Abstract
Dietary lipids and their metabolites activate members of the peroxisome proliferative-activated receptor (PPAR) family of transcription factors and are critical for colonic health. The PPARα isoform plays a vital role in regulating inflammation in various disease settings, but its role in intestinal inflammation, commensal homeostasis and mucosal immunity in the gut are unclear. Here, we demonstrate that the PPARα pathway in innate immune cells orchestrates gut mucosal immunity and commensal homeostasis by regulating the expression of IL-22 and the anti-microbial peptides RegIIIβ, RegIIIγ and calprotectin. In addition, the PPARα pathway is critical for imparting regulatory phenotype in intestinal macrophages. PPARα deficiency in mice resulted in commensal dysbiosis in the gut resulting in microbiota-dependent increase in the expression of inflammatory cytokines and enhanced susceptibility to intestinal inflammation. Pharmacological activation of this pathway decreased the expression of inflammatory cytokines and ameliorated colonic inflammation. Together, these findings identify a new important innate immune function for the PPARα signaling pathway in regulating intestinal inflammation, mucosal immunity and commensal homeostasis. Thus, the manipulation of the PPARα pathway could provide novel opportunities for enhancing mucosal immunity and treating intestinal inflammation.
Introduction
Dietary components regulate microbial composition and immunity in the intestine(1). Accumulating evidence also suggests that intestinal inflammation is inextricably linked to altered microbial composition and loss of immune homeostasis (1, 2). Dietary lipids such as saturated and unsaturated long chain fatty acids are widely present in the intestine and are critical for colonic health and for limiting inflammation (3–5). The PPAR family of nuclear receptors are lipid ligand-activated transcription factors that mediate the effects of dietary lipids and their metabolites (6). The PPAR family includes three isoforms, namely PPARα, PPARβ/δ and PPARγ, that are differentially expressed by various immune cells. For example, macrophages express high levels of PPARα, whereas activated T cells, dendritic cells and intestinal epithelial cells express high levels of the PPARγ isoform (7, 8). Upon activation, all three isoforms bind to the peroxisome proliferator response element (PPRE) as a heterodimer with retinoid X receptor (RXR) and regulate target gene expression (7, 8). However, little is known about the roles of these transcription factors in regulating the balance between immune response and tolerance to commensal microbiota in the gut. In addition, the molecular mechanisms by which they regulate commensal homeostasis and intestinal inflammation are still unknown.
The most widely studied functions of PPARα is its ability to regulate key genes involved in lipid and glucose metabolism (9–11). Emerging evidence from genome-wide analysis in the gut on the PPARα pathway has revealed that this pathway is highly active in both human and mouse intestine (9–11). PPARα agonist treatment was shown to suppress inflammation in different disease settings such as psoriasis (12), atherosclerosis (13), and EAE (14) and in chemically-induced colitis (6, 11). Similarly, genetic ablation of PPARα in mice resulted in increased susceptibility to chemically-induced intestinal inflammation (15–18). In contrast, other studies have shown an inflammatory role for PPARα in the intestine(19, 20). Whether and how the PPARα pathway impacts commensal and immune homeostasis in the intestine is unclear. Furthermore, the role of PPARα in the regulation of commensal homeostasis and mucosal immunity has not been studied before. We hypothesized that the PPARα signaling pathway in the intestine is critical for maintaining a delicate balance between immune tolerance and immune response to commensal microbiota in the gut.
In the current study we show that the PPARα pathway in innate immune cells play an important role in maintaining barrier immunity, commensal homeostasis and in suppressing intestinal inflammation. This is mediated through the expression of IL-22 in innate immune cells, which in turn promotes the expression of anti-microbial peptides that are critical for limiting inflammatory responses to commensal microbiota. Furthermore, our data also show that the PPARα pathway in intestinal macrophages is critical for suppressing the expression of inflammatory cytokines that drive Th17/Th1 responses thereby suppressing inflammation to gut microbiota. Taken together, these findings demonstrate an important role for the PPARα pathway in innate immune cells in shaping the balance between immunity and tolerance towards intestinal microbiota, which is critical for optimal host health.
Methods
Mice
C57BL/6 (B6), PPARα−/− (αKO) B6 mice and Rag1−/− B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), OT-II (Rag 2−/−) B6 mice were purchased from Taconic and PPRE-Luc transgenic mice (21) were originally obtained from Charles River Laboratories and bred on-site. PPARα+/− B6 mice were crossed to obtain littermates αKO PPARα+/+ (WT) and PPARα−/−(αKO), and were caged separately upon weaning. Likewise, Rag1/ PPARα+/ − B6 mice were crossed to obtain littermates Rag1−/− PPARα+/+ (Rag1) and Rag1−/−/ PPARα−/− (Rag1/αKO), and were caged separately upon weaning. All the experiments were carried out with age matched littermate controls unless specified otherwise. All the mice were housed under specific pathogen-free conditions in the Laboratory Animal Services of Georgia Regents University. Animal care protocols were approved by the Institutional Animal Care and Use Committee of Augusta University.
Antibodies and reagents
Antibodies against mouse CD3 (145-2C11), CD4 (GK1.5), CD8a (53-6.7), NK1.1 (PK136), CD335 (29A1.4), CD19 (1D3), CD45 (30-F11), Foxp3 (FJK-16s), IL-10 (JES5-16E3), CD11c (N418), CD11b (M1/70), I-Ab ( 25-9-17), CD90.1 (HIS51), V alpha 2 TCR (B20.1), V beta 5.1/5.2 TCR (MR9-4), IFN-γ (XMG1.2), IL-22 (1H8PWSR) and IL17A (17B7) were purchased from eBioscience. PPARα antibody was obtained from Cell Signaling Technology. PPARα antagonist GW6471 and orally active PPARα agonists GW7647 were purchased from Tacoris. OVA323−339 (ISQVHAAHAEINEAGR) peptide was purchased from Anaspec.
CD45RBhigh CD4+ T cells transfers
CD4+ T cells from WT mouse spleen and lymph nodes (inguinal and axillary) were enriched using CD4-specific microbeads and MACS column (Miltenyi Biotech; Auburn, CA), and then naïve CD4+ T cells were further purified by FACS-sorting to collect a population of cells that were CD4+ CD45RBhigh CD25− and CD4+ CD25+. Approximately 3×105 CD4+ CD45RBhigh CD25− cells were injected i.p. into the indicated recipient Rag1−/− or Rag1−/− PPARα−/− mice. In some experiments, 3×105 CD4+ CD45RBhigh CD25− cells were co-transferred with 3×105 CD4+ CD25+ cells. Mice were then monitored for body weight twice a week and any mice showing >15% decrease in initial weight were considered to have reached the experimental end point and were humanely euthanized. In some experiments two week post transfer of T cells, mice were treated orally with PPARα agonist (GW7647; 10mg/kg) twice weekly at indicated time points.
Induction of DSS-induced colonic inflammation
Rag1−/− or Rag1−/− PPARα−/− mice were fed with 3% (w/v) DSS in their drinking water for 6 days and sacrificed at day 9. Mice were monitored for weight changes, diarrhea and rectal bleeding as previously described(22, 23). Diarrhea was scored as (0) normal stool; (1) soft but formed pellet; (2) very soft pellet; (3) diarrhea (no pellet), or; (4) dysenteric diarrhea. Rectal bleeding was recorded as (0) no bleeding; (2) presence of occult blood in stool, or; (4) gross macroscopic bleeding.
