Abstract
Urolithiasis affects around 10% of the US population with an increasing rate of prevalence, recurrence and penetrance. The causes for the formation of most urinary calculi remain poorly understood, but obtaining the chemical composition of these stones might help identify key aspects of this process and new targets for treatment. The majority of urinary stones are composed of calcium that is complexed in a crystalline matrix with organic and inorganic components. Surprisingly, mitigation of urolithiasis risk by altering calcium homeostasis has not been very effective. Thus, studies to identify other therapeutic stone-specific targets, using proteomics, metabolomics and microscopy techniques, have been conducted, revealing a high level of complexity. The data suggest that numerous metals other than calcium and many nonmetals are present within calculi at measurable levels and several have distinct distribution patterns. Manipulation of the levels of some of these elemental components of calcium-based stones has resulted in clinically beneficial changes in stone chemistry and rate of stone formation. The elementome—the full spectrum of elemental content—of calcium-based urinary calculi is emerging as a new concept in stone research that continues to provide important insights for improved understanding and prevention of urinary stone disease.
Introduction
Urinary stone disease is a considerable burden on public health worldwide. In the USA, urolithiasis is estimated to occur in 8–15% of the population, resulting in an annual cost of approximately 4 billion dollars to the US national healthcare system.1,2 In developing countries, urinary stone disease affects up to 25% of the population and can result in death when adequate urological care is lacking.3 Stone recurrence rates are approximately 10% at 1 year, 33% at 5 years and 50% at 10 years.4 The disease also increasingly occurs in previously less affected populations, including children and black and Hispanic individuals.5,6 Furthermore, the prevalence of urinary calculi is increasing in the USA and many other countries in parallel with the rising rates of obesity and metabolic syndrome.7,8 Yet, after decades of research, little progress has been made in defining the aetiology of urolithiasis or designing strategies for the prevention of urinary stones in susceptible patients. Analysis of the chemical components within calcium-based urinary stones is one approach that is being used by researchers to gain insights into the disease process.
Compositional analysis of urinary calculi is not a new strategy; component analysis of stones has been suggested to have begun as far back as the end of the 18th century.9 Currently, we appreciate that urinary stones can be classified based on several specific chemical components, including oxalate, phosphate, apatite, struvite, uric acid, cystine and a few other rare categories.2 Mixtures of these chemical compositions in a single stone are also common, resulting in a spectrum of different stone chemistries. However, 80–90% of calculi are calcium-based concretions, in which the calcium component is usually complexed to organic or inorganic matrices in specific crystalline formations.10
Although many of the uncommon stone types have defined aetiologies, the calcium-based stones are mostly idiopathic in nature. Undoubtedly, the urinary concentrations of Ca2+ and its binding partners, such as oxalate, are important, but this measure alone is not sufficient to enable prediction of who will ultimately form stones, or how frequently.11 Thus, a large amount of research has been performed to discover which other components within the calculus could be measured and possibly altered to reduce stone formation. Several studies of calcium-based stones found matrix proteins, organic acids, polysaccharides and a variety of metals other than calcium within the calculi, revealing a more complex composition than originally expected.12–14
Of the stone components, the metal constituents are arguably the most well studied; overall however, only few studies investigating the effects of various metals on stone formation and physical properties have been reported. Understanding the full range of elements that can be components of calcium-based urinary stones—the elementome—is a key goal of our group. In addition to the important knowledge basis formed by previous studies,15 several important publications in the past 5 years have added to our understanding of the complex roles of the various elemental components of calcium-based stones.
Key points.
The majority of human urinary stones are primarily composed of crystalline calcium salts but many other metals and nonmetals are detectable with concentrations ranging over 10 orders of magnitude
The contribution of elements other than calcium to the formation, recurrence or physical properties of human urinary stones is generally poorly defined
Over the past 50 years, 20–30 studies of elemental stone content have been published and their findings can be summarized to produce a working elementome of the human calcium-based urinary stone
The amount of some elements within human calcium-based urinary stones does not correlate with their normal urinary concentrations, suggesting that accumulation or other processes affect the elemental composition of stones
Further refinement of the elementome of calcium-based urinary stones is warranted because it is likely to reveal novel opportunities for monitoring lithogenesis and new targets for therapeutic intervention
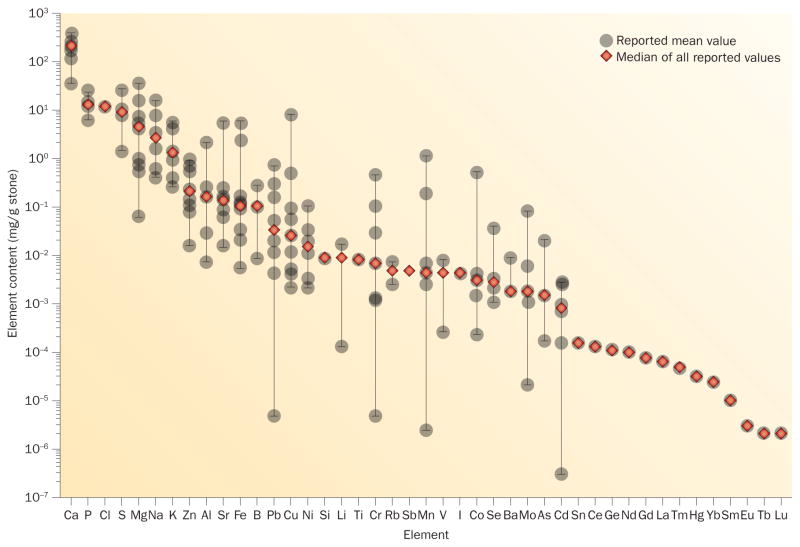
Several studies have reported the presence of a wide range of elements within human urinary stones (Table 1). Taken together, these studies offer an overview of the elementome across a diverse set of stone types and patients, which has been discovered using various methodologies and instruments. The evaluation of the reported content ranges of different elements found in calcium-based stones revealed an interesting rank order of weight-normalized elemental content over 10 orders of magnitude (Figure 1). The values of many elements with high concentrations (for example, calcium, phosphorus, sodium and potassium) were generally similar among studies. However, the values found for many elements that occur in low concentrations (for example, manganese and lead) were highly variable between studies. Importantly, even within a single study, the variation around the mean value of any one reported element was very high (often >100% of the mean value itself) regardless of whether the element occurred at a high or low concentration. Although some of the large differences between studies could be related to biological variability between patients or differing analytical tools, we suspect that the strongest contribution to the variability of values comes from the fact that specific types of calcium-based stones were used in each of the studies. These calculi can be generally classified into different subtypes based on their mineral composition, including whewellite (CaC2O4·H2O), weddellite (CaC2O4·2H2O), apatite (Ca5(PO4)3(F,Cl,OH)), brushite (CaHPO4·2H2O) or a mix of these different types. These different minerals might have differing amounts of trace metals within their crystal structures, which has been noted in a few previous reports.16–20
Table 1.
Studies defining elemental content within human urinary stones*
| Study [PMID] | Stone type(s)‡ | Stones analysed (n) | Elements analysed (n) | Identity of evaluated elements | Analysis method(s) |
|---|---|---|---|---|---|
| Ohta et al. (1957)177 [no PMID] | A, B, C, D, E, F, M | 8 | 10 | Ca, Cu, Fe, K, Mg, Mn, Na, P, Pb, Zn | Colorimetric and FP |
| Nagy et al. (1963)20 [13937198] | A, M | 85 | 21 | Ag, Al, Ba, Bi, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, Si, Sn, Sr, Zn | LA-OES |
| Meyer et al. (1977)18 [844995] | A, M | 10 | 40 | Ag, Al, Au, As, B, Ba, Be, Bi, Cd, Ce, Co, Cr, Cu, Fe, Ga, Ge, Hg, In, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Pd, Pt, Rb, Sb, Si, Sn, Sr, Ti, U, V, W, Zn, Zr | ICP-OES |
| Levinson et al. (1978)178 [627468] | A, B, C, D, F, M | 186 | 20 | Ag, Al, Be, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Pb, Si, Sr, Ti, V, Zn | ICP-OES |
| Wandt et al. (1984)179 [6486462] | A, F, M | 37 | 3 | Ca, Mg, P | ICP-OES |
| Durak et al. (1988)180 [3191333] | A, C, F, M | 29 | 5 | Cd, Cu, Fe, Mg, Pb | AAS |
| Wandt et al. (1988)181 [3401656] | A, D, F, M | 102 | 13 | Al, Ca, Cu, Fe, K, Mg, Mo, Na, P, Pb, S, Sr, Zn | ICP-OES |
| Durak et al. (1990)42 [2351193] | A, C, M | 47 | 5 | Cd, Cu, Fe, Mg, Zn | AAS |
| Komleh et al. (1990)80 [2354889] | M | 60 | 6 | Ca, Cu, Mg, Mn, P, Zn | Colorimetric and AAS |
| Durak et al. (1991)182 [1889967] | A, M | 31 | 3 | Cl, K, Na | Colorimetric and FP |
| Hofbauer et al. (1991)128 [1984106] | A, D, M | 25 | 14 | Al, Cd, Co, Cr, Cu, Fe, Li, Mn, Mo, Ni, Pb, Sr, Ti, Zn | ICP-OES |
| Durak et al. (1992)81 [1736483] | A, B, C, D, M | 37 | 4 | Ca, Cu, Fe, Zn | AAS |
| Höbarth et al. (1993)183 [8212413] | A, B, C | 10 | 10 | Ce, Eu, Gd, La, Lu, Nd, Sm, Tb, Tm, Yb | NAA |
| Küpeli et al. (1993)112 [8508898] | A | 20 | 4 | Cu, Fe, Mg, Zn | AAS |
| Perk et al. (2002)164 [12053034] | A, B, C, D, M | 45 | 5 | Al, Ca, Cd, Ni, Pb | AAS |
| Fang et al. (2005) 184 [16193228] | A, B, C, D, F | 7 | 6 | Ca, K, Mg, Na, Pb, Sm | LIBS |
| Atakan et al. (2007)46 [17203355] | A | 104 | 4 | Cu, Fe, Mg, Zn | AAS |
| Bazin et al. (2007)110 [17492279] | A, B, C, D, F, M | 78 | 7 | Cu, Fe, Pb, Rb, Se, Sr, Zn | XRF |
| Abboud et al. (2008)185 [17476575] | A, B, F, M | 110 | 16 | Al, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, S, Sr, Zn | AAS |
| Turgut et al. (2008)49 [18176803] | A | 38 | 7 | Cr, Cu, Fe, Mg, Mn, Pb, Zn | AAS |
| Słojewski et al. (2010)96 [20024629] | A, B, C, D | 219 | 29 | Al, As, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, Ge, Hg, I, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Se, Si, Sn, Sr, V, Zn | ICP-OES |
| Bazin et al. (2011)100 [21997917] | M | 3 | 1 | Sr | XANES |
| Blaschko et al. (2013)101 [23260568] | A, B, D, M | 4 | 5 | Ca, Fe, Pb, Sr, Zn | XRF |
| Giannossi et al. (2013)186 [23141501] | A, B, C, D, F, M | 48 | 9 | Ca, Cr, Cu, Fe, K, Mg, Mn, Pb, Zn | AAS and ICP-OES |
| Abdel-Gawad et al. (2014)176 [25155408] | A, B, C, D, E, F | 74 | 21 | Al, As, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mo, Mn, Na, Ni, P, S, Se, Sr, Zn | ICP-OES |
| Bazin et al. (2014)99 [24365928] | M | 6 | 1 | Sr | XANES |
| Keshavarzi et al. (2015)187 [25433503] | A, B, D, F, M | 39 | 23 | Al, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, Rb, S, Sb, Se, Sr, V, Zn | ICP-MS |
Elemental studies including evaluation of calcium-based urinary stones are listed, excluding nonpublished thesis data or articles that could not be easily evaluated owing to their age or other factors.
