Abstract
Background
While many studies have evaluated the association between acute childhood leukemia and environmental factors, knowledge is limited. Ambient air pollution has been classified as a Group 1 carcinogen, but studies have not established whether traffic-related air pollution is associated with leukemia. The goal of our study was to determine if children with acute leukemia had higher odds of exposure to traffic-related air pollution at birth compared to controls.
Methods
We conducted a case-control study using the Oklahoma Central Cancer Registry to identify cases of acute leukemia in children diagnosed before 20 years of age between 1997 and 2012 (n=307). Controls were selected from birth certificates and matched to cases on week of birth (n=1,013). Using a novel satellite-based land-use regression model of nitrogen dioxide (NO2) and estimating road density based on the 2010 US Census, we evaluated the association between traffic-related air pollution and childhood leukemia using conditional logistic regression.
Results
The odds of exposure to the fourth quartile of NO2 (11.19–19.89 ppb) were similar in cases compared to controls after adjustment for maternal education (OR: 1.08, 95% CI: 0.75, 1.55). These estimates were stronger among children with acute myeloid leukemia (AML) than acute lymphoid leukemia, with a positive association observed among urban children with AML (4th quartile odds ratio: 5.25, 95% confidence interval: 1.09, 25.26). While we observed no significant association with road density, male cases had an elevated odds of exposure to roads at 500 m from the birth residence compared to controls (OR: 1.39, 95% CI: 0.93, 2.10), which was slightly attenuated at 750 m.
Conclusions
Although we observed no association overall between NO2 or road density, this was the first study to observe an elevated odds of exposure to NO2 among children with AML compared to controls suggesting further exploration of traffic-related air pollution and AML is warranted.
Keywords: children, leukemia, traffic, air pollution, nitrogen dioxide
1. Introduction
Acute leukemia is the most common type of childhood cancer, accounting for approximately 26% of all childhood cancers (United States Department of Health and Human Services et al. 2016). Many studies have evaluated the association between acute childhood leukemia and various environmental factors, with conflicting results (Belson et al. 2007; Smith et al. 1999). One environmental exposure of interest is air pollution, a component of which is motor vehicle emissions. According to the International Agency for Research on Cancer, ambient air pollution has been classified as a Group 1 carcinogen (International Agency for Research on Cancer 2013).
Although studies have used different methodologies to measure nitrogen dioxide (NO2) at different time points of exposure, several have detected a positive association between NO2 and childhood leukemia (Amigou et al. 2011; Badaloni et al. 2013; Feychting et al. 1998; Ghosh et al. 2013; Raaschou-Nielsen et al. 2001; Weng et al. 2008). Studies occurred in varying regions of the world, with associations observed at different levels of NO2 exposure ranging from 6.5 parts per billion (ppb) to 42.6 ppb (Amigou et al. 2011; Ghosh et al. 2013; Weng et al. 2008). In addition to NO2, investigators have also evaluated other traffic-related measures to assess the relationship with leukemia and any childhood cancer with mixed results (Abdul Rahman et al. 2008; Amigou et al. 2011; Badaloni et al. 2013; Crosignani et al. 2004; Harrison et al. 1999; Heck et al. 2013; Langholz et al. 2002; Nordlinder and Jarvholm 1997; Pearson et al. 2000; Raaschou-Nielsen et al. 2001; Reynolds et al. 2001; Reynolds et al. 2002; Reynolds et al. 2004; Savitz and Feingold 1989; Spycher et al. 2015; Steffen et al. 2004; Von Behren et al. 2008). Of the seven studies that measured exposure to main roads or gas stations within 50 meters (m) to 500 m of the residence, five observed positive associations with leukemia (Abdul Rahman et al. 2008; Amigou et al. 2011; Harrison et al. 1999; Spycher et al. 2015; Steffen et al. 2004), while Badaloni et al. (2013) and Houot et al. (2015) observed no association.
Studies have not established whether a specific pollutant, such as benzene, or the combination of pollutants from traffic is carcinogenic. Therefore, it is important to evaluate exposure to traffic as a source of complex chemical mixtures in relation to childhood leukemia in addition to specific pollutants. NO2 is considered a good indicator of traffic exposure and is commonly used to measure traffic density (Beckerman et al. 2008). As one of the six criteria pollutants established by the Clean Air Act, the Environmental Protection Agency (EPA) set the National Ambient Air Quality Standard of NO2 at a one-hour limit of 100 ppb and an annual average limit of 53 ppb (United States Environmental Protection Agency 2011). Although many dispersion models exist to estimate NO2, Novotny et al. (2011) developed a novel method of estimating NO2 using satellite-based estimates in combination with ground-level estimates in a land-use regression (LUR) model for the entire United States, resulting in spatially precise estimates. This model accounts for NO2 from multiple sources, although variables in the LUR model are specifically related to traffic.
Our study incorporated a novel method of estimating NO2 for the contiguous United States using a satellite-based LUR model at the census block level, which we compared with a standard individual-level measure of road density. Only one other study has incorporated satellite-based methods to estimate NO2 in Italy (Badaloni et al. 2013). While the authors observed no association with childhood leukemia, others have detected a positive association with NO2 estimated from traffic or monitoring stations, indicating the importance of further evaluating this methodology in the US. The goal of our study was to determine if children with acute leukemia had higher odds of exposure to traffic-related air pollution, measured through NO2 and road density, compared to controls.
2. Methods
2.1. Study Design and Data Sources
To evaluate the association between traffic-related air pollution and acute leukemia, we conducted a case-control study using the Oklahoma Central Cancer Registry (OCCR) as our source for acute leukemia cases (n=360) diagnosed between 1997 and 2012 prior to the age of 20 years. Controls were selected from birth certificate records and matched to cases on week of birth (n=1,440). We initially matched controls to cases in a 4:1 ratio, but because some addresses failed to geocode, 307 cases and 1,013 controls were available for analysis resulting in a ratio of approximately 3.3:1. Data on covariates, including address, were obtained from the birth certificate records for all children and data related to the leukemia diagnosis were obtained from OCCR. To determine urbanization of the child’s residence, we used the 2000 US Census, which classified census blocks as urban or rural. We obtained IRB approval from the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
2.2. Measurement of the Outcome
We included children diagnosed with acute leukemia under 20 years of age between the years of 1997 and 2012 in Oklahoma. To define acute leukemia, we included children with International Classification of Diseases of Oncology, Third Edition histology codes for both acute lymphoid leukemia (ALL) (9820, 9823, 9826, 9827, 9831–9837, 9940, 9948) and acute myeloid leukemia (AML) (9840, 9861, 9866, 9867, 9870–9874, 9891, 9895–9897, 9910, 9920, 9931) as classified by the International Classification of Childhood Cancers, Third edition (Steliarova-Foucher et al. 2005). We linked acute leukemia cases to birth certificates using Registry Plus™ Link Plus software v. 2.0 (CDC, Atlanta, GA). To link the databases we used name and date of birth since a unique identifier was not available, resulting in 72% of leukemia cases linking to birth records. Because we matched controls to cases on week of birth, all children born in the same week who were at risk of developing leukemia at the time the index case was diagnosed were eligible to be selected as a control.