Lymphocyte preparation and flow cytometry
Lamina propria (LP) lymphocytes from colons were isolated as described in our previous study (24). Isolated LP lymphocytes were collected, washed, and stained with antibodies specific for mouse CD4 and Foxp3, and analyzed by FACS. Briefly, single-cell suspensions from lymph nodes, spleen and LP were resuspended in PBS containing 5% FBS. After incubation for 15 min at 4ºC with the blocking Ab 2.4G2 (anti-FcγRIII/I), the cells were stained with the appropriately labeled Abs. Samples were then washed twice in PBS containing 5% FBS. In some experiments, mononuclear cells from colonic LP or spleen were cultured with PMA plus ionomycin in the presence of GolgiStop and Golgiplug for 5 hr. The cells were then stained for CD4 followed by intracellular staining of IFN-γ, IL-17A, IL-22 and IL-10.
Antibiotic-treatment of mice
Antibiotic-treatment of mice was performed as described in our previous study (24). In brief, WT, αKO, Rag1 or Rag1/αKO mice were fed with an antibiotic cocktail (ampicilin-1g/L, 1 g/l metronidazole, neomycin sulfate-1g/L and vancomycin 0.5g/L ) in drinking water for six weeks. All antibiotics were purchased from Sigma-Aldrich.
Ex vivo colon culture and ELISAs
Approximately, 1 cm-long sections of the ascending colon were excised, removed of feces, washed three times with sterile HBSS, and then longitudinally opened. The colon sections were then placed into culture in complete RPMI media (2% FBS, L-glutamine, penicillin, streptomycin and tetracycline) and cultured at 37ºC with 5% CO2. Supernatants were collected after two days, and the cytokine concentrations determined by ELISA. IL-17, IL-6, IL-12 (p40), IL-12 (p70), IL-10, TNF-α, IFN-γ and IL-1β in culture supernatants were quantitated using BD Biosciences ELISA kits, and IL-22 was measured using a Bio-Legend ELISA kit.
Cell Culture
Colonic macrophage and OT-II CD4+ T cell co-culture experiments were preformed as described previously(24). Sorted colonic macrophages (Mφs) (105) were cultured together with naive CD4+CD62L+OT-II T cells (105) and OVA (5 μg/ml) in 200 μl RPMI 1640 complete medium in 96-well round-bottom plates. Cell culture supernatants were analyzed after 90 h for indicated cytokine production by ELISA, and cells were restimulated with PMA/Ionomycin for intracellular cytokine staining. In some experiments sorted colonic Mφs (104) were cultured together with colonic CD3− CD19− NK1.1− NKp46+ ILC3 cells (104) in 200 μl RPMI 1640 complete medium in 96-well round-bottom plates. Cell culture supernatants were analyzed after 48 h for IL-22 production by ELISA
Luciferase Enzyme assay
Luciferase enzyme reporter assay on intestinal tissues was performed as described in previous study(25). Luciferase enzyme activity in the tissue extract was measured by the Luciferase system, according to the manufacturer’s instructions (Promega).
Histopathology and Immunohistochemistry
Sections (5 μm) from formalin-fixed and paraffin-embedded colons were placed onto glass slides. H&E-stained sections were blindly scored for severity of colonic inflammation as described previously(26). The parameter used were (a) LP inflammation (0–3); (b) goblet cell loss (0–2); (c) abnormal crypts (0–3); (d) crypt abscesses (0–1); (e) mucosal erosion or ulceration (0–1) and (f) submucosal spread to transmural inflammation (0–4). The individual scores from each parameter were summed to derive histological score for colonic inflammation (maximum score 14).
Bacterial DNA Extraction
Quantification of indicated bacterial groups in feces of WT, Rag1, αKO and Rag1/αKO mice by qPCR were performed as described previously (23, 27). Fecal pellets were collected from mice and bacterial DNA was extracted with the QIAamp DNA Stool Kit (QIAGEN). Quantitative PCR for the 16S rRNA gene was performed with SYBR Green (Bio-Rad). Amounts of indicated bacteria groups were first normalized to that of total bacterial DNA. Abundance of each bacterial group in the feces from WT or Rag1 mice was taken as 1 to calculate the relative abundances of corresponding bacterial group in feces from αKO or Rag1/αKO mice. Reactions were run with the MyiQ5 ICycler Real-Time PCR Detection System (Bio-Rad). Primers used in this study have been described previously(23, 27).
Real-time PCR
Total mRNA was isolated from colon or indicated cell type using the Omega Total RNA Kit according to the manufacturer’s protocol. cDNA was generated using the RNA to cDNA Ecodry Premix Kit (Clontech) according to the manufacturer’s protocol. cDNA was used as a template for quantitative real-time PCR using SYBR Green Master Mix (Roche), and gene-specific primers(27, 28). PCR analysis was performed using a MyiQ5 ICycler (BioRad). Gene expression was calculated relative to Gapdh.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software. An unpaired one -tailed Student’s t test was used to determine statistical significance for mRNA expression levels, Treg percentages and cytokines released by various cell types between different groups. A P value less than 0.05 (*) was considered to be significant, a P value less than 0.01 (**) was considered to be very significant, and a P value less than 0.001 (***) was considered to be extremely significant.
Results
PPARα deficiency enhances susceptibility to colonic inflammation
Since ligands that activate the PPARα pathway are widely present in the gut, we sought to determine whether the PPARα pathway is active in the intestine using PPRE-luciferase (PPRE-Luc) reporter mice (21). We observed much higher levels of reporter gene expression in the small intestine and colon compared to the spleen (Fig 1A). Interestingly, the colon showed significantly higher reporter activity compared to the small intestine (Fig 1A). Also, PPARα inhibitor (GW 6471) treatment markedly reduced the reporter gene activity both in the small intestine and colon. Thus, suggesting that the PPARα signaling pathway is highly active in the intestine under homeostatitc conditions (Fig 1A).
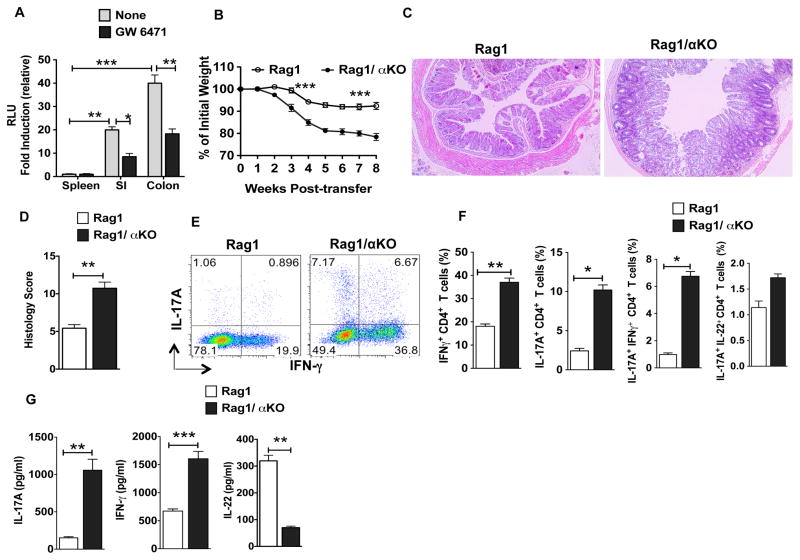
Figure 1. The absence of PPARα signaling exacerbates susceptibility to T cell-mediated colitis.
