Abstract
Aims
High body mass index (BMI) is a risk factor for atrial fibrillation (AF). The aim of this study was to determine whether lean body mass (LBM) predicts AF.
Methods and results
The Women's Health Initiative is a study of post-menopausal women aged 50–79 enrolled at 40 US centres from 1994 to 1998. A subset of 11 393 participants at three centres underwent dual-energy X-ray absorptiometry. Baseline demographics and clinical histories were recorded. Incident AF was identified using hospitalization records and diagnostic codes from Medicare claims. A multivariable Cox hazard regression model adjusted for demographic and clinical risk factors was used to evaluate associations between components of body composition and AF risk. After exclusion for prevalent AF or incomplete data, 8832 participants with an average age of 63.3 years remained for analysis. Over the 11.6 years of average follow-up time, 1035 women developed incident AF. After covariate adjustment, all measures of LBM were independently associated with higher rates of AF: total LBM [hazard ratio (HR) 1.24 per 5 kg increase, 95% confidence intervals (CI) 1.14–1.34], central LBM (HR 1.51 per 5 kg increase, 95% CI 1.31–1.74), and peripheral LBM (HR 1.39 per 5 kg increase, 95% CI 1.19–1.63). The association between total LBM and AF remained significant after adjustment for total fat mass (HR 1.22 per 5 kg increase, 95% CI 1.13–1.31).
Conclusion
Greater LBM is a strong independent risk factor for AF. After adjusting for obesity-related risk factors, the risk of AF conferred by higher BMI is primarily driven by the association between LBM and AF.
Keywords: Atrial fibrillation, Epidemiology, Arrhythmia
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in women, who comprise the majority of individuals with AF over age 75 years.1 The prevalence of AF is projected to increase 2.5-fold by the year 2050.2 Women with AF are at higher risk of stroke and death compared with men.3 Several independent risk factors for AF have been identified from large epidemiologic studies.4 However, a better understanding of the mechanisms by which these risk factors are associated with AF is necessary to inform novel preventative and therapeutic interventions.
Obesity, as defined by elevated body mass index (BMI), has emerged as one of the most important independent predictors of incident AF in women, accounting for 12–18% of the population attributable risk.5,6 It has been assumed that adiposity is responsible for this association, possibly via inflammatory mechanisms.7,8 These theories have been fueled by observations that adiposity in epicardial and pericardial distributions is associated with higher rates of AF.9
However, a recent Danish study suggested that greater lean body mass (LBM), estimated using bioelectrical impedance measurements, was associated with AF risk.10 No other studies have investigated the association between LBM and risk of incident AF. Bioelectrical impedance measures are prone to inaccuracy and wide within-subject variability.11,12 Dual-energy X-ray absorptiometry (DXA) provides high-quality, detailed body composition data, permitting a more accurate distinction between lean, fat, and bone mass and quantification of regional mass. The Women's Health Initiative (WHI) comprises one of the largest cohorts with DXA scans and provides a unique opportunity to study the relationship between lean body mass and incident AF.
The aim of this study was to identify the independent association between LBM and risk of incident AF.
Methods
Study population
Details of study design methods have been previously published.13 From 1994 to 1998, 161 808 women were enrolled at 40 US clinical centres. Participants were recruited through mass mailings to age-eligible women. Women were eligible if they were post-menopausal, with ages between 50 and 79 years at time of enrolment. Women were ineligible if they were unlikely to survive or remain in the vicinity of a WHI clinic for 3 years, or had alcoholism, drug dependency, or dementia. Eligible participants were invited to enroll in randomized clinical trials of dietary modification, hormone therapy, and calcium plus vitamin D. Those who did not participate in the trials were enrolled in the observational study. Three clinical centres participating in the WHI were designated as DXA scan centres, and nearly all (>99%) WHI participants enrolled at those sites underwent whole-body DXA scanning. This study includes the subset of women in the WHI observational study and clinical trials who received DXA scans.
Study procedure
At time of study enrolment, all participants completed self- or interviewer-administered questionnaires. Baseline characteristics including demographic, reproductive, personal, and family histories were obtained. Participants underwent measurement of height, weight, blood pressure, and complete physical examination. Women completed annual questionnaires to update their lifestyle and medical information. The study was reviewed and approved by the institutional review boards at each clinical centre, and all participants provided written informed consent.
Assessment of baseline variables
Details of study questionnaires, physical measurements, and quality assurance have been previously described.13 Baseline demographic and medical history information, including age, race/ethnicity, income, education, history of hypertension, diabetes, hyperlipidaemia, coronary heart disease, stroke, congestive heart failure, peripheral artery disease, smoking, and alcohol use, were ascertained by self-report on baseline questionnaires. Participants with measured resting systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at initial clinic visit were also classified as hypertensive. Height and weight were measured by study personnel at initial enrolment visit. Body mass index was calculated as weight (kg) divided by the square of measured height (m2).
