Abstract
Neisseria gonorrhoeae , the causative agent of the sexually transmitted infection, gonorrhea, has developed resistance to most conventional antibiotics. Safe and effective vaccines against gonorrhea are needed urgently. A candidate vaccine that targets a lipooligosaccharide (LOS) epitope recognized by monoclonal (mAb) 2C7 attenuates gonococcal burden in the mouse vaginal colonization model. Glycan extensions from the LOS core heptoses (HepI and HepII) are controlled by phase-variable LOS glycosyltransferase (lgt) genes; we sought to define how HepI glycan extensions affect mAb 2C7 function. Isogenic gonococcal mutants in which the lgt required for mAb 2C7 reactivity (lgtG) was genetically locked ‘ON’ and the lgt loci required for HepI variation (lgtA, lgtC and lgtD) were genetically locked ‘ON’ or ‘OFF’ in different combinations were created. We observed 100% complement-dependent killing by mAb 2C7 of a mutant that expressed lactose (Gal-Glc) from HepI, while a mutant that expressed Gal-Gal-Glc-HepI fully resisted killing (>100% survival). Mutants that elaborated 4- (Gal-GlcNAc-Gal-Glc-HepI) and 5-glycan (GalNAc-Gal-GlcNAc-Gal-Glc-HepI) structures displayed ‘intermediate’ phenotypes (<50% killing with 2 μg/ml and >95% killing with 4 μg/ml of mAb 2C7). The contrasting phenotypes of the lactose-HepI and the Gal-Gal-Glc-HepI LOS structures were recapitulated with phase-variants of a recently isolated clinical strain. Despite lack of killing of the Gal-Gal-Glc-HepI mutants, mAb 2C7 deposited sufficient C3 on these bacteria for opsonophagocytic killing by human neutrophils. In conclusion, mAb 2C7 showed functional activity against all gonococcal HepI LOS structures defined by various lgtA/C/D ‘ON/OFF’ combinations, thereby providing further impetus for use of the 2C7 epitope in a gonococcal vaccine.
Keywords: Neisseria gonorrhoeae, vaccine, lipooligosaccharide, complement, opsonophagocytosis
Introduction
Gonorrhea, caused by the gram-negative diplococcus Neisseria gonorrhoeae (the gonococcus) is the most common bacterial sexually transmitted infection (STI) worldwide (2nd most common in the U.S.). While most cases result in ‘uncomplicated’ infections of the lower genital tract (urethritis in men and cervicitis in women), gonorrhea may sometimes lead to complications such as pelvic inflammatory disease and disseminated gonococcal infection. Serious sequelae of gonorrhea include infertility and ectopic pregnancy. Infected individuals who are asymptomatic or minimally symptomatic constitute an important reservoir for the transmission of infection.
Globally, about 78 million new cases of gonorrhea occur annually (1). As a result of the emergence of antibiotic resistant strains, including strains resistant to third-generation cephalosporins such as cefixime and ceftriaxone (2) and the lack of vaccines (3, 4) or novel anti-infective therapeutics, gonorrhea has become a major public health concern. A safe and effective vaccine would be a key step in curbing the spread of multidrug-resistant gonorrhea.
An obstacle to gonococcal vaccine development is the wide antigenic variation and/or variable expression of antigens that may elicit a protective response (e.g., pilin, opacity proteins, porin (Por) B, lipooligosacharides [LOSs]) (3–5). In addition, certain conserved antigens elicit non-protective, and in some instances subversive responses; an example of the latter is Reduction modifiable protein (Rmp) (6).
Despite its phase-variable nature (7), gonococcal LOS has been considered as a potential vaccine antigen (8, 9). Men who were experimentally infected with N. gonorrhoeae were less likely to become infected upon rechallenge if they elicited an anti-LOS IgG response following the initial infection (10). Previous work by our group identified an epitope on gonococcal LOS that is recognized by a monoclonal antibody (mAb) called 2C7 (and therefore referred to as the ‘2C7 epitope) and was expressed on 94% of gonococci (64 out of 68) recovered directly from human cervical secretions (11). Gonococcal infection in humans elicits an antibody response against the 2C7 epitope (11). Expression of a lactose residue from heptose (Hep) II is required for binding of mAb 2C7 (12). Addition of an α-linked Glc residue at the 3-position of HepII represents the first step in synthesis of the lactose extension from HepII and is mediated by the phase-variable LOS glycosyltransferase G (lgtG) (13).
Expression of LgtG is important for murine infection (14). Passive administration of mAb 2C7, as well as active immunization with a peptide mimic (mimitope) of the 2C7 epitope that was configured as a ‘multi-antigen peptide’ on a poly-lysine ‘backbone’ significantly shortened the duration and burden of infection in the murine vaginal colonization model of gonorrhea (14). Taken together, these data suggest that the 2C7 epitope represents a promising gonococcal vaccine candidate.
Phase variation of LOS glycan extensions is mediated by slipped-strand mispairing at homopolymeric tracts within the coding regions of the lgt genes; lgtA, lgtC, lgtD modify glycan extensions from HepI; lgtG permits glycan extensions from HepII, as discussed above. Phase variation permits gonococci to express several distinct LOS structures that differ in their glycan composition (7, 15). Modulation of mAb 2C7 function by variations in HepI glycans has not been studied, is an important consideration that may impact the efficacy of a 2C7 epitope-based vaccine and forms the basis of this study.
Materials and Methods
Bacterial strains and culture conditions
The Neisserial strains used in this study are described in Table 1. N. gonorrhoeae MS11 4/3/1 is a variant of MS11 VD300 with an IPTG inducible pilE that controls pilus expression (16). UMNJ60_06UM was recovered in 2013 from a symptomatic male with urethritis in Nanjing, PRC (17), and shows intermediate resistance to ceftriaxone (Etest MIC = 0.38 μg/ml and disc = 35 mm (sensitive ≥ 35mm). UMNJ60_06UM belongs to NG-MAST sequence type (ST) 3289 and MLST ST 1600.
Table 1.
Bacterial strains used in this study
| Strain | Expected LOS phenotype | Description | Ref. |
|---|---|---|---|
| MS11 4/3/1 | LOS structure not defined | MS11 with an IPTG inducible pilE | (16) |
|
| |||
| Derivatives of MS11 4/3/1 | |||
|
| |||
| 5-Hex/G+ | GalNAc-Gal-GlcNAc-Gal-Glc-HepI Gal-Glc-HepII |
lgtA-ON A lgtD-ON B lgtC-OFF C lgtG-ON D |
This study |
|
| |||
| 5-Hex/G− | GalNAc-Gal-GlcNAc-Gal-Glc-HepI Unsubstituted HepII |
lgtA-ON A lgtD-ON B lgtC-OFF C lgtG::kan |
This study |
|
| |||
| 4-Hex/G+ | Gal-GlcNAc-Gal-Glc-HepI Gal-Glc-HepII |
lgtA-ON A lgtD-del E lgtC-OFF C lgtG-ON D |
This study |
|
| |||
| 4-Hex/G− | Gal-GlcNAc-Gal-Glc-HepI Unsubstituted HepII |
lgtA-ON A lgtD-del E lgtC-OFF C lgtG::kan |
This study |
|
| |||
| 3-Hex/G+ | Gal-Gal-Glc-HepI Gal-Glc-HepII |
lgtA-del F lgtC-ON G lgtG-ON D |
This study |
|
| |||
| 3-Hex/G− | Gal-Gal-Glc-HepI Unsubstituted HepII |
lgtA-del F lgtC-ON G lgtG::kan |
This study |
|
| |||
| 2-Hex/G+ | Gal-Glc-HepI Gal-Glc-HepII |
lgtA-del F lgtC-OFF C lgtG-ON D |
This study |
|
| |||
| 2-Hex/G− | Gal-Glc-HepI Unsubstituted HepII |
lgtA-del F lgtC-OFF C lgtG::kan |
This study |
|
| |||
| 2 → 3-Hex/G+ | Gal-Gal-Glc-HepI; Gal-Glc-HepII |
2-Hex/G+ with lgtC-ON G | This study |
|
| |||
| 3 → 2-Hex/G+ | Gal-Glc-HepI Gal-Glc-HepII |
3-Hex/G+ with lgtC-OFF C | This study |
|
| |||
| UMNJ60_06UM | LOS structure not defined | Nanjing, PRC 2013; symptomatic male with urethritis. Intermediate resistance to ceftriaxone (E-test MIC = 0.38 μg/ml and disc = 35 mm (sensitive ≥ 35mm) | (17) |
| Derivatives of UMNJ60_06UM | |||
|
| |||
| UMNJ60 2-Hex | Gal-Glc-HepI Gal-Glc-HepII |
UMNJ60_06UM lgtA::kan; expresses 2-Hex on HepI | This study |
|
| |||
| UMNJ60 3-Hex | Gal-Gal-Glc-HepI Gal-Glc-HepII |
UMNJ60_06UM lgtA::kan; expresses 3-Hex on HepI | This study |
lgtA-ON; G12 → GGGCGGAGGTGG
lgtD-ON; G13 → GGGCGGAGGTG
lgtC-OFF; G14 → GGTGAGGGGGGGGG
lgtG-ON; C11 → CCCCTCCGCCA
lgtD-del; 744 base pair (64 – 808 of coding sequence) deletion from lgtD
lgtA-del; 417 base pair (50 – 467 of coding sequence) deletion from lgtA
lgtC-ON; G14 → GGGGCGGAGG
Gonococcal strains were routinely cultured at 37°C in an atmosphere of 5% CO2 on chocolate agar enriched with a chemically defined supplement (termed isovitalex) used as an additive for cultivation of nutritionally fastidious microorganisms. For growth in liquid culture Morse A supplemented with Morse B and isovitalex were used (18). When used, antibiotics were added to GC agar plates at the following concentrations: erythromycin (Erm) 5 μg/ml, kanamycin (Kan) 100 μg/ml and streptomycin (Sm) 10 mg/ml. To induce pilus expression and enable transformation, strain MS11 4/3/1 was cultured on GC agar plates supplemented with 0.25 mM isopropyl-beta-D-thiogalactopyranoside (IPTG).
E. coli Top10, XL-10 gold and INVαF′ (Invitrogen) were cultured on LB agar supplemented, as needed, with antibiotics at the following concentrations; ampicillin (125 μg/ml), Kan (50 μg/ml), Erm (400 μg/ml) or Cm (50 μg/ml). INVαF′, a naturally streptomycin sensitive strain, was used for propagation of all plasmids containing the ErmR-SmS streptomycin sensitivity cassette.