2.3. Geocoding
After standardizing all addresses with the United States Postal Service (United States Postal Service 2014), we used ArcGIS (ESRI®, Redlands, CA) to geocode residence at birth using 2014 TIGER/Line files. Because ArcGIS can only geocode complete street addresses, we used Melissa Data® to geocode rural routes and any addresses that did not geocode in ArcGIS (Melissa Data 2014). This allowed us to geocode 25% of children with rural route addresses. However, we were unable to geocode Highway Contract (HC) Boxes or post office (PO) Boxes since a physical address was not available from birth certificate records. We obtained 2000 US census blocks from the US Census TIGER/Line files (United States Census Bureau 2014).
2.4. Satellite Measurement of NO2
To evaluate NO2, we used the satellite-based LUR model developed by Novotny et al. (2011). The authors used a linear regression model to predict NO2 for the contiguous United States for 2006, which included data from NO2 monitors, land-use characteristics from geographic information systems (GIS), and data on satellite-based NO2 from the Ozone Monitoring Instrument (OMI).
Monitored ground-level annual-mean NO2 levels were obtained from 369 of the EPA’s 423 monitors in the contiguous United States. Monitors missing >25% of hourly measurements were excluded per the EPA’s reliability criteria. The final LUR model was determined using stepwise linear regression (Novotny et al. 2011). Variables included in the final annual mean model were impervious surface, annual OMI NO2, tree canopy, major roads, minor roads, elevation, and distance to the coast, in addition to the monitoring station parameters (distance to major road, annual NO2, latitude, and longitude). The authors applied the final model to all census blocks within the contiguous United States using the 2000 US Census. While the LUR model included all sources of NO2, including industry, airports, and harbors, the model estimated traffic-related NO2 better than industrial sources. In validating the LUR model, the authors observed high correlation between the data used to build the model (90%) and the model-testing data (10%) (R2=0.78 for model-building data, R2=0.76 for model-testing data).
2.5. Road Density
Roads were classified by the 2010 US Census as primary, secondary, and tertiary. Primary roads generally include limited access highways; secondary roads generally include US, State, or County Highways systems; and tertiary roads are local roads (Table 1). However, some limited access highways may have a lower road classification if traffic is generally lower (i.e., toll roads). Using 2014 TIGER/Line® road files we created buffers of 500 m and 750 m around the birth residence and included all road classifications within the buffers to define exposure (United States Census Bureau 2015). High exposure was classified as a residence within 500 m (750 m) of:
Primary roads only
Primary and secondary roads, with or without tertiary roads
Primary and tertiary roads only
Secondary and tertiary roads only
Table 1.
Road classifications included in our study using 2014 TIGER/Line® files (2010 US Census).
| Road Classification |
Description | Study Classification |
|---|---|---|
| S1100 | Generally divided, limited-access highways (interstate highways, including toll highways) |
Primary |
| S1200 | Generally US, State, or County Highway system, may have multiple lanes, may or may not be divided, generally include intersections with other roads/highways (Main arteries) |
Secondary |
| S1400 | Local neighborhood road, rural road, or city street | Tertiary |
| S1500 | Vehicular Trail 4WD | Tertiary |
| S1630 | Ramp | Tertiary |
| S1640 | Service drive usually along a limited access highway |
Tertiary |
| S1710 | Walkway or pedestrian trail | Tertiary |
| S1730 | Alley | Tertiary |
| S1740 | Private road for service vehicles | Tertiary |
| S1750 | Internal US Census Bureau use | Tertiary |
| S1780 | Parking lot road | Tertiary |
Low exposure was classified as a residence within 500 m (750 m) of:
Secondary roads only
Tertiary roads only
No roads
In addition, we conducted a sensitivity analysis evaluating the sum of total miles of road within 500 m of the child’s birth residence, divided into quartiles of exposure (1st Quartile: 0.61-<4.70 miles, 2nd Quartile: 4.70-<6.34 miles, 3rd Quartile, 6.34-<7.96 miles, 4th Quartile: 7.96–16.02 miles), allowing us to evaluate dose response.
2.6. Statistical Analysis
We used conditional logistic regression to evaluate the association between both NO2 and road density and childhood acute leukemia, accounting for the matching variable, week of birth. In our evaluation of the association between traffic-related air pollution and acute leukemia, we used a directed acyclic graph to identify potentially confounding variables. The minimally sufficient set of confounding variables included urbanization and maternal education. However, the relationship between urbanization and socioeconomic status is unclear. Degree of urban development may be influenced by socioeconomic status, but is often used as a surrogate of socioeconomic status as it may be related to income (Tselios 2013). Urbanization may also be a surrogate for traffic-related air pollution and may represent other sources of pollution and geographic factors separate from and in addition to traffic (Liu and Diamond 2005). Therefore, we considered models including both urbanization and maternal education and maternal education only. We used backwards selection to assess confounding in our multivariate model, evaluating NO2 and road density in separate models, and defined confounding as greater than 20% change in the OR after removal of covariates from the model. We also evaluated effect modification of the association between traffic-related air pollution and leukemia by urbanization and other covariates from the birth certificate using tests of interaction in the regression model.
We observed that the logit was not linear for the independent variable of NO2 after categorizing the data into eight categories, defined by approximately equal ppb intervals, and plotting them against the estimated logit. Thus, we categorized exposure into quartiles based on the distribution among the controls for analysis. Additionally, we fitted a locally weighted scatterplot smoothing (LOESS) curve to inform the selection of exposure cutpoints. The LOESS curve plotted the estimated log-odds of being a case against all values of NO2 exposure. The pattern was relatively constant above the 10th percentile (5.20 ppb) and below the 95th percentile (14.33 ppb). Thus, in addition to exposure quartiles, we also examined three categories of exposure representing low, moderate, and high exposure with cutpoints at 5.20 ppb and 14.33 ppb.
Because older children may be less likely to live in their birth residence compared to younger children, we stratified our analysis by age at diagnosis/index age among matched controls to evaluate the potential effect of residential mobility (0–4, 5–9, 10–14, 15–19 years). Additionally, we compared cases in regard to exposure to NO2 and road density who did and did not change residence between birth and leukemia diagnosis. To assess potential temporal misclassification of traffic-related air pollution, we conducted a stratified analysis by year of birth (1979–1989, 1990–1994, 1995–1999, 2000–2004, and 2005–2010). We also conducted a sensitivity analysis restricted to non-Hispanic (NH) white children to evaluate differences by race/ethnicity and limited to children under 15 years of age at leukemia diagnosis, a common cutpoint for childhood leukemia, excluding adolescents. Additionally, in order to evaluate whether exposure to traffic-related air pollution differed by leukemia type, we examined associations with the specific sub-groups of leukemia, ALL and AML, in an exploratory analysis.
In our data, 316 children (n=53 [14.7%] cases and n=263 [18.3%] controls) did not geocode due to incomplete address data, PO Boxes, HC boxes, and some rural route addresses. However, we conducted a sensitivity analysis that assigned the census block with the highest population within the child’s ZIP code. We then assigned NO2 levels based on the designated census block of these children (n=314), which allowed us to evaluate the association between NO2 and acute leukemia among all but two children originally identified for the study (n=1,798). Because we did not have a physical address, we were unable to evaluate road density in this sensitivity analysis. In addition, among those who were born in Oklahoma but did not link to a birth record, we compared the cancer covariates of type of cancer, year of birth, year of diagnosis, and age at diagnosis to those who linked to birth records using a Chi-Square Test.
