Abstract
Checkpoint blockade-based immunotherapies are effective in cancers with high numbers of non-synonymous mutations. In contrast, current paradigms suggest that such approaches will be ineffective in cancers with few non-synonymous mutations. To examine this issue we made use of a murine model of BCR-ABL+ B-lineage Acute Lymphoblastic Leukemia. Using a principal component analysis, we found that robust MHC-II expression, coupled with appropriate costimulation, correlated with lower leukemic burden. We next assessed whether checkpoint blockade or therapeutic vaccination could improve survival in mice with pre-established leukemia. Consistent with the low mutation load in our leukemia model, we found that checkpoint blockade alone had only modest effects on survival. In contrast, robust heterologous vaccination with a peptide derived from the BCR-ABL fusion (BAp), a key driver mutation, generated a small population of mice that survived long-term. Checkpoint blockade strongly synergized with heterologous vaccination to enhance overall survival in mice with leukemia. Enhanced survival did not correlate with numbers of BAp:I-Ab-specific T cells, but rather with increased expression of IL10, IL17, and Granzyme B and decreased expression of Programmed Death 1 on these cells. Our findings demonstrate that vaccination to key driver mutations cooperates with checkpoint blockade and allows for immune control of cancers with low non-synonymous mutation loads.
Introduction
Patients with B-cell acute lymphoblastic leukemia (B-ALL) harboring the BCR-ABL chromosomal translocation have very poor outcomes (1, 2). Current therapies for BCR-ABL+ BALL include cytotoxic chemotherapeutics, tyrosine kinase inhibitors, and bone marrow transplantation. These treatments are often transiently effective, indicating that new treatment options are urgently needed. One such option is immunotherapy. Recent work in cancers with frequent non-synonymous mutations, such as melanomas, has demonstrated that immunotherapy involving neutralization of programmed death 1 (PD1) and cytotoxic T lymphocyte antigen 4 (CTLA4) (checkpoint blockade) is an effective treatment option (3, 4). It remains unclear whether immunotherapy involving checkpoint blockade strategies will also be effective in cancers with few non-synonymous mutations, such as B-ALL (5).
To determine whether immunotherapy is an effective option for treating B-ALL we used a syngeneic mouse model of BCR-ABL+ B-ALL to characterize the host immune response to this leukemia in immune-competent recipient animals (6–8). We previously demonstrated that the host adaptive immune system responds to BCR-ABL+ B-ALL (9). Although B-ALL cells have been shown to have low numbers of non-synonymous mutations (5), the fusion between BCR and ABL does generate an MHC class II restricted peptide antigen that can be recognized by a small population of endogenous BAp:I-Ab-specific T cells in mice (9). Transfer of BCR-ABL+ leukemic cells into C57BL/6 mice resulted in proliferation of BAp:I-Ab-specific T cells, although 50% of these cells differentiated into FOXP3+ Treg cells (10). Thus, T cells do respond to BCR-ABL+ leukemia in this mouse model, but the response was immune suppressive in nature, and detrimental to host survival. Herein, we address if the immune response to leukemia could be modulated thus making BCR-ABL+ B-ALL malleable to checkpoint blockade-based T cell immunotherapy.
Materials and Methods
Mice
C57BL/6 mice and Cdkn2a−/− (strain 01XF6, B6, 129-Cdkn2atm1Cjs/Nci (11)) mice came from the National Cancer Institute. Foxp3-GFP (stock# 006772) and Ifng−/− (stock# 002287) came from Jackson Laboratories (Bar Harbor, ME). OT-IxRag2−/− mice were generated locally as previously described (12). Mice were housed at the University of Minnesota in specific pathogen free conditions or BSL-2 facilities, and all experiments were approved by IACUC.
Listeria monocytogenes generation
Acta- Listeria monocytogenes strain 1942 (from Dr. Sing Sing Way) expressing BAp peptide from a plasmid was constructed as previously described (13–15).
Infections and Immunizations
107 Colony-forming Units (CFU) of L. monocytogenes (LM) expressing BAp (LM+BAp) were injected intravenously through the tailvein. Mice vaccinated with LCMV-Armstrong received 105 PFU i.p. at day 0. Vesicular Stomatitis Virus-Indiana was used at 5×105 PFU i.v. at day 0. At day 3 and day 5, mice were injected i.v. with 200μg BAp. Mice were harvested at indicated timepoints.
Leukemia model
Cdkn2a−/− mouse bone marrow cells were transduced with viral supernatant containing a BCR-ABL (P190)-IRES-GFP retrovirus (16) and cultured for adoptive transfer as previously described (7, 9).
Tetramer production
Purified monomer was tetramerized with SA-PE or SA-APC and cells were enriched as previously described (9, 17).
In-vivo antibody treatment
Unvaccinated mice were treated with 100 μg anti-PDL1 and anti-CTLA i.p. every-other-day. Vaccinated mice received and 200μg anti-PDL1 and anti-CTLA i.p. twice per week. Mice treated with anti-CD40 received 200μg i.p. every-other-day.
Antibodies
Antibodies for flow cytometry include CD3 PE, CD4 (RM4-5) PerCPCy5.5, CD8 (53-6.7) BV650, CD11c (N418) PE, FOXP3 (FJK16S) PE, CD80 (16-10A1) APC, CD86 (GL1) PE-Cy7, CD19 BV605, B220 (RA3-6B2) Horizon V500, IFNgamma (XMG1.2) BV650, LAP (TW7-16B4) PE, TNF alpha (MP6-XT22) BV421, IL17A (TC11-18H10) AlexaFluor488, and PSGL1 (2PH1) BV421 purchased from BD Biosciences (San Jose, CA); NK1.1 (PK136), CD11b (M1/70), CD11c (N418), B220 (RA3-6B2), and F4/80 in APC-eFluor780; PD1 (J43) FITC, CD73 eFluor450, FR4 PE-Cy7, PDL1 PerCP-eFluor710, MHC-II I-Ab eFluor450, IL10 (JESS-16E3) PE, Granzyme B (NGZB) PE-Cy7, GARP (YGIC86) eFluor450, and all ELISpot antibodies were purchased from eBiosciences (San Diego, CA), and IgM (Fab′) APC was purchased from Jackson Immunoresearch (West Grove, PA). Rat IgG1 (HRPN) PerCP-Cy5.5 Isotype and Rat IgG2a (2A3) violetFluor450 Isotype were purchased from Tonbo Biosciences (San Diego, CA). Cells from enriched fractions were analyzed on an LSR-II Fortessa cytometer (BD Biosciences, San Jose CA) and data was analyzed in FlowJo (Treestar, Ashland OR).
Statistics and principal components analysis description
Standard normality tests suggested departures from normality, so non-parametric tests (Mann-U Whitney test for two groups, Kruskal-Wallace & Dunns’ Test for more than two groups) were used unless otherwise stated. Normality assessments, non-parametric tests, and survival analyses were done in GraphPad Prism (LaJolla, CA). For the Cox-Mantel tests, we report hazard ratios, which describe the multiplicative change in risk when moving from the baseline group to the treatment group. Principal component analysis (PCA) was conducted in R (prcomp function) (18). Linear regressions and correlation coefficients were estimated in GraphPad Prism and R.
A detailed description of the PCA and corresponding linear regression are included herein. We performed a PCA on the following five phenotype metrics collected on each mouse: PDL1, MHC-II, CD40, CD80, CD86. We added one to each metric, and then log-transformed the resulting value so that our data met the PCA assumption of joint normality. Components were estimated using the prcomp function in the stats package in R.