(A) Relative fold induction of luciferase reporter gene activity (representing PPAR activity) in the SI and colon as compared to spleen of PPRE-luciferase reporter mice treated with or without PPARα inhibitor (GW6471) (n= 3). (B) Percent weight change compared to initial weight for Rag1KO and Rag1/αKO mice at indicated time points post CD4+CD25−CD45RBhi T cell adoptive transfer (n=8). (C) Representative colon histology of Rag1KO and Rag1/αKO mice on 6 weeks post naïve CD4+ T cell transfer (H&E staining with original magnification, 100X). Histology scores are shown in (D) and data are representative of two independent experiments. (E, F) Representative FACS plots or frequencies of colonic IL-17A+, IFN-γ+, IL-17A+IFN-γ+ and IL-22+ IL-17A+ CD4+ T cells from Rag1KO and Rag1/αKO mice on 6 weeks post-transfer of naïve CD4+ T cell (n=8). (G) Excised colon samples in panel D were cultured for 2 days ex vivo, and the secreted IL-17A, IFN-γ and IL-22 cytokine levels in the culture supernatants were quantified by ELISA. The error bars indicate mean ± SEM of 8 mice/group. *p<0.05; **p<0.01; ***p<0.001.
Next, we tested whether PPARα regulates intestinal inflammation using a well-characterized CD45RBhiCD4+ T-cell-transfer colitis model in which the colitis is caused by disruption of T cell homeostasis with uncontrolled Th1 and Th17 responses to commensal microbiota (29, 30). Thus, we adoptively transferred wild-type CD45RBhiCD4+ T-cells into Rag1KO (Rag1−/−) and Rag1/αKO (Rag1−/− X PPARα−/−) mice, and monitored for clinical signs of colitis. When compared to Rag1 mice, Rag1/αKO mice displayed early onset of wasting disease starting by 2 weeks post-T cell transfer (Fig 1B). In addition, Rag1/αKO mice showed increased colitis severity, as revealed by diarrhea, anal inflammation, and severe destruction of colonic tissues (Fig 1C, D). Consistent with these observations, the percentages of IFNγ+ and IL-17A+ CD4+ T cells were markedly increased in the colon of Rag1/αKO mice compared to those of control mice (Fig 1E, F). Further examination of effector CD4+ T cells revealed that the percentage of IFNγ+-co- expressing IL-17A+ cells was substantially higher in Rag1/αKO mice than in Rag1KO mice (Fig 1E, F). In contrast, the percentage of IL-17A+ CD4+ T cells co-expressing IL-22 was not significantly different between the two groups (Fig 1F). Consistent with these results, colons of Rag1/αKO colitic mice produced higher levels of IL-17A and IFN-γ ex vivo but also produced much lower levels of IL-22 compared to control colons (Fig 1G). In contrast, transfer of CD4+ CD25+ T cells with CD45RBhiCD4+ T cells from WT mice into Rag1KO and Rag1/αKO mice suppressed the colitis development (Supplemental Fig. 1A). In addition, adoptive transfer of WT or αKO CD45RBhiCD4+ T-cells into Rag1KO mice resulted in similar levels of disease severity suggesting that PPARα expression in T cells is dispensable in this model of intestinal inflammation (data not shown).
PPARα signaling promotes IL-10 expression and suppresses proinflammatory cytokine expression in the colon
We next used chemical-(DSS) induced colitis model to determine whether or not the PPARα pathway in innate immune cells regulates intestinal inflammation, as inflammatory cytokines produced by innate immune cells present in the gut microenvironment drives colitis in this model of intestinal inflammation (31). As shown in Fig 2A–C, Rag1/αKO deficient mice were more susceptible to DSS-induced colitis compared to Rag1KO mice. Accordingly, Rag1/αKO showed more severe weight loss, diarrhea and rectal bleeding compared to the Rag1 mice (Fig. 2A–C). Moreover, DSS treatment of Rag1/αKO resulted in significant reduction in colon length compared to colons of the Rag1KO mice (Fig. 2D). Likewise, histopathological analysis of colons of DSS-treated Rag1/αKO mice showed extensive damage to mucosa with epithelial erosion, loss of crypts and infiltration of immune cells compared to colons of DSS-treated Rag1 mice (Fig. 2E).
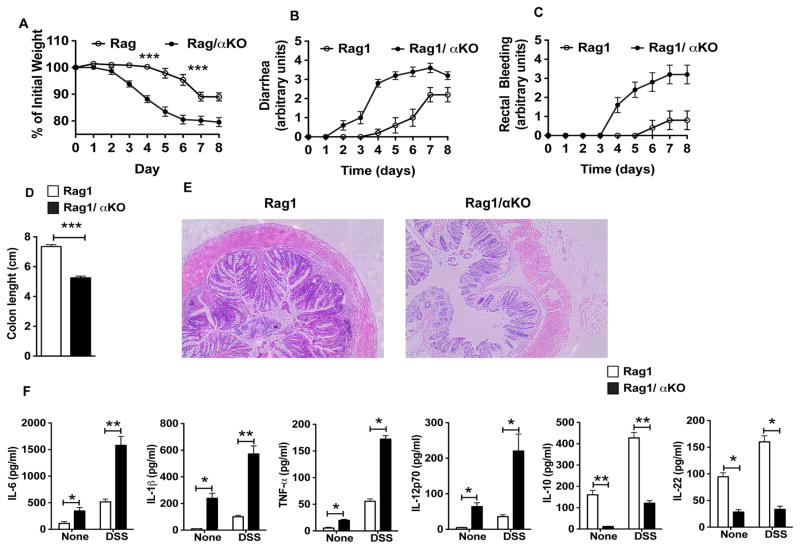
Figure 2. Increased susceptibility of Rag1/αKO mice to DSS-induced colonic inflammation.
Rag1KO and Rag1/αKO mice were treated with 3% DSS in drinking water for 6 days and at day 9 colons of mice were analyzed for inflammation. (A–D) Change in body weight, diarrhea, rectal bleeding and colon length (day 9) of Rag1KO and Rag1/αKO mice (n≥6). (E) Representative images of H&E-stained colonic sections from DSS treated Rag1KO and Rag1/αKO mice (day 9, original magnification, 100X). (F) Excised colon samples in panel D were cultured for 2 days ex vivo, and the secreted IL-6, TNF-α, IL-1β, IL-12p70, IL-10 and IL-22 cytokine levels in the culture supernatants were quantified by ELISA. The error bars indicate mean ±SEM of 5–6 mice/group. *p<0.05; **p<0.01; ***p<0.001.
We analyzed the expression levels of various inflammatory and anti-inflammatory cytokines in the colon of Rag1/αKO and Rag1 mice treated with or without DSS. Colon explant culture showed that αKO mouse colons released lower levels of IL-10 and IL-22, and higher levels of inflammatory cytokines, such as IL-1β, IL-6 and TNF-α compared to Rag1KO mouse colons under homeostatic condition as well as upon DSS treatment (Fig 2F). Collectively, these results suggest that the absence of PPARα leads to an imbalance in the expression of pro-inflammatory versus immune regulatory cytokines in the intestine.
