancreata were collected from 1–2 day old B6.MIP-LUC mice and approximately 7 to 8 newborn pancreata were transplanted per recipient.
Transplantation
For some experiments, pancreatic progenitor cells or MIN6 cells were transplanted under the kidney capsule as reported previously.17,18 For subcutaneous transplants, pancreatic progenitor cells or MIN6 cells were pelleted in a PE50 polyethylene tubing, which was threaded through a skin incision into a subcutaneous space created by blunt dissection and the cells were deposited into the space. Recipient mice of pancreatic progenitors were monitored for possible tumor growth. The tumors that formed were large, cystic, expanded rapidly and were easily palpable. When a nodule was detected at the transplant site, mice were killed. Presence of tumor tissue was confirmed by necropsy. Mice that did not have palpable tumor by the end of experiments (60 to 120 days after transplant) were killed and absence of large cystic tumor was confirmed. For real-time monitoring of islet development, embryonic pancreata from B6.MIP-GFP mice were transplanted into the anterior chamber of the eye of syngeneic recipients using a procedure previously described.19 The transplanted mice were monitored using confocal microscopy for tissue morphology and GFP fluorescence for evidence of development of insulin-producing cells.
Encaptra macroencapsulation devices
Encaptra macroencapsulation devices were provided by ViaCyte (San Diego, CA). The device is a sandwich of polymeric membranes that has been sealed at the periphery to create a pouch. One end of the pouch includes a short tubing that serves as a port for loading cells into the lumen of the device. The polymeric membranes have a nominal pore size of 0.45μm and are impermeable to cells. This device is built in a similar planar design and with similar membrane properties as the Theracyte device,20 but using more modern manufacturing and quality control methods with proper design controls as required for developing a medical device for eventual use in human subjects. Encaptra devices have passed all the biocompatibility tests required by the International Standards Organization and are currently being used in a phase I/II clinical trial (NCT02239354).
Subcutaneous implantation and removal of Encaptra
Embryonic pancreata, newborn pancreata, or MIN6 cells were loaded into Encaptra and the loading port was sealed with medical-grade silicone glue (Nusil, Carpinteria CA). The cell-loaded devices were kept in media when mice were prepared for implantation. Mice were anesthetized with isoflurane and an incision was made in the skin of the flank and a subcutaneous space was created using blunt dissection. Loaded devices were inserted into the space and the incision was closed using surgical clips. At the end of some experiments, devices were surgically removed through an incision in the skin of anesthetized mice.
Bioluminescence imaging
Mice transplanted with luciferase-expressing cells were injected IP with 15 mg/Kg D-luciferin solution (Goldbio Biotechnology, St. Louis, MO) 8 minutes before imaging on a Xenogen IVIS 200 imaging system (Perkin Elmer, Waltham, MA). Signals were acquired with 1-minute exposure and analyzed using the Living Image analysis software (Xenogen Corp., Alameda, CA). Circular regions of interests (ROI) were manually drawn to encircle all the signals and same sized circles were used for the analysis of all data points within the same experiment to ensure consistency in signal quantification. Photons emitted over the time of exposure within the ROI were quantified. Identical protocol was strictly followed for sequential imaging of the same mice over a period of up to three months to ensure comparability of assay performance over time. Background signals in an area distal to the transplants were similarly analyzed and found to be consistent over time.
Immunofluorescence microscopy
Tissues were fixed in 4% paraformaldehyde for 4 hours, followed by submersion in 30% sucrose overnight before embedding in Tissue Tek OCT® (Sakura, Torrance, CA) and freezing in vapor phase of liquid nitrogen. Sections were treated with Power Block (BioGenex, San Ramon, CA), incubated with polyclonal guinea pig anti-insulin antibody (Dako, Carpinteria, CA) and rabbit polyclonal anti-glucagon antibody (BioGenex), and then stained with Alexa Fluor® 568 goat anti-guinea pig IgG, Alexa Fluor® 488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA), and DAPI (0.2 mg/mL). The slides were mounted in Crystal Mount (Biomeda, Foster City, CA) and dried before image capture on a Leica DM6000 CS upright confocal microscope (Leica Microsystem, Buffalo Grove, IL).
Cell transfer
One day after cell transplantation, mice received carboxyfluorescein succinimidyl ester (CFSE) -labeled lymph node (LN) cells from congenic 4C, TCR75, and 2C TCR transgenic mice via retro-orbital injection as previously described.7 Seven and 12 days after the transplant, axillary (if the transplant was subcutaneous) or renal draining LN (if the transplant was under the kidney capsule) were harvested and the proliferation of transferred T cells was determined using flow cytometry by measuring the dilution of CSFE. A Fortessa flow cytometer (BD Biosciences, San Jose, CA) and FACSDiva software (BD Biosciences) were used for flow cytometric analysis. For sensitization of BALB/c mice, B6 splenocytes were cultured overnight in presence of 10 μg/mL LPS (Escherichia coli 026:B6, Sigma, St. Louis, MO),21 washed, and 20 × 106 cells were injected IP into each recipient animal.
