Abstract
Membrane-type matrix metalloproteinase 1 (MT1-MMP) is a transmembrane zinc-endopeptidase that breaks down extracellular matrix components, including several collagens, during tissue development and physiological remodeling. MT1-MMP-deficient mice (MT1-MMP−/−) feature severe defects in connective tissues, such as impaired growth, osteopenia, fibrosis, and conspicuous loss of molar tooth eruption and root formation. In order to define the functions of MT1-MMP during root formation and tooth eruption, we analyzed the development of teeth and surrounding tissues in the absence of MT1-MMP. In situ hybridization showed that MT1-MMP was widely expressed in cells associated with teeth and surrounding connective tissues during development. Multiple defects in dentoalveolar tissues were associated with loss of MT1-MMP. Root formation was inhibited by defective structure and function of Hertwig's epithelial root sheath (HERS). However, no defect was found in creation of the eruption pathway, suggesting that tooth eruption was hampered by lack of alveolar bone modeling/remodeling coincident with reduced periodontal ligament (PDL) formation and integration with the alveolar bone. Additionally, we identified a significant defect in dentin formation and mineralization associated with the loss of MT1-MMP. To segregate these multiple defects and trace their cellular origin, conditional ablation of MT1-MMP was performed in epithelia and mesenchyme. Mice featuring selective loss of MT1-MMP activity in the epithelium were indistinguishable from wild type mice, and importantly, featured a normal HERS structure and molar eruption. In contrast, selective knock-out of MT1-MMP in Osterix-expressing mesenchymal cells, including osteoblasts and odontoblasts, recapitulated major defects from the global knock-out including altered HERS structure, short roots, defective dentin formation and mineralization, and reduced alveolar bone formation, although molars were able to erupt. These data indicate that MT1-MMP activity in the dental mesenchyme, and not in epithelial-derived HERS, is essential for proper tooth root formation and eruption. In summary, our studies point to an indispensable role for MT1-MMP-mediated matrix remodeling in tooth eruption through effects on bone formation, soft tissue remodeling and organization of the follicle/PDL region.
Keywords: Extracellular matrix, matrix metalloproteinases, dentin, bone formation, periodontal
1. INTRODUCTION
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases capable of degrading extracellular matrix (ECM) structural proteins and processing a number of bioactive molecules. MMPs play important roles in tissue development, physiological remodeling, and pathological processes when normal matrix remodeling is co-opted by pathogens or invasive tissue destructive processes [1-3]. Membrane-type 1 matrix metalloproteinase (MT1-MMP; also known as MMP14) is a cell-associated, membrane-bound MMP with substrate specificity for collagens (I, II, and III), gelatin, fibronectin, and other matrix molecules [4, 5]. The widespread functions of MT1-MMP were revealed by targeted gene deletion in mice where severe deficits in connective tissue remodeling leads to dwarfism, soft tissue fibrosis, osteopenia, skeletal dysplasia, joint disease, and premature death [6].
The dentoalveolar complex is comprised of multiple connective tissues including dentin, cementum, periodontal ligament (PDL), and alveolar bone, with each tissue composed of a distinct ECM [7-9]. Tooth development encompasses several dynamic processes directed by and dependent on ECM production and modification, including cell-cell signaling and cell migration through ECM [10]. Odontogenesis requires secretion and mineralization of dentin matrix whereas root-periodontal formation depends on construction of a complex dentin-cementum-PDL-bone composite structure of mineralized and unmineralized ECM. Eruption of the tooth, in turn, requires that the tooth bud breaches through the mineralized ECM of its bony crypt to assume its position in the oral cavity [11]. The function of the tooth over time further requires that the PDL-bone ECM is remodeled in response to occlusal loads [12].
MT1-MMP deficient mice (MT1-MMP−/−) feature disrupted molar root formation and lack of eruption [13], though underlying mechanisms for these defects are not well understood. In order to define the functions of MT1-MMP during root formation and tooth eruption, we analyzed the development of teeth and surrounding tissues in the absence of MT1-MMP. Additionally, we further elucidated the dentoalveolar defects from loss of MT1-MMP by studying two mouse models with cell-specific ablation of the protease in distinct tissue compartments.