Construction of mutants
We created eight LOS mutants in MS11 4/3/1 (Table 1), in which expression of the four phase variable lgt genes (lgtG, lgtA, lgtC and lgtD [shown schematically in Fig. 1A]) was genetically fixed either ‘ON’ or ‘OFF’ (or deleted) .
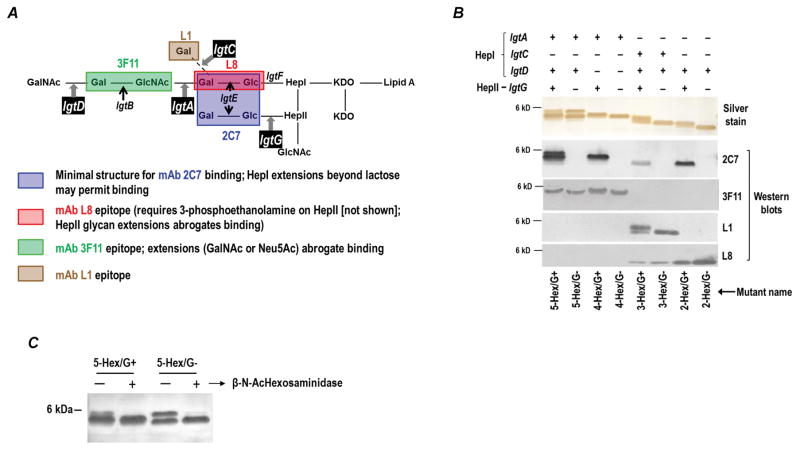
Figure 1.
Characterization of the LOS of the MS11 mutants used in this study. A. Schematic representation of gonococcal LOS, relevant LOS glycosyltransferase (lgt) genes involved in its biosynthesis and the glycan structures recognized by anti-LOS mAbs. The four phase variable genes involved in glycan extensions lgtA, C, D and G are shown in black boxes. Specific structural requirements for binding of each of the four anti-LOS mAbs are indicated below the figure. B. Phenotypic characterization of the LOS mutants. Proteinase K-treated bacterial lysates were separated on 12% Bis-tris gels and subsequently either stained with silver or transferred to PVDF by Western blotting and probed with specific anti-LOS mAbs. The genotypes of the mutants are indicated above the silver stain, while the simplified nomenclature for each of the mutants is indicated below the mAb L8 western blot. C. β-N-acetyl hexosaminidase treatment of 5-Hex mutants results in loss of the highest molecular mass LOS species. Bacterial lysates were treated with β-N-acetyl hexosaminidase (lanes marked “+”) or buffer alone (lanes marked “−”) and LOS was separated on 16.5% Criterion™ Tricine gel (Bio-Rad) and visualized by silver staining.
lgtG was insertionally inactivated (G− mutants; HepII unsubstituted) by amplifying lgtG::kan from FA19 lpt6A lptA lgtG (kindly provided by Dr. William Shafer, Emory University) using lgtG_F and lgtG_R primers (Supplemental Table S1), and subsequently transforming MS11 4/3/1 with the purified PCR product. The kanamycin marker in lgtG in FA19 was derived from pCK49 (19). Inactivation of lgtG in kanamycin resistant MS11 transformants was confirmed by PCR and DNA sequencing.
LgtG was fixed ‘ON’ (G+ mutants; HepII substituted with lactose) by first exchanging the wild type lgtG with lgtG containing the ermC′-rpsLF62 cassette (pRYGW2ES1; Supplemental Table S2) that encodes resistance to Erm and sensitivity to Sm (20). Erm-resistant transformants were subsequently transformed with an lgtG-‘ON’ construct (plgtG+; Supplemental Table S2) in which the C11 homopolymer had been changed to the non-phase variable sequence CCCCTCCGCCA. lgtG-‘ON’ (G+) mutants were selected for resistance to streptomycin and screened for sensitivity to erythromycin (20).
HepI glycan mutants were made in both the MS11 G+ and G− backgrounds, by first exchanging each of the three phase variable HepI lgt genes (lgtA, lgtC, lgtD) with an ermC′-rpsLF62 cassette (plgtA-ES, plgtC-ES, plgtD-ES; Supplemental Table S2), followed by transformation with the respective locked ‘ON’ (plgtA-’ON’, plgtC-’ON’, plgtD-’ON’; Supplemental Table S2), locked ‘OFF’ (plgtC-’OFF’; Supplemental Table S2) or mutated (segment deleted) form of each gene (plgtA-del and plgtD-del; Supplemental Table S2).
To insert the ermC′-rpsLF62 cassette into each lgt, the homopolymeric phase variation sequence in each lgt was deleted and a SmaI restriction site was incorporated by overlap extension PCR (using the respective F-Ext/ R-Int and F-Int / R-Ext primers; Supplemental Table S1). Each mutated (homopolymer deleted and SmaI incorporated)) lgt was amplified (F-Ext and R-Ext; Supplemental Table S1) and cloned (separately) into pCR2.1 TOPO TA (Invitrogen, USA). The ermC′-rpsLF62 cassette was extracted from pFLOB4300 (provided by Dr. Janne G. Cannon, University of North Carolina, Chapel Hill)) with pvuII and inserted into the SmaI site of each phase variable HepI lgt gene (See plasmids plgtA-ES, plgtC-ES and plgtD-ES; Supplemental Table S2). Plasmids carrying ermC′-rpsLF62 were maintained in the streptomycin sensitive E. coli INVαF′ (Life Technologies, USA).
Wildtype lgtA, lgtC and lgtD were amplified from MS11 4/3/1 chromosomal DNA by PCR using the corresponding F-Ext and R-Ext primers (Supplemental Table S1) and the amplicons ligated with pCR2.1 TOPO TA cloning vector (Life Technologies, USA) and transformed into chemically competent E. coli TOP10 (Life Technologies, USA) per the manufacturer’s instructions (Supplemental Table S2). Plasmids with lgtA, lgtC and lgtD locked ‘ON’ and lgtC locked ‘OFF’ were generated using Quick Change Lightning Multi Site-Directed Mutagenesis kit (Agilent Technologies, USA) with the corresponding mutagenic primers (Supplemental Table S1) and transformed into chemically competent XL-10 Gold E. coli cells as per manufacturer’s recommendations (Supplemental Table S2). Double digestion and subsequent ligation of plasmids with wild type lgtA and lgtD with BbsI and SspI, and NotI and SpeI respectively, yielded plasmids with deletion mutations in lgtA and lgtD (Supplemental Table S2).
Replacement of the lgtC-locked ‘ON’ gene in the mutant that expressed the 3-Hex HepI/lgtG+ LOS structure with lgtC locked-’OFF’ yielded the 2-Hex-HepI/lgtG+ mutant. Conversely, locking lgtC ‘ON’ in the 2-Hex-HepI/lgtG+ mutant yielded a lgtG+ mutant that expressed 3-Hex from HepI.
UMNJ60_06UM lgtA::kan was constructed as previously described (21). Inactivation of lgtA was confirmed by PCR and western blot using mAb 3F11 (mAb 3F11 described below). UMNJ60_06UM lgtA::kan 2-Hex and UMNJ60_06UM lgtA::kan 3-Hex were identified by western blot; UMNJ60_06UM lgtA::kan 2-Hex reacted with mAb L8 but not mAb L1 (both mAbs are described below) and UMNJ60_06UM lgtA::kan 3-Hex reacted with mAb L1 (recognizes the globotriose Galα1,4-Galβ1,4-Glc structure, also called the PK-like structure) but not mAb L8 (data not shown). All UMNJ60_06UM strains reacted with mAb 2C7 by western blot (data not shown) and by flow cytometry (see Results).
Mass spectrometry
Fresh chocolate agar plates were inoculated with bacteria harvested from cultures grown overnight for 15 h and bacteria were grown for 6 h. LOS was extracted, de-O-acylated and analyzed by MS as described previously (22).
Antibodies
Anti-LOS mAbs 2-1-L8 (henceforth referred to as mAb L8) (23), 17-1-L1 (referred to as mAb L1) (24), 3F11 (25) and 2C7 (11) have been described previously. A schematic of the epitopes recognized by these mAbs is provided in Fig. 1. mAb 2C7 was purified from tissue culture supernatants over protein A/G (Pierce). Affinity-isolated goat anti-human factor H (FH) was prepared from anti-FH antiserum (Complement Technology, Inc., Tyler, TX) by passage over FH-sepharose as described previously (26). Alkaline phosphatase conjugated anti-mouse IgG and anti-mouse IgM, and FITC-conjugated anti-mouse IgG and anti-goat IgG were from Sigma. mAb 104 that binds to domains 1 and 2 of the α chain of human C4b-binding protein (C4BP) (27) was provided by Dr. Anna M. Blom (Lund University, Malmö, Sweden). mAb 104 blocks C4BP function (27) and also blocks C4BP binding to gonococcal PorB (28) when pre-incubated with serum. However, mAb 104 does not displace C4BP already bound to the gonococcal surface and was used as the detection reagent for C4BP binding, as previously described (28). C3 deposited on gonococci was detected with FITC-conjugated anti-human C3c (AbD Serotec / BioRad), which detects both C3b as well as iC3b, at a dilution of 1:100. In order to demonstrate that mAb 104 blocked C4BP binding to bacteria, complement was incubated with mAb 104 (9 μg of mAb 104 was added to 30 μl of complement) on ice for 10 min, added to bacteria. C4BP bound to bacteria was detected with anti-human C4BP mAb 67 (provided by Dr. Anna M. Blom) that recognizes domain 4 of the α chain of C4BP, followed by anti-mouse IgG A647 (Sigma) both at a dilution of 1:100.
SDS-PAGE and Western blotting
Protease K-digested bacterial lysates were separated on 12% Bis-Tris gels (Invitrogen) with MES running buffer (Invitrogen) and LOS was visualized by Silver Stain (Bio-Rad). LOS was transferred to PVDF (Millipore) by western blotting; membranes were blocked with PBS/1% milk for 1 h at 37 °C and probed with tissue culture supernatants containing anti-LOS mAbs 2C7, 3F11, L1 and L8 (described above) for 15 h at 4 °C, as described previously (29). mAb-reactive LOS bands were visualized with anti-mouse IgG-alkaline phosphatase (for mAbs 2C7, L1 and L8) or anti-mouse IgM alkaline phosphatase (for mAb 3F11).