To assess model fit, we calculated Pearson and Deviance residual measures to identify observations that were not fit well by the model and observed no poorly fit observations (residual values <5). Unless otherwise specified, all statistical analyses used an alpha of 0.05 to define significance and were conducted using SAS v. 9.3 (Cary, NC).
3. Results
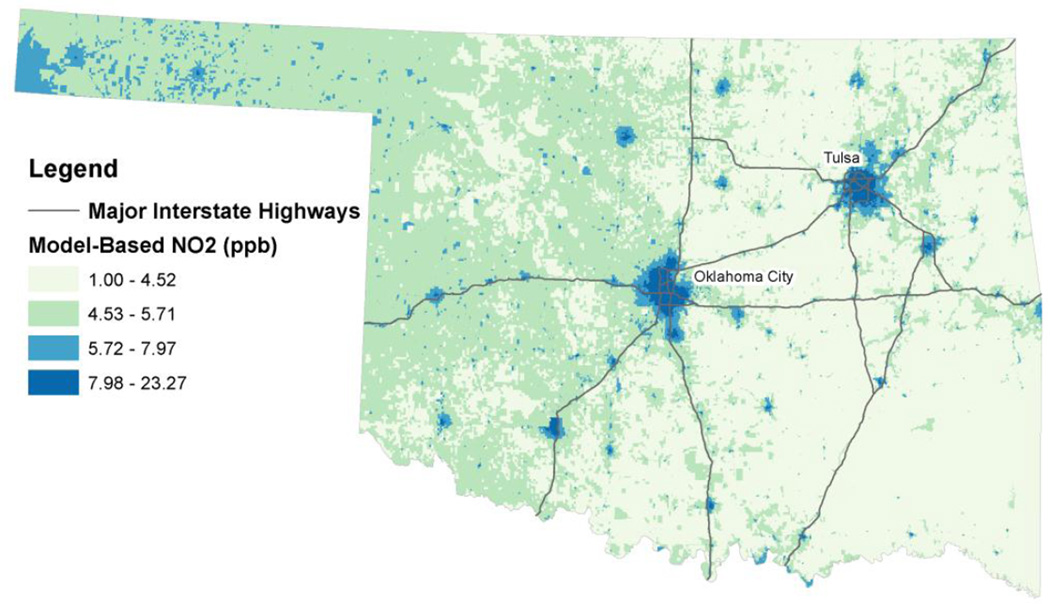
In Oklahoma, the average annual NO2 level in 2006 was 6.65 ppb (range: 22.27 ppb), which was highest in Oklahoma (mean: 11.74 ppb) and Tulsa (mean: 10.65 ppb) counties, with the largest cities being Oklahoma City and Tulsa in these counties, respectively (Figure 1). Levels in the highest quartile also occurred in other larger cities in Oklahoma. In general, NO2 levels were lowest in the eastern half of the state and in the second quartile in the western half.
Figure 1.
Distribution of NO2 in Oklahoma using 2006 estimates from the satellite-based land-use regression model developed by Novotny et al. (2011).
Source: Major interstate highways were obtained from the National Transportation Atlas Databases for the United States published by the Federal Highway Administration.
Of the 1,800 children originally included in our study (n=360 cases, n=1,440 controls), 82% had a complete address (n=1,481), 8% had rural route addresses (n=137), <1% had HC Boxes (n=17), and 9% had PO Boxes (n=165). Nearly all children with a complete physical street address were geocoded (98%, n=1,450), whereas 25% of rural route addresses were geocoded (n=34). We were able to geocode 1,484 birth addresses to a latitude and longitude, which included 307 cases and 1,177 controls. However, because some of these geocoded controls were matched to a case who did not geocode, we excluded an additional 165 controls ensuring there was at least one and up to four geocoded controls for each geocoded case. This resulted in 1,013 controls, with a total sample size of 1,320. A higher percentage of controls did not geocode (18%) compared to cases (15%) (p<0.0001). Among both cases and controls, those who did not geocode were more likely to be NH-white (cases: p=0.03, controls: p<0.0001) and live in a rural census block at birth based on ZIP code (p<0.0001 for cases and controls) compared to those who geocoded. Additionally, maternal age at the child’s birth was lower among cases who did not geocode compared to geocoded cases (p=0.02), but no difference was observed among controls.
Cases were more likely to be male and Hispanic ethnicity and less likely to be NH-African American (Table 2). ALL was the most common type of acute leukemia among cases (74.3%) and nearly half of all cases were diagnosed with leukemia between birth and age 4 (48.5%). A high percentage of NH-African American (95.6%) and Hispanic (94.8%) cases resided in an urban compared to a rural census block at delivery, which was less pronounced in NH-white (78.8%) and NH-American Indian (69.3%) children. All children in the NH-Other category resided in an urban census block at delivery. Approximately 81% of children with ALL and AML resided in an urban census block.
Table 2.
Distribution of birth characteristics of the child, mother, and residence by case/control status.
| Cases (n=307) |
Controls (n=1,013) |
||||
|---|---|---|---|---|---|
| N | % | N | % | p-valuea | |
| Female (v. Male) | 130 | 42.4 | 518 | 51.1 | 0.02 |
| Race/Ethnicityb | 0.001 | ||||
| Non-Hispanic (NH) White | 207 | 67.4 | 702 | 69.3 | |
| NH African American | 20 | 6.5 | 122 | 12.0 | |
| NH American Indian | 29 | 9.5 | 88 | 8.7 | |
| NH Other | 14 | 4.6 | 22 | 2.2 | |
| Hispanic | 37 | 12.1 | 79 | 7.8 | |
| Maternal Age at Child’s Delivery | 0.34 | ||||
| <20 years | 40 | 13.0 | 162 | 16.0 | |
| 20–34 years | 240 | 78.2 | 778 | 76.8 | |
| ≥35 years | 27 | 8.8 | 73 | 7.2 | |
| Parity | 0.05 | ||||
| 0 previous deliveries | 112 | 36.5 | 430 | 42.5 | |
| 1 previous delivery | 91 | 29.6 | 320 | 31.6 | |
| 2 previous deliveries | 67 | 21.8 | 159 | 15.7 | |
| 3+ previous deliveries | 37 | 12.1 | 104 | 10.3 | |
| Tobacco Use During Pregnancy | 0.87 | ||||
| (1991–2010) | |||||
| Yes | 36 | 13.8 | 126 | 14.4 | |
| No | 198 | 75.9 | 689 | 78.7 | |
| Unknown | 27 | 10.3 | 60 | 6.9 | |
| Maternal Education | 0.33 | ||||
| >High School Education | 118 | 38.4 | 406 | 40.1 | |
| Completed High School | 103 | 33.6 | 361 | 35.6 | |
| <High School Education | 81 | 26.4 | 229 | 22.6 | |
| Unknown | 5 | 1.6 | 17 | 1.7 | |
| Urban Census Block at Birth | |||||
| Residence (v. Rural) | 249 | 81.1 | 830 | 81.9 | 0.63 |
| Complete Street Address (v. Rural Route) |
300 | 97.7 | 989 | 97.6 | 0.94 |
p-value based on univariate conditional logistic regression
Unknown Hispanic ethnicity classified as non-Hispanic, NH Other ethnicity includes Asian, Pacific Islander, other unclassified ethnicity, and unknown ethnicity.
