Abstract
There is an ongoing need for effective materials that can replace autologous bone grafts in the clinical treatment of bone injuries and deficiencies. In recent years, research efforts have shifted away from a focus on inert biomaterials to favor scaffolds that mimic the biochemistry and structure of the native bone extracellular matrix (ECM). The expectation is that such scaffolds will integrate with host tissue and actively promote osseous healing. To further enhance the osteoinductivity of bone graft substitutes, ECM-mimetic scaffolds are being engineered with a range of growth factors (GFs). The technologies used to generate GF-modified scaffolds are often inspired by natural processes that regulate the association between endogenous ECMs and GFs. The purpose of this review is to summarize research centered on the development of regenerative scaffolds that replicate the fundamental collagen-hydroxyapatite structure of native bone ECM, and the functionalization of these scaffolds with GFs that stimulate critical events in osteogenesis.
Keywords: Bone, scaffold, biomimetic, growth factor, extracellular matrix, regenerative medicine
Introduction
Bone has an inherent capacity for repair and regeneration, however there are many clinical conditions that require intervention in the form of bone grafting. Bone graft procedures are employed in a range of settings including dentistry, orthopaedics, and craniofacial medicine. Autologous bone offers the most effective material for grafting, as it contains osteoprogenitor cells, osteoinductive molecules, and a structured mineral component. However, there are considerable drawbacks associated with autologous bone grafting including the limited supply of donor bone, the need for a second surgery, and the significant incident of long-term pain and morbidity at the donor bone site [1,2]. These issues are driving factors in the ongoing search for alternative graft substrates. Allograft, xenograft, and alloplast materials are common replacements for autologous bone, however these lack the robust osteogenic and osteoinductive potential of autograft sources [1,3]. As well, allograft and xenograft pose risks of immunogenic reaction or pathogen transfer. Hence, a major focus of current research is on developing more advanced biomaterials that are effective in stimulating new bone growth. In many cases, these materials are designed to mimic the biochemistry and/or structure of native bone extracellular matrix (ECM).
The natural ECM within bone has a well-defined organization consisting of oriented collagen I fibers with intervening nanocrystals of carbonated hydroxyapatite (HA), a type of calcium phosphate (CaP) mineral [4]. Collagen I, which comprises over 90% of the organic phase of bone, has both structural and cell signaling functions. Interactions between collagen I and cell surface receptors such as integrins regulate cell adhesion to the ECM, and also promote differentiation of mesenchymal stem cells (MSCs) along the osteoblastic lineage [4]. The remaining 10% of the organic phase is composed of more than a hundred different proteins [5,6] including a number of proteoglycans. Although less abundant than collagen I, these play critical roles in the organization and homeostasis of bone. Noncollagenous bone matrix proteins contribute to collagen fibrillogenesis, matrix mineralization, cell signaling, and sequestration and release of growth factors (GFs) and morphogens.
In addition to its distinct architecture and biochemistry, bone matrix has greater mechanical strength than most other ECMs, but is also resorbable, allowing for continuous bone remodeling. These unique features present a barrier with regard to synthesizing bone-mimetic matrices. Integrating the appropriate biochemistry, 3-dimensional structure, and biomechanical properties into a unified scaffold is a challenging endeavor. Much effort has been directed toward developing scaffolds that reproduce either the biochemistry or mechanical properties of bone matrix, but more limited attention has been paid to addressing both of these parameters. Another point of consideration is whether mature bone matrix is, in fact, the best model for a regenerative scaffold. It has been suggested that a scaffold composed of molecules found within provisional wound-healing types of matrices may better recapitulate the natural process of bone repair. For these latter types of scaffolds, the delivery of bone-inductive molecules from a temporary scaffold is of greater priority than scaffold mechanical strength. One very promising avenue of research centers on the use of fibrin, hyaluronan, or heparin-derived scaffolds or hydrogels with embedded GFs or cytokines. GF-embedded hydrogels have been extensively reviewed elsewhere [7–10], and will not be a major focus of this report. Ultimately, the optimal scaffold for bone repair will likely depend upon the specific therapeutic application; for example, scaffold mechanical properties are important for graft sites that require structural support. Additionally, in some instances, the scaffold must be produced in a well-defined 3-dimensional geometry, which limits the types of materials and technologies that can be employed.
The current review will focus on the synthesis of scaffolds that mimic the collagen-HA structure of native bone matrix, as well as the further functionalization of such scaffolds with reparative bone-inducing GFs (the term “growth factor” will be used broadly herein to include molecules with pleiotropic functions). The overall objective is to highlight how our understanding of the structure and composition of native bone ECM can be leveraged to design bioinspired scaffolds that evoke critical regenerative processes.
Scaffolds that mimic native bone matrix
A bone-mimetic scaffold composed of collagen fibers with interspersed HA nanocrystals offers a biochemical and topographical landscape that is, optimally, perceived as native bone matrix by infiltrating MSCs and other osteoprogenitor cells. These cells must attach to the scaffold surface, survive and proliferate, and then undergo osteoblastic differentiation, leading to the synthesis of a new mineralized matrix. Simultaneously, the scaffold should degrade at a rate that is well-matched to the cell-mediated deposition of new bone matrix. Another scaffold property important for effective tissue regeneration is the capacity of the material to foster cell infiltration, which correspondingly depends upon scaffold porosity and degradability. Cell infiltration is a critical requirement for scaffold vascularization, turnover and integration with native tissue in the wound bed. The synthesis of scaffolds constructed from collagen I and/or CaP represents an area of intense research focus, however, to date there is no single scaffold that epitomizes an ideal bone graft substitute. The following section summarizes the advantages and disadvantages of scaffolds derived from collagen I, CaP, and collagen/CaP composites.
Scaffolds composed of collagen I
Scaffolds comprised of collagen I support cell adhesion through engaging cell surface integrin receptors. The activation of collagen-binding integrins, such as α2β1, on the surface of MSCs and osteoprogenitor cells initiates signaling cascades that induce osteoblastic differentiation, which then promotes formation of a mineralized matrix [11–13]. As other advantageous features, collagen-based scaffolds often have an ECM-like nanofibrous topography, and can be readily degraded by endogenous enzymes, facilitating scaffold remodeling. However, as a shortcoming, collagen scaffolds typically have poor mechanical properties, and resorb too quickly for optimal tissue development. For this reason, collagen scaffolds are frequently used as temporary carriers for GFs or other biomodifiers.
Many avenues are being pursued to increase the mechanical strength of collagen scaffolds and enable tuning of scaffold degradation rate. In the body, collagen I fibers are highly cross-linked, which provides strength and flexibility to the ECM. Modeling this process, methods have been developed to introduce cross-links into scaffold collagen fibers [14]. A plethora of chemical methods has been employed with good success, although a caveat is that many cross-linking agents are cytotoxic. Negative effects on tissue healing could ensue if residual cross-linker remains in the scaffold. UV irradiation and other types of non-chemical cross-linking protocols have also shown efficacy in improving scaffold mechanical properties. As an alternative, synthetic polymers are often mixed with collagen to increase scaffold tensile properties. There are several FDA-approved biodegradable polymers that have an established history of clinical use including poly(lactic acid) (PLA) and polycaprolactone (PCL). These polymers have greater tensile strength and slower resorption times than collagen I. By blending varying ratios of collagen I to synthetic polymer, the biomechanics and biodegradability of the scaffold can be tailored to suit the specific therapeutic application. One downside of this approach is that the breakdown products of some synthetic polymers are acidic, and can provoke an inflammatory reaction. As well, many polymers have relatively slow degradation times, which can impede scaffold turnover and replacement with newly-formed tissue.
Scaffolds composed of CaP
Materials containing CaP from either a biologic or synthetic origin are the most commonly used substrates for bone grafting. Allograft from bone bank or cadaveric sources is the second most frequently implanted graft material after autograft [1]. Allograft is sterilized and processed to remove cellular material as well as some of the proteinaceous matrix, leaving the mineral component intact. Xenograft materials such as anorganic bovine bone (ABB) are also used clinically, however these are treated to remove all organic components, yielding a scaffold that is essentially pure CaP, mostly in the form of HA. Allograft and ABB can be isolated from cancellous or cortical bone sources, offering substrates with an array of architectures, porosities and degradation times. Supplementing allograft and xenograft products, numerous alloplast materials are available. These are characteristically derived from synthetic HA, or other CaPs including β-tricalcium phosphate (β-TCP). β-TCP resorbs more quickly than HA [15], and thus β-TCP is sometimes mixed with HA to control graft resorption time. A major advantage of synthetic CaPs is that they are highly biocompatible, abundant, and can be synthesized at relatively low cost, facilitating translation to the clinic. One disadvantage is that CaPs such as HA are brittle and prone to fracture.
Historically, CaP biomaterials have been considered to serve primarily as supportive, osteoconductive matrices. However recent evidence implicates a more active role for CaPs in regulating osteogenic cell behavior and bone tissue development than previously thought. Some investigators now suggest that certain CaPs may be osteoinductive [16–18]. The mechanisms by which CaPs promote bone healing are still poorly-understood, but are likely to involve multiple pathways. CaP biomaterials may contribute to the regulation of extracellular pools of calcium and phosphate ions, similar to the activity of native bone mineral, in turn modulating osteogenic cell response. Additionally, CaPs could indirectly regulate cell differentiation state through adsorbing and releasing critical osteoinductive factors such as Bone Morphogenetic Proteins (BMPs). Another possibility is that the mechanical properties of CaPs, particularly stiffness, may have effects on cells independent of the scaffold’s biochemical composition. Compelling work by several groups has established the importance of matrix mechanical properties in controlling cell behaviors including cell differentiation [19–22]. Elegant work by Discher’s group has shown that MSCs seeded onto stiff matrices preferentially differentiate along the osteoblastic lineage [19]. Taken together, these findings support the concept that CaPs may have some osteoinductive capacity, however further investigation is needed to elucidate the molecular mechanisms by which the inorganic component of ECM contributes to cell activity and osteogenesis.
