Abstract
A recently identified mechanism for oligomeric Aβ-induced glutamate release from astrocytes involves intracellular Ca2+ elevation, potentially via Ca2+-dependent vesicular release. Evidence suggests that levetiracetam (LEV, Keppra®), an antiepileptic drug, can improve cognitive performance in both humans suffering mild cognitive impairment and animal models of Alzheimer disease (AD). Because LEV acts by modulating neurotransmitter release from neurons via interaction with synaptic vesicles, we tested the effect of LEV on Aβ-induced astrocytic release of glutamate. We used a FRET-based glutamate sensor (termed SuperGluSnFR), whose structure is based on the ligand-binding site of glutamate receptors, to monitor glutamate release from primary cultures of human astrocytes exposed to oligomeric amyloid-β peptide 1-42 (Aβ42). We found that LEV (10 μM) inhibited oligomeric Aβ-induced astrocytic glutamate release. Additionally, we show that this Aβ-induced glutamate release from astrocytes is sensitive to tetanus neurotoxin (TeNT), an inhibitor of the vesicle release machinery. Taken together, our evidence suggests that LEV inhibits Aβ-induced vesicular glutamate release from astrocytes and thus may underlie, at least in part, the ability of LEV to reduce hyperexcitability in AD.
Keywords: Alzheimer's disease, Levetiracetam, Amyloid-β, Synaptic vesicle glycoprotein 2A, astrocyte
Introduction
Levetiracetam (LEV) is an antiepileptic drug with known pro-cognitive properties [1]. Preclinical experiments have shown that LEV improves memory retrieval [2] and restores cognitive function in mouse models of Alzheimer's disease (AD) [3,4]. In humans, clinical studies suggest that LEV, at doses as low as 250 mg/day, can improve cognitive performance in patients with amnestic mild cognitive impairment (aMCI) [5], a condition that often precedes the onset of AD. LEV pro-cognitive effects likely result from its ability to reduce abnormal neuronal hyperactivity in regions of the brain involved in learning and memory [5]. In the brain, LEV reportedly interacts with synaptic vesicles in neurons [6] to modulate Ca2+-dependent vesicular release of neurotransmitter such as glutamate. Importantly, glutamate levels are also tightly controlled by astrocytes [7], and impaired astrocytic metabolism and clearance of extracellular glutamate have been linked to AD pathophysiology [8,9]. Thus, we examined the effect of LEV on astrocytic handling of glutamate in the presence of oligomeric amyloid-β peptide 1-42 (Aβ42), which is thought to be a key mediator of AD [10]. It was recently shown that picomolar to nanomolar oligomeric Aβ42 can trigger Ca2+-dependent glutamate release from astrocytes, contributing to increased extracellular glutamate in the brain, with consequent overactivation of extrasynaptic NMDA receptors and synaptic loss [11]; other amino acids were not released by oligomeric Aβ42 in this paradigm. In the present study, we show that therapeutically-relevant concentrations of LEV can sig nificantly reduce this oligomeric Aβ-induced astrocytic release of glutamate, most likely via interaction with the vesicular release machinery.
Materials and methods
Cell cultures
To utilize the glutamate-sensor SuperGluSnFR in astrocyte cultures, the probe was engineered in HEK 293T “interrogator” “cells,” as previously described [11]. For this purpose, HEK 293T cells were grown in DMEM supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin in a humidified 5% CO2/balance air atmosphere incubator that was kept at 37 °C. Once confluent, cells were enzymatically dissociated with trypsin-EDTA, seeded at 1×105 cells/well, co-transfected with SuperGluSnFR and neuroligin plasmids (1 μg each/well) using 1 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and used for glutamate FRET assays within 24-48 h of transfection. Normal human astrocytes (NHA™, Lonza) were cultured in astrocyte growth medium (AGM™, Lonza) in a humidified 5%CO2/air atmosphere incubator at 37 °C [12].