When evaluating NO2 at the census block level, we observed no association with any acute leukemia or ALL with odds ratios (OR) near 1.0 and little difference after adjustment for maternal education (Table 3). While the ORs for children with AML were elevated and stronger in the fourth quartile after adjustment for maternal education, the 95% confidence interval (CI) included the null value. We observed a marginal interaction between NO2 and urbanization for children with AML (p=0.09), but no interaction in our analysis with any leukemia (p=0.99) or ALL (p=0.93). Because there were no rural children with NO2 levels above the median in our analysis of AML, we conducted a sub-analysis restricted to urban children (Table 4). We observed increased odds of exposure to higher levels of NO2 among urban children with AML compared to matched controls, with a significant, but imprecise, estimate for the fourth quartile of exposure (OR: 5.25, 95% CI: 1.09, 25.26) after adjustment for maternal education.
Table 3.
OR and 95% CI for the association between NO2 and childhood acute leukemia.
| All Leukemia | Acute Lymphoid Leukemia |
Acute Myeloid Leukemia |
||||||
|---|---|---|---|---|---|---|---|---|
| Cases N |
Controls N |
Unadjusted OR (95% CI) |
Adjusted for Maternal Education OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted for Maternal Education OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted for Maternal Education OR (95% CI) |
|
|
Quartiles of NO2 |
||||||||
| 1.00–7.04 ppb |
80 | 252 | Referent | Referent | Referent | Referent | Referent | Referent |
| 7.05–8.82 ppb |
73 | 251 | 0.94 (0.65, 1.34) |
0.88 (0.61, 1.27) |
0.88 (0.58, 1.32) |
0.85 (0.56, 1.29) |
1.16 (0.55, 2.45) |
1.17 (0.54, 2.56) |
| 8.83–11.18 ppb |
64 | 256 | 0.79 (0.54, 1.14) |
0.75 (0.52, 1.09) |
0.66 (0.42, 1.02) |
0.65 (0.42, 1.01) |
1.24 (0.61, 2.53) |
1.28 (0.60, 2.71) |
| 11.19– 19.89 ppb |
90 | 254 | 1.11 (0.78, 1.58) |
1.08 (0.75, 1.55) |
1.15 (0.77, 1.71) |
1.09 (0.72, 1.64) |
1.04 (0.50, 2.20) |
1.28 (0.59, 2.80) |
|
Cutpoints at 10th and 95th percentiles |
||||||||
| 1.00–5.19 ppb |
23 | 101 | Referent | Referent | Referent | Referent | Referent | Referent |
| 5.20–14.32 ppb |
274 | 860 | 1.34 (0.83, 2.17) |
1.30 (0.81, 2.10) |
1.19 (0.71, 2.00) |
1.15 (0.68, 1.93) |
2.36 (0.67, 8.29) |
2.64 (0.74, 9.43) |
| 14.33- 19.89 ppb |
10 | 52 | 0.80 (0.35, 1.84) |
0.79 (0.34, 1.84) |
0.76 (0.29, 2.00) |
0.74 (0.28, 1.98) |
1.24 (0.21, 7.23) |
1.62 (0.27, 9.74) |
OR=odds ratio, CI=confidence interval
Table 4.
OR and 95% CI for the association between NO2 and childhood acute myeloid leukemia among urban children.
| Quartiles of NO2 | Cases | Controls | Unadjusted OR (95% CI) |
Adjusted for Maternal Education OR (95% CI) |
|---|---|---|---|---|
| 1.00–7.04 ppb | <5a | 26 | Referent | Referent |
| 7.05–8.82 ppb | 19 | 58 | 4.54 (0.96, 21.46) | 4.73 (0.97, 23.04) |
| 8.83–11.18 ppb | 23 | 66 | 4.54 (0.97, 21.35) | 4.82 (1.00, 23.31) |
| 11.19–19.89 ppb | 20 | 68 | 4.09 (0.88, 19.03) | 5.25 (1.09, 25.26) |
Categories with <5 observations suppressed due to confidentiality reasons.
OR=odds ratio, CI=confidence interval
Regarding road density, we observed no association between road density and acute leukemia when exposure was assessed within 500 m or 750 m from the residence at birth (Table 5). We detected an interaction between road density and gender at 500 m from the birth residence (p=0.01), resulting in a higher unadjusted odds of exposure for male cases compared to controls, but a lower unadjusted odds of exposure among females. For comparison, we also included results stratified by gender for road density within 750 m of the birth residence, although the interaction achieved only marginal statistical significance (p=0.08). The OR for both males and females was stronger when road density was assessed at a narrower distance of 500 m from the residence, with a larger OR for males and a more protective OR for females at 500 m. In contrast to the results with NO2, we observed no interaction between road density and urbanization (750 m: p=0.76, 500 m: p=0.81). When stratified by subtype of leukemia, we observed a higher, but non-significant unadjusted OR for males (OR: 1.53, 95% CI: 0.96, 2.44) than females (OR: 0.57, 95% CI: 0.27, 1.02) for ALL, but no differences for AML (Table 6). In our sensitivity analysis evaluating the sum of total miles of road within 500 m of the child’s birth residence, we observed results similar to those in our primary analysis of road density (data not shown).
Table 5.
OR and 95% CI for the association between road density and childhood acute leukemia.
| Cases | Controls | Unadjusted | Adjusted for Maternal Education |
Adjusted for Urbanization and Maternal Education |
|
|---|---|---|---|---|---|
| N | N | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
|
Road Density within 750 m of Birth Residencea |
|||||
| Overall | |||||
| Low Exposure | 171 | 568 | Referent | Referent | Referent |
| High Exposure | 136 | 445 | 1.01 (0.78, 1.31) | 0.99 (0.77, 1.29) | 0.99 (0.76, 1.29) |
| Males | |||||
| Low Exposure | 90 | 280 | Referent | Referent | Referent |
| High Exposure | 87 | 215 | 1.24 (0.84, 1.83) | 1.16 (0.77, 1.73) | 1.14 (0.76, 1.71) |
| Females | |||||
| Low Exposure | 81 | 288 | Referent | Referent | Referent |
| High Exposure | 49 | 230 | 0.93 (0.58, 1.49) | 0.86 (0.53, 1.41) | 0.86 (0.53, 1.41) |
|
Road Density within 500 m of Birth Residencea |
|||||
| Overall | |||||
| Low Exposure | 204 | 682 | Referent | Referent | Referent |
| High Exposure | 103 | 331 | 1.03 (0.79, 1.35) | 1.01 (0.77, 1.33) | 1.00 (0.76, 1.32) |
| Males | |||||
| Low Exposure | 110 | 346 | Referent | Referent | Referent |
| High Exposure | 67 | 149 | 1.39 (0.93, 2.10) | 1.33 (0.88, 2.02) | 1.31 (0.85, 1.99) |
| Females | |||||
| Low Exposure | 94 | 336 | Referent | Referent | Referent |
| High Exposure | 36 | 182 | 0.72 (0.43, 1.20) | 0.64 (0.38, 1.09) | 0.64 (0.37, 1.08) |
High exposure: Both primary and secondary roads (with or without tertiary roads), primary roads only, both primary and tertiary roads, both secondary and tertiary roads within the specified buffer around the birth residence. Low exposure: Secondary roads only and tertiary roads only within the specified buffer around the birth residence.
OR=odds ratio, CI=confidence interval
Table 6.
