Abstract
Marrow adipose tissue (MAT) is a unique fat depot, located in the skeleton, that has the potential to contribute to both local and systemic metabolic processes. In this review we highlight several recent conceptual developments pertaining to the origin and function of MAT adipocytes; consider the relationship of MAT to beige, brown, and white adipose depots; explore MAT expansion and turnover in humans and rodents; and discuss future directions for MAT research in the context of endocrine function and metabolic disease. MAT has the potential to exert both local and systemic effects on metabolic homeostasis, skeletal remodeling, hematopoiesis, and development of bone metastases. The diversity of these functions highlights the breadth of MAT’s potential impact on health and disease.
Keywords: adipose tissue, marrow fat, beige fat, adiponectin, obesity, anorexia
Bone marrow adipose tissue; does it matter?
Research on white and brown adipose tissues (WAT and BAT respectively, see glossary) has accelerated in recent years in response to the rising incidence of obesity in most developed nations [1]. Perhaps coincidentally, there has been a resurgence in the study of the adipose tissue within the skeleton [2]. Our knowledge of this so-called marrow adipose tissue (MAT) generally lags behind that of the other adipose depots. In this review we summarize MAT composition and variation between species and place it within the context of the other adipose tissues. We also explore MAT’s endocrine and paracrine secretory properties, and expansion and regulation in metabolic disease. Lastly, we propose future directions for MAT research in the context of endocrine function and metabolic disease.
MAT composition and distribution
Marrow adipose tissue formation begins at or slightly before birth at distal skeletal sites including the tail, hands, and feet in mice and humans (reviewed in [2]). This pattern of early fatty marrow conversion in the distal skeleton in conserved in vertebrate species including mice, rats, rabbits, and humans. After this initial change, marrow adipocytes continue to form throughout life in areas of hematopoietic marrow [3]. MAT that is concentrated in the distal skeletal gives this bone marrow its characteristic ‘yellow’ appearance [4] and is referred to as constitutive MAT (cMAT) [5]. The ‘red’ marrow in regions including the lumbar/thoracic vertebrae, proximal limb skeleton, hip and ribs contains the majority of the hematopoietic cells, but can still retain a high volumetric proportion of adipocytes [2,6], known as regulated MAT (rMAT) (Box 1). Historic observations in rabbits [7] and humans [3,8] and recent work in mice [5] and rabbits [4] support the concept that MAT undergoes differential development and regulation depending on where it is located in the skeleton [5].
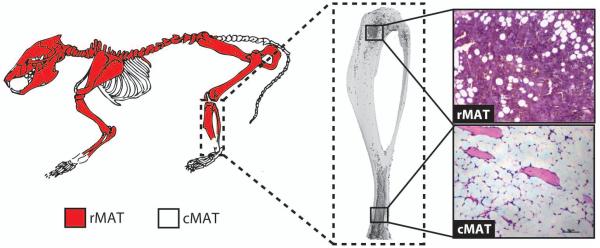
Box 1. Regulated and constitutive marrow adipose tissue.
Work by Tavassoli in 1976 identified a population of marrow adipocytes that were stable in response to phenylhydrazine, and another that was labile. He also observed differential staining of marrow adipocytes with performic acid Schiff (PFAS) [7]. Similar results were recently reported in the context of cold exposure, in which the adipocytes within the red marrow decrease in number and size while those within the yellow marrow do not [5,7]. Based on these results, single adipocytes interspersed within red marrow have been termed regulated marrow adipocytes (rMAT), and those within yellow marrow, constitutive (cMAT) (Figure IA-C) [5]. This may have a genetic basis, as knock-out of PTRF in mice, a model of congenital generalized lipodystrophy-4, results in selective loss of the rMAT, but not cMAT, adipocytes [5].
In addition to differences in regulation and development, rMAT and cMAT adipocytes are currently defined by their unique gene expression profile and lipid composition. Expression of Pparg is similar between rat rMAT and cMAT adipocytes, however, Cebpa and Cebpb are selectively elevated in cMAT – suggesting that these adipocytes may undergo alternative transcriptional regulation. Relative to rMAT and WAT, cMAT adipocytes also have increased lipid unsaturation, driven by decreases in palmitate and stearate and increases in their monounsaturated derivatives [5]. Indirect 1H-MRS analyses of the femur and tibia suggests that the same may be true in humans [5]. Future work is needed to refine the properties of rMAT and cMAT adipocytes and their conservation between species.
Though we know that rMAT-like adipocytes are enriched in the proximal, hematopoietic skeleton and cMAT-like cells are enriched in the distal areas, there is possibility for both subtypes to exist in the same region [5]. In rabbits, for example, the adipocytes near the hematopoietically active, endocortical surface of the femur are PFAS-positive, while those clustered in the center of the femoral marrow are PFAS-negative [7]. Like this central core of femoral MAT, the cMAT adipocytes in the distal tibia are also PFAS-negative.
There is much that remains unknown about rMAT and cMAT. Though they appear to be developmentally distinct, it is unclear whether they derive from distinct progenitors, or rather represent a different state of maturation of the MAT adipocyte from a common lineage. Identification of microenvironmental and cell-autonomous differences are needed to understand why rMAT is preferentially regulated. Lastly, we need to determine the impact of each subtype on bone loss and systemic metabolism in health and disease.
BOX 1 Figure I. Regulated and constitutive marrow adipose tissue (MAT) in the mouse.
(A) Proposed distribution of regulated MAT (rMAT) and constitutive MAT (cMAT) in the mouse skeleton when marrow is present. Regulated MAT is found in the more proximal regions including the mid- to proximal-tibia, femur, and lumbar vertebrae. Constitutive MAT is found in the most distal portion of the tibia and tail vertebrae. (B) Three-dimensional reconstruction of an osmium-stained mouse tibia. (C) Representative histology of rMAT and cMAT adipocytes within the bone marrow.