2. RESULTS
2.1 MT1-MMP mRNA is widely expressed in the developing tooth and surrounding tissues
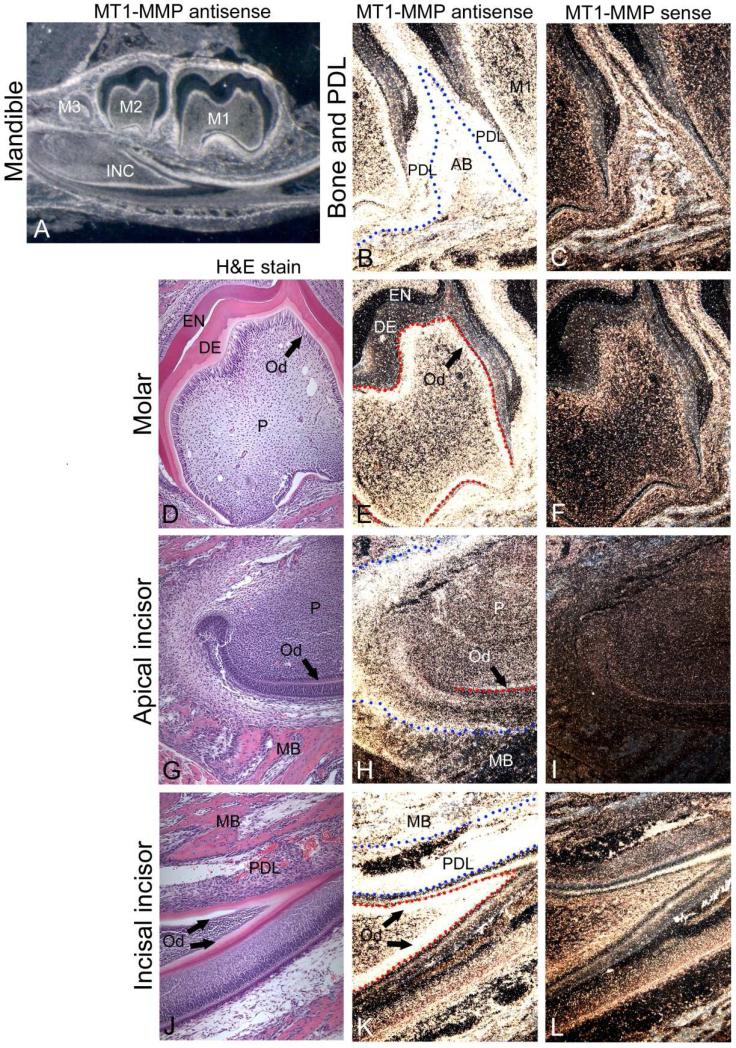
As a first step to determine the role(s) of MT1-MMP in tooth root formation, we identified mRNA localization in dentoalveolar tissues by in situ hybridization (ISH) in mice at 10 days postnatal (dpn), while the molar root is actively forming. Intense MT1-MMP mRNA expression was observed in several cell populations associated with the developing dentoalveolar complex, including cells in or around Hertwig's epithelial root sheath (HERS), the mesenchyme surrounding the root tip, odontoblasts in both molar and incisor teeth, developing periodontal ligament (PDL), and alveolar bone osteoblasts (Figure 1).
Figure 1. MT1-MMP mRNA is widely expressed in the developing tooth and surrounding tissues.
In situ hybridization was used to localize MT1-MMP mRNA expression within mouse dentoalveolar tissues at 10 dpn (A, B, E, H, K), with H&E (D, G, J) shown for morphological reference, and sense panels (C, F, I, L) included as negative controls. (A) MT1-MMP expression is observed in molar (M1-M3) and incisor (INC) teeth and surrounding tissues. (B, C) Intense expression of MT1-MMP is present in developing Hertwig's epithelial root sheath (HERS), periodontal ligament (PDL), and alveolar bone (AB) around molar teeth. The blue dotted line marks the PDL-AB border. (D-F) Odontoblasts (Od) of the molar tooth express high levels of MT1-MMP, though little expression is found in dental pulp (P). The red dotted line outlines the odontoblast cell layer. Odontoblasts in the (G-I) apical incisor begin expressing MT1-MMP mRNA coincident with differentiation and cell layer organization. Osteoblasts (Ob) on the surface of mandibular bone (MB) exhibit MT1-MMP expression. Red dotted lines indicate the odontoblast layer and blue dotted lines demarcate bone surfaces. (J-L) Odontoblasts of the incisal incisor tooth retain strong MT1-MMP mRNA expression, and cells of the PDL and mandibular bone exhibit intense MT1-MMP gene expression.
2.2 Deletion of MT1-MMP prevents root growth and molar tooth eruption
MT1-MMP−/− mice were subsequently analyzed to correlate the observed MT1-MMP expression profile with potential functions of the enzyme during molar root formation. Radiography and micro-CT were used to survey bone and tooth development prior to root initiation (5 dpn), during active root growth, intra-osseous tooth eruption (14 dpn), and after completion of root formation with subsequent molar eruption into the oral cavity (26 dpn).
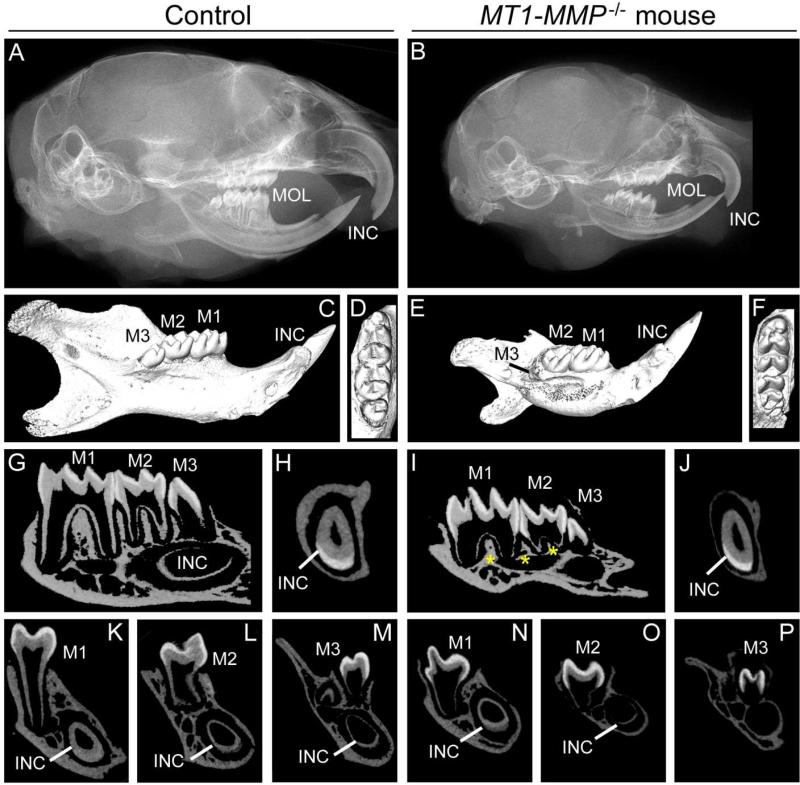
At 5 dpn, skulls and mandibles of MT1-MMP−/− mice were overtly smaller than controls (Supplementary Figure 1). Corresponding radiography and microCT analysis revealed bone mineralization defects, however, molar crown formation appeared unperturbed. Defects in both mandible size and bone mineralization of MT1-MMP−/− mice became more apparent by 14 dpn (Supplementary Figure 2) and 26 dpn (Figure 2). At 14 dpn, first and second molar root development was delayed in MT1-MMP−/− mice, and third molar development also delayed. Basal bone formation apical to the molars as well as alveolar bone height in both the interradicular and interdental areas were reduced compared to WT. By 26 dpn, bone and tooth aberrancies in MT1-MMP−/− mice were even more accentuated (Figure 2). All molars failed to erupt and had incomplete root development with diminished alveolar bone support. The third molar root also failed to form. By 26 dpn, all molars were fully erupted with alveolar bone support in control littermates.