OR and 95% CI for the association between road density and childhood acute leukemia, stratified by leukemia type.
| Acute Lymphoid Leukemia | Acute Myeloid Leukemia | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Maternal Education |
Adjusted for Urbanization and Maternal Education |
Unadjusted | Adjusted for Maternal Education |
Adjusted for Urbanization and Maternal Education |
|
| OR (95% CI) | OR (95% CI) |
OR (95% CI) | OR (95% CI) | OR (95% CI) |
OR (95% CI) | |
|
Road Density within 750 m of Birth Residencea |
||||||
| High Exposure (v. Low) | 0.98 (0.72 1.32) |
0.96 (0.71, 1.31) |
0.96 (0.71, 1.31) |
1.11 (0.68, 1.83) |
1.14 (0.68, 1.89) |
1.11 (0.67, 1.86) |
| Stratified by Gender |
||||||
| Males (High v. Low) | 1.31 (0.84, 2.05) |
1.19 (0.75, 1.89) |
1.19 (0.74, 1.89) |
1.02 (0.45, 2.32) |
0.89 (0.38, 2.10) |
0.86 (0.36, 2.05) |
| Females (High v. Low) | 0.83 (0.46, 1.48) |
0.80 (0.43, 1.46) |
0.80 (0.44, 1.48) |
1.17 (0.52, 2.63) |
0.93 (0.39, 2.21) |
0.95 (0.40, 2.27) |
|
Road Density within 500 m of Birth Residencea |
||||||
| High Exposure (v. Low) | 1.02 (0.74, 1.41) |
1.01 (0.73, 1.39) |
1.01 (0.73, 1.39) |
1.05 (0.63, 1.76) |
1.02 (0.61, 1.72) |
1.00 (0.60, 1.69) |
| Stratified by Gender |
||||||
| Males (High v. Low) | 1.53 (0.96, 2.44) |
1.43 (0.88, 2.32) |
1.44 (0.88, 2.38) |
1.01 (0.43, 2.38) |
0.88 (0.36, 2.17) |
0.92 (0.37, 2.27) |
| Females (High v. Low) | 0.57 (0.30, 1.09) |
0.52 (0.27, 1.02) |
0.52 (0.26, 1.02) |
1.11 (0.48, 2.60) |
0.89 (0.35, 2.24) |
0.91 (0.36, 2.34) |
OR=odds ratio, CI=confidence interval
After stratifying by age at diagnosis for cases/index age for matched controls, we observed little difference in road density (500 m OR: 0.92, 95% CI: 0.62, 1.36; 750 m OR: 0.87, 95% CI: 0.60, 1.26) (Supplemental Table 1), but an elevated OR among cases exposed to the fourth quartile of NO2 compared to controls (OR: 1.19, 95% CI: 0.72, 1.97) (Supplemental Table 2) in children under five years of age at diagnosis/index age. In addition, we observed a stronger association between road density and acute leukemia among children aged 15–19 years at diagnosis/index age (750 m OR: 1.69, 95% CI: 0.84, 3.40; 500 m OR: 1.78, 95% CI: 0.87, 3.63). However, there was a significant protective, though imprecise, association between NO2 and acute leukemia for children aged 10–14 years (3rd quartile unadjusted OR: 0.12, 95% CI: 0.03, 0.59), but no association observed in the fourth quartile. In the third quartile there were fewer than five cases, with 37 total cases aged 10–14 years. Approximately two-thirds (n=199, 67.2%) of geocoded cases changed residence, which increased with age at diagnosis (0–4 years: 58.3%, 5–9 years: 64.9%, 10–14 years: 85.7%, 15–19 years: 86.1%). At diagnosis, a higher percentage of case residences were in a rural census block compared to birth residence (22.6% v. 12.6%, McNemar’s Test p=0.003). In general, there was a higher proportion of cases in the low exposure category of road density and the first quartile of NO2 exposure at diagnosis compared to birth (data not shown).
In a sensitivity analysis evaluating the impact of potential temporal exposure misclassification, we observed a positive association between NO2 and leukemia among those born from 2005–2010 (4th quartile unadjusted OR: 2.94, 95% CI: 1.22, 7.09). However, results varied with no clear trend in our analysis of road density by year of birth (Supplemental Tables 3 and 4). Furthermore, the results of our sensitivity analysis to evaluate the impact of excluding children who did not geocode, which included all but two children (n=360 cases, n=1,438 controls) was similar to our analysis including only those who geocoded (n=1,320) (data not shown).
Because we were unable to link all leukemia cases to birth certificates, we evaluated the potential under-ascertainment of leukemia cases. Approximately 70% of children who did not link were either born outside of Oklahoma or had an unknown state of residence at birth based on social security number. Among the 43 children (8% of children eligible for linkage) who were born in Oklahoma but did not link to a birth certificate, we observed no differences regarding cancer-related characteristics, including type of leukemia, year of birth, year of diagnosis, and age at diagnosis compared to those who did link (data not shown).
Additionally, we evaluated the association between traffic-related air pollution and acute leukemia restricted to NH-white children and restricted to children under 15 years of age at diagnosis/index age. Among NH-white children, estimates for NO2 were similar to those observed in our primary analysis including all racial/ethnic groups, although less precise (data not shown). The OR for NH-white urban children with AML remained elevated, but reduced (4th quartile OR adjusted for maternal education: 2.10, 95% CI: 0.18, 25.03). For analyses by road density, the ORs among NH-white children were slightly elevated, but with wider confidence intervals compared to the results observed when including all children (750 m OR: 1.19, 95% CI: 0.85, 1.67; 500 m OR: 1.21, 95% CI: 0.85, 1.72). When restricting our analysis to children under 15 years of age, results were essentially unchanged for NO2 and road density overall (data not shown). However, when the analysis of urban children with AML was restricted to children under 15 years, the magnitude of the ORs for NO2 increased but the width of the confidence intervals were also substantially larger than observed in our primary analysis (e.g., 4th quartile OR adjusted for maternal education: 9.46, 95% CI: 1.11, 80.67).
4. Discussion
In our analysis of road density and NO2, we observed higher odds of exposure to the fourth quartile of NO2 among urban children with AML compared to matched controls after adjustment for maternal education, indicating a possible dose-response relationship. However, this result should be interpreted with caution due to small sample size. Three previous studies have evaluated NO2 stratified by leukemia subtype, with none observing significant differences in exposure among children with AML (Amigou et al. 2011; Ghosh et al. 2013; Houot et al. 2015).
Consistent with the overall results of our study for any leukemia and ALL, several studies observed no association between NO2 and leukemia (Badaloni et al. 2013; Feychting et al. 1998; Houot et al. 2015; Raaschou-Nielsen et al. 2001). In Denmark, no association with leukemia was observed when NO2 was measured at the mother’s residence during pregnancy or at the childhood residence, with NO2 levels ranging from 1.5 to 28.1 ppb (Raaschou-Nielsen et al. 2001). Badaloni et al. (2013) also reported no association with NO2 ranging from 3.6 to 42.5 ppb at the birth residence in Italy. Furthermore, Houot et al. (2015) observed no association with NO2 exposure above the median of 28 ppb in France. While Feychting et al. (1998) observed an association with all childhood cancer cases for NO2 levels greater than 42.56 ppb for the address at diagnosis/recruitment, they did not detect an association with leukemia. NO2 levels estimated by Novotny et al. (2011) were less than 24 ppb at the highest levels, which were considerably lower than the current National Ambient Air Quality Standards limit for annual exposure. Although we used NO2 as a surrogate for general traffic exposure, this may indicate lower traffic in Oklahoma compared to other parts of the US. Our observations, however, did not support the results of several studies that observed a positive association between NO2 and any acute leukemia (Amigou et al. 2011; Ghosh et al. 2013; Weng et al. 2008). These studies measured exposure at various time points, including during pregnancy and childhood, with no consistent trend in results.