Assessment of body composition
Body composition data were obtained from DXA scans performed at time of study enrolment. Details of DXA procedures have been previously published.14 All DXA operators completed a central training session and were certified on demonstration of satisfactory scanning and analysis techniques. Scans were performed at three WHI clinical centres: Birmingham, AL, Tuscon/Phoenix, AZ, and Pittsburgh, PA, USA. Whole-body scans using fan-beam mode were obtained using the QDR 2000, 2000+, or 4500 devices (Hologic, Inc., Waltham, MA, USA). The same standardized protocol was employed for all scans at all sites. Densitometry technicians at the DXA coordinating centre reviewed a random sample of all scans, those reporting extremes of bone mineral density values, and those identified as problematic by the DXA clinical centres. In the event that reviewers deemed study quality or technique to be unsatisfactory, feedback was provided to DXA clinical centres where re-scanning or re-analysis was performed when indicated. Each DXA clinical centre and the DXA coordinating centre performed routine monitoring of scanner performance using daily spine and three times weekly whole-body phantom scans. Data were reviewed for possible changes in scanner calibration using a standardized quality control method.
Total body and regional lean and fat masses were obtained from DXA scans. Non-bone LBM was determined by subtracting bone mass from total lean mass. The LBM index (LBMI) was calculated by dividing LBM by height in meter squared. The central body compartment was defined as comprising the chest, abdomen, and pelvis. The peripheral body compartment was defined as comprising the four extremities. Two different measures of central fat percentages were calculated, using total body fat mass and total central mass as the denominators.
Ascertainment of incident atrial fibrillation
Ascertainment of incident AF in the WHI has been previously described.6 At years 3–8 of study follow-up, all participants completed annual questionnaires that inquired about new medical diagnoses and intervening hospitalizations. If women reported a new diagnosis of AF or a hospitalization, medical records were obtained and the International Classification of Disease-version 9 (ICD-9) code for AF (427.31) was extracted over the time period since the prior questionnaire. In 97% of Medicare-eligible WHI participants, WHI data were successfully linked with Centers for Medicare and Medicaid Services (CMS) data using social security numbers, birth dates, and death dates. Incident AF was identified by first occurrence of ICD-9 code 427.31 in any diagnosis position in the inpatient (MEDPAR), outpatient, and carrier files during years 1994–2011 for all participants with Medicare coverage. A time-dependent indicator variable of Medicare coverage was added to the Cox hazard models described below to adjust for possible ascertainment bias related to differential exposure to CMS. Medicare time eligible for analysis included those intervals where participants were enrolled in fee-for-service Medicare and not simultaneously enrolled in a Medicare-managed care plan. The outcome of incident AF was defined as any single ICD-9 code of 427.31 from review of Medicare claims or hospital records.
Statistical analysis
Baseline demographic and medical characteristics were compared across tertiles of total LBM using analysis of variance (ANOVA) for continuous variables and the χ2 test for categorical variables. We constructed a correlation matrix showing the Pearson's correlation coefficients between body composition variables of interest. The associations between baseline body composition characteristics and incident AF were assessed using Cox hazard regression analyses. Multivariable analyses were performed using a primary model (Model 1) containing baseline covariates with known or suspected association with AF: age, race/ethnicity, education, hypertension, diabetes, hyperlipidaemia, coronary artery disease, heart failure, peripheral artery disease, and smoking. Models were also adjusted for clinical trial enrolment and intervention status, and time-dependent exposure to Medicare coverage. To further adjust for total body size, multivariable analyses were also performed using a secondary model (Model 2) containing all covariates included in Model 1 with the addition of BMI. Sensitivity analyses were performed substituting alternative measures of body size for BMI in Model 2: height, weight, waist-to-hip ratio, total LBM, and total fat mass. We performed a sensitivity analysis adjusting only for age, race/ethnicity, and education, eliminating covariates that could act as biologic intermediates in the associations between body composition variables and incident AF. We also performed sensitivity analyses including the ICD-9 code for atrial flutter (427.32) in our incident AF outcome.
Spline regression analysis, using four knots, was performed to examine the relationship between body composition and risk of incident AF. In the model where a non-linear relationship was suggested by the spline regression analysis, a sensitivity analysis was performed by adding a quadratic term (fat mass2) to the primary regression model. To determine whether the AF risk conferred by LBM differs across baseline characteristics, we performed a subgroup analysis across predetermined categories of covariates included in Model 1 (age, ethnicity, BMI, hypertension, diabetes, coronary heart disease, congestive heart failure, smoking, and alcohol use). Interactions between LBM and subgroup variables were assessed by adding interaction terms to the primary multivariable Cox hazard regression model.