Regional differences have been observed between rMAT and cMAT (Box 1). Experiments in mice and/or rats have shown that rMAT adipocytes preferentially develop within the red marrow throughout life, are smaller in size (~31-33 μm diameter), contain more saturated lipids and express lower levels of the adipogenic transcription factors Cebpa and Cebpb. Conversely, cMAT adipocytes develop shortly after birth, are larger in size (~38-39 μm diameter), contain more unsaturated lipids, and have elevated Cebpa and Cebpb [4,5]. Regulated MAT lipid saturation and expression of adipocyte genes is reminiscent of WAT – thus the critical comparison is that cMAT has elevated expression of these genes and increased lipid unsaturation relative to rMAT or WAT depots.
Based on histology and gross anatomic assessment of human specimens, roughly 70% of the adult bone marrow volume is MAT [2,3]. Total marrow volume is, on average, 1632+/−587 cm3 based on whole body assessment with PET/CT [9]. Combined, this would predict 1142+/−410 cm3 of total MAT, equivalent to 1.03+/−0.37 kg (fat density = 0.9 g/cm3). Indirect MRI-based techniques concur, revealing that the amount of total MAT in the skeleton of an average size human is ~1.35 kg (ranging from 0.5 to 3 kg) [8,10,11], accounting for approximately 8% of total fat mass. Depending on peripheral fat volume, this proportion can range from as low as 1% to as high as ~30% [2,12]. One can therefore speculate that the average human skeleton contains enough MAT to directly influence local and systemic metabolic processes, and that the balance between total WAT and MAT volume has the potential to contribute to these relationships.
MAT volume varies between species
As a general rule, the MAT content, by percent volume, in the red marrow is directly proportional to the size of the animal (i.e. humans > rabbits > rats > mice) [2]. Mice have a significantly reduced proportion of MAT in areas of red marrow, as quantified in three-dimensions using osmium tetroxide staining combined with micro-computed tomography [5,8,12,13]. For example, 12-week-old C57Bl/6J (B6) male mice have on average 0.7% MAT by volume in the bone marrow of the tibial diaphysis [5]. MAT content and its distribution also vary between mouse strains. For example, the proximal tibial MAT in C3H/HeJ mice is 12.7-fold higher than B6 mice at 12-weeks of age, though the total tibial MAT volume is only increased by 1.7-fold [5]. This is explained by increased MAT storage in the distal tibia of B6 mice. In humans, based on biopsies of the iliac crest, the amount of MAT in the red marrow is roughly proportional to the age of the patient (i.e. 30 years old = 30% MAT; 70 years old = 70% MAT) [14]. Though both observations follow the well-documented increase in MAT with age [3], the absolute amount of MAT varies considerably. This may have important implications for the relationship of MAT to surrounding tissues. For example, during caloric restriction (CR) in mice, tibial MAT volume expands to 40% of diaphyseal volume; however, when this MAT expansion was limited to only 15%, systemic adaptations to CR were impaired [12]. Systemic effects of MAT may therefore occur only when MAT volume surpasses a critical threshold, in which case even a 17-fold increase in tibial MAT volume in an aged B6 mouse may not result in a detectable metabolic or skeletal phenotype (e.g. insulin resistance or bone loss). In contrast, the ~2.3-fold increase from 30% to 70% MAT in the red marrow of humans with age, or the reported 1.3-fold increase with anorexia, may be more likely to achieve ‘critical mass’ with significant downstream consequences in humans, including contributions to bone turnover and metabolic adaptation of peripheral tissues [2,12].
It is currently unknown whether the decreased MAT within the red marrow of small animals such as mice means that the proportion of total MAT relative to peripheral WAT is also lower. Rodents, unlike humans, have a tail, the vertebrae of which are filled almost completely with MAT [5,15]. This may compensate for the decrease in MAT at other sites and warrants further characterization. Until this is clarified, it is worth keeping in mind that MAT-related findings in rodents may become more significant in larger species with greater MAT volume (such as humans) or in conditions of marked MAT expansion (such as CR), especially in the red marrow.
MAT versus other adipose tissues
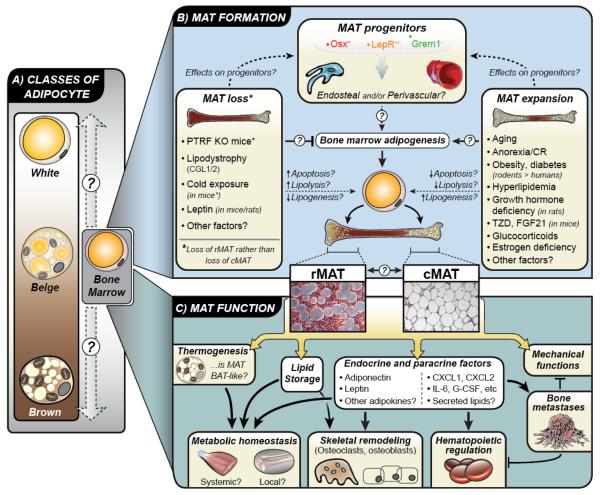
Examination of marrow fat in the context of the rapidly expanding literature on adipose tissues provides clues about its function. Current classifications include white, brown, beige/brite, and more recently even lactation-associated ‘pink’ adipocytes [16,17]. Where does MAT fall on this spectrum – if at all (Figure 1)?
Figure 1. KEY FIGURE. The knowns and unknowns of MAT.
