Abstract
Objective
To evaluate a central line care maintenance bundle to reduce central line-associated bloodstream infection (CLABSI) in non-ICU settings.
Design
Before-after trial with 12 month follow-up period.
Setting
1250-bed teaching hospital.
Participants
Patients with central lines on eight general medicine wards. Four wards received the intervention and four served as controls.
Intervention
A multifaceted catheter care maintenance bundle consisting of educational programs for nurses, update of hospital policies, visual aids, a competency assessment, process monitoring, regular progress reports, and consolidation of supplies necessary for catheter maintenance.
Results
Data were collected for 25,542 catheter-days including 43 CLABSI (rate = 1.68 per 1,000 CL-days) and 4,012 catheter dressing observations. Following the intervention, a 2.5% monthly decrease in the CLABSI incidence density was observed on intervention floors, but this was not statistically significant (95% confidence interval (CI); −5.3 – 0.4). On control floors, there was a smaller, but marginally significant decrease in CLABSI incidence during the study (change in monthly rate = −1.1%; 95% CI, −2.1 - −0.1). Implementation of the bundle was associated with improvement in catheter dressing compliance on intervention wards (78.8% compliance pre-intervention vs. 87.9% during intervention/follow-up; p<0.001) but improvement was also observed on control wards (84.9% compliance pre-intervention vs. 90.9% during intervention/follow-up; P = .001).
Conclusions
A multi-faceted program to improve catheter care was associated with improvement in catheter dressing care, but no change in CLABSI rates. Additional study is needed to determine strategies to prevent CLABSI in non-ICU patients.
INTRODUCTION
Central line-associated bloodstream infections (CLABSI) are common healthcare-associated infections that can lead to longer hospital stays and increased healthcare costs.1,2 Improved central line insertion practices have led to reductions in CLABSI rates in intensive care units (ICUs), but a substantial number of CLABSI occur among patients in non-ICU inpatient wards.3 Of an estimated 250,000 CLABSI that occur in U.S. hospitals annually, only about 80,000 occur in ICUs.4 Increased recognition of the problem of CLABSI outside of ICUs has led hospitals to expand CLABSI surveillance to non-ICU settings.
Central lines are commonly used outside ICUs. While the proportion of patients with central lines is generally lower in non-ICU wards than in ICUs, the total number of non-ICU patients with central lines in any given hospital is often higher.5-7 In addition, many patients in non-ICU settings have central lines in place for prolonged periods of time, which supports the use of interventions to improve central line use and maintenance practices as a means to prevent infection.8 Reported CLABSI rates in non-ICU settings range from 2 to 6 per 1000 line-days,7-14 which is similar to rates observed in ICUs before implementation of interventions to reduce CLABSI.10
Adherence to best practices for central line care after insertion is a well-established method to prevent CLABSI. Several organizations have noted the importance of central line maintenance practices in CLABSI prevention and have recommended that central line care be a focus of performance improvement and quality assurance in all programs.15,16 Nationally-published guidelines provide specific catheter care recommendations including: education of healthcare personnel responsible for catheter maintenance; disinfection of hubs, needleless connectors, and injection ports before catheter access; use of chlorhexidine skin prep with alcohol for skin antisepsis; and routinely changing transparent dressings every 5-7 days or every 2 days for gauze dressings or whenever soiled, loose, or damp.15-17 Yet, despite recognition of the importance of central line maintenance in relation to CLABSI prevention, adherence to best practices may be inadequate in non-ICU wards, which have not generally been included in CLABSI prevention efforts.6
The purpose of this study was to develop, implement, and evaluate a central line care maintenance bundle designed to optimize central line maintenance practices and reduce CLABSI in non-ICU settings at a large, academic medical center. Prior studies have demonstrated the effectiveness of bundled educational and behavioral interventions in improving compliance with recommended catheter care practices and reducing CLABSI incidence.18-24 However, most of these studies have focused exclusively on ICUs. It was hypothesized that similar bundled interventions might reduce CLABSI incidence in non-ICU settings.