PPARα limits Th1 and Th17 effector responses against gut microflora
The type of cytokine milieu present in the gut microenvironment drives the differentiation and expansion of effector T cells and regulatory T cells(1, 32). Therefore based on the above results, we sought to determine whether PPARα signaling is critical for intestinal homeostasis. Because Th1/Th17 cells in the colon promote inflammation(32), we quantified the frequency of Th1/Th17 cells in the intestine of αKO and WT mice under steady state conditions. Remarkably, αKO mice displayed higher frequencies of CD4+ cells producing IFNγ or IL-17A in the colonic LP compared to WT mice (Fig. 3A, B). Further analysis of T cells reveled that the frequency of IL-17-expressing cells co-expressing IFN-γ among CD4+ cells was substantially higher in αKO mice than in WT mice (Fig. 3A). Consistent with these results, we also observed significantly higher levels of IFN-γ and IL-17A cytokines, and lower levels of IL-10 and IL-22 in the αKO mouse colon compared to the WT mouse colon (Fig. 3C). Interestingly, there was no significant difference in the frequencies of Th1 and Th17 cells in the spleens of αKO versus WT mice (data not shown). These observations suggest that an increase in Th1 and Th17 cells in αKO mice is specific to the intestine.
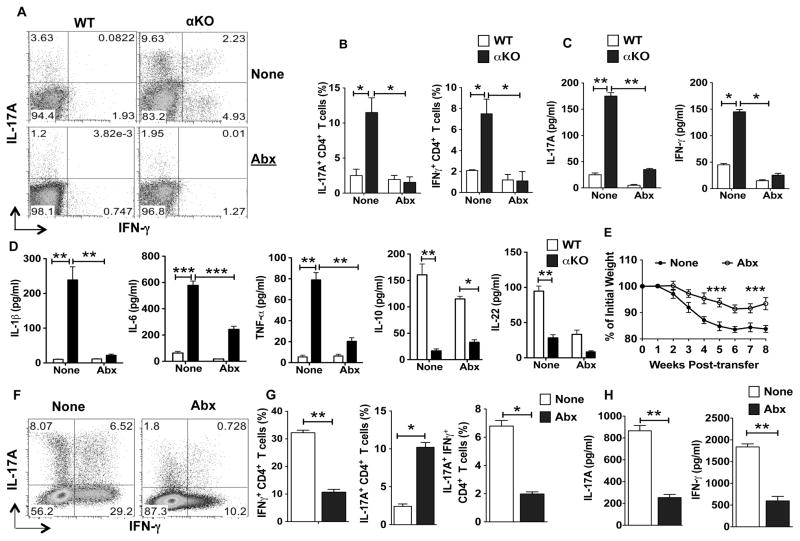
Figure 3. PPARα signaling limits inflammatory responses to commensal microbiota.
(A, B) FACS plot representing percentages or cumulative frequencies of CD4+ T cells positive for IL-17A and IFN-γ cells isolated from colon of WT (littermate control) and αKO mice treated with (Abx, bottom panels) or without (None, top panels) antibiotics treatment (n=8). (C, D) Excised colon samples in panel A were cultured for 2 days ex vivo, and then the secreted IL-17A, IFN-γ, IL-1β, TNF-α, IL-6, IL-10 and IL-22 amounts in the culture supernatants were quantified by ELISA (n=5). (E–H) Rag1/αKO mice adoptively transferred with FACS sorted CD4+ CD25−CD45RBhi T cells from WT mice and were left untreated or treated with antibiotics for 6 weeks. (E) Percent weight change as compared to initial weight for antibiotic treated and untreated Rag1/αKO mice at various weeks post naïve CD4+ T cell adoptive transfer (n=8). (F,G) Representative FACS plots or frequencies of colonic IL-17A+, IFN-γ+ and IL-17A+IFN-γ+ CD4+ T cells from Rag1/αKO mice on 6 weeks post-transfer of naïve CD4+ T cell from panel I (n=8). (H) Excised colon samples in panel F were cultured for 2 days ex vivo, and the secreted IL-17A and IFN-γ cytokine levels in the culture supernatants were quantified by ELISA. The bar indicates mean ±SEM of 8 mice/group *p<0.05; **p<0.01; ***p<0.001.
Microflora in the gut play a critical role in the induction of CD4+ effector T cells and regulatory T cells (1, 2). Also, loss of immune tolerance to intestinal microflora or commensal dysbiosis results in increased levels of Th17 and Th1 cells in the intestine (2, 32). Therefore, we next assessed whether increased Th17 /Th1 cell frequencies and inflammatory cytokine expression in the colon of αKO mice is due to gut microflora by performing microbiota depletion studies using antibiotics (24). Antibiotic treatment resulted in marked reduction in the frequencies of Th17 and Th1 cells in the colon of αKO mice as compared to untreated control mice (Fig 3A, B). Consistent with this observation, colons of antibiotic-treated αKO mice produced significantly less IL-17A and IFN-γ compared to control colons (Fig 3C). Likewise, the depletion of intestinal microflora resulted in a significant decrease in the inflammatory cytokines IL-1β, IL-6 and TNF-α in the αKO colons compared to control colons (Fig 3D). Interestingly, antibiotic treatment in WT mice resulted in marked reduction in IL-22 and IL-10 cytokine levels compared to untreated controls (Fig 3D). These observations suggest that under steady state, commensal microfloral induces IL-22 and IL-10 production in the gut. In addition, these observations provide evidence that the PPARα pathway is critical for limiting the expression of inflammatory factors in response to commensal microbiota.
Since the absence of PPARα caused differences in gut Th17/Th1 levels in an antibiotic (i.e. microflora)-dependent manner, we reasoned that the more severe colitis of Rag1/αKO mice in the Rag1KO mouse model was due to differences in gut microflora. To test this, we treated Rag1/αKO mice with or without antibiotic cocktail and then induced colitis as above. Antibiotic treatment significantly delayed the onset of colitis, with reduced weight loss and disease severity in Rag1/αKO mice compared to the untreated mice (Fig 3E). Consistent with this observation, the percentages of IFNγ+ and IL-17A+ CD4+ T cells were markedly reduced in antibiotic-treated mice compared to those of control mice (Fig 3F, G). Furthermore, colons of antibiotic-treated Rag1/αKO mice produced lower levels of IL-17A and IFN-γ compared to control colons (Fig 3H). These observations suggests that increased inflammatory response to the commensals in Rag1/αKO mice leads to more severe intestinal inflammation and explains the reduction in intestinal inflammation upon antibiotic treatment in Rag1/αKO mice. Taken together our data suggest that lack of PPARα results in increased production of inflammatory cytokines that induce effector Th17/Th1 cell responses in against gut microflora.