(A) Adipose tissue is typically classified as white, brown, or beige. Bone marrow adipocytes are morphologically similar to white adipocytes; however, it is unclear where they fall on this ‘white-beige-brown’ spectrum, if at all. (B) Lineage tracing studies demonstrate that bone marrow adipocyte progenitors express osterix (Osx), but not Grem1. Some marrow adipocytes are also derived from progenitors that express the leptin receptor (LepR). Based on these findings it is unclear if marrow adipocyte progenitors are endosteal and/or perivascular in origin, although they are clearly distinct to progenitors for white and brown adipocytes. Also unclear is how these progenitors are driven toward adipogenesis to generate bone marrow adipocytes, which can be classed as two distinct subtypes: regulated (rMAT) and constitutive (cMAT). Do these subtypes derive from distinct progenitors, and can rMAT and cMAT interconvert? In addition to physiological MAT formation, various conditions are associated with MAT loss or MAT expansion, predominantly in rMAT. However, the mechanisms linking these conditions to MAT loss or gain remain largely uncertain. (C) The function of MAT is also yet to be firmly established. Some reports suggest that MAT has BAT-like properties, though this remains controversial. Instead, MAT may have more WAT-like properties such as lipid storage and endocrine functions. It is now clear that MAT can release adipokines such as adiponectin and leptin, as well as paracrine factors such as cytokines and lipids. These secreted factors may allow MAT to exert both local and systemic effects on metabolic homeostasis, skeletal remodeling, hematopoiesis, and development of bone metastases. The diversity of these functions highlights the breadth of MAT’s potential impact on health and disease.
Formation
Adipocytes are formed after differentiation of their progenitor cells. The complexities of the WAT progenitor have been reviewed previously [18]. Like WAT, MAT preadipocytes gradually accumulate lipid that coalesces into a unilocular droplet and displaces the nucleus and cytoplasm peripherally [19]. Electron microscopy suggests that the ‘fibroblast-like’ WAT progenitors have dense profiles of rough endoplasmic reticulum (ER) and are closely-associated with collagen fibers [19–21]. Though MAT progenitors may contain occasional rough-ER profiles, they are not associated with collagen. When compared to mature BAT adipocytes using EM, MAT generally lacks glycogen and has reduced mitochondrial content [19]. Lineage tracing in ‘mT/mG’ mice has also been used to characterize the origins of the MAT adipocyte. These mice constitutively express a floxed, membrane-targeted tdTomato cassette (mT) upstream of an eGFP cassette (mG). When cre-recombinase is expressed, mT is excised allowing expression of the membrane-targeted eGFP. Unlike gonadal WAT and intramuscular fat, MAT adipocytes are uniformly traced in Osterix-cre:mT/mG mice [22] (Figure 1). These ultrastructural and lineage tracing findings suggest that MAT adipocytes are derived from a unique progenitor cell that is distinct from that of WAT and BAT.
A proportion of the developing MAT adipocytes are closely associated with the endothelium of the marrow sinuses [19]. These progenitors may have a shared identity with perivascular CXCL12-abundant reticular (CAR) cells and/or Nestin+ mesenchymal stem cells [23,24]. However, it remains unknown whether they are distinct in origin and/or function from adipocytes that develop near the endosteal surface of the bone. There is recent evidence, for example, that distinct waves of embryonic and adult Osterix+ progenitor cells organize the developing bone and marrow [25]. Additional experiments have revealed that LepR+ perisinusoidal cells give rise to adult, but not developmental, osteo-adipogenic lineages [26]. Both LepR+ and Osterix+ progenitor cells contribute to irradiation-induced MAT expansion [25]. In contrast, Grem1 identifies a population of osteochondroreticular cells near the growth plate and trabecular bone that can give rise to osteoblasts, but not adipocytes [27]. In WAT, adipocyte progenitors that promote homeostasis of the adult WAT are lineage positive for smooth muscle actin and reside in a perivascular niche [28]. In contrast, progenitors responsible for initial WAT organogenesis lack a vascular niche and are negative for smooth muscle actin [28]. Like these skeletal and WAT-progenitors, MAT subpopulations may be derived from multiple types of precursor cells (e.g. endosteal vs perivascular, tissue development vs maintenance, etc). Clarification of the identity of the MAT progenitor, or progenitors, will be necessary to accurately model the nuances of MAT development and function both in vitro and in vivo.
Characterization
It has been proposed that MAT is ‘beige’ or ‘brown-like’ in character (Box 2). BAT adipocytes function to convert stored energy into heat. They have a multilocular lipid droplet, express UCP-1, and the majority of cells are derived from a Myf5+ progenitor [16,29]. Though Myf5 is thought to be a BAT-specific lineage marker, it also traces unilocular adipocytes within the anterior subcutaneous WAT and the retroperitoneal WAT [29]. Beige/brite adipocytes are derived from a subset of pre-existing cells within the WAT that can be reversibly differentiated into ‘brown-like’ multilocular cells that express UCP-1 [30–33]. Adult MAT is not histologically [2] or ultrastructurally [19] similar to traditional BAT. However, there has been one published image of several UCP-1+, BAT-like cells in the lumbar vertebral marrow of a young 3-week-old mouse [34]. These reported BAT-like cells may be distinct from unilocular, adult MAT. The concept of a beige-like unilocular MAT adipocyte is attractive, with proposed functional implications [35]. However, the interpretation of current research is limited by the technical challenges of working with MAT; hence, more work is clearly needed to explore this possibility (Box 2).
Box 2. Is MAT beige or brown fat?