Glutamate FRET imaging
FRET microscopy was performed with the SuperGluSnFR probe to monitor glutamate release from pure human astrocyte cultures. To detect release of glutamate, astrocytes were plated on top of HEK-293 cells expressing the SuperGluSnFR probe and neuroligin. In this assay, neuroligin is used to bring the HEK-interrogator cell into close apposition with the overlying astrocytes [11]. Under these conditions, changes in extracellular glutamate can be detected within a wide dynamic range of glutamate concentrations (300 nM to 100 μM) [13]. Experiments were performed on an inverted microscope (Axiovert 100M, Zeiss) equipped for epifluorescence microscopy. Coverslips were placed on the stage and light delivered to the sample through a 63× oil immersion objective (1.4 numerical aperture, NA). Cells were continuously superfused at room temperature (RT, 22 °C) with extracellular solution (in mM): NaCl 146, KCl 2.5, NaOH 4, CaCl2 1, D-glucose 20, sucrose 20, HEPES 10, adjusted to pH 7.4. Drugs and reagents were added manually using a micropipette. Cells were epi-illuminated alternately at 434 and 514 nm, and emitted light collected at 527 and 476 nm for yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP), respectively. In a typical experiment, 3-7 regions of interest (ROIs) were imaged, each containing an HEK-FRET sensor cell and one or more astrocytes. Peak CFP/YFP ratio was divided by baseline CFP/YFP ratio and plotted after baseline normalization to a value of 1. Images were obtained every second following a 400 ms stimulus and processed using SlideBook software (Intelligent Imaging Innovations, Santa Monica, CA). A 2×2 binning method was used to improve the signal-to-noise ratio and minimize photobleaching.
Oligomeric Aβ preparation
Oligomeric Aβ was prepared as previously described [11]. Synthetic human Aβ42 peptide (GenicBio, Ltd., Shanghai, China, or Anaspec, Inc., Freemont, CA) was suspended to an initial concentration of 1 mM in hexafluoroisopropanol, incubated for 2 h at RT, and solvent evaporated in a SpeedVac. Peptide was re-suspended in dry DMSO as monomers and frozen at -80 °C until use. For oligomerization, Aβ42 peptide was incubated at 4 °C for ≥24 h and sonicated prior to use. In the present study and in prior studies, the oligomeric, but not the monomeric, form of Aβ42 was found to exert the effects studied here [11]. Total concentration of Aβ42 was monitored by ELISA after centrifugation at 11,000 g for 2 min. To characterize the degree of oligomerization of the preparations, western blot and light scattering analyses were performed before and after centrifugation as previously described [11]. For experiments, both monomeric and oligomeric synthetic Aβ42 preparations were diluted in physiological buffer.
Statistical analysis
Results are reported from at least five independent observations and expressed as mean ± SEM. Statistical analysis and representation was prepared using GraphPad Prism 6 (GraphPad Software, Inc. San Diego, California, USA). Comparison of values was made using a two-tailed Student's t-test for statistical significance. A P-value of less than 0.05 was considered to be statistically significant.