Colonic macrophages from αKO mice are potent inducers of Th1 and Th17 differentiation
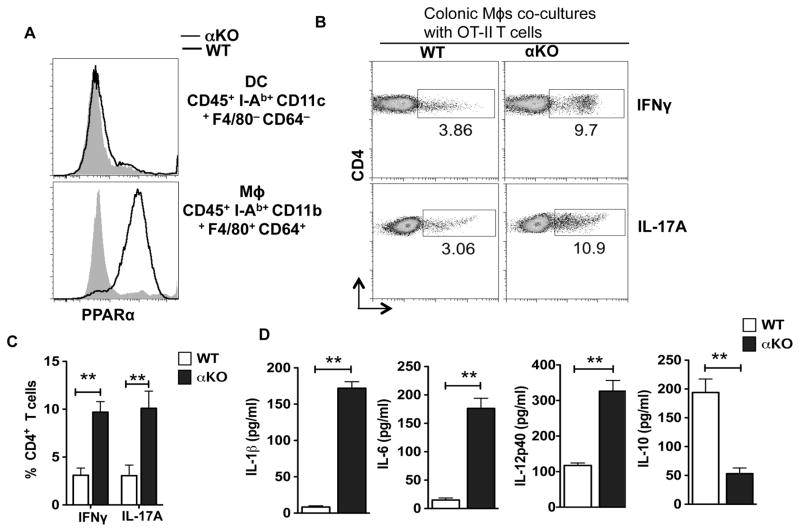
Intestinal DCs and macrophages play a critical role in maintaining balance a between regulatory and effector T cells (32–34). To gain insights into the mechanisms of increased Th1 and Th17 cells, and enhanced expression of pro-inflammatory cytokines in the intestine of αKO mice, we evaluated the expression of PPARα by intestinal antigen presenting cells such as DCs (CD45+ I-Ab+ CD11c+ F4/80− CD64−) and macrophages (CD45+ I-Ab+ CD11b+ F4/80+ CD64+ ) (35). We observed that WT colonic macrophages express high levels of PPARα compared to αKO colonic macrophages (Fig 4A). In contrast, colonic DCs did not show any detectable amounts of PPARα (Fig 4A). Intestinal macrophages express immune regulatory factors such as IL-10 and retinoic acid that preferentially drive T regulatory responses while suppressing Th1/Th17 responses (36). Since intestinal macrophages express high levels of PPARα, we reasoned that increased colonic Th1 and Th17 cells in αKO mice are due to the loss of anti-inflammatory phenotype of colonic macrophages. Therefore, we tested the ability of colonic macrophages isolated from αKO and WT mice to promote the differentiation of naïve OT-II CD4 cells into Th1/Th17 cells. Colonic macrophages isolated from αKO mice were more potent in inducing inflammatory IFNγ and IL-17A producing T cells as compared to control macrophages from WT mice (Fig. 4B, C). Consistent with this observation, colonic macrophages from αKO mice produced higher levels of inflammatory cytokines such as IL-6, IL-1β and IL-12 that drive differentiation of Th1 and Th17 cells (Fig 4D). In contrast, PPARα-deficient colonic macrophages produced low levels of IL-10, which suppresses the differentiation of Th1/Th17 cells. Taken together, these results suggest that PPARα-signaling in intestinal macrophages imparts an immune regulatory phenotype and limits the expression of inflammatory cytokines.
Figure 4. PPARα signaling in colonic macrophages limits the expression of inflammatory cytokines and suppresses Th1/Th17 cells differentiation.
(A) FACS plot showing intracellular expression levels of PPARα protein in DCs and macrophages isolated from the colon of the WT and αKO mice. Data are from one experiment representative of three. (B) Intracellular expression of IL-17 and IFN-γ in naïve CD4+OT-II T cells stimulated to differentiate in vitro by colonic macrophages isolated from WT and αKO mice, in the presence of TGF-β (1 ng/ml). Numbers in FACS plots represent percentage of cells positive for the indicated protein. Data are from one experiment representative of three (C) Cumulative frequencies of CD4+OT-II T cells positive for IL-17, and IFN-γ as described in panel B (n=3). (D) Sorted colonic macrophages from WT and αKO mice were cultured for 2 days ex vivo, and IL-1β, IL-12p40, IL-6 and IL-10 cytokine amounts in the culture supernatants were quantified by ELISA (n=4). The bar indicates mean ±SEM. *p<0.05; **p<0.01; ***p<0.001.
PPARα regulates IL-22 expression and anti-microbial peptide expression in the gut
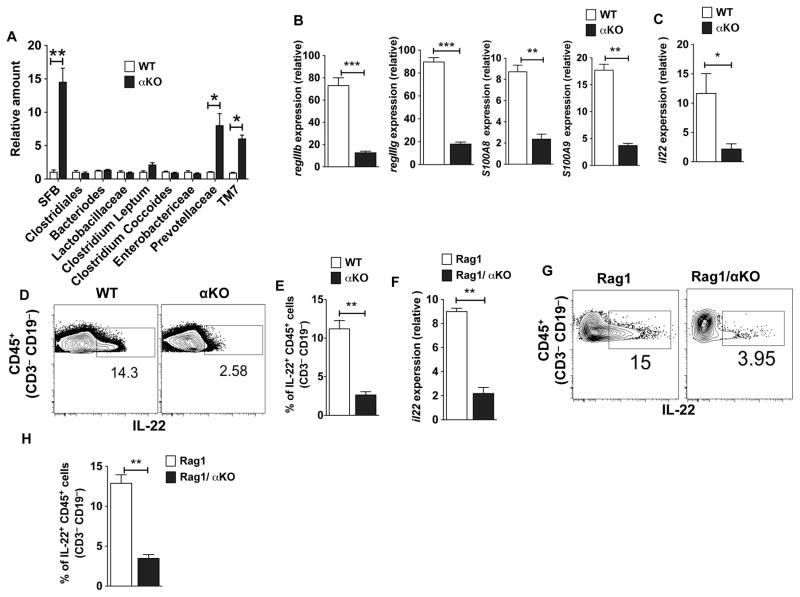
Since depleting microbiota reduced both Th17/Th1 cells and inflammatory cytokine levels in the colon of αKO mice, we investigated whether an increase in CD4+ inflammatory T cells in the colon of αKO mice is due to commensal dysbiosis. To study this, we quantified the relative levels of different bacterial species in the feces of αKO and WT mice. Strikingly, we observed an increased presence of SFB, Prevotellaceae and TM7 groups of commensal bacteria in αKO as mice compared to the WT mice (Fig 5A). Bacteroides and Clostridiales groups of bacteria were present in comparable numbers in feces of both WT and αKO mice. We also observed an increase in the presence of SFB, Prevotellaceae and TM7 groups of commensal bacteria in the feces of Rag1/αKO mice (Supplementary Fig. 1B). This result provides evidence for microbial dysbiosis in αKO mice and explains enhanced susceptibility of αKO mice to colonic inflammation.
Figure 5. PPARα regulates the expression of IL-22 and anti-microbial peptides in the intestine.
(A) Relative quantification of different bacterial species in the fecal material from αKO mice compared with WT mice as analyzed by quantitative RT-PCR analysis (n= 5). (B, C) Quantitative real-time PCR analysis of regIIIb, regIIIg, S100A8, S100A9 and il22 mRNA expression relative to GAPDH in colon of αKO mice and WT. (D, E) FACS plot representing percentages or cumulative frequencies of IL-22 producing cells gated on CD45+ CD3− CD19− cells isolated from colons of WT (littermate control) and αKO mice. (F) Quantitative real-time PCR analysis of il22 mRNA expression relative to GAPDH in colon of Rag1 (littermate control) and Rag1/ αKO mice. (G) Representative FACS plot and (H) frequencies for IL-22 producing cells gated on CD45+ CD3− CD19− cells isolated from colons of Rag1 and Rag1/αKO mice. Error bars show mean values ± SEM. *p<0.05; **p<0.01; ***p<0.001.
The expression of anti-microbial proteins, such as RegIIIβ, RegIIIγ and calprotecin (S100A8/S100A9), by intestinal epithelial cells is critical for barrier immunity and commensal homeostasis. Thus, we next investigated whether commensal dysbiosis in αKO mice is due to changes in the expression levels of these antimicrobial peptides. Accordingly, colon of αKO mouse expressed significantly lower levels of RegIIIβ, RegIIIγ, S100A8 and S100A9 compared to the colon of WT mouse (Fig 5B).