Scaffolds composed of collagen I/CaP mixtures
A wealth of technologies has been developed for synthesizing scaffolds that combine collagen I with HA and other forms of CaP [23–25]. Of these, electrospinning and 3D-printing are especially adept at producing scaffolds that mimic bone ECM. In the electrospinning process, a voltage is applied to an ejected collagen-containing solution, generating a nanofiber that has a diameter comparable to the size of a native collagen bundle [26]. The nanofibers are collected on a grounding plate to create a degradable mesh with interconnecting pores, similar to native ECM. Interconnecting pores are important for nutrient transport, cellular waste removal, and establishment of cell-cell junctions. Adding another level of biomimicry, collagen-based electrospun scaffolds have been engineered to incorporate nanoparticles of HA [27–34]. Hence, the electrospinning process holds potential to construct scaffolds that duplicate the general topography and biochemistry of the natural bone matrix. However, collagen I can be denatured during electrospinning [35], and the collagen fibers lack the degree of cross-linking present in endogenous collagen I-rich matrices. To increase mechanical strength, electrospun scaffolds are often fabricated with blended nanofibers composed of collagen I and a synthetic polymer such as PCL or PLA. Another limitation of electrospun scaffolds is that the relatively small pore sizes within these matrices can hinder cell infiltration, which could compromise scaffold vascularization and remodeling in vivo. There are also significant challenges associated with scaling up the electrospinning process to produce large 3-dimensional constructs. Nonetheless, these caveats aside, electrospun collagen/HA composite scaffolds hold considerable promise with regard to replicating the activity of native bone ECMs in stimulating cell behaviors important for new bone synthesis [28,30–32,36,37]. Compared with scaffolds formulated from only one ECM component, composite collagen/HA electrospun scaffolds stimulate greater cell proliferation, activation of integrin-related signaling pathways, and scaffold mineralization [28,30,36].
3D-printing (also referred to as additive manufacturing or rapid prototyping) is another exciting technology for synthesizing bone-mimetic scaffolds [38]. There are several protocols used for 3D-printing, however all involve the layer-by-layer deposition of 2-dimensional material sheets that are laid down sequentially to form a 3-dimensional construct. 3D-printing offers unparalleled control of scaffold design, porosity, topography and overall geometry. 3D-printers readily interface with computer-assisted design programs and imaging systems to create scaffolds of specified sizes and shapes, which is beneficial for generating implants for patient-specific defects. As other positive attributes, 3D-printing can be executed with a broad assortment of biologic molecules, and cells can be printed along with matrix molecules in defined patterns. Both organic and inorganic molecules are amenable to 3D-printing, and numerous scaffolds have been printed from either collagen I or CaPs. However, 3D-printed scaffolds that combine collagen I and CaP are markedly less abundant. In one innovative study, a 3D porous scaffold composed of HA and collagen I was made under low temperature conditions using a collagen I/phosphoric acid mixture as a binder material to print on HA powder [39]. The inclusion of collagen in scaffolds 3D-printed from HA enhanced cell viability, and when placed into murine femoral defects, the composite scaffolds elicited bone formation equivalent to that induced by allograft materials [39].
Functionalizing bone-mimetic scaffolds with growth factors
The functionalization of biomimetic scaffolds with GFs represents a key objective in the tissue engineering field. In many instances, the methods used to couple GFs to scaffolds have been inspired by knowledge of how GFs interact with the native ECM. Endogenous ECMs play a vital role as a reservoir for GFs, regulating the intensity and duration of GF signaling. ECM molecules and GFs have reciprocal binding sites that contribute to GF sequestration [40,41]. Several GFs have heparin-binding domains that direct GF association with matrix glycosaminoglycans. Alternatively, some GFs have binding sites for collagen I, fibronectin or other ECM proteins. The binding of GFs to ECM can have variable effects on GF activity. This raises a question regarding the best approach for coupling GFs to engineered scaffolds. In seminal work by Griffith’s group, it was shown that covalent immobilization of Epidermal Growth Factor (EGF) onto a scaffold potentiated EGF-induced cell activation, and prolonged downstream signaling by preventing internalization of EGF receptors following stimulation [42,43]. This study provided critical proof of concept for covalent tethering of GFs to scaffolds. On the other hand, some GF/receptor complexes require internalization for directing the appropriate cell response. Furthermore, molecules such as Vascular Endothelial Growth Factor (VEGF) function as chemotactic gradients and thus, controlled release from the ECM may be critical for activity. In view of these considerations, strategies aimed at coupling GFs to scaffolds should take into account the unique features of each individual GF signaling axis.
Degradation of the ECM constitutes another fundamental mechanism regulating the degree of GF/matrix association. Matrix-localized proteases are dynamically regulated during normal wound healing and pathologic processes including cancer and fibrosis. Changes in matrix degradability can have a significant impact on GF bioavailability. Engineered scaffolds composed of biologic materials, such as collagen or fibrin, are susceptible to the local proteolytic environment, whereas scaffolds generated from synthetic polymers degrade through other pathways. Considering both matrix degradation and the diverse binding mechanisms that control GF-ECM interactions, the complexities associated with GF delivery from an engineered scaffold are apparent. There are many challenges that must be addressed when developing a GF-modified scaffold. As examples, the coupling protocol must be effective in anchoring sufficient quantities of GF without causing GF denaturation, and release of the GF from the scaffold in vivo must occur with the correct timing and dosage.
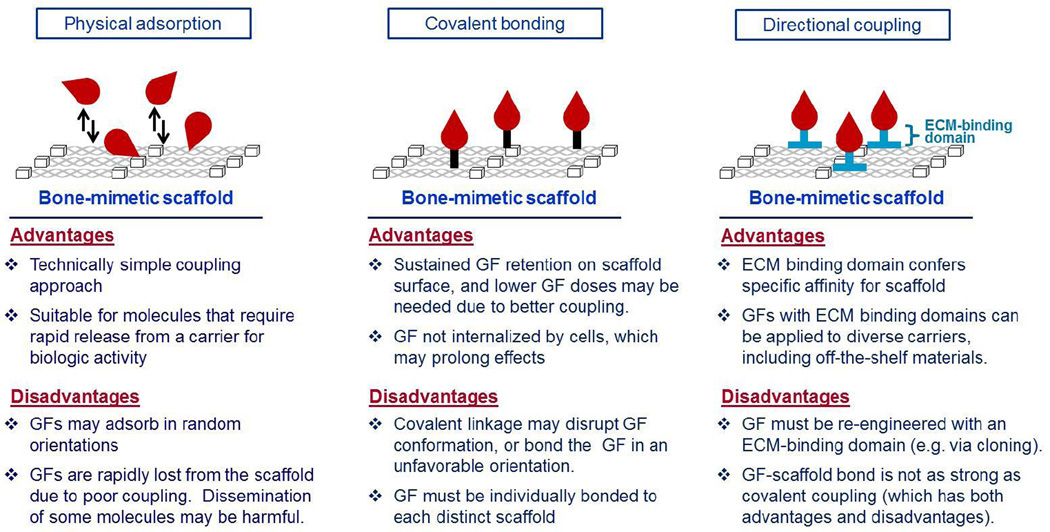
Methods for functionalizing scaffolds with GFs fall into two broad categories, incorporation of the GF into the biomaterial itself, or attachment of the GF to the scaffold surface. In the first approach, GFs are embedded or encapsulated within microspheres, hydrogels or other 3-dimensional matrices. Once implanted, the GFs are released through diffusion and/or gradual breakdown of the carrier material by endogenous hydrolytic or enzymatic processes. Most of the materials used for encapsulation derive from synthetic polymers or natural polymers such as hyaluronan, gelatin or fibrin. In keeping with this review’s focus on collagen-CaP derived scaffolds, this approach will not be further discussed. As a second strategy, GFs are engineered onto the scaffold surface. Many technologies are available for surface functionalization of either collagen or CaP scaffolds, however GF conjugation to hybrid collagen/CaP scaffolds has been less-investigated. One potential area of future research is to exploit the disparate chemistries of collagen and CaP to conjugate different GFs to these distinct matrix molecules, thus enriching the amount of biological information encoded within the scaffold. There are three common approaches for surface functionalization (Figure 1): (1) passive or physical adsorption of the GF onto the scaffold (e.g., dip-coating or soak-coating); (2) covalent immobilization of the GF onto the biomaterial; and (3) directional, or site-specific, coupling procedures, such as engineering a GF with an ECM-specific binding domain.
Figure 1. Methods for coupling GFs to bone-mimetic scaffolds.
Advantages and disadvantages of three common coupling approaches: physical adsorption; covalent bonding; and directional coupling. Growth factors are depicted in red.
Physical adsorption
In this method, scaffolds are soaked in a GF-containing solution and the GF becomes passively adsorbed to the material surface. The amount of GF that adsorbs is dependent upon many factors including the biochemical and biophysical properties of the scaffold, as well as the unique characteristics of each distinct GF (amino acid sequence, isoelectric point, post-translational modifications, etc.) [44]. Although physical adsorption offers the benefit of technical simplicity, a common problem is the weak coupling between the GF and the carrier. When implanted, most of the GF is rapidly released from the carrier. To address this issue, very high GF doses must be initially adsorbed so that the residual GF on the scaffold following bolus release is high enough to influence tissue healing. GFs are often physically adsorbed to scaffolds in supraphysiologic doses, which can have deleterious effects, and also greatly increase the cost of treatment. The INFUSE® product, composed of BMP2 adsorbed to a collagen sponge, serves as an example. INFUSE® is used therapeutically in spinal fusions, and the applied BMP2 is very effective in stimulating new bone synthesis. However, clinical studies of this product, along with animal studies of related BMP2-collagen scaffolds, have shown that the release and subsequent dissemination of high dose BMP2 can elicit chronic inflammation, edema, bone resorption, heterotopic bone formation and increased cancer risk [45–48].