Figure 2. Knockout of MT1-MMP prevents root growth and molar tooth eruption.
Radiographs (A, B) and micro-CT (C-P) were used to analyze dentoalveolar development at 26 dpn (Ages 5 and 14 dpn are shown in Supplementary Figures 1 and 2). (A-F) MT1-MMP−/− mice feature a smaller cranium and mandible compared to WT controls at 26 dpn. The radiograph shows that molars (M1-3) in MT1-MMP−/− mice have not erupted, though the coronal eruption pathway has been cleared (shown in D and F). (G-P) MT1-MMP−/− mice exhibit failure of molar root formation compared to WT, with short roots and thin dentin. Third molar and incisor development are delayed compared to WT. MT1-MMP−/− mouse mandibles show severe lack of formation of tooth-associated bone of the alveolar ridge, interalveolar septum and interradicular septum regions (yellow stars in I).
Based on these aggregate observations of mandibular and radicular developmental defects in the absence of MT1-MMP, we sought to define the effects of MT1-MMP deletion on first molar tissue compartments including HERS, dentin, cementum, PDL, and surrounding alveolar bone.
2.3 Loss of MT1-MMP results in altered HERS structure and signaling
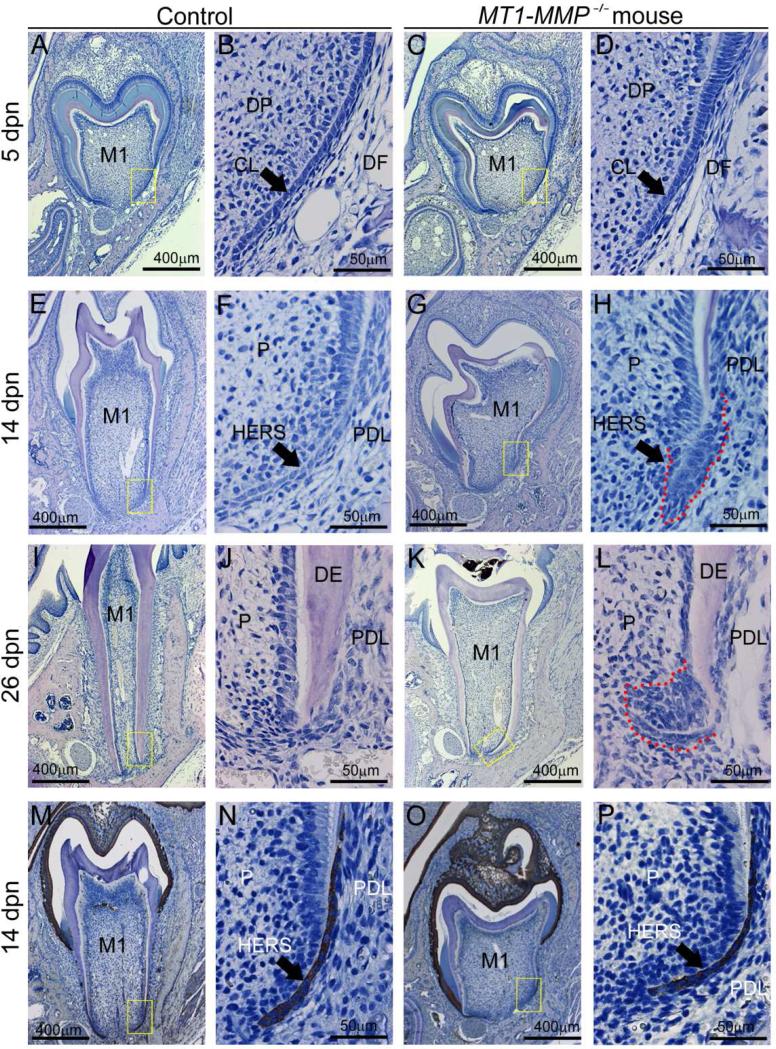
During root formation, growth of HERS defines the size and shape of the tooth root [14]. In WT mice, a well-defined apical HERS migration between 5 and 14 dpn precedes normal root maturation, which is complete by 26 dpn (Figure 3). In contrast, HERS in MT1-MMP−/− mice was disorganized and surrounded by a dense mass of cells from the dental papilla and follicle along its perimeter, with a diffuse boundary between mesenchyme and (HERS) epithelium. The mean HERS length in MT1-MMP−/− mice was reduced by 58% on the buccal molar aspect (p = 0.02) and 26% on the lingual molar aspect (p = 0.26). Furthermore, the characteristic inflection of HERS in relation to the root and apical foramen was blunted in the MT1-MMP−/− mouse (132° on the buccal aspect, 112° on the lingual aspect) compared to controls (146° on the buccal aspect, 143° on the lingual aspect), showing significant changes (p=0.03 for the buccal aspect, p<0.0001 for the lingual aspect).