IL-22 is a key cytokine that regulates the expression of RegIIIβ, RegIIIγ, S100A8 and S100A9 (37–39). Since αKO mice expressed low levels of these antimicrobial proteins, we reasoned that PPARα signaling might regulate IL-22 expression in the colon. Therefore, we examined colonic IL-22 expression levels in αKO and WT mice. Accordingly, there were lower levels of IL-22 expression in αKO mouse colon compared to control mouse colon (Fig 5C). Past studies have shown that both innate and adaptive immune cells express IL-22 (39–42). Innate immune cells (CD45+ CD3− CD19− ) isolated from the colon of αKO mice produced lower levels of IL-22 compared to those from WT mice (Fig 5D, E), indicating a critical role for PPARα in IL-22 expression by innate immune cells. In line with these observations, innate immune cells (CD45+ CD3− CD19− ) isolated from the colon of Rag1−/−αKO mice expressed low levels of IL-22 compared to those of controls (Fig. 5F–H). Collectively, these results demonstrate that the PPARα signaling pathway is critical for IL-22 expression by innate immune cells in the gut.
PPARα regulates IL-22 produced by NKp46+ innate lymphoid cells
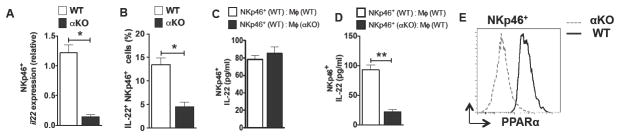
NKp46+ ILC3 cells are the major producers of IL-22 under homeostatic conditions in the gut(43). So, we determined whether PPARα regulates IL-22 expression in these innate cells. Colonic NKp46+ ILC3 (CD45+ CD3− CD19− NK1.1−) cells isolated from αKO mice expressed markedly lower levels of IL-22 mRNA compared to WT NKp46+ ILC3 cells (Fig 6A). Consistent with this observation, the percentages of colonic IL-22+ NKp46+ ILC3 cells were markedly reduced in αKO mice compared to those of control mice (Fig 6B).
Figure 6. PPARα regulates IL-22 produced by NKp46+ innate lymphoid cells.
(A) Quantitative real-time PCR analysis of il22 mRNA expression relative to GAPDH in NKp46+ cells (CD3− CD19− NK1.1− NKp46+) isolated from colon of αKO and WT mice. (B) Frequencies for IL-22 producing cells gated on CD45+ CD3− CD19− NK1.1− NKp46+cells isolated from colons of αKO and WT mice (n=5). (C, D) Sorted colonic NKp46+ innate lymphoid cells and macrophages from WT and αKO mice were co-cultured for 2 days ex vivo, and IL-22 cytokine amounts in the culture supernatants were quantified by ELISA (n=4). (E) FACS plot showing intracellular expression levels of PPARα protein in NKp46+ innate cells isolated from the colon of WT (solid) and αKO (dashed) mice. Error bars show mean values ± SEM. *p<0.05; **p<0.01.
Recent studies have highlighted a critical role for intestinal macrophages in supporting IL-22 expression by innate lymphoid cells(44–46). Thus, we next tested whether PPARα regulates IL-22 produced by NKp46+ ILC3 cells directly or indirectly via macrophages. WT NKp46+ ILC3 cells co-cultured with WT or αKO colonic macrophages produced similar levels of IL-22 (Fig 6C). In contrast, αKO NKp46+ ILC3 cells co-cultured with WT colonic macrophages produced significantly lower levels of IL-22 compared to WT NKp46+ ILC3 cells (Fig 6D). Next, we evaluated whether colonic NKp46+ ILC3 cells express of PPARα. NKp46+ ILC3 cells isolated from the colon of WT mouse express markedly high levels of PPARα (Fig 6E). These results demonstrate that the PPARα signaling pathway is critical for IL-22 expression by NKp46+ ILC3 cells in the gut.
Pharmacological activation of PPARα ameliorates intestinal inflammation and enhances mucosal immunity
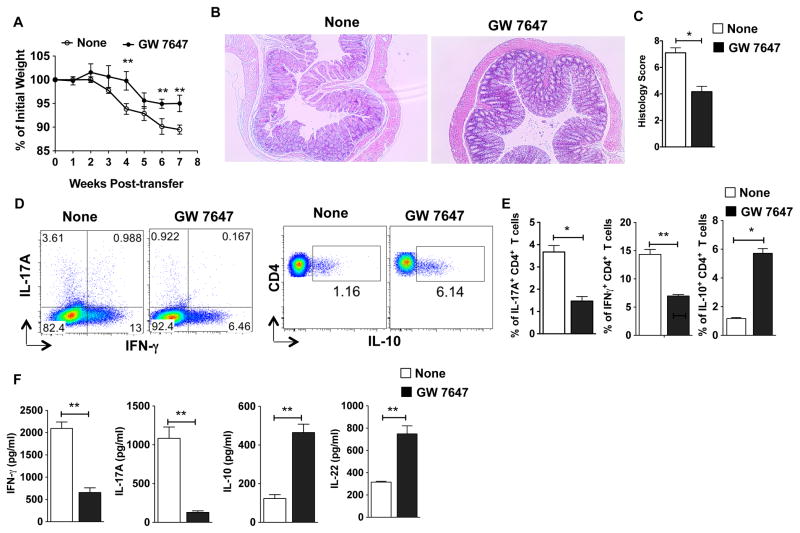
Next, we examined the effects of pharmacological activation of PPARα in the Rag-deficient T-cell-transfer model of colitis by treating mice with PPARα agonist with treatments starting 2 weeks post T cell transfer. As expected, control Rag1 mice adoptively transferred with naïve T cells showed rapid body weight loss around 4 weeks post-T cell transfer (Fig 7A). In contrast, PPARα agonist treatment significantly delayed disease onset and reduced disease severity (Fig 7B, C). In line with these observations, the percentages of IFNγ+ and IL-17A+ CD4+ T cells, as well as IL-17A and IFN-γ cytokine levels were markedly reduced in PPARα-agonist treated mice as compared to control mice (Fig 7D, E, F). In contrast, we observed a significant increase in IL-10+ CD4+ Tr1 cells , as well as colonic IL-10 and IL-22 cytokine levels upon PPARα-agonist treatment (Fig 6D, E, F). Consistent with this observation, PPARα agonist treatment resulted in increased expression of IL-22 responsive genes RegIIIβ, RegIIIγ, S100A8 and S100A9 in the colon (data not shown). Furthermore, innate cells isolated from PPARα agonist-treated mice had higher expression of IL-22 as compared to WT mice (data not shown). However, agonist treatment had no effect on diseases severity in Rag1/αKO (Supplemental Fig. 1C). Collectively, these results suggest that activation of the PPARα pathway promotes mucosal immunity and suppresses intestinal inflammation by regulating innate immune cell functions.
Figure 7. Pharmacological activation of PPARα ameliorates mucosal inflammation.
(A–F) CD45RBhiCD4+ T cells from WT mice were adoptively transferred into Rag1 mice. Animals were treated with PPARα agonist orally (GW7647; 10mg/kg; on Weeks 2,3,4) and monitored over a period of time for percent weight loss compared to initial weight. (A) Percent weight change for Rag1 mice treated with PPARα agonist compared with untreated mice at various weeks post naïve CD4+ T cell adoptive transfer (n=6). (B, C) Representative colon histology (H&E staining with original 10X magnification) and histology scores of Rag1 mice on 6 weeks post naïve CD4+ T cell transfer treated with or without PPARα agonist. (D, E) Representative FACS plots and frequencies of colonic IL-17A+, IFN-γ+ and IL-10+ cells from Rag1 treated with PPARα agonist compared with untreated mice on week 6 post naïve CD4+ T cell adoptive transfer (n=6). (G) Excised colon samples in panel C were cultured for 2 days ex vivo, and then the secreted IL-17A, IFN-γ, IL-10 and IL-22 cytokine amounts in the culture supernatants were quantified by ELISA. The bar indicates mean ±SEM of 6 mice/group. *p<0.05; **p<0.01; ***p<0.001.