Covalent immobilization
On the other end of the spectrum, GFs have been covalently linked to scaffolds [49,50]. Although this has most often been achieved with synthetic polymers and other non-biologic materials, covalent immobilization has been successful with some types of collagen and CaP substrates. In an illustrative study, covalent bonding of VEGF and angiopoietin to porous collagen scaffolds was accomplished using 1-ethyl-3-(3-dimethylamino propyl) carbodiimide hydrochloride (EDC) chemistry [51,52]. Alternatively, VEGF and Transforming Growth Factor β2 (TGFβ2) have been conjugated to collagen scaffolds using various bifunctional cross-linkers [53,54]. As another promising approach that models native GF-ECM interactions, collagen scaffolds have been covalently decorated with heparin, followed by the noncovalent attachment of GFs that naturally harbor heparin-binding domains [55–57]. In contrast to collagen-derived scaffolds, CaPs offer few functional groups for covalent bonding, and thus a surface activation procedure, such as aminosilanization, is usually required prior to conjugation. Moon et al. utilized this technology to couple Fibroblast Growth Factor 2 (FGF2) to biphasic calcium phosphate (a material that combines HA and β-TCP) [58]. Compared with physical adsorption, covalent GF immobilization can enhance GF stability and availability, reduce the amount of GF required, and facilitate spatial control of the GF. However, there are some potential drawbacks to covalent coupling. First, the chemical procedures required to achieve covalent linkage must not denature or degrade the GF. Secondly, the covalently-bound molecule should be oriented in such a manner that preserves the capacity of the GF to bind and activate cell surface receptors. Finally, as noted above, some GFs require release from a solid substrate in order to elicit a biologically relevant response.
Directional binding
As a third option, many investigators have pursued a directional, or site-specific, coupling strategy, in which GFs are engineered with domains that exhibit high affinity binding to select ECM molecules. Many of these domains are modeled after native amino acid sequences known to function in the tethering of endogenous proteins to the ECM. Naturally-derived amino acid sequences have been identified that confer specific binding to collagen I, glycosaminoglycans or HA, providing a facile mechanism for immobilization of GFs onto engineered scaffolds that incorporate these matrix molecules. ECM-binding sequences are attached to a GF through either recombinant protein engineering or chemical coupling procedures. In addition to native ECM-binding motifs, other types of domains have been used to tether GFs to bone-mimetic ECMs, including bisphosphonate moieties or amino acid sequences identified through phage display technology. Compared with other methods for GF conjugation, a directional coupling approach offers several advantages. ECM-binding domains typically mediate stronger GF anchoring to a scaffold than is achievable with physical adsorption, and the orientation of the bound GF may be better controlled, facilitating accessibility of the active site. Furthermore, the coupling of GFs via ECM-binding domains offers some potential translational benefits relative to scaffolds with covalently-immobilized GFs. For example, one envisions that recombinant GFs engineered with an ECM-binding domain could be developed as a lyophilized product for coating onto off-the-shelf scaffolds or clinical bone graft materials.
The most common directional coupling approach for GF functionalization of collagen scaffolds is to add a collagen-binding domain (CBD) onto the GF. A number of different CBDs have been employed [59–64]. In an early study, a CBD was identified within the Clostridium histolyticum collagenase, and this domain was subsequently engineered onto EGF. Collagen scaffolds conjugated with CBD-EGF fusion proteins were implanted subcutaneously and it was found that EGF remained at the implant site for 10 days, compared with only 24 hours for scaffolds passively coated with EGF lacking the CBD [65]. This study established proof of principle for using ECM-binding domains to enhance retention of GFs in vivo. In subsequent studies, CBDs from several other protein sources have been exploited as tools for GF delivery. CBDs derived from von Willebrand factor have been fused to BMP2 [66], TGF-β1 [67], and EGF [68], whereas fibronectin-derived CBDs have been engineered onto EGF [62] and Hepatocyte Growth Factor (HGF) [69]. These collective studies have unequivocally established the efficacy of CBDs in enhancing GF coupling to scaffolds, as well as GF stimulation of cells that come into contact with the functionalized scaffolds.
A variety of approaches has been pursued to achieve directional coupling of GFs to CaP-containing scaffolds. Indeed, the use of CaP-binding domains to couple GFs to CaP materials is an inherently biomimetic approach. Many of the native proteins that localize preferentially to bone are anchored specifically through endogenous CaP-binding domains. Bone-localized proteins including bone sialoprotein, osteocalcin, osteonectin, and osteopontin have distinct amino acid sequences that confer specificity for CaP [70–74]. These domains are enriched in the negatively-charged amino acids, glutamate, γ-carboxyglutamate, or aspartate, which have affinity for the positively-charged calcium within bone mineral. In addition, bone-localized proteins are often highly modified with other negatively-charged groups including phosphate and sialic acid. The determination that negatively-charged motifs function as CaP-binding domains was established many decades ago [75–77]. More recently, these motifs have been adapted for use as anchoring domains to attach biomodifiers to CaP biomaterials.
Polyglutamate and polyaspartate sequences have been extensively used to functionalize CaP materials, mainly with biomimetic peptides derived from cell adhesive ligands or GFs [78–84]. Acidic amino acid domains bind rapidly and tightly to a diversity of CaP substrates including commercial bone allograft and xenograft, as well as synthetic HA and β-TCP [85,86]. Notably, the strength of polyglutamate binding to CaP can be tailored by altering the number of glutamates within the polyglutamate domain, offering a practical method for controlling the dose and kinetics of GF delivery [85,86]. Another important characteristic of acidic amino acid domains is that they appear to be surprisingly selective for bone tissue. Polyglutamate-modified peptides overlaid onto murine tissue sections bind only to bone and not soft tissue [79], and following intravenous injection, polyglutamate or polyaspartate-containing entities accumulate preferentially in the skeleton [79,87,88].
Polyglutamate/polyaspartate domains have been utilized in preclinical studies as a vehicle for systemic delivery of recombinant therapeutic proteins. A domain comprised of six aspartate residues was engineered onto estradiol and delivered to osteoporotic rats either intranasally [88] or through intravenous injection [87]. Both of these treatment modalities elicited an increase in bone mineral density, ameliorating the osteoporotic phenotype. In another investigation, a deca-aspartate sequence was fused to the tissue-nonspecific isozyme of alkaline phosphatase (TNALP), a protein critical for phosphate homeostasis [89]. TNALP-null mice are a model for infantile hypophosphatasia. Subcutaneous injections of the deca-aspartate-TNALP fusion protein into TNALP-null mice prevented hypophosphatasia [89]. These studies support the potential utility of polyglutamate/polyaspartate domains for bone-targeting therapies.
In addition to bioinspired CaP-binding domains, amino acid sequences with specificity for CaP have been identified using combinatorial phage display or other high throughput screening approaches. Phage display was used to discover peptide sequences with selective affinity for HA [90–93] or β-TCP [94]. In the latter study, a β-TCP binding domain was engineered onto EGF and the fusion protein was then anchored onto β-TCP disks. MSCs seeded onto the EGF-conjugated disks exhibited enhanced Extracellular Signal-Regulated Kinase (ERK) signaling, consistent with EGF-mediated cell activation [94].
Bisphosphonate groups have also been used successfully as CaP anchoring domains. These are typically attached to biomodifiers via chemical coupling. In initial studies using model proteins such as bovine serum albumin and fetuin, Uludag’s group showed that the addition of bisphosphonate to the model protein enhanced binding to CaP-containing materials, as well as enrichment in bone tissue following systemic injection [95]. More recently, bisphosphonate groups have been conjugated to anti-cancer drugs, antibiotics, imaging agents and hormones to enable specific therapeutic targeting of bone [95,96]. Bisphosphonates have been fused to several GFs, including BMP2, to promote CaP binding [97]. In a complementary study, bisphosphonate groups were covalently linked to heparin; the heparin-modified bisphosphonates were then adsorbed onto CaP materials and bFGF and BMP2 were bound via the GF heparin binding domains [98].
GFs commonly used for bone tissue engineering applications
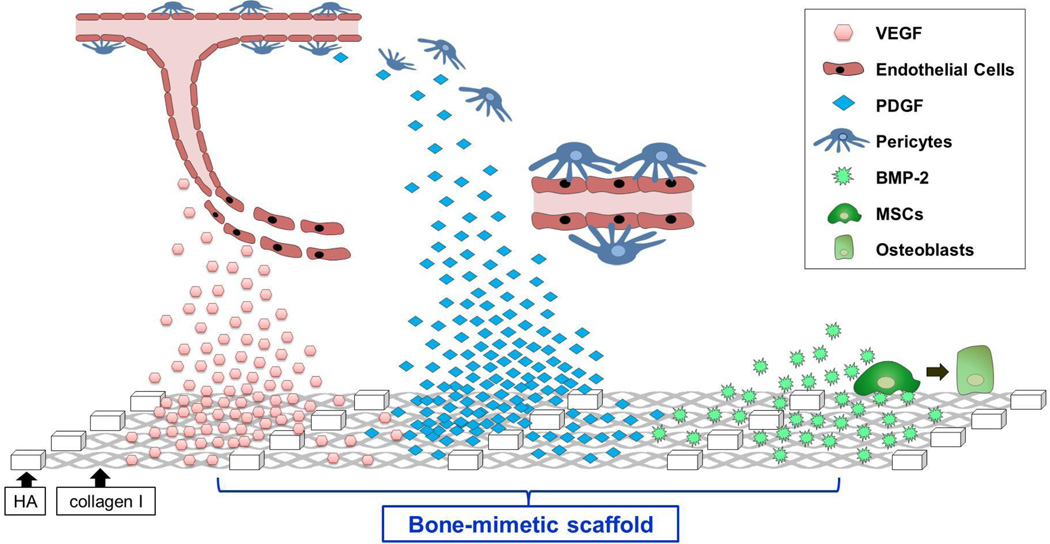
Multiple GFs have been considered for potential activity in promoting bone regeneration including members of the BMP, TGFβ, Insulin-like Growth Factor (IGF), FGF, Platelet-Derived Growth Factor (PDGF) and VEGF families [99]. Of these, BMP, VEGF, and PDGF are among the most widely-studied and will be further discussed. BMP, VEGF, and PDGF are all rapidly degraded in vivo, and must be delivered on some type of carrier for optimal activity. BMP, VEGF, and PDGF contribute to osteogenesis through different mechanisms (Figure 2). Selected BMPs act on MSCs and other osteoprogenitor cells to stimulate differentiation along the osteoblastic lineage. In turn, osteoblasts secrete osteoid, followed by cell-mediated mineralization of the osteoid matrix. VEGF enhances bone development by promoting angiogenesis. VEGF directs endothelial cell recruitment to sites of bone injury, leading to neovascularization of the regenerating tissue. The effect of PDGF on osteogenesis is multifaceted. PDGF acts as a mitogen for multiple cell types involved in bone healing, including osteoprogenitor cells and cells involved in blood vessel formation. PDGF complements the angiogenic function of VEGF by regulating the activity of pericytes and smooth muscle cells that support and stabilize nascent vessels. Because of the distinct functions mediated by BMPs, VEGF, and PDGF, delivery of combinations of these GFs constitutes a promising direction in the tissue engineering field [99]. Some of the prevalent GF combinations currently being investigated include: (1) BMP2 plus VEGF [100–104], which aims to promote both osteogenesis and angiogenesis, and (2) VEGF with either bFGF or PDGF [105–107]. The VEGF acts to stimulate activity of endothelial cells, while bFGF or PDGF attracts supporting pericytes to help stabilize the neovasculature.