Urinary stone types are identified as A, calcium oxalate; B, calcium phosphate or brushite; C, magnesium phosphate or struvite; D, uric acid; E, xanthine; F, cystine; M, mixed stone type.
Abbreviations: AAS, atomic absorption spectroscopy; FP, flame photometry; ICP-MS, inductively coupled plasma mass spectrometry; ICP-OES, inductively coupled plasma optical emission spectroscopy; LA-OES, laser-ablation optical emission spectroscopy; LIBS, laser-induced breakdown spectroscopy; NAA, neutron activation analysis; PMID, PubMed unique identifier; XANES, X-ray absorption near edge structure; XRF, X-ray fluorescence.
Figure 1.
Elemental content of calcium-based urinary stones. The mean values of the elemental content of human urinary calculi reported in 15 studies summarized in Table 1 are represented in rank order of the overall median values. Only values for calcium-based stones were analysed, apart from the data reported by Abdel-Gawad et al.176 in which 78% were calcium-based stones.
Given that various stone types were analysed in these studies and many had only sampled a small number of calculi, the finding that reported trace mineral content was highly variable is not surprising. Some of the elements were only measured in one or a few studies (for example, chlorine, titanium and the lanthanide metals); therefore, the ranking of those constituents has lower confidence in the overall elementome profile. In addition, a few reported values for some elements (for example, chromium and cobalt) seem unrealistically high, suggesting metal contamination of the samples or typographical errors in the report. Other, low-mass elements such as carbon and nitrogen are likely to be present in the stones as well, particularly in the protein matrix, but cannot be easily measured using conventional elemental analysis techniques. These issues will be better resolved when additional studies become available that use next-generation technologies and cover a wider range of elements and larger sample sets with more discrete mineral types than the current studies.
The proposed composition of the elementome of calcium-based stones (Figure 1) also raises several questions that should be the basis of future studies. For example, previous investigations demonstrated that many metals other than calcium are present in these stones but the reasons for this diversity are unclear. Many metals that occur in calculi in small amounts could simply reflect urinary clearance of these metals but mechanisms that specifically concentrate these metals within a stone could also exist. In addition, the implications of the new knowledge of the elementome of urinary calculi for urolithiasis therapy need to be addressed. Metals other than calcium could affect stone formation rates or the physical properties of stones. Therapeutic alterations in the urinary levels of some of these metals could possibly change the chemical content of developing stones and alter the amenability of the stones to treatment.
This Review summarizes our current knowledge of the elementome of calcium-based urinary calculi. First, we provide a brief overview of the history of research and techniques used in elemental analysis of stones. We then discuss the findings of studies investigating those elements that are found in calcium-based stones and note when data published for those elements have not been extensively investigated. Throughout, we highlight results that have clinical relevance and could be important in shaping the future therapeutic strategies in urinary stone disease.
Measuring the elementome
Compositional analysis of urinary stones began in the 18th century,21 but the available methods would have been limited to chemical reactivity tests after laborious separation procedures. Furthermore, only the most abundant elements within the stone would have been detectable with these approaches. In the 1950s, a number of optical techniques for elemental analysis were developed and soon adopted by biomedical researchers, including atomic absorption spectroscopy, atomic emission spectroscopy and fluorescence spectroscopy.22 Further technical developments made mass spectrometric atomic spectroscopy and X-ray fluorescence techniques available for routine elemental analysis.17 These techniques have unique operational advantages but they are also accompanied by challenges that can affect the quality of elemental analysis.23,24
All of these methods have been used to study urinary stones and have resulted in a refined understanding of the elemental composition to part-per-trillion levels. Furthermore, improvements in proteomics, metabolomics and next-generation microscopy techniques have revealed that the elemental content of the stone is highly complex and heterogeneously distributed.25–27 These insights have generated new hypotheses regarding the key effectors of stone formation and recurrence, resulting in studies in which urinary stone disease was attenuated through alteration of the homeostasis of some of these effectors.
The elementome of urinary stones
For many elements, little detail regarding their normal urinary levels and effects in urological and renal physiology and pathology is known (Table 2);28 in the field of stone research, only a limited number of studies have assessed elemental content of urinary calculi, and of calcium-based stones in particular. Other elements, apart from the ones reported to date, might be present in stones. These would, for example, include unreactive gases, radioactive elements and elements rarely encountered in our environment, which would probably not be relevant in urolithiasis. The elements calcium, phosphorus and chloride constitute the bulk of most urinary stones. In this Review, we focus on the data that are available for the stone components that generally make up <1% of the stone mass, which we discuss following their order of abundance in the proposed stone elementome (Table 1).
Table 2.
Summary of urinary content and regulation of the elements identified within human kidney stones*
| Element | Proportion renally excreted | Hormonal regulation | Normal urinary levels range [mg/dl]‡ | Normal urinary amounts range [mg daily] | Urinary stone levels range (median) [mg/g] | Lithogenic potential |
|---|---|---|---|---|---|---|
| Ca | Major | Yes | NR | 100–300 | 34.5–383 (217) | Prolithogenic |
| P | Major | Unknown | 2.30–4.10 | NR | 6.10–23.5 (13.3) | Prolithogenic |
| Cl | Major | Yes | NR | 3,890–8,850 | 11.5 | Prolithogenic |
| S | Major | Unknown | NR | NR | 1.40–26.0 (8.60) | Unknown |
| Mg | Major | Yes | NR | 72.9–122 | 0.0576–35.0 (4.38) | Antilithogenic |
| Na | Major | Yes | NR | 920–6,600 | 0.402–15.0 (2.50) | Prolithogenic |
| K | Major | Yes | NR | 975–4,880 | 0.245–5.60 (1.30) | Antilithogenic |
| Zn | Minor | Unknown | 0.018–0.085 | 0.15–1.20 | 0.0157–0.891 (0.200) | Unknown |
| Al | Major | Unknown | 3×10−4–0.001 | NR | 0.00718–2.10 (0.156) | Unknown |
| Sr | Unknown | Unknown | NR | NR | 0.0145–5.60 (0.130) | Unknown |
| Fe | Minor | Unknown | 2×10−4–0.007 | 0.003–0.098 | 0.00530–5.60 (0.102) | Unknown |
| B | Major | Unknown | NR | NR | 0.00847–0.272 (0.100) | Antilithogenic |
| Pb | Major | Unknown | 0.008 | NR | 4.64×10−6–0.720 (0.0329) | Unknown |
| Cu | Minor | Unknown | 2×10−4–0.008 | 0.003–0.035 | 0.00220–7.49 (0.0245) | Unknown |
| Ni | Major | Unknown | NR | 1×10−4–0.01 | 0.00205–0.100 (0.0144) | Unknown |
| Si | Major | Unknown | NR | NR | 0.00846 | Unknown |
| Li | Major | Unknown | NR | NR | 1.28×10−4–0.0166 (0.00834) | Unknown |
| Ti | Minor | Unknown | NR | NR | 0.00760 | Unknown |
| Cr | Major | Unknown | 1×10−5–2×10−4 | NR | 4.55×10−6–0.450 (0.00690) | Unknown |
| Rb | Major | Unknown | NR | 1.0–3.0 | 0.00250–0.007 (0.00475) | Unknown |
| Sb | Major | Unknown | 0.001 | NR | 0.0043 | Unknown |
| Mn | Minor | Unknown | 5×10−5–9.8×10−4 | NR | 2.31×10−6–1.10 (0.0043) | Unknown |
| V | Major | Unknown | 8×10−6–2.4×10−5 | NR | 2.38×10−4–0.0082 (0.00422) | Unknown |
| I | Major | Unknown | 0.011–0.0129 | NR | 0.00414 | Unknown |
| Co | Major | Unknown | 1×10−4–2×10−4 | NR | 2.16×10−4–0.500 (0.00302) | Unknown |
| Se | Major | Unknown | 7×10−4–0.016 | NR | 0.001–0.0362 (0.00266) | Unknown |
| Ba | Major | Unknown | NR | NR | 0.00174–0.00873 (0.00180) | Unknown |
| Mo | Major | Unknown | 8×10−4–0.0034 | NR | 2.03×10−5–8.00×10−2 (1.74×10−3) | Unknown |
| As | Major | Unknown | NR | 0.005–0.05 | 1.59×10−4–0.0202 (0.00148) | Unknown |
| Cd | Major | Unknown | 5×10−5–4.7×10−4 | NR | 2.81×10−7–2.68×10−3 (8.02×10−4) | Prolithogenic |
| Sn | Major | Unknown | 0.004 | NR | 1.56×10−4 | Unknown |
| Ce | Major | Unknown | NR | NR | 1.24×10−4 | Unknown |
| Ge | Major | Unknown | NR | NR | 1.04×10−4 | Unknown |
| Nd | Major | Unknown | NR | NR | 9.5×10−5 | Unknown |
| Gd | Major | Unknown | NR | NR | 7.3×10−5 | Unknown |
| La | Major | Unknown | NR | NR | 5.9×10−5 | Unknown |
| Tm | Major | Unknown | NR | NR | 4.6×10−5 | Unknown |
| Hg | Major | Unknown | 0.002 | NR | 2.95×10−5 | Unknown |
| Yb | Major | Unknown | NR | NR | 2.4×10−5 | Unknown |
| Sm | Major | Unknown | NR | NR | 1×10−5 | Unknown |
| Eu | Major | Unknown | NR | NR | 3×10−6 | Unknown |
| Tb | Major | Unknown | NR | NR | 2×10−6 | Unknown |
| Lu | Major | Unknown | NR | NR | 2×10−6 | Unknown |
Elements are listed in rank order of weight-normalized elemental content within calcium-based stones (Figure 1). The clinical reference range values are listed depending on whether they are usually measured as urinary concentration or daily urinary content.173
For some metals only a single value, which should not be exceeded under normal circumstances, is listed.
Abbreviation: NR, not reported.
Sulphur
Sulphur is an essential nonmetal nutrient that is obtained either from sulphur-containing amino acids or from sulphate in the diet. Sulphur is required to make cysteine, methionine, glutathione and many other sulphur-containing compounds within the body.29,30 The effects of elemental sulphur on calcium-based stones are mostly unknown. However, convincing evidence exists that the increased sulphate load in kidneys of individuals with high animal protein consumption is associated with an increased risk of stone formation.31 This effect is primarily driven by elevated uric acid and reduced citrate levels in the urine, which are both known risk factors that promote formation of calcium-based stones.32 In addition, urinary sulphate excretion has been shown to be higher in patients who have urinary stones than in individuals who do not form stones, although this effect might be related to increased levels of urinary Ca2+ in stone formers: sulphate can bind Ca2+, competing with sites in a growing stone that can bind Ca2+, which keeps the Ca2+ ions in solution and increases the amounts of both Ca2+ and sulphate that are excreted in the urine.33 For this reason, other investigators have argued that therapeutically increasing urinary sulphate concentrations could actually reduce the risk of calcium-based stones.34
Sulphur-containing compounds have been found in calcium-based stones, but the exact identities and amounts of such molecules have not been well established.35 Free sulphate is not known to directly bind to crystalline formations found in calcium-based stones; however, small amounts of proteins are found within these stones, so any cysteine or methionine residues would account for the sulphur content. In addition, other sulphur-compounds that are present in urine at low levels, including thioesters and mercaptans, could react with components within a stone.36 Although reduction of protein consumption has been shown to reduce the risk of stone formation,37 no direct proof exists that this risk reduction specifically relates to decreased levels of sulphur-containing amino acids or other sulphur-containing compounds. On the one hand, metabolism of sulphur-containing amino acids increases the acid levels in the urine but, on the other hand, sulphate and other sulphur-compounds might act as inhibitors of stone formation by competing for free urinary Ca2+ with the Ca2+ binding sites of a stone, inhibiting crystallization. To our knowledge, no studies have been reported that prospectively altered sulphur homeostasis to determine whether such a change has an effect on calcium-based lithogenic risk.