METHODS
Setting and Design
This before-after study with control group was conducted at Barnes-Jewish Hospital (BJH), a 1,250-bed urban tertiary care academic medical center. The study included all adult inpatients on eight general medicine wards who had central lines in place for one or more days between July 1, 2012 and December 31, 2013. There were no exclusion criteria. Four wards were randomly selected to receive the intervention and four wards served as controls. Intervention and control wards had separate nursing leadership and staff nurses did not rotate between wards.
CLABSI prevention policies at BJH follow nationally published guidelines and emphasize proper dressing change procedure, chlorhexidine skin antisepsis, disinfection of catheter hubs/needleless connectors/injection ports prior to access, and daily reassessment of the need for continued central line access.1 Education and training on central line care policies and procedures is provided to staff on an annual basis.
Intervention
A multifaceted, central line care maintenance bundle was developed focusing on maximizing aseptic technique for accessing catheters for blood draws and medication/fluid administration. The intervention bundle consisted of educational programs for nurses emphasizing catheter/dressing care, enhancement of hospital catheter-care policies, visual aids illustrating proper catheter care techniques, including accessing hubs, competency assessment, process monitoring, and consolidation/standardization of the supplies necessary for optimal central line maintenance into a convenient package located in a standard location on the study wards. Key components of the bundle included photo slides with pictures showing proper procedure for changing catheter dressings and regular feedback of data summarizing direct observations of catheter dressings and insertion sites for ward patients. Other measures associated with CLABSI prevention (i.e., hand hygiene observations and training on catheter insertion practices) were already in place throughout the hospital prior to the start of the study.
The intervention bundle was implemented between October and December, 2012. A three-month pre-intervention period (July-September, 2012) preceded bundle implementation and was used to establish baseline catheter care practices and CLABSI rates. We collected data for the 12-months following the intervention period to determine the impact of the intervention once it had been fully implemented.
Data Collection
Patients with central lines were identified using hospital electronic medical records. Demographic and hospitalization data were also abstracted from electronic records, including age, race, sex, dates of hospital admission, and catheter start and end dates. For patients who had catheter-days on more than one study ward during their admission, the total number of catheter-days on intervention and control wards was calculated. Pharmacy records were used to determine the use of chemotherapy (all antineoplastic agents excluding hydroxyurea) and corticosteroids (i.e., prednisone, hydrocortisone, or dexamethasone). Laboratory data was used to identify patients with neutropenia (i.e., absolute neutrophil count <500 cells/microliter).
Throughout the study period, trained research assistants conducted twice weekly observations of existing central line dressings on each study ward. Observations were standardized and included: whether the dressing was secured and intact; whether the dressing was clean and dry; whether the dressing was expired (48 hours for gauze dressings, 7 days for transparent); whether there was purulent discharge; whether the central line was secured; and whether the dressing was dated (date written on the dressing or recorded in the electronic medical record). These measures were used to calculate an overall dressing compliance score, with observations that met all six criteria being labeled as 100% compliant.
Reports summarizing dressing observation data and CLABSI rate data were regularly shared with staff on intervention wards as part of the intervention. These reports included data for each intervention ward and the combined data from all four intervention wards. Reports were shared with staff at monthly meetings during the intervention period and given to the nursing managers on each intervention ward every two months during the follow-up period.
Incident CLABSI data were collected via the hospital's automated Non-ICU CLABSI Surveillance system (NICER).25 The NICER surveillance system applies an algorithm to electronic patient and microbiology data to identify CLABSI in non-ICU wards. The algorithm identifies positive blood cultures that are: hospital-acquired (occur ≥ 48 hours after admission); positive for a non-common skin contaminant; associated with a patient who had a central line in place in the 48 hours before the culture; and not associated with a positive culture of the same organism from another body site.25 A study comparing the NICER surveillance algorithm to manual chart review using NHSN definitions to identify CLABSI determined that NICER had 95% sensitivity and 97% specificity.25
CLABSI were attributed to the ward where the patient was admitted at the time of infection. Infections were labeled early CLABSI if they occurred <14 days after the first recorded catheter-day; infections that occurred 14 or more days after line insertion were labeled late-onset CLABSI. This cutoff was based on the observation that intra-luminal contamination increases after a catheter is in place for two weeks.26 Microbiological data were obtained for each CLABSI to identify microbiological etiology. Catheter-day denominator data were abstracted from medical informatics using midnight census data.