Figure 2. Bone-mimetic scaffolds functionalized with GFs.
VEGF released from collagen I/HA bone-mimetic scaffolds attracts endothelial cells to promote neovascularization. PDGF released from the scaffold stimulates recruitment of pericytes, which then bind to infiltrating endothelial cells to stabilize nascent vessels. BMP2 presented on scaffolds binds to Mesenchymal Stem Cells (MSCs) to induce differentiation along the osteoblast lineage.
BMP
BMPs are the predominant GF utilized in bone regenerative medicine [108–111]. At least 20 members of the BMP family have been identified, and of these, BMP 2,4,6,7, and 9 appear to have the greatest osteoinductive potential [112,113]. BMPs function throughout the bone reparative process, from stimulating MSC recruitment to promoting osteoblast-mediated matrix mineralization [101]. BMPs bind to BMP receptors on osteoprogenitor cells to activate SMAD-dependent, and other, intracellular signaling cascades, leading to upregulation and activation of the Runx2 transcription factor [114]. Runx 2 induces expression of genes responsible for driving osteoblastogenesis [115,116]. There is compelling evidence from both animal and human studies showing that recombinant BMPs (rBMPs) are highly effective in inducing bone formation [99,117]. Clinically, rBMPs are utilized in orthopaedic applications including spinal fusion and fracture nonunions, and in dentistry for bone augmentation and enhancement of implant integration.
BMPs are secreted from cells and associate with the native ECM through multiple interactions. BMPs have heparin-binding domains as well as binding sites for collagen I [108,118]. Therapeutic delivery of rBMP has most often been accomplished by soaking the BMP onto a collagen sponge. There are currently two FDA-approved BMP products for orthopaedic applications, INFUSE®, as described above (BMP2 on a collagen sponge), and Op-1®. Op-1® consists of a collagen sponge soaked in rBMP7, and is used primarily for fracture nonunions. Both INFUSE® and Op-1® have well-established bone-inductive activity, however side effects related to high dose, disseminated BMP (e.g. inflammation and edema), have been documented for these products, especially for INFUSE® [119]. This issue underscores the need for better BMP-carrier coupling.
To improve BMP anchoring to collagen-based scaffolds, rBMPs have been engineered with CBDs derived from collagen-binding proteins including von Willebrand factor, fibronectin and others [66,120–122]. CBDs have been incorporated into recombinant BMP2 [60,61,66], BMP3 [120], and BMP4 [121,122]. All of these CBD-BMP fusion proteins, when coupled to collagen carriers, were found to stimulate greater bone formation when compared with scaffolds impregnated with unmodified rBMP. More specifically, greater osteoinduction was elicited by: (1) CBD-BMP3-collagen scaffolds implanted into rat cranial defects [120]; (2) CBD-BMP4-collagen sponges placed into rabbit femurs [122]; and (3) CBD-BMP2-demineralized bone matrix (which is rich in collagen I) implanted into rabbit mandibular defects [66].
As with collagen, CaP materials have been widely used for therapeutic delivery of rBMP [99,111]. Extensive animal studies support the efficacy of both synthetic and biologic CaPs (e.g., allograft) as BMP carriers [123–130]. While rBMPs appear to bind more efficiently to CaPs than a collagen sponge [131,132], concerns remain regarding insufficient BMP-CaP coupling. Moreover, as a conjugation strategy, physical adsorption to a carrier offers minimal control over the dose and timing of rBMP delivery. Only a few studies have aimed to engineer rBMP with a CaP binding domain. In one such study, rBMP4 was conjugated with an HA-binding sequence [133]. This was accomplished by enzymatically ligating a peptide enriched in phosphoserine (pSpS) to the rBMP4. In vitro studies suggested that the addition of the pSpS domain enhanced rBMP4 binding to HA beads, and the pSpS-rBMP4-coupled HA beads stimulated some degree of osteoblastogenesis of murine osteoprogenitor cells [133].
BMP mimetic peptides
A developing area of investigation in the field of BMP therapeutics is the use of small synthetic BMP-derived peptides designed to replicate the activity of the full-length BMP molecule. There are many attractive features associated with synthetic peptides when compared with recombinant proteins. First, large quantities of relatively pure peptides can be produced in a cost-effective manner by a commercial peptide synthesizer. Secondly, the use of synthetic peptides eliminates the risk of immunogenic or pathogenic contaminants that may be introduced by the host cell systems required for production of recombinant proteins. Finally, the activity of shorter synthetic peptides is not usually as dependent upon 3-dimensional conformation as intact recombinant proteins, and hence there is less concern about denaturation upon conjugation to a biomaterial surface.
Most BMP-mimetic peptides are derived from a sequence within native BMP called the “knuckle” domain [78,134–140]. Full-length BMPs contain two important functional domains, a “wrist” domain that binds type I BMP receptors, and the knuckle domain, which binds type II BMP receptors [141]. In contrast to the knuckle domain, the wrist domain is composed of noncontiguous amino acids, and is therefore not as amenable to creating a synthetic mimetic peptide. Multiple investigators have shown that knuckle domain-derived BMP mimetic peptides have osteoinductive activity [137,140,142]. BMP2 mimetic peptides have been delivered to diverse implant sites on a range of carriers including α-tricalcium phosphate [138] and ABB [140].
Engineering BMP-mimetic peptides with a CaP-binding domain to improve anchoring to CaP carriers is an active area of research. Murphy’s group developed a modular peptide encompassing a BMP2 mimetic sequence fused to an HA-binding domain modeled upon a γ-carboxyglutamate-rich motif found within osteocalcin [143]. HA biomaterials functionalized with this modular peptide stimulated osteogenic differentiation of MSCs in vitro [143], and enhanced bone repair in vivo [144,145]. In a related approach, a heptaglutamate-modified BMP2 mimetic peptide (E7-BMP2pep) was conjugated onto human allograft [79] or ABB [78]. ABB particles with bound E7-BMP2pep were implanted into rat subcutaneous pouches (ectopic bone formation model) or mandibular critical defects (bony implant model) [78]. The E7-BMP2pep/ABB implants elicited equivalent, and by some measures greater, bone formation than ABB passively-adsorbed with full-length rBMP2 [78]. Furthermore, in the mandibular defect model, rBMP2/ABB samples elicited extensive inflammation, whereas no side effects were noted with the E7-BMP2pep/ABB group [78].
VEGF
One of the key barriers hindering bone regenerative therapy is the lack of rapid vascularization into implanted scaffolds, and therefore functionalization of scaffolds with angiogenic molecules is a high priority [146]. Most research efforts have focused on delivery of VEGF, either singly or in combination with other angiogenic factors such as bFGF or PDGF. The VEGF family includes five members which are secreted as homodimers: VEGF-A through VEGF-D, as well as placental growth factor [147]. VEGF-A, also referred to as VEGF165, is the principal isoform used to functionalize bone-mimetic scaffolds. During physiological bone healing, VEGF expression peaks around 10 days, and then gradually declines [148,149]. For therapeutic administration of recombinant VEGF (rVEGF), both the timing and concentration of rVEGF delivery are critical. In excess, rVEGF increases vascular permeability, which can lead to systemic hypotension and edema [150,151].
Mechanisms for rVEGF delivery have largely centered on either hydrogel vehicles or physical adsorption to various scaffolds [9,152]. CaPs with adsorbed rVEGF have been tested in multiple bone defect models for angiogenic and osteogenic potential [153–155]. β-TCP implants with adsorbed rVEGF stimulated greater healing of rabbit ulnar defects than β-TCP alone [154]. To improve the coupling of rVEGF to CaP carriers, Wernike et al. developed a co-precipitation method for rVEGF deposition onto biphasic calcium phosphate (BCP) ceramics [153]. Compared with BCP superficially adsorbed with rVEGF, BCP scaffolds with the co-precipitated rVEGF exhibited reduced rVEGF burst release, and when implanted into murine cranial defects, the co-precipitated samples showed enhanced vascularization and osseointegration [153].
rVEGF has also been covalently-linked to carriers [53,156–158]. For rVEGF immobilization on collagen scaffolds, both homobifunctional linkers [53] and EDC chemistry [156] have been utilized. As an alternative, carriers have been covalently modified with heparin, and then rVEGF attached to the carriers through VEGF’s heparin-binding domain. In one notable study, Steffens et al. covalently immobilized heparin onto a collagen scaffold and then rVEGF was physically adsorbed [159]. Scaffolds with adsorbed rVEGF had increased angiogenic potential compared with scaffolds lacking rVEGF.