First, pairs plots of the raw manifest variables and log-transformed manifest variables were created. Principal component analysis is a method for reducing the dimension of a dataset by transforming an initial set of possibly-correlated manifest variables into a set of new orthogonal variables, which are referred to as “principal components” (PC). These components describe the multivariate correlation in the dataset. While the method generates as many components as there are measured variables in the dataset, most of the variation can usually be captured with only a few components. Each component consists of a value that describes the proportion of variation in the original dataset explained by the component, and a set of loadings that describe the extent to which each manifest variable correlates with that component. Let X be an n × k matrix containing measurements of k different manifest variables for n sampled individuals. Estimation is obtained through an eigen-decomposition of the square matrix X′X. Eigenvalues correspond to proportions of variance in the original dataset captured by each component, and eigenvectors describing correlations between each manifest variable and each component. Although the method constructs as many principal components as there are manifest variables in the dataset, interpretation is limited to those components that explain the preponderance of variation. The number of components to interpret is often determined using a screeplot, showing the proportion of variance explained by each component.
A screeplot for the components identified for the log-transformed immunogenicity phenotype metrics is shown in Figure 1D. Based on the screeplot, we interpreted the first two components. Each manifest variable’s loading on each component is shown in Table 1.
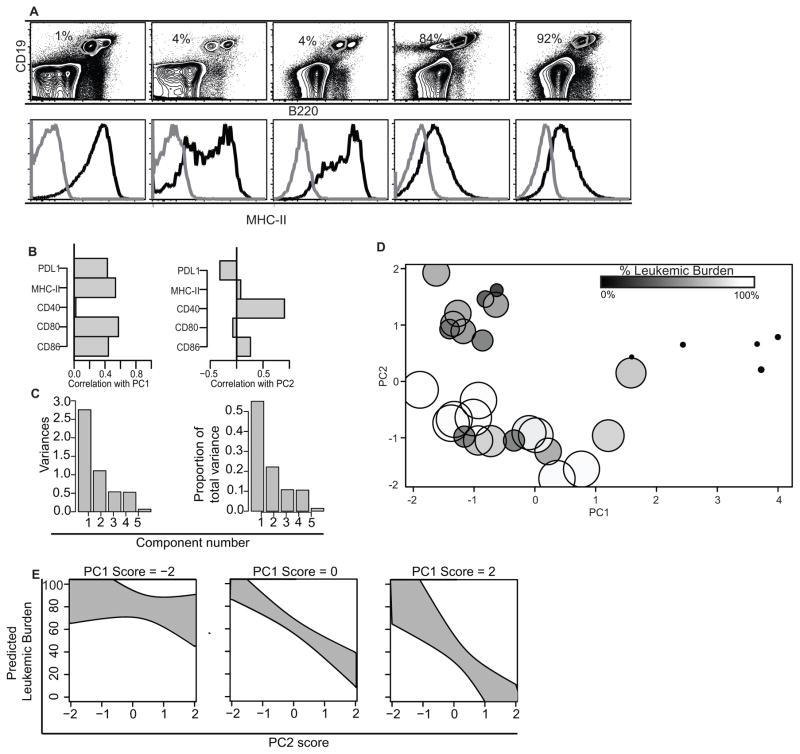
Figure 1. Adaptive immunity plays a role in the anti-BCR-ABL+ B-ALL response.
A. Representative dot plots and histograms from five mice with varying leukemic burden (gated as live, singlet, CD19+, B220low cells. Black curves are MHC-II, grey curves are isotype. Listed are the percentage of Live singlet events which fall into the CD19+, B220low gate. B. Correlations of measured variables with first two principal components. PDL1, MHC-II, CD80, and CD86 correlate positively with PC1; CD40 and (to a lesser extent) CD86 correlate positively, while PDL1 correlates negatively, with PC2. C. Screeplots of Principal Components (PC). Y-axis shows proportion of variance accounted for by each PC. D. Distribution of individual mouse scores on PCs 1 and 2. Mouse leukemic burden is indicated by dot size and dot shade, larger white dots indicate mice with higher leukemic burden, and smaller black dots indicate mice with lower leukemic burden. E. Predicted leukemic burden as a function of PC2 scores at three separate levels of PC1 (low, average, and high). Grey regions denote 95% confidence bounds. Principal components were derived from 27 separate mice in 3 experiments.
Table I.
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| Standard Deviation | 1.67 | 1.05 | 0.73 | 0.73 | 0.26 |
| Proportion of Variance | 0.55 | 0.22 | 0.11 | 0.11 | 0.014 |
| Cumulative Proportion | 0.55 | 0.77 | 0.88 | 0.99 | 1.00 |
Shown is the standard deviation, proportion of variance, and cumulative proportion accounted for by each principal component for the data plotted in figure 1. PC 1 and PC2 together described 77% of the variation in leukemic burden.
We extracted PC scores for PC1 and PC2 for each mouse in our dataset. We used a linear regression model to relate these PC scores to percent leukemic burden measured on these same mice. The regression model consisted of four terms: an intercept (β0), main effects for each PC, (β1 and β2), and an interaction term between the two PCs (β3). Let yi be percent leukemic burden in the ith studied mouse, let X1,i be the ith mouse’s PC1 score, and let X2,i be the ith mouse’s PC2 score. Then the regression model we fit can be written as:
The model was fit using the lm function in R (1). An overall F-test clearly suggested that at least some of the coefficients in the model differed significantly from zero (F-statistic = 23.76 on 3 and 26 degrees of freedom; p < 0.0001). Specifically, the model detected strong relationships between the first two PCs and leukemic burden, and a marginally significant interaction effect in our dataset.
In general, the intercept term corresponds to expected leukemic burden for mice with average scores on both PC1 and PC2. Specifically, under this model we expect that mice with average PC1 and PC2 scores have an average leukemic burden of 62.64 percent. For mice with an average score of PC2, but who are one unit above average on PC1, we expect an average leukemic burden of 52.64 percent (62.64 – 10.00). For mice with an average score on PC1, but who are one unit about average on PC2, we expect an average leukemic burden of 43.34 percent (62.64 – 19.30). For mice that are one unit above average on both PC1 and PC2, we expect an average leukemic burden of 27.38 percent (62.64 – 10.00 – 19.30 – 5.96).
Results
Adaptive immunity plays a role in the anti-BCR-ABL+ B-ALL response
We previously showed that there was a higher fraction of live leukemic cells in the BM and secondary lymphoid organs of OT-I x Rag2−/− mice than in C57BL/6 mice. Further, the range of leukemic burdens was quite broad in the C57BL/6 hosts (IQR=11%–69%), but less-so in the OT-I x Rag2−/− hosts (IQR=90%–98%) (9). To understand why there was such a range in leukemic burden, we looked for characteristic differences in the leukemic cells from mice with low versus high leukemic burden. Since B cells can function as antigen presenting cells, we examined the expression of MHC-II to stratify the leukemic burden based on expression of surface markers. MHC-II expression inversely correlated with leukemic burden (mice that had a low percentage of leukemic cells in the bone marrow had high MHC-II expression on the leukemic cells, Fig 1A). We also examined the expression of the costimulatory molecules CD40, CD80, CD86, and PDL1. None of these costimulatory molecules individually correlated with leukemic burden. Therefore, we used Principal Components Analysis (PCA) to identify if there was an ensemble of costimulatory molecules that correlated with leukemic burden (Fig 1B). The first component described a positive correlation between MHC-II, CD80, CD86, and PDL1. The second component was driven by a negative correlation between CD40 and PDL1 (Fig 1B). These first two components described 77% of the variation in leukemic burden that we observed in mice (Table 1, Fig 1C). Mice that had low leukemic burden tended to have high scores for both PC1 and PC2 (Fig 1D). Therefore, we used linear regression to examine relationships between the first two PC scores and leukemic burden (Fig 1E). Both high PC1 and high PC2 scores were associated with significantly decreased leukemic burden (p<0.001, Fig 1E). A low PC1 score (score = −2) was predictive of high leukemic burden (left panel) regardless of PC2 score. In contrast, leukemias with higher PC1 scores (score = 0–2), showed dependence on PC2 in predicting leukemic burden (Fig 1E). Taken together this analysis supports the conclusion that robust antigen presentation combined with CD80/86 costimulation (PC1), and a high ratio of CD40:PDL1 (PC2), correlates with improved disease outcome.