Discussion
The current study defines an essential role for PPARα in the innate immune cell compartment, in regulating commensal homeostasis and suppressing colonic inflammation. In αKO mice, we observed substantial upregulation of Th17 and Th1 cells in the intestine and this is due to commensal dysbiosis. Accordingly, antibiotic treatment in αKO mice markedly decreased Th17 and Th1 cells in the intestine. The present study also implicates PPARα as a critical regulator of IL-22 production in NKp46+ ILC3 innate cells, which in turn influences the expression of anti-microbial peptides by epithelial cells and restores commensal homeostasis. In addition, PPARα signaling imparts regulatory phenotype in intestinal macrophages and limits the expression of inflammatory cytokines under homeostatic conditions.
Recent studies in mice have shown that loss of immune homeostasis or genetic modification of the host leads to microbial dysbiosis resulting in host susceptibility to colonic inflammation (23, 27, 47, 48). In addition, it is well documented that commensal microbiota-induced cytokine production by innate immune cells drives Th1/Th17/T regulatory responses in the gut (49–52). Our study demonstrates that genetic deletion of PPARα resulted in enhanced Th17/Th1 response in the intestine under steady-state conditions. In addition, PPARα-deficiency resulted in increased expression of pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α and IL-12 that drive Th1/Th17 cell differentiation. This is dependent on commensal microbiota, as antibiotics treatment markedly reduced the expression of inflammatory cytokines and Th1/Th17 cells in the intestine of αKO mice. Further analysis of microbial species in the stool revealed increased representation of SFB, Prevotellaceae and TM7 groups of commensal bacteria in αKO and Rag1/αKO mice compared to WT littermate controls. Just as in αKO mice here, past studies have shown that increased representation of SFB, Prevotellaceae and TM7 groups of commensal bacteria is associated with enhanced risk of colitis in mice(23, 27, 47). These observations provide evidence that increased inflammatory responses observed in PPARα-deficient mice is due to commensal dysbiosis.
IL-22 is also critical for maintaining epithelial cell barrier integrity, mucous production, and epithelial cell regeneration and repair (53). In mice, IL-22-deficiency is associated with commensal dysbiosis and aberrant expansion of SFB, Prevotellaceae and TM7 groups of commensal bacteria(27, 54). Under homeostatic conditions, NKp46+ ILC3 cells are the major producers of IL-22 in the gut and IL-22 deficiency alters colonic microbiota (54). NKp46+ ILC3 cells isolated from the intestine of αKO mice produced low levels of IL-22 compared to littermate controls. In addition, αKO mice expressed low levels of anti-microbial peptides RegIIIβ and RegIIIγ. IL-22 is a bifunctional cytokine with both pro-inflammatory and protective functions (53). IL-22 was shown to protect mice from DSS-induced colitis (55), CD45RBhigh T cell driven colitis (55, 56) and Th2 driven ulcerative colitis (57). Prior studies using DSS-induced colitis models have shown that αKO mice are more susceptible to intestinal inflammation whereas PPARα agonist treatment protected mice from colitis. However, the role of PPARα in regulating IL-22 was not studied in this system as well as in CD45RBhigh T cell driven colitis. In the current study we observed that PPARα-deficiency results in increased susceptibility to both DSS-induced colitis, whether on a Rag1KO background (Fig. 2) or not (data not shown), and CD45RBhigh T cell-driven colitis. PPARα agonist treatment of mice conferred protection against intestinal inflammation in both models. Consistent with these observations, deletion of PPARα resulted in significantly lower levels of IL-22 during inflammation whereas PPARα agonist treatment resulted in a significant increase in IL-22 during inflammation in both the disease settings.
An important unresolved question is how the PPARα pathway regulates IL-22 expression in innate immune cells. Recent studies have highlighted a critical role for intestinal macrophages in regulating IL-22 expression by ILC3 cells (44–46). WT NKp46+ ILC3 cells cultured with αKO macrophages produced IL-22 similar to the levels cultured with WT macrophages. However, culture of αKO NKp46+ ILC3 cells with WT macrophages resulted in reduced IL-22 expression compared to the WT NKp46+ ILC3 cells. These observations provide evidence that PPARα might directly regulate the transcription of IL-22 in NKp46+ ILC3 cells in the colon under steady- state conditions. Recent studies have shown that in ILC3 cells, Ahr regulates IL-22 expression and promotes mucosal immunity against SFB and C. rodentium (27, 58). Mice deficient in Ahr contain high levels of SFB in the intestine and are susceptible to C. rodentium infection (27). In addition, prior studies have shown that PPARα transcriptionally regulates Ahr expression(59, 60). So, it possible that PPARα might indirectly regulate IL-22 expression in ILC3 cells by regulating aryl hydrocarbon receptor (Ahr) expression. However, further studies are warranted to confirm a direct link between PPARα and Ahr in the regulation of IL-22 production.
Microbiota-induced IL-1β and IL-6 are critical for the development of intestinal Th-17 cells, and the gut-resident macrophage population is the main source of IL-1β (61). Since colonic macrophages express PPARα, it is possible that PPARα might play a critical role in regulating IL-1β and other inflammatory cytokines in macrophages in response to microbes under steady-state conditions. Accordingly, we observed that the absence of PPARα resulted in increased expression of IL-1β and other inflammatory cytokines. Consistent with this observation, depletion of microbiota in αKO mice markedly lowered inflammatory cytokines levels in the colon. Our study demonstrates that PPARα suppresses the expression of inflammatory cytokines in macrophages and promotes IL-22 expression in innate cells. This observation is consistent with a study that showed PPARα agonist treatment protects mice from DSS-induced colitis by suppressing the expression of inflammatory cytokines such as IL-1β, IL-6 and IFNγ (18). In addition, PPARα agonists are shown to inhibit NF-Kβ and AP-1 transcription factors critical for the induction of inflammatory cytokines that drive Th1/Th17 cell differentiation (62). However, other studies have shown an inflammatory role for PPARα in the intestine (19, 20). These differences might due to differences in the microbiota, housing conditions and the genetic background of the mice.
In summary, the current study indicates that the PPARα pathway plays a pivotal role in regulating commensal homeostasis by regulating the expression of IL-22 and anti-microbial peptides in the gut. In addition, deletion or disruption of the PPARα pathway altered colonic macrophage function, resulting in increased expression of inflammatory cytokines that drive Th1/Th17 responses and intestinal inflammation. Taken together, targeting the PPARα pathway may represent a promising strategy for the treatment of colitis and for enhancing mucosal immunity.
Supplementary Material
Acknowledgments
We thank Jeanene Pihkala, Jeane Silva and Ningchun Xu for technical help with FACS sorting and analysis; Janice Randall with mouse husbandry, as well as our colleagues in the Augusta University Cancer Immunology, Inflammation and Tolerance program for constructive comments on various aspects of this study.
This work was supported by National Institutes of Health Grants DK097271 (to SM).