Intracellular IFNγ detection
Draining LN cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of 10 μg/mL brefeldin A for 3 hours, stained for surface markers, fixed with 4% paraformaldehyde, permeabilized in 0.1% saponin, and stained with anti-IFNγ (XMG1.2; eBioscience, San Diego, CA). The samples were analyzed by using a Fortessa flow cytometer (BD Biosciences, San Jose, CA) and FACSDiva software (BD Biosciences).
Statistical analysis
Statistical analyses were performed with the aid of Prism GraphPad software (La Jolla, CA).
Results
Pancreatic progenitor cells are alloimmunogenic
There are two general approaches to model alloimmune responses to human cellular products in mice: using allogeneic mouse cells that are similar to the human cellular product or using the actual human cellular product in a humanized mouse model. We choose take the former approach to take advantages of immunological tools available in mouse models. Progenitor cells derived from mouse ESC using a protocol similar to that used for human cells would be an ideal cell type to model hESC-derived pancreatic progenitor cells. However, deriving islet progenitor cells from mouse ESC is not a minor undertaking and most work focuses on hESC for their potential clinical utility. To circumvent this challenge, we used more than one type of cells to better approximate the different characteristics of ESC-derived progenitor cells. Cells extracted from embryonic mouse pancreata contain multiple developing endocrine progenitor cells that simulate the ESC-derived progenitor cells currently tested in the clinics and we used them for immunogenicity experiments. However, much like ESC-derived progenitor cells, these mouse embryo-derived cells take three months to show detectable insulin production. For experiments that need faster graft function, we also used digested pancreata from newborn mice or an insulinoma cell line MIN6 cells. The rate of engraftment of embryonic and newborn pancreata varies from experiment to experiment, making it challenging to assess their rejection in sensitized hosts. MIN6 cells, on the other hand, provided consistent engraftment. Low-passage MIN6 cells as the ones used in this study have been shown to have similar profile of insulin release upon glucose stimulation as isolated mouse islets.15,22 Although MIN6 cells are tumor cells and may be less immunogenic than islet progenitor cells, in our experiences other published reports, they are rejected in allogeneic recipients.23
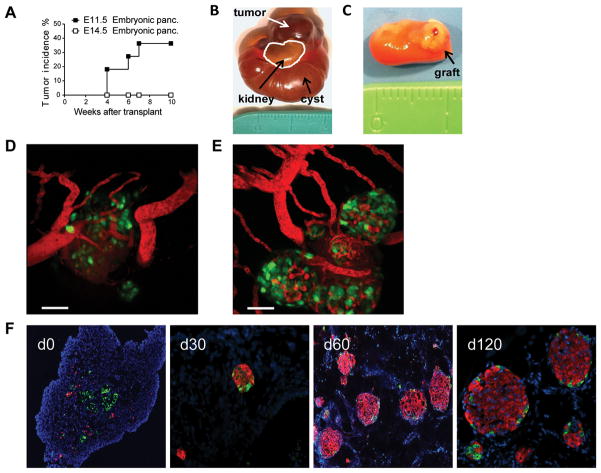
Previous reports have shown that pancreata micro-dissected from the embryonic pancreas contain progenitor cells for mouse islets.24 For this study, we want to use mouse embryonic pancreata from a developmental stage that is similar to the human pancreatic progenitors generated in vitro from hESC.25,26 We isolated embryonic pancreata from B6 fetuses between E11.5 and E16.5 of age and transplanted under the kidney capsule of syngeneic animals. Approximately 40% of the E11.5 embryonic pancreata showed uncontrolled growth after transplant, suggesting that the tissue has not sufficiently differentiated and retained tumorigenic potential (Fig. 1A and B), whereas no tumor was observed with E14.5 tissue (Fig. 1C). Moreover, transplanting MIP.GFP embryonic pancreata into the anterior chamber of the eye of syngeneic recipients, we also observed excessive growth from E11.5, but not E14.5, tissue in some recipients. Moreover, in the recipient that did not develop tumor, the E11.5 tissue had lower potential to develop insulin-producing cells than E14.5 tissue (Fig. 1D and E), in agreement with previous reports. 27 On the other hand, E16.5 embryonic pancreatic tissue contained exocrine tissue and was unsuitable for our experiments.6,28 E13.5 to E14.5 embryonic pancreata developed into mature islets between 60 to 120 days after transplant without tumor formation (Fig. 1C and F). Thus, we used E14.5 embryonic pancreata for the rest of our study because of its low tendency to generate tumors and high potential to develop into insulin-producing cells.
Fig 1. Mouse embryonic pancreata as a model for pancreatic progenitor cell transplantation.