We then tested whether or not LBM mediates the known association between height and incident AF.15 First we confirmed a positive correlation between height and LBM using Pearson's correlation. Then we measured the association between height and incident AF using a multivariable Cox hazard regression analysis adjusted for the covariates from the primary model, with and without adjustment for LBM. We compared the hazard ratios (HR) measuring the association between height and AF derived from these two models. We performed sensitivity analyses including the ICD-9 code for atrial flutter (427.32) in our incident AF outcome.
Associations were reported as HR with 95% confidence intervals (CI). The proportional hazards assumption was verified by visual inspection of the log-likelihood plots of developing AF over time. Analyses were performed using SAS statistical software, version 9.1.
Results
Baseline characteristics
A total of 11 393 participants in the WHI underwent DXA scanning: 6415 from the observational study and 4978 from the clinical trials. Of the total participants, 2561 were excluded for analysis: 586 with prevalent AF and 1975 with missing data. A total of 8832 participants remained for analysis. The mean follow-up time was 11.6 years. Over the study duration, 1035 women developed incident AF, with an annual cumulative incidence of 1.1%.
Baseline demographic and medical characteristics of the study group by tertiles of total LBM are presented in Table 1. The average age was 63.3 years and 77.3% were white. Compared with the lowest tertile of total LBM, participants in the highest tertile were younger (61.6 vs. 64.8, P < 0.001) and more likely to be African American (25.8 vs. 6.3%, P < 0.001). Higher LBM was associated with a higher prevalence of multiple AF risk factors, including hypertension (36.3 vs. 21.8%, P < 0.001), diabetes (12.2 vs. 2.2%, P < 0.001), coronary artery disease (4.2 vs. 2.9%, P = 0.029), heart failure (1.0 vs. 0.3%, P = 0.007), peripheral artery disease (2.6 vs. 1.7%, P = 0.036), and smoking (49.3 vs. 42.2%, P < 0.001).
Table 1.
Baseline characteristics by tertiles of total lean body mass
| Characteristics | Overall |
Tertile 1 |
Tertile 2 |
Tertile 3 |
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | n | % | n | % | n | % | n | % | |
| Age, mean (SD) | 63.3 (7.4) | 64.8 (7.4) | 63.5 (7.3) | 61.6 (7.0) | <0.001 | ||||
| Ethnicity | <0.001 | ||||||||
| White | 6828 | 77.3 | 2469 | 83.0 | 2372 | 80.9 | 1987 | 67.9 | |
| African American | 1292 | 14.6 | 186 | 6.3 | 350 | 11.9 | 756 | 25.8 | |
| Hispanic | 513 | 5.8 | 231 | 7.8 | 156 | 5.3 | 126 | 4.3 | |
| Other/unknown | 199 | 2.3 | 90 | 3.0 | 53 | 1.8 | 56 | 1.9 | |
| Income | <0.001 | ||||||||
| <$20 000 | 2212 | 25.0 | 693 | 23.3 | 689 | 23.5 | 830 | 28.4 | |
| $20 000–$49 999 | 3820 | 43.3 | 1305 | 43.9 | 1286 | 43.9 | 1229 | 42.0 | |
| $50 000–$74 999 | 1276 | 14.4 | 443 | 14.9 | 424 | 14.5 | 409 | 14.0 | |
| ≥$75 000 | 910 | 10.3 | 314 | 10.6 | 336 | 11.5 | 260 | 8.9 | |
| Education | 0.261 | ||||||||
| ≤High school/GED | 2769 | 31.4 | 930 | 31.3 | 882 | 30.1 | 957 | 32.7 | |