It has been hypothesized that MAT has BAT-like characteristics. This is based on gene expression in whole tibia bones showing that BAT markers Prdm16, Dio2, FoxC2 and Pgc1α (but not Ucp1) are highly elevated relative to epididymal WAT (eWAT) and expressed at comparable levels to whole BAT - when normalized to the reference gene Fabp4/aP2 [47]. In a separate study, Fabp4 was expressed at only 2.4-4.3% of what is observed in eWAT [82]. Thus, its use as a normalization tool in a complex tissue such as the tibia may significantly elevate predicted gene expression. Indeed, expression of Prdm16, Pgc1α, and FoxC2 were not consistently elevated in purified MAT when compared to eWAT adipocytes from mice at 6-, 14-, and 18-months of age [82]. Deiodinase2 (Dio2)-mediated thyroid hormone conversion from thyroxine (T4) to triiodothyronine (T3) has the potential to regulate adipose tissue thermogenesis. Expression of Dio2 was elevated by 237-fold in whole tibia when normalized to Fabp4/aP2 [47] and by 1.3- to 12.1-fold in isolated MAT adipocytes [82].
Skeletal MAT volume is regulated by temperature [2,5], however, the ability of MAT adipocytes to undergo stimulated thermogenesis and produce heat is not known [36]. UCP-1 is the canonical regulator of thermogenic uncoupling [16]. Thus, it is worth noting that in both of the above studies Ucp1 expression was not elevated in MAT relative to eWAT at baseline [47,82]. However, treatment of B6 mice with rosiglitazone increased MAT and caused a 2- to 3-fold elevation of BAT-markers including Ucp1, Pgc1α, and Prdm16 [47]. PGC-1α regulates the expression of thermogenic genes in BAT, it also regulates mitochondrial biogenesis and oxidative metabolism in other cell types [84]. PRDM16 is a co-activator that promotes thermogenic gene expression and forms a complex with multiple adipogenic transcription factors, including PPARγ and PGC-1α [85]. Though interesting, use of the whole tibia makes these changes challenging to interpret [47]. In addition, it is unclear at what point Ucp1 expression indicates uncoupling of respiration [86] and Ucp1 at the RNA level does not necessarily correlate with UCP-1 protein and adaptive thermogenesis [87]. For this reason analysis of intact MAT obtained from rabbits [4,12] or from isolated MAT adipocytes [5] might be preferable. While the concept of a BAT/beige-like MAT is intriguing, clearly more work is needed to definitively address this hypothesis and its implications.
Function
Unlike WAT, which can increase seemingly without limit, MAT is constrained spatially within the boundaries set by the skeleton. MAT adipocytes are also situated in a unique microenvironment, surrounded by hematopoietic and skeletal lineage cells. This likely contributes to its differential regulation and points to the local microenvironment, in addition to endocrine mediators, as a major regulators of MAT function.
WAT is the primary site for long-term storage of excess energy and, as a consequence, is tightly controlled in response to whole-body energy balance (see [16] for review). Energy storage by WAT is principally regulated by insulin, which promotes glucose and lipid uptake while inhibiting lipolysis. Current evidence suggests that MAT expresses components of the insulin signaling machinery [36]. MAT also responds positively to rosiglitazone, a thiazolidinedione, by inducing genes involved in insulin signaling, fatty acid and carbohydrate metabolism [36,37]. Thus, like WAT, is MAT used for energy storage and mobilization? When the current evidence is considered, the results are puzzling. For example, the observation that both CR [12,38] and high-fat diets [39] can increase skeletal MAT in mice is counterintuitive if the role of MAT, like WAT, is dynamically related to systemic energy demands. Recent work suggests that increased MAT with CR is due to expansion of adipocyte numbers through adipogenesis rather than increases in adipocyte size, which may further disassociate MAT and energy metabolism [4,12]. Accumulation of MAT in mouse models of T1DM also demonstrates that hypoinsulinemia is insufficient to block MAT expansion [40].
It may be that some MAT adipocytes are linked to systemic energy demand while others provide support for surrounding cells. For example, MAT may serve as a lipid reservoir, a storage site for ectopic lipid that protects skeletal osteoblasts from lipotoxicity [41]. The liver and skeletal muscle, for example, efficiently store lipid in times of excess circulating triglyceride [42]. This also prompts the question – are all MAT-appearing cells within the bone marrow actually adipocytes? Expression of key adipogenic transcription factors Pparg, Cebpa, and Cebpb in isolated MAT cells from healthy animals supports this identity [5,43]. However, the storage of marrow lipid in non-MAT populations, especially in states of disease, has not been thoroughly explored.
MAT may function to provide support to surrounding cells and tissues. For example, there is evidence that MAT may contribute to the mechanical properties of the skeleton [44,45] (Figure 1). MAT is also intimately associated with the blood-forming marrow. Primary human MAT adipocytes, purified from the iliac crest, have the ability to support differentiation of CD34+ hematopoietic progenitor cells in vitro [43]. However, in vivo evidence in mice suggests that MAT adipocytes are predominantly negative regulators of the hematopoietic microenvironment, inducing both quiescence and loss of progenitor cells [15]. Indeed, genetic or pharmacologic inhibition of MAT expansion enhances hematopoietic engraftment and recovery after irradiation and bone marrow transplant in mice [15]. It remains unclear whether MAT is always a negative component of the hematopoietic niche. For example, thiazolidinedione (TZD)-induced MAT expansion in mice did not alter hematopoietic progenitor frequency within the bone marrow in vivo [46]. Future work is needed to extend these studies and to determine if MAT can serve as a supportive member of the niche or an energy source for hematopoietic differentiation in some contexts (Figure 1).
MAT as a secretory tissue
The secretion profile of MAT and its functional endocrine and paracrine implications remain largely unexplored. MAT secreted factors have been analyzed in conditioned media from three types of cell preparations: isolated bone mesenchymal stem cells (BMSCs) that are differentiated to adipocytes (in vitro), primary MAT adipocytes purified by collagenase digestion (ex vivo), and whole MAT tissue explants (explant). Each method has limitations. In vitro models lack micro-environmental programming during differentiation and often rely on potent adipogenic stimulants such as TZDs, which can independently regulate MAT [47]. Ex vivo analysis of primary adipocytes overcomes these limitations, but chemical processing and loss of surrounding microenvironmental signals can limit interpretation of results. Explants maintain MAT adipocytes in their native microenvironment but are complicated by heterogeneity and cell breakdown. Future comparison and utilization of multiple techniques will help to accurately refine MAT’s secretory properties.