C57BL/6 embryonic pancreata were isolated from embryonic age 11.5 (E11.5, n=12) and 14.5 (E14.5, n=17) days and transplanted under the kidney capsule of syngeneic recipients. (A) Tumor incidence of E11.5 and E14.5. The curves were compared using the log-rank (Mantel-Cox) statistical method. E11.5 versus E14.5 embryonic pancreata p = 0.0335. Representative pictures of (B) E11.5 and (C) E14.5 embryonic pancreata grafts 10 weeks after transplant under the kidney capsule. (D) E11.5 (n=6) and (E) E14.5 (n=6) tissue transplanted into the anterior chamber of the eye for 60 days. Insulin expression is reported by the MIP.GFP (green) and blood vessel are visualized by Evan’s Blue (red) injected just before imaging. Scale bar=50μm. (F) Time course of islet development from E14.5 embryonic pancreata. Immunofluorescence staining for insulin (red), glucagon (green), and dapi (blue) were performed on the graft sections before transplant (d0) and on days 30, 60 and 120 after transplant. Images represent results from 8 recipient mice in three independent experiments. Original magnification 10x.
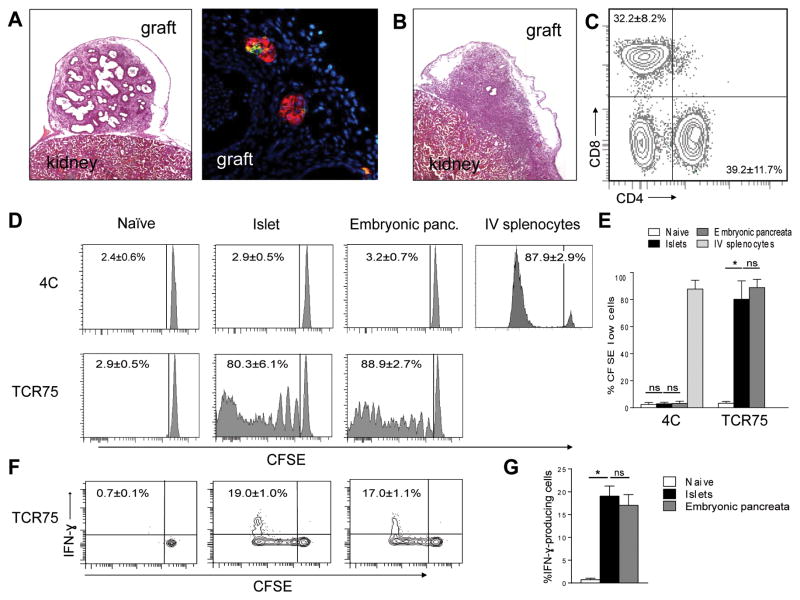
We analyzed MHC class I and class II expression on cells from dissociated BALB/c E14.5 tissue using flow cytometry. Despite the presence of CD11c+ dendritic cells in the tissue (Supplemental Fig. S1), MHC expression was very low when compared with cells from adult islets or spleen (Supplemental Fig. S2). We next transplanted BALB/c embryonic pancreata into syngeneic BALB/c or allogeneic B6 recipients and analyzed the grafts and graft infiltrating cells 12 days later using histology and flow cytometry. H&E and immunofluorescence staining of the syngeneic grafts showed that the tissue contained duct-like structures with small clusters of insulin and glucagon positive cells without immune infiltrates (Fig. 2A). In contrast, allogeneic grafts were dominated by lymphocytic infiltration (Fig. 2B). Flow cytometric analysis of the dissociated graft cells showed that the infiltrates were predominately CD4+ (39.2 ± 11.7%) and CD8+ (32.2 ± 8.5%) T cells (Fig. 2C). This is preceded by an increase of MHC expression on the graft cells (Supplemental Fig. S2). This suggests that embryonic pancreatic tissue is alloimmunogenic.
Fig 2. Immunogenicity of embryonic pancreata in vivo.
(A to C) B6 mice were transplanted with syngeneic or allogeneic BALB/c embryonic pancreata and grafts were analyzed 12 days later. Representative H&E (5x original magnification) and immunofluorescence micrograph (insulin red, glucagon green, 40x original magnification) of syngeneic embryonic pancreatic grafts are shown in A. Representative H&E (5x original magnification) micrograph of allogeneic grafts collected is shown in B. Flow cytometric analysis of enzymatically dissociated graft cells is shown in C. Events shown have been gated on live cells. Results in A, B and C are representative of 5 independent experiments with 1 syngeneic and one allogeneic recipient in each experiment. (D to G) B6 mice were transplanted with allogeneic BALB/c embryonic pancreata under the kidney capsule followed by an injection of CFSE-labeled 4C and TCR75 cells one day later. Positive control mice received islet transplant from adult BALB/c donors and naïve control did not receive transplant. For 4C cells, an additional positive control of intravenously infusion of BALB/c splenocytes 7 days before analysis was included to demonstrate their capability to respond to BALB/c antigens. Representative histograms of CFSE dilution of 4C and TCR75 cells in naïve, mature islet recipients, and embryonic pancreata recipients are shown in D and quantitative summary of results is shown in E. Two-way ANOVA followed by Tukey’s multiple comparison test was used to determine statistical significance (n=5 mice in each group, p<0.0001). (F) Representative flow plots depicting IFN-γ production by CFSElow TCR75 cells in a naïve, an islet recipient, and an embryonic pancreas recipient. (G) Quantitative summary of results in F. The p values were determined using one-way ANOVA followed by Tukey’s multiple comparison test (n=5 mice in each group, p<0.0001).