| Some college | 3308 | 37.5 | 1130 | 38.0 | 1111 | 37.9 | 1067 | 36.5 | |
| ≥College degree | 2755 | 31.2 | 916 | 30.8 | 938 | 32.0 | 901 | 30.8 | |
| Medical history | |||||||||
| Hypertension | <0.001 | ||||||||
| Never treated | 4784 | 54.2 | 1800 | 60.5 | 1681 | 57.4 | 1303 | 44.5 | |
| Currently untreated | 1578 | 17.9 | 526 | 17.7 | 492 | 16.8 | 560 | 19.1 | |
| Treated | 2470 | 28.0 | 650 | 21.8 | 758 | 25.9 | 1062 | 36.3 | |
| Diabetes mellitus | 537 | 6.1 | 65 | 2.2 | 116 | 4.0 | 356 | 12.2 | <0.001 |
| Hyperlipidaemia | 1154 | 13.1 | 383 | 12.9 | 378 | 12.9 | 393 | 13.4 | 0.768 |
| Coronary artery disease | 313 | 3.5 | 86 | 2.9 | 105 | 3.6 | 122 | 4.2 | 0.029 |
| MI | 235 | 2.7 | 65 | 2.2 | 82 | 2.8 | 88 | 3.0 | 0.188 |
| CABG/PTCA | 171 | 1.9 | 49 | 1.6 | 54 | 1.8 | 68 | 2.3 | 0.437 |
| Stroke | 113 | 1.3 | 39 | 1.3 | 32 | 1.1 | 42 | 1.4 | 0.380 |
| Heart failure | 57 | 0.6 | 9 | 0.3 | 20 | 0.7 | 28 | 1.0 | 0.007 |
| Peripheral artery disease | 186 | 2.1 | 50 | 1.7 | 59 | 2.0 | 77 | 2.6 | 0.036 |
| Systolic BP, mean (SD) | 128.3 (18.3) | 127.2 (18.6) | 127.4 (18.1) | 130.2 (18.1) | <0.001 | ||||
| Diastolic BP, mean (SD) | 74.5 (9.3) | 73.3 (9.3) | 74.4 (9.2) | 75.9 (9.2) | <0.001 | ||||
| Heart rate, mean (SD) | 69.0 (9.9) | 68.7 (9.2) | 68.5 (10.0) | 69.9 (10.4) | <0.001 | ||||
| Habits | |||||||||
| Smoking | <0.001 | ||||||||
| Never | 4792 | 54.3 | 1720 | 57.8 | 1591 | 54.3 | 1481 | 50.6 | |
| Past | 3326 | 37.7 | 1023 | 34.4 | 1109 | 37.8 | 1194 | 40.8 | |
| Current | 714 | 8.1 | 233 | 7.8 | 231 | 7.9 | 250 | 8.5 | |
| Alcohol | <0.001 | ||||||||
| Never | 1494 | 16.9 | 520 | 17.5 | 477 | 16.3 | 497 | 17.0 | |
| Past | 1919 | 21.7 | 595 | 20.0 | 548 | 18.7 | 776 | 26.5 | |
| Current | 5371 | 60.8 | 1848 | 62.1 | 1888 | 64.4 | 1635 | 55.9 | |
Central fat percentage out of total body fat mass. All values represent the total and percentage of participants unless otherwise indicated. P-value is for the comparison of all subjects across central fat percentage tertiles using ANOVA.
BMI, body mass index; GED, general educational development; MI, myocardial infarction; CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty.
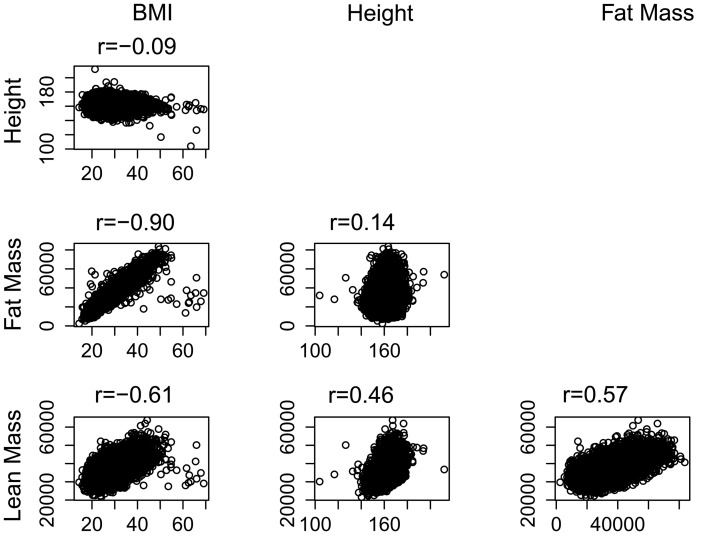
Mean body mass was 70.7 kg. Of total body mass, an average of 46.7% (33 kg) comprised LBM and 44.7% (31.6 kg) comprised fat mass. Total LBM and fat mass were normally distributed. Figure 1 displays a correlation matrix of height, BMI, and body composition characteristics. The Pearson correlation coefficient between total LBM and fat mass was r = 0.57, P < 0.001.
Figure 1.
Correlation matrix of body composition characteristics. P-value for all comparisons <0.001. Body mass index (BMI) reported as kilograms per height in metres squared. Height reported in centimetres. Fat and lean mass reported in kilograms.
Body composition and risk of incident atrial fibrillation
The univariate- and multivariable-adjusted HRs for incident AF by body composition variables are shown in Table 2. Higher BMI was associated with an increased risk of incident AF after multivariable adjustment (HR 1.07 per each 5 kg/m2 increase, 95% CI 1.01, 1.13, P = 0.014, Model 1).6
Table 2.