Magnesium
Magnesium is an essential metal micronutrient required as a cofactor in a range of metabolic, bioenergetic, regulatory and cell signalling functions. Magnesium also has structural roles during biomineralization, with 50–60% of the total amount of magnesium in the body found in bone.38 The effects of magnesium status on urinary stone disease have been the subject of several investigations. Studies have shown decreased calcium oxalate crystallization and growth in the presence of high concentrations of Mg2+,39–41 and others have demonstrated increased lithogenesis when urinary Mg2+ levels are low.42 Magnesium has an important role as a nephrolithiasis inhibitor, acting more effectively in combination with citrate—magnesium citrate complexes slow the nucleation and growth rate of stones.41 Mg2+ also competes with Ca2+ for binding to oxalate in the urine, therefore, reducing the number of calcium oxalate crystals.40 Moreover, severe restriction of dietary magnesium has been reported to cause nephrocalcinosis and stone formation in rats.43 By contrast, other studies have shown that urinary magnesium excretion was not significantly different between patients with stones and healthy controls,39,44,45 yet another group found urinary Mg2+ levels to be higher in healthy controls compared with patients with stones.46
Although not conclusive, the current evidence suggests that urinary magnesium can generally be antilithogenic within the renal system. Thus, urinary magnesium concentration is regularly measured in 24-h urine analysis, and the Mg2+:Ca2+ ratio in the urine is sometimes used as an estimate of stone risk, with a higher ratio being more antilithogenic.47
The effects of magnesium within urinary calculi have been investigated in a few studies. A study using molecular dynamics computer simulations showed that the presence of Mg2+ reduces the average size of the calcium oxalate and calcium phosphate aggregates; for calcium oxalate aggregates, Mg2+ destabilized the ionic pairing of Ca2+ and oxalate.48 Moreover, authors of another report found that calcium oxalate monohydrate stones that contained lower levels of magnesium (around 3.3 g/kg) were more resistant to fragmentation in shock-wave therapy than stones containing higher levels of magnesium (around 6.1 g/kg).49
Despite the encouraging results in experimental studies, the usefulness of manipulating magnesium homeostasis as a treatment strategy for urinary stone disease is unclear, as clinical studies have generated conflicting results.41,50,51 One review published in 2005 concluded that the available evidence does not justify the use of magnesium salts alone as a therapy for calcium oxalate urinary stones for most patients, but that the addition of magnesium supplementation to conventional therapeutic modalities is useful, especially in patients who are at risk of magnesium deficiency.52 Importantly, although the kidneys are the primary determinant of magnesium homeostasis, the gastrointestinal tract also has a major role through its ability to regulate the absorption of Mg2+ from the diet. Thus, the effectiveness of magnesium supplementation might be complicated by changes in alimentary absorption. In addition, no sensitive measure of adequacy exists to monitor changes in dietary magnesium intake or overall magnesium balance, making it difficult to assess magnesium status in a patient. Given the demonstrated benefits of magnesium in multiple aspects of stone disease, further investigation of the usefulness of magnesium supplementation in well controlled studies is highly warranted.
Sodium
Sodium is an essential metal micronutrient and Na+ acts as the principle cation of extracellular fluid, with key functions in control of osmotic balance, body fluid volume regulation, ionic gradients and signal transduction. Na+ also has a structural role, as it interacts with and neutralizes many anionic chemical molecules, proteins and membrane structures.53 Elevated urinary sodium levels result in increased calcium excretion and high urinary calcium excretion is known to be one of the main risk factors for developing calcium-based urinary stones.54,55 Curhan et al.56 reported a strong epidemiological connection linking high Na+ intake and nephrolithiasis in the Nurses’ Health Study I of 91,731 women, showing a clear increasing trend in the risk of developing stones as the quintiles of salt intake rise (relative risk 1.30 for sodium intake >4 g/day). Others have shown similar results.57,58 Elevated urinary Na+ excretion can also lead to hypocitraturia, resulting in the reduction in levels of the natural stone inhibitor citrate and a concomitant increase in calcium-based urinary stone risk.59,60
Interventional studies investigating the role of dietary sodium restriction have mostly demonstrated favourable results. Dietary salt reduction resulted in decreased urinary Ca2+ excretion.61,62 Borghi et al.37 prospectively followed male idiopathic calcium oxalate stone formers for 5 years who were instructed to maintain a diet that was low in salt and animal protein and found that the diet lowered urinary calcium levels and was associated with a 50% reduction of calcium oxalate stone recurrence rates. Taylor et al.63 demonstrated a lower recurrence of all urinary stone types in individuals consuming a healthier diet (lower amounts of salt and dairy fat, but higher intake of fruits, vegetables and whole grains) compared with individuals consuming a less healthy diet. Nouvenne et al.64 reported that a reduction in salt intake of 8 g/day returned elevated urinary calcium levels to normal levels in a cohort of hypertensive patients followed for 3 months. Dietary sodium reduction seems to be an effective way to reduce calcium-based urinary stone recurrence rates.
By contrast, our group found that dietary sodium supplementation resulted in an increased voided urine volume and decreased the relative risk supersaturation ratio for calcium oxalate stones in patients with a history of hypocitraturic calcium oxalate nephrolithiasis.65 Urinary excretion of calcium, oxalate and uric acid were not changed, suggesting that sodium restriction is inappropriate in patients with hypocitraturia and recurrent urinary stones. Sodium supplementation might be beneficial in these patients because it can promote fluid intake. However, the role of sodium in stone formation remains unclear and, to our knowledge, no studies on how the sodium content of stones correlates with stone formation risk or physical properties of stones have been published.
Potassium
Potassium is an essential metal micronutrient and K+ acts as the principle cation of intracellular fluid, with a key role in osmotic balance, body fluid volume regulation, ionic gradients and signal transduction. Similar to Na+, K+ also has a structural role as it interacts with and neutralizes many anionic chemical molecules, proteins and membrane structures.65 High urinary potassium levels can alter the excretion of sodium and calcium, owing to interrelated reabsorption transporters in the nephron.66,67 As with sodium, potassium excretion is sensitive to urinary pH and osmolarity. In two large prospective studies from the USA, the incidence of urinary stones correlated strongly and negatively with dietary potassium intake.68,69 Another study performed outside the USA has reported similar findings.70 Once calcium-based urinary stones form, potassium can be found within a stone at levels approaching 0.5% of the stone mass. The potassium content in stones has historically been treated as an epiphenomenon that occurs simply as a consequence of the abundance of K+ ions in the urine, which leads to interaction of K+ with the crystalline matrix of the stone, resulting in K+ ions becoming trapped within the mineral.
To our knowledge, no studies have been published that investigated whether the amount of potassium within a stone correlates to its physical properties or the behaviour of the stone during clinical procedures, such as how sensitive the stone is to shockwave lithotripsy. Despite the strong association between low potassium status and high risk of urinary calculi, few studies that directly tested the use of potassium supplementation for urinary stone disease have been performed. Investigators of one study from Finland in male smokers reported that men who had elevated dietary K+ intake did not have a detectable risk reduction of stones.70 Of note, the average potassium intake in Finland was considerably higher than in the USA at that time.70 However, numerous studies on the combination of K+ and citrate exist. Potassium citrate is a common therapeutic agent for urinary stone disease that is efficacious in reducing the recurrence of calcium-based stones,71 particularly in patients with distal renal tubular acidosis.72 It is generally believed that the citrate, and not the potassium, is the key factor that reduces the recurrence of urinary stones, but potassium might also have an unappreciated benefit. However, the exact role of potassium in stone formation has not been fully elucidated.
Zinc
Zinc is an essential metal micronutrient required for hundreds of enzymatic processes in which it can have catalytic, regulatory, structural and signalling roles. In addition, zinc participates in biomineralization; approximately 30% of the total zinc content of the human body is found in bone.73 The effects of zinc on the formation of calcium-based stones have been investigated in several model systems. In an in vitro model, Zn2+ ions acted as potent inhibitors of calcium phosphate mineralization.74 Another study showed that Zn2+ ions at low concentrations inhibited the growth of calcium apatite crystals; however, high concentrations promoted the formation of amorphous calcium phosphate or zinc-substituted calcium phosphate.75 Other researchers demonstrated that substituting calcium with zinc made apatites less prone to crystallize, finding that increases in Zn2+ concentration caused calcium apatite crystals to be smaller and more irregular in shape.76,77
Epidemiological studies have also supported an association between zinc and lithogenesis. Two studies using data from the Third National Health and Nutrition Examination Survey (NHANES III) and the European Prospective Investigation into Cancer and Nutrition (EPIC) reported an association between high dietary zinc intake and increased risk of urinary stones.78,79 However, attempts to assess zinc status in individual patients have resulted in conflicting information. Data from a report published in 1963 and one publication from 2007 showed that calcium-based stone formers had lower urinary zinc levels compared with controls, consistent with an inhibitory effect of zinc on calcium-based stone formation.16,46 Similarly, a study from 1990 found that urinary zinc excretion was higher in healthy controls than in patients with calcium-based stones.80 Other translational and clinical studies have reported varying behaviour of zinc in stone formation.81–83 Of note, none of the clinical studies determined the form in which zinc was present in the urine samples. For example, Zn2+ can bind directly to oxalates and phophates84 or with higher binding affinity to small thiols and peptides,85 which might influence the effects of zinc on stone formation. Hence, understanding the role of zinc in lithogenesis in vivo will require more careful analysis of chemical forms of zinc.
Zinc is found in many sites of mineral formations within the body, including bone, vascular plaques, teeth and urinary stones.86–90 Within bone hydroxyapatite, calcium was preferentially substituted by zinc at the Ca-2 crystal position.91 However, within urinary stones, the distribution of zinc is not as well understood. One study found that zinc was concentrated in Randall plaques, which are thought to be the starting structure of a urinary stone.92 According to another study, zinc, along with iron and copper, was more concentrated in the interior than the crust of a stone, suggesting a role for zinc and other metals in the early phase of lithogenesis.81 Our group observed similar distributions using synchrotron X-ray fluorescence microscopy to map the location of zinc and other elements at high resolution within calcium-based urinary stones (S. Ho, personal communication). However, another study has not found differences in zinc distribution within stones.18 Two further reports, investigating biomineralization at other sites of the body, demonstrated accumulation of zinc at sites of mineralized tissue formation in bone and teeth.88,93
The usefulness of manipulating zinc homeostasis to affect stone formation remains unclear. To our knowledge, no studies have been reported that prospectively alter zinc homeostasis to determine whether such manipulation has an effect on lithogenic risk. However, using a Drosophila melanogaster model of stone formation, our group has found that increased dietary zinc intake increased the rate of stone formation, whereas addition of the zinc-selective chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine to the food decreased the rate.94 In addition, manipulating zinc levels in stones might change the physical properties of the stones. Researchers found that low concentrations of zinc, along with magnesium and manganese, made calcium oxalate monohydrate stones more resistant to fragmentation in shockwave therapy compared with stones containing higher levels of zinc.49 Interest in the role of zinc in urinary stone disease seems to be growing based on the increasing number of studies that focus on this metal in the context of urolithiasis.