VEGF mimetic peptides
Similar to BMP mimetic peptides, VEGF-derived peptides have been developed with the goal of replacing full-length rVEGF therapeutics. The design of these peptides was enabled by knowledge of the 3-dimensional structure of native VEGF. D’Andrea et al. examined the crystal structure of VEGF bound to its cognate receptor and identified a region in the VEGF binding interface [160]. The authors then produced a synthetic peptide encompassing this domain, termed the “QK” peptide, and showed that this peptide could activate VEGF receptors [160]. In fact, the QK peptide appears to be able to reproduce all of the key cellular responses elicited by full-length VEGF including endothelial proliferation and sprouting, as well as vessel formation in in vivo angiogenesis models [160–163].
To enhance coupling to collagen carriers, QK peptides have been modified with CBDs. Yu’s group synthesized a CBD-QK peptide and coated the peptide onto composite collagen/gelatin scaffolds [164]. The scaffolds with CBD-QK stimulated in vitro endothelial network formation [164,165]. QK peptides have also been engineered with CaP-binding domains. Murphy and colleagues synthesized a QK peptide with an osteocalcin-derived HA-binding domain and dip-coated this peptide onto HA microparticles [166]. The QK-coupled HA substrates stimulated endothelial cell proliferation and migration. This same HA-binding QK peptide was coated onto β-TCP disks and the disks were then implanted intramuscularly into sheep [104]. Disks with the anchored QK peptide stimulated significantly greater blood vessel ingrowth.
PDGF
PDGF is one of the first factors released following a bone injury, and its chemotactic and mitogenic activities are crucial for osseous repair [167,168]. The PDGF family includes PDGF-A, B, C and D isoforms, and these assemble into either homodimers or heterodimers [169]. PDGF-BB has shown significant potential for stimulating bone regeneration, evidenced by its success in enhancing the healing of fractures and critical-size defects [169–171]. PDGF-BB is produced by, and acts on, a diversity of cell types. Early in the wound cascade, PDGF-BB stimulates recruitment of neutrophils and macrophages, which participate in the formation of granulation tissue. PDGF-BB also attracts osteoprogenitor cells, including MSCs, as well as several cell types involved in angiogenesis. Thus, PDGF-BB activity is central to both the vascularization and synthesis of mineralized matrix required for bone healing.
The therapeutic use of rPDGF for bone repair has been the focus of substantial research [168,172]. rPDGF has been delivered on an array of carriers including allogeneic bone [173,174], collagen I [171], ABB [175–177], and synthetic CaPs such as β-TCP [170,178–180], and BCP [181]. In particular, β-TCP has been frequently used as a rPDGF carrier in both animal and human studies. β-TCP with adsorbed PDGF was reported to improve or accelerate bone healing in multiple animal models [168] including rat tibial fractures [170] and sheep ilium defects [180]. rPDGF/β-TCP products are currently used in the clinic for orthopedic and periodontal applications. A PDGF/β-TCP matrix (marketed as GEM21S® by BioMimetic Therapeutics) was one of the first FDA-approved dental products combining a recombinant GF with a synthetic bone graft material. In a multicenter clinical trial involving 180 patients, participants receiving rPDGF/β-TCP in intraosseous periodontal defects had significantly greater bone fill compared with the β-TCP carrier alone [179]. A related rPDGF/β-TCP product, AUGMENT® (BioMimetic Therapeutics) has been evaluated as a healing adjunct in foot and ankle surgeries and distal radius fractures. A randomized, controlled pilot study suggested that AUGMENT® was at least as effective as autologous bone graft in stimulating hindfoot and ankle fusion [182].
To enhance anchoring to collagen-based scaffolds, PDGF has been engineered with a CBD. Compared to collagen scaffolds with passively adsorbed rPDGF-BB, CBD-PDGF-BB-loaded collagen scaffolds stimulated increased fibroblast proliferation in vitro, and greater vascular in-growth when implanted into rat dorsal skin wounds [63]. In a second study, heparin was crosslinked onto collagen-rich demineralized bone matrix (DBM), and then rPDGF was attached to the heparin-DBM scaffold via the heparin binding domain within PDGF [183]. The DBM-heparin scaffolds with bound rPDGF stimulated fibroblast proliferation in vitro and greater cell infiltration upon implantation into rat subcutaneous pockets [183].
Conclusions and Future Directions
Intensive research is currently focused on regenerative scaffolds that replicate the structure and biochemistry of native ECM, with the hope that such scaffolds will better integrate with host tissue, and induce greater osteogenesis when compared with bioinert materials. The further functionalization of ECM surrogates with GFs offers a scaffold with high potential to control cell and tissue response. The past decade has witnessed a remarkable expansion in the range of bioinspired scaffolds, as well as methods for incorporating GFs into these matrix mimetics. Some of these products and methods are relatively straightforward to move into the clinic, while others are quite complex and will be costly and difficult to scale-up for therapeutic administration. Furthermore, obtaining regulatory approval for many of the scaffolds under investigation may be challenging. Nonetheless, even scaffolds that face translational hurdles have significant value. Bone-like scaffolds, with or without attached GFs, serve as important 3-dimensional culture matrices for probing osteogenic cell signaling. Such scaffolds also provide critical platforms for both drug testing and mechanistic studies of matrix assembly. Finally, studies of mimetic scaffolds implanted into animal models offer vital information regarding host response to defined features of engineered matrices, as well as requirements for GF delivery. Thus, going forward, there is a need to maintain a balanced focus on the development of scaffolds that have feasible potential to impact patient care with less-translatable, but elegantly-designed, scaffolds that advance our fundamental knowledge of how the ECM regulates osteogenic cell behavior and bone tissue development.
Highlights.
Scaffolds that mimic bone extracellular matrix hold promise for improving bone repair therapies.
The attachment of growth factors to bone mimetic scaffolds potentiates regenerative capacity.
A variety of technologies has been developed to couple growth factors to scaffolds.
Osteoinductive and angiogenic growth factors are key scaffold cargo for enhancing osteogenesis.
Acknowledgments
The authors gratefully acknowledge support from NIH grant R01 DE024670 (SLB), as well as predoctoral fellowships from NASA (NWP) and NIH T32 DE017607 (ASC).
Abbreviations
- ABB
anorganic bovine bone
- BCP
Bicalcium Phosphate
- BMP
Bone Morphogenetic Protein
- CaP
Calcium Phosphate
- CBD
Collagen Binding Domain
- DBM
Demineralized Bone Matrix
- ECM
Extracellular Matrix
- EGF
Epidermal Growth Factor
- ERK
Extracellular Signal-Regulated Kinase
- FGF
Fibroblast Growth Factor
- GF
Growth Factor
- HA
Hydroxyapatite
- HGF
Hepatocyte Growth Factor
- IGF
Insulin-Like Growth Factor
- MSCs
Mesenchymal Stem Cells
- PDGF
Platelet-Derived Growth Factor
- PCL
polycaprolactone
- PLA
poly(lactic acid)
- TGFβ
Transforming Growth Factor β
- β-TCP
βTricalcium Phosphate
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Bostrom MP, Seigerman DA. The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study. HSS J. 2005;1:9–18. doi: 10.1007/s11420-005-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alford AI, Kozloff KM, Hankenson KD. Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol. 2015;65:20–31. doi: 10.1016/j.biocel.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Salmon CR, Tomazela DM, Ruiz KG, Foster BL, Paes Leme AF, et al. Proteomic analysis of human dental cementum and alveolar bone. J Proteomics. 2013;91:544–555. doi: 10.1016/j.jprot.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang X, Ye M, Jiang X, Liu G, Feng S, et al. Method development of efficient protein extraction in bone tissue for proteome analysis. J Proteome Res. 2007;6:2287–2294. doi: 10.1021/pr070056t. [DOI] [PubMed] [Google Scholar]
- 7.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Silva AK, Richard C, Bessodes M, Scherman D, Merten OW. Growth factor delivery approaches in hydrogels. Biomacromolecules. 2009;10:9–18. doi: 10.1021/bm801103c. [DOI] [PubMed] [Google Scholar]
- 9.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129:133–138. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 13.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 14.Walters BD, Stegemann JP. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014;10:1488–1501. doi: 10.1016/j.actbio.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981;157:259–278. [PubMed] [Google Scholar]
- 16.Chai YC, Carlier A, Bolander J, Roberts SJ, Geris L, et al. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 2012;8:3876–3887. doi: 10.1016/j.actbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Yuan H, Yang Z, Li Y, Zhang X, De Bruijn JD, et al. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med. 1998;9:723–726. doi: 10.1023/a:1008950902047. [DOI] [PubMed] [Google Scholar]
- 18.Habibovic P, Sees TM, van den Doel MA, van Blitterswijk CA, de Groot K. Osteoinduction by biomaterials--physicochemical and structural influences. J Biomed Mater Res A. 2006;77:747–762. doi: 10.1002/jbm.a.30712. [DOI] [PubMed] [Google Scholar]
- 19.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoldan J, Karagiannis ED, Lee CY, Anderson DG, Langer R, et al. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials. 2011;32:9612–9621. doi: 10.1016/j.biomaterials.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Lai JH, Han LH, Tong X, Yang F. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng Part A. 2014;20:2131–2139. doi: 10.1089/ten.tea.2013.0531. [DOI] [PubMed] [Google Scholar]