Results
Levetiracetam inhibits glutamate release from human astrocytes
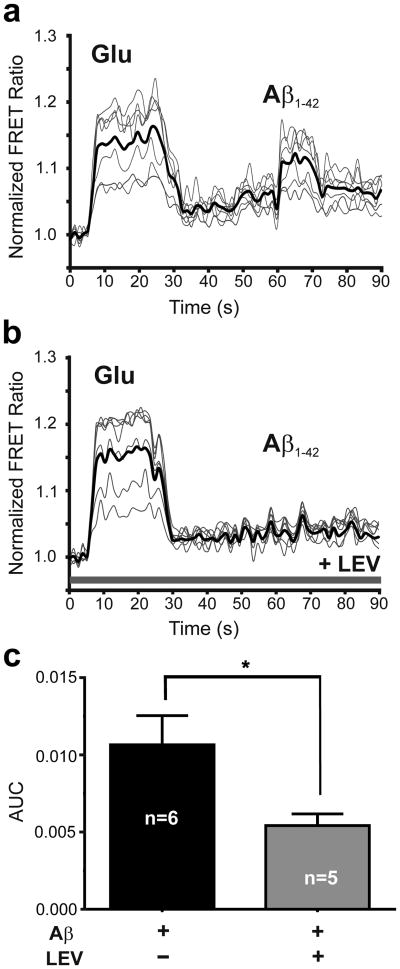
We and others have previously shown that preparations of Aβ42 oligomers can trigger glutamate release from astrocytes; importantly, however, monomeric Aβ42 did not result in glutamate release [11]. Specifically, nanomolar concentrations of synthetic Aβ42 oligomers or picomolar concentrations of natural Aβ42 oligomers prepared from human AD brain were shown to trigger glutamate release [11]. In the present study, to test the effect of LEV on oligomeric Aβ-induced astrocytic glutamate release, we incubated primary human astrocytes in LEV (10 μM) for 30 min and measured glutamate release using the SuperGluSnFR, a FRET-based glutamate sensor probe [13]. Before exposure to synthetic Aβ oligomers, probe sensitivity and maximal level of response to glutamate were assessed by bath application of saturating concentrations of glutamate (100-300 μM), as previously described [13]. As shown in Fig. 1a, the change in FRET fluorescence intensity, reflecting glutamate concentration, was significantly increased after bath application of 100 μM glutamate (as a control) or 250 nM oligomeric Aβ42. In contrast, preincubation in LEV (10 μM) significantly inhibited oligomeric Aβ42–induced glutamate release but not the response to control glutamate application (Fig. 1b). These responses are quantified in Fig. 1c.
Fig. 1.
Levetiracetam reduces Aβ-induced glutamate release from human astrocytes. (a) Change in FRET ratio when co-cultures containing astrocytes and HEK sensor cells expressing SuperGluSnFR were exposed to exogenously applied glutamate (Glu, 100 μM) or synthetic Aβ42 (250 nM oligomers). Values shown are mean from each experiment plus overall mean (dark line; n = 40 ROIs in 6 experiments). (b) When co-cultures were pre-incubated with levetiracetam (LEV, 10 μM), sensor cells were still sensitive to exogenously applied glutamate, but no longer detected any Aβ-induced glutamate release from astrocytes (n = 31 ROIs in 5 experiments). (c) Bar graph showing area under the curve (AUC) for the FRET responses to Aβ42 in panels A and B for equal epochs of time in the presence and absence of levetiracetam (P < 0.014 by two-tailed Student's t-test).
Oligomeric Aβ induces vesicular glutamate release from astrocytes
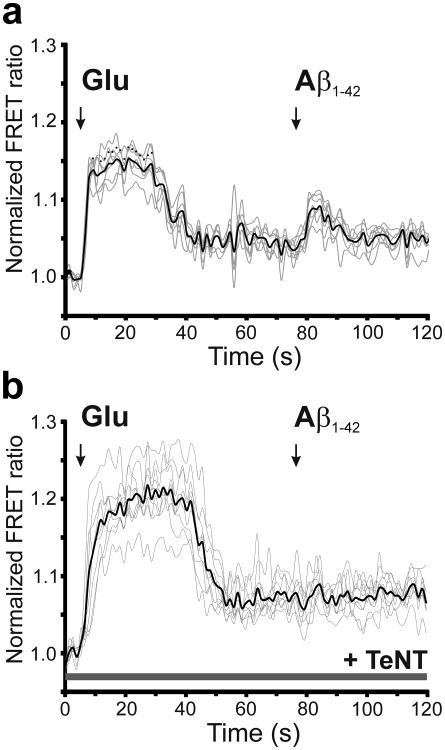
To determine if Aβ-induced astrocytic glutamate release is indeed dependent on vesicular release, we applied oligomeric human Aβ42 to human astrocytes that had been pre-incubated in tetanus toxin light chain (TeNT-LC) peptide for 24 h vs. control conditions (Fig. 2, b and c) [14]. TeNT-LC inhibited Aβ-induced astrocytic glutamate release. Taken together, our data suggest that oligomerized Aβ triggers vesicular release of glutamate from human astrocytes. Moreover, this conclusion is consistent with prior evidence that astrocytic release of glutamate can be mediated by Ca2+-dependent vesicular exocytosis [15].