Aluminium
Aluminium is a metal that is not considered essential, as no metabolic role specific for this element has ever been described. Rather, aluminium is regarded as a toxic metal, which is abundant in the environment and might cause disease when intake levels are elevated; however, this claim is controversial.95 One study found an association of the aluminium levels in stones with the aluminium levels in hair and urine of the same patient, suggesting that stone aluminium content reflects environmental exposures.96 In an in vitro study, researchers found that physiological concentrations of Al3+ affected calcium phosphate crystal growth; however, its inhibitory activity towards calcium phosphate crystal growth in urine seemed to be insufficient to support a regulatory role in stone growth.18 To our knowledge, no studies investigating whether aluminium content of a stone is correlated to its physical properties have been published. Equally, no studies have been published that prospectively altered aluminium homeostasis to determine whether such modification has an effect on lithogenic risk.
Strontium
Strontium is a metal that is considered nonessential, as no specific metabolic role has been described for it. However, this element is sometimes considered beneficial because it can strengthen the hydroxyapatite crystal structure in bone.97,98 Similar to zinc, strontium is found in many types of biomineralization processes in the body, including bone, teeth and urinary stones, and is thought to generally substitute for calcium.99–101 Within bone hydroxyapatite, strontium preferentially replaces calcium at the Ca-2 crystal position.102 Strontium ranelate is used clinically to increase bone volume and trabecular thickness,103 although one study suggests that the actual effect of strontium on crystal structure in hydroxyapatite is highly variable depending on dose.98 In vitro experiments using nanobacteria revealed strontium incorporation in early stone formation,104 but few other studies on strontium incorporation into human urinary stones exist. In a Drosophila larval model, researchers discovered that strontium could accumulate into urinary stone concretions to a much greater degree than calcium, when supplemental Sr2+ was added to the diet.105 However, the exact role of strontium in calcium-based lithogenesis is still unclear.
Whether the presence of elevated strontium has an effect on the physical properties of urinary calculi is also unclear, but it is known that strontium strengthens hydroxyapatite,106 so some calcium-based stones might be similarly affected. Data from a study by our group suggest that strontium co-localizes with calcium in calcium-based stones, in which it is present primarily as strontium apatite.101 This finding suggests that strontium hydroxyapatite, similar to calcium hydroxyapatite, might be a nidus in calcium-based lithogenesis. We are aware of no published studies in which strontium homeostasis was prospectively altered to determine a possible effect on lithogenic risk. As strontium ranelate has been clinically used for several years to increase bone mineral density and decrease fractures,106 determining whether lithogenesis rates change in patients on strontium ranelate therapy would be interesting.
Iron
Iron is an essential metal micronutrient required as a cofactor for numerous proteins in which it can have catalytic, regulatory and ligand-binding roles;107 however, the effect of iron on the lithogenic process is undefined. Iron can bind oxalates and phosphates, which might interfere with calcium-based crystallization.19,108,109 However, iron can also form complexes with citrate, a natural inhibitor of stone formation, rendering the citrate unavailable for binding with calcium and, thus, might promote stone formation.19 Although one earlier study failed to detect iron in calcium oxalate stones,18 several newer reports have found considerable concentrations of iron in calcium oxalate and calcium phosphate stones.46,110,111 Investigators in one report noted that decreased urinary excretion of iron correlated with low shockwave lithotripsy success rates in patients with calcium oxalate stones.112 No studies that prospectively altered iron homeostasis to determine effects on lithogenic risk currently exist.
Boron
Boron is considered to be a metalloid that is nonessential, as no specific metabolic role for this element in animals has ever been described. By contrast, boron is essential for plants. However, boron has been proposed as a micro-nutrient in humans, owing to developmental defects in boron-deficient animals, as well as associations between decreased risk of certain cancers and increased dietary boron intake.113
No studies have been reported that evaluate boron status and lithogenic risk. Boron might promote bone development and could possibly be used as a therapeutic agent for osteoarthritis and osteoporosis.114 The mechanism by which boron improves bone health is not fully understood but it might affect magnesium and phosphorus homeostasis or cellular membrane integrity.115,116 Boron is present within calcium-based urinary stones, but little understanding of the physiological relevance of boron content exists; also, no direct association between the physical properties of stones and boron levels in urine or a stone has been reported. However, some studies on boron supplementation and risk of urinary stones have been published. One report found that supplemental boron resulted in decreased urinary calcium and oxalate levels in postmenopausal women, but only when magnesium intake was low.117 In preliminary studies, a small number of patients treated with boron had decreased urinary calcium levels without serious adverse effects.118,119 However, more evidence is needed to be able to fully evaluate the clinical utility of boron supplementation. Given the increasing popularity of boron supplements for bone health, investigation of the effect of boron on lithogenesis would be worthwhile.
Lead
Lead is a nonessential, toxic metal that causes disease when intake levels are elevated.120 No studies have been reported that evaluate lead status and lithogenic risk. Lead has biophysical characteristics similar to calcium, so the finding that lead can accumulate in bone is not surprising.121 Lead has also been detected in urinary stones and correlations of lead content with other metals and stone chemistry have been studied.96,110 One study found an association of the lead levels in stones with the lead levels in hair and urine of the same patient,96 suggesting that stone lead content reflects environmental exposures. No additional studies have been reported that evaluate lead content within calcium-based stones.
Copper
Copper is an essential metal micronutrient required as a cofactor for numerous proteins in which it can have catalytic and regulatory roles. Copper homeostasis affects bone biomineralization, as severe copper deficiency results in osteoporosis.122 Four studies from one group demonstrated that copper salts inhibited the crystallization of calcium oxalate in vitro and in rat models.123–126 By contrast, another report suggested that copper could inhibit certain types of crystal aggregation (particularly calcium phosphate crystal formation) but not calcium oxalate crystallization.18 Similarly, one study found that copper excretion was decreased in patients who form calcium-based stones compared with controls,80 but other investigators have reported increased copper excretion in patients prone to developing calcium-based stones.111 Currently, the effects of copper on lithogenesis and on the physical properties of stones are unclear. In one study, variation of copper concentrations in the organizational matrix of calcium oxalate stones affected stone hardness; hence, the authors proposed that low concentrations of copper in calcium oxalate stones might confer resistance to shock wave lithotripsy.112
Nickel
Nickel is a metal that is not considered essential, but a role as a potential micronutrient has been proposed, owing to the effects of nickel on glucose metabolism and amino acid synthesis in animal models.127 Nickel has not been studied extensively with regards to lithogenesis. One study demonstrated significantly lower urinary and serum nickel levels for active calcium stone formers compared with healthy individuals,128 but overall the effects of nickel on stone formation remain unclear. No studies of how nickel content of stones might be correlated with their physical properties or whether altering nickel homeostasis affects lithogenic risk have been published.
Silicon
Silicon is considered to be a nonessential metalloid, but it has been proposed to be a micronutrient, owing to observations of adverse changes in bone formation, collagen deposition, and acid phosphatase activity under experimental silicon deficiency in animal models.129,130 Most of the silicon in the body is present in bone and connective tissue and, therefore, can be incorporated into biomineralized formations. However, we are not aware of any published studies evaluating the relationship between silicon balance and lithogenic risk. Furthermore, the effect of silicon content on stone properties in calcium-based stones has not been studied. Silicon has been proposed to help regulate calcium and magnesium homeostasis, but mechanistic details are lacking.131 Of note, chronic use of antacid trisilicates can lead to increased urinary stone formation, and these stones are rich in silicon and have less calcium content than commonly found in calcium-based stones.132,133 No evidence exists that altering silicon homeostasis affects lithogenic risk.
Lithium
Lithium is a metal that is not considered to be an essential nutrient and it has not been studied in detail with regards to lithogenesis. Lithium is a potent inhibitor of the Na+/dicarboxylate cotransporters 1 and 3 that reabsorb citrate from the urine; Zhang et al.134 have proposed that lithium might be useful as a therapeutic to increase urinary citrate, an endogenous inhibitor of calcium-based stone formation. However, elevated lithium levels can be toxic, so the benefits of lithium use to treat urinary stone disease might not outweigh the risks.
Chromium
Chromium (as Cr3+) is an essential metal micronutrient required as a cofactor for glucose tolerance and insulin regulation, whereas other forms of chromium (as Cr6+) are toxic.135,136 No studies that evaluate the influence of chromium status on lithogenic risk have been reported. Chromium is known to accumulate in several tissues including biomineralized tissue, such as bone.137 Chromium has also been detected in urinary stones and correlations of stone chromium content with the amount of other metals, including calcium and vanadium, in hair and urine have been reported.96 We are not aware of any studies that have reported a specific role for chromium in the stone formation processes or in the physical properties of calcium-based stones.
Rubidium
Rubidium is a metal that is not considered essential for metabolism, although it has been proposed to be a micronutrient, as it has unique neurophysiological activity.138 The effects of rubidium on lithogenesis of calcium-based stones are mostly unknown. Similar to potassium, the rubidium content in stones has historically been considered an epiphenomenon, resulting from interaction with and entrapment in the crystalline matrix of a calculus.110 Possible effects of rubidium content of a stone on its physical properties have not been studied to date and no studies in which rubidium homeostasis has been prospectively altered to determine an effect on lithogenic risk have been published.
Manganese
Manganese is an essential metal micronutrient required as a cofactor for key steps in carbohydrate and cholesterol metabolism, amino acid synthesis, bioenergetics and oxidant defence.139 Manganese has an important role in bone biomineralization: changes in manganese homeostasis can affect this process, as this element is a cofactor needed in proteoglycan synthesis, which is required for bone matrix formation.140 A study in rats demonstrated that elevated manganese intake increased stone formation rate, as rats receiving high doses of manganese had viscous, gritty urine in the urinary bladder and developed urinary stones.141 Multiple clinical studies have shown that blood and urinary manganese levels in patients with calcium-based stones were lower than in controls.80,128,142 The effects of manganese on the physical properties of stones are unclear. Results of one study suggested that low manganese levels in urinary stones might alter their fragility by making them more susceptible to shockwave therapy.49 Further investigation of the role of this metal in lithogenesis is warranted.
Vanadium
Vanadium is a metal that is not essential for metabolism, yet has been proposed to be a micronutrient as it has insulinogenic and neurophysiological activity.143 The effects of vanadium on the lithogenesis of calcium-based stones are mostly unknown; however, this metal is known to accumulate in bone, so incorporation into biomineralized tissues might be possible. Whether vanadium content of a stone influences its physical properties is not known.
Cobalt
Cobalt is an essential trace element, as a key constituent of vitamin B12, however free cobalt can cause toxicity after occupational or environmental exposure.144 The effect of cobalt on stone formation remains unclear. Słojewski and colleagues96 noted cobalt to be one of five elements, along with vanadium, aluminium, lead and molybdenum, whose content positively correlated with each other in calcium phosphate stones and hair. These findings might reflect environmental exposure to common industrial heavy metals, but the actual reasons for these metal correlations remain unclear. No studies have been published in which cobalt homeostasis was prospectively manipulated to determine whether an effect on lithogenic risk exists.
Selenium
Selenium, which has been classified as a nonmetal or a metalloid, is an essential micronutrient in humans. The role of selenium in lithogenesis is not quite clear and is under investigation. One study in rats treated with ethylene glycol showed that selenium administration inhibited calcium oxalate stone formation; the authors suggested that selenium is incorporated on the crystal surface and inhibits aggregation or induction of new crystals.145 Another group showed that selenium plus vitamin E also protected from hyperoxaluria-induced renal damage in lithogenic rats.146 In canine renal tubular cells, selenium inhibited the development of calcium oxalate monohydrate crystals.147 Although selenium supplementation in experimental models has proved therapeutic, the effects of selenium on calcium-based stone formation in humans have not been reported.