- 23.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 24.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9629. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venugopal J, Prabhakaran MP, Zhang Y, Low S, Choon AT, et al. Biomimetic hydroxyapatite-containing composite nanofibrous substrates for bone tissue engineering. Philos Trans A Math Phys Eng Sci. 2010;368:2065–2081. doi: 10.1098/rsta.2010.0012. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 27.Venugopal J, Vadgama P, Sampath Kumar TS, Ramakrishna S. Biocomposite nanofibres and osteoblasts for bone tissue engineering. Nanotechnology. 2007;18:1–8. [Google Scholar]
- 28.Phipps MC, Clem WC, Catledge SA, Xu Y, Hennessy KM, et al. Mesenchymal stem cell responses to bone-mimetic electrospun matrices composed of polycaprolactone, collagen I and nanoparticulate hydroxyapatite. PLoS One. 2011;6:e16813. doi: 10.1371/journal.pone.0016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phipps MC, Clem WC, Grunda JM, Clines GA, Bellis SL. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials. 2012;33:524–534. doi: 10.1016/j.biomaterials.2011.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venugopal J, Low S, Choon AT, Sampath Kumar TS, Ramakrishna S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J Mater Sci Mater Med. 2008;19:2039–2046. doi: 10.1007/s10856-007-3289-x. [DOI] [PubMed] [Google Scholar]
- 31.Phipps MC, Xu Y, Bellis SL. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS One. 2012;7:e40831. doi: 10.1371/journal.pone.0040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balaji Raghavendran HR, Puvaneswary S, Talebian S, Raman Murali M, Vasudevaraj Naveen S, et al. A comparative study on in vitro osteogenic priming potential of electron spun scaffold PLLA/HA/Col, PLLA/HA, and PLLA/Col for tissue engineering application. PLoS One. 2014;9:e104389. doi: 10.1371/journal.pone.0104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandakumar A, Fernandes H, de Boer J, Moroni L, Habibovic P, et al. Fabrication of bioactive composite scaffolds by electrospinning for bone regeneration. Macromol Biosci. 2010;10:1365–1373. doi: 10.1002/mabi.201000145. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Reddy VJ, Wong SY, Li X, Su B, et al. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng Part A. 2010;16:1949–1960. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- 35.Zeugolis DI, Khew ST, Yew ES, Ekaputra AK, Tong YW, et al. Electro-spinning of pure collagen nano-fibres - just an expensive way to make gelatin? Biomaterials. 2008;29:2293–2305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Prabhakaran MP, Venugopal J, Ramakrishna S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009;5:2884–2893. doi: 10.1016/j.actbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Song JH, Kim HE, Kim HW. Electrospun fibrous web of collagen-apatite precipitated nanocomposite for bone regeneration. J Mater Sci Mater Med. 2008;19:2925–2932. doi: 10.1007/s10856-008-3420-7. [DOI] [PubMed] [Google Scholar]
- 38.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Clark RA. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014;134:895–901. doi: 10.1038/jid.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt MO, Roman AJ, Wells A, Lauffenburger DA, Griffith LG. Sustained epidermal growth factor receptor levels and activation by tethered ligand binding enhances osteogenic differentiation of multi-potent marrow stromal cells. J Cell Physiol. 2009;221:306–317. doi: 10.1002/jcp.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 44.King WJ, Krebsbach PH. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv Drug Deliv Rev. 2012;64:1239–1256. doi: 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu SX, Fiorini T, Lee J, Prasad HS, Buxton AN, et al. Evaluation of a compression resistant matrix for recombinant human bone morphogenetic protein-2. J Clin Periodontol. 2013;40:688–697. doi: 10.1111/jcpe.12109. [DOI] [PubMed] [Google Scholar]
- 46.McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2) J Spinal Disord Tech. 2006;19:483–486. doi: 10.1097/01.bsd.0000211231.83716.4b. [DOI] [PubMed] [Google Scholar]
- 47.Lee KB, Taghavi CE, Murray SS, Song KJ, Keorochana G, et al. BMP induced inflammation: a comparison of rhBMP-7 and rhBMP-2. J Orthop Res. 2012;30:1985–1994. doi: 10.1002/jor.22160. [DOI] [PubMed] [Google Scholar]
- 48.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8:1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Hajimiri M, Shahverdi S, Kamalinia G, Dinarvand R. Growth factor conjugation: strategies and applications. J Biomed Mater Res A. 2015;103:819–838. doi: 10.1002/jbm.a.35193. [DOI] [PubMed] [Google Scholar]
- 50.Masters KS. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol Biosci. 2011;11:1149–1163. doi: 10.1002/mabi.201000505. [DOI] [PubMed] [Google Scholar]
- 51.Chiu LL, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 52.Chiu LL, Weisel RD, Li RK, Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J Tissue Eng Regen Med. 2011;5:69–84. doi: 10.1002/term.292. [DOI] [PubMed] [Google Scholar]
- 53.Koch S, Yao C, Grieb G, Prevel P, Noah EM, et al. Enhancing angiogenesis in collagen matrices by covalent incorporation of VEGF. J Mater Sci Mater Med. 2006;17:735–741. doi: 10.1007/s10856-006-9684-x. [DOI] [PubMed] [Google Scholar]
- 54.Bentz H, Schroeder JA, Estridge TD. Improved local delivery of TGF-beta2 by binding to injectable fibrillar collagen via difunctional polyethylene glycol. J Biomed Mater Res. 1998;39:539–548. doi: 10.1002/(sici)1097-4636(19980315)39:4<539::aid-jbm6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 55.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64:103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 56.Wissink MJ, Beernink R, Scharenborg NM, Poot AA, Engbers GH, et al. Endothelial cell seeding of (heparinized) collagen matrices: effects of bFGF pre-loading on proliferation (after low density seeding) and pro-coagulant factors. J Control Release. 2000;67:141–155. doi: 10.1016/s0168-3659(00)00202-9. [DOI] [PubMed] [Google Scholar]
- 57.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 58.Moon K-S, Choi E-J, Oh S, Kim S. The effect of covalently immobilized FGF-2 on biphasic calcium phosphate bone substitute on enhanced biological compatibility and activity. BioMed Res Int. 2015;2015:742192. doi: 10.1155/2015/742192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrades JA, Wu LT, Hall FL, Nimni ME, Becerra J. Engineering, expression, and renaturation of a collagen-targeted human bFGF fusion protein. Growth Factors. 2001;18:261–275. doi: 10.3109/08977190109029115. [DOI] [PubMed] [Google Scholar]
- 60.Chen B, Lin H, Wang J, Zhao Y, Wang B, et al. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007;28:1027–1035. doi: 10.1016/j.biomaterials.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Han X, Zhang W, Gu J, Zhao H, Ni L, et al. Accelerated postero-lateral spinal fusion by collagen scaffolds modified with engineered collagen-binding human bone morphogenetic protein-2 in rats. PLoS One. 2014;9:e98480. doi: 10.1371/journal.pone.0098480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishikawa T, Terai H, Kitajima T. Production of a biologically active epidermal growth factor fusion protein with high collagen affinity. J Biochem. 2001;129:627–633. doi: 10.1093/oxfordjournals.jbchem.a002900. [DOI] [PubMed] [Google Scholar]
- 63.Lin H, Chen B, Sun W, Zhao W, Zhao Y, et al. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials. 2006;27:5708–5714. doi: 10.1016/j.biomaterials.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Zhao Y, Chen B, Han Q, Sun W, et al. Collagen-binding human epidermal growth factor promotes cellularization of collagen scaffolds. Tissue Eng Part A. 2009;15:3589–3596. doi: 10.1089/ten.TEA.2008.0648. [DOI] [PubMed] [Google Scholar]
- 65.Nishi N, Matsushita O, Yuube K, Miyanaka H, Okabe A, et al. Collagen-binding growth factors: production and characterization of functional fusion proteins having a collagen-binding domain. Proc Natl Acad Sci U S A. 1998;95:7018–7023. doi: 10.1073/pnas.95.12.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen B, Lin H, Zhao Y, Wang B, Zhao Y, et al. Activation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. J Biomed Mater Res A. 2007;80:428–434. doi: 10.1002/jbm.a.30900. [DOI] [PubMed] [Google Scholar]
- 67.Andrades JA, Han B, Becerra J, Sorgente N, Hall FL, et al. A recombinant human TGF-beta1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp Cell Res. 1999;250:485–498. doi: 10.1006/excr.1999.4528. [DOI] [PubMed] [Google Scholar]
- 68.Hall FL, Kaiser A, Liu L, Chen ZH, Hu J, et al. Design, expression, and renaturation of a lesion-targeted recombinant epidermal growth factor-von Willebrand factor fusion protein: efficacy in an animal model of experimental colitis. Int J Mol Med. 2000;6:635–643. doi: 10.3892/ijmm.6.6.635. [DOI] [PubMed] [Google Scholar]
- 69.Ota T, Gilbert TW, Schwartzman D, McTiernan CF, Kitajima T, et al. A fusion protein of hepatocyte growth factor enhances reconstruction of myocardium in a cardiac patch derived from porcine urinary bladder matrix. J Thorac Cardiovasc Surg. 2008;136:1309–1317. doi: 10.1016/j.jtcvs.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldberg HA, Warner KJ, Li MC, Hunter GK. Binding of bone sialoprotein, osteopontin and synthetic polypeptides to hydroxyapatite. Connect Tissue Res. 2001;42:25–37. doi: 10.3109/03008200109014246. [DOI] [PubMed] [Google Scholar]
- 71.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 72.Wazen RM, Tye CE, Goldberg HA, Hunter GK, Smith CE, et al. In vivo functional analysis of polyglutamic acid domains in recombinant bone sialoprotein. J Histochem Cytochem. 2007;55:35–42. doi: 10.1369/jhc.6A7046.2006. [DOI] [PubMed] [Google Scholar]
- 73.Bolander ME, Young MF, Fisher LW, Yamada Y, Termine JD. Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid) Proc Natl Acad Sci U S A. 1988;85:2919–2923. doi: 10.1073/pnas.85.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujisawa R, Wada Y, Nodasaka Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta. 1996;1292:53–60. doi: 10.1016/0167-4838(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 75.Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975;72:3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poser JW, Price PA. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979;254:431–436. [PubMed] [Google Scholar]