Hexosaminidase treatment
To ascertain whether a terminal hexosamine (in this instance, GalNAc) was present on the lgtD-’ON’ (D+) mutants, bacteria were suspended in water, frozen at -20 °C and thawed at 37 °C to osmotically lyse them and treated with 10 U DNAse I in DNAse buffer (Ambion) for 60 min at 37 °C. Treatment with DNAse I was carried out to reduce viscosity of the sample prior to electrophoresis. Proteins were digested with 1 mg/ml protease K (Calbiochem) in SDS (final concentration 0.01%) for 1 h at 50 °C. Protease K activity was destroyed by heating at 100 °C for 20 min. Terminal N-acetyl hexosamine from LOS was released by treating the sample with 30 U β-N-acetylhexosaminidase in G2 buffer (both from New England Biolabs) for 15 h at 37 ° C. Samples were electrophoresed on a 16.5% Criterion™ Tricine gel (Bio-Rad) at 100 V at 4 °C and LOS was visualized with silver staining as described above.
Human complement
Blood was obtained from human volunteers (informed consent approved by the University of Massachusetts Institutional Review Board) and serum immunodepleted of IgG and IgM by passage over Protein A/G plus agarose (Pierce, USA) and anti-human IgM agarose columns (Sigma) to prepare complement (30). The flow through was spin concentrated, equilibrated with PBS/0.1 mM EDTA and sterilized by passage through a 0.22 μm filter (Millipore, USA). Hemolytic activity was determined using the Total Haemolytic Complement Kit (Binding Site, UK). Flow cytometry using FITC-conjugated anti-human IgG and anti-human IgM (Sigma) showed no detectable IgG or IgM binding in the depleted serum to strains that were used in experiments. Antibody depleted serum (henceforth referred to as “complement” or C′) was aliquoted and stored at -80 °C until use. In some experiments C4BP function and binding to gonococci was blocked by adding mAb 104 (28, 31) to complement (30 μg of mAb 104 / 100 μl of complement).
Flow cytometry
Flow cytometry was used to measure binding of mAb 2C7, C4b-binding protein (C4BP) and deposition of complement C3 to bacteria as described previously (32–34). All Abs were diluted in Hanks Balanced Salt Solution containing 2 mM each of Ca2+ and Mg2+ (HBSS++). Data were collected from a BD LSRII or FACSCalibur instrument (BD Biosciences, Franklin Lakes, NJ) and analyzed using a FlowJo analysis software program (version 7.2.5; Tree Star, Ashland, MA).
Serum bactericidal assays
Serum bactericidal assays were performed as described previously (18, 29). Briefly, bacteria harvested from an overnight culture on chocolate agar plates were re-passaged onto fresh chocolate agar and grown for 6 h at 37°C in an atmosphere of 5% CO2. Approximately 2000 CFU gonococci in HBSS++ were incubated with complement (concentration specified for each experiment) either in the presence or absence of mAb 2C7 (concentration specified for each experiment). In some experiments, C4BP function was blocked by preincubating complement with 30 μg/ml of mAb 104 as described above. Final bactericidal reaction volumes were maintained at 150 μl. Aliquots of 10 μl were plated onto chocolate agar plates in duplicate at the beginning of the assay (t0) and again after incubation at 37°C for 30 min (t30). Survival was calculated as the number of viable colonies at t30 relative to t0.
Opsonophagocytosis assay using human PMNs
Human neutrophils were isolated from human blood over a Percoll gradient and opsonophagocytosis assays performed using freshly isolated IL-8 primed adherent neutrophils as previously described (35). Briefly, bacteria were incubated with mAb 2C7 (4 μg/ml) and/or human complement (20%), or with HBSS++ alone (controls) for 15 min at 37 °C to permit IgG binding and C3 deposition. Reaction mixtures were added to IL-8 primed, adherent PMNs at an MOI of 1:1 and centrifuged at 400 g for 4 min at 10 °C to achieve synchronous infection (35). Cells were washed once with PBS/0.5% BSA, placed into RPMI with 10% heat-inactivated FBS and warmed to 37 °C. Cells were washed and lysed using 1% saponin in PBS at 0 min (taken immediately after the 10 °C centrifugation step) and parallel wells were similarly treated at 60 min, serially diluted in GC broth and plated to determine viable CFU. Survival was expressed as the percent of CFU at 60 min relative to CFU at 0 min.
Statistical analysis
Comparisons between two groups were made using the two-tailed unpaired t test. One-way ANOVA was used to compare multiple groups; pairwise comparisons were made by Tukey’s post-hoc test, while comparisons with a control group were made by Dunnett’s test. Two-way ANOVA was employed to compare groups when time or concentrations were independent variables.
Results
Characterization of the LOS of the mutant strains
A schematic of potential gonococcal LOS structures, the relevant enzymes involved in biosysthesis of the outer core and the specificity of anti-LOS mAbs used to characterize LOS glycan extensions are shown in Fig. 1A. Phase variable expression of lgtA, C and D leads to variation in the HepI glycan extensions; HepI 2-Hex (lgtA, C and D all ‘OFF’), HepI 3-Hex (lgtA ‘OFF’, lgtC ‘ON’ and lgtD ‘ON’ or ‘OFF’; expression of lgtD is extraneous in an lgtA ‘OFF’ background), HepI 4-Hex (lgtA ‘ON’, lgtC and D ‘OFF’) and HepI 5-Hex (lgtA and D ‘ON’, lgtC ‘OFF’). Phase variable expression of lgtG controls expression of lactose on HepII. To investigate the role of HepI glycan extensions on the function of mAb 2C7, we constructed a series of mutants in the background of MS11 4/3/1 in which the phase variable lgt loci (lgtA, C, D and G) were genetically fixed either ‘ON’ or ‘OFF’. Lgt loci were fixed ‘ON’ by mutating the repetitive homopolymeric sequence found in each gene such that the homopolymer was removed but the coding sequence was not altered, as previously described (36). Lgt loci were fixed ‘OFF’’ by deletion (lgtA, lgtD), insertional inactivation (lgtG) or by removing the homopolymeric sequence and inserting stop codons in all three reading frames (lgtC).
The LOS structures expressed by individual mutants were characterized by western blotting using the anti LOS mAbs described in Fig. 1A; relative masses of the LOSs were determined by SDS-PAGE (Fig. 1B). For simplicity, we refer to the mutants used in this study by their longest predicted HepI structures assuming activity of all expressed Lgt enzymes. The ‘ON’ and ‘OFF’ status of lgtG is indicated as G+ and G-, respectively. For example, in lane 1 (Fig. 1B), the mutant with lgtA and lgtD ‘ON’ is expected to have a 5-Hex HepI structure (note the use ‘Hex’ in the text includes both hexoses and N-acetyl hexosamines). lgtG in this mutant is fixed ‘ON’, so the mutant is referred to as 5-Hex/G+. This simplified designation for each mutant is provided at the bottom of Fig. 1B and in Table 1.
Note that fixing an lgt ‘ON’ does not ensure that all of the LOS displayed on the bacterial surface will be substituted with the glycan added only by the encoded lgt enzyme(s) because transport of ‘incomplete’ LOS molecules to the outer membrane from the site of assembly on the cytoplasmic side of the inner membrane may occur prior to the addition of a glycan by all Lgts that are fixed ‘ON’. The amount and efficiency of each lgt enzyme will determine the ratio of ‘complete’ to ‘incomplete’ LOS expressed (37). An example of the transport of ‘incomplete’ LOS, shows that >50% of the LOS expressed by the two strains in which lgtD has been locked ‘ON’ (5-Hex/G+ and 5-Hex/G-) reacts with mAb 3F11 and represent 4-Hex structures with a terminal lactosamine (the lower, more prominent band in lanes 1 and 2 shown in the Silver stain row; Fig. 1B) indicating that, despite expression of lgtD, the majority of LOS in these mutants is exported to the surface prior to the addition of the terminal GalNAc to LOS. Another example is provided by mAb L8, which reacts specifically with LOS structures that contain a lactose on HepI and no glycans from the 3-position of HepII (i.e., lgtA ‘OFF’ and lgtG ‘OFF’ respectively) (23). Thus if all the LOSs expressed by mutants with lgtA ‘OFF’ and lgtC and/or lgtG ‘ON’ were substituted with a terminal α(1,4)-linked Gal on HepI and/or a proximal Glc on HepII, these mutants should not react with mAb L8. In fact, mAb L8 reacted with all three mutants that had lgtA ‘OFF’ and lgtC and/or lgtG ‘ON’ (lanes 5, 6 and 7 in the L8 blot in Fig. 1B), indicating export of LOS structures to the surface in these mutants prior to addition of: Glc on HepII by LgtG (lanes 5 and 7) and/or the distal α(1,4)-linked Gal on HepI (LgtC; lane 5). By contrast, fixing lgtA ‘ON’ (lgtA+) did not result in any detectable ‘short’ LOS structures (no L8 reactive bands seen in lanes 2 and 4 (Fig. 1B; mutants with lgtA ‘ON’ and lgtG ‘OFF’), suggesting that LgtA efficiently added GlcNAc to the proximal lactose on HepI.
Mass spectrometric analysis confirmed loss of HepII glycan extensions in the lgtG ‘OFF’ mutants and the presence of HepII glycans in the lgtG ‘ON’ mutants (Supplemental Table S3). Mass spectrometry also confirmed that all mutants expressed the expected HepI glycan extensions shown in Table 1, as well as ‘incomplete’ structures as noted above. Further evidence that supported the presence of a terminal HexNAc residue in the 5-Hex/G+ and 5-Hex/G− mutants was provided by β-N-acetyl hexosaminidase treatment, which resulted in almost complete disappearance of the highest molecular mass band on silver staining of their LOS (Fig. 1C).