Molybdenum
Molybdenum is an essential metal micronutrient and cofactor required for enzymes involved in catabolism of sulphur-containing amino acids and heterocyclic metabolites, such as purines.148 No studies have been published that specifically evaluate molybdenum status and lithogenic risk. Excess dietary molybdenum has been associated with increased urinary uric acid levels, which can promote urinary stone formation, but other studies failed to show the same effect.149,150 Hence, the effects of molybdenum on lithogenesis remain unclear.
Arsenic
Arsenic is a metalloid that is not considered essential and is generally regarded as toxic. This element is known to be concentrated in specific geographical locations, which can cause disease when intake levels are elevated.151 Some studies have found higher concentrations of arsenic in urinary calculi made of calcium apatite compared with other calcium-based stones, postulating that arsenic replaces phosphorus in the apatite, owing to its similar charge and size.111 Another study found that dietary arsenic deprivation increased kidney calcium concentrations in female rats fed a standard America Institute of Nutrition-76 diet.152 However, the effect of arsenic on stone formation remains unclear and no studies of how arsenic content of a stone correlates with its physical properties and whether changes in arsenic homeostasis could affect stone risk have been published.
Cadmium
Cadmium is a metal that is considered nonessential and toxic.153 Cadmium is associated with several renal changes that favour stone formation, including hypercalciuria, hyperphosphaturia, elevated urinary uric acid levels, reduced urinary citrate levels and renal tubular acidosis.154 Epidemiological studies indicate that elevated cadmium exposure is associated with a higher incidence of urinary stones.155–157 In addition, multiple studies have shown a higher prevalence of urinary stones among individuals with elevated urinary cadmium levels.158–160 By contrast, another study with a 12-year follow-up period did not find a strong association between dietary cadmium intake and urinary stone risk at the exposure levels seen in the general population.161
Cadmium might also affect the physical properties of stones: for example, researchers of one study proposed that cadmium might inhibit calcium oxalate crystallization based on known cadmium toxicity mechanisms.128 However, the effects of cadmium on lithogenesis are still not clear. When levels are elevated, cadmium can interfere with calcium deposition in bone and make bones brittle.162,163 One study found higher levels of cadmium in the nucleus than in the outer crust of calcium-based stones, suggesting that cadmium has a role in the early formation of calcium-based stones.164 To our knowledge, no studies have been published that evaluate cadmium content and stone physical properties.
Tin
Tin is a metal that is not considered essential, as no metabolic role has been described specifically for it.165 Tin has been measured in urinary stones and correlations of its content with other metals and stone chemistry have been studied.96 However, the effect of tin on stone formation and stone physical properties and its potential lithogenic risk remain unclear.
Lanthanides
The lanthanide group of elements are a series of 15 metals that are not considered essential, as no metabolic role has been described for them to date.166 These metals are increasingly used in modern technologies, hence, exposures to lanthanides are increasing each year.167 In addition, lanthanum carbonate has been used as a therapeutic to control elevated phosphate levels during renal failure, owing to the highly selective binding of lanthanum to free phosphate.168–170 Hyperphosphataemia is a risk factor for nephrolithiasis (especially in children)171 but, to date, no reports determining the effect of lanthanum levels on stone formation have been published. In addition, no studies have been reported that evaluate the other lanthanide elements and their effects on lithogenic risk or stone physical properties.
Elements lacking study on lithogenic effect
For several elements whose content ranges in calcium-based stones have been analysed and reported (Table 1, Figure 1) no studies have been published that evaluate their effects on lithogenic risk. These elements are titanium, antimony, iodine, barium, germanium and mercury. In addition, no studies have been published that investigated whether their presence in a urinary stone alters the stone’s physical properties. Some of these elements, such as barium, have similar biophysical characteristics to calcium; hence, it is not surprising that they can accumulate in bone.110,172
The elementome and urinary levels
Although the elemental content of urinary stones is certainly related to the concentration of elements in the surrounding urine, several investigators have reported that this relationship is complex and might be strongly affected by other factors in the stone formation processes.18,19,46,96,111 Hence, we analysed the relationship between the reported concentrations of elements within calcium-based stones and normal urinary values of these elements provided by clinical reference guides (Figure 2, Table 2).173 Importantly, our analysis was limited by the characterization of the stone mineral types provided in the previously published reports. Many of these studies aggregated the different types of calcium-based stones into a single category, which could contribute to larger variances in elemental content. Moreover, modern analytical techniques have shown that urinary stones often have a mix of mineral types within the same stones, which would further increase elemental content differences. Despite these limitations, a positive correlation between urinary and stone elemental content exists, with elements that are more abundant in urine also having higher concentrations in urinary stones; however, some elements do show the ability to accumulate in stones, with concentrations in the calculi exceeding urinary levels (for example, aluminium, vanadium and zinc). In addition, many elements (for example, chromium, manganese and molybdenum) have very large ranges of elemental content in stones. Studies designed to determine the reasons for the accumulation or variability of these specific elements could provide important insights into the role of these elements in stone formation. The resulting data could be a starting point for new strategies in the treatment of stone disease, for example through dietary manipulation or pharmacological intervention. Further studies are needed to determine if measuring the levels of specific elements in the urine of individuals at risk of urolithiasis could be a useful way to monitor the development of urinary stone disease.
Figure 2.
Relationships between the elemental content in calcium-based urinary stones and normal urine. The reported elemental content of human urinary calculi was plotted against the clinical reference ranges of these elements in normal human urine.173 The reference range values are listed as a | daily amounts or b | concentrations, depending on how these values are usually reported. The bars in the X-axis dimension reflect standard deviations around the mean. The bars in the Y-axis dimension reflect upper and lower range limits around the median.
Conclusions
The previous studies that have analysed the elemental content of stone samples are an important first step in understanding the elementome of human urinary stones. The next step is to begin high-throughput analysis with sensitive elemental detection strategies, focused on chemically defined stone types and involving high numbers of stones, so that the full range and biological variance of the elementome within these calculi can be defined. The resulting data will enable us to connect analytical descriptions of stone composition to criteria that drive clinical impact, and to ultimately answer questions about the aetiology of stone formation in the patient population. For example, for the USA, it is estimated that more than half of the population regularly consumes a diet that is inadequate in magnesium,174 but whether deficiency in magnesium or any other specific metal micronutrient promotes stone disease is currently unknown.45,51,52 Similarly, whether excessive supplementation of specific metal micronutrients could also promote lithogenesis is unclear but requires study, as, for example, an estimated 15% of men in the USA use zinc supplements at a level that is higher than the established upper limit of safety.175 In addition, exposure to specific metal toxins such as cadmium might increase the risk of developing stone disease.158–160 Additional studies are needed to clarify the chemical mechanisms underlying stone formation and to elucidate whether some specific elements are key in providing the nidus for crystallisation.
Classifying calcium-based stones into subdivisions according to the amounts of specific elements they contain might be useful to better define therapy for a specific individual. Furthermore, the use of targeted micronutrient supplementation or specific metal chelators might be discovered as new therapeutic possibilities in treating stone disease. Further studies are needed to define whether measurement of levels of specific stone constituents (or levels of specific elements in the urine) can be used to predict stone recurrence once treatment has been successful. Hence, resolving the complete elemental profile of human urinary stones and of stones on a patient-specific basis might be the next key step in improving treatment of urolithiasis.
Acknowledgments
The authors’ research is supported by the NIH NIDDK RFA-DK-12-003: Planning Centers for Interdisciplinary Research in Benign Urology (IR-BU)(P20).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
K.R. and D.W.K. researched data for the article. K.R., D.W.K. and M.L.S. wrote the article. All authors contributed to discussion of the content and reviewed and edited the manuscript before submission.
References
- 1.Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000;13(Suppl 3):S45–S50. [PubMed] [Google Scholar]
- 2.Pearle MS, et al. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316–324. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–e96. [PMC free article] [PubMed] [Google Scholar]
- 4.Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic Diseases in America Project: urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer ME, et al. Temporal trends in incidence of kidney stones among children: a 25-year population based study. J Urol. 2012;188:247–252. doi: 10.1016/j.juro.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 8.West B, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Ohta Y, Suzuki KT. Methylation and demethylation of intermediates selenide and methylselenol in the metabolism of selenium. Toxicol Appl Pharmacol. 2008;226:169–177. doi: 10.1016/j.taap.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Sutor DJ, Scheidt S. Identification standards for human urinary calculus components, using crystallographic methods. Br J Urol. 1968;40:22–28. doi: 10.1111/j.1464-410x.1968.tb11808.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewandowski S, Rodgers AL. Idiopathic calcium oxalate urolithiasis: risk factors and conservative treatment. Clin Chim Acta. 2004;345:17–34. doi: 10.1016/j.cccn.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Evan AP, Coe FL, Lingeman JE, Worcester E. Insights on the pathology of kidney stone formation. Urol Res. 2005;33:383–389. doi: 10.1007/s00240-005-0488-0. [DOI] [PubMed] [Google Scholar]