Figure 3. Loss of MT1-MMP alters the structure of Hertwig's epithelial root sheath (HERS).
HERS was analyzed by H&E staining of the first mandibular molar at 5, 14, and 26 dpn. (A-D) At 5 dpn, the shape of the cervical loop (CL) separating dental papilla (DP) and dental follicle (DF) is similar in WT controls and MT1-MMP−/− mice, though the structure appears shorter in total length. (E-H) At 14 dpn, the WT molar root is lengthening while the tooth erupts. In the MT1-MMP−/− mouse, root length is severely reduced. Compared to the elongated bilayer of HERS in WT, the HERS structure in MT1-MMP−/− mice is short, blunted, and surrounded by an accumulated mass of cells (indicated by red dotted line) from pulp (P) and/or periodontal ligament (PDL). (I-L) By 26 dpn, the WT molar has completed root growth and the HERS structure has disappeared. In contrast, in the MT1-MMP−/− mouse, a dysmorphic HERS structure exists and is surrounded by a dense mass of cells (indicated by red dotted line). (M-P) Cytokeratin 14 immunostaining was used to identify epithelial cells, revealing that the mass of cell surrounding the HERS structure in MT1-MMP−/− mice are mesenchymal in origin.
Based on structural alterations in HERS of MT1-MMP−/− mice, we analyzed cellular processes and signaling pathways previously suggested to be important for HERS function and root formation including proliferation and apoptosis [15-17], Wnt signaling [18], and NFIC expression [19]. PCNA immunohistochemistry indicated similar proliferation potential in 14 dpn WT and MT1-MMP−/− mouse molars, and similar numbers of TUNEL-positive apoptotic cells in the region of WT and MT1-MMP−/− HERS (Supplementary Figure 3A-D). However, a clear difference in apoptotic cell number was observed in the alveolar bone of MT1-MMP−/− mandibles. Wnt-signaling appeared unaffected, as no difference was observed in the pattern of ß-catenin immunostaining in odontoblasts (Supplementary Figure 3E-H). However, NFIC localization was reduced in the dental papilla immediately surrounding HERS in MT1-MMP−/− mice coincident with abnormal cellular aggregation (Supplementary Figure 3 I-L).
2.4 MT1-MMP−/− mice feature defective dentin formation and mineralization
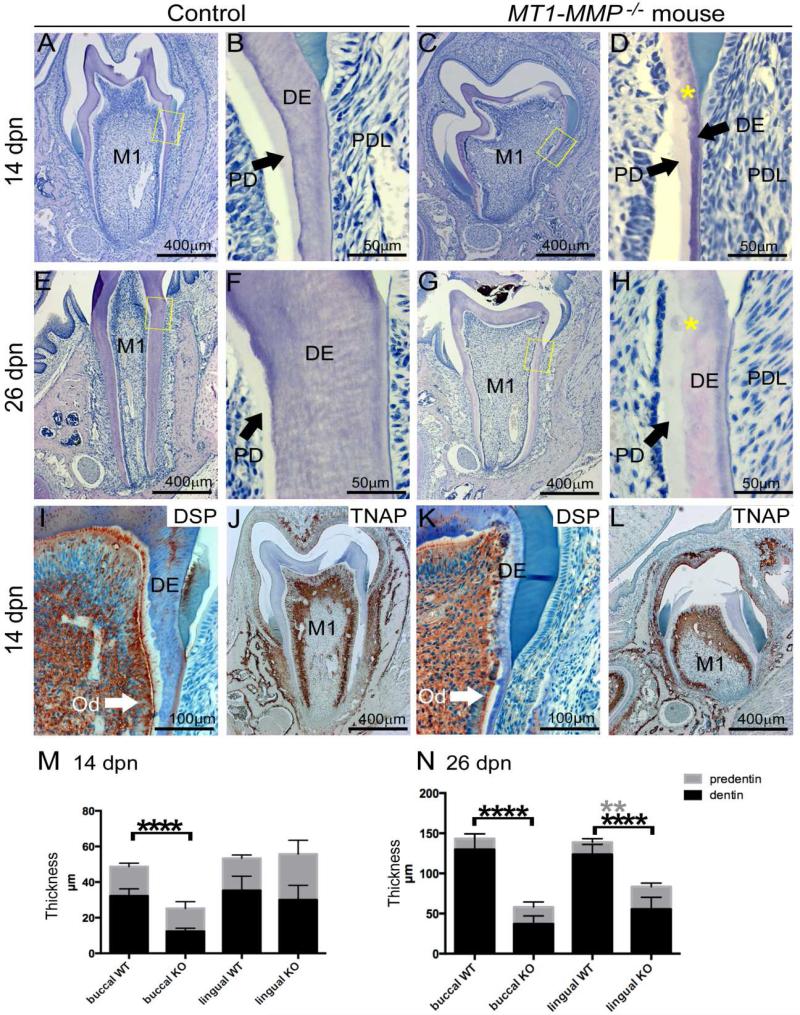
Dentinogenesis is required for crown formation as well as root development and elongation during tooth eruption. Considering the diminished root formation in MT1-MMP−/− mice, we further analyzed dentinogenesis. WT mouse molars at 14 dpn displayed a highly organized odontoblast cell layer and well developed dentin with a smooth, regular border demarcating the mineralized dentin from the unmineralized predentin (Figure 4A, B). In contrast, MT1-MMP−/− mouse molars displayed regions of grossly disorganized odontoblasts forming a dystrophic dentin matrix with protein deposition in coronal dentin (Figure 4C, D). Coronal odontoblasts here were entrapped in an ECM composed of types I and XII collagen with unusually organized and oriented fibers extending into the pulp (Supplementary Figure 4C, D, asterisks). Increased interglobular dentin was also characteristic of this area. Ectopic calcification visualized by von Kossa and Goldner's trichrome staining were found in the pulp and associated with disorganized odontoblasts (Supplementary Figure 4K, L, asterisks). These histologic aberrations were associated with significantly reduced total dentin thickness (by 55-71%) in molar roots (p<0.0001), significantly increased lingual predentin layer (by 56-85%; p=0.002), and an increased predentin:dentin ratio (increased by 4- to 7-fold) by 26 dpn (Figure 4E-H and M, N). Despite this diminution in dentin content, odontoblast differentiation appeared normal initially as induction of the markers dentin sialoprotein (DSP) and tissue nonspecific alkaline phosphatase (TNAP) [20, 21] were unaltered (Figure. 4 I-L).