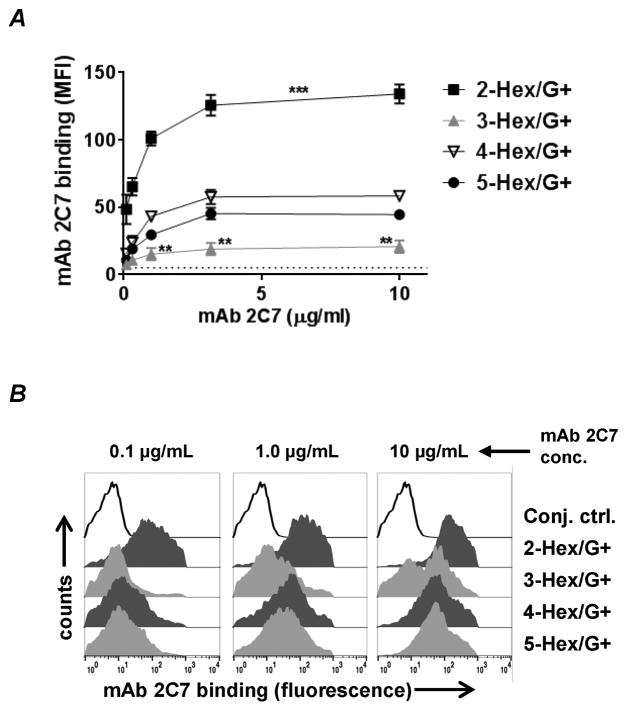
HepI glycan substitutions modulate binding of mAb 2C7
Binding of mAb 2C7 (concentrations ranging from 0.1 to 10 μg/ml) to the LOS mutants was studied by flow cytometry (FCM). The amount of mAb 2C7 bound to bacteria measured by FCM varied across the mutants (Fig. 2). The 2-Hex/G+ mutant showed maximum binding and 3-Hex/G+ the least; 4- and 5-Hex/G+ mutants bound ‘intermediate’ amounts of 2C7. Binding of mAb 2C7 requires lactose extension from HepII. As expected, none of the lgtG deletion (“G−”) mutants showed binding above conjugate control levels (data not shown).
Figure 2.
HepI LOS glycan extensions modulate binding of mAb 2C7. The LOS mutants were incubated at 37 °C with increasing concentrations of mAb 2C7 for 30 minutes. Surface-bound mAb 2C7 was detected by flow cytometry (FCM) using FITC-conjugated anti-mouse IgG. A. Binding of mAb 2C7 (concentrations ranging from 0.1 to 10 μg/ml) to LOS mutants. Each data point represents the mean of the median fluorescence intensities of 3 separate experiments (± SEM). Comparisons between the mutants at each dilution of mAb 2C7 were performed by two-way ANOVA with Tukey’s post test. ***, P<0.001 for the 2-Hex/G+ versus all other mutants at each of the five concentrations tested. **, P<0.01 for the 3-Hex/G+ mutant versus the 4- and 5-Hex/G+ mutants at the three concentrations indicated. Overall P values for interaction, row factor and column factors were all <0.0001. B. Representative histograms depicting mAb 2C7 binding at 0.1, 1.0 and 10 μg/mL. X-axis, fluorescence on a log10 scale; Y-axis, counts.
HepI glycan extensions modulate bactericidal efficacy of mAb 2C7
The ability of mAb 2C7 to kill each of the four G+ mutants was studied next. Bacteria were incubated with either 2 μg/ml or 4 μg/ml of mAb 2C7 and 20% human complement (normal human serum depleted of IgG and IgM); survival at 30 min was measured by bacterial CFUs relative to CFUs at 0 min (Fig. 3). As expected, control reactions (no mAb 2C7 added) showed no killing (>100% survival). Additional controls with mAb 2C7 alone (no added complement) or heat-inactivated complement also showed no killing (data not shown). The 2-Hex/G+ mutant showed >90% killing in the presence of 2 μg/ml of mAb 2C7; the 3-Hex/G+ mutant was fully resistant (>100% survival) to 4 μg/ml of mAb 2C7. The 4-Hex/G+ and 5-Hex/G+ mutants showed an intermediate pattern – i.e., resistance (≥ 50% survival) to 2 μg/ml of 2C7, but sensitivity (<50% survival) to 4 μg/ml of 2C7 (in this instance, >90% killing was observed). The bactericidal data followed a hierarchy similar to that seen with mAb 2C7 binding (Fig. 2).
Figure 3.
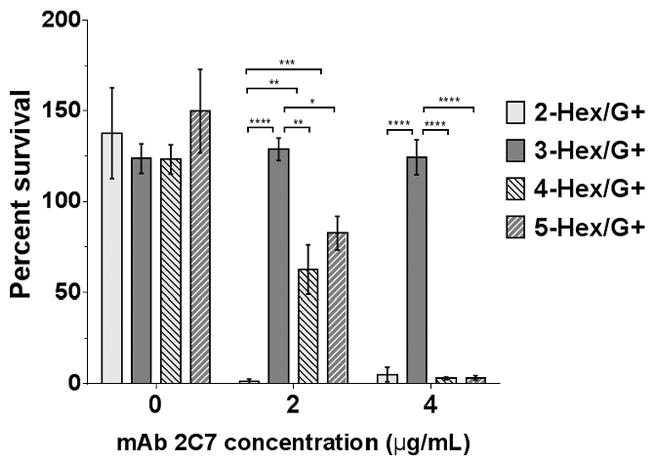
HepI glycan extensions affect complement-dependent bactericidal activity by mAb 2C7. Each mutant was incubated with 20% (v/v) human complement (C′) in HBSS++ at 37 °C for 30 minutes either in the absence of or presence of 2 or 4 μg/ml mAb 2C7. Percent survival was calculated as the number of CFUs at 30 min relative to the number of CFUs at 0 min. Each bar represents the percent survival (mean of 3 independent experiments ± SEM). Comparisons among the mutants at each of the mAb 2C7 concentrations tested were made by two-way ANOVA and pairwise comparisons were made with Tukey’s post-hoc analysis. Overall P values for interaction, row factor and column factors were all <0.0001. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001.
Binding of the classical pathway inhibitor C4b-binding protein (C4BP) to gonococci modulates the efficacy of mAb 2C7 (29) and could have contributed to differences in susceptibility to mAb 2C7. We measured binding of C4BP to the four G+ mutants using heat-inactivated serum as a source of C4BP and found that all G+ mutants bound high and similar amounts of C4BP (Supplemental Fig. S1). These findings are consistent with prior data showing that MS11 and its LOS derivatives that expressed at least 2 hexoses from HepI bound C4BP well (28, 38).
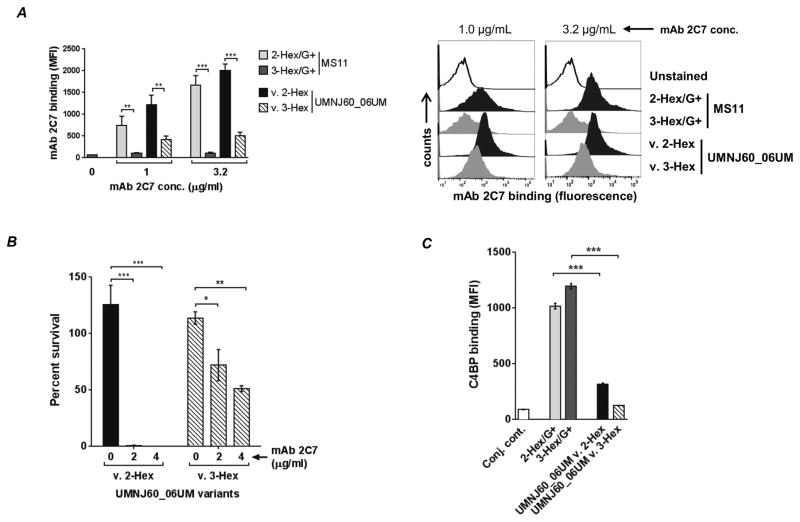
The gonococcal genome contains over 100 phase variable genes. To confirm that differences in the binding of mAb 2C7 and killing between the 3-Hex/G+ and 2-Hex/G+ mutants were specifically related to LOS structure; lgtC was fixed ‘ON’ in the 2-Hex/G+ strain permitting addition of Gal-α(1,4) to HepI (strain designated as 2→3-Hex/G+) and lgtC was fixed ‘OFF’ in the 3-Hex/G+ strain, which blocked addition of Gal-α(1,4) to HepI (strain designated as 3→2-Hex/G+). The LOSs expressed by the mutants were verified by silver staining and with western blots using mAbs L8 and L1 (Fig. 4A). The two mutants, 2→3-Hex/G+ and 3→2-Hex/G+, were next examined for their ability to bind and be killed by mAb 2C7 (Fig. 4B and 4C). The results recapitulated those seen with the 3-Hex/G+ and 2-Hex/G+ mutants, respectively.
Figure 4.
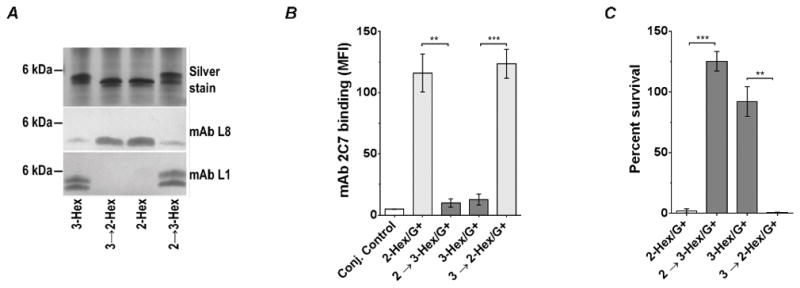
Genetic conversion of MS11 2-Hex/G+ to 3-Hex/G+ (2→3-Hex/G+) and 3-Hex/G+ to 2-Hex/G+ (3→2-Hex/G+) confirms decreased binding mAb 2C7 and increased resistance of 3-Hex/G+ to mAb 2C7-mediated complement-dependent killing. A. Verification of LOS expression by the mutants by silver staining and western blotting with mAbs L1 and L8. B. mAb 2C7 (4 μg/ml) binding to the 2- and 3-Hex ‘conversion ‘ mutants. mAb 2C7 binding was performed by FCM as described in Fig. 2. Each bar represents the mean (± SEM) of three independent experiments. An unpaired two-tailed t-test was used to compare the mutant pairs. C. Susceptibility of the 2- and 3-Hex ‘conversion’ mutants. Serum bactericidal assays were performed as described in Fig. 3 in the presence of 4 μg/mL of mAb 2C7 and 20% human complement. Each bar represents the mean (± SEM) of three independent experiments. An unpaired two-tailed t-test was used to compare the mutant pairs. **, P<0.01; ***, P<0.001.