The study protocol was reviewed and approved by the Washington University Human Research Protection Office. The need for written informed consent was waived because this was determined to be a quality improvement project.
Analysis
The characteristics of patients on intervention versus control wards were compared using Chi Square and t-tests. Central line duration for intervention versus control wards and for patients who did versus did not develop CLABSI was compared using Mann-Whitney U tests. Monthly device utilization ratios (catheter-days/patient-days) were calculated to identify potential differences in use of central lines over time. Dressing observation data from the pre-intervention period versus the combined intervention/follow-up period were compared using Chi Square tests to evaluate the impact of the intervention bundle on catheter care practices. CLABSI incidence densities were calculated for the combined intervention and control wards, both pre-intervention and during intervention/follow-up. The effect of the intervention on the monthly CLABSI rate was evaluated using regression models with autoregressive integrated moving average (ARIMA) errors, to account for the auto correlation of errors over time.27 Microbiology data were evaluated to identify the types of organisms associated with CLABSI. The proportion of early vs. late CLABSI during the pre-intervention vs. intervention/follow-up periods were compared using Fisher's Exact Tests. Data were analyzed using IBM SPSS Statistics, Version 21.0 (IBM Corp., Armonk, NY) and R Statistical Software, Version 3.2.3 (R Core Team, Vienna Austria).
RESULTS
There were 25,542 catheter-days associated with 5,054 admissions during the study period; 13,394 catheter-days occurred on intervention wards and 12,148 on control wards. There were 4,410 catheter-days during the pre-intervention period, 4,249 during the intervention period, and 16,883 during the follow-up period. Forty-three CLABSI were identified, 26 (60.5%) on intervention wards and 17 (39.5%) on control wards. Ten CLABSI occurred during the pre-intervention period, 6 during the intervention period, and 27 during follow-up. Eighteen infections (41.9%) were classified as late-onset CLABSI.
There were no significant differences in patient demographics or chemotherapy or corticosteroid use for patients on intervention versus control wards (Table 1). The median number of catheter-days per admission was three for both intervention and control wards, (p = .085) and remained stable throughout the study period (data not shown). The device utilization ratio was also similar for intervention and control wards (0.31 for intervention floors and 0.32 for control floors) and remained stable throughout the study (0.30 before the intervention versus 0.31 during/after the intervention on the intervention wards and 0.32 both before and during/after the intervention on the control wards).
Table 1.
Comparison of patient demographics, by intervention status
| Control wards n = 2432 n (%) | Intervention wards n = 2738 n (%) | P | |
|---|---|---|---|
| Female sex | 1331 (54.7%) | 1556 (56.8%) | .129 |
| Race | .185 | ||
| Caucasian | 1332 (54.8%) | 1439 (52.6%) | |
| Black | 926 (38.1%) | 1111 (40.6%) | |
| Other/Unknown | 174 (7.2%) | 188 (6.9%) | |
| Age - mean (SDa) | 55.5 (17.1) | 54.9 (16.7) | .212 |
| Neutropenia | 67 (2.8%) | 56 (2.0%) | .095 |
| Chemotherapy | 161 (6.6%) | 163 (6.0%) | .323 |
| Corticosteroids | 597 (24.5%) | 694 (25.3%) | .508 |
| Line days per admission - median (IQRb) | 3 (2-6) | 3 (2-6) | .085 |
SD, Standard deviation
IQR, Interquartile range
Males were more likely to develop CLABSI than females (60.5% of CLABSI patients were male versus 44.0% of non-CLABSI patients; p =.031) (Table 2). Patients who developed CLABSI also had a longer median duration of catheterization than patients who did not develop CLABSI (10 versus 3 days; p <.001).
Table 2.