Fig. 2.
Effect of tetanus toxin on Aβ-induced glutamate release from astrocytes. (a and b) FRET ratio observed when co-cultures containing primary human astrocytes and HEK sensor cells expressing SuperGluSnFR were exposed to exogenously applied glutamate (Glu, 100 μM) or synthetic Aβ42 (250 nM oligomers) in the absence (a, n = 7 ROIs) and presence (b, n = 9 ROIs) of tetanus toxin light chain (TeNT-LC). For these experiments, cultures were pre-incubated with TeNT-LC (100 nM) for 24 h. Note that after TeNT exposure, sensor cells were still sensitive to exogenously applied glutamate but no longer detected release of glutamate from astrocytes in response to oligomerized Aβ.
Discussion
Recent evidence has suggested in rodent brain, studied by microdialysis, that LEV can reduce extracellular levels of the excitatory neurotransmitter glutamate in vivo [16]. To account for this effect, it was previously assumed that LEV prevented glutamate release from the synaptic endings of neurons. Unexpectedly, we found here that LEV also inhibits release of glutamate from human astrocytes. Importantly, our group and others previously showed that oligomeric Aβ can induce astrocytic glutamate release via α7 nicotinic receptors in a calcium-dependent manner to contribute to synaptic damage [11,17]. Here, we report that this Aβ-induced glutamate release is vesicular in nature because it can be blocked by TeNT-LC. Moreover, LEV can abrogate the release of glutamate. This new finding may account, at least in part, for the therapeutic benefit of LEV that has been observed in mouse models of AD [3,4].
Mechanistically, it has been posited that LEV and similar drugs such as brivaracetam bind to the synaptic vesicle-related protein SV2A in neurons, accounting for their ability to reduce excitation, possibly via inhibition of glutamate release from presynaptic terminals. One caveat with this reasoning concerns the fact that SV2A is expressed in both excitatory glutamatergic and inhibitory GABAergic neurons [18,19]. Inhibition of SV2A activity might therefore be expected to affect both glutamatergic and GABAergic synaptic transmission. Moreover, deletion of the SV2A gene has been shown to manifest opposite effects on glutamatergic and GABAergic neurotransmission, with decreased GABAergic synaptic currents [20] but increased glutamatergic transmission [21]. Thus, LEV might be acting as a positive allosteric modulator of SV2A, resulting in decreased glutamate release. Additionally, LEV appears to lack a significant effect on other neuronal parameters that might influence glutamate release, including passive and active neuronal membrane properties [22], neuronal fast or persistent Na+ currents [23,24], neuronal low voltage-activated calcium currents [23,24], and evoked excitatory postsynaptic potentials (EPSPs) [24].
In this background, and with the recent report that the molecular target of LEV, SV2A, is present in acutely isolated cortical astrocytes [25], our new findings suggest that LEV can also reduce excitation emanating from gliotransmission, i.e., vesicular release of glutamate from astrocytes (Fig. 3). This opens up an entirely new avenue for drug action and future drug development, taking advantage of an alternative mechanism of action based on LEV-mediated inhibition of glutamate release from astrocytes rather than only from neurons. Recently, the contribution of astrocytes has come to the forefront of neuroscience research, not only in mediating normal neuronal-glial communication in the brain, but also in contributing to neurological diseases, including Alzheimer's disease [11]. In this light, it is likely that glial release of transmitters like glutamate, as studied here, may well play a role in both physiological and pathophysiological processes mediated by astrocytes in the central nervous system. Moreover, LEV and related drugs may prove to be a useful tool as well as therapeutic to modulate the astrocytic release mechanism.