To determine if embryonic pancreatic grafts activate direct or indirect alloantigen-reactive cells in vivo, we analyzed the activation of adoptively transferred TCR transgenic T cells from 4C (directly alloreactive) and TCR75 (indirectly alloreactive) mice. We transferred CFSE-labeled 4C and TCR75 cells 1 day after transplanting BALB/c embryonic pancreata to B6 recipients. A group of B6 mice were transplanted with islets from adult BALB/c donors as a positive control. Negative control B6 mice did not receive grafts and were designated as naive. Flow cytometric analysis of draining LN 12 days after cell transplant showed that 4C T cells did not proliferate (1.9 ± 1.2% naïve, 2.95 ± 0.85% islet, and 2.25 ± 1.75% embryonic pancreata) (Fig. 2D and 2E), consistent with our previous report that islets do not induce strong direct responses7. The 4C T cells responded vigorously to iv infusion of BALB/c splenocytes, suggesting that the absence of proliferation to islets and pancreatic progenitors was likely due to lack of stimulation by the grafts. TCR75 cells showed extensive proliferation in islet and embryonic pancreata recipients but not in the naïve control group (2.7 ± 0.7% naïve, 73.4 ± 12.0% islets, and 86.7 ± 4.4% embryonic pancreata) (Fig. 2D and 2E). In addition, the TCR75 T cells that displayed extensive proliferation also differentiated into IFN-γ-producing effectors (Fig. 2F and 2G). The T cell response elicited by embryonic pancreatic grafts were indistinguishable to that induced by mature islets, demonstrating that allogeneic embryonic pancreatic grafts are fully capable of inducing indirect T cell responses.
Encaptra™ blocks alloimmune T cell response
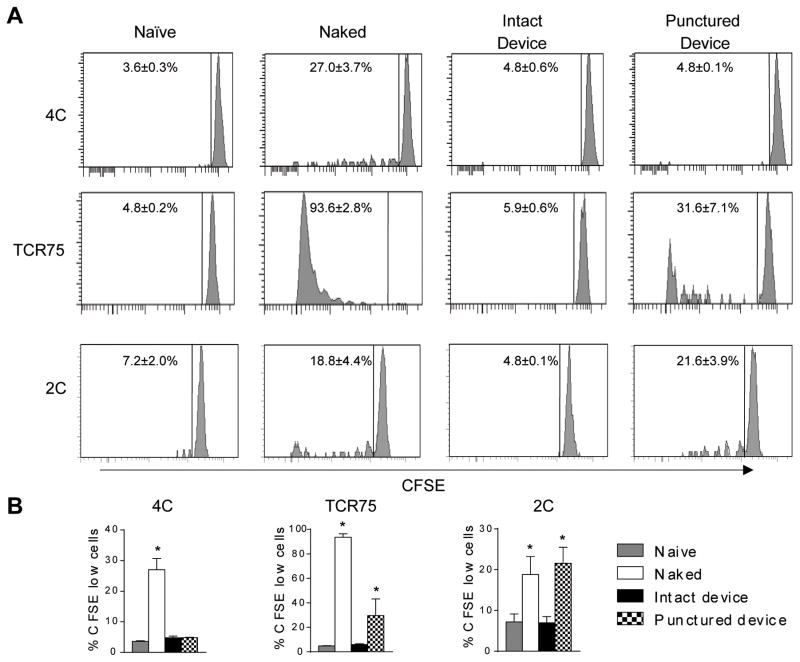
We then evaluated the immune isolation property of a new macroencapsulation device Encaptra™. We challenged the device with a more robust alloantigen by loading them with adult mouse splenocytes that contain professional antigen presenting cells with higher levels of MHC class I and II expression. We loaded 20 x 106 BALB/c x B6 F1 splenocytes into Encaptra™ and transplanted them subcutaneously in B6 mice. As positive controls, F1 splenocytes were injected directly subcutaneously or loaded into previously punctured devices. We then monitored T cell responses in the draining LN to these cellular grafts using the same approach described for Figure 2, except that T cells from 2C TCR transgenic mice were added to monitor direct CD8+ T cell response. Our previous experiments analyzing responses to allogeneic skin transplants using the same system shows that T cell proliferative responses peaked between days 5 and 15. Considering that the cells are transplanted into the subcutaneous space of the mice, we estimate that the kinetics of the responses should be similar. We therefore analyzed T cell proliferation on days 7 and 14 after transplant. In the group of naked splenocytes, 27.0 ± 6.1% of 4C T cells, 18.8 ± 4.4% of 2C cells, and 93.6 ± 2.8% of TCR75 cells had diluted CFSE 7 days after transplant. In the group that received splenocytes in intact Encaptra™, the CFSE profile of 4C, 2C and TCR75 cells were indistinguishable from the naive group that did not receive splenocytes (4.8 ± 0.6% 4C, 5.9 ± 0.6% TCR75, and 7.0 ± 1.5% 2C for the encapsulated group vs. 3.5 ± 0.4% 4C, 4.8 ± 0.2% TCR75, and 7.1 ± 3.4% 2C for the naïve group). When the devices were previously perforated 9 times using 29G needles, proliferation of TCR75 and 2C T cells could be detected (29.6 ± 13.6% of TCR75 cells and 20.7 ± 5.5% for 2C) whereas 4C proliferation remained low (5.0 ± 0.1%, Fig. 3). Similar results were observed on day 14 after transplant (data not shown). These results show that Encaptra™ can effectively prevent sensing of encapsulated allogeneic cells by the host T cells.