Comparison of patients by CLABSI status
| No CLABSI 5127 (%) n (%) | CLABSI 43 (%) n (%) | P | |
|---|---|---|---|
| Female sex | 2870 (56.0%) | 17 (39.5%) | .031 |
| Race | .131 | ||
| Caucasian | 2749 (53.6%) | 22 (51.2%) | |
| Black | 2016 (39.3%) | 21 (48.8%) | |
| Other/Unknown | 362 (7.1%) | 0 | |
| Age - mean (SDa) | 55.2 (16.9) | 53.0 (15.6) | .734 |
| Neutropenia | 123 (2.4%) | 0 | .304 |
| Chemotherapy | 322 (6.3%) | 2 (4.7%) | .661 |
| Corticosteroids | 1278 (24.9%) | 13 (30.2%) | .423 |
| Line days per admission - median (IQRb) | 3 (2-6) | 10 (7-17) | <.001 |
SD, Standard deviation
IQR, Interquartile range
A total of 4,012 central line dressing observations were made for the study: 801 during the pre-intervention period, 579 during the intervention period, and 2,632 during the follow-up period. Approximately 55% of observed lines were single or double lumen peripherally inserted central catheters (PICC). The remainder were triple lumen non-tunneled central lines or dialysis catheters. There was no significant difference in the proportion of PICCs on the intervention versus control wards (54.1% versus 56.1%, p = 0.183). The results of the dressing observations are shown in Table 3. The proportion of dressings observed to be 100% compliant (meeting all 6 compliance measures) was higher on control wards than on intervention wards during the pre-intervention period (84.9% vs. 78.8%, p = .026) and remained higher during the intervention/follow-up period (90.9% vs. 87.9%, p = .007). On intervention wards, the proportion of dressings achieving 100% compliance increased from 78.8% pre-intervention to 87.9% during intervention/follow-up (difference = +9.1%; p<.001) (Figure 1). However, dressing compliance on control wards also improved during the study period, with the proportion of 100% compliant dressings increasing from 84.9% pre-intervention to 90.9% during intervention/follow-up (difference = +6.0%; p = .001).
Table 3.
Comparison of dressing observations, by period and intervention status
| Study Period | Dressing Intact |
Dressing Clean/Dry |
Dressing Dated |
Dressing not Expired |
Non-Purulent Insertion Site |
Dressing Secured |
100% Compliancea |
|---|---|---|---|---|---|---|---|
| Intervention Wards (2,091 observations) | |||||||
| Pre-Intervention | 384 (86.7%) | 409 (92.3%) | 443 (100%) | 429 (96.8%) | 424 (95.7%) | 438 (98.9%) | 349 (78.8%) |
| Intervention/follow-up | 1540 (93.4%) | 1602 (97.2%) | 1639 (99.5%) | 1610 (97.7%) | 1623 (98.5%) | 1635 (99.2%) | 1449 (87.9%) |
| P-valueb | <.001 | <.001 | .119 | .305 | <.001 | .492 | <.001 |
| Control Wards (1,921 observations) | |||||||
| Pre-Intervention | 329 (91.9%) | 344 (96.1%) | 356 (99.4%) | 354 (98.9%) | 349 (97.5%) | 354 (98.9%) | 304 (84.9%) |
| Intervention/follow-up | 1492 (95.5%) | 1517 (97.1%) | 1548 (99.0%) | 1553 (99.4%) | 1539 (98.5%) | 1554 (99.4%) | 1420 (90.9%) |
| P-valueb | .006 | .342 | .465 | .338 | .199 | .260 | .001 |
Composite score incorporating all 6 of the individual dressing quality measures; dressings observed to have 6 of 6 measures compliant were defined as being 100% compliant.
Comparison of pre-intervention to intervention/follow-up period.
Figure 1.