Serum resistance of 3-Hex/G+ is overcome by increasing complement concentrations or blocking C4BP binding
We next asked whether serum resistance of the 3-Hex/G+ mutant could be overcome by either increasing complement concentrations or by blocking C4BP binding to bacteria. As shown in Fig. 5A, in the presence of 4 μg/ml of 2C7, killing of 3-Hex/G+ was enhanced in a dose dependent manner by increasing the concentration of complement. Complement alone (mAb 2C7 absent), even at the highest concentration tested (70%), did not result in killing. Similar to our prior observations with strain MS11 (28), mAb 104 blocked C4BP binding to the 3-Hex/G+ mutant (Fig. 5B), which resulted in enhanced killing by mAb 2C7 compared to control reactions that lacked mAb 104 (Fig. 5C). Thus, increasing complement activation on the 3-Hex/G+ mutant either by increasing the concentration of complement or by decreasing complement inhibition by C4BP overcame its serum-resistant phenotype.
Figure 5.
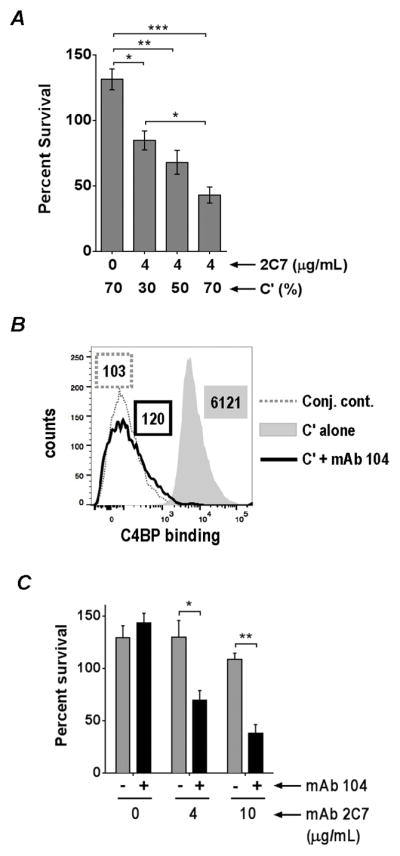
Serum resistance of MS11 3-Hex/G+ can be overcome by increasing complement concentrations or by inhibiting C4BP binding to bacteria. A. Increasing complement concentrations enhances killing of the 3-Hex/G+ mutant in a dose-dependent manner. The 3-Hex/G+ mutant was incubated with 4 μg/ml of mAb 2C7 and increasing concentrations of human complement (C′; 30%, 50% and 70%) and bactericidal assays were performed as described in Fig. 2. The control reaction contained only the highest concentration of C′ used (70%) without any added mAb 2C7. The Y-axis shows percent survival. Each bar represents the mean (± SEM) of three independent experiments. Comparisons across different conditions of incubation were made by one-way ANOVA and pairwise comparisons were made with Tukey’s post-test. The overall P value for the ANOVA was 0.0002. B. mAb 104 blocks C4BP binding to 3-Hex/G+. 3-Hex/G+ was incubated with either 20% C′ alone or 20% C′ plus mAb 104 (final concentration of 30 μg/ml in the reaction mixture). C4BP bound to bacteria was detected with anti-C4BP mAb 67 followed by anti-mouse IgG A647. X-axis, fluorescence on a log10 scale; Y-axis, counts. Numbers alongside histograms represents median fluorescence of the entire population and outlines or shading correspond to the histograms. C. Bactericidal efficacy of mAb 2C7 when C4BP binding to bacteria and function was blocked using mAb 104. C′ was incubated with anti-C4BP mAb 104 to a final concentration of 30 μg/ml at 4 °C for 15 min. The 3-Hex mutant was then incubated with (4 μg/ml or 10 μg/ml) or without mAb 2C7, followed by the addition of mAb 104-treated serum to a final concentration of 20%. Parallel control reactions included bacteria, mAb 2C7 and C′ (no added mAb 104). The Y-axis denotes percent survival at 30 min. Comparisons between reactions that did or did not contain mAb 104 at each concentration of mAb 2C7 were made with a two-way ANOVA, with Sidak’s multiple comparison test. Overall P values for interaction, row factor and column factor were 0.009, 0.0009 and 0.0014, respectively. Each bar represents the mean (± SEM) of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001.
mAb 2C7 enhances C3 deposition and facilitates opsonophagocytosis of the 3-Hex/G+ mutant
C3 fragments – in particular iC3b – deposited on bacteria enhance opsonophagocytosis. mAb 2C7 did not promote direct killing by complement of the 3-Hex/G+ mutant in serum bactericidal assays that used 20% complement (Fig. 3). However, we reasoned that mAb 2C7 mediated C3 deposition on the 3-Hex/G+ mutant supports opsonophagocytic killing and constitutes a potential mechanism of protection by vaccine Ab.
Total C3 (C3b and iC3b) deposition on the 3-Hex/G+ was measured by FCM; the three other G+ mutants were included as comparators. Bacteria were incubated with either 2 μg/ml or 4 μg/ml of mAb 2C7 and 20% complement; C3 deposited at 15 and 30 min was measured. In the absence of mAb 2C7 there was minimal C3 deposition on all mutants (median fluorescence <2-fold above baseline conjugate control levels [Fig. 6]). As expected, the 2-Hex/G+ mutant that was highly susceptible to complement-dependent killing showed the most rapid accumulation and the highest levels of C3 deposition. An intermediate amount of C3 was deposited on the 4-Hex mutant. The 3- and 5-Hex/G+ mutants bound the least; there was a trend toward less C3 on 3-Hex/G+ compared to 5-Hex/G+, however the differences were not significant.
Figure 6.
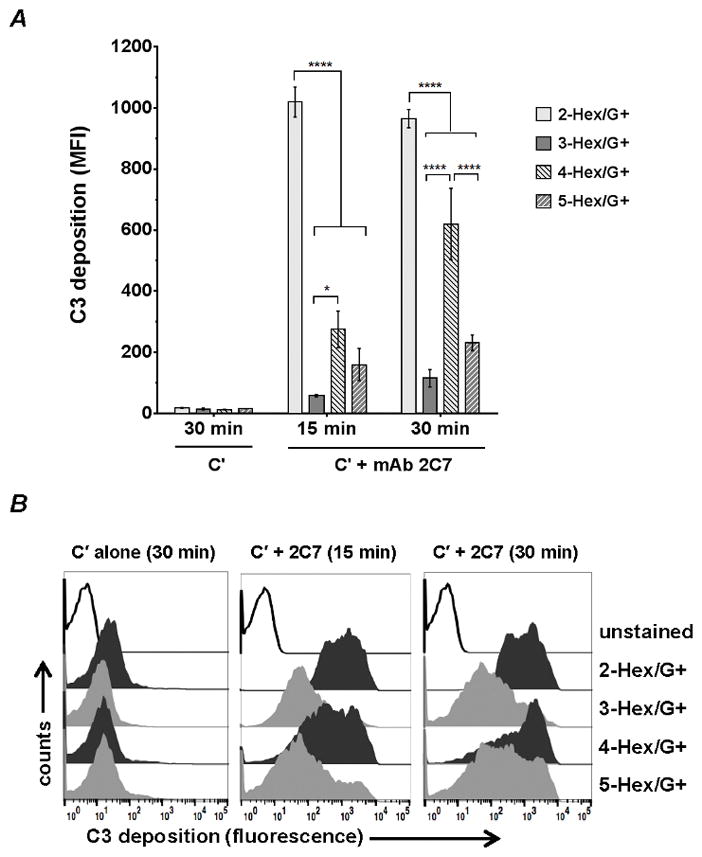
C3 deposition on the lgtG-‘ON’ (G+) HepI glycan LOS mutants. The four G+ LOS mutants with varying HepI glycan extensions were incubated with 20% C′ and 4 μg/ml mAb 2C7 in HBSS++ at 37 °C and total C3 (C3b and iC3b fragments) deposited on the bacterial surface at 15 or 30 min was measured by FCM with FITC-conjugated sheep anti-human C3c Ab (detects both C3b and iC3b). Control reactions included bacteria incubated with 20% Cμ alone (no mAb 2C7 present) for 15 and 30 min. The median fluorescence intensity was recorded. Similar C3 deposition was seen on bacteria incubated with C′ alone for 15 min (not shown). Differences in C3 deposition across the mutants within each group was measured by two-way ANOVA and pairwise comparisons were made with Tukey’s post-test. The data represent the mean (± SEM) from three independent experiments. P values for interaction, row factor and column factors were all <0.0001. *, P<0.05; ****, P<0.0001. B. Representative histograms of a representative experiment in A is shown. X-axis, fluorescence on a log10 scale; Y-axis, counts. .
The opacity (Opa) proteins of N. gonorrhoeae encompass a phase-variable family of proteins (gonococci possess 11 opa genes and can express three or four Opa proteins simultaneously (39) that can engage CEACAM3 on PMNs and mediate opsonophagocytic killing independent of antibody and complement (40, 41). To address the potential role of mAb 2C7-dependent complement activation in facilitating opsonophagocytosis of the 3-Hex/G+ mutant, we recreated the 3-Hex/G+ LOS structure in the background of an Opa-negative MS11 strain (42). The 3-Hex/G+ Opa-negative MS11 mutant strain bound similar (low) levels of mAb 2C7 and was fully resistant (>100% survival) to killing by 4 μg/ml of mAb 2C7 plus 20% complement (Fig. 7; bar at far left), analogous to the 3-Hex/G+ in MS11 with its native opa genes intact. In the presence of both mAb 2C7 and complement (bar to the extreme right), PMNs caused a 60% decrease in bacterial survival (P<0.01 compared to the control reaction with bacteria alone plus PMNs [second bar from left]). Compared to the control with bacteria and PMNs (second bar from left), reactions that contained bacteria, PMNs and 2C7 (third bar from the left) or bacteria, PMNs and complement (fourth bar from the left) did not show increased killing.
Figure 7.