Modulation of individual costimulatory molecules modestly improves survival of leukemic mice
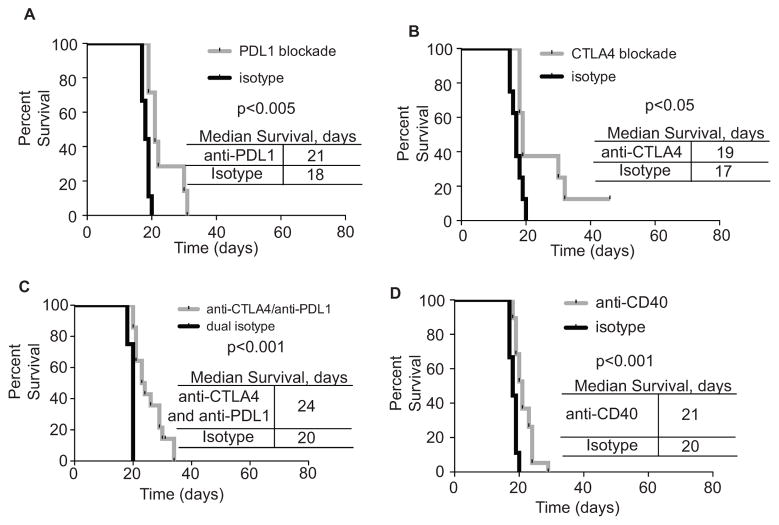
Our PCA suggested that an ensemble of costimulatory molecules (CD80, CD86, PDL1, CD40) functioned as a cohesive unit to modulate anti-leukemia immunity. Nonetheless, it was possible that individual targeting of antigen presentation and costimulatory molecules might change the disease course. We tested if antibody targeting of PDL1, CTLA4, or CD40 would be sufficient to change leukemia progression. Antibody blockade of PDL1 and CTLA4 (either individually, or in combination) led to a modest, but significant increase in survival of leukemic mice (Fig 2 A–C). Additionally, treatment of leukemic mice with an anti-CD40 antibody that is characterized as an agonist also led to a modest, yet significant increase in survival (19) (Fig 2D). Thus, the components defined by our PCA do not individually identify very effective therapeutic targets in this model. These results support the concept that the molecules identified by our PCA are best addressed as an ensemble.
Figure 2. Modulation of individual antigen presentation and costimulation molecules improves survival of leukemic mice.
A. C57BL/6 mice were inoculated with 2500 leukemic cells and treated every-other-day with 100μg anti-PDL1 until moribund. B. Mice were treated as in A, except with 100μg anti-CTLA4. C. Mice were treated as in A, except with 100μg anti-PDL1 plus 100μg anti- CTLA4. D. Mice were treated as in A, except with 200μg anti-CD40. Two or more independent experiments with 4 or more replicates shown in each group, Log-Rank (Mantel-Cox) test was used to establish significance in all panels.
BAp-specific T cells can be primed by acute infection plus exogenous peptide
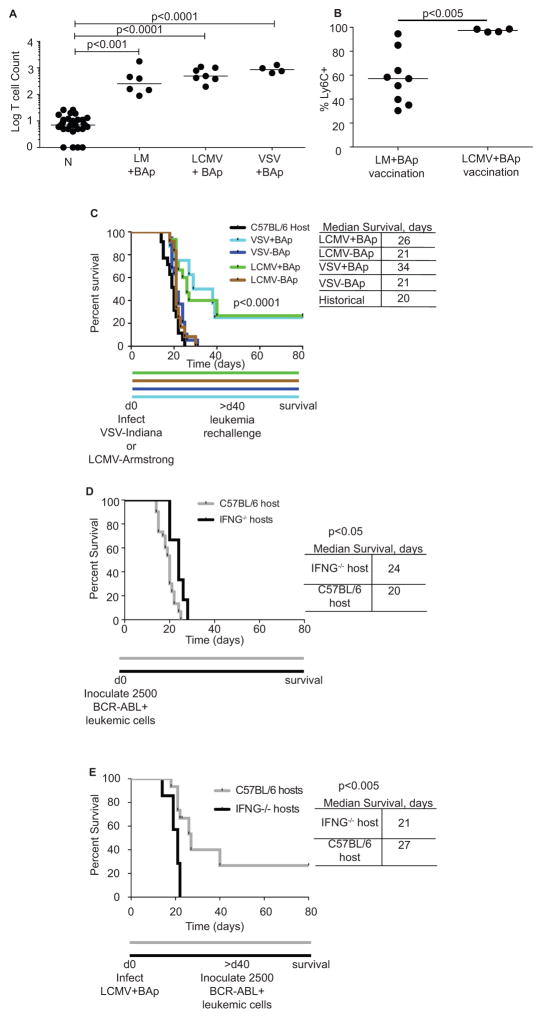
None of the immune checkpoint modulations we tested substantially improved survival of leukemic mice. However, we have previously shown that Ly6C+ BAp:I-Ab-specific T cells correlate with anti-leukemia immunity upon Treg depletion (9). We attempted to re-create an inflammatory environment to generate many Ly6C+ BAp:I-Ab-specific T cells. To do this, we infected mice with Listeria monocytogenes (LM) expressing the BAp peptide (termed LM+BAp), which caused a 65-fold increase in BAp:I-Ab-specific T cell numbers. In parallel, we infected mice with either LCMV-Armstrong or VSV-Indiana and then delivered 200μg BAp peptide i.v. at 3 and 5 days post-infection. This allowed us to utilize the inflammation caused by acute viral infection to induce a strong BAp:I-Ab-specific CD4+ T cell response (termed LCMV+BAp or VSV+BAp). At peak infection LCMV+BAp caused a 74-fold proliferation of BAp:I-Ab-specific T cells, while VSV+BAp caused a 114-fold proliferation of BAp:I-Ab-specific T cells (Fig 3A). These results show that BAp:I-Ab-specific T cell proliferation can be initiated by immunization. Additionally we found that LCMV+BAp induced a high frequency of Ly6C+ memory BAp:I-Ab-specific T cells following leukemia re-challenge, while LM+BAp induced substantially fewer Ly6C+ memory BAp:I-Ab-specific T cells following leukemia re-challenge (Fig 3B). Since our previous work showed that Ly6C was expressed on the majority of BAp:I-Ab-specific T cells upon Treg depletion (which also resulted in significantly less leukemic burden and significantly longer survival of leukemic mice), we reasoned that acute viral infections that result in increased Ly6C expression might induce protective BAp-specific immunity.
Figure 3. Prophylactic vaccination with BAp peptide allows long-term survival in leukemic mice.
A. Naïve mice (N) were immunized with CFA+BAp, LM+BAp, LCMV+BAp or VSV+BAp. Secondary lymphoid organs were harvested at peak infection or two weeks post- immunization (CFA+BAp) and BAp:I-Ab-specific T cells were enumerated. More than two independent experiments are shown for each infection. Kruskal-Wallace and Dunn’s test used to establish significance. B. Percent Ly6C+ BAp:I-Ab-specific T cells from mice vaccinated with either LM+BAp or LCMV+BAp at d0 and then rechallenged with leukemia at d30. Two or more independent experiments with 4 or more replicates conducted for each infection. Mann-Whitney U Test used to establish significance. C. Mice were vaccinated with LCMV-Arm+/−BAp or VSV-I+/−BAp. >40 days later, mice were re-challenged with 2500 leukemic cells and survival assessed by Log-Rank (Mantel-Cox) test. Shown are three or more independent experiments with 8 or more replicates used for each group. D. 2500 BCR-ABL+ leukemic cells were adoptively transferred into control C57BL/6 mice or Ifng−/− mice. Survival was analyzed using the Log-Rank (Mantel-Cox) test. E. Control C57BL/6 mice or Ifng−/− mice were vaccinated with LCMV-Armstrong + BAp peptide and challenged with 2500 leukemic cells as in Figure 3B. Log-Rank (Mantel-Cox) test was used to establish significance. Shown are two or more independent experiments with 7 or more replicates used in each group.