Multivariate-adjusted hazard ratios of incident atrial fibrillation
| Characteristics | Univariate adjusted |
Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| BMI (kg/m2) | 1.04 (0.99, 1.10) | 0.108 | 1.07 (1.01, 1.13) | 0.014 | ||
| Lean mass | ||||||
| Total body lean mass (kg) | 1.10 (1.03, 1.16) | 0.003 | 1.21 (1.14, 1.29) | <0.001 | 1.24 (1.14, 1.34) | <0.001 |
| Central lean mass (kg) | 1.28 (1.14, 1.44) | <0.001 | 1.49 (1.31, 1.69) | <0.001 | 1.51 (1.31, 1.74) | <0.001 |
| Peripheral lean mass (kg) | 1.12 (1.00, 1.26) | 0.049 | 1.37 (1.21, 1.56) | <0.001 | 1.39 (1.19, 1.63) | <0.001 |
| Lean body mass index | ||||||
| Total LBMI (kg/m2) | 1.28 (1.08, 1.52) | 0.005 | 1.39 (1.15, 1.67) | <0.001 | ||
| Central LBMI (kg/m2) | 2.01 (1.43, 2.82) | <0.001 | 2.03 (1.41, 2.91) | <0.001 | ||
| Peripheral LBMI (kg/m2) | 1.34 (0.97, 1.86) | 0.077 | 1.74 (1.22, 2.50) | 0.002 | ||
| Fat mass | ||||||
| Total body fat mass (kg) | 1.02 (0.99, 1.05) | 0.230 | 1.04 (1.01, 1.07) | 0.007 | 1.04 (0.97, 1.10) | 0.256 |
| Central fat mass (kg) | 1.03 (0.98, 1.09) | 0.187 | 1.05 (1.00, 1.11) | 0.059 | 0.99 (0.90, 1.09) | 0.809 |
| Peripheral fat mass (kg) | 1.03 (0.97, 1.08) | 0.341 | 1.10 (1.04, 1.16) | 0.001 | 1.12 (1.01, 1.24) | 0.032 |
| Fat % | ||||||
| Total body fat % | 0.99 (0.95, 1.04) | 0.777 | 1.00 (0.95, 1.04) | 0.909 | 0.92 (0.86, 0.98) | 0.006 |
| Central fat % (of central mass) | 0.99 (0.95, 1.04) | 0.728 | 0.98 (0.94, 1.03) | 0.451 | 0.89 (0.84, 0.95) | <0.001 |
| Central fat % (of total fat mass) | 1.00 (0.96, 1.05) | 0.920 | 0.95 (0.90, 0.99) | 0.025 | 0.92 (0.88, 0.97) | 0.002 |
Model 1: Adjusted for age, ethnicity, education, hypertension, diabetes, hyperlipidaemia, coronary artery disease, heart failure, peripheral artery disease, smoking, dietary modification intervention, and hormone therapy intervention. Model 2: Adjusted for all variables in model 1, in addition to body mass index. HR reported per 5 unit increase in each predictor.
HR, hazard ratios; CI, confidence intervals.
There were strong and significant associations between all measures of LBM and increased AF risk in all models. After multivariable adjustment including BMI, all measures of LBM were associated with increased AF risk: total LBM (HR 1.24 per 5 kg increase, 95% CI 1.14, 1.34, P < 0.001), central LBM (HR 1.51 per 5 kg increase, 95% CI 1.31, 1.74, P < 0.001), and peripheral LBM (HR 1.39 per 5 kg increase, 95% CI 1.19, 1.63, P < 0.001). Height-indexed measures of LBM adjusted for all covariates (except BMI) were also associated with an increased incidence of AF: total LBMI (HR 1.39 per 5 kg/m2 increase, 95% CI 1.15, 1.67, P < 0.001), central LBMI (HR 2.03 per 5 kg/m2 increase, 95% CI 1.41, 2.91, P < 0.001), and peripheral LBMI (HR 1.74 per 5 kg/m2 increase, 95% CI 1.22, 2.50, P = 0.002). Sensitivity analyses performed testing the association between measures of LBM with incident AF including the ICD-9 code for atrial flutter (427.32) did not change the results.
Greater total fat mass was associated with a small increase in AF risk in the primary model (HR 1.04 per 5 kg increase, 95% CI 1.01, 1.07, P = 0.007), though the association was non-significant after adjusting for BMI (HR 1.04 per 5 kg increase, 95% CI 0.97, 1.10, P = 0.256). The association between central fat mass and incident AF in the primary model was non-significant (HR 1.05 per 5 kg increase, 95% CI 1.00, 1.11, P = 0.059) and remained non-significant after adjusting for BMI (HR 0.99 per 5 kg increase, 95% CI 0.90, 1.09, P = 0.809). Peripheral fat mass was associated with increased AF risk in the multivariable model (HR 1.10 per 5 kg increase, 95% CI 1.04, 1.16, P = 0.001) and remained significant after adjusting for BMI (HR 1.12 per 5 kg increase, 95% CI 1.01, 1.24, P = 0.032).