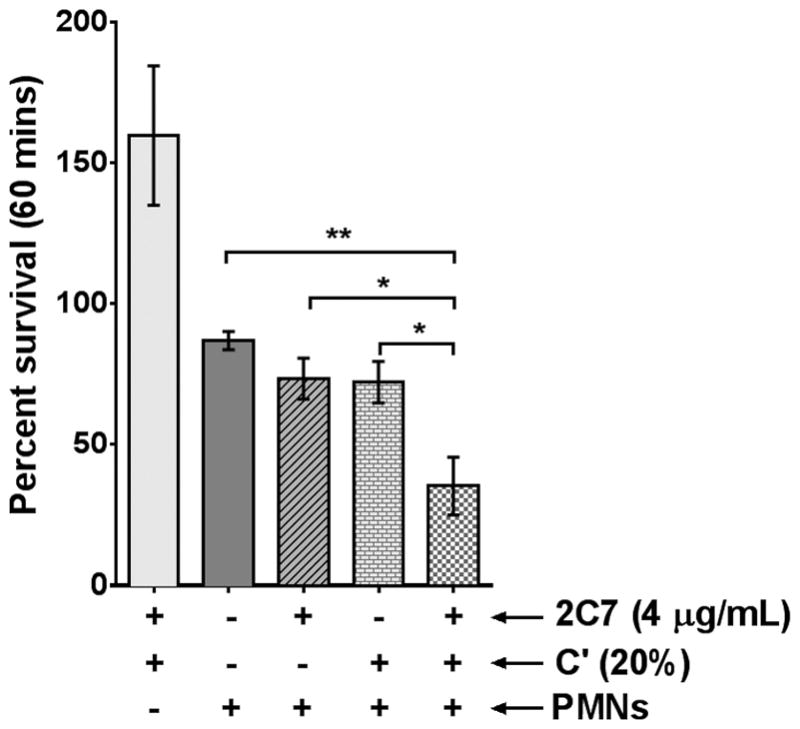
mAb 2C7 facilitates opsonophagocytosis of 3-Hex/G+ by PMNs. Freshly isolated IL-8 primed human PMNs adherent on plastic coverslips were synchronously infected with the 3-Hex/G+ mutant that had been pre-incubated with mAb 2C7 (4 μg/ml) and/or C′ for 15 min 37 °C at a MOI of 1. A reaction that contained bacteria, mAb 2C7 and C′ (no PMNs) was also included (bar closest to the Y-axis). Percent survival (CFUs at 60 min relative to CFUs at 0 min) is shown on the Y-axis. Each bar represents the mean of the percent survival of 3 separate experiments (± SEM). A comparison of killing across the four groups that contained PMNs was performed by one-way ANOVA, with Dunnett’s post-test used to make comparisons with the control reaction (bacteria plus PMNs; second bar from left). The overall P value for the ANOVA was 0.0064. *, P<0.05; **, P<0.01.
Relative resistance of a 3-Hex expressing lgtC phase-variant of a clinical isolate to killing by mAb 2C7
To ascertain if the decreased mAb 2C7 binding and increased resistance of the 3-Hex/G+ mutant was generalizable and not unique to strain MS11 alone, we identified 2-Hex and 3-Hex phase variants of an lgtA mutant (ΔlgtA) of a minimally passaged clinical isolate called UMNJ60_O6UM. Two natural variants of UMNJ60_O6UM ΔlgtA were selected – one that expressed lactose on HepI (UMNJ60_O6UM 2-Hex; analogous with lgtC phase varied ‘OFF’ and therefore did not react with mAb L1) and one that expressed 3-Hex’ Pk-like LOS on HepI (UMNJ60_O6UM 3-Hex; analogous with lgtC phase varied ‘ON’ and therefore reacted with mAb L1; Ref. (43)). Both variants had lgtG phase-’ON’ and therefore expressed lactose from HepII. The two variants were examined for mAb 2C7 binding and killing in a complement-dependent bactericidal assay. UMNJ60_O6UM 2-Hex variant bound more mAb 2C7 than the UMNJ60_O6UM 3-Hex variant (Fig. 8A). UMNJ60_O6UM 3-Hex was also more resistant to complement-dependent killing by mAb 2C7 (Fig. 8B). The 2-Hex variant was killed >99% and 100% by complement in the presence of 2 μg/ml and 4 μg/ml of 2C7 respectively; the 3-Hex variant survived ~70% and ~50% under similar conditions.
Figure 8.
Validation of the MS11 3-Hex/G+ phenotype with a 3-Hex/G+ phase variant of UMNJ60_06UM, a recent clinical isolate of N. gonorrhoeae. Phase variants of an lgtA mutant of UMNJ60_06UM that expressed 3-Hex HepI (UMNJ60 v. 2-Hex; lgtC ‘ON’, reacts with mAb L1, but not mAb L8) and that expressed 2-Hex HepI (UMNJ60 v. 3-Hex; lgtC ‘OFF’, reacted with mAb L8 but not mAb L1) were selected. Both strains reacted with mAb 2C7 (consistent with lgtG ‘ON’). A. The 3-Hex expressing variant of UMNJ60_06UM binds less 2C7 than the 2-Hex expressing variant. Bacteria were incubated with mAb 2C7 (1 μg/ml or 3.2 μg/ml) and bound mAb was detected by FCM as described in Figure 2. The MS11 2-Hex/G+ and 3-Hex/G+ mutants were included as comparators. Each bar represents the mean of the median fluorescence intensities of 3 independent experiments (± SEM). Comparisons between the HepI 2-Hex and HepI 3-Hex-expressing isolates were made by the unpaired two-tailed t-test. Histograms from a representative experiment are shown on the right of the bar graphs. B. A 3-Hex variant of UMNJ60_06UM is more resistant to killing by mAb 2C7 than a 2-Hex variant. UMNJ60 v. 2-Hex and v. 3-Hex were incubated with increasing concentrations of mAb 2C7 (either 0, 2 or 4 μg/ml) plus 20% human complement and survival of bacteria at 30 min relative to CFU at time 0 min, was measured in serum bactericidal assays. Y-axis, percent survival. One-way ANOVA was used to compare killing of each of the variants at the different concentrations of mAb 2C7 tested; the v. 2-Hex and the v. 3-Hex groups were compared separately. The overall P values for the v. 2-Hex and v. 3-Hex groups were 0.0002 and 0.006, respectively. C. The HepI LOS phase-variants of UMNJ60_06UM are weak C4BP binders. UMNJ60 v. 2-Hex and UMNJ60 v. 3-Hex were incubated with heat-inactivated NHS and C4BP bound to bacteria was detected by FCM. MS11 2-Hex/G+ and 3-Hex/G+ were included as comparators. The conjugate control (“Conj. cont.”) represents a reaction mixture that lacked serum. Each bar represents the mean of 3 independent experiments ± SEM. A two-tailed unpaired t-test was used to compare C4BP binding to the 2-Hex and 3-Hex expressing strain pairs. Histograms from a representative experiment are shown to the right of the bar graph. The X-axis shows fluorescence on a log10 scale and the Y-axis the events. *, P<0.05; **, P<0.01; ***, P<0.001, ****, P<0.0001.
Unlike the MS11 3-Hex/G+ mutant that was fully resistant (>100% survival) to killing by even 4 μg/ml of mAb 2C7 plus 20% complement (Fig. 3), the UMNJ60_O6UM 3-Hex variant was partly susceptible to killing under similar conditions. As noted above, C4BP binds to certain N. gonorrhoeae strains, including MS11, and promotes serum resistance (Ref. (28) and Supplemental Fig. S1). To determine if UMNJ60_O6UM bound C4BP, we compared C4BP binding to the two UMNJ60_O6UM LOS variants with the corresponding MS11 LOS mutants. Both UMNJ60_O6UM variants bound significantly lower amounts of C4BP than the corresponding MS11 LOS mutants (Fig. 8C), thus providing a probable explanation for the greater sensitivity to complement of UMNJ60_O6UM variant 3-Hex compared to MS11 3-Hex/G+. Collectively, these data suggest that the 3-Hex HepI structure negatively affects mAb 2C7 binding and function.
Discussion
The role of LOS glycan extensions in the pathogenesis of N. gonorrhoeae, including their role in immune evasion, is well recognized. Much attention has been directed to LOS that expresses the lacto-N-neotetraose (LNnT) structure on HepI, which mimics host paraglobosides (44). Unsialylated LNnT interacts with the asialoglycoprotein receptor and facilitates adhesion of gonococci to male urethral epithelial cells (45). A lectin-like interaction between the terminal lactosamine residue of LNnT and gonococcal opacity proteins (Opa) plays a role in inter-gonococcal adhesion and the degree of colony opacity (46). Gonococci possess a surface-exposed LOS sialyltransferase (47) that catalyzes the transfer of N-acetylneuraminic acid (Neu5Ac) from the donor molecule CMP-Neu5Ac present in host secretions (48, 49), on to the 3-position of the terminal Gal residue of LNnT. LNnT sialylation is involved in the inhibition of all three pathways of complement and enables gonococci to resist killing by natural IgG/IgM and complement in normal human serum (NHS), called serum resistance (50–52). Sialylation of gonococcal LNnT in vivo has been demonstrated by electron micrographs of organisms in human secretions (53). Schneider and colleagues also demonstrated the importance of phase variation of gonococcal LOS and LNnT sialylation in humans. They inoculated male volunteers intraurethrally with a variant of strain MS11 that expressed a (non-sialylated) 2-Hex HepI structure predominantly. At the onset of symptoms, several days later, almost all men shed bacteria that expressed LOS with predominantly longer (including the 4-Hex HepI), sialylatable HepI structures (54). Recently, McLaughlin and colleagues found that gonococci present in urethral exudates of infected men displayed an lgt genotype that predicted sialylation of terminal lactosamine; lgtA was in-frame, while lgtC and lgtD were out-of-frame in most cases (55). The importance of LOS sialylation in pathogenesis has also been demonstrated in the mouse vaginal colonization/infection model; gonococcal mutants that lack LOS sialyltransferase (Lst) are outcompeted by their wild-type counterparts (32, 56). The efficacy of mAb 2C7 against phase variant and sialylated bacteria has been demonstrated both in vitro (organisms grown in media containing 2 μg/ml of CMP-Neu5Ac; (29)) and in vivo in mice, where LOS sialylation occurs (57)).
Diversity of surface antigens generated by phase variation confers a survival advantage to microbes and enables them to adapt to different niches in the host. Phase-variation of LOS also modulates resistance of N. gonorrhoeae to killing by NHS, independent of LOS siaylation (58–60). Expression of GalNAc distal to LNnT (i.e., 5-Hex HepI; lgtA and lgtD ‘ON’) permits binding of natural IgM present in NHS (61) and enhances bacterial killing by complement (58). Although the terminal GalNAc enhances killing by NHS, gonococci that possess LOS with this terminal residue interact with macrophage galactose-type lectin (MGL) on dendritic cells. This may result in more pronounced Th2 and Th17 responses (62), instead of protective Th1 responses (4, 63). Expression of a truncated 3.6 kDa LOS (the 2-Hex (lactosyl) HepI), which also is a host glycan mimic, is also associated with increased resistance to NHS because most humans lack natural (IgG/IgM) Ab against this epitope (59).