Proportion of Central line Insertion sites/Dressings that were 100% Compliant (6/6 measures) for the combined intervention and control wards
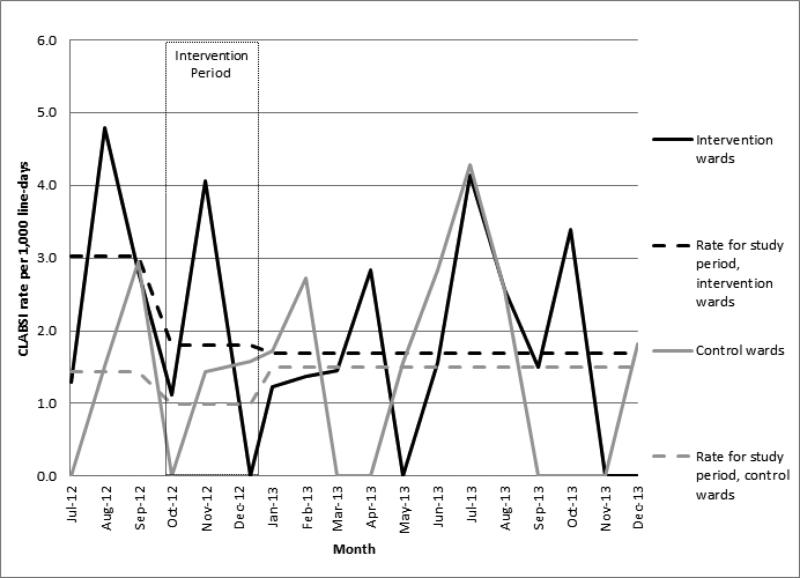
On the intervention wards, the pre-intervention CLABSI incidence density was 3.02 per 1000 catheter-days versus 1.72 per 1000 catheter-days during/after the intervention (incidence rate ratio (IRR) = 0.57). On the control wards, CLABSI incidence was 1.43 per 1000 catheter-days pre-intervention versus 1.39 per 1000 catheter-days during/after the intervention (IRR = 0.97) (Figure 2). ARIMA analysis indicted that, during the study period, the monthly CLABSI incidence density decreased at a rate of 2.5% per month on the intervention floors (−2.5; 95% confidence interval (CI): −5.3 - 0.4), though this decrease was not statistically significant. On control floors, the monthly CLABSI rate decreased at a rate of 1.1% (−1.1; 95% CI, −2.1 - −0.1), which was marginally significant. There was no statistically significant difference in mean CLABSI incidence rates for the intervention versus control wards during the pre-intervention period (IRR = 2.10; 95% CI, 0.56 – 7.89) or during the intervention/follow-up period (IRR = 1.23; 95% CI, 0.62-2.45).
Figure 2.
CLABSI rates over time by intervention status for the combined intervention and control wards, with mean rate by study period.
For the CLABSI that occurred during the study period, the types of organisms identified upon microbiological analysis included: Gram positive aerobes (37.7%), Gram negative aerobes (31.9%), anaerobes (15.9%), and fungi (13.0%). There was no association between early versus late CLABSI status and the types of organisms identified (p = 0.651). Eighteen CLABSI (41.8%) were polymicrobial. The proportion of CLABSI that were late-onset in the pre-intervention period versus the intervention/follow-up period were similar in the intervention wards (4/7 (57.1%) vs. 9/19 (47.4%); p = 1.00) and in the control wards (0/3 (0%) vs. 5/14 (35.7%); p =.515).
DISCUSSION
We found that implementation of the central-line care maintenance bundle was associated with improved insertion-site care on both intervention and control wards. The intervention was also associated with a decline in CLABSI incidence on the intervention floors, although this decrease was not statistically significant, and a smaller, but marginally significant decrease on control wards.
The dressing observation data revealed gaps in catheter care practices on both intervention and control wards. Problems with improperly dated or undated dressings have been reported in other studies of catheter care practices.28 In the survey of nurses that we conducted to inform this study, nurses frequently mentioned undated dressings as being a barrier to performing dressing changes (data not shown). Further work is needed to facilitate accurate recording of dressing changes and to integrate reminders into electronic medical record systems.
We also found that baseline compliance with optimal dressing care practices was higher on control wards than on intervention wards. In addition, while there was a 9.1% improvement in compliance on intervention wards between the pre-intervention and intervention/follow-up periods, there was a 6.0% improvement in compliance on control wards over the same periods. This indicates that larger secular trends in improved dressing care hospital-wide may potentially account for some of the observed improvement. Overall baseline compliance with optimal dressing care practices was high on both intervention and control wards even at baseline (78.8% and 84.9%, respectively). This is in contrast to previous observational studies at other teaching hospitals, where dressing and insertion site care non-compliance was reported to be 31-44.8%.28,29
Improvement in catheter care practices on intervention wards following introduction of the central line care maintenance bundle was correlated with a 43% decrease in CLABSI on intervention floors; however, this decrease did not achieve statistical significance There was a smaller but marginally significant decrease in the CLABSI rate on control wards, along with improvement in catheter care practices during the study period. It is possible that, because compliance with catheter care practices in these wards was already high at baseline, the impact of further improvements was minimized. It is also possible that hub disinfection practices did not change in response to the intervention, reducing the impact on CLABSI incidence. However, it is also possible that the observation period was too short to detect a significant change in rates over time, especially because the rates in some wards were zero over some months. Further study is needed to determine why better catheter maintenance did not lead to lower CLABSI rates.