BAp-specific adaptive immunity confers long-term survival for leukemic mice
Our PCA suggested that MHC-II expression, and thus antigen presentation, was important in describing the immune response to leukemia. We have identified one peptide from BCR-ABL (BAp) that is processed and presented on MHC-II in vivo (9). Thus, we hypothesized that immunization with BAp plus strong adjuvants might mediate protection from BCR-ABL+ B-ALL in mice. To test this, we infected mice with either LCMV-Armstrong (+/− BAp peptide) or VSV-Indiana (+/− BAp peptide) and re-challenged mice with BCR-ABL+ leukemia >40 days later, a memory timepoint when no acute inflammation remained (Fig 3C). Mice that were infected with an acute viral pathogen plus BAp peptide survived long-term. In contrast, mice that were infected with an acute viral pathogen in the absence of BAp succumbed to leukemia rapidly. The hazard ratio comparing all “+BAp” vaccinations to all “−BAp” vaccinations in Figure 3C was 0.24 with a 95% confidence interval from 0.12 to 0.46 (p<0.0001). Thus, BAp-specific adaptive immunity confers long-term survival in this model. Since BAp only binds to MHC-II and not MHC-I (data not shown), our results support the conclusion that BAp:I-Ab-specific T cells are critical for protecting against BCR-ABL+ leukemia in our prophylactic vaccination studies.
Interferon-γ potentiates anti-leukemia immunity during prophylactic vaccination
In CD4+ T cells, IFNγ is normally produced by Th1 cells, which can have a role in anti-tumor immunity (20). We have previously shown that in unvaccinated mice most BAp:I-Ab-specific T cells responding to BCR-ABL+ leukemia are Treg cells and thus are likely not making IFNγ. Consistent with this idea, we found that the ability of host T cells to make IFNγ in unvaccinated mice did not affect survival following leukemia inoculation, since Ifng−/− hosts succumbed to leukemia similarly to C57BL/6 hosts (Fig 3D). However, we hypothesized that IFNγ might play a role in the adaptive immune response to leukemia following prophylactic vaccination. To test this, we vaccinated Ifng−/− mice with LCMV-Arm+BAp and re-challenged with leukemia at more than 40 days post-vaccination. Vaccination of IFNγ-deficient mice did not increase survival following challenge with leukemia, when compared with unvaccinated control mice. In contrast, vaccination of Ifng-replete mice resulted in significant long-term survival when compared with unvaccinated controls (Fig 3E). Thus, IFNγ production was one mechanism required for effective anti-leukemia immunity following prophylactic vaccination.
Specific pathogens mediate effective prophylactic vaccination for BCR-ABL+ B-ALL
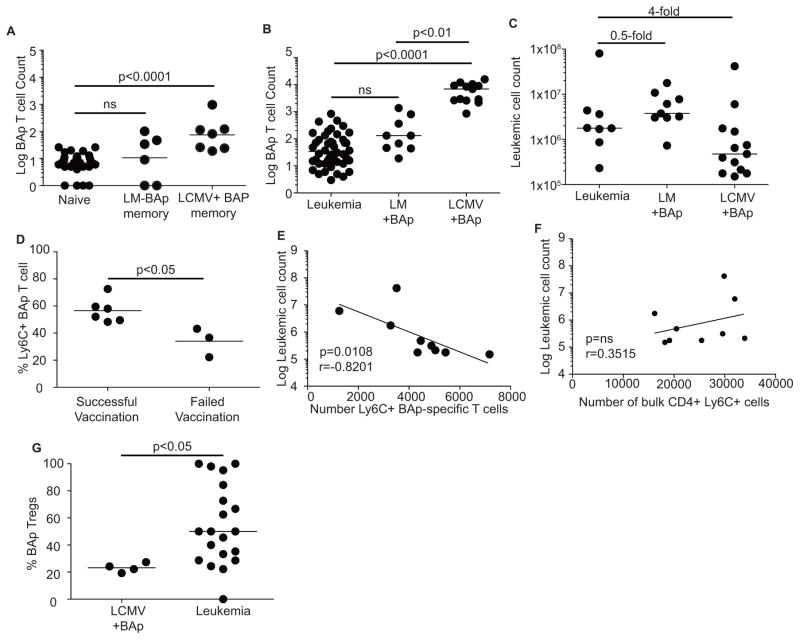
Immune memory is a critical component of prophylactic vaccination. To determine if effective BAp:I-Ab-specific memory T cells were formed by vaccination, we infected mice with LM+BAp or LCMV+BAp and waited 40 days to enumerate BAp:I-Ab-specific memory T cells. We recovered significantly more memory BAp:I-Ab-specific T cells from LCMV+BAp infected mice than from LM+BAp infected mice (Fig 4A). We then vaccinated mice with either LCMV+BAp or LM+BAp and re-challenged by transferring 2500 leukemic cells into the mice 30 days later. Vaccination with LCMV+BAp, but not LM+BAp, led to a significant increase in the number of BAp:I-Ab-specific T cells following leukemia challenge and decreased leukemic burden (4-fold, Fig 4 B,C). Thus, the increase in BAp:I-Ab memory T cell numbers following LCMV+BAp but not LM+BAp vaccination correlated with disease outcome.
Figure 4. Prophylactic vaccination induces protective immune responses against BCR-ABL+ leukemia.
A. Mice were infected as in 3A and rested 30 days, when BAp:I-Ab-specific T cells counts were compared to those in naïve mice. Shown are BAp:I-Ab-specific Log (Y+1) T cell counts of BAp:I-Ab-specific memory cells following vaccinations, gated on CD11ahighCD44high cells. B. Mice were unvaccinated or vaccinated with LCMV+BAp or LM+BAp, and 2500 BCR-ABL+ cells were transferred 30 days post-infection. Shown are BAp:I-Ab-specific Log (Y+1) T cell counts; two or more independent experiments shown for each infection. C. Mice were treated as in 4B, and leukemic burden was analyzed. Lines are median values; numbers represent fold changes in median. D. Percent Ly6C+ on BAp:I-Ab-specific T cells harvested from LCMV+BAp-vaccinated mice.
E. Ly6C+ BAp:I-Ab-specific T cell count negatively correlates with leukemic burden from secondary lymphoid organs. Spearman correlation r=−0.8201, p<0.05. F. Ly6C+CD4+ T cell count does not correlate with leukemic burden from secondary lymphoid organs. Spearman correlation r=0.3515, p>0.05. G. Percent BAp:I-Ab-specific Treg cells recovered from leukemic Foxp3-GFP mice unvaccinated or vaccinated with LCMV+BAp. All comparisons done by Kruskal-Wallace and Dunn’s test (more than two groups) or Mann-Whitney U test (two groups). Two or more independent experiments with 4 or more replicates are shown for each group.
The quality and quantity of BAp:I-Ab-specific memory T cells was different comparing LM+BAp vaccination to LCMV+BAp vaccination. We observed that the inter-quartile range of leukemic burdens in the mice vaccinated with LCMV+BAp was broad (IQR=1.4 ×106, Fig 4C), showing that protection mediated by LCMV+BAp vaccination was more effective in some mice than in others. We previously observed that Ly6C expression was increased on BAp:I-Ab-specific T cells when Tregs were depleted (9). Therefore, we examined whether Ly6C expression on BAp:I-Ab-specific T cells correlated with leukemic burden. Mice with high leukemic burden despite prophylactic vaccination (and thus considered ‘failed vaccinated mice’), had a significantly lower percent of Ly6C+ BAp:I-Ab-specific T cells than the ‘successfully vaccinated mice’ (Fig 4D). Additionally, significantly more BAp:I-Ab-specific T cells expressed Ly6C after LCMV+BAp vaccination (which lowered leukemic burden) than LM+BAp (which had no effect on leukemic burden) (Fig 3B). Importantly, the number of Ly6C+ BAp:I-Ab-specific T cells was inversely correlated with leukemic burden in LCMV+BAp vaccinated mice (Fig 4E). In contrast, leukemic burden did not correlate with total CD4+Ly6C+ cells in these mice (Fig 4F). We also observed that Treg cells made up a smaller portion of the BAp:I-Ab-specific T cell population in mice that were prophylactically vaccinated with LCMV+BAp than unvaccinated mice (Fig 4G). These results support a functional role for Ly6C+FOXP3− BAp:I-Ab-specific T cells during the immune response to leukemia following prophylactic vaccination.