- 13.Fleisch H. Inhibitors and promoters of stone formation. Kidney Int. 1978;13:361–371. doi: 10.1038/ki.1978.54. [DOI] [PubMed] [Google Scholar]
- 14.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 15.Słojewski M. Major and trace elements in lithogenesis. Cent European J Urol. 2011;64:58–61. doi: 10.5173/ceju.2011.02.art1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird ED, Thomas WC., Jr Effect of various metals on mineralization in vitro. Proc Soc Exp Biol Med. 1963;112:640–643. doi: 10.3181/00379727-112-28126. [DOI] [PubMed] [Google Scholar]
- 17.Caruso JA, Montes-Bayon M. Elemental speciation studies—new directions for trace metal analysis. Ecotoxicol Environ Saf. 2003;56:148–163. doi: 10.1016/s0147-6513(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 18.Meyer JL, Angino EE. The role of trace metals in calcium urolithiasis. Invest Urol. 1977;14:347–350. [PubMed] [Google Scholar]
- 19.Muñoz JA, Valiente M. Effects of trace metals on the inhibition of calcium oxalate crystallization. Urol Res. 2005;33:267–272. doi: 10.1007/s00240-005-0468-4. [DOI] [PubMed] [Google Scholar]
- 20.Nagy Z, Szabo E, Kelenhei M. Spectrum analysis of kidney calculi for metal trace elements [German] Z Urol. 1963;56:186–190. [PubMed] [Google Scholar]
- 21.Richet G. Nephrolithiasis at the turn of the 18th to 19th centuries: biochemical disturbances. A genuine cascade giving rise to clinical chemistry. Am J Nephrol. 2002;22:254–259. doi: 10.1159/000063770. [DOI] [PubMed] [Google Scholar]
- 22.Rarback H, et al. Elemental analysis using differential absorption techniques. Biol Trace Elem Res. 1987;13:103–113. doi: 10.1007/BF02796625. [DOI] [PubMed] [Google Scholar]
- 23.Morrison GH, Risby TH. Elemental trace analysis of biological materials. Crit Rev Anal Chem. 1979;8:287–320. [Google Scholar]
- 24.Parsons PJ, Barbosa F., Jr Atomic spectrometry and trends in clinical laboratory medicine. Spectrochim Acta Part B At Spectros. 2007;62:992–1003. [Google Scholar]
- 25.Thongboonkerd V. Proteomics and kidney stone disease. Contrib Nephrol. 2008;160:142–158. doi: 10.1159/000125972. [DOI] [PubMed] [Google Scholar]
- 26.Vezzoli G, Terranegra A, Arcidiacono T, Soldati L. Genetics and calcium nephrolithiasis. Kidney Int. 2011;80:587–593. doi: 10.1038/ki.2010.430. [DOI] [PubMed] [Google Scholar]
- 27.Gambaro G, et al. Genetics of hypercalciuria and calcium nephrolithiasis: from the rare monogenic to the common polygenic forms. Am J Kidney Dis. 2004;44:963–986. doi: 10.1053/j.ajkd.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Smith KS, Huyck HL. An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals. In: Plumlee GS, Logsdon MJ, Filipek LF, editors. The environmental geochemistry of mineral deposits: Part A, Processes, techniques, and health issues. 6A. Society of Economic Geologists; 1999. pp. 29–70. [Google Scholar]
- 29.Hamadeh MJ, Schiffrin A, Hoffer LJ. Sulfate production depicts fed-state adaptation to protein restriction in humans. Am J Physiol Endocrinol Metab. 2001;281:E341–E348. doi: 10.1152/ajpendo.2001.281.2.E341. [DOI] [PubMed] [Google Scholar]
- 30.Magee EA, Curno R, Edmond LM, Cummings JH. Contribution of dietary protein and inorganic sulfur to urinary sulfate: toward a biomarker of inorganic sulfur intake. Am J Clin Nutr. 2004;80:137–142. doi: 10.1093/ajcn/80.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Heilberg IP, Goldfarb DS. Optimum nutrition for kidney stone disease. Adv Chronic Kidney Dis. 2013;20:165–174. doi: 10.1053/j.ackd.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid–base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–274. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 33.Tschöpe W, Ritz E. Sulfur-containing amino acids are a major determinant of urinary calcium. Miner Electrolyte Metab. 1985;11:137–139. [PubMed] [Google Scholar]
- 34.Rodgers A, et al. Sulfate but not thiosulfate reduces calculated and measured urinary ionized calcium and supersaturation: implications for the treatment of calcium renal stones. PLoS ONE. 2014;9:e103602. doi: 10.1371/journal.pone.0103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faragalla FF, Gershoff SN. Interelations among magnesium, vitamin B6, sulfur and phosphorus in the formation of kidney stones in the rat. J Nutr. 1963;81:60–66. doi: 10.1093/jn/81.1.60. [DOI] [PubMed] [Google Scholar]
- 36.White RH. Occurrence of S-methyl thioesters in urines of humans after they have eaten asparagus. Science. 1975;189:810–811. doi: 10.1126/science.1162354. [DOI] [PubMed] [Google Scholar]
- 37.Borghi L, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 38.Romani AM. Cellular magnesium homeostasis. Arch Biochem Biophys. 2011;512:1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohri K, Garside J, Blacklock NJ. The role of magnesium in calcium oxalate urolithiasis. Br J Urol. 1988;61:107–115. doi: 10.1111/j.1464-410x.1988.tb05057.x. [DOI] [PubMed] [Google Scholar]
- 40.Oka T, Yoshioka T, Koide T, Takaha M, Sonoda T. Role of magnesium in the growth of calcium oxalate monohydrate and calcium oxalate dihydrate crystals. Urol Int. 1987;42:89–93. doi: 10.1159/000281861. [DOI] [PubMed] [Google Scholar]
- 41.Kato Y, et al. Changes in urinary parameters after oral administration of potassium-sodium citrate and magnesium oxide to prevent urolithiasis. Urology. 2004;63:7–11. doi: 10.1016/j.urology.2003.09.057. discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 42.Durak I, et al. Iron, copper, cadmium, zinc and magnesium contents of urinary tract stones and hair from men with stone disease. Eur Urol. 1990;17:243–247. doi: 10.1159/000464048. [DOI] [PubMed] [Google Scholar]
- 43.Kasaoka S, Kitano T, Hanai M, Futatsuka M, Esashi T. Effect of dietary magnesium level on nephrocalcinosis and growth in rats. J Nutr Sci Vitaminol (Tokyo) 1998;44:503–514. doi: 10.3177/jnsv.44.503. [DOI] [PubMed] [Google Scholar]
- 44.Schmiedl A, Schwille PO. Magnesium status in idiopathic calcium urolithiasis—an orientational study in younger males. Eur J Clin Chem Clin Biochem. 1996;34:393–400. doi: 10.1515/cclm.1996.34.5.393. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz BF, Bruce J, Leslie S, Stoller ML. Rethinking the role of urinary magnesium in calcium urolithiasis. J Endourol. 2001;15:233–235. doi: 10.1089/089277901750161638. [DOI] [PubMed] [Google Scholar]
- 46.Atakan IH, et al. Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. Int Urol Nephrol. 2007;39:351–356. doi: 10.1007/s11255-006-9050-4. [DOI] [PubMed] [Google Scholar]
- 47.Oreopoulos DG, Soyannwo MA, McGeown MG. Magnesium–calcium ratio in urine of patients with renal stones. Lancet. 1968;2:420–422. doi: 10.1016/s0140-6736(68)90464-9. [DOI] [PubMed] [Google Scholar]
- 48.Riley JM, Kim H, Averch TD, Kim HJ. Effect of magnesium on calcium and oxalate ion binding. J Endourol. 2013;27:1487–1492. doi: 10.1089/end.2013.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turgut M, et al. The concentration of Zn, Mg and Mn in calcium oxalate monohydrate stones appears to interfere with their fragility in ESWL therapy. Urol Res. 2008;36:31–38. doi: 10.1007/s00240-007-0133-1. [DOI] [PubMed] [Google Scholar]
- 50.Ettinger B, Citron JT, Livermore B, Dolman LI. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol. 1988;139:679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 51.Johansson G, et al. Effects of magnesium hydroxide in renal stone disease. J Am Coll Nutr. 1982;1:179–185. doi: 10.1080/07315724.1982.10718985. [DOI] [PubMed] [Google Scholar]
- 52.Massey L. Magnesium therapy for nephrolithiasis. Magnes Res. 2005;18:123–126. [PubMed] [Google Scholar]
- 53.Whelton PK, He J. Health effects of sodium and potassium in humans. Curr Opin Lipidol. 2014;25:75–79. doi: 10.1097/MOL.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 54.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 55.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290–2298. doi: 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 56.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 57.Meschi T, et al. Dietary habits in women with recurrent idiopathic calcium nephrolithiasis. J Transl Med. 2012;10:63. doi: 10.1186/1479-5876-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al Zahrani H, Norman RW, Thompson C, Weerasinghe S. The dietary habits of idiopathic calcium stone-formers and normal control subjects. BJU Int. 2000;85:616–620. doi: 10.1046/j.1464-410x.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 59.Massey LK, Whiting SJ. Dietary salt, urinary calcium, and kidney stone risk. Nutr Rev. 1995;53:131–139. doi: 10.1111/j.1753-4887.1995.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 60.Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982;22:292–296. doi: 10.1038/ki.1982.168. [DOI] [PubMed] [Google Scholar]
- 61.Burtis WJ, Gay L, Insogna KL, Ellison A, Broadus AE. Dietary hypercalciuria in patients with calcium oxalate kidney stones. Am J Clin Nutr. 1994;60:424–429. doi: 10.1093/ajcn/60.3.424. [DOI] [PubMed] [Google Scholar]
- 62.Silver J, Rubinger D, Friedlaender MM, Popovtzer MM. Sodium-dependent idiopathic hypercalciuria in renal-stone formers. Lancet. 1983;2:484–486. doi: 10.1016/s0140-6736(83)90513-5. [DOI] [PubMed] [Google Scholar]
- 63.Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009;20:2253–2259. doi: 10.1681/ASN.2009030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nouvenne A, et al. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2010;91:565–570. doi: 10.3945/ajcn.2009.28614. [DOI] [PubMed] [Google Scholar]
- 65.Stoller ML, Chi T, Eisner BH, Shami G, Gentle DL. Changes in urinary stone risk factors in hypocitraturic calcium oxalate stone formers treated with dietary sodium supplementation. J Urol. 2009;181:1140–1144. doi: 10.1016/j.juro.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 66.Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol. 1993;264:F181–F198. doi: 10.1152/ajprenal.1993.264.2.F181. [DOI] [PubMed] [Google Scholar]
- 67.Cirillo M, Laurenzi M, Panarelli W, Stamler J. Urinary sodium to potassium ratio and urinary stone disease. The Gubbio Population Study Research Group. Kidney Int. 1994;46:1133–1139. doi: 10.1038/ki.1994.376. [DOI] [PubMed] [Google Scholar]
- 68.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 69.Curhan GC. Dietary calcium, dietary protein, and kidney stone formation. Miner Electrolyte Metab. 1997;23:261–264. [PubMed] [Google Scholar]
- 70.Hirvonen T, Pietinen P, Virtanen M, Albanes D, Virtamo J. Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am J Epidemiol. 1999;150:187–194. doi: 10.1093/oxfordjournals.aje.a009979. [DOI] [PubMed] [Google Scholar]
- 71.Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993;150:1761–1764. doi: 10.1016/s0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 72.Preminger GM, Sakhaee K, Skurla C, Pak CY. Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis. J Urol. 1985;134:20–23. doi: 10.1016/s0022-5347(17)46963-1. [DOI] [PubMed] [Google Scholar]
- 73.King JC, et al. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr. 2001;74:116–124. doi: 10.1093/ajcn/74.1.116. [DOI] [PubMed] [Google Scholar]
