Abstract
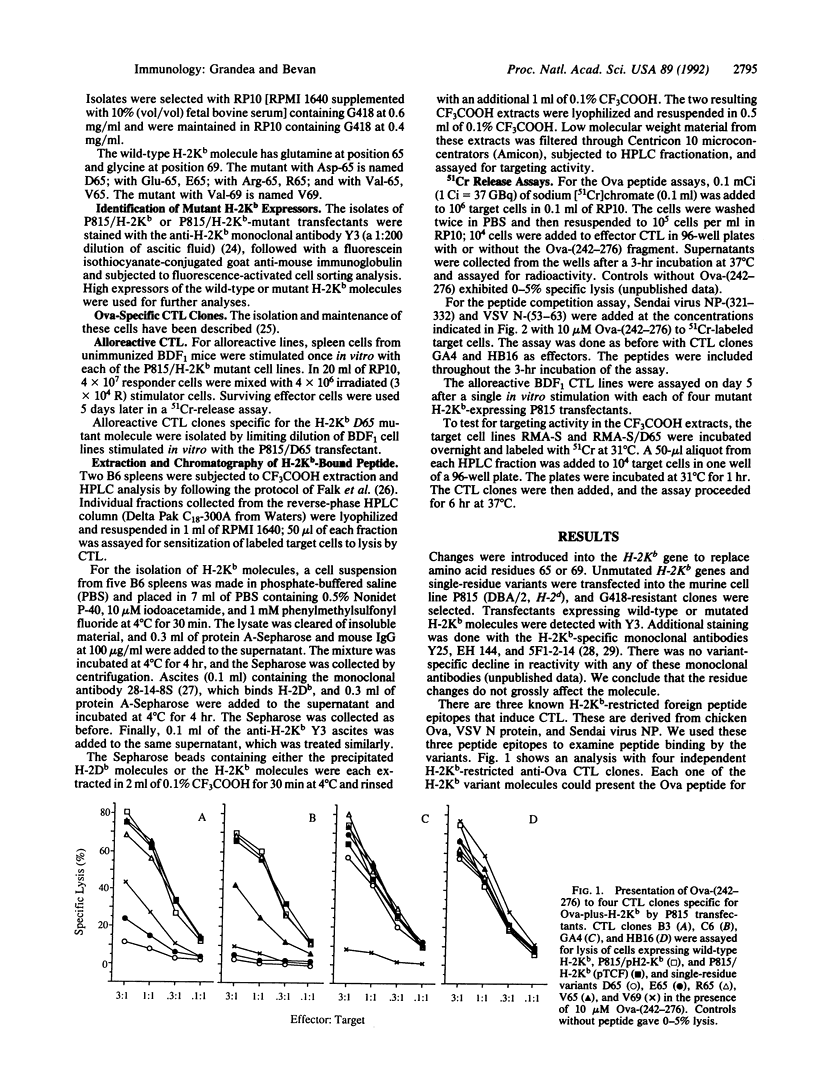
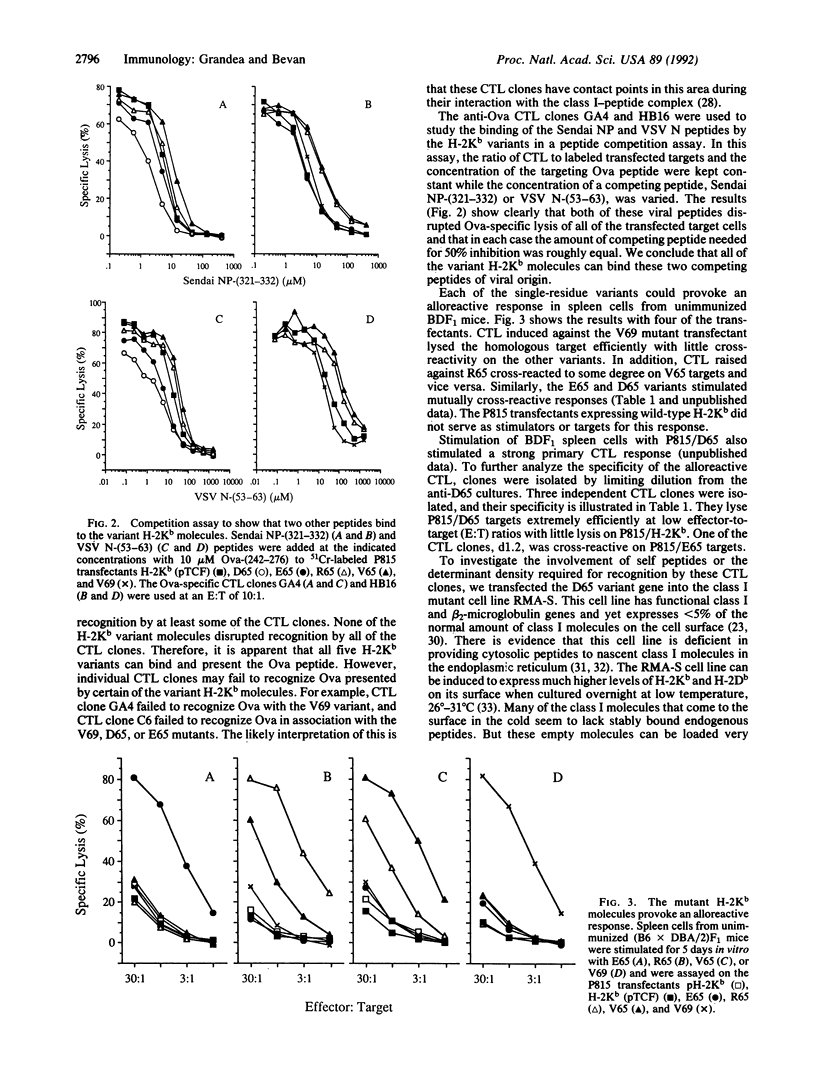
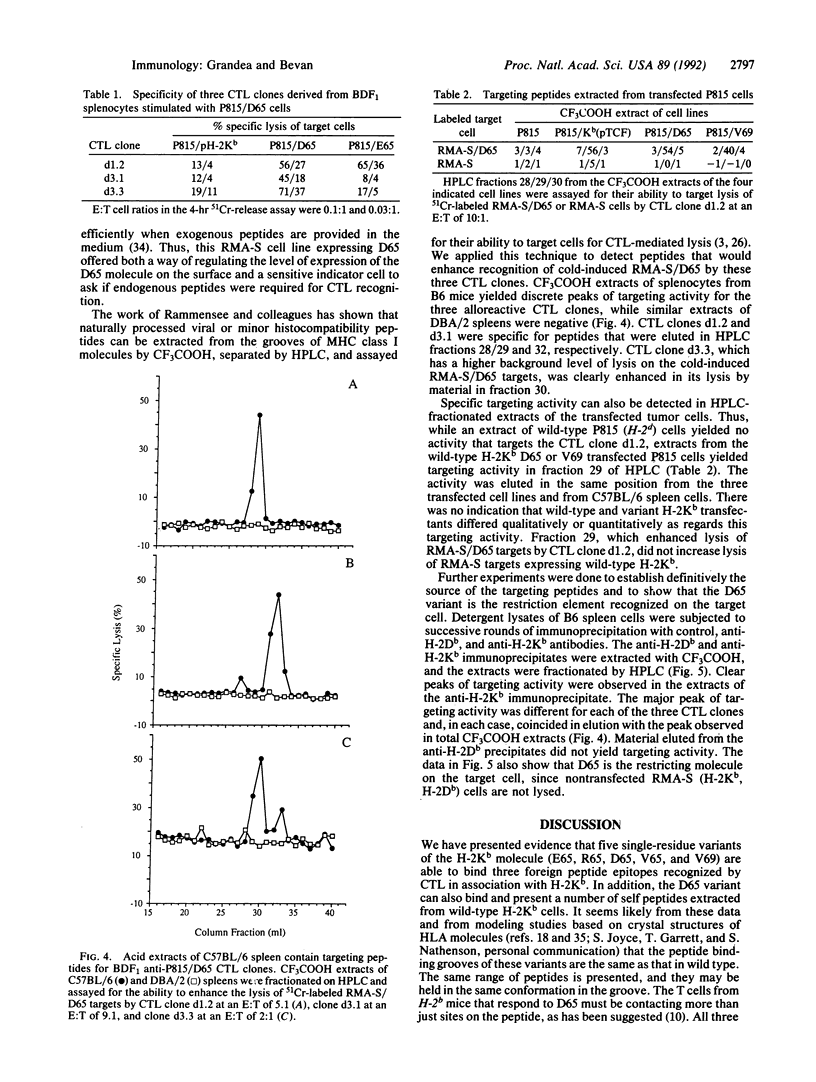
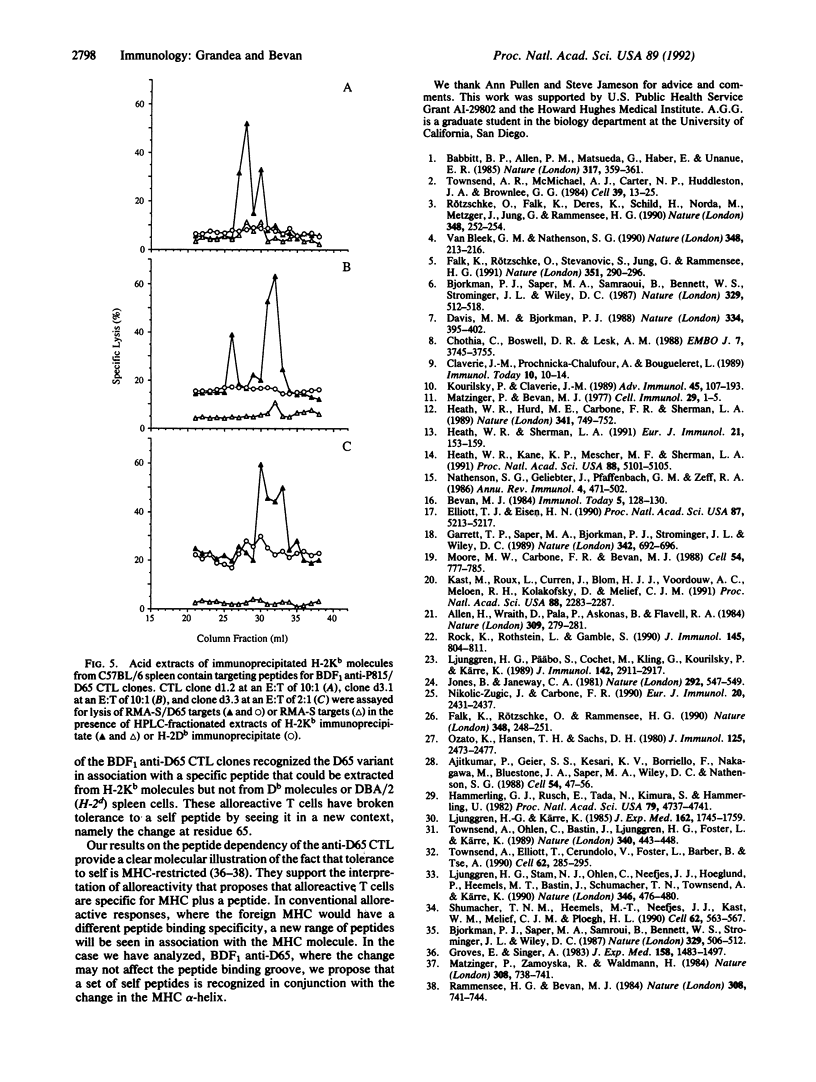
Single-residue changes were introduced into the murine major histocompatibility complex class I molecule H-2Kb at positions 65 and 69, which are predicted to point up from the alpha-helix of the alpha 1 domain and not into the peptide binding groove. Mutated and wild-type genes were transfected into the murine cell line P815 (H-2d). We present evidence that the changes did not affect the binding of three foreign peptides that are recognized by cytotoxic T lymphocytes (CTL) in association with H-2Kb. Additionally, the mutants provoked strong alloreactive responses in T cells from mice expressing unmutated H-2Kb. The alloreactive CTL were specific for self peptides, which could be extracted from wild-type H-2Kb molecules, recognized in the context of the mutant class I.