In vitro, human adipocytes derived from sternal BMSCs secrete the cytokines IL-6, MIP-1α, G-CSF, and GM-CSF [48]. In mice, in vitro BMSC-derived adipocytes also produce CXCL1 and CXCL2 [49]. Ex vivo primary human MAT adipocytes from the iliac crest also secrete detectable levels of IL-6 and G-CSF, in addition to IL-8, after 7-days of ceiling culture [43]. It has been proposed that secretion of cytokines by MAT stimulates local differentiation and activation of osteoclasts [49]. This suggests that, at least in some contexts, MAT may promote increased bone remodeling - contributing to osteoporotic bone loss and even bone destruction in the setting of skeletal metastasis [49] (Figure 1).
MAT adipocytes also secrete adipokines such as leptin (in vitro) [50] and adiponectin (explant) [12]. Paradoxically, CR in rodents and humans causes elevation of circulating adiponectin despite loss of WAT [12]. However, CR also drives MAT expansion [4,12,38]. To determine whether MAT contributes to circulating adiponectin with CR, MAT expansion was inhibited by overexpressing the Wnt10b transgene from the osteocalcin promoter in mice (OCN-Wnt10b) [12]. Inhibition of MAT expansion was sufficient to suppress CR-associated hyperadiponectinemia [12]. Similarly, in rabbits, neither MAT expansion nor hyperadiponectinemia occured during moderate- or extensive-CR, despite loss of WAT [4]. These results demonstrate that CR-induced MAT expansion is necessary for maximal increases in circulating adiponectin. These observations support the ability of MAT to function as an endocrine organ in the context of CR. While further research is needed, these studies also revealed that impaired MAT expansion and/or hyperadiponectinemia is associated with blunted induction of Pgc1a, Tfam and Acadm in skeletal muscle during CR in OCN-Wnt10b mice [12], further implying that MAT may contribute to skeletal muscle adaptation during CR and suggesting that, as an endocrine organ, MAT can exert systemic effects.
Endocrine regulation of MAT expansion in metabolic disease
As with CR in humans and mice, MAT expansion is a common response to many clinical conditions and pharmacotherapies, including diabetes, obesity, anorexia, aging, estrogen deficiency and glucocorticoid use (Table 1). In general, MAT expansion can occur through increases in cell number and/or cell size [51]. Though MAT can contribute to skeletal muscle adaptation with CR [12], it remains unclear whether MAT accumulation is uniformly beneficial. Alternatively, it might be a passive, inconsequential phenomenon, or a pathological response that negatively impacts local or systemic health [52]. For example, MAT formation and bone loss are often inversely associated, though this relationship remains unclear despite significant research efforts over the last 30 years (reviewed in [53]) (Figure 1). The relationship between MAT and metabolic health remains almost entirely unknown.
Table 1.
Changes in marrow adipose tissue in selected metabolic conditions
| Human MAT | Rodent MAT | Ref | |
|---|---|---|---|
| Lipodystrophy | |||
| CGL-1 | Absent | NA | [2] |
| CGL-2 | Absent | NA | [2] |
| CGL-3 | Retained* | Retained | [2, 5] |
| CGL-4 | Retained* | rMAT decreased, cMAT no change |
[2, 5] |
| Diabetes | |||
| Type 1 | No change | Increased (tibia/femur) or no change (vertebrae) |
[40, 54] |
| Type 2 | Increase or No Change |
Increased | [53, 55, 56] |
| Obesity/HFD | Increase or No Change |
Increased | [39, 57, 58] |
| Anorexia/CR | Increased | Increased | [4, 12, 38] |
| Aging | Increased | Increased | [5, 14] |
| Estrogen Deficiency |
Increased | Increased | [66, 67] |
| Glucocorticoi ds |
Increased | Increased | [68, 69] |
CGL-X = Congenital Generalized Lipodystrophy where X represents the subtype of the disease, CR = Calorie Restriction, HFD = High Fat Diet, NA = Not Available,
Limited patient data available
The fold-increase of MAT in rodent models of metabolic disease generally exceeds what is observed in humans. In mice with type 1 diabetes mellitus (T1DM), MAT selectively increases in the appendicular, but not axial, skeleton [40,54]. In contrast, neither MAT loss nor gain has been observed in humans with T1DM [54] (Table 1). In rodent models of T2DM, such as the ob/ob mouse, a significant increase in MAT occurs [55]. However, in humans with type 2 diabetes, only a slight increase (or no change) in MAT has been reported [53,56]. Similar results have been noted with obesity in which MAT expansion is much more dramatic in mice fed a high-fat diet when compared to obese humans [39,57,58]. The diminished expansion of MAT in humans, relative to rodents, may be related to the higher baseline MAT content of human marrow. Once formed, MAT is also more persistent in humans than in mice – lasting for years after prolonged bed rest or radiation therapy [59,60]. This is also true for WAT adipocytes, which have an estimated lifespan of 10-years in humans [61] but less than 1-year in mice [62]. The implications of these differences are unknown, though recent work has shown that increases in MAT lipid saturation are related to fracture risk in postmenopausal women with T2DM [63]. Thus it may be the quality, rather than the quantity, of MAT that contributes to skeletal fragility and metabolic adaptation in humans.
There are many local and systemic factors with the potential to regulate MAT formation and function, thereby contributing to MAT expansion. For example, there is emerging clinical evidence that MAT formation is driven by increases in circulating lipids such as triglycerides [54,64]. Thus, inhibition of elevated serum lipid levels (ex. treatment with statins) could potentially decrease MAT.