The association between high density roads and acute leukemia was stronger among males, particularly when restricting to cases with ALL and using a buffer of 500 m around the child’s birth residence, which may indicate a more refined measure of exposure to motor vehicle exhaust. However, no other studies evaluated the association between traffic density and leukemia by gender and this requires further investigation. While a higher incidence of ALL has been reported among US males compared to females, few gender differences have been reported for AML (Smith et al. 1999; United States Department of Health and Human Services et al. 2016).
Among studies evaluating the association between exposure to main roads or gas stations and leukemia among children under 15 years of age, results are mixed. While Steffen et al. (2004) observed a positive association between exposure to repair garages or gas stations collected through interview within 50 m of the childhood residence and leukemia (OR: 4.0, 95% CI: 1.5, 10.3), there was no association between exposure to high density roads. Harrison et al. (1999), Badaloni et al. (2013), Houot et al. (2015), and Abdul Rahman et al. (2008), using exposure information collected through objective measures, also observed no association when evaluating exposure to major roads or gas stations. However, Amigou et al. (2011) measured proximity to roads within 500 m of the residence at diagnosis/recruitment for controls using a GIS and incorporating different road classifications, similar to that in our study, and observed marginally elevated ORs (Low exposure: OR 1.1, 95% CI 0.9, 1.4; Intermediate exposure: OR 1.2, 95% CI 0.8, 1.8; High exposure OR: 2.0, 95% CI 1.0, 3.6). Furthermore, Spycher et al. (2015) observed an elevated, but non-significant hazard ratio for leukemia (Hazard Ratio: 1.45, 95% CI: 0.72, 2.94) among those residing <100 m from a highway compared to ≥500 m using existing records, which was stronger among children under five years of age (Hazard Ratio: 2.62, 95% CI: 0.96, 7.16) with stable residence adjusted for multiple factors, including urbanization.
We were unable to fully evaluate effect modification by urbanization in our analysis of NO2 and AML due to limited variability in exposure among rural children. We observed a significantly elevated association between NO2 and AML among urban children; however, the estimate was imprecise due to a small number of children with AML (n=64). Although we did not observe differences in the analysis of road density by urbanization status, our measure of exposure was dichotomized and may not reflect the true variability within road classifications. However, we also observed no interaction by urbanization status in our sensitivity analysis analyzing the sum of total miles of road within 500 m of the child’s birth residence (p=0.58). No other studies evaluated potential effect modification by urbanization and our results suggest further investigation is warranted.
In two separate meta-analyses, Boothe et al. (2014) and Filippini et al. (2015) suggested postnatal residence may be the etiologically relevant time for exposure measurement based on studies of traffic-related air pollution and childhood leukemia. However, these meta-analyses did not directly address exposure misclassification due to residential mobility during pregnancy or childhood. Two air pollution studies evaluating maternal residential mobility during pregnancy observed that approximately 13%-30% of mothers changed residence during pregnancy, with minimal exposure misclassification (Chen et al. 2010; Lupo et al. 2010). In evaluating mobility between birth and cancer diagnosis, Urayama et al. (2009) observed that approximately one-third of children moved during the first year of life and nearly two-thirds moved between birth and cancer diagnosis. Furthermore, Reynolds et al. (2001) observed that 55% of children with cancer in their study changed residence within the first five years of life. Our study evaluated residence at birth and the extent to which this would reflect exposures during childhood depends on the family’s residential mobility after birth. Furthermore, measuring exposure at birth may not represent exposure during pregnancy in our study if the mother changed residence prior to delivery. While we did not measure residential mobility directly, we observed lower exposure to traffic-related air pollution at the residence at diagnosis compared to the residence at birth for cases. This suggests that the perinatal exposures assessed in this study may not be good proxies for postnatal traffic exposure. Additionally, we stratified our results by age at leukemia diagnosis/index age for controls. Residential mobility may be reduced among the youngest group of children resulting in less exposure misclassification, but we observed no association among children under five years of age. Among children aged 10–14 years, we observed a significant protective association in the third quartile of NO2 exposure (8.83–11.18 ppb). However, it is possible that the small sample size for this analysis produced results by chance.
In combination with land-use variables, monitored NO2, and satellite data from OMI, this LUR model provided comprehensive estimates of NO2 with improved coverage compared to a model using only ground estimates of NO2 (Novotny et al. 2011). While misclassification remains a concern, a validation study conducted by Raaschou-Nielsen et al. (2001) indicated exposure misclassification was minor and occurred between adjacent categories when modeled NO2 levels from the Operational Street Pollution Model in Denmark were compared to personal exposure measurements. However, individuals in our study may have more or less exposure than that of the census block in which they reside due to differing activity patterns; thus, misclassification could result in either an over- or underestimate of the association if one truly exists. Future studies that adjust for exposure misclassification, a major limitation of air pollution studies, using estimates from validation studies are critical to advancing our understanding of the effects of exposure to air pollution on rare diseases with long latency periods.
Regarding road density, road classifications were obtained from the US Census and are the same regardless of geographic location; thus, classifications in a high-traffic state such as California are the same as in Oklahoma where traffic density differs. This limited our ability to compare observations with geographic locations where traffic density was different from that in Oklahoma. However, it is important to determine if traffic-related air pollution is a risk factor for childhood leukemia in locations with differing traffic patterns. Although the road density data only indicate proximity to roads and potential exposure to engine exhausts, these data provide individual exposure levels at the place of residence at birth.
Another limitation of this study was the risk for temporal misclassification of exposure. Although the LUR model accounted for variability by season, weekday/weekend, and time of day, it only estimated average NO2 for the year 2006, preventing the ability to incorporate variability during the child’s window of exposure. Furthermore, road density data were only obtained for 2014 (based on 2010 US Census). Our birth data ranged from 1979 through 2010 and traffic-related air pollution has changed over time, with increases in traffic and decreases in NO2 concentration (Oklahoma Department of Transportation 2011; United States Environmental Protection Agency 2013). For children born in the earlier years of the study period, exposure to NO2 was likely underestimated using the 2006 LUR model data, but also may have greater misclassification than those born closer to 2006. In a sensitivity analysis, we observed a statistically significant positive association between NO2 and acute leukemia in the most recent years of the study (2005–2010), which suggests our observations among children born during the entire study period (1979–2010) may be due to exposure misclassification, biasing results towards the null.
We were also concerned with the potential for selection bias in our study due to the lack of complete geocoding and potential under-ascertainment of cases arising from the inability to link all cases with birth certificates. Although controls were less likely to geocode than cases, results from our sensitivity analysis assigning the census block with the highest population within the child’s ZIP code for nearly all children available for the study (n=1,798) were similar to the results for the geocoded dataset (n=1,320), demonstrating that selection bias is an unlikely explanation for our results. Furthermore, most cases who did not link to Oklahoma birth certificates were born outside of the state and, thus, were not eligible members of the target population. Reasons for non-linkage among those born in Oklahoma may be due to name changes, but without a unique identifier available in both birth certificate data and OCCR we were unable to determine residence at birth for these children. Among the cases born in Oklahoma without birth certificate linkage, we observed no differences regarding cancer-related characteristics, including type of leukemia, year of birth, year of diagnosis, and age at diagnosis when compared to cases who did link. Future studies could have increased power if all available cases of acute leukemia had available birth data and complete address information, which may be improved as vital statistics registries gain additional resources.