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajitkumar P., Geier S. S., Kesari K. V., Borriello F., Nakagawa M., Bluestone J. A., Saper M. A., Wiley D. C., Nathenson S. G. Evidence that multiple residues on both the alpha-helices of the class I MHC molecule are simultaneously recognized by the T cell receptor. Cell. 1988 Jul 1;54(1):47–56. doi: 10.1016/0092-8674(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Elliott T. J., Eisen H. N. Cytotoxic T lymphocytes recognize a reconstituted class I histocompatibility antigen (HLA-A2) as an allogeneic target molecule. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5213–5217. doi: 10.1073/pnas.87.13.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Groves E. S., Singer A. Role of the H-2 complex in the induction of T cell tolerance to self minor histocompatibility antigens. J Exp Med. 1983 Nov 1;158(5):1483–1497. doi: 10.1084/jem.158.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W. R., Hurd M. E., Carbone F. R., Sherman L. A. Peptide-dependent recognition of H-2Kb by alloreactive cytotoxic T lymphocytes. Nature. 1989 Oct 26;341(6244):749–752. doi: 10.1038/341749a0. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Kane K. P., Mescher M. F., Sherman L. A. Alloreactive T cells discriminate among a diverse set of endogenous peptides. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5101–5105. doi: 10.1073/pnas.88.12.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W. R., Sherman L. A. Cell-type-specific recognition of allogeneic cells by alloreactive cytotoxic T cells: a consequence of peptide-dependent allorecognition. Eur J Immunol. 1991 Jan;21(1):153–159. doi: 10.1002/eji.1830210123. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourilsky P., Claverie J. M. MHC-antigen interaction: what does the T cell receptor see? Adv Immunol. 1989;45:107–193. doi: 10.1016/s0065-2776(08)60693-8. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985 Dec 1;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Päbo S., Cochet M., Kling G., Kourilsky P., Kärre K. Molecular analysis of H-2-deficient lymphoma lines. Distinct defects in biosynthesis and association of MHC class I heavy chains and beta 2-microglobulin observed in cells with increased sensitivity to NK cell lysis. J Immunol. 1989 Apr 15;142(8):2911–2917. [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Bevan M. J. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977 Mar 1;29(1):1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Zamoyska R., Waldmann H. Self tolerance is H-2-restricted. Nature. 1984 Apr 19;308(5961):738–741. doi: 10.1038/308738a0. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Nikolić-Zugić J., Carbone F. R. The effect of mutations in the MHC class I peptide binding groove on the cytotoxic T lymphocyte recognition of the Kb-restricted ovalbumin determinant. Eur J Immunol. 1990 Nov;20(11):2431–2437. doi: 10.1002/eji.1830201111. [DOI] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Rammensee H. G., Bevan M. J. Evidence from in vitro studies that tolerance to self antigens is MHC-restricted. Nature. 1984 Apr 19;308(5961):741–744. doi: 10.1038/308741a0. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L., Gamble S. Generation of class I MHC-restricted T-T hybridomas. J Immunol. 1990 Aug 1;145(3):804–811. [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]