Figure 4. MT1-MMP−/− mice feature defective dentin formation and mineralization.
Dentinogenesis was analyzed by H&E and immunostaining of the first mandibular molar at 14 and 26 dpn. Yellow boxes in A, C, E, and G indicate locations of higher magnification images in B, D, F, and H. (A-D, M) At 14 dpn, compared to WT control, MT1-MMP−/− molar root dentin (DE) thickness is significantly reduced (on the buccal aspect, p < 0.05), and regions of increased interglobular dentin (yellow stars) are observed in the crown. (E-H, N) At 26 dpn, MT1-MMP−/− dentin defects are more pronounced, including significantly reduced thickness of dentin, increased predentin (PD), and residual interglobular dentin patterns (yellow stars) compared to controls. (I-L) Two odontoblast markers, dentin sialoprotein (DSP) and tissue-nonspecific alkaline phosphatase (TNAP), display comparable localization in controls and MT1-MMP−/− mice, indicating odontoblast differentiation is not initially defective. *indicates significant difference, p < 0.05. M1=first molar; PDL=periodontal ligament.
2.5 Loss of MT1-MMP disrupts periodontal organization
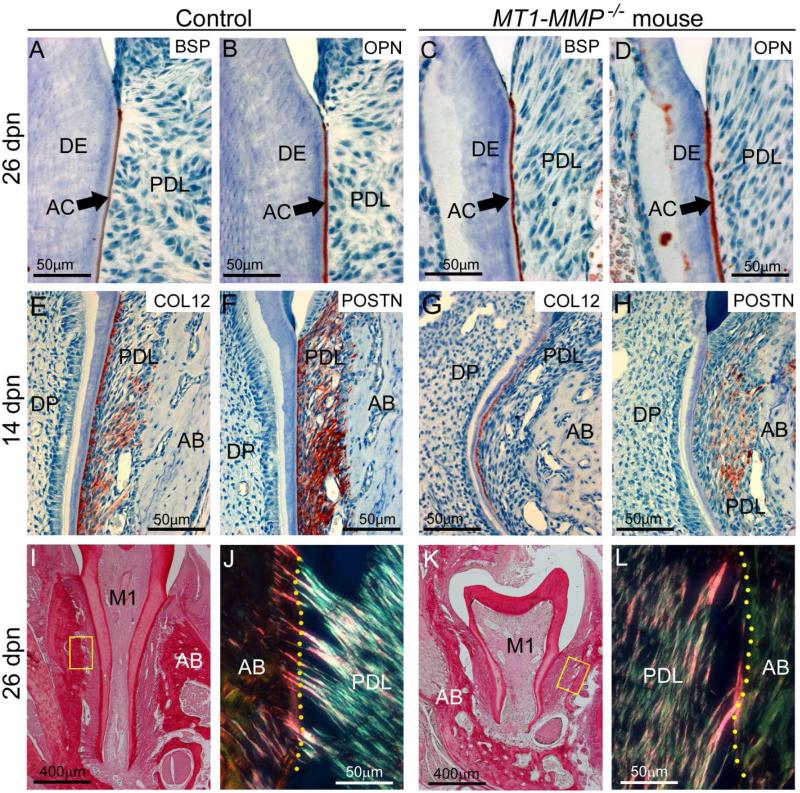
The periodontium forms in step with the root, and we therefore examined cementogenesis and PDL formation in MT1-MMP−/− mice. Acellular cementum was present on the cervical root aspects of WT and MT1-MMP−/− mice (Figure 5A-D), as indicated by immunostaining for bone sialoprotein (BSP) and osteopontin (OPN) [22]. The acellular cementum layer in MT1-MMP−/− mice was thicker in WT controls at 14 and 26 dpn (Figure 5A, B vs. C, D), whereas cellular cementum was absent on the shortened molars of MT1-MMP−/− mice by 26 dpn (data not shown).
Figure 5. Loss of MT1-MMP disrupts periodontal structure.