Fig 3. Effect of Encaptra™ on T cell priming by allogeneic cells.
Groups of B6 mice were transplanted with non-encapsulated BALB/c x B6 F1 splenocytes (naked) or BALB/c x B6 F1 splenocytes in intact or punctured Encaptra™. One day later all the mice alone with a group on non-transplanted (naïve) mice received CFSE-labeled 4C, TCR75 and 2C cells. (A) Representative histograms of CFSE dilution of 4C, TCR75 and 2C cells are shown. (B) Quantitative summary of results on A from 5 independent experiments are shown. The p values were determined using Kruskal-Wallis test followed by Dunn’s multiple comparisons test (4C: Naked vs. Intact device p=0.04; TCR75: Naked vs. Intact device p=0.005, Intact device vs. Punctured device p=0.04; 2C: Naked vs. Intact device p<0.05, Intact device vs. Punctured device p=0.04).
Encaptra™ protects allogeneic cells from immune rejection
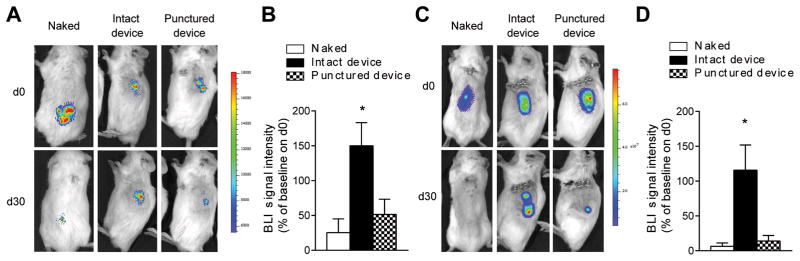
An effective encapsulation device not only has to prevent activation of the host immune system by the grafts, but also protect the grafts against rejection by previously activated effectors cells especially when recipients have been sensitized. We next determined if Encaptra™ could protect β cell against attack by fully activated alloimmune effectors. For these experiments, we transplanted luciferase expressing β cells and monitored allograft survival in real time in live animals using bioluminescence imaging. In the first set of experiments, we transplanted newborn pancreata from B6.MIP-LUC mice into NODRag2−/− and allowed the islets to stably engraft for 30 days before transferring 20 x 106 splenocytes from male NOD mice to initiate an alloimmune response. The bioluminescent signal from naked grafts and punctured devices were greatly diminished 30 days after splenocyte transfer, however the signal was maintained and even increased with intact Encaptra™ devices (Fig. 4A and B).
Fig 4. Effect of Encaptra™ on allogeneic β cell rejection.
(A and B) NOD.Rag2−/− mice were transplanted with newborn MIP-LUC pancreata with or without Encaptra™ and allowed to engraft for 30 days. The mice then received 20 X 106 NOD splenocytes on day 30 after transplant. Representative bioluminescence images taken on days 0 and 30 after splenocyte transfer is shown in A and quantitative summary of the normalized bioluminescence signal 30 days after cell transfer is shown in B (Naked n=4; Intact device n=4; Punctured device n=6). One-way ANOVA followed by Dunn’s multiple comparisons test was used to determine the p values (Naked vs. Intact device p=0.008). (C and D) BALB/c recipients were sensitized with an injection of LPS-treated B6 splenocytes 1 week before subcutaneous MIN6.Luc cells transplant with or without Encaptra™. Representative bioluminescence images days 0 and 30 of transplant are shown in C and quantitative summary of the normalized bioluminescence signal 30 days after transplant is shown in D (Naked n=7; Intact device n=9; Punctured device n=9). The p values were determined using one-way ANOVA followed by Dunn’s multiple comparisons test (Naked vs. Intact device p=0.001).