- 74.Wu LN, Genge BR, Wuthier RE. Differential effects of zinc and magnesium ions on mineralization activity of phosphatidylserine calcium phosphate complexes. J Inorg Biochem. 2009;103:948–962. doi: 10.1016/j.jinorgbio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 75.LeGeros RZ, Bleiwas CB, Retino M, Rohanizadeh R, LeGeros JP. Zinc effect on the in vitro formation of calcium phosphates: relevance to clinical inhibition of calculus formation. Am J Dent. 1999;12:65–71. [PubMed] [Google Scholar]
- 76.Fujii E, et al. Selective protein adsorption property and characterization of nano-crystalline zinc-containing hydroxyapatite. Acta Biomater. 2006;2:69–74. doi: 10.1016/j.actbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Ren F, Xin R, Ge X, Leng Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009;5:3141–3149. doi: 10.1016/j.actbio.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Tang J, McFann K, Chonchol M. Dietary zinc intake and kidney stone formation: evaluation of NHANES III. Am J Nephrol. 2012;36:549–553. doi: 10.1159/000345550. [DOI] [PubMed] [Google Scholar]
- 79.Turney BW, et al. Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur J Epidemiol. 2014;29:363–369. doi: 10.1007/s10654-014-9904-5. [DOI] [PubMed] [Google Scholar]
- 80.Komleh K, Hada P, Pendse AK, Singh PP. Zinc, copper and manganese in serum, urine and stones. Int Urol Nephrol. 1990;22:113–118. doi: 10.1007/BF02549826. [DOI] [PubMed] [Google Scholar]
- 81.Durak I, Kilic Z, Sahin A, Akpoyraz M. Analysis of calcium, iron, copper and zinc contents of nucleus and crust parts of urinary calculi. Urol Res. 1992;20:23–26. doi: 10.1007/BF00294330. [DOI] [PubMed] [Google Scholar]
- 82.Gupta A, Srivastava DK, Kumar S. Role of zinc in nephrolithiasis. J Indian Med Assoc. 1984;82:235–237. [PubMed] [Google Scholar]
- 83.Ozgurtas T, Yakut G, Gulec M, Serdar M, Kutluay T. Role of urinary zinc and copper on calcium oxalate stone formation. Urol Int. 2004;72:233–236. doi: 10.1159/000077122. [DOI] [PubMed] [Google Scholar]
- 84.Chou AH, LeGeros RZ, Chen Z, Li Y. Antibacterial effect of zinc phosphate mineralized guided bone regeneration membranes. Implant Dent. 2007;16:89–100. doi: 10.1097/ID.0b013e318031224a. [DOI] [PubMed] [Google Scholar]
- 85.Winterbourn CC, Peskin AV, Parsons-Mair HN. Thiol oxidase activity of copper, zinc superoxide dismutase. J Biol Chem. 2002;277:1906–1911. doi: 10.1074/jbc.M107256200. [DOI] [PubMed] [Google Scholar]
- 86.Storrie H, Stupp SI. Cellular response to zinc-containing organoapatite: an in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials. 2005;26:5492–5499. doi: 10.1016/j.biomaterials.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 87.Hadley KB, Newman SM, Hunt JR. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J Nutr Biochem. 2010;21:297–303. doi: 10.1016/j.jnutbio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Cerovic A, et al. Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol Trace Elem Res. 2007;116:61–71. doi: 10.1007/BF02685919. [DOI] [PubMed] [Google Scholar]
- 89.Yang YJ, et al. Dietary zinc intake is inversely related to subclinical atherosclerosis measured by carotid intima-media thickness. Br J Nutr. 2010;104:1202–1211. doi: 10.1017/S0007114510001893. [DOI] [PubMed] [Google Scholar]
- 90.Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91:177–181. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 91.Ma X, Ellis DE. Initial stages of hydration and Zn substitution/occupation on hydroxyapatite (0001) surfaces. Biomaterials. 2008;29:257–265. doi: 10.1016/j.biomaterials.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Carpentier X, et al. High Zn content of Randall’s plaque: a μ-X-ray fluorescence investigation. J Trace Elem Med Biol. 2011;25:160–165. doi: 10.1016/j.jtemb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Kalicanin BM, Nikolić RS. Potentiometric stripping analysis of zinc and copper in human teeth and dental materials. J Trace Elem Med Biol. 2008;22:93–99. doi: 10.1016/j.jtemb.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Chi T, et al. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE. 2015;10:e0124150. doi: 10.1371/journal.pone.0124150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greger JL, Sutherland JE. Aluminum exposure and metabolism. Crit Rev Clin Lab Sci. 1997;34:439–474. doi: 10.3109/10408369709006422. [DOI] [PubMed] [Google Scholar]
- 96.Słojewski M, et al. Microelements in stones, urine, and hair of stone formers: a new key to the puzzle of lithogenesis? Biol Trace Elem Res. 2010;137:301–316. doi: 10.1007/s12011-009-8584-6. [DOI] [PubMed] [Google Scholar]
- 97.Pors Nielsen S. The biological role of strontium. Bone. 2004;35:583–588. doi: 10.1016/j.bone.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 98.Li ZY, et al. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials. 2007;28:1452–1460. doi: 10.1016/j.biomaterials.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Bazin D, et al. The status of strontium in biological apatites: an XANES/EXAFS investigation. J Synchrotron Radiat. 2014;21:136–142. doi: 10.1107/S1600577513023771. [DOI] [PubMed] [Google Scholar]
- 100.Bazin D, et al. The status of strontium in biological apatites: an XANES investigation. J Synchrotron Radiat. 2011;18:912–918. doi: 10.1107/S0909049511032651. [DOI] [PubMed] [Google Scholar]
- 101.Blaschko SD, et al. Strontium substitution for calcium in lithogenesis. J Urol. 2013;189:735–739. doi: 10.1016/j.juro.2012.08.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vezzoli G, et al. Strontium absorption and excretion in normocalciuric subjects: relation to calcium metabolism. Clin Chem. 1998;44:586–590. [PubMed] [Google Scholar]
- 103.Ammann P, Badoud I, Barraud S, Dayer R, Rizzoli R. Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res. 2007;22:1419–1425. doi: 10.1359/jbmr.070607. [DOI] [PubMed] [Google Scholar]
- 104.Ciftçioglu N, Bjorklund M, Kuorikoski K, Bergström K, Kajander EO. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 1999;56:1893–1898. doi: 10.1046/j.1523-1755.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 105.Wessing A, Zierold K. Metal-salt feeding causes alterations in concretions in Drosophila larval Malpighian tubules as revealed by X-ray microanalysis. J Insect Physiol. 1992;38:623–632. [Google Scholar]
- 106.Reginster JY, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 107.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 108.Meyer JL, Thomas WC., Jr Trace metal-citric acid complexes as inhibitors of calcification and crystal growth II Effects of Fe(III), Cr(III) and Al(III) complexes on calcium oxalate crystal growth. J Urol. 1982;128:1376–1378. doi: 10.1016/s0022-5347(17)53511-9. [DOI] [PubMed] [Google Scholar]
- 109.Meyer JL, Thomas WC., Jr Trace metal-citric acid complexes as inhibitors of calcification and crystal growth I Effects of Fe(III), Cr(III) and Al(III) complexes on calcium phosphate crystal growth. J Urol. 1982;128:1372–1375. doi: 10.1016/s0022-5347(17)53510-7. [DOI] [PubMed] [Google Scholar]
- 110.Bazin D, Chevallier P, Matzen G, Jungers P, Daudon M. Heavy elements in urinary stones. Urol Res. 2007;35:179–184. doi: 10.1007/s00240-007-0099-z. [DOI] [PubMed] [Google Scholar]
- 111.Joost J, Tessadri R. Trace element investigations in kidney stone patients. Eur Urol. 1987;13:264–270. doi: 10.1159/000472792. [DOI] [PubMed] [Google Scholar]
- 112.Küpeli S, et al. Efficiency of extracorporeal shockwave lithotripsy on calcium-oxalate stones: role of copper, iron, magnesium and zinc concentrations on disintegration of the stones. Eur Urol. 1993;23:409–412. doi: 10.1159/000474640. [DOI] [PubMed] [Google Scholar]
- 113.Naghii MR, Samman S. The role of boron in nutrition and metabolism. Prog Food Nutr Sci. 1993;17:331–349. [PubMed] [Google Scholar]
- 114.Benderdour M, Bui-Van T, Dicko A, Belleville F. In vivo and in vitro effects of boron and boronated compounds. J Trace Elem Med Biol. 1998;12:2–7. doi: 10.1016/S0946-672X(98)80014-X. [DOI] [PubMed] [Google Scholar]
- 115.Hunt CD. Dietary boron: progress in establishing essential roles in human physiology. J Trace Elem Med Biol. 2012;26:157–160. doi: 10.1016/j.jtemb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Nielsen FH. Update on human health effects of boron. J Trace Elem Med Biol. 2014;28:383–387. doi: 10.1016/j.jtemb.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 117.Hunt CD, Herbel JL, Nielsen FH. Metabolic responses of postmenopausal women to supplemental dietary boron and aluminum during usual and low magnesium intake: boron, calcium, and magnesium absorption and retention and blood mineral concentrations. Am J Clin Nutr. 1997;65:803–813. doi: 10.1093/ajcn/65.3.803. [DOI] [PubMed] [Google Scholar]
- 118.Naghii MR, Einollahi B, Rostami Z. Preliminary evidence hints at a protective role for boron in urolithiasis. J Altern Complement Med. 2012;18:207–209. doi: 10.1089/acm.2011.0865. [DOI] [PubMed] [Google Scholar]
- 119.Naghii MR. Boron and antioxidants complex: a new concept for the treatment of kidney stones without rigorous pain. Endocr Regul. 2014;48:120–125. doi: 10.4149/endo_2014_03_120. [DOI] [PubMed] [Google Scholar]
- 120.Guidotti TL. Toxicity and poisoning: the example of lead. Arch Environ Occup Health. 2014;69:64–65. doi: 10.1080/19338244.2014.811995. [DOI] [PubMed] [Google Scholar]
- 121.Sanín LH, González-Cossío T, Romieu I, Hernández-Avila M. Accumulation of lead in bone and its effects on health [Spanish] Salud Publica Mex. 1998;40:359–368. [PubMed] [Google Scholar]
- 122.Turnlund JR. Human whole-body copper metabolism. Am J Clin Nutr. 1998;67:960S–964S. doi: 10.1093/ajcn/67.5.960S. [DOI] [PubMed] [Google Scholar]
- 123.Tawashi R, Cousineau M. Growth retardation of weddellite (calcium oxalate dihydrate) by sodium copper chlorophyllin. Invest Urol. 1980;18:86–89. [PubMed] [Google Scholar]
- 124.Tawashi R, Cousineau M, Denis G. Crystallisation of calcium oxalate dihydrate in normal urine in presence of sodium copper chlorophyllin. Urol Res. 1982;10:173–176. doi: 10.1007/BF00255940. [DOI] [PubMed] [Google Scholar]
- 125.Desjardins A, Tawashi R. Growth retardation of calcium oxalate by sodium copper chlorophyllin. Eur Urol. 1978;4:294–297. doi: 10.1159/000473975. [DOI] [PubMed] [Google Scholar]
- 126.Tawashi R, Cousineau M, Sharkawi M. Effect of sodium copper chlorophyllin on the formation of calcium oxalate crystals in rat kidney. Invest Urol. 1980;18:90–92. [PubMed] [Google Scholar]
- 127.Sarkar B. Nickel metabolism. IARC Sci Publ. 1984;53:367–384. [PubMed] [Google Scholar]
- 128.Hofbauer J, et al. Trace elements and urinary stone formation: new aspects of the pathological mechanism of urinary stone formation. J Urol. 1991;145:93–96. doi: 10.1016/s0022-5347(17)38256-3. [DOI] [PubMed] [Google Scholar]
- 129.Nielsen FH. Update on the possible nutritional importance of silicon. J Trace Elem Med Biol. 2014;28:379–382. doi: 10.1016/j.jtemb.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 130.Carlisle EM. The nutritional essentiality of silicon. Nutr Rev. 1982;40:193–198. doi: 10.1111/j.1753-4887.1982.tb05307.x. [DOI] [PubMed] [Google Scholar]
- 131.Najda J, Gmiński J, Drózdz M, Danch A. The action of excessive, inorganic silicon (Si) on the mineral metabolism of calcium (Ca) and magnesium (Mg) Biol Trace Elem Res. 1993;37:107–114. doi: 10.1007/BF02783786. [DOI] [PubMed] [Google Scholar]
- 132.Haddad FS, Kouyoumdjian A. Silica stones in humans. Urol Int. 1986;41:70–76. doi: 10.1159/000281165. [DOI] [PubMed] [Google Scholar]