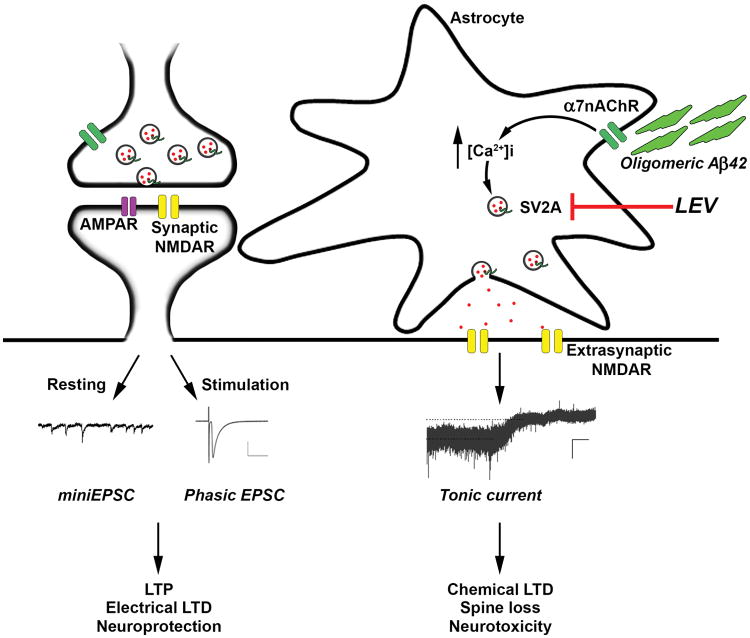
Fig. 3.
Schema depicting possible mode of action of LEV on astrocytes. Oligomeric Aβ triggers glutamate release from astrocytes in an α7 nicotinic receptor-mediated manner, resulting in tonic extrasynaptic NMDA-type glutamate receptors with consequent synaptic damage. In contrast, phasic synaptic NMDA receptor activity fosters events associated with neuroprotection. Here, we report that LEV blocks the oligomeric Aβ-induced release of glutamate from human astrocytes, presumably via its interaction with SV2A. This finding provides mechanistic insight into the beneficial effects of LEV in mouse models of AD.
Conclusion
The antiepileptic drug LEV and similar drugs such as brivaracetam [26] have been reported to ameliorate cognitive deficits in animal models of Alzheimer's disease (AD) or to improve performance in human patients with mild cognitive impairment. LEV reduces potentially harmful hyperexcitability in brain by inhibiting vesicular release of neurotransmitters from neurons. However, heretofore there has been no evidence that LEV can target neurotransmitter release from glial cells, which also use vesicular exocytosis. Here we show that LEV inhibits glutamate release from astrocytes exposed to toxic levels of oligomeric amyloid-β peptide (Aβ42), thought to be a key mediator of AD. Our evidence suggests the positive effects of LEV and similar drugs in transgenic mouse models of AD may result from the drug's ability to target both dysfunctional neurotransmission and gliotransmission.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 HD29587, R01 NS086890, and P30 NS076411 (to S.A.L.). We thank Andrew Hires (University of Southern California) for providing the SuperGluSnFR construct and Traci Fang Niemeyer for excellent technical assistance.
Source of funding: This work was supported by National Institutes of Health Grants P01 HD29587, R01 NS086890, and P30 NS076411 (to S.A.L.).
Footnotes
Conflicts of Interest: None declared.