References
- 1.Spencer SP, Belkaid Y. Dietary and commensal derived nutrients: shaping mucosal and systemic immunity. Current opinion in immunology. 2012;24:379–384. doi: 10.1016/j.coi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annual review of immunology. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viladomiu M, Hontecillas R, Yuan L, Lu P, Bassaganya-Riera J. Nutritional protective mechanisms against gut inflammation. The Journal of nutritional biochemistry. 2013;24:929–939. doi: 10.1016/j.jnutbio.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassaganya-Riera J, Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Current opinion in clinical nutrition and metabolic care. 2010;13:569–573. doi: 10.1097/MCO.0b013e32833b648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutrition journal. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends in endocrinology and metabolism: TEM. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nature reviews Immunology. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 8.Bertin B, Dubuquoy L, Colombel JF, Desreumaux P. PPAR-gamma in ulcerative colitis: a novel target for intervention. Current drug targets. 2013;14:1501–1507. doi: 10.2174/13894501113149990162. [DOI] [PubMed] [Google Scholar]
- 9.Bunger M, van den Bosch HM, van der Meijde J, Kersten S, Hooiveld GJ, Muller M. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiological genomics. 2007;30:192–204. doi: 10.1152/physiolgenomics.00198.2006. [DOI] [PubMed] [Google Scholar]
- 10.de Vogel-van den Bosch HM, Bunger M, de Groot PJ, Bosch-Vermeulen H, Hooiveld GJ, Muller M. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC genomics. 2008;9:231. doi: 10.1186/1471-2164-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riccardi L, Mazzon E, Bruscoli S, Esposito E, Crisafulli C, Di Paola R, Caminiti R, Riccardi C, Cuzzocrea S. Peroxisome proliferator-activated receptor-alpha modulates the anti-inflammatory effect of glucocorticoids in a model of inflammatory bowel disease in mice. Shock. 2009;31:308–316. doi: 10.1097/SHK.0b013e31818339e7. [DOI] [PubMed] [Google Scholar]
- 12.Dubrac S, Schmuth M. PPAR-alpha in cutaneous inflammation. Dermato-endocrinology. 2011;3:23–26. doi: 10.4161/derm.3.1.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochimica et biophysica acta. 2007;1771:972–982. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Gocke AR, Lovett-Racke A, Drew PD, Racke MK. PPAR Alpha Regulation of the Immune Response and Autoimmune Encephalomyelitis. PPAR research. 2008;2008:546753. doi: 10.1155/2008/546753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, Di Paola R, Mazzon E, Genovese T, Muia C, Centorrino T, Caputi AP. Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-alpha) in the development of inflammatory bowel disease in mice. Laboratory investigation; a journal of technical methods and pathology. 2004;84:1643–1654. doi: 10.1038/labinvest.3700185. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Kohno H, Yoshitani S, Takashima S, Okumura A, Murakami A, Hosokawa M. Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer research. 2001;61:2424–2428. [PubMed] [Google Scholar]
- 17.Lee JW, Bajwa PJ, Carson MJ, Jeske DR, Cong Y, Elson CO, Lytle C, Straus DS. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133:108–123. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 18.Azuma YT, Nishiyama K, Matsuo Y, Kuwamura M, Morioka A, Nakajima H, Takeuchi T. PPARalpha contributes to colonic protection in mice with DSS-induced colitis. International immunopharmacology. 2010;10:1261–1267. doi: 10.1016/j.intimp.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Qi Y, Jiang C, Tanaka N, Krausz KW, Brocker CN, Fang ZZ, Bredell BX, Shah YM, Gonzalez FJ. PPARalpha-dependent exacerbation of experimental colitis by the hypolipidemic drug fenofibrate. Am J Physiol Gastrointest Liver Physiol. 2014;307:G564–573. doi: 10.1152/ajpgi.00153.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G, Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 21.Ciana P, Biserni A, Tatangelo L, Tiveron C, Sciarroni AF, Ottobrini L, Maggi A. A novel peroxisome proliferator-activated receptor responsive element-luciferase reporter mouse reveals gender specificity of peroxisome proliferator-activated receptor activity in liver. Molecular endocrinology. 2007;21:388–400. doi: 10.1210/me.2006-0152. [DOI] [PubMed] [Google Scholar]
- 22.Manicassamy S, Manoharan I. Mouse models of acute and chronic colitis. Methods in molecular biology. 2014;1194:437–448. doi: 10.1007/978-1-4939-1215-5_25. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedusi V, Martorana F, Brambilla L, Maggi A, Rossi D. The peroxisome proliferator-activated receptor gamma (PPARgamma) controls natural protective mechanisms against lipid peroxidation in amyotrophic lateral sclerosis. The Journal of biological chemistry. 2012;287:35899–35911. doi: 10.1074/jbc.M112.366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlick KP, Ostanin DV, Furr KL, Laroux FS, Brown CM, Gray L, Kevil CG, Grisham MB. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. International immunology. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suryawanshi A, Manoharan I, Hong Y, Swafford D, Majumdar T, Taketo MM, Manicassamy B, Koni PA, Thangaraju M, Sun Z, Mellor AL, Munn DH, Manicassamy S. Canonical wnt signaling in dendritic cells regulates Th1/Th17 responses and suppresses autoimmune neuroinflammation. Journal of immunology. 2015;194:3295–3304. doi: 10.4049/jimmunol.1402691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. The Journal of experimental medicine. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. The Journal of experimental medicine. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiesler P, I, Fuss J, Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Flannigan KL, Geem D, Harusato A, Denning TL. Intestinal Antigen-Presenting Cells: Key Regulators of Immune Homeostasis and Inflammation. The American journal of pathology. 2015;185:1809–1819. doi: 10.1016/j.ajpath.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nature immunology. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 35.Harusato A, Flannigan KL, Geem D, Denning TL. Phenotypic and functional profiling of mouse intestinal antigen presenting cells. Journal of immunological methods. 2015;421:20–26. doi: 10.1016/j.jim.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 38.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature immunology. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 39.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? Journal of immunology. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 42.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Killig M, Glatzer T, Romagnani C. Recognition strategies of group 3 innate lymphoid cells. Front Immunol. 2014;5:142. doi: 10.3389/fimmu.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, Niess JH. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal immunology. 2013;6:177–188. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. The Journal of experimental medicine. 2014;211:1571–1583. doi: 10.1084/jem.20140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satoh-Takayama N, Serafini N, Verrier T, Rekiki A, Renauld JC, Frankel G, Di Santo JP. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 2014;41:776–788. doi: 10.1016/j.immuni.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouyang W, Valdez P. IL-22 in mucosal immunity. Mucosal immunology. 2008;1:335–338. doi: 10.1038/mi.2008.26. [DOI] [PubMed] [Google Scholar]
- 54.Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L, Flavell RA. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. Journal of immunology. 2013;190:5306–5312. doi: 10.4049/jimmunol.1300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eken A, Singh AK, Treuting PM, Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal immunology. 2014;7:143–154. doi: 10.1038/mi.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. The Journal of clinical investigation. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fallone F, Villard PH, Decome L, Seree E, Meo M, Chacon C, Durand A, Barra Y, Lacarelle B. PPARalpha activation potentiates AhR-induced CYP1A1 expression. Toxicology. 2005;216:122–128. doi: 10.1016/j.tox.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 60.Villard PH, Caverni S, Baanannou A, Khalil A, Martin PG, Penel C, Pineau T, Seree E, Barra Y. PPARalpha transcriptionally induces AhR expression in Caco-2, but represses AhR pro-inflammatory effects. Biochem Biophys Res Commun. 2007;364:896–901. doi: 10.1016/j.bbrc.2007.10.084. [DOI] [PubMed] [Google Scholar]
- 61.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. The Journal of experimental medicine. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bougarne N, Paumelle R, Caron S, Hennuyer N, Mansouri R, Gervois P, Staels B, Haegeman G, De Bosscher K. PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7397–7402. doi: 10.1073/pnas.0806742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.