Antigen presentation and costimulation on leukemic cells are modulated by prophylactic vaccination
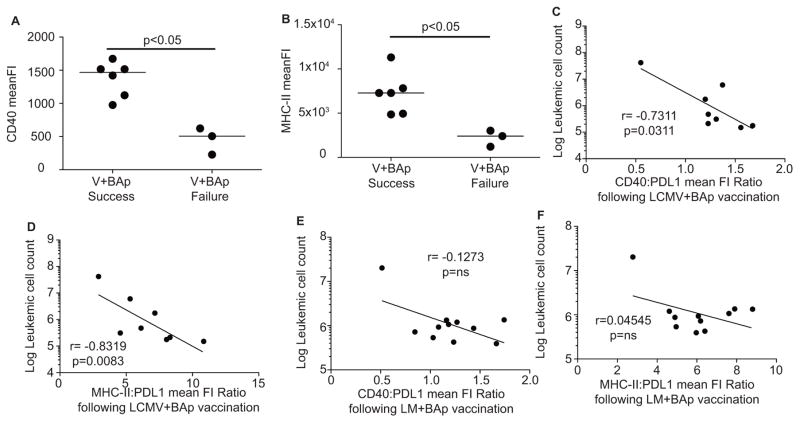
Our principal component analysis suggests that high ratios of CD40:PDL1 and MHC-II:PDL1 may be predictive of low leukemic burden. We examined the leukemic cells from LCMV+BAp vaccinated mice and LM+BAp-vaccinated mice. First, we found that leukemias in mice that were successfully vaccinated with LCMV+BAp had higher expression of CD40 and MHC-II than their “failed vaccination” counterparts (Fig 5A, B). Second, we found that CD40:PDL1 and MHC-II:PDL1 increased on LCMV+BAp vaccinated mice (which was an effective vaccination regimen, Fig 5C, D) but not significantly on LM-BAp vaccinated mice (an ineffective vaccination regimen, Fig 5E, F). Thus, prophylactic vaccination with acute viral pathogens plus BAp peptide results in protection from leukemia and correlates with expression of the biomarkers that we previously demonstrated were linked to strong anti-leukemia immune responses (Fig 1).
Figure 5. Prophylactic vaccination induces antigen presentation and costimulation on leukemic cells.
A. Mice were vaccinated with LCMV+BAp and inoculated with leukemia 30 days later. CD40 Mean Fluorescence Intensity from leukemic cells harvested from Successful vaccinated mice or Failed vaccinated mice derived from Figure 4C. Mann-Whitney U Test used to establish significance. B. MHC-II Mean Fluorescence Intensity from leukemic cells harvested from Successful vaccinated mice or Failed Vaccinated mice. Mann-Whitney U test used to establish significance. C. Ratio of Mean Fluorescence Intensity of CD40:PDL1 on leukemic cells was calculated from mice vaccinated with LCMV+BAp, and a correlation was calculated between this ratio (X-axis) and the log leukemic cell count (Y-axis). D. Ratio of Mean Fluorescence Intensity of MHC-II: PDL1 on leukemic cells was calculated from mice vaccinated with LCMV+BAp, and a correlation was calculated between this ratio (X-axis) and the log leukemic cell count (Y- axis). E. Ratio of Mean Fluorescence Intensity of CD40: PDL1 on leukemic cells was calculated from mice vaccinated with LM+BAp, and a correlation was calculated between this ratio (X-axis) and the log leukemic cell count (Y-axis). F. Ratio of Mean Fluorescence Intensity of MHC-II: PDL1 on leukemic cells was calculated from mice vaccinated with LM+BAp, and a correlation was calculated between this ratio (X-axis) and the log leukemic cell count (Y-axis). The values on graphs are from the Spearman Correlation test. Two or more independent experiments with 4 or more replicates are shown for each group.
Therapeutic heterologous vaccination drives long-term survival
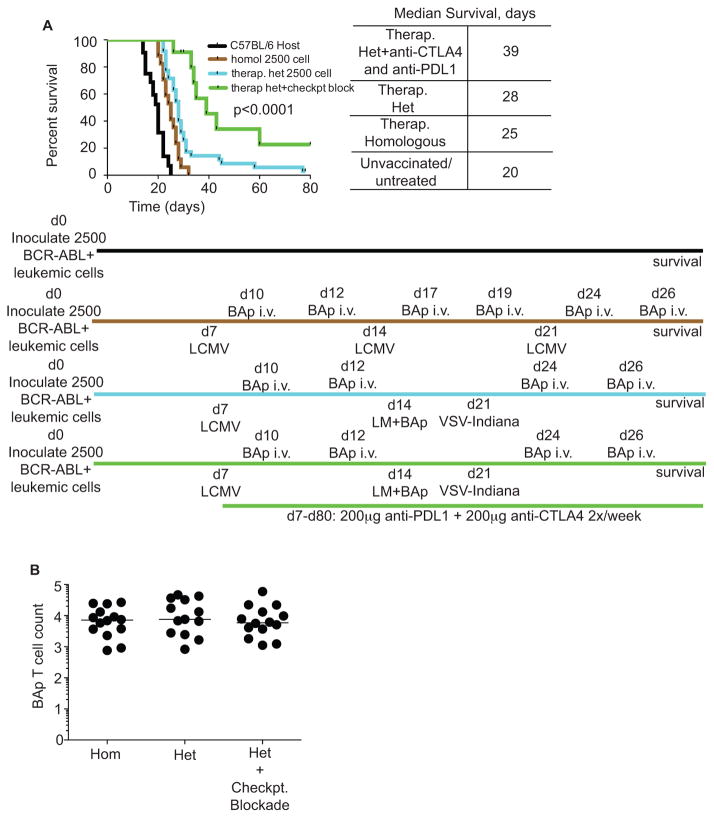
We hypothesized that a pro-inflammatory environment might counter leukemia-derived immune suppression, while also inducing BAp-specific adaptive immunity, and thus inhibit leukemia progression. To test this hypothesis, we therapeutically vaccinated mice, which had established leukemia, using either homologous vaccinations with LCMV-Armstrong+BAp peptide or heterologous vaccinations with LCMV-Armstrong+BAp, LM+BAp, and VSV-Indiana+BAp (Fig 6A). Homologous vaccination with LCMV+BAp significantly prolonged survival although all mice ultimately succumbed to leukemia. Heterologous vaccination should create a more robust pro-inflammatory response, since antibodies created during the primary infection will not neutralize the secondary and tertiary infections. Indeed, heterologous vaccination was significantly more effective and led to long-term survival (>twice the median untreated survival) in ~10% of mice. Thus, repeated vaccination with heterologous agents was an effective treatment strategy in mice with BCR-ABL+ B-ALL.
Figure 6. Therapeutic vaccination synergizes with checkpoint blockade to improve outcome.