Immunostaining and selective staining approaches were used to analyze periodontal development in WT control and MT1-MMP−/− mice. (A-D) BSP and OPN immunostaining indicate the presence the acellular cementum (AC) layer on the molar root surface in controls and MT1-MMP−/− mice. The PDL begins to organize in WT by 14 dpn, as shown by increased localization of ECM markers (E) collagen type XII (COL12) and (F) periostin (POSTN), whereas the MT1-MMP−/− mouse PDL shows little localization of (G) COL12 or (H) POSTN, though strong COL12 staining near the root surface reflects collagen fiber insertion into cementum. Picrosirius red staining (under non-polarized light) indicates the presence of fibrillar collagen in 26 dpn (I) WT and (K) MT1-MMP−/− first molars (M1), PDL, and alveolar bone (AB). Under polarized light, (J) WT PDL shows organized collagen fibrils in the PDL, and numerous insertions of Sharpey's fibers into the alveolar bone (yellow dotted line indicates the PDL-bone border). (K) MT1-MMP−/− PDL shows less organization, with loose collagen fibers running mostly parallel to the root surface, and few Sharpey's fibers that are also poorly embedded.
Immunostaining for two ECM proteins associated with PDL organization, collagen type XII (COL XII) [23] and periostin (POSTN) [24], revealed reduced localization of both markers in the PDL of the MT1-MMP−/− mice (Figure 5E-H). Collagen fibers of the PDL remained poorly organized at 26 dpn, with near absence of insertion of Sharpey's fibers at the alveolar bone surface (Figure 5K, L and Supplementary Figure 5).
2.6 Loss of MT1-MMP results in decreased alveolar bone formation
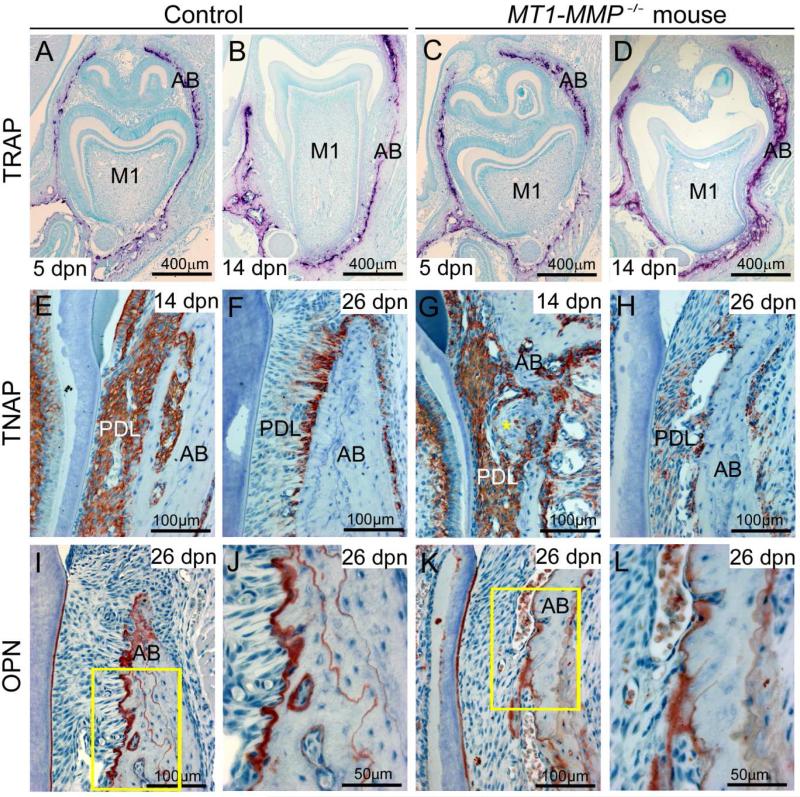
Directed processes of basal bone apposition and coronal resorption are required for tooth eruption. Given the dramatic differences in bone architecture between control and MT1-MMP-deficient mice (Figure 2 and Supplementary Figures 1, 2), we examined mandibular and alveolar bone in further detail by histology. Deficient bone resorption coronal to the tooth can impede the formation of the eruption pathway and contribute to failure of tooth eruption [25]. Tartrate resistant acid phosphatase (TRAP) staining at 5 and 14 dpn, however, revealed numerous osteoclasts coronal to the forming tooth in both WT and MT1-MMP−/− mice (Figure 6A-D). The TRAP staining pattern in conjunction with 3-dimensional images generated by microCT analysis showed clearance of bone coronal to the tooth (Supplementary Figures 1 and 2; Figure 2), consistent with an eruption pathway for the developing molars in MT1-MMP−/− mice.
Figure 6. Alveolar bone dysplasia in MT1-MMP−/− mice.
Immunostaining and selective staining were used to analyze alveolar bone modeling and remodeling in WT and MT1-MMP−/− mice. (A-D) TRAP staining (red-purple color) at 5 and 14 dpn reveals the presence of numerous osteoclasts on alveolar bone (AB) coronal to the forming tooth in both WT and MT1-MMP−/− mice, corresponding to the open eruption pathway observed by micro-CT and histology. (E, F) Immunostaining indicates widespread TNAP in the WT periodontium at 14 dpn, and selectively localized TNAP to alveolar bone surfaces at 26 dpn. (G, H) In MT1-MMP−/− mice, TNAP localization in the PDL space is disrupted in large fibrotic areas at the bone surface (yellow star), and by 26 dpn, TNAP localization adjacent to bone is severely decreased compared to WT controls. (I, J) Matrix protein OPN localizes to reversal lines in bone, indicating cycles of remodeling in WT alveolar bone. (K, L) In MT1-MMP−/− mice, OPN immunostaining reveals a conspicuous lack of remodeling and little osteoblastic activity, resulting in an adynamic appearance to alveolar bone.