Endocrine factors also contribute to the balance between formation of new MAT cells through adipogenesis and turnover of mature MAT adipocytes by lipolysis or apoptosis (Figure 1). Pituitary-derived growth hormone, for example, actively suppresses MAT expansion [65]. Specifically, removal of the pituitary gland in rats causes robust increases in MAT that are rescued by administration of exogenous growth hormone, but not intermittent PTH, 17β-estradiol, or IGF-1 [65]. Estrogen deficiency [66,67], FGF-21 [68] and glucocorticoids [69,70] also promote MAT formation. Of these, increases in circulating glucocorticoids may be necessary for MAT accumulation with CR [4].
Several other endocrine factors have been postulated to regulate MAT formation. Notable among these is leptin, with hypoleptinemia suggested to promote MAT expansion. Indeed, pituitary excision in rats [65] and WAT loss with CR in mice [4] can both lead to decreased circulating leptin and increased MAT formation. Conversely, direct administration of leptin into the intracerebroventricular (ICV) space leads to profound MAT loss in mice and rats [71–73]. These effects, however, require administration of supra-physiological doses of leptin [74], and it remains unclear if changes in leptin concentrations, within physiological limits, are sufficient to impact MAT turnover. For example, estrogen deficiency is associated with increases in MAT despite increases in circulating leptin [75,76], demonstrating that increased leptin per se is not sufficient to ablate MAT. In contrast, CR in rabbits leads to hypoleptinemia without MAT expansion, while in female mice CR leads to MAT expansion without causing hypoleptinemia [4]. Together, these observations suggest that hypoleptinemia is neither sufficient nor necessary for MAT expansion. When administered peripherally, elevated circulating leptin may inhibit MAT progenitor differentiation [77]. In addition, both ICV and peripheral administration likely cause sustained activation of the sympathetic nervous system (SNS) [78]. Sympathetic drive coordinates metabolic responses to energy deficits with respect to nutrient partitioning, release, and utilization [16]. Activation of the SNS could promote local release of norepinephrine and induction of lipolysis in mature MAT adipocytes through β-adrenergic receptors. There is also evidence that leptin-induced loss of MAT can occur through apoptosis [55,71] (Figure 1).
With the exception of certain lipodystrophies, MAT is either retained or increased in metabolic diseases including diabetes, obesity, anorexia, and gonadal dysfunction (Table 1). MAT expansion is dynamically regulated by endogenous circulating endocrine factors such as growth hormone, estrogen, and glucocorticoids and is also readily modified by pharmacotherapies that impact any of these systems. However, despite its prevalence, the role of MAT expansion in metabolic disease remains relatively unknown.
Concluding Remarks and Future Perspectives
As a field, we know the location of the MAT and how much of it we have. This has been aided by the development and refinement of skeletal imaging techniques in rodents [13] and humans [10,79,80]. We also know how MAT changes with a relatively large variety of systemic conditions and medications (Table 1); that relative MAT volume and expansion differ between rodents and humans; that MAT behaves differently depending on where we look in the skeleton (e.g. rMAT vs cMAT); and that MAT is capable of secreting endocrine factors, such as adiponectin. However, many questions remain (see outstanding questions box).
Outstanding Questions Box.
Biology of the MAT adipocyte
What is the identity of the MAT progenitor(s)?
Do regulated and constitutive MAT adipocytes derive from distinct progenitors, or do they represent unique differentiation states within the same lineage?
Can regulated and constitutive MAT adipocytes interconvert? Physiology of MAT
What metabolites and endocrine factors does MAT secrete?
What are the metabolic implications of MAT’s endocrine action?
Does MAT have functionally significant BAT or beige-like adipocyte characteristics?
Can MAT serve as an energy source for hematopoietic or skeletal cells in some contexts?
Pathology of MAT
Are certain types of MAT adipocytes more likely to have deleterious local or systemic effects?
Are all MAT-appearing cells within the bone marrow actually adipocytes? Can marrow lipid be acutely stored in non-MAT populations in some situations?
How is MAT expansion regulated in metabolic disease? What are the implications?
It is unknown whether MAT can become dysfunctional or unhealthy, or whether certain types of MAT adipocytes are more likely to have deleterious effects [35,81]. In humans in particular, it may be the quality rather than the quantity of marrow fat that matters [63]. For example, MAT lipid saturation, rather than total MAT, is a novel biomarker of skeletal fragility in diabetes [63]. This is particularly intriguing since rMAT is defined, in part, by its increased lipid saturation relative to cMAT [5]. Regarding dysfunction, gene expression in isolated B6 mouse MAT adipocytes suggests potential for mitochondrial dysfunction and altered fatty acid synthesis with aging [82]. Similar analyses in whole tibiae from B6 mice reveal age-related decreases in BAT-marker genes Prdm16, FoxC2, Adrb3, and Dio2 that may also indicate a shift in the nature of the MAT adipocyte [47].
Quantitative analysis of the metabolic processes occurring within the MAT adipocytes (e.g. lipid synthesis, uptake, turnover and breakdown) in response to local and systemic stimulants will be necessary to place MAT into the context of whole-body energy balance. To do this the field will need to establish standardized protocols for molecular analysis of MAT in order to produce meaningful comparisons between adipocyte subpopulations across species and strains of animals.
Future work is also needed to identify the mechanisms underlying MAT expansion and to characterize its benefits or consequences in metabolic disease states. In the context of hematopoiesis, ablation of MAT promotes hematopoietic recovery post-irradiation [15]. However, more MAT isn’t necessarily a bad thing. For example, increases in MAT with CR promote metabolic adaptation [12]. In addition, the cMAT-rich distal tibia, despite being nearly filled with adipocytes, actually has more trabecular bone with increased mineral density relative to the proximal tibia [83]. Metastasis of tumors to cMAT-rich areas is also very rare when compared to hematopoietic marrow [3]. In contrast, areas with rMAT can more readily undergo MAT accumulation, for example with HFD, which may promote prostate tumor progression by increasing osteoclast activity and bone turnover [49]. It will be important to consider MAT’s site-specific differences in future studies on the relationships between MAT, bone, hematopoiesis, and metabolism. In addition, it will be equally important to shift our focus away from what we know about WAT and toward what makes MAT unique in its own context and microenvironment to truly advance the field.