A. Mice were inoculated with 2500 BCR-ABL+ leukemic cells at d0, and then rechallenged with one of four different treatments. 1) No treatment (Black); 2) Homologous vaccination with Lymphocytic Choriomeningitis Virus (LCMV) Armstrong at day 7, day 14, and day 21, with 200μg exogenous BAp peptide delivered i.v. at three and five days post-infection (days 10, 12, 17, 19, 24, 26) (Brown); 3) Heterologous vaccination with LCMV-Armstrong at day 7, LM-BAp at day 14, and Vesicular Stomatitis Virus (VSV) Indiana at day 21, with with 200μg exogenous BAp peptide delivered i.v. at three and five days post-infection (days 10, 12, 17, 19, 24, 26), (Blue); 4) as in 3, except with 200μg of anti-PDL1 and 200μg of anti-CTLA4 twice per week from day 7 to day 80, (Green). Surviving mice were euthanized at day 80 post-leukemia inoculation. Shown are survival curves; Log-Rank (Mantel-Cox) test was used to analyze statistics. B. Mice were treated as in A, but treatment was started on the same day as leukemia challenge. Mice were euthanized at day 21 and BAp:I-Ab-specific T cells were harvested and enumerated. Shown are BAp:I-Ab-specific Log (Y+1) T cell counts; two or more independent experiments shown for each infection. Groups were compared with Kruskal-Wallace and Dunn’s test; no significant differences were found. Two or more independent experiments with 10 or more replicates are shown for each group.
The immune response to acute viral and bacterial infection is canonically pro-inflammatory. However, since mice with active leukemia have high doses of leukemia antigens during this pro-inflammatory state, this may cause chronic antigen stimulation, a situation where PDL1 signaling is highly expressed (21). Additionally, our initial findings showing that CD44 was not highly expressed on all BAp:I-Ab-specific T cells responding to leukemia suggest that BAp-specific T cell priming is not optimal (9). CTLA4 blocks interaction of CD28 with B7-1 and B7-2 molecules, thereby reducing T cell functionality (22–24). Thus, we hypothesized that therapeutically vaccinated mice that were treated with dual PDL1/CTLA4 checkpoint blockade might show improved survival. This treatment strategy led to a significant increase in survival beyond that seen for either PDL1+CTLA4 blockade (Fig 2C) or therapeutic vaccination (Fig 6A), with 31% of mice surviving long-term. Since this long-term survival is far past the timepoint when inflammation would remain from the therapeutic vaccination, it suggests that an adaptive immune response is mediating long-term survival.
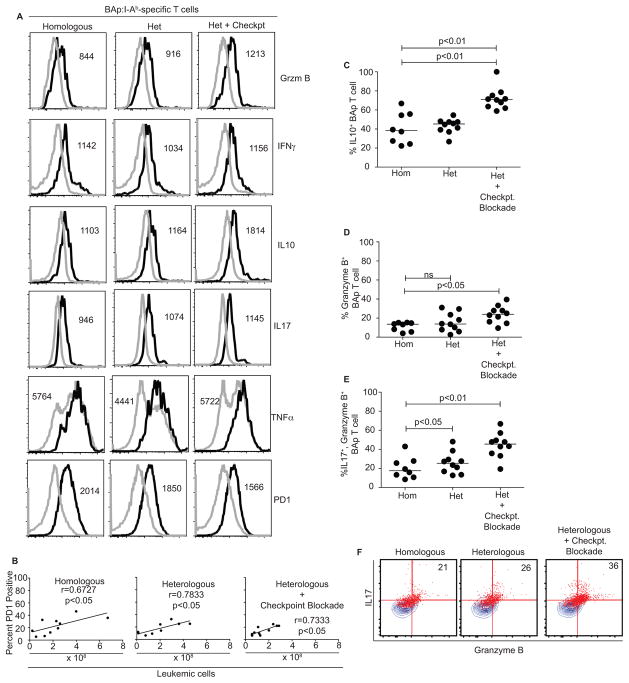
To understand some of the mechanisms allowing effective therapeutic vaccination, we compared homologous, heterologous, and heterologous plus checkpoint blockade treatments and assessed BAp:I-Ab-specific T cell expansion and effector function. To do this, we inoculated mice with BCR-ABL+ leukemia and started therapeutic vaccination at the same timepoint. We then harvested the mice 21 days later and enumerated BAp:I-Ab-specific T cells. We found that all regimens induced robust proliferation (~1250-fold over naïve precursor numbers); however, there was no difference in the number of BAp:I-Ab-specific T cells recovered between any of the three treatment groups (Fig 6B). Since total numbers of BAp:I-Ab-specific T cells did not help give insight into the mechanisms that allowed effective therapeutic vaccination, we examined the phenotype of the BAp:I-Ab-specific T cells. We reasoned that BAp:I-Ab-specific T cells should take on a more Th1-like phenotype in response to therapeutic vaccination, and that this phenotype should correlate with improved disease outcome. However, we found no significant increase in IFNγ or TNFα (two canonical Th1 cytokines) when comparing all three treatment groups. Interestingly, in mice that received heterologous vaccination plus checkpoint blockade, we found that a larger fraction of BAp:I-Ab-specific T cells produced more IL10, Granzyme B, and both IL17 and Granzyme B together (Fig 7A, C–F), all of which have previously been associated with pro-inflammatory tumor clearance(25–28). Additionally, we found that PD1 expression on BAp:I-Ab-specific T cells positively correlated with leukemic burden in all therapeutically vaccinated mice (Fig 7B), and that PD1 expression was lowest on these cells in heterologous vaccination plus checkpoint blockade-treated mice (Fig 7A). Together, these results demonstrate that poly-functional CD4+ leukemia-specific T cells produce a combination of IL10, IL17, and Granzyme B, and this correlated with effective anti-leukemia adaptive immunity.
Figure 7. BAp:I-Ab-specific T cell derived cytokines change in response to therapeutic vaccination.
A. BAp:I-Ab-specific T cells were harvested from mice as in Figure 6B, and re-stimulated ex-vivo with PMA and Ionomycin to analyze potential cytokine production. Shown is concatenated data from 10 individual mice in two or more independent experiments. B. PD1 expression on BAp:I-Ab-specific T cells was analyzed and linear regression was used to compare to leukemic burden in the same mouse. Data from Spearman Correlation shown. C. Based on concatenated histograms from Figure 7A, gates were drawn to delineate “positive” vs. “negative” fraction of cells and applied to individual mice. Percent positive is shown on the Y-axis for IL10. D. Data was acquired as in Figure 7C and Granzyme B was analyzed. E. Percent of BAp:I-Ab-specific T cells which are double-positive for IL17 and Granzyme B is shown and analyzed as in Figure 7C and 9D. Results in 7C–E were analyzed by Kruskal-Wallace and Dunn’s test. F. BAp:I-Ab-specific T cells were harvested as in Figure 7A and concatenated events from 10 mice were gated to show IL17+, Granzyme B+ percentages of BAp:I-Ab-specific T cells. Numbers on the graph are percentage of double-positive events in the gates as shown. Two or more independent experiments with 10 or more replicates are shown for each group.
Discussion
BCR-ABL+ B-ALL is only transiently responsive to current therapies (29), with a five year survival of approximately 35% (30, 31). Given this low survival rate, additional therapies are needed. One approach that has not been well-explored in this disease is checkpoint blockade-based immunotherapy. Checkpoint blockade works well in malignancies with many non-synonymous mutations and can lead to improved long-term survival in patients with such cancers (4, 32–36). Presumably, this is because the increased number of non-synonymous mutations allows for a concurrent increase in the number of neo-antigen specific T cells. Comparatively, in cancers with low numbers of non-synonymous mutations, like B-ALL (36), checkpoint blockade-based immunotherapy targeting the endogenous immune response is relatively unstudied. In fact, current paradigms suggest that cancers with low numbers of non-synonymous mutations may not be effectively treated using anti-CTLA4 and anti-PD1 checkpoint blockade (35). Herein, we show that an endogenous T cell response can be effective in controlling BCR-ABL+ B-ALL, but this requires both checkpoint blockade and an intensive heterologous vaccination strategy.