To simulate the condition of a sensitized host, we transplanted B6-derived luciferase-expressing insulinoma MIN6 (MIN6-Luc) cells into BALB/c recipients that had been pre-sensitized by IP injection of 20 x 106 LPS-treated B6 splenocytes 1 week before transplant. Naked MIN6-Luc cells and those transplanted in punctured devices were rejected by day 30 post-transplant, demonstrating that recipients are capable of rejecting MIN6-Luc cells. In contrast, mice that received MIN6-Luc cells in intact Encaptra™ devices maintained the signal for the entire duration of the experiment (Fig. 4C and D). These results demonstrate that the device can protect allogeneic grafts in fully sensitized hosts.
Encapsulated MIN6 cells are protected from alloimmune and autoimmune responses
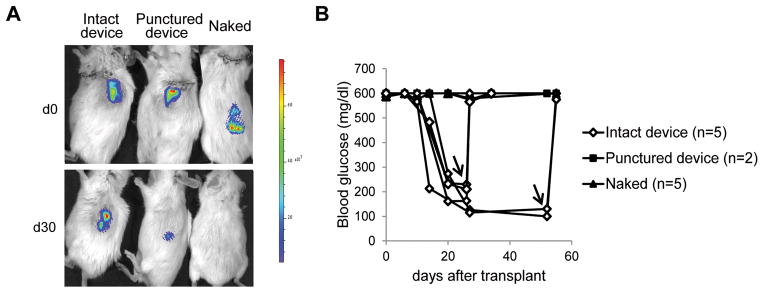
In addition to alloimmune attacks, transplanted pancreatic progenitor cells could be rejected by recurrent autoimmune responses. To address whether Encaptra™ can protect against both autoimmune and alloimmune responses, we transplanted MIN6-Luc cells into spontaneously diabetic NOD mice to better mimic a clinical scenario where a patient with T1D receives an allogeneic graft. Rejection in spontaneously diabetic NOD mice is more difficult to control than that in chemical induced diabetic models.29 The encapsulated grafts maintained bioluminescence signal (Fig. 5A) and reversed hyperglycemia within 2 to 3 weeks (Fig. 5B). Explanting the device led to a sharp rise of blood glucose, demonstrating the function of the grafts in Encaptra™. The animals in the control groups that received naked cells or cells in punctured devices lost luciferase signal and remained hyperglycemic (Fig. 5B). Together, the results show that Encaptra can protect allogeneic β cells against alloimmune and recurrent autoimmune responses.
Fig 5. Effect of Encaptra™ on allogeneic β cells survival in autoimmune diabetic recipients.
Female spontaneous diabetic NOD mice were transplanted with luciferase transduced MIN6 cells without encapsulation or loaded intact or punctured Encaptra™. Graft survival was measured using bioluminescence imaging and graft function was assessed by blood glucose monitoring. Representative bioluminescence results on days 0 and 30 of transplant is shown in A. Blood glucose results of all mice are summarized in B. Arrows indicate the time point at which grafts were explanted.
Discussion
We investigated the immune isolating property of a new macroencapsulation device, Encaptra™.
We used different sources of mouse tissue to approximate the characteristics of hESC-derived islet progenitor cells that will be used for clinical applications. Initial publications reported that stem cells would benefit from an immune-privileged status.30 Our results show that pancreatic progenitor cells are immunogenic in vivo and elicit mainly an indirect alloimmune response, which is fully capable of rejecting pancreatic progenitor grafts. Thus immune protection is necessary to ensure function of the transplanted cells.
An effective encapsulation device should block both afferent and efferent immune responses to prevent recipient sensitization and rejection by pre-formed effectors in sensitized patients. Indirect T cell responses elicited by pancreatic progenitor cells are more difficult to block since cellular debris and protein fragments that may leak out of the encapsulation devices may be sufficient to prime the immune system. By our rigorous challenge of Encaptra™ using allogeneic splenocytes, we found the Encaptra™ completely prevented priming of the indirect CD4+ T cells. Blocking efferent immune responses will likely be more demanding since islet killing can be mediated by effector cytokines such as IFN-γ, IL-1β, and TNF-α in addition to direct contact of immune cells with the grafts.31–33 These cytokines have molecular weights of 17 to 51 kDa and have hydrodynamic radii of 2 to 3 nm, close to that of insulin (MW 5.8 kDa, radius 1.3 nm). Despite these concerns, we found that Encaptra™ robustly prevented rejection in fully sensitized and autoimmune recipients while supporting β cell function as shown by normalization of blood glucose in spontaneously diabetic NOD recipients.