5. Conclusions
Traffic exhaust is composed of multiple pollutants, including nitrogen oxides, carbon monoxide, and benzene, in addition to particulate matter (Benbrahim-Tallaa et al. 2012). In studies of air pollution and childhood leukemia, multiple hazardous air pollutants have been evaluated, with benzene as the primary pollutant of interest. The primary studies examining benzene and adult AML have been occupational studies, thus, assessing higher doses of benzene exposure than that of the general population (Khalade et al. 2010; Rushton and Romaniuk 1997). Associations between ambient benzene and childhood leukemia have also been observed, but results are conflicting (Crosignani et al. 2004; Raaschou-Nielsen et al. 2001; Vinceti et al. 2012; Whitworth et al. 2008). NO2 is a widely used marker for traffic-related air pollution (Briggs et al. 2000), and has the additional benefit of spatial heterogeneity. Other markers such as intra-urban ambient particulate matter >2.5 microns (PM2.5) are fairly homogenous (Wilson et al. 2005), and less useful for capturing variation in traffic exposures across a population. The research paradigm for understanding the health effects of air pollution is moving from a one pollutant model to a multiple pollutant model (Greenbaum and Shaikh 2010) and we consider this to be future work. As a next step, we are evaluating exposure to benzene in relation to childhood leukemia in Oklahoma.
In summary, we observed an association between NO2 and AML among urban children after adjustment for maternal education, while associations with any acute leukemia and ALL were near the null. We also observed stronger ORs for males with high exposure to road density for children with any leukemia and ALL. Modeling traffic-related air pollution exposure and NO2 is complex, making it difficult to measure individual exposure without the use of personal monitors or detailed activity patterns over time. Future studies would benefit from incorporating more precise measures of traffic, including traffic counts in addition to density of the roads. Additionally, these results suggest further evaluation of this novel method of estimating NO2 using satellite data in other geographical areas and further exploration of the potential association with AML while incorporating adjustments for misclassification based on validation studies.
Supplementary Material
Highlights.
Association between traffic-related air pollution and childhood leukemia.
Novel measurement of nitrogen dioxide using satellite-based model.
First to observe association between nitrogen dioxide and acute myeloid leukemia.
Acknowledgments
We thank Dr. Derek Pate and Ryan Webb of the Oklahoma State Department of Health for their support in providing data for this study.
Funding sources
This project was supported in part by Grant Number UB6HP27874 from the U.S. Department of Health and Human Services, Health Resources and Services Administration, Affordable Care Act (ACA) Public Health Training Centers Program and by Grant Number 1 U54GM104938 from the National Institutes of Health, National Institute of General Medical Sciences, an IDeA-CTR to the University of Oklahoma Health Sciences Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Health Resources and Services Administration or of the U.S. Department of Health and Human Services. The authors declare no competing financial interests.
Abbreviations
- AML
acute myeloid leukemia
- ALL
acute lymphoid leukemia
- CI
confidence interval
- EPA
Environmental Protection Agency
- GIS
Geographic Information System
- HC
Highway Contract
- LOESS
locally weighted scatterplot smoothing
- LUR
land-use regression
- m
meters
- NO2
nitrogen dioxide
- OCCR
Oklahoma Central Cancer Registry
- OMI
Ozone Monitoring Instrument
- OR
odds ratio
- PO
Post Office
- PPB
parts per billion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics
We obtained IRB approval for this research from the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
Contributor Information
Amanda E. Janitz, Email: amanda-janitz@ouhsc.edu.
Janis E. Campbell, Email: janis-campbell@ouhsc.edu.
Sheryl Magzamen, Email: sheryl.magzamen@colostate.edu.
Anne Pate, Email: anne.pate@swosu.edu.
Julie A. Stoner, Email: julie-stoner@ouhsc.edu.
Jennifer D. Peck, Email: jennifer-peck@ouhsc.edu.
References
- Abdul Rahman HI, Shah SA, Alias H, Ibrahim HM. A case-control study on the association between environmental factors and the occurrence of acute leukemia among children in klang valley, malaysia. Asian Pac J Cancer Prev. 2008;9:649–652. [PubMed] [Google Scholar]
- Amigou A, Sermage-Faure C, Orsi L, Leverger G, Baruchel A, Bertrand Y, et al. Road traffic and childhood leukemia: The ESCALE study (SFCE) Environ Health Perspect. 2011;119:566–572. doi: 10.1289/ehp.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaloni C, Ranucci A, Cesaroni G, Zanini G, Vienneau D, Al-Aidrous F, et al. Air pollution and childhood leukaemia: A nationwide case-control study in italy. Occupational & Environmental Medicine. 2013;70:876–883. doi: 10.1136/oemed-2013-101604. doi: http://dx.doi.org/10.1136/oemed-2013-101604. [DOI] [PubMed] [Google Scholar]
- Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmospheric Environment. 2008;42:275–290. doi: http://dx.doi.org/10.1016/j.atmosenv.2007.09.042. [Google Scholar]
- Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: A review. Environ Health Perspect. 2007;115:138–145. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Baan RA, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13:663–664. doi: 10.1016/s1470-2045(12)70280-2. [DOI] [PubMed] [Google Scholar]
- Boothe VL, Boehmer TK, Wendel AM, Yip FY. Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am J Prev Med. 2014;46:413–422. doi: 10.1016/j.amepre.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DJ, de Hoogh C, Gulliver J, Wills J, Elliott P, Kingham S, et al. A regression-based method for mapping traffic-related air pollution: Application and testing in four contrasting urban environments. Sci Total Environ. 2000;253:151–167. doi: 10.1016/s0048-9697(00)00429-0. doi: http://dx.doi.org/10.1016/S0048-9697(00)00429-0. [DOI] [PubMed] [Google Scholar]
- Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environmental Research. 2010;110:162–168. doi: 10.1016/j.envres.2009.11.001. doi: http://dx.doi.org/10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Crosignani P, Tittarelli A, Borgini A, Codazzi T, Rovelli A, Porro E, et al. Childhood leukemia and road traffic: A population-based case-control study. Int J Cancer. 2004;108:596–599. doi: 10.1002/ijc.11597. [DOI] [PubMed] [Google Scholar]
- Feychting M, Svensson D, Ahlbom A. Exposure to motor vehicle exhaust and childhood cancer. Scand J Work Environ Health. 1998;24:8–11. doi: 10.5271/sjweh.272. [DOI] [PubMed] [Google Scholar]
- Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. Journal of environmental science and health Part C, Environmental carcinogenesis & ecotoxicology reviews. 2015;33:36–66. doi: 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JK, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol. 2013;178:1233–1239. doi: 10.1093/aje/kwt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D, Shaikh R. First steps toward multipollutant science for air quality decisions. Epidemiology. 2010;21:195–197. doi: 10.1097/EDE.0b013e3181ccc52a. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Leung PL, Somervaille L, Smith R, Gilman E. Analysis of incidence of childhood cancer in the West Midlands of the United Kingdom in relation to proximity to main roads and petrol stations. Occup Environ Med. 1999;56:774–780. doi: 10.1136/oem.56.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Wu J, Lombardi C, Qiu J, Meyers TJ, Wilhelm M, et al. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect. 2013;121:1385–1391. doi: 10.1289/ehp.1306761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot J, Marquant F, Goujon S, Faure L, Honoré C, Roth M-H, et al. Residential proximity to heavy-traffic roads, benzene exposure, and childhood leukemia—the GEOCAP study, 2002–2007. Am J Epidemiol. 2015;182:685–693. doi: 10.1093/aje/kwv111. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Air pollution and cancer. France: Lyon; 2013. (IARC Scientific Publication No 161) [Google Scholar]
- Khalade A, Jaakkola MS, Pukkala E, Jaakkola JJ. Exposure to benzene at work and the risk of leukemia: A systematic review and meta-analysis. Environ Health. 2010;9:31. doi: 10.1186/1476-069X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langholz B, Ebi KL, Thomas DC, Peters JM, London SJ. Traffic density and the risk of childhood leukemia in a Los Angeles case-control study. Ann Epidemiol. 2002;12:482–487. doi: 10.1016/s1047-2797(01)00317-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Diamond J. China's environment in a globalizing world. Nature. 2005;435:1179–1186. doi: 10.1038/4351179a. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Symanski E, Chan W, Mitchell LE, Waller DK, Canfield MA, et al. Differences in exposure assignment between conception and delivery: The impact of maternal mobility. Paediatr Perinat Epidemiol. 2010;24:200–208. doi: 10.1111/j.1365-3016.2010.01096.x. doi: http://dx.doi.org/10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- Melissa Data. [[accessed December 8, 2014]];Melissa data: Lookups. 2014 Available: http://www.melissadata.com/lookups/index.htm.