- 77.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bain JL, Bonvallet PP, Abou-Arraj RV, Schupbach P, Reddy MS, et al. Enhancement of the regenerative potential of anorganic bovine bone graft utilizing a polyglutamate-modified BMP2 peptide with improved binding to calcium-containing materials. Tissue Eng A. 2015;21:2426–2436. doi: 10.1089/ten.tea.2015.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Culpepper BK, Bonvallet PP, Reddy MS, Ponnazhagan S, Bellis SL. Polyglutamate directed coupling of bioactive peptides for the delivery of osteoinductive signals on allograft bone. Biomaterials. 2013;34:1506–1513. doi: 10.1016/j.biomaterials.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawyer AA, Weeks DM, Kelpke SS, McCracken MS, Bellis SL. The effect of the addition of a polyglutamate motif to RGD on peptide tethering to hydroxyapatite and the promotion of mesenchymal stem cell adhesion. Biomaterials. 2005;26:7046–7056. doi: 10.1016/j.biomaterials.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 81.Fujisawa R, Mizuno M, Nodasaka Y, Kuboki Y. Attachment of osteoblastic cells to hydroxyapatite crystals by a synthetic peptide (Glu7-Pro-Arg-Gly-Asp-Thr) containing two functional sequences of bone sialoprotein. Matrix Biol. 1997;16:21–28. doi: 10.1016/s0945-053x(97)90113-x. [DOI] [PubMed] [Google Scholar]
- 82.Itoh D, Yoneda S, Kuroda S, Kondo H, Umezawa A, et al. Enhancement of osteogenesis on hydroxyapatite surface coated with synthetic peptide (EEEEEEEPRGDT) in vitro. J Biomed Mater Res. 2002;62:292–298. doi: 10.1002/jbm.10338. [DOI] [PubMed] [Google Scholar]
- 83.Culpepper BK, Phipps MC, Bonvallet PP, Bellis SL. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials. 2010;31:9586–9594. doi: 10.1016/j.biomaterials.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy MB, Hartgerink JD, Goepferich A, Mikos AG. Synthesis and in vitro hydroxyapatite binding of peptides conjugated to calcium-binding moieties. Biomacromolecules. 2007;8:2237–2243. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- 85.Culpepper BK, Webb WM, Bonvallet PP, Bellis SL. Tunable delivery of bioactive peptides from hydroxyapatite biomaterials and allograft bone using variable-length polyglutamate domains. J Biomed Mater Res A. 2014;102:1008–1016. doi: 10.1002/jbm.a.34766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bain JL, Culpepper BK, Reddy MS, Bellis SL. Comparing variable-length polyglutamate domains to anchor an osteoinductive collagen-mimetic peptide to diverse bone graft materials. Int J Oral Maxillofac Implants. 2014;29:1437–1445. doi: 10.11607/jomi.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yokogawa K, Miya K, Sekido T, Higashi Y, Nomura M, et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–1233. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- 88.Yokogawa K, Toshima K, Yamoto K, Nishioka T, Sakura N, et al. Pharmacokinetic advantage of an intranasal preparation of a novel anti-osteoporosis drug, L-Asp-hexapeptide-conjugated estradiol. Biol Pharm Bull. 2006;29:1229–1233. doi: 10.1248/bpb.29.1229. [DOI] [PubMed] [Google Scholar]
- 89.Millan JL, Narisawa S, Lemire I, Loisel TP, Boileau G, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jin HE, Chung WJ, Lee SW. Phage display for the discovery of hydroxyapatite-associated peptides. Methods Enzymol. 2013;532:305–323. doi: 10.1016/B978-0-12-416617-2.00014-X. [DOI] [PubMed] [Google Scholar]
- 91.Gungormus M, Fong H, Kim IW, Evans JS, Tamerler C, et al. Regulation of in vitro calcium phosphate mineralization by combinatorially selected hydroxyapatite-binding peptides. Biomacromolecules. 2008;9:966–973. doi: 10.1021/bm701037x. [DOI] [PubMed] [Google Scholar]
- 92.Segvich SJ, Smith HC, Kohn DH. The adsorption of preferential binding peptides to apatite-based materials. Biomaterials. 2009;30:1287–1298. doi: 10.1016/j.biomaterials.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiger MC, Park JJ, Roy MD, Stafford CM, Karim A, et al. Quantification of the binding affinity of a specific hydroxyapatite binding peptide. Biomaterials. 2010;31:2955–2963. doi: 10.1016/j.biomaterials.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Alvarez LM, Rivera JJ, Stockdale L, Saini S, Lee RT, et al. Tethering of epidermal growth factor (EGF) to beta tricalcium phosphate (betaTCP) via fusion to a high affinity, multimeric betaTCP-binding peptide: Effects on human multipotent stromal cells/connective tissue progenitors. PLoS One. 2015;10:e0129600. doi: 10.1371/journal.pone.0129600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S, Gangal G, Uludag H. ‘Magic bullets’ for bone diseases: progress in rational design of bone-seeking medicinal agents. Chem Soc Rev. 2007;36:507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]
- 96.Ossipov DA. Bisphosphonate-modified biomaterials for drug delivery and bone tissue engineering. Expert Opin Drug Deliv. 2015;12:1443–1458. doi: 10.1517/17425247.2015.1021679. [DOI] [PubMed] [Google Scholar]
- 97.Schuessele A, Mayr H, Tessmar J, Goepferich A. Enhanced bone morphogenetic protein-2 performance on hydroxyapatite ceramic surfaces. J Biomed Mater Res A. 2009;90:959–971. doi: 10.1002/jbm.a.31745. [DOI] [PubMed] [Google Scholar]
- 98.Gittens SA, Bagnall K, Matyas JR, Lobenberg R, Uludag H. Imparting bone mineral affinity to osteogenic proteins through heparin-bisphosphonate conjugates. J Control Release. 2004;98:255–268. doi: 10.1016/j.jconrel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Gothard D, Smith EL, Kanczler JM, Rashidi H, Qutachi O, et al. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur Cell Mater. 2014;28:166–207. doi: 10.22203/ecm.v028a13. [DOI] [PubMed] [Google Scholar]
- 100.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 102.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang W, Zhu C, Wu Y, Ye D, Wang S, et al. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cell Mater. 2014;27:1–11. doi: 10.22203/ecm.v027a01. [DOI] [PubMed] [Google Scholar]
- 104.Suarez-Gonzalez D, Lee JS, Diggs A, Lu Y, Nemke B, et al. Controlled multiple growth factor delivery from bone tissue engineering scaffolds via designed affinity. Tissue Eng Part A. 2014;20:2077–2087. doi: 10.1089/ten.tea.2013.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 106.De la Riva B, Sanchez E, Hernandez A, Reyes R, Tamimi F, et al. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J Control Release. 2010;143:45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 107.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 108.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 109.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 110.Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27:S16–S23. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 111.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 112.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 113.Wang RN, Green J, Wang Z, Deng Y, Qiao M, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 115.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene. 2004;23:4232–4237. doi: 10.1038/sj.onc.1207131. [DOI] [PubMed] [Google Scholar]
- 117.Hustedt JW, Blizzard DJ. The controversy surrounding bone morphogenetic proteins in the spine: a review of current research. Yale J Biol Med. 2014;87:549–561. [PMC free article] [PubMed] [Google Scholar]
- 118.Blanquaert F, Barritault D, Caruelle JP. Effects of heparan-like polymers associated with growth factors on osteoblast proliferation and phenotype expression. J Biomed Mater Res. 1999;44:63–72. doi: 10.1002/(sici)1097-4636(199901)44:1<63::aid-jbm7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 119.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 120.Han B, Perelman N, Tang B, Hall F, Shors EC, et al. Collagen-targeted BMP3 fusion proteins arrayed on collagen matrices or porous ceramics impregnated with Type I collagen enhance osteogenesis in a rat cranial defect model. J Orthop Res. 2002;20:747–755. doi: 10.1016/S0736-0266(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 121.Lu H, Kawazoe N, Kitajima T, Myoken Y, Tomita M, et al. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials. 2012;33:6140–6146. doi: 10.1016/j.biomaterials.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 122.Shiozaki Y, Kitajima T, Mazaki T, Yoshida A, Tanaka M, et al. Enhanced in vivo osteogenesis by nanocarrier-fused bone morphogenetic protein-4. Int J Nanomedicine. 2013;8:1349–1360. doi: 10.2147/IJN.S44124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Donati D, Di Bella C, Lucarelli E, Dozza B, Frisoni T, et al. OP-1 application in bone allograft integration: preliminary results in sheep experimental surgery. Injury. 2008;39(Suppl 2):S65–S72. doi: 10.1016/S0020-1383(08)70017-2. [DOI] [PubMed] [Google Scholar]
- 124.Pluhar GE, Manley PA, Heiner JP, Vanderby R, Jr, Seeherman HJ, et al. The effect of recombinant human bone morphogenetic protein-2 on femoral reconstruction with an intercalary allograft in a dog model. J Orthop Res. 2001;19:308–317. doi: 10.1016/S0736-0266(00)90002-0. [DOI] [PubMed] [Google Scholar]
- 125.Li RH, Bouxsein ML, Blake CA, D’Augusta D, Kim H, et al. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J Orthop Res. 2003;21:997–1004. doi: 10.1016/S0736-0266(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 126.Yuan H, De Bruijn JD, Zhang X, Van Blitterswijk CA, De Groot K. Use of an osteoinductive biomaterial as a bone morphogenetic protein carrier. J Mater Sci Mater Med. 2001;12:761–766. doi: 10.1023/a:1013957431372. [DOI] [PubMed] [Google Scholar]
- 127.Edwards RB, 3rd, Seeherman HJ, Bogdanske JJ, Devitt J, Vanderby R, Jr, et al. Percutaneous injection of recombinant human bone morphogenetic protein-2 in a calcium phosphate paste accelerates healing of a canine tibial osteotomy. J Bone Joint Surg Am. 2004;86-A:1425–1438. doi: 10.2106/00004623-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 128.Miranda DA, Blumenthal NM, Sorensen RG, Wozney JM, Wikesjo UM. Evaluation of recombinant human bone morphogenetic protein-2 on the repair of alveolar ridge defects in baboons. J Periodontol. 2005;76:210–220. doi: 10.1902/jop.2005.76.2.210. [DOI] [PubMed] [Google Scholar]