Various islet macro-encapsulation devices have been developed and very few have progressed to clinical trials.34 Encaptra™ evaluated in this study is similar to the Theracyte device first developed in 1990 with proprietary modifications made by Viacyte Inc. Our findings extend previous functional studies of the Theracyte device to demonstrate that Encaptra™ macroencapsulation devices can be effective in preventing alloimmune sensitization and rejection in mouse models.35,36 These results are supportive of moving forward to evaluate Encaptra™ as an immune isolation device in human patients. In this regard, a phase 1/2 clinical trial (NCT02239354) was initiated in September 2014 to assess the safety, tolerability, and efficacy of (VC-01™), a combination product of hESC-derived progenitor cells encapsulated in Encaptra™ devices. This trial will shed light on the efficacy of this device in immune isolation in human subjects.
Supplementary Material
Acknowledgments
We would like to thank ViaCyte for providing the Encaptra™ devices and Dr. Michael McManus for providing MIN6 cells for the experiments. We are grateful to Drs. Peter Stock, Jeffrey Bluestone, and Greg Szot for helpful discussions and Dr. Steven Wisel for critical review of the manuscript. We thank Vi Dang and Ninnia Lescano for their assistance with mouse husbandry.
This work was supported by a grant from CIRM (DR1-01423, QT subcontract) and the UCSF Diabetes Endocrinology Research Center grant from NIDDK (P30 DK063720).
Abbreviations
- B6
C57BL/6
- CFSE
Carboxyfluorescein succinimidyl ester
- DMEM
Dulbecco’s Modified Eagle Medium
- ESC
Embryonic stem cell
- FBS
Fetal Bovine Serum
- FITC
Fluorescein isothiocyanate
- H&E
Hematoxylin and eosin
- IF
Immune Fluorescence
- IP
Intraperitoneal
- LN
Lymph nodes
- NOD
Non-obese diabetic
- PE
Phycoerythrin
- PE-Cy7
Phycoerythrin-cyanine 7
- PMA
Phorbol 12-myristate 13-acetate
- T1D
Type 1 diabetes
- ROI
Regions of interests
- Tg
Transgenic
Footnotes
The authors declare no conflicts of interest.
Authorship: GF, KL, and VN conducted the experiments and analyzed the results; QT directed the research and reviewed all the experimental findings and conclusions; GF and QT wrote the manuscript.
References
- 1.Gerber PA, Pavlicek V, Demartines N, et al. Simultaneous islet-kidney vs pancreas-kidney transplantation in type 1 diabetes mellitus: a 5 year single centre follow-up. Diabetologia. 2008;51(1):110–119. doi: 10.1007/s00125-007-0860-4. [DOI] [PubMed] [Google Scholar]
- 2.Vantyghem MC, Balavoine AS, Caiazzo R, Kerr-Conte J, Noel C, Pattou F. Diabetes cell therapy: a decade later. Minerva endocrinologica. 2011;36(1):23–39. [PubMed] [Google Scholar]
- 3.Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes/metabolism research and reviews. 2004;20(6):479–486. doi: 10.1002/dmrr.482. [DOI] [PubMed] [Google Scholar]
- 4.Kao DI, Chen S. Pluripotent stem cell-derived pancreatic beta-cells: potential for regenerative medicine in diabetes. Regenerative medicine. 2012;7(4):583–593. doi: 10.2217/rme.12.27. [DOI] [PubMed] [Google Scholar]
- 5.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature biotechnology. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 6.Guo T, Hebrok M. Stem cells to pancreatic beta-cells: new sources for diabetes cell therapy. Endocrine reviews. 2009;30(3):214–227. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan TV, Jaigirdar A, Hoang V, et al. Preferential priming of alloreactive T cells with indirect reactivity. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(4):709–718. doi: 10.1111/j.1600-6143.2009.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugliese A, Reijonen HK, Nepom J, Burke GW., 3rd Recurrence of autoimmunity in pancreas transplant patients: research update. Diabetes management. 2011;1(2):229–238. doi: 10.2217/dmt.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SY, Wang X, Chen Z, et al. Optical imaging of pancreatic beta cells in living mice expressing a mouse insulin I promoter-firefly luciferase transgene. Genesis. 2005;43(2):80–86. doi: 10.1002/gene.20157. [DOI] [PubMed] [Google Scholar]
- 10.Khanna KM, Blair DA, Vella AT, McSorley SJ, Datta SK, Lefrancois L. T cell and APC dynamics in situ control the outcome of vaccination. Journal of immunology. 2010;185(1):239–252. doi: 10.4049/jimmunol.0901047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan TV, Hoang V, Garrod KR, et al. A new T-cell receptor transgenic model of the CD4+ direct pathway: level of priming determines acute versus chronic rejection. Transplantation. 2008;85(2):247–255. doi: 10.1097/TP.0b013e31815e883e. [DOI] [PubMed] [Google Scholar]
- 12.Udaka K, Tsomides TJ, Eisen HN. A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 1992;69(6):989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 13.Honjo K, Xu X, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004;77(3):452–455. doi: 10.1097/01.TP.0000112937.12491.42. [DOI] [PubMed] [Google Scholar]