Based on the evidence supporting eruption pathway formation, we considered other factors essential to eruption, such as basal bone apposition [26]. Mandibular bone did not exhibit radiographic or histologic distinctions in MT1-MMP−/− mice compared to WT at 5 dpn, prior to root formation (Figure 3A, C and Supplementary Figure 1). In contrast to bone of WT mice at 14 or 26 days of age, basal and alveolar bone in MT1-MMP−/− littermates remained immature and poorly organized, and in particular, alveolar bone surrounding molars was thin, with a woven appearance (Figure 3E, G, I, K, Figure 2, and Supplementary Figure 2). Immunostaining for TNAP, which is abundant in WT osteoblasts, revealed decreased TNAP in alveolar bone osteoblasts in MT1-MMP−/− mice, as well as fibrotic areas at the PDL-bone interface where cells were disorganized and no TNAP was detected (Figure 6E-H). The matrix protein OPN localizes to reversal lines in bone [27] and immunohistochemistry clearly revealed cycles of apposition in WT alveolar bone (Figure 6I, J). However, in MT1-MMP−/− mice, OPN immunostaining demonstrated a conspicuous lack of apposition and an adynamic appearance of alveolar bone (Figure 6K, L).
2.7 Conditional ablation of MT1-MMP in dental mesenchyme but not epithelium affects root and alveolar bone formation
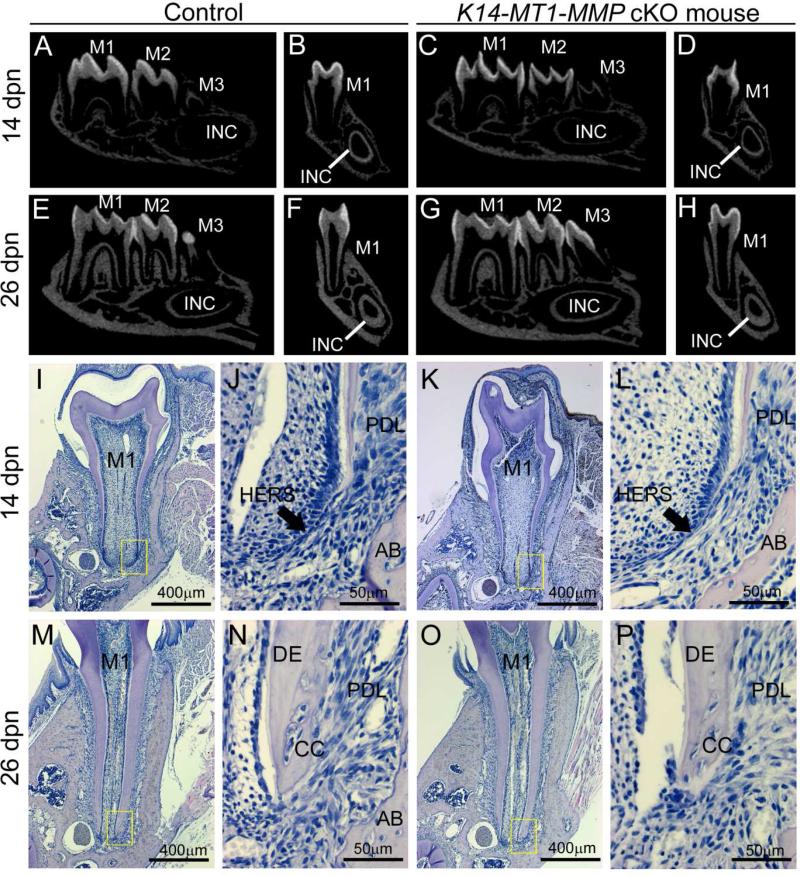
In light of diminished molar root growth and lack of eruption, and the observed aberrations of HERS structure, dentinogenesis, periodontal, and bone formation in MT1-MMP−/− mice, we next aimed to determine which tissue compartment(s) required MT1-MMP enzyme activity. To this end, we generated two types of MT1-MMP conditional knockout mice. The keratin 14 (K14)-Cre line has been used to selectively delete floxed alleles from the oral epithelium and its derived tissues, including HERS [19, 28, 29]. Micro-CT and histology revealed that K14-Cre+; MT1-MMP flox/flox (K14-MT1-MMP cKO) mice displayed normal HERS structure, root development and size, and tooth eruption in molars (Figure 7).
Figure 7. Conditional knockout of MT1-MMP in the dental epithelium does not affect root formation or eruption.
Tooth root development and eruption in K14-MT1-MMP cKO mice was analyzed by micro-CT and histology at 14 and 26 dpn. Yellow boxes in I, K, M, and O are shown at higher magnification in J, L, N, and P, respectively. (A-H) Micro-CT reveals comparable size and mineralization in WT and K14-MT1-MMP cKO mandibles and teeth at 14 and 26 dpn. (I-P) H&E staining of the first mandibular molar of WT and K14-MT1-MMP cKO mice shows comparable tooth morphology at 14 and 26 dpn stage, including structure of Hertwig's epithelial root sheath (HERS), root length, dentin (DE) structure and thickness, periodontal ligament (PDL) organization, alveolar bone (AB), and presence of cellular cementum (CC).