Examination of microbiology data identified Gram positive and Gram negative aerobes as the most common sources of infection for the CLABSI identified during the study period, with many patients having multiple organisms identified upon blood culture. There was no statistical difference in the proportion of CLABSI that were late-onset during the pre-intervention versus intervention/follow-up periods on either intervention or control wards. However, the small number of CLABSI patients in this sample would have made any differences difficult to detect.
In our study population, patients with central lines who developed CLABSI were more likely to be male and had longer average catheter duration than patients with central lines who did not develop CLABSI. Male gender has previously been identified as a risk factor for catheter-related BSI in studies involving ICU patients, 30,31 and longer catheter duration is well-known to increase risk for CLABSI due to extended exposure to risk.26,32
Strengths of this study include: a focus on CLABSI prevention in non-ICU settings, direct observation of catheter insertion site care practices, and detailed microbiology data. The main limitation of this study was the small number of patients who developed CLABSI, which made it difficult to determine the impact of the central line care maintenance bundle on CLABSI incidence.
Reduction of CLABSI incidence within intensive care units in the United States over the last decade has been a major success in the field of healthcare-associated infection prevention.33 Translation of these benefits to patients outside the ICU is needed. Additional studies are still needed to determine the optimal strategy to make this occur.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by a CDC Prevention Epicenters Grant (1U54CK000162-01). All authors report no conflicts of interest relevant to this article. The authors would like to thank Candice Cass and Rachel Hartzell for their assistance in conducting this study, and the nursing staff at Barnes-Jewish Hospital for their cooperation, without which this study would not be possible.
Footnotes
This original research has not previously been presented in a preliminary report or abstract.
REFERENCES
- 1.Han Z, Liang SY, Marschall J. Current strategies for the prevention and management of central line-associated bloodstream infections. Infect Drug Resist. 2010;3:147–163. doi: 10.2147/IDR.S10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whited A, Lowe JM. Central line-associated bloodstream infection: not just an intensive care unit problem. Clin J Oncol Nurs. 2013;17:21–24. doi: 10.1188/13.CJON.21-24. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease C Prevention. Vital signs: central line-associated blood stream infections--United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–248. [PubMed] [Google Scholar]
- 4.O'Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52:1087–1099. doi: 10.1093/cid/cir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Climo M, Diekema D, Warren DK, et al. Prevalence of the use of central venous access devices within and outside of the intensive care unit: results of a survey among hospitals in the prevention epicenter program of the Centers for Disease Control and Prevention. Infection Control & Hospital Epidemiology. 2003;24:942–945. doi: 10.1086/502163. [DOI] [PubMed] [Google Scholar]
- 6.Trick WE, Vernon MO, Welbel SF, Wisniewski MF, Jernigan JA, Weinstein RA. Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25:266–268. doi: 10.1086/502390. [DOI] [PubMed] [Google Scholar]
- 7.Ajenjo MC, Morley JC, Russo AJ, et al. Peripherally inserted central venous catheter-associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32:125–130. doi: 10.1086/657942. [DOI] [PubMed] [Google Scholar]
- 8.Kallen AJ, Patel PR, O'Grady NP. Preventing catheter-related bloodstream infections outside the intensive care unit: expanding prevention to new settings. Clin Infect Dis. 2010;51:335–341. doi: 10.1086/653942. [DOI] [PubMed] [Google Scholar]
- 9.Memish ZA, Arabi Y, Cunningham G, et al. Comparison of US and non-US central venous catheter infection rates: evaluation of processes and indicators in infection control study. Am J Infect Control. 2003;31:237–242. doi: 10.1067/mic.2003.5. [DOI] [PubMed] [Google Scholar]