Our studies used mAb 2C7 and NHS depleted of IgG/IgM in bactericidal assays. In contrast to previous studies where mutants with short HepI glycan extensions (e.g., lgtA ‘OFF’) were more resistant to killing by NHS (58, 60), the 2-Hex/G+ mutant studied herein was more susceptible to killing by mAb 2C7 and complement (i.e., NHS depleted of IgG/IgM) than the three remaining mutants because it bound the most mAb 2C7, which resulted in overwhelming complement activation. By comparison, the 5-Hex/G+ strain, ordinarily more sensitive to killing by IgM in NHS (61), was relatively resistant to killing by mAb 2C7 plus complement because it bound less mAb 2C7. We replicated previous studies and showed killing (100% killing in 10% serum [IgG and IgM intact]) of the 5-Hex/G+ mutant; the 2- and 3-Hex/G+ strains were fully serum resistant (>100% survival in 10% NHS).
The 2C7 LOS epitope represents a promising vaccine candidate. Separately, we have shown that HepII glycan extension, required to generate the epitope, is also important in the mouse model of gonococcal colonization/infection, where an lgtG deletion mutant of strain FA1090 was shown to be less ‘fit’ (14). A role for HepII glycans in gonococcal pathogenesis is supported by the observation that >95% (96 of 101) of minimally passaged clinical isolates reacted with mAb 2C7 (11). A contemporary analysis of over 70 gonococcal isolates recovered from men with urethritis attending an STD clinic in Nanjing, China, has substantiated these findings; all the recovered isolates bind mAb 2C7 (our unpublished observations).
A potential reason for (2C7) vaccine resistance would be selection of LOS variants that show decreased binding of Ab. One explanation would be natural selection of variant(s) expressing LOS with lgtG ‘OFF’. However, loss of LgtG expression may reduce fitness and therefore not be favored (14). The hypothesis addressed in our study was that other different HepI LOS structures may affect binding and function of mAb 2C7, an important consideration in predicting vaccine efficacy and coverage. Strains that expressed the Pk-like LOS structure (represented by the 3-Hex/G+ mutant) bound the least amount of mAb 2C7 and were relatively resistant to complement-mediated killing by mAb 2C7. With the exception of lgtC, which adds the terminal Gal on HepI of the 3-Hex/G+ mutant via an α-linkage, glycans added by enzymes encoded by the lgtABCDE operon are β-linked (58, 64–66). The α-linked terminal Gal on HepI of the 3-Hex/G+ may hinder access of mAb 2C7 to its epitope.
Several microbes bind C4BP, an inhibitor of the classical pathway, to evade killing by complement (67). MS11 binds C4BP and mAb 2C7 must surmount this barrier to mediate killing through the membrane attack complex insertion. The inhibiting role of C4BP was evident when C4BP binding to bacteria and C4BP function were blocked using mAb 104 resulting in increased killing of bacteria. Moreover, the 3-Hex variant of a clinical strain that bound low levels of C4BP (UMNJ60_06UM) was more susceptible to killing by mAb 2C7 than the MS11 3-Hex/G+ that binds high levels of C4BP.
Despite the absence of killing through membrane attack complex, mAb 2C7 deposited sufficient C3 on 3-Hex/G+ bacteria to enable human PMNs to decrease CFUs by >50%. Of note, maximal opsonophagocytic killing by PMNs required both Ab and complement; either one alone did not result in killing above baseline levels when bacteria only were incubated with PMNs. The Neisserial Pk-like LOS structure can also be sialylated (43, 68). Unlike sialylation of LNnT LOS, sialylation of Pk-like LOS does not enhance FH binding to bacteria and confers resistance to only low, but not high, concentrations of NHS (43). While McLaughlin and colleagues found that lgtC was out-of-frame in all 7 samples of N. gonorrhoeae obtained directly from male urethras (55), Mandrell reported that as many as 36 of 70 (51%) of strains surveyed in vitro bound mAb 3D9, which reacts with the Pk antigen (69). The advantage conferred by the gonococcal Pk-like LOS in vivo remains to be elucidated.
How mAb 2C7 decreases gonococcal burden and duration of infection in vivo – i.e., the specific contributions of direct complement-mediated killing, opsonophagocytosis or other novel mechanism(s) of Ab-mediated clearance – remain to be elucidated. Notwithstanding the differences in direct killing of the mutants by mAb 2C7 and complement, enhanced C3 deposition occurred on all the HepI/G+ mutants; in particular the 3-Hex/G+ mutant, which was not killed directly, was opsonophagocytosed and killed. Our findings support the inclusion of the 2C7 LOS epitope in a vaccine candidate against N. gonorrhoeae.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health / National Institutes of Allergy and Infectious Diseases, AI111728, AI118161 and AI119327 (to S.R.), AI114710 (to P.A.R) and AI114790 (to P.A.R and S.R.).
We thank Ashild Vik and Michael Koomey (University of Oslo) for providing strain MS11 4/3/1, Daniel C. Stein (University of Maryland) for the Opa-negative MS11 mutant and F62 ΔlgtA lgtG+, William M. Shafer (Emory University) for providing FA19 lgtG::kan, Anna M. Blom (Lund University) for mAbs 67 and 104, Alison K. Criss (University of Virginia) for advice with opsonophagocytosis assays. We thank Sunita Gulati for mAb 2C7, and Nancy Nowak and Bo Zheng (all from the University of Massachusetts, Worcester) for expert technical assistance.
Nonstandard Abbreviation used
- LOS
lipooligosaccharide
- lgt
LOS glycosyltransferase
- FH
factor H
- C4BP
C4b-binding protein
- LNnT
lacto-N-neotetraose
- PK antigen
a human blood group antigen of the P series
- HBSS
Hanks’ balanced salt solution
- PMN
polymorphonuclear neutrophil
- C′
complement
References
- 1.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jerse AE, Bash MC, Russell MW. Vaccines against gonorrhea: current status and future challenges. Vaccine. 2014;32:1579–1587. doi: 10.1016/j.vaccine.2013.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu W, Chen CJ, Thomas CE, Anderson JE, Jerse AE, Sparling PF. Vaccines for gonorrhea: can we rise to the challenge? Front Microbiol. 2011;2:124. doi: 10.3389/fmicb.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake MS, Wetzler LM. Vaccines for gonorrhea: where are we on the curve? Trends Microbiol. 1995;3:469–474. doi: 10.1016/s0966-842x(00)89012-5. [DOI] [PubMed] [Google Scholar]
- 6.Joiner KA, Scales R, Warren KA, Frank MM, Rice PA. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985;76:1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotschlich EC. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J Exp Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngampasutadol J, Rice PA, Walsh MT, Gulati S. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine. 2006;24:157–170. doi: 10.1016/j.vaccine.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki R, Maruyama T, Yabe U, Asuka S. Normal human sera contain bactericidal IgG that binds to the oligosaccharide epitope expressed within lipooligosaccharides of Neisseria gonorrhoeae. J Biochem. 2005;137:487–494. doi: 10.1093/jb/mvi061. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt KA, Schneider H, Lindstrom JA, Boslego JW, Warren RA, Van de Verg L, Deal CD, McClain JB, Griffiss JM. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis. 2001;28:555–564. doi: 10.1097/00007435-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids [published erratum appears in J Infect Dis 1997 Apr;175(4):1027] J Infect Dis. 1996;174:1223–1237. doi: 10.1093/infdis/174.6.1223. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki R, Koshino H, Kurono S, Nishinaka Y, McQuillen DP, Kume A, Gulati S, Rice PA. Structural and immunochemical characterization of a Neisseria gonorrhoeae epitope defined by a monoclonal antibody 2C7; the antibody recognizes a conserved epitope on specific lipo-oligosaccharides in spite of the presence of human carbohydrate epitopes. J Biol Chem. 1999;274:36550–36558. doi: 10.1074/jbc.274.51.36550. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee A, Wang R, Uljon SN, Rice PA, Gotschlich EC, Stein DC. Identification of the gene (lgtG) encoding the lipooligosaccharide beta chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1998;95:10872–10877. doi: 10.1073/pnas.95.18.10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati S, Zheng B, Reed GW, Su X, Cox AD, St Michael F, Stupak J, Lewis LA, Ram S, Rice PA. Immunization against a Saccharide Epitope Accelerates Clearance of Experimental Gonococcal Infection. PLoS Pathog. 2013;9:e1003559. doi: 10.1371/journal.ppat.1003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong Y, Arking D, Ye S, Reinhold B, Reinhold V, Stein DC. Neisseria gonorrhoeae strain PID2 simultaneously expresses six chemically related lipooligosaccharide structures. Glycobiology. 2002;12:523–533. doi: 10.1093/glycob/cwf047. [DOI] [PubMed] [Google Scholar]
- 16.Wolfgang M, van Putten JP, Hayes SF, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;19:6408–6418. doi: 10.1093/emboj/19.23.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaughnessy J, Gulati S, Agarwal S, Unemo M, Ohnishi M, Su XH, Monks BG, Visintin A, Madico G, Lewis LA, Golenbock DT, Reed GW, Rice PA, Ram S. A Novel Factor H-Fc Chimeric Immunotherapeutic Molecule against Neisseria gonorrhoeae. Journal of immunology. 2016 doi: 10.4049/jimmunol.1500292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuillen DP, Gulati S, Rice PA. Complement-mediated bacterial killing assays. Methods Enzymol. 1994;236:137–147. doi: 10.1016/0076-6879(94)36013-8. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng YL, Datta A, Ambrose K, Lo M, Davies JK, Carlson RW, Stephens DS, Kahler CM. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. The Journal of biological chemistry. 2004;279:35053–35062. doi: 10.1074/jbc.M401433200. [DOI] [PubMed] [Google Scholar]
- 20.Johnston DM, Cannon JG. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene. 1999;236:179–184. doi: 10.1016/s0378-1119(99)00238-3. [DOI] [PubMed] [Google Scholar]
- 21.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement. PLoS Pathog. 2010;6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchet V, Hood DW, Li J, Brisson JR, Randle GA, Martin A, Li Z, Goldstein R, Schweda EK, Pelton SI, Richards JC, Moxon ER. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A. 2003;100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor ET, Swanson KV, Cheng H, Fluss K, Griffiss JM, Stein DC. Structural requirements for monoclonal antibody 2-1-L8 recognition of neisserial lipooligosaccharides. Hybridoma (Larchmt) 2008;27:71–79. doi: 10.1089/hyb.2007.0552. [DOI] [PubMed] [Google Scholar]