In this study, we found that a strong immune response correlated with decreased leukemic burden. MHC-II expression on leukemic cells correlated with disease outcome, which hinted that CD4+ T cells were important for anti-leukemia immunity. We have previously shown that the leukemia antigen BAp is presented on MHC-II (9). In contrast, BAp does not bind to MHC-I in C57BL/6 mice (data not shown). These findings, in combination with our studies showing that the presence of BAp during prophylactic vaccination is required for protection from leukemia (Fig 3C), provide evidence supporting a role for MHC-II mediated presentation of BAp in anti-leukemia immunity. Moreover, an effective anti-leukemia immune response requires IFNγ and correlates with increased induction of Ly6C on the BAp:I-Ab-specific T cells (Fig 3D,E).
Our PCA strongly suggested a role for targeting the immune checkpoint molecules PDL1 and CTLA4 as part of a therapeutic strategy for BCR-ABL+ B-ALL. Costimulatory and co-inhibitory molecules play a role in cancer progression (4, 37–42). In our model we observed statistically significant increases in survival upon monotherapy with either anti-PDL1 or anti-CTLA4, or dual checkpoint blockade therapy with both anti-PDL1 and anti-CTLA4. However, despite achieving statistical significance, the effects of checkpoint blockade alone were modest (increased survival of 2–4 days), and thus possibly of limited biological impact. The limited impact of checkpoint blockade treatment alone in BCR-ABL+ leukemia fits prevailing concepts regarding checkpoint blockade. Current models suggest that cancers with low numbers of non-synonymous mutations will not be susceptible to checkpoint blockade (35, 36). Our leukemia model likely has few non-synonymous mutations, and checkpoint blockade is not very effective in this leukemia model. Thus, our observations are consistent with the idea that small numbers of non-synonymous mutations result in poor anti-cancer responses following checkpoint blockade therapy.
Immune checkpoint blockade therapy alone was only minimally effective in treating leukemic mice in our model. Thus, we explored therapeutic vaccination immunotherapy. Two lines of evidence precipitated this strategy. First, previous reports show that therapeutic heterologous vaccination can be effective in other cancers, albeit those with higher mutation rates (43).
Second, it was clear that MHC-II mediated antigen presentation was important for leukemia outcome, and the pathogens used in our therapeutic vaccination scheme all induce MHC-II expression on APCs (44–46). When mice were therapeutically vaccinated with these MHC-II inducing pro-inflammatory pathogens, we saw increased survival (Fig 6). We used heterologous vaccination since this approach has previously been shown to be effective at inducing a robust T cell response (47–49). In this approach, we used multiple infectious adjuvants to generate a pro-inflammatory environment that should promote robust adaptive immune activation. Similar approaches have been used prophylactically (47) and therapeutically for cancer (43, 49). However, our study examines therapeutic heterologous vaccination in combination with checkpoint blockade specifically to target CD4+ T cells in cancer - an underexplored field.
We found that therapeutic vaccination synergized with anti-PDL1 and anti-CTLA4 therapies to improve long-term survival in mice with BCR-ABL+ leukemia. Thirty-one percent of the mice that received therapeutic heterologous vaccination in combination with anti-PDL1 and anti-CTLA4 checkpoint blockade exhibited long-term survival. In contrast, only 10% of mice treated with therapeutic heterologous vaccination alone survived long-term. Furthermore, no leukemia bearing mice treated therapeutically with checkpoint blockade alone exhibited long-term survival. These results suggest that even malignancies with few non-synonymous mutations (like B-ALL) can be responsive to immunotherapies that classically work well only in malignancies with high levels of non-synonymous mutations (35). Importantly, such results are contingent upon intensive therapeutic vaccination approaches. One possible explanation for the synergistic effect of vaccination plus checkpoint blockade is that leukemia-derived antigen is available for the entire duration of the therapeutic vaccination regimen. This chronic antigen stimulation may lead to continual high expression of PDL1 and CTLA4 on leukemic cells, which may explain the synergy between therapeutic vaccination (which is susceptible to inhibition by PDL1/PD1 and CTLA4 pathways) and dual checkpoint blockade (which inhibits those pathways). Finally, it is important to note that oncolytic viruses (which include VSV, used in our scheme) have been used for anti-cancer immunotherapy in the past (50) and are currently being used in clinical trials as a treatment option cancer (51, 52). Thus, the approach taken to treat leukemia in our murine model is feasible to consider for human patients with BCR-ABL+ leukemia.
Our data provide initial mechanistic insights into how therapeutic vaccination therapy plus checkpoint blockade can lead to leukemia rejection by the C57BL/6 host. First, we saw a trend towards decreased PD1 expression on BAp:I-Ab-specific T cells that correlated significantly with decreased leukemic burden (Fig 7A, B). Checkpoint blockade could interfere with this potential mechanism of tolerance induction. Second, during the therapeutic vaccination response, we saw that many BAp:I-Ab-specific T cells were poly-functional (producing Granzyme B and multiple cytokines such as IFNγ, TNFα, IL10, and IL17). Importantly, in the most-effective vaccination regimen (therapeutic heterologous vaccination + checkpoint blockade), we saw a significantly increased fraction of BAp:I-Ab-specific T cells that produced Granzyme B, IL10 and a combination of Granzyme B + IL17. This observation demonstrates that effective therapeutic vaccination induces formation of poly-functional leukemia-specific CD4+ T cells. Future studies will be needed to delineate the importance of these cytokines and Granzyme B expression. However, it is intriguing to note that although IL10 is more typically associated with immunosuppression, previous literature supports a role for T cell-derived IL10 in anti-tumor immunotherapy (25, 53, 54). As well, IL17 and Granzyme B have both been implicated in T cell responses to cancer (26, 28, 55). Thus, we envision two possibilities for how and when BAp:I-Ab-specific T cells might elicit anti-leukemia immunity after therapeutic vaccination. First, since the most effective therapeutic vaccination regimen we used (heterologous vaccination + checkpoint blockade) yielded the greatest fraction of Granzyme B producing BAp:I-Ab-specific T cells, it is possible that these cells directly kill MHC-II+ BCR-ABL+ leukemic cells. Second, it is possible these poly-functional BAp:I-Ab-specific T cells induce BAp:I-Ab-specific memory T cells, which may be required for long-term leukemia control. In support of this idea, Th17 cells responding to tumors in other models have a long lifespan, which may be associated with memory formation (28, 56). Therefore, our observations support the idea that poly-functional BAp:I-Ab-specific T cells are induced by intensive therapeutic vaccination, and that these cells contribute to effective leukemia control.
The current paradigm suggests that neo-antigen specific T cells respond better to tumors because the repertoires of these cells have not been pruned by thymic central tolerance (36). This idea implies that cross-reactive T cells will respond poorly to tumors since the repertoires of these cells have been limited by thymic central tolerance. We have previously shown that BAp:I-Ab-specific T cells are cross-reactive with self antigen and that the BAp:I-Ab-specific T cell repertoire is limited by thymic central tolerance (9). Nonetheless, we observed here that BAp-specific adaptive immunity is crucial for anti-leukemia immunity following prophylactic vaccination. Thus, our observations provide a counterpoint to the idea that neo-antigen specific T cells are a prerequisite for effective endogenous anti-cancer T cell responses (36). Taken broadly, our observations suggest that fusion proteins created by chromosomal translocations may be viable immunotherapy targets even when the fusions do not create neo-antigens. This is particularly relevant since chromosomal translocations often result in “driver” mutations, thus leaving minimal opportunity for cancer immunoediting to occur.
Checkpoint blockade is thought to work best in tumors with high numbers of non-synonymous mutations (35). Our results support this concept, as checkpoint blockade was only minimally effective in B-ALL, a leukemia that generally has lower numbers of non-synonymous mutations (5). However, we also demonstrate that intensive heterologous vaccination synergizes with checkpoint blockade to unmask a strong immune response that is capable of controlling this highly aggressive and uniformly fatal form of leukemia in mice. In conclusion, our work establishes that immunotherapy approaches can induce long-term survival with B-ALL, even though mice with B-ALL are refractory to checkpoint blockade-based immunotherapy (36).