Trends Box.
Marrow adipose tissue (MAT) is dynamically regulated in metabolic diseases including diabetes, obesity, and anorexia, and by pharmacotherapies including estrogen, growth hormone, and glucocorticoids.
MAT is an endocrine organ that during caloric restriction can contribute to circulating adipokines, such as adiponectin.
MAT exists in two forms, regulated and constitutive. Regulated and constitutive marrow adipocytes are defined based on their surrounding microenvironment and response to external stimuli. They have distinct developmental patterns, adipocyte size, lipid saturation, and transcription factor expression.
The fold-increase of MAT in rodent models of metabolic diseases such as diabetes and obesity exceeds what is observed in humans. The persistence of MAT adipocytes after their formation is also significantly reduced in mice.
Acknowledgments
This work was supported by grants from the National Institutes of Health including R24-DK092759 (O.A.M., M.C.H.), K99-DE024178 (E.L.S.), and T32-HD007505 (A.A.B.). W.P.C. is supported by a Career Development Award (MR/M021394/1) from the Medical Research Council (United Kingdom) and by a Chancellor’s Fellowship from the University of Edinburgh.
Glossary
- Adiponectin
A secreted adipocyte-derived hormone that is produced by both marrow and white adipose tissues. Has the potential to regulate metabolic and cardiovascular health.
- Brown adipose tissue (BAT)
a distinct type of adipose tissue found in mammals that is specialized for mediating non-shivering thermogenesis.
- Constitutive marrow adipose tissue (cMAT)
a subtype of marrow adipocytes found in the yellow bone marrow that develops early in life, primarily in the distal regions of the skeleton.
- Lineage tracing
is a technique used for the identification of progeny of a single cell; single cells and their progeny are marked based on expression of fluorescent proteins (ex. tdTomato and eGFP) in vivo.
- mT/mG
A lineage tracing system in which all cells constitutively express a floxed, membrane-targeted tdTomato (mT) cassette. When exposed to CRE-recombinase, generally expressed under the control of a genetic promoter, the tdTomato cassette is excised allowing the cells to express eGFP. This switches the color of the cells within the traced lineage from ‘red’ to ‘green’.
- Myogenic factor 5 (Myf5)
a transcriptional activator that promotes transcription of muscle-specific target genes. The Myf5 promoter has also been used to genetically trace BAT adipocytes, and a subset of WAT, based on its expression in their progenitor cells.
- Osmium tetroxide staining
a method by which radio-dense osmium is incorporated into the lipid droplet of the marrow adipocyte. The stained adipocytes are then visualized in three-dimensions using high-resolution computed tomography.
- Osterix (Osx)
a transcriptional activator that is essential for osteoblast differentiation. The osterix promoter is expressed by skeletal mesenchymal precursor cells and can be used to genetically trace osteoblasts and MAT adipocytes.
- Positron emission tomography - computed tomography (PET/CT)
a radiological technique that uses a specialized camera to detect and image radioactive tracers within the body. This information is then overlaid on the three-dimensional CT scan to pinpoint the exact anatomic location of the tracer.
- Regulated marrow adipose tissue (rMAT)
A subtype of marrow adipocytes found within the red, blood-cell forming portions of the bone marrow.
- White adipose tissue (WAT)
a metabolically active endocrine organ found in discrete depots and specialized to store excess energy as triacylglycerols, and release fatty acid (FA)s and glycerol when energy expenditure exceeds energy intake.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 4.Cawthorn WP, et al. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia. Endocrinology. 2016;157:508–521. doi: 10.1210/en.2015-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheller EL, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichardo JC, et al. Method for estimating skeletal spongiosa volume and active marrow mass in the adult male and adult female. J Nucl Med. 2007;48:1880–1888. doi: 10.2967/jnumed.107.044354. [DOI] [PubMed] [Google Scholar]
- 7.Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976;100:16–18. [PubMed] [Google Scholar]
- 8.Emery JL, Follett GF. Regression of bone-marrow haemopoiesis from the terminal digits in the foetus and infant. Br J Haematol. 1964;10:485–489. doi: 10.1111/j.1365-2141.1964.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 9.Sambuceti G, et al. Estimating the whole bone-marrow asset in humans by a computational approach to integrated PET/CT imaging. Eur J Nucl Med Mol Imaging. 2012;39:1326–1338. doi: 10.1007/s00259-012-2141-9. [DOI] [PubMed] [Google Scholar]
- 10.Shen W, et al. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W, et al. Ethnic and sex differences in bone marrow adipose tissue and bone mineral density relationship. Osteoporos Int. 2012;23:2293–2301. doi: 10.1007/s00198-011-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cawthorn WP, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheller EL, et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Meth Enzymol. 2014;537:123–139. doi: 10.1016/B978-0-12-411619-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Justesen J, et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 15.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peirce V, et al. The different shades of fat. Nature. 2014;510:76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- 17.Giordano A, et al. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170:R159–R171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 18.Cawthorn WP, et al. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab. 2012;23:270–277. doi: 10.1016/j.tem.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavassoli M. Ultrastructural development of bone marrow adipose cell. Acta Anat (Basel) 1976;94:65–77. doi: 10.1159/000144545. [DOI] [PubMed] [Google Scholar]
- 20.Tavassoli M. Marrow adipose cells. Ultrastructural and histochemical characterization. Arch Pathol. 1974;98:189–192. [PubMed] [Google Scholar]
- 21.Tran KV, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, et al. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS ONE. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omatsu Y, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Mizoguchi T, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou BO, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worthley DL, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, et al. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014;9:1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contreras GA, et al. Inducible brown adipocytes in subcutaneous inguinal white fat: the role of continuous sympathetic stimulation. Am J Physiol Endocrinol Metab. 2014;307:E793–E799. doi: 10.1152/ajpendo.00033.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenwald M, et al. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 32.Cinti S. The Adipose Organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishio M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394–406. doi: 10.1016/j.cmet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Adler BJ, et al. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10:737–748. doi: 10.1038/nrendo.2014.169. [DOI] [PubMed] [Google Scholar]