- 129.Salkeld SL, Patron LP, Barrack RL, Cook SD. The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg Am. 2001;83-A:803–816. doi: 10.2106/00004623-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 130.Fukuroku J, Inoue N, Rafiee B, Sim FH, Frassica FJ, et al. Extracortical bone-bridging fixation with use of cortical allograft and recombinant human osteogenic protein-1. J Bone Joint Surg Am. 2007;89:1486–1496. doi: 10.2106/JBJS.F.00290. [DOI] [PubMed] [Google Scholar]
- 131.Hsu EL, Ghodasra JH, Ashtekar A, Nickoli MS, Lee SS, et al. A comparative evaluation of factors influencing osteoinductivity among scaffolds designed for bone regeneration. Tissue Eng Part A. 2013;19:1764–1772. doi: 10.1089/ten.tea.2012.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16:329–345. doi: 10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 133.Sakuragi M, Kitajima T, Nagamune T, Ito Y. Recombinant hBMP4 incorporated with non-canonical amino acid for binding to hydroxyapatite. Biotechnol Lett. 2011;33:1885–1890. doi: 10.1007/s10529-011-0637-1. [DOI] [PubMed] [Google Scholar]
- 134.Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, et al. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res. 2000;50:405–409. doi: 10.1002/(sici)1097-4636(20000605)50:3<405::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 135.Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta. 2003;1651:60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 136.Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide. J Biomed Mater Res A. 2004;70:115–121. doi: 10.1002/jbm.a.30071. [DOI] [PubMed] [Google Scholar]
- 137.Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J Biomed Mater Res A. 2005;72:77–82. doi: 10.1002/jbm.a.30208. [DOI] [PubMed] [Google Scholar]
- 138.Saito A, Suzuki Y, Kitamura M, Ogata S, Yoshiharak Y, et al. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A. 2006;77:700–706. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- 139.Bergeron E, Marquis ME, Chretien I, Faucheux N. Differentiation of preosteoblasts using a delivery system with BMPs and bioactive glass microspheres. J Mater Sci Mater Med. 2007;18:255–263. doi: 10.1007/s10856-006-0687-4. [DOI] [PubMed] [Google Scholar]
- 140.Park JB, Lee JY, Park HN, Seol YJ, Park YJ, et al. Osteopromotion with synthetic oligopeptide-coated bovine bone mineral in vivo. J Periodontol. 2007;78:157–163. doi: 10.1902/jop.2007.060200. [DOI] [PubMed] [Google Scholar]
- 141.Nickel J, Dreyer MK, Kirsch T, Sebald W. The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S7–S14. [PubMed] [Google Scholar]
- 142.Choi JY, Jung UW, Kim CS, Eom TK, Kang EJ, et al. The effects of newly formed synthetic peptide on bone regeneration in rat calvarial defects. J Periodontal Implant Sci. 2010;40:11–18. doi: 10.5051/jpis.2010.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu Y, Lee JS, Nemke B, Graf BK, Royalty K, et al. Coating with a modular bone morphogenetic peptide promotes healing of a bone-implant gap in an ovine model. PLoS One. 2012;7:e50378. doi: 10.1371/journal.pone.0050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu Y, Markel MD, Nemke B, Lee JS, Graf BK, et al. Influence of hydroxyapatite-coated and growth factor-releasing interference screws on tendon-bone healing in an ovine model. Arthroscopy. 2009;25:1427–1434. doi: 10.1016/j.arthro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38:258–268. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 148.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 149.Uchida S, Sakai A, Kudo H, Otomo H, Watanuki M, et al. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone. 2003;32:491–501. doi: 10.1016/s8756-3282(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 150.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 151.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 152.Belair DG, Le NN, Murphy WL. Design of growth factor sequestering biomaterials. Chem Commun. 2014;50:15651–15668. doi: 10.1039/c4cc04317k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, et al. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 154.Clarke SA, Hoskins NL, Jordan GR, Marsh DR. Healing of an ulnar defect using a proprietary TCP bone graft substitute, JAX, in association with autologous osteogenic cells and growth factors. Bone. 2007;40:939–947. doi: 10.1016/j.bone.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 155.Du B, Liu W, Deng Y, Li S, Liu X, et al. Angiogenesis and bone regeneration of porous nano-hydroxyapatite/coralline blocks coated with rhVEGF165 in critical-size alveolar bone defects in vivo. Int J Nanomedicine. 2015;10:2555–2565. doi: 10.2147/IJN.S78331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 157.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 158.Guex AG, Hegemann D, Giraud MN, Tevaearai HT, Popa AM, et al. Covalent immobilisation of VEGF on plasma-coated electrospun scaffolds for tissue engineering applications. Colloids Surf B Biointerfaces. 2014;123:724–733. doi: 10.1016/j.colsurfb.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 159.Steffens GC, Yao C, Prevel P, Markowicz M, Schenck P, et al. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng. 2004;10:1502–1509. doi: 10.1089/ten.2004.10.1502. [DOI] [PubMed] [Google Scholar]
- 160.D’Andrea LD, Iaccarino G, Fattorusso R, Sorriento D, Carannante C, et al. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci U S A. 2005;102:14215–14220. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Finetti F, Basile A, Capasso D, Di Gaetano S, Di Stasi R, et al. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochem Pharmacol. 2012;84:303–311. doi: 10.1016/j.bcp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 162.Cai L, Dinh CB, Heilshorn SC. One-pot synthesis of elastin-like polypeptide hydrogels with grafted VEGF-mimetic peptides. Biomater Sci. 2014;2:757–765. doi: 10.1039/C3BM60293A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials. 2011;32:5782–5789. doi: 10.1016/j.biomaterials.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 164.Chan TR, Stahl PJ, Yu SM. Matrix-bound VEGF mimetic peptides: Design and endothelial cell sctivation in collagen scaffolds. Adv Funct Mater. 2011;21:4252–4262. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chan TR, Stahl PJ, Li Y, Yu SM. Collagen-gelatin mixtures as wound model, and substrates for VEGF-mimetic peptide binding and endothelial cell activation. Acta Biomater. 2015;15:164–172. doi: 10.1016/j.actbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee JS, Wagoner Johnson AJ, Murphy WL. A modular, hydroxyapatite-binding version of vascular endothelial growth factor. Adv Mater. 2010;22:5494–5498. doi: 10.1002/adma.201002970. [DOI] [PubMed] [Google Scholar]
- 167.Civinini R, Macera A, Nistri L, Redl B, Innocenti M. The use of autologous blood-derived growth factors in bone regeneration. Clin Cases Miner Bone Metab. 2011;8:25–31. [PMC free article] [PubMed] [Google Scholar]
- 168.Kaigler D, Avila G, Wisner-Lynch L, Nevins ML, Nevins M, et al. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther. 2011;11:375–385. doi: 10.1517/14712598.2011.554814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 170.Hollinger JO, Onikepe AO, MacKrell J, Einhorn T, Bradica G, et al. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26:83–90. doi: 10.1002/jor.20453. [DOI] [PubMed] [Google Scholar]
- 171.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, et al. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–208. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 172.Friedlaender GE, Lin S, Solchaga LA, Snel LB, Lynch SE. The role of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and regeneration. Curr Pharm Des. 2013;19:3384–3390. doi: 10.2174/1381612811319190005. [DOI] [PubMed] [Google Scholar]
- 173.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74:1282–1292. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 174.Nevins M, Hanratty J, Lynch SE. Clinical results using recombinant human platelet-derived growth factor and mineralized freeze-dried bone allograft in periodontal defects. Int J Periodontics Restorative Dent. 2007;27:421–427. [PubMed] [Google Scholar]
- 175.Simion M, Rocchietta I, Kim D, Nevins M, Fiorellini J. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26:415–423. [PubMed] [Google Scholar]
- 176.Nevins ML, Camelo M, Schupbach P, Kim DM, Camelo JM, et al. Human histologic evaluation of mineralized collagen bone substitute and recombinant platelet-derived growth factor-BB to create bone for implant placement in extraction socket defects at 4 and 6 months: a case series. Int J Periodontics Restorative Dent. 2009;29:129–139. [PubMed] [Google Scholar]
- 177.Simion M, Rocchietta I, Dellavia C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: report of two cases. Int J Periodontics Restorative Dent. 2007;27:109–115. [PubMed] [Google Scholar]
- 178.McAllister BS, Haghighat K, Prasad HS, Rohrer MD. Histologic evaluation of recombinant human platelet-derived growth factor-BB after use in extraction socket defects: a case series. Int J Periodontics Restorative Dent. 2010;30:365–373. [PubMed] [Google Scholar]
- 179.Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 180.Choo T, Marino V, Bartold PM. Effect of PDGF-BB and beta-tricalcium phosphate (beta-TCP) on bone formation around dental implants: a pilot study in sheep. Clin Oral Implants Res. 2013;24:158–166. doi: 10.1111/j.1600-0501.2011.02345.x. [DOI] [PubMed] [Google Scholar]
- 181.Schwarz F, Sager M, Ferrari D, Mihatovic I, Becker J. Influence of recombinant human platelet-derived growth factor on lateral ridge augmentation using biphasic calcium phosphate and guided bone regeneration: a histomorphometric study in dogs. J Periodontol. 2009;80:1315–1323. doi: 10.1902/jop.2009.090034. [DOI] [PubMed] [Google Scholar]
- 182.Digiovanni CW, Baumhauer J, Lin SS, Berberian WS, Flemister AS, et al. Prospective, randomized, multi-center feasibility trial of rhPDGF-BB versus autologous bone graft in a foot and ankle fusion model. Foot Ankle Int. 2011;32:344–354. doi: 10.3113/FAI.2011.0344. [DOI] [PubMed] [Google Scholar]
- 183.Sun B, Chen B, Zhao Y, Sun W, Chen K, et al. Crosslinking heparin to collagen scaffolds for the delivery of human platelet-derived growth factor. J Biomed Mater Res B Appl Biomater. 2009;91:366–372. doi: 10.1002/jbm.b.31411. [DOI] [PubMed] [Google Scholar]