Acknowledgments
We thank Gregory Hubbard, Alyssa Kne, Christopher Reis, Amy Mack and Emilea Sykes for assistance with mouse husbandry, Justin Taylor for experimental design, Markus Muschen for BCR-ABL-IRES-GFP retrovirus, Christine Henzler for statistical advice, and Lynn Heltemes-Harris, Casey Katerndahl, and Dan Kaplan for commentary on the manuscript. David Masopust and Marc Jenkins contributed ideas and reagents, particularly in support of the vaccination regimens.
NIH P30CA77598 supports the University of Minnesota Flow Cytometry Resource. LSM and JMS are supported by NIH fellowships (F31CA183226 & F30DK100159, respectively). KRM is supported by the Pennsylvania State University Academic Computing fellowship. KEP is supported by the Robertson Foundation/Cancer Research Institute Irvington Fellowship. MAF is supported by R01CA151845, R01CA154998, R56AI113138, and R01CA185062 from the National Institutes of Health, the University of Minnesota Masonic Cancer Center, and by a Leukemia & Lymphoma Scholar Award.
References
- 1.Gleissner B, Gokbuget N, Bartram CR, Janssen B, Rieder H, Janssen JW, Fonatsch C, Heyll A, Voliotis D, Beck J, Lipp T, Munzert G, Maurer J, Hoelzer D, Thiel E. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99:1536–1543. doi: 10.1182/blood.v99.5.1536. [DOI] [PubMed] [Google Scholar]
- 2.Ravandi F, O’Brien S, Thomas D, Faderl S, Jones D, Garris R, Dara S, Jorgensen J, Kebriaei P, Champlin R, Borthakur G, Burger J, Ferrajoli A, Garcia-Manero G, Wierda W, Cortes J, Kantarjian H. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van ‘t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manlove LS, Berquam-Vrieze KE, Pauken KE, Williams RT, Jenkins MK, Farrar MA. Adaptive immunity to leukemia is inhibited by cross-reactive induced regulatory T cells. The Journal of Immunology. 2015 doi: 10.4049/jimmunol.1501291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RT, Roussel MF, Sherr CJ. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6688–6693. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams RT, den Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes & development. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manlove LS, Berquam-Vrieze KE, Pauken KE, Williams RT, Jenkins MK, Farrar MA. Adaptive Immunity to Leukemia Is Inhibited by Cross-Reactive Induced Regulatory T Cells. Journal of immunology (Baltimore, Md: 1950) 2015;195:4028–4037. doi: 10.4049/jimmunol.1501291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riva G, Luppi M, Barozzi P, Quadrelli C, Basso S, Vallerini D, Zanetti E, Morselli M, Forghieri F, Maccaferri M, Volzone F, Del Giovane C, D’Amico R, Locatelli F, Torelli G, Comoli P, Potenza L. Emergence of BCR-ABL-specific cytotoxic T cells in the bone marrow of patients with Ph+ acute lymphoblastic leukemia during long-term imatinib mesylate treatment. Blood. 2010;115:1512–1518. doi: 10.1182/blood-2009-06-230391. [DOI] [PubMed] [Google Scholar]
- 11.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 12.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 13.Brundage RA, Smith GA, Camilli A, Theriot JA, Portnoy DA. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr MT, Orgun NN, Wilson CB, Way SS. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. Journal of immunology (Baltimore, Md: 1950) 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertelt JM, Rowe JH, Johanns TM, Lai JC, McLachlan JB, Way SS. Selective priming and expansion of antigen-specific Foxp3- CD4+ T cells during Listeria monocytogenes infection. Journal of immunology (Baltimore, Md: 1950) 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 17.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nature protocols. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Team, R. C. R: A language and environment for statistical computing. Computing. 2012:14. [Google Scholar]
- 19.Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Haring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Rocken M. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 21.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 22.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. The Journal of experimental medicine. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The Journal of experimental medicine. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, Sheppard C, Hong K, Cutler C, Turner S, LaFace D, Kleinschek M, Judo M, Ayanoglu G, Langowski J, Gu D, Paporello B, Murphy E, Sriram V, Naravula S, Desai B, Medicherla S, Seghezzi W, McClanahan T, Cannon-Carlson S, Beebe AM, Oft M. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. The Journal of experimental medicine. 2013;210:743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 28.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarjian HM, Walters RS, Keating MJ, Smith TL, O’Brien S, Estey EH, Huh YO, Spinolo J, Dicke K, Barlogie B, et al. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1990;8:994–1004. doi: 10.1200/JCO.1990.8.6.994. [DOI] [PubMed] [Google Scholar]
- 30.Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, Garcia-Manero G, Borthakur G, Kadia T, Rytting M, Konopleva M, Kantarjian H, O’Brien S. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100:653–661. doi: 10.3324/haematol.2014.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, Fischer T, O’Brien SG, Stone RM, Gambacorti-Passerini CB, Russell NH, Reiffers JJ, Shea TC, Chapuis B, Coutre S, Tura S, Morra E, Larson RA, Saven A, Peschel C, Gratwohl A, Mandelli F, Ben-Am M, Gathmann I, Capdeville R, Paquette RL, Druker BJ. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 32.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 33.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, NY) 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 37.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nature medicine. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 38.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nature medicine. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 39.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nature medicine. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 40.Murphy KA, Lechner MG, Popescu FE, Bedi J, Decker SA, Hu P, Erickson JR, O’Sullivan MG, Swier L, Salazar AM, Olin MR, Epstein AL, Ohlfest JR. An in vivo immunotherapy screen of costimulatory molecules identifies Fc-OX40L as a potent reagent for the treatment of established murine gliomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:4657–4668. doi: 10.1158/1078-0432.CCR-12-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (New York, NY) 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 42.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer research. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 43.Hodge JW, Higgins J, Schlom J. Harnessing the unique local immunostimulatory properties of modified vaccinia Ankara (MVA) virus to generate superior tumor-specific immune responses and antitumor activity in a diversified prime and boost vaccine regimen. Vaccine. 2009;27:4475–4482. doi: 10.1016/j.vaccine.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boudreau JE, Bridle BW, Stephenson KB, Jenkins KM, Brunelliere J, Bramson JL, Lichty BD, Wan Y. Recombinant vesicular stomatitis virus transduction of dendritic cells enhances their ability to prime innate and adaptive antitumor immunity. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:1465–1472. doi: 10.1038/mt.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. Journal of immunology (Baltimore, Md: 1950) 1997;158:2259–2267. [PubMed] [Google Scholar]
- 46.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. The Journal of clinical investigation. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. Journal of immunology (Baltimore, Md: 1950) 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 48.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 49.Irvine KR, Chamberlain RS, Shulman EP, Surman DR, Rosenberg SA, Restifo NP. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. Journal of the National Cancer Institute. 1997;89:1595–1601. doi: 10.1093/jnci/89.21.1595. [DOI] [PubMed] [Google Scholar]
- 50.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Science translational medicine. 2014;6:226ra232. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, Moon A, Mun JH, Sommermann EM, Maruri Avidal L, Patt R, Pelusio A, Burke J, Hwang TH, Kirn D, Park YS. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Molecular therapy: the journal of the American Society of Gene Therapy. 2015;23:1532–1540. doi: 10.1038/mt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, Goldberg S, Gross P, O’Neil JD, Groene WS, Roberts MS, Rabin H, Bamat MK, Lorence RM. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 53.Dennis KL, Saadalla A, Blatner NR, Wang S, Venkateswaran V, Gounari F, Cheroutre H, Weaver CT, Roers A, Egilmez NK, Khazaie K. T-cell Expression of IL10 Is Essential for Tumor Immune Surveillance in the Small Intestine. Cancer immunology research. 2015;3:806–814. doi: 10.1158/2326-6066.CIR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Current opinion in oncology. 2013;25:637–645. doi: 10.1097/CCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGeachy MJ. Th17 memory cells: live long and proliferate. Journal of leukocyte biology. 2013;94:921–926. doi: 10.1189/jlb.0313113. [DOI] [PubMed] [Google Scholar]