- 133.May M, et al. Silica-containing urinary stones —clinical issues to keep in mind [German] Urologe A. 2005;44:68–72. doi: 10.1007/s00120-004-0730-3. [DOI] [PubMed] [Google Scholar]
- 134.Zhang X, et al. The role of lithium carbonate and lithium citrate in regulating urinary citrate level and preventing nephrolithiasis. Int J Biomed Sci. 2009;5:215–222. [PMC free article] [PubMed] [Google Scholar]
- 135.Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969;49:163–239. doi: 10.1152/physrev.1969.49.2.163. [DOI] [PubMed] [Google Scholar]
- 136.Anderson RA, et al. Effects of chromium supplementation on urinary Cr excretion of human subjects and correlation of Cr excretion with selected clinical parameters. J Nutr. 1983;113:276–281. doi: 10.1093/jn/113.2.276. [DOI] [PubMed] [Google Scholar]
- 137.Lim TH, Sargent T, 3rd, Kusubov N. Kinetics of trace element chromium(III) in the human body. Am J Physiol. 1983;244:R445–R454. doi: 10.1152/ajpregu.1983.244.4.R445. [DOI] [PubMed] [Google Scholar]
- 138.Spinedi A, Pacini L, Luly P, Lombardi U, Nisticò G. Rubidium shows effects different from lithium on phosphatidylinositol metabolism in a cell line of human neuroblastoma. Funct Neurol. 1992;7:305–308. [PubMed] [Google Scholar]
- 139.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leach RM, Jr, Gay CV. Role of epiphyseal cartilage in endochondral bone formation. J Nutr. 1987;117:784–790. doi: 10.1093/jn/117.4.784. [DOI] [PubMed] [Google Scholar]
- 141.Ponnapakkam T, Iszard M, Henry-Sam G. Effects of oral administration of manganese on the kidneys and urinary bladder of Sprague-Dawley rats. Int J Toxicol. 2003;22:227–232. doi: 10.1080/10915810305103. [DOI] [PubMed] [Google Scholar]
- 142.Trinchieri A, Mandressi A, Luongo P, Longo G, Pisani E. The influence of diet on urinary risk factors for stones in healthy subjects and idiopathic renal calcium stone formers. Br J Urol. 1991;67:230–236. doi: 10.1111/j.1464-410x.1991.tb15124.x. [DOI] [PubMed] [Google Scholar]
- 143.French RJ, Jones PJ. Role of vanadium in nutrition: metabolism, essentiality and dietary considerations. Life Sci. 1993;52:339–346. doi: 10.1016/0024-3205(93)90146-t. [DOI] [PubMed] [Google Scholar]
- 144.Heinrich HC, Gabbe EE. Metabolic behavior of inorganic cobalt and of vitamin B-12 as well as vitamin B-12 coenzyme structure of organically bound cobalt in mammals [German] Z Naturforsch B. 1964;19:1032–1042. [PubMed] [Google Scholar]
- 145.Sakly R, Chaouch A, el Hani A, Najjar MF. Effects of intraperitoneally administered vitamin E and selenium on calcium oxalate renal stone formation: experimental study in rat. Ann Urol (Paris) 2003;37:47–50. doi: 10.1016/s0003-4401(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 146.Santhosh Kumar M, Selvam R. Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem. 2003;14:306–313. doi: 10.1016/s0955-2863(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 147.Lahme S, Feil G, Strohmaier WL, Bichler KH, Stenzl A. Renal tubular alteration by crystalluria in stone disease —an experimental study by means of MDCK cells. Urol Int. 2004;72:244–251. doi: 10.1159/000077124. [DOI] [PubMed] [Google Scholar]
- 148.Mendel RR. Metabolism of molybdenum. Met Ions Life Sci. 2013;12:503–528. doi: 10.1007/978-94-007-5561-1_15. [DOI] [PubMed] [Google Scholar]
- 149.Karring M, Pohjanvirta R, Rahko T, Korpela H. The influence of dietary molybdenum and copper supplementation on the contents of serum uric acid and some trace elements in cocks. Acta Vet Scand. 1981;22:289–295. doi: 10.1186/BF03548654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Deosthale YG, Gopalan C. The effect of molybdenum levels in sorghum (Sorghum vulgare Pers) on uric acid and copper excretion in man. Br J Nutr. 1974;31:351–355. doi: 10.1079/bjn19740043. [DOI] [PubMed] [Google Scholar]
- 151.Shen S, Li XF, Cullen WR, Weinfeld M, Le XC. Arsenic binding to proteins. Chem Rev. 2013;113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Uthus EO. Arsenic essentiality: a role affecting methionine metabolism. J Trace Elem Exper Med. 2003;16:345–355. [Google Scholar]
- 153.Nawrot TS, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 2010;23:769–782. doi: 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- 154.Järup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- 155.Friberg L. Health hazards in the manufacture of alkaline accumulators with special reference to chronic cadmium poisoning; a clinical and experimental study. Acta Med Scand Suppl. 1950;240:1–124. [PubMed] [Google Scholar]
- 156.Elinder CG, Edling C, Lindberg E, Kågedal B, Vesterberg O. Assessment of renal function in workers previously exposed to cadmium. Br J Ind Med. 1985;42:754–760. doi: 10.1136/oem.42.11.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scott R, Paterson PJ, Burns R, Fell GS, Ottoway JM. The effects of treatment on the hypercalciuria of chronic cadmium poisoning. Urol Res. 1979;7:285–289. doi: 10.1007/BF00256610. [DOI] [PubMed] [Google Scholar]
- 158.Ferraro PM, et al. Cadmium exposure and kidney stone formation in the general population—an analysis of the National Health and Nutrition Examination Survey III data. J Endourol. 2011;25:875–880. doi: 10.1089/end.2010.0572. [DOI] [PubMed] [Google Scholar]
- 159.Swaddiwudhipong W, et al. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environ Res. 2012;112:194–198. doi: 10.1016/j.envres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Kaewnate Y, et al. Association of elevated urinary cadmium with urinary stone, hypercalciuria and renal tubular dysfunction in the population of cadmium-contaminated area. Bull Environ Contam Toxicol. 2012;89:1120–1124. doi: 10.1007/s00128-012-0856-8. [DOI] [PubMed] [Google Scholar]
- 161.Thomas LD, Elinder CG, Tiselius HG, Wolk A, Akesson A. Dietary cadmium exposure and kidney stone incidence: a population-based prospective cohort study of men & women. Environ Int. 2013;59:148–151. doi: 10.1016/j.envint.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 162.Chen X, et al. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone. 2014;63:76–80. doi: 10.1016/j.bone.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 163.Bhattacharyya MH. Cadmium osteotoxicity in experimental animals: mechanisms and relationship to human exposures. Toxicol Appl Pharmacol. 2009;238:258–265. doi: 10.1016/j.taap.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Perk H, Serel TA, Koşar A, Deniz N, Sayin A. Analysis of the trace element contents of inner nucleus and outer crust parts of urinary calculi. Urol Int. 2002;68:286–290. doi: 10.1159/000058452. [DOI] [PubMed] [Google Scholar]
- 165.Kappas A, Maines MD. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976;192:60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- 166.Esposito M, et al. Plasma and tissue levels of some lanthanide elements in malignant and non-malignant human tissues. Sci Total Environ. 1986;50:55–63. doi: 10.1016/0048-9697(86)90351-7. [DOI] [PubMed] [Google Scholar]
- 167.Sojka B, et al. Hydrophobic sodium fluoride-based nanocrystals doped with lanthanide ions: assessment of in vitro toxicity to human blood lymphocytes and phagocytes. J Appl Toxicol. 2014;34:1220–1225. doi: 10.1002/jat.3050. [DOI] [PubMed] [Google Scholar]
- 168.de Freitas D, Donne RL, Hutchison AJ. Lanthanum carbonate--a first line phosphate binder? Semin Dial. 2007;20:325–328. doi: 10.1111/j.1525-139X.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 169.Barton Pai A, Conner TA, McQuade CR. Therapeutic use of the phosphate binder lanthanum carbonate. Expert Opin Drug Metab Toxicol. 2009;5:71–81. doi: 10.1517/17425250802614886. [DOI] [PubMed] [Google Scholar]
- 170.Guo H, Zhang X, Tang S, Zhang S. Effects and safety of lanthanum carbonate in end stage renal disease patients with hyperphosphatemia: a meta-analysis—system review of lanthanum carbonate. Ren Fail. 2013;35:1455–1464. doi: 10.3109/0886022X.2013.828365. [DOI] [PubMed] [Google Scholar]
- 171.Cameron MA, Sakhaee K, Moe OW. Nephrolithiasis in children. Pediatr Nephrol. 2005;20:1587–1592. doi: 10.1007/s00467-005-1883-z. [DOI] [PubMed] [Google Scholar]
- 172.Tandon L, Iyengar GV, Parr RM. A review of radiologically important trace elements in human bones. Appl Radiat Isot. 1998;49:903–910. doi: 10.1016/s0969-8043(97)10102-6. [DOI] [PubMed] [Google Scholar]
- 173.Tietz NW. Clinical guide to laboratory tests. Saunders; 1995. [Google Scholar]
- 174.Moshfegh A, Goldman J, Cleveland L. What we eat in America, NHANES 2001–2002: usual nutrient intakes from food compared to dietary reference intakes. US Department of Agriculture, Agriculture Research Service; 2005. [online], http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/0102/usualintaketables2001-02.pdf. [Google Scholar]
- 175.Briefel RR, et al. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. J Nutr. 2000;130:1367S–1373S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- 176.Abdel-Gawad M, Ali-El-Dein B, Mehta S, Al-Kohlany KM, Elsobky E. A correlation study between macro- and micro-analysis of pediatric urinary calculi. J Pediatr Urol. 2014;10:1267–1272. doi: 10.1016/j.jpurol.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 177.Ohta N. Studies on inorganic constituents in biological materials—On the inorganic constituents in human stones. Bull Chem Soc Jpn. 1957;30:833–841. [Google Scholar]
- 178.Levinson AA, et al. Trace elements in kidney stones from three areas in the United States. Invest Urol. 1978;15:270–274. [PubMed] [Google Scholar]
- 179.Wandt MA, Pougnet MA, Rodgers AL. Determination of calcium, magnesium and phosphorus in human stones by inductively coupled plasma atomic-emission spectroscopy. Analyst. 1984;109:1071–1074. doi: 10.1039/an9840901071. [DOI] [PubMed] [Google Scholar]
- 180.Durak I, Yasar A, Yurtarslani Z, Akpoyraz M, Tasman S. Analysis of magnesium and trace elements in urinary calculi by atomic absorption spectrophotometry. Br J Urol. 1988;62:203–205. doi: 10.1111/j.1464-410x.1988.tb04318.x. [DOI] [PubMed] [Google Scholar]
- 181.Wandt MA, Underhill LG. Covariance biplot analysis of trace element concentrations in urinary stones. Br J Urol. 1988;61:474–481. doi: 10.1111/j.1464-410x.1988.tb05083.x. [DOI] [PubMed] [Google Scholar]
- 182.Durak I, Akpoyraz M, Sahin A. Sodium, potassium and chloride concentrations in the inner nucleus and outer crust parts of urinary tract calculi. Int Urol Nephrol. 1991;23:221–226. doi: 10.1007/BF02550415. [DOI] [PubMed] [Google Scholar]
- 183.Höbarth K, Koeberl C, Hofbauer J. Rare-earth elements in urinary calculi. Urol Res. 1993;21:261–264. doi: 10.1007/BF00307707. [DOI] [PubMed] [Google Scholar]
- 184.Fang X, Ahmad SR, Mayo M, Iqbal S. Elemental analysis of urinary calculi by laser induced plasma spectroscopy. Lasers Med Sci. 2005;20:132–137. doi: 10.1007/s10103-005-0356-8. [DOI] [PubMed] [Google Scholar]
- 185.Abboud IA. Concentration effect of trace metals in Jordanian patients of urinary calculi. Environ Geochem Health. 2008;30:11–20. doi: 10.1007/s10653-007-9103-3. [DOI] [PubMed] [Google Scholar]
- 186.Giannossi ML, Summa V, Mongelli G. Trace element investigations in urinary stones: a preliminary pilot case in Basilicata (Southern Italy) J Trace Elem Med Biol. 2013;27:91–97. doi: 10.1016/j.jtemb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 187.Keshavarzi B, et al. Trace elements in urinary stones: a preliminary investigation in Fars province, Iran. Environ Geochem Health. 2015;37:377–389. doi: 10.1007/s10653-014-9654-z. [DOI] [PubMed] [Google Scholar]