- Nordlinder R, Jarvholm B. Environmental exposure to gasoline and leukemia in children and young adults--an ecology study. Int Arch Occup Environ Health. 1997;70:57–60. doi: 10.1007/s004200050186. [DOI] [PubMed] [Google Scholar]
- Novotny EV, Bechle MJ, Millet DB, Marshall JD. National satellite-based land-use regression: NO2 in the United States. Environmental Science & Technology. 2011;45:4407–4414. doi: 10.1021/es103578x. [DOI] [PubMed] [Google Scholar]
- Oklahoma Department of Transportation. [[accessed November 12, 2013]];Oklahoma traffic count information system. 2011 Available: http://www.okladot.state.ok.us/aadtcnt/
- Pearson RL, Wachtel H, Ebi KL. Distance-weighted traffic density in proximity to a home is a risk factor for leukemia and other childhood cancers. J Air Waste Manag Assoc. 2000;50:175–180. doi: 10.1080/10473289.2000.10463998. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol. 2001;153:433–443. doi: 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Elkin E, Scalf R, Von Behren J, Neutra RR. A case-control pilot study of traffic exposures and early childhood leukemia using a geographic information system. Bioelectromagnetics Suppl. 2001;5:S58–S68. doi: 10.1002/1521-186x(2001)22:5+<::aid-bem1024>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith D. Traffic patterns and childhood cancer incidence rates in California, United States. Cancer Causes Control. 2002;13:665–673. doi: 10.1023/a:1019579430978. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A. Residential exposure to traffic in California and childhood cancer. Epidemiology. 2004;15:6–12. doi: 10.1097/01.ede.0000101749.28283.de. [DOI] [PubMed] [Google Scholar]
- Rushton L, Romaniuk H. A case-control study to investigate the risk of leukaemia associated with exposure to benzene in petroleum marketing and distribution workers in the united kingdom. Occup Environ Med. 1997;54:152–166. doi: 10.1136/oem.54.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Feingold L. Association of childhood cancer with residential traffic density. Scand J Work Environ Health. 1989;15:360–363. doi: 10.5271/sjweh.1848. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ries LAG, Gurney JG, Ross JA. Leukemia. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL Jr, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. pp. 17–34. [Google Scholar]
- Spycher BD, Feller M, Roosli M, Ammann RA, Diezi M, Egger M, et al. Childhood cancer and residential exposure to highways: A nationwide cohort study. Eur J Epidemiol. 2015;30:1263–1275. doi: 10.1007/s10654-015-0091-9. [DOI] [PubMed] [Google Scholar]
- Steffen C, Auclerc MF, Auvrignon A, Baruchel A, Kebaili K, Lambilliotte A, et al. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons; a case-control study. Occup Environ Med. 2004;61:773–778. doi: 10.1136/oem.2003.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, Third edition. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- Tselios V. Urbanization and socioeconomic status in the european regions: The role of population ageing and capital city regions. European Planning Studies. 2013;22:1879–1901. [Google Scholar]
- United States Census Bureau. [[accessed December 8, 2014]];TIGER/Line® shapefiles and TIGER/Line® files. 2014 Available: http://www.census.gov/geo/maps-data/data/tiger-line.html.
- United States Census Bureau. [[accessed October 21, 2014]];TIGER/Line® shapefiles and TIGER/Line® files. 2015 Available: http://www.census.gov/geo/maps-data/data/tiger-line.html.
- United States Department of Health and Human Services, Centers for Disease Control and Prevention. United States Cancer Statistics. National Cancer Institute; 2016. 1999 – 2011 incidence, WONDER On-line Database. [Google Scholar]
- United States Environmental Protection Agency. [[accessed December 3, 2015]];Air quality guide for nitrogen dioxide (epa-456/f-11-003) 2011 Available: http://www3.epa.gov/airnow/no2.pdf.
- United States Environmental Protection Agency. [[accessed November 12, 2013]];Airdata: Download daily data. 2013 Available: http://www.epa.gov/airdata/ad_data_daily.html.
- United States Postal Service. [[accessed December 8, 2014]];Look up a ZIP Code™. 2014 Available: https://tools.usps.com/go/ZipLookupAction_input.
- Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. Factors associated with residential mobility in children with leukemia: Implications for assigning exposures. Ann Epidemiol. 2009;19:834–840. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, et al. Leukemia risk in children exposed to benzene and pm(10) from vehicular traffic: A case-control study in an italian population. Eur J Epidemiol. 2012;27:781–790. doi: 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Behren J, Reynolds P, Gunier RB, Rull RP, Hertz A, Urayama KY, et al. Residential traffic density and childhood leukemia risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2298–2301. doi: 10.1158/1055-9965.EPI-08-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HH, Tsai SS, Chen CC, Chiu HF, Wu TN, Yang CY. Childhood leukemia development and correlation with traffic air pollution in Taiwan using nitrogen dioxide as an air pollutant marker. J Toxicol Environ Health A. 2008;71:434–438. doi: 10.1080/15287390701839042. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Symanski E, Coker AL. Childhood lymphohematopoietic cancer incidence and hazardous air pollutants in southeast texas, 1995–2004. Environ Health Perspect. 2008;116:1576–1580. doi: 10.1289/ehp.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Kingham S, Pearce J, Sturman AP. A review of intraurban variations in particulate air pollution: Implications for epidemiological research. Atmospheric Environment. 2005;39:6444–6462. doi: http://dx.doi.org/10.1016/j.atmosenv.2005.07.030. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.