- 24.McLeod Griffiss J, Brandt BL, Saunders NB, Zollinger W. Structural relationships and sialylation among meningococcal L1, L8, and L3,7 lipooligosaccharide serotypes. J Biol Chem. 2000;275:9716–9724. doi: 10.1074/jbc.275.13.9716. [DOI] [PubMed] [Google Scholar]
- 25.Mandrell RE, Griffiss JM, Macher BA. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis LA, Carter M, Ram S. The relative roles of factor H binding protein, neisserial surface protein A, and lipooligosaccharide sialylation in regulation of the alternative pathway of complement on meningococci. J Immunol. 2012;188:5063–5072. doi: 10.4049/jimmunol.1103748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardig Y, Hillarp A, Dahlback B. The amino-terminal module of the C4b-binding protein alpha-chain is crucial for C4b binding and factor I-cofactor function. Biochem J. 1997;323:469–475. doi: 10.1042/bj3230469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ram S, Cullinane M, Blom A, Gulati S, McQuillen D, Monks B, O’Connell C, Boden R, Elkins C, Pangburn M, Dahlback B, Rice PA. Binding of C4b-binding Protein to Porin: A molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–296. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulati S, Agarwal S, Vasudhev S, Rice PA, Ram S. Properdin is critical for antibody-dependent bactericidal activity against Neisseria gonorrhoeae that recruit C4b-binding protein. J Immunol. 2012;188:3416–3425. doi: 10.4049/jimmunol.1102746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray TD, Lewis LA, Gulati S, Rice PA, Ram S. Novel blocking human IgG directed against the pentapeptide repeat motifs of Neisseria meningitidis Lip/H.8 and Laz lipoproteins. J Immunol. 2011;186:4881–4894. doi: 10.4049/jimmunol.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardig Y, Dahlback B. The amino-terminal module of the C4b-binding protein beta-chain contains the protein S-binding site. J Biol Chem. 1996;271:20861–20867. doi: 10.1074/jbc.271.34.20861. [DOI] [PubMed] [Google Scholar]
- 32.Lewis LA, Gulati S, Burrowes E, Zheng B, Ram S, Rice PA. alpha-2,3-Sialyltransferase Expression Level Impacts the Kinetics of Lipooligosaccharide Sialylation, Complement Resistance, and the Ability of Neisseria gonorrhoeae to Colonize the Murine Genital Tract. MBio. 2015;6:e02465–02414. doi: 10.1128/mBio.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis LA, Shafer WM, Dutta Ray T, Ram S, Rice PA. Phosphoethanolamine residues on the lipid A moiety of Neisseria gonorrhoeae lipooligosaccharide modulate binding of complement inhibitors and resistance to complement killing. Infect Immun. 2013;81:33–42. doi: 10.1128/IAI.00751-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. Factor H-Dependent Alternative Pathway Inhibition Mediated by Porin B Contributes to Virulence of Neisseria meningitidis. MBio. 2013;4:e00339–00313. doi: 10.1128/mBio.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stohl EA, Criss AK, Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol. 2005;58:520–532. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song W, Ma L, Chen R, Stein DC. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med. 2000;191:949–960. doi: 10.1084/jem.191.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun DC, Stein DC. The lgtABCDE gene cluster, involved in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae, contains multiple promoter sequences. J Bacteriol. 2004;186:1038–1049. doi: 10.1128/JB.186.4.1038-1049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ram S, Ngampasutadol J, Cox AD, Blom AM, Lewis LA, St Michael F, Stupak J, Gulati S, Rice PA. Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect Immun. 2007;75:4071–4081. doi: 10.1128/IAI.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. The Journal of experimental medicine. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol. 2007;9:2167–2180. doi: 10.1111/j.1462-5822.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 41.Sarantis H, Gray-Owen SD. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun. 2012;80:345–358. doi: 10.1128/IAI.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein DC, LeVan A, Hardy B, Wang LC, Zimmerman L, Song W. Expression of Opacity Proteins Interferes with the Transmigration of Neisseria gonorrhoeae across Polarized Epithelial Cells. PLoS One. 2015;10:e0134342. doi: 10.1371/journal.pone.0134342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulati S, Cox A, Lewis LA, Michael FS, Li J, Boden R, Ram S, Rice PA. Enhanced factor H binding to sialylated Gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in Gonococci. Infect Immun. 2005;73:7390–7397. doi: 10.1128/IAI.73.11.7390-7397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey HA, Swords WE, Apicella MA. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic neisseria and haemophilus. J Autoimmun. 2001;16:257–262. doi: 10.1006/jaut.2000.0477. [DOI] [PubMed] [Google Scholar]
- 45.Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol. 2001;42:659–672. doi: 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 46.Blake MS, Blake CM, Apicella MA, Mandrell RE. Gonococcal opacity: lectin-like interactions between Opa proteins and lipooligosaccharide. Infect Immun. 1995;63:1434–1439. doi: 10.1128/iai.63.4.1434-1439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shell DM, Chiles L, Judd RC, Seal S, Rest RF. The Neisseria lipooligosaccharide-specific alpha-2,3-sialyltransferase is a surface-exposed outer membrane protein. Infect Immun. 2002;70:3744–3751. doi: 10.1128/IAI.70.7.3744-3751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nairn CA, Cole JA, Patel PV, Parsons NJ, Fox JE, Smith H. Cytidine 5′-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988;134:3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- 49.Parsons NJ, Patel PV, Tan EL, Andrade JRC, Nairn CA, Goldner M, Cole JA, Smith H. Cytidine 5′-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human red blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb Pathog. 1988;5:303–309. doi: 10.1016/0882-4010(88)90103-9. [DOI] [PubMed] [Google Scholar]
- 50.Devyatyarova-Johnson M, I, Rees H, Robertson BD, Turner MW, Klein NJ, Jack DL. The lipopolysaccharide structures of Salmonella enterica serovar Typhimurium and Neisseria gonorrhoeae determine the attachment of human mannose-binding lectin to intact organisms. Infect Immun. 2000;68:3894–3899. doi: 10.1128/iai.68.7.3894-3899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elkins C, Carbonetti NH, Varela VA, Stirewalt D, Klapper DG, Sparling PF. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol Microbiol. 1992;6:2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 52.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apicella MA, Mandrell RE, Shero M, Wilson M, Griffiss JM, Brooks GF, Fenner C, Breen CF, Rice PA. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990;162:506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- 54.Schneider H, Griffiss JM, Boslego JW, Hitchcock PJ, Zahos KM, Apicella MA. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991;174:1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin SE, Cheng H, Ghanem KG, Yang Z, Melendez J, Zenilman J, Griffiss JM. Urethral exudates of men with Neisseria gonorrhoeae infections select a restricted lipooligosaccharide phenotype during transmission. J Infect Dis. 2012;206:1227–1232. doi: 10.1093/infdis/jis481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H, Jerse AE. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect Immun. 2006;74:4094–4103. doi: 10.1128/IAI.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu H, Jerse AE. In: Caugant DA, Wedege E, editors. Sialylation of gonococcal LOS occurs during experimental murine gonococcal genital tract infection; 13th International Pathogenic Neisseria Conference; Oslo, Norway. 2002. p. 228. [Google Scholar]
- 58.Balthazar JT, Gusa A, Martin LE, Choudhury B, Carlson R, Shafer WM. Lipooligosaccharide Structure is an Important Determinant in the Resistance of Neisseria Gonorrhoeae to Antimicrobial Agents of Innate Host Defense. Front Microbiol. 2011;2:30. doi: 10.3389/fmicb.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider H, Griffiss JM, Mandrell RE, Jarvis GA. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985;50:672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shafer WM, Datta A, Kolli VS, Rahman MM, Balthazar JT, Martin LE, Veal WL, Stephens DS, Carlson R. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J Endotoxin Res. 2002;8:47–58. [PubMed] [Google Scholar]
- 61.Griffiss JM, Jarvis GA, O’Brien JP, Eads MM, Schneider H. Lysis of Neisseria gonorrhoeae initiated by binding of normal human IgM to a hexosamine-containing lipooligosaccharide epitope(s) is augmented by strain-specific, properdin-binding-dependent alternative complement pathway activation. J Immunol. 1991;147:298–305. [PubMed] [Google Scholar]
- 62.van Vliet SJ, Steeghs L, Bruijns SC, Vaezirad MM, Snijders Blok C, Arenas Busto JA, Deken M, van Putten JP, van Kooyk Y. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog. 2009;5:e1000625. doi: 10.1371/journal.ppat.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol. 2011;2:52. doi: 10.3389/fmicb.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.John CM, Griffiss JM, Apicella MA, Mandrell RE, Gibson BW. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991;266:19303–19311. [PubMed] [Google Scholar]
- 65.Yamasaki R, Kerwood DE, Schneider H, Quinn KP, Griffiss JM, Mandrell RE. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of gonococcal lipooligosaccharide. J Biol Chem. 1994;269:30345–30351. [PubMed] [Google Scholar]
- 66.Yamasaki R, Nasholds W, Schneider H, Apicella MA. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae: IgM monoclonal antibodies (3F11 and 1-1-M) recognize non-reducing termini of the LOS components. Mol Immunol. 1991;28:1233–1242. doi: 10.1016/0161-5890(91)90010-h. [DOI] [PubMed] [Google Scholar]
- 67.Blom AM, Hallstrom T, Riesbeck K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol. 2009;46:2808–2817. doi: 10.1016/j.molimm.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 68.Wakarchuk WW, Gilbert M, Martin A, Wu Y, Brisson JR, Thibault P, Richards JC. Structure of an alpha-2,6-sialylated lipooligosaccharide from Neisseria meningitidis immunotype L1. Eur J Biochem. 1998;254:626–633. doi: 10.1046/j.1432-1327.1998.2540626.x. [DOI] [PubMed] [Google Scholar]
- 69.Mandrell RE. Further antigenic similarities of Neisseria gonorrhoeae lipooligosaccharides and human glycosphingolipids. Infect Immun. 1992;60:3017–3020. doi: 10.1128/iai.60.7.3017-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.