- 10.Marschall J, Leone C, Jones M, Nihill D, Fraser VJ, Warren DK. Catheter-associated bloodstream infections in general medical patients outside the intensive care unit: a surveillance study. Infection Control & Hospital Epidemiology. 2007;28:905–909. doi: 10.1086/519206. [DOI] [PubMed] [Google Scholar]
- 11.Wischnewski N, Kampf G, Gastmeier P, et al. Prevalence of primary bloodstream infections in representative German hospitals and their association with central and peripheral vascular catheters. Zentralbl Bakteriol. 1998;287:93–103. doi: 10.1016/s0934-8840(98)80152-7. [DOI] [PubMed] [Google Scholar]
- 12.Vonberg RP, Behnke M, Geffers C, et al. Device-associated infection rates for non-intensive care unit patients. Infect Control Hosp Epidemiol. 2006;27:357–361. doi: 10.1086/503339. [DOI] [PubMed] [Google Scholar]
- 13.Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73:41–46. doi: 10.1016/j.jhin.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Trick WE, Miranda J, Evans AT, Charles-Damte M, Reilly BM, Clarke P. Prospective cohort study of central venous catheters among internal medicine ward patients. Am J Infect Control. 2006;34:636–641. doi: 10.1016/j.ajic.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Chopra V, Krein SL, Olmsted RN, Safdar N, Saint S. Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Agency for Healthcare Research and Quality; Rockville, MD: 2013. Prevention of Central Line-Associated Bloodstream Infections: Brief Update Review. pp. 88–109. [Google Scholar]
- 16.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S22–30. doi: 10.1086/591059. [DOI] [PubMed] [Google Scholar]
- 18.Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59–64. doi: 10.1097/00003246-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139:131–136. doi: 10.1001/archsurg.139.2.131. [DOI] [PubMed] [Google Scholar]
- 20.Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355:1864–1868. doi: 10.1016/S0140-6736(00)02291-1. [DOI] [PubMed] [Google Scholar]
- 21.Fakih MG, Jones K, Rey JE, et al. Sustained improvements in peripheral venous catheter care in non-intensive care units: a quasi-experimental controlled study of education and feedback. Infect Control Hosp Epidemiol. 2012;33:449–455. doi: 10.1086/665322. [DOI] [PubMed] [Google Scholar]
- 22.Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29:1171–1173. doi: 10.1086/591862. [DOI] [PubMed] [Google Scholar]
- 23.Shedlarski A, White-Williams C. Research Rounds: An evidence-based project to decrease catheter-related bloodstream infections. Nursing Critical Care. 2013;8:39–43. [Google Scholar]
- 24.Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126:1612–1618. doi: 10.1378/chest.126.5.1612. [DOI] [PubMed] [Google Scholar]
- 25.Woeltje KF, McMullen KM, Butler AM, Goris AJ, Doherty JA. Electronic surveillance for healthcare-associated central line-associated bloodstream infections outside the intensive care unit. Infect Control Hosp Epidemiol. 2011;32:1086–1090. doi: 10.1086/662181. [DOI] [PubMed] [Google Scholar]
- 26.Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14:212. doi: 10.1186/cc8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyndman RJ, Athanasopoulos G. Forecasting: principles and practice. OTexts. 2013 [Google Scholar]
- 28.Rupp ME, Cassling K, Faber H, et al. Hospital-wide assessment of compliance with central venous catheter dressing recommendations. Am J Infect Control. 2013;41:89–91. doi: 10.1016/j.ajic.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Shapey IM, Foster MA, Whitehouse T, Jumaa P, Bion JF. Central venous catheter-related bloodstream infections: improving post-insertion catheter care. J Hosp Infect. 2009;71:117–122. doi: 10.1016/j.jhin.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37:2167–2173. doi: 10.1097/CCM.0b013e3181a02d8f. quiz 2180. [DOI] [PubMed] [Google Scholar]
- 31.Lissauer ME, Leekha S, Preas MA, Thom KA, Johnson SB. Risk factors for central line-associated bloodstream infections in the era of best practice. The journal of trauma and acute care surgery. 2012;72:1174–1180. doi: 10.1097/TA.0b013e31824d1085. [DOI] [PubMed] [Google Scholar]
- 32.Klevens RM, Edwards JR, Richards CL, Jr., et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagan RP, Edwards JR, Park BJ, Fridkin SK, Magill SS. Incidence trends in pathogen-specific central line-associated bloodstream infections in US intensive care units, 1990-2010. Infect Control Hosp Epidemiol. 2013;34:893–899. doi: 10.1086/671724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.