Sensitivity analyses were performed substituting alternative measures of total body size for BMI in the multivariable regressions: height, weight, waist-to-hip ratio, total LBM, and total fat mass. The strength and significance of the associations between measures of LBM and incident AF were consistent across all sensitivity models. Total LBM was significantly associated with increased AF risk in a multivariable model adjusting for total fat mass (HR 1.22 per 5 kg increase, 95% CI 1.13, 1.31, P < 0.001). However, total fat mass was not a significant predictor of AF risk in a multivariable model adjusting for total LBM (HR 1.00 per 5 kg increase, 95% CI 0.96, 1.03, P = 0.801). Sensitivity analyses including cases of atrial flutter in the incident AF outcome did not alter these results. Sensitivity analyses were also performed using a regression model adjusting only for age, race/ethnicity, and education, eliminating covariates that could act as biologic intermediates in the associations between body composition variables and incident AF. In these analyses, central fat mass was a significant predictor of AF risk (HR 1.10 per 5 kg increase, 95% CI 1.04, 1.16, P < 0.001). The associations between body composition variables and AF risk were otherwise unchanged.
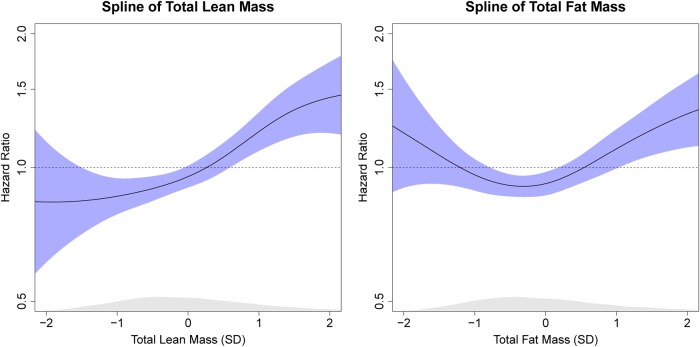
Spline regression curves demonstrating the multivariable-adjusted HRs of total LBM and fat mass for risk of incident AF are displayed in Figure 2. The spline of total fat mass suggested a non-linear relationship between fat mass and incident AF. The quadratic term (fat mass2) was associated with incident AF. However, sensitivity analyses demonstrated that LBM was still associated with incident AF independent of fat mass after accounting for this quadratic term.
Figure 2.
Splines of multivariable association between total body lean mass, fat mass, and incident atrial fibrillation. Adjusted for age, ethnicity, education, body mass index, hypertension, diabetes, hyperlipidaemia, coronary artery disease, heart failure, peripheral artery disease, smoking, dietary modification intervention, and hormone therapy intervention. Shaded areas enclose 95% confidence intervals.
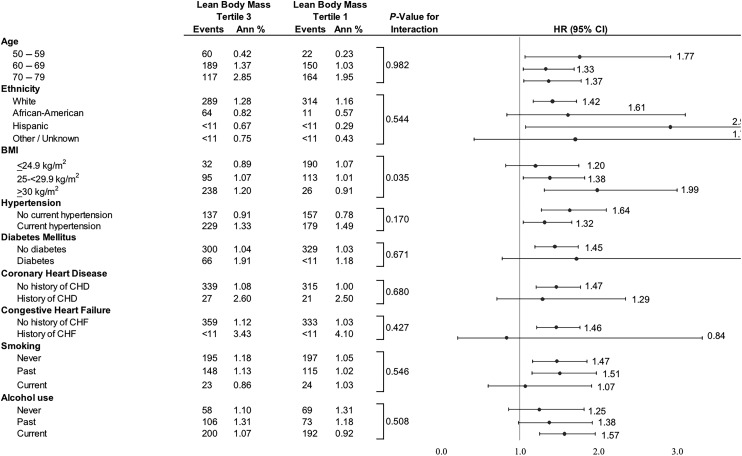
The association between the highest and lowest tertiles of LBM and AF risk stratified by baseline subgroups is displayed in Figure 3. The highest tertile of LBM conferred a greater risk of incident AF in participants in higher BMI categories (interaction P = 0.035). Compared with participants in the lowest tertile of LBM, those in the highest tertile of LBM had increased risk of incident AF in the highest BMI category (BMI ≥ 30 kg/m2: HR 1.99, 95% CI 1.31, 3.00, P = 0.001).
Figure 3.
Association of total body lean body mass with incident atrial fibrillation, stratified by baseline subgroups.