- 14.Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122(2):439–447. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara H, Asano T, Tsukuda K, et al. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia. 1993;36(11):1139–1145. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- 16.Day CP, Carter J, Bonomi C, et al. Lentivirus-mediated bifunctional cell labeling for in vivo melanoma study. Pigment cell & melanoma research. 2009;22(3):283–295. doi: 10.1111/j.1755-148X.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. Journal of visualized experiments : JoVE. 2007;(9):404. doi: 10.3791/404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juang JH, Hsu BR, Kuo CH. Islet transplantation at subcutaneous and intramuscular sites. Transplantation proceedings. 2005;37(8):3479–3481. doi: 10.1016/j.transproceed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Abdulreda MH, Faleo G, Molano RD, et al. High-resolution, noninvasive longitudinal live imaging of immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12863–12868. doi: 10.1073/pnas.1105002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafael E, Wernerson A, Arner P, Tibell A. In vivo studies on insulin permeability of an immunoisolation device intended for islet transplantation using the microdialysis technique. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes. 1999;31(3):249–258. doi: 10.1159/000008700. [DOI] [PubMed] [Google Scholar]
- 21.Monos D, Gray I, Cooper HL. Glycogen regulation in LPS-stimulated murine splenocytes. Experimental cell research. 1984;151(2):306–313. doi: 10.1016/0014-4827(84)90381-1. [DOI] [PubMed] [Google Scholar]
- 22.Cheng K, Delghingaro-Augusto V, Nolan CJ, et al. High passage MIN6 cells have impaired insulin secretion with impaired glucose and lipid oxidation. PloS one. 2012;7(7):e40868. doi: 10.1371/journal.pone.0040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura F, Gotoh M, Tanaka T, et al. Locally expressed CTLA4-Ig in a pancreatic beta-cell line suppresses accelerated graft rejection response induced by donor-specific transfusion. Diabetologia. 2002;45(6):831–840. doi: 10.1007/s00125-002-0844-3. [DOI] [PubMed] [Google Scholar]
- 24.Gittes GK, Rutter WJ. Onset of cell-specific gene expression in the developing mouse pancreas. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(3):1128–1132. doi: 10.1073/pnas.89.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature biotechnology. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 26.Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PloS one. 2012;7(5):e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White P, May CL, Lamounier RN, Brestelli JE, Kaestner KH. Defining pancreatic endocrine precursors and their descendants. Diabetes. 2008;57(3):654–668. doi: 10.2337/db07-1362. [DOI] [PubMed] [Google Scholar]
- 28.Slack JM. Developmental biology of the pancreas. Development. 1995;121(6):1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 29.Markees TG, Serreze DV, Phillips NE, et al. NOD mice have a generalized defect in their response to transplantation tolerance induction. Diabetes. 1999;48(5):967–974. doi: 10.2337/diabetes.48.5.967. [DOI] [PubMed] [Google Scholar]
- 30.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 31.Pickersgill LM, Mandrup-Poulsen TR. The anti-interleukin-1 in type 1 diabetes action trial--background and rationale. Diabetes/metabolism research and reviews. 2009;25(4):321–324. doi: 10.1002/dmrr.960. [DOI] [PubMed] [Google Scholar]
- 32.Angaswamy N, Fukami N, Tiriveedhi V, Cianciolo GJ, Mohanakumar T. LMP-420, a small molecular inhibitor of TNF-alpha, prolongs islet allograft survival by induction of suppressor of cytokine signaling-1: synergistic effect with cyclosporin-A. Cell transplantation. 2012;21(6):1285–1296. doi: 10.3727/096368911X637371. [DOI] [PubMed] [Google Scholar]
- 33.Blaabjerg L, Christensen GL, Matsumoto M, et al. CRFR1 activation protects against cytokine-induced beta-cell death. Journal of molecular endocrinology. 2014;53(3):417–427. doi: 10.1530/JME-14-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Loudovaris T, Jacobs S, Young S, Maryanov D, Brauker J, Johnson RC. Correction of diabetic nod mice with insulinomas implanted within Baxter immunoisolation devices. Journal of molecular medicine. 1999;77(1):219–222. doi: 10.1007/s001090050340. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai-Braesch M, Jacobson S, Mori H, et al. The TheraCyte device protects against islet allograft rejection in immunized hosts. Cell transplantation. 2013;22(7):1137–1146. doi: 10.3727/096368912X657486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.