References
- 1.Helmstaedter C, Witt JA. The effects of levetiracetam on cognition: a non-interventional surveillance study. Epilepsy Behav. 2008;13:642–649. doi: 10.1016/j.yebeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Sara SJ. Memory retrieval deficits: alleviation by etiracetam, a nootropic drug. Psychopharmacology (Berl) 1980;68:235–241. doi: 10.1007/BF00428109. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci USA. 2012;109:E2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verloes R, Scotto AM, Gobert J, Wulfert E. Effects of nootropic drugs in a scopolamine-induced amnesia model in mice. Psychopharmacology (Berl) 1988;95:226–230. doi: 10.1007/BF00174514. [DOI] [PubMed] [Google Scholar]
- 5.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Arco A, Segovia G, Fuxe K, Mora F. Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem. 2003;85:23–33. doi: 10.1046/j.1471-4159.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- 8.Steele ML, Robinson SR. Reactive astrocytes give neurons less support: implications for Alzheimer's disease. Neurobiol Aging. 2012;33:423 e421–413. doi: 10.1016/j.neurobiolaging.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 10.Klein WL. Synaptotoxic amyloid-β oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer's disease? J Alzheimers Dis. 2013;33 Suppl 1:S49–65. doi: 10.3233/JAD-2012-129039. [DOI] [PubMed] [Google Scholar]
- 11.Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, et al. Abe induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110:E2518–2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hires SA, Zhu Y, Tsien RY. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc Natl Acad Sci U S A. 2008;105:4411–4416. doi: 10.1073/pnas.0712008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 16.Ueda Y, Doi T, Takaki M, Nagatomo K, Nakajima A, Willmore LJ. Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: in vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 2009;1266:1–7. doi: 10.1016/j.brainres.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Pirttimaki TM, Codadu NK, Awni A, Pratik P, Nagel DA, Hill EJ, et al. α7 Nicotinic receptor-mediated astrocytic gliotransmitter release: Aβ effects in a preclinical Alzheimer's mouse model. PLoS One. 2013;8:e81828. doi: 10.1371/journal.pone.0081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bragina L, Fattorini G, Giovedi S, Melone M, Bosco F, Benfenati F, et al. Analysis of Synaptotagmin, SV2, and Rab3 Expression in Cortical Glutamatergic and GABAergic Axon Terminals. Front Cell Neurosci. 2011;5:32. doi: 10.3389/fncel.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci USA. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesan K, Alix P, Marquet A, Doupagne M, Niespodziany I, Rogister B, et al. Altered balance between excitatory and inhibitory inputs onto CA1 pyramidal neurons from SV2A-deficient but not SV2B-deficient mice. J Neurosci Res. 2012;90:2317–2327. doi: 10.1002/jnr.23111. [DOI] [PubMed] [Google Scholar]
- 22.Birnstiel S, Wulfert E, Beck SG. Levetiracetam (ucb LO59) affects in vitro models of epilepsy in CA3 pyramidal neurons without altering normal synaptic transmission. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:611–618. doi: 10.1007/pl00005097. [DOI] [PubMed] [Google Scholar]
- 23.Zona C, Niespodziany I, Marchetti C, Klitgaard H, Bernardi G, Margineanu DG. Levetiracetam does not modulate neuronal voltage-gated Na+ and T-type Ca2+ currents. Seizure. 2001;10:279–286. doi: 10.1053/seiz.2000.0504. [DOI] [PubMed] [Google Scholar]
- 24.Costa C, Martella G, Picconi B, Prosperetti C, Pisani A, Di Filippo M, et al. Multiple mechanisms underlying the neuroprotective effects of antiepileptic drugs against in vitro ischemia. Stroke. 2006;37:1319–1326. doi: 10.1161/01.STR.0000217303.22856.38. [DOI] [PubMed] [Google Scholar]
- 25.Lalo U, Rasooli-Nejad S, Pankratov Y. Exocytosis of gliotransmitters from cortical astrocytes: implications for synaptic plasticity and aging. Biochem Soc Trans. 2014;42:1275–1281. doi: 10.1042/BST20140163. [DOI] [PubMed] [Google Scholar]
- 26.Nygaard HB, Kaufman AC, Sekine-Konno T, Huh LL, Goin H, Feldman SJ, et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer's disease mouse model. Alzheimer's Res Therap. 2015;7:25. doi: 10.1186/s13195-015-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]