We determined whether LBM mediated the well-known association between height and incident AF.16,17 Height was correlated with total LBM (r = 0.46, P < 0.001). Total LBM was associated with AF after multivariable adjustment (HR 1.21 per 5 kg increase, 95% CI 1.14, 1.29, P < 0.001). Height was an independent predictor of incident AF after multivariable adjustment for all covariates in the primary model, substituting weight in kilograms for BMI to avoid over-adjustment for height (HR 1.22 per 10 cm increase, 95% CI 1.09, 1.35, P < 0.001). When adding total LBM to the multivariable model, the increased hazard of AF due to a 10 cm increase in height is reduced by 36.4% (HR 1.14, 95% CI 1.01, 1.28, P = 0.027). Sensitivity analyses including cases of atrial flutter in the incident AF outcome did not alter these results.
Discussion
In this multi-ethnic cohort of 8832 women, we report robust associations between all measures of total and regional LBM with higher risk of incident AF after multivariable adjustment for demographic and clinical risk factors. Total LBM partially mediates the well-known association between height and AF.
Frost et al. recently reported an association between bioelectrical impedance-derived measures of LBM and AF risk.10 However, bioelectrical impedance is prone to wide intra-observer variability and lacks precision in individuals outside the normal range of body fat.11,12 In the present study, we used superior DXA-derived measures of body composition in a larger population of well-characterized women with incident AF and performed sensitivity analyses adjusting for measures of total body size to validate and explore the association between LBM and AF. Further investigation of the novel association of LBM with incident AF may provide new insights into AF pathophysiology and ultimately inform preventative and therapeutic strategies. A few potential mechanisms of association between LBM and AF risk may be considered.
Greater LBM may predict changes in the size and structure of cardiac chambers implicated in AF pathophysiology. Lean body mass is the measure of body size and composition most closely correlated with left ventricular mass.18 There are two purported explanations for this association.18 First, skeletal muscle produces the majority of total metabolic demand, thus determining total oxygen demand and cardiac output. Second, genetic and hormonal influences may similarly affect skeletal and cardiac muscle. Left ventricular mass is an independent risk factor for incident AF.19
Similarly, several studies report associations between increased body size, as measured by BMI, height, and body surface area, with left atrial enlargement.20,21 Left atrial dilatation and resultant interstitial fibrosis and electrical remodelling are key developments in the pathogenesis of AF, and left atrial enlargement is a strong independent predictor of incident AF.19 The excess AF risk associated with obesity may be mediated by left atrial enlargement.22 Studies of the association between LBM and left atrial size are lacking. However, it is possible that greater LBM is in-part responsible for the associations between larger body size and left atrial enlargement.
Additionally, skeletal muscle may exert direct influence on AF risk through the secretion of a diverse collection of myokines that may promote atrial arrhythmogenesis.23 The myokine myostatin is a highly conserved member of the TGF-β superfamily that is expressed in cardiac myocytes and is implicated in the regulation of cardiac myocyte growth in animal models.24 A transgenic mouse model with cardiac-specific expression of the inhibitory N-terminus of myostatin pro-peptide develops ventricular hypertrophy, atrial enlargement and fibrosis, and spontaneous AF.25 Additional study of myokine-mediated cardiac pathophysiology is necessary to elucidate potential implications in AF.
Height is a well-established AF risk factor independent of body weight.21 Height may predict AF risk through its strong association with left atrial size.26 Atrial fibrillation-associated genes27,28 are associated with growth pathways and may exert pleomorphic effects to increase risk of AF.15 However, these explanations do not fully account for the association between height and AF. Mont et al.17 demonstrate that height remains a significant independent risk factor for AF after adjustment for left atrial size. Rosenberg et al.15 investigated potential anatomic and physiologic parameters that might mediate the association between height and AF, including left atrial diameter, left ventricular mass and diastolic dimension, peak E and A velocities, NT-proBNP, and hypertension, but none attenuated the effect of height on increased incident AF. Ours is the first study to show that LBM is an important mediator of the association of height with incident AF.
The finding that LBM is associated with incident AF has several potential clinical and public health implications. Although obesity is detrimental to cardiovascular health, Mendelian randomization studies suggest that much of this effect may be mediated through known risk factors like hypertension and diabetes.29 The residual association between BMI and AF after adjusting for these risk factors, on the other hand, appears to be primarily due to higher LBM. Given the novelty of these findings, the pathophysiologic basis for this relationship must be further explored with new lines of basic mechanistic research. Though in the present study we did not investigate biomarkers that may serve as mediators of the association between LBM and AF risk, this is an area that merits further exploration. The effects of public health and environmental exposures that influence LBM, such as excess protein consumption, will also need to be investigated. Furthermore, our findings have potential applications for the identification of vulnerable populations at high risk of incident AF, who may benefit from intensified AF surveillance or preventative interventions.