- 36.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–539. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shockley KR, et al. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106:232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devlin MJ, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doucette CR, et al. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J Cell Physiol. 2015;230:2032–2037. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin LM, McCabe LR. Type I diabetic bone phenotype is location but not gender dependent. Histochem Cell Biol. 2007;128:125–133. doi: 10.1007/s00418-007-0308-4. [DOI] [PubMed] [Google Scholar]
- 41.Gunaratnam K, et al. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155:108–116. doi: 10.1210/en.2013-1712. [DOI] [PubMed] [Google Scholar]
- 42.Browning JD, et al. The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J Lipid Res. 2012;53:577–586. doi: 10.1194/jlr.P020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poloni A, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41:558–566.e2. doi: 10.1016/j.exphem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Ma HT, et al. A simulation study of marrow fat effect on bone biomechanics. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4030–4033. doi: 10.1109/EMBC.2014.6944508. [DOI] [PubMed] [Google Scholar]
- 45.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 46.Spindler TJ, et al. Adipocytic cells augment the support of primitive hematopoietic cells in vitro but have no effect in the bone marrow niche under homeostatic conditions. Stem Cells Dev. 2014;23:434–441. doi: 10.1089/scd.2013.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krings A, et al. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corre J, et al. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol. 2006;208:282–288. doi: 10.1002/jcp.20655. [DOI] [PubMed] [Google Scholar]
- 49.Hardaway AL, et al. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32:353–368. doi: 10.1007/s10585-015-9714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laharrague P, et al. High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J. 1998;12:747–752. doi: 10.1096/fasebj.12.9.747. [DOI] [PubMed] [Google Scholar]
- 51.Rozman C, et al. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17:34–37. [PubMed] [Google Scholar]
- 52.Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23:577–585. doi: 10.1002/ajhb.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz AV. Marrow fat and bone: review of clinical findings. Front Endocrinol (Lausanne) 2015;6:40. doi: 10.3389/fendo.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slade JM, et al. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complicat. 2012;26:1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Hamrick MW, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 56.Baum T, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–124. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bredella MA, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bredella MA, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97:4115–4122. doi: 10.1210/jc.2012-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trudel G, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol. 2009;107:540–548. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 60.Casamassima F, et al. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood. 1989;73:1677–1681. [PubMed] [Google Scholar]
- 61.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 62.Berry DC, et al. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patsch JM, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28:1721–1728. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bredella MA, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. 2013;269:534–541. doi: 10.1148/radiol.13130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menagh PJ, et al. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25:757–768. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwaniec UT, Turner RT. Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone. 2013;53:145–153. doi: 10.1016/j.bone.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syed FA, et al. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–1330. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei W, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vande Berg BC, et al. Fat conversion of femoral marrow in glucocorticoid-treated patients: a cross-sectional and longitudinal study with magnetic resonance imaging. Arthritis Rheum. 1999;42:1405–1411. doi: 10.1002/1529-0131(199907)42:7<1405::AID-ANR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 70.Ko JY, et al. MicroRNA-29a mitigates glucocorticoid induction of bone loss and fatty marrow by rescuing Runx2 acetylation. Bone. 2015;81:80–88. doi: 10.1016/j.bone.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 71.Hamrick MW, et al. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007;327:133–141. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 72.Ambati S, et al. Central leptin versus ghrelin: effects on bone marrow adiposity and gene expression. Endocrine. 2010;37:115–123. doi: 10.1007/s12020-009-9274-z. [DOI] [PubMed] [Google Scholar]
- 73.Bartell SM, et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–1720. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 74.Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223:T83–T96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ainslie DA, et al. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 76.Martin RB, et al. Bone marrow fat content in relation to bone remodeling and serum chemistry in intact and ovariectomized dogs. Calcif Tissue Int. 1990;46:189–194. doi: 10.1007/BF02555043. [DOI] [PubMed] [Google Scholar]
- 77.Scheller EL, et al. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28:1071–1080. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 79.Bredella MA, et al. Marrow Adipose Tissue Quantification of the Lumbar Spine by Using Dual-Energy CT and Single-Voxel (1)H MR Spectroscopy: A Feasibility Study. Radiology. 2015;277:230–235. doi: 10.1148/radiol.2015142876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bredella MA, et al. Marrow fat composition in anorexia nervosa. Bone. 2014;66:199–204. doi: 10.1016/j.bone.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 82.Liu LF, et al. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. doi: 10.1186/1471-2164-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma YF, et al. Prostaglandin E2 adds bone to a cancellous bone site with a closed growth plate and low bone turnover in ovariectomized rats. Bone. 1994;15:137–146. doi: 10.1016/8756-3282(94)90700-5. [DOI] [PubMed] [Google Scholar]
- 84.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29(Suppl 1):S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 85.Seale P, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keipert S, Jastroch M. Brite/beige fat and UCP1 - is it thermogenesis? Biochim Biophys Acta. 2014;1837:1075–1082. doi: 10.1016/j.bbabio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013;1831:943–949. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]