Several limitations to this study should be acknowledged. The regional body composition data obtained from DXA scans in this study did not distinguish between visceral and subcutaneous adipose tissues. Therefore, the participants with greater lean mass may have had greater visceral and pericardial fat mass that confounded the association with AF risk. However, DXA measurements of central fat have been demonstrated to be strongly associated with visceral fat.30 Approximately 10% of the sample was excluded due to missing data; however, we did not find clinically meaningful differences in rates of AF or body composition between the study sample and the sample that was excluded. The subjects of our study were post-menopausal women; our findings should be validated in pre-menopausal women and in men.
In a large, multi-ethnic cohort of post-menopausal women, greater LBM was a novel and strong independent risk factor for increased risk of incident AF. The increased AF risk conferred by obesity, commonly attributed to the effects of greater adiposity, may be partially driven by the association between LBM and AF.
Acknowledgements
A full listing of Women's Health Initiative investigators can be found at http://whiscience.org/publications/WHI_investigators_longlist. We thank the Women's Health Initiative investigators, staff and study participants for their outstanding dedication and commitment.
Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services (HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C). M.V.P. receives funding from a Fellow to Faculty American Heart Association award.
Conflict of interest: none declared.
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155:469–473. [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 3.Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol 2004;94:889–894. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–844. [PubMed] [Google Scholar]
- 5.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, Wassertheil-Smoller S, Stefanick ML. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women's Health Initiative Observational Study. Heart 2013;99:1173–1178. [DOI] [PubMed] [Google Scholar]
- 7.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol 2010;56:784–788. [DOI] [PubMed] [Google Scholar]
- 10.Frost L, Benjamin EJ, Fenger-Grøn M, Pedersen A, Tjønneland A, Overvad K. Body fat, body fat distribution, lean body mass, and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring) 2014;22:1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neovius M, Hemmingsson E, Freyschuss B, Uddén J. Bioelectrical impedance underestimates total and truncal fatness in abdominally obese women. Obesity (Silver Spring) 2006;14:1731–1738. [DOI] [PubMed] [Google Scholar]
- 12.Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie YG, Mathews M, Barron JR, Fitzpatrick DG, Gulliver W, Zhang H. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr 2005;81:74–78. [DOI] [PubMed] [Google Scholar]
- 13.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 14.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(9 Suppl):S98–S106. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg MA, Patton KK, Sotoodehnia N, Karas MG, Kizer JR, Zimetbaum PJ, Chang JD, Siscovick D, Gottdiener JS, Kronmal RA, Heckbert SR, Mukamal KJ. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J 2012;33:2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 17.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A, Brugada J, Investigators GGIdReF-lA. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace 2008;10:15–20. [DOI] [PubMed] [Google Scholar]
- 18.Bella JN, Devereux RB, Roman MJ, O'Grady MJ, Welty TK, Lee ET, Fabsitz RR, Howard BV. Relations of left ventricular mass to fat-free and adipose body mass: the strong heart study. The Strong Heart Study Investigators. Circulation 1998;98:2538–2544. [DOI] [PubMed] [Google Scholar]
- 19.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994;89:724–730. [DOI] [PubMed] [Google Scholar]
- 20.McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation 2010;121:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J 2009;30:1113–1120. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457–465. [DOI] [PubMed] [Google Scholar]
- 24.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 2006;99:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg MA, Das S, Pinzon PQ, Knight AC, Sosnovik DE, Ellinor PT, Rosenzweig A. A novel transgenic mouse model of cardiac hypertrophy and atrial fibrillation. J Atr Fibrillation 2012;2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol 2003;41:1036–1043. [DOI] [PubMed] [Google Scholar]
- 27.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Müller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dörr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Völker U, Völzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, Kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann HE, Witteman JC, Kao WH, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjögren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BH, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kääb S. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 2012;44:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448:353–357. [DOI] [PubMed] [Google Scholar]
- 29.Holmes MV, Lange LA, Palmer T, Lanktree MB, North KE, Almoguera B, Buxbaum S, Chandrupatla HR, Elbers CC, Guo Y, Hoogeveen RC, Li J, Li YR, Swerdlow DI, Cushman M, Price TS, Curtis SP, Fornage M, Hakonarson H, Patel SR, Redline S, Siscovick DS, Tsai MY, Wilson JG, van der Schouw YT, FitzGerald GA, Hingorani AD, Casas JP, de Bakker PI, Rich SS, Schadt EE, Asselbergs FW, Reiner AP, Keating BJ. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet 2014;94:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clasey JL, Bouchard C, Teates CD, Riblett JE, Thorner MO, Hartman ML, Weltman A. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res 1999;7:256–264. [DOI] [PubMed] [Google Scholar]