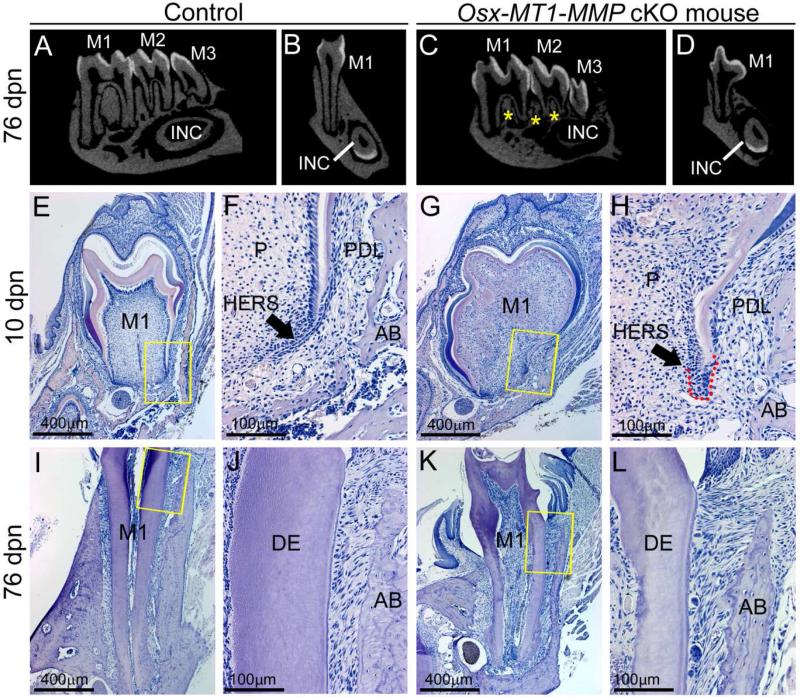
The Osterix (Osx)-Cre line has been used to selectively delete floxed alleles in committed osteoblasts and odontoblasts [30, 31], though the spatiotemporal expression of Osx in the wider periodontium has not been completely resolved [32-34]. While the Osx-Cre transgene has been reported to cause delayed or defective skeletal and craniofacial mineralization resulting from Osterix loss-of-function [35-37], studies including analysis of molar teeth have not identified similar dental defects [30, 38, 39]. To rule out dental alterations from the Osx-Cre transgene, several control genotypes were analyzed (Supplementary Figure 6). Osx-Cre+; MT1-MMP flox/flox (Osx-MT1-MMP cKO) mice displayed nearly all of the phenotypic characteristics of the MT1-MMP−/−, including short molar roots and reduced alveolar bone (Figure 8A-L). Notably, in Osx-MT1-MMP cKO, the HERS structure was defective and surrounded by a mass of accumulated cells strongly resembling the phenotype of MT1-MMP−/− mice (Figure 8F, H). When considered along with the lack of HERS phenotype in K14-MT1-MMP cKO mice, these data strongly implicate the mesenchymal component in dentin and root formation defects observed in the absence of MT1-MMP. Additionally, Osx-MT1-MMP cKO featured overt defects in crown and root dentin, including abnormal coronal morphology, defective circumpulpal dentin production, thin dentin, disorganized dentin-pulp border, disrupted odontoblast layer, and numerous cells embedded in the osteodentin-like matrix (Figure 8E-L). Despite crown and root defects and alveolar bone alterations, molar teeth in Osx-MT1-MMP cKO erupted into the oral cavity.
Figure 8. Conditional knockout of MT1-MMP in the dental mesenchyme affects root and alveolar bone formation.
Tooth root development and eruption in Osx-MT1-MMP cKO mice was analyzed by micro-CT and histology at 10 and 76 dpn. Yellow boxes in E, G, I, and K are shown at higher magnification in F, H, J, and L, respectively. (A-D) Micro-CT reveals lack of alveolar bone formation (yellow stars) and shorter molar roots in the Osx-MT1-MMP cKO mice, compared to controls. (E-H) At 10 dpn, Osx-MT1-MMP cKO mice display severely deficient crown and root dentin, including abnormal crown shape, defective circumpulpal dentin production, thin dentin, dysregulated dentin-pulp border, disrupted odontoblast layer, and numerous cells embedded in the osteodentin-like matrix in the pulp space (P). Compared to the elongated bilayer of HERS in WT, the HERS structure in Osx-MT1-MMP cKO mice is short, blunted, and surrounded by an accumulated mass of cells (red dotted line). (I-L) At 76 dpn, Osx-MT1-MMP cKO mice display reduced mandible alveolar bone (AB) formation, short roots, and thin and aberrant dentin (DE). However, molar teeth in Osx-MT1-MMP cKO are noted to erupt into the oral cavity.
3. DISCUSSION
MT1-MMP is essential during development in both humans and mice for dynamic remodeling of connective tissues, which in turn display profound defects in MT1-MMP-deficiency [3, 6, 40]. We document here that MT1-MMP is widely expressed in the tooth and surrounding connective tissues during development and postnatal growth. Consistent with this expression, we demonstrate that loss of MT1-MMP in mice impairs tooth root formation and eruption in association with multiple defects in dentoalveolar tissues. Defective root formation is associated with aberrant structure and function of Hertwig's epithelial root sheath (HERS) [19, 41], and is further disrupted by lack of alveolar bone apposition/remodeling, or periodontal ligament (PDL) formation and integration into the alveolar bone [11]. For the first time, we have identified a significant defect in dentin formation and mineralization caused by the loss of MT1-MMP. Conditional ablation of MT1-MMP from the dental epithelium did not recapitulate root or eruption defects seen in MT1-MMP−/− mice, while selective ablation of MT1-MMP from the mesenchyme did recapitulate root and bone development, and dentinogenesis defects, indicating important functional roles for MT1-MMP activity in the dental mesenchyme for proper tooth root formation.
3.1 Defective root formation resulting from the loss of MT1-MMP activity
Previous work has demonstrated the general importance of MT1-MMP in tooth root growth and tooth eruption in mice [13], however, the extent of pathological changes and cellular involvement remained unclear to date. Here we systematically analyzed tissue compartments contributing to root development and eruption in the absence of MT1-MMP. Additionally, we employed selective epithelial and mesenchymal ablation of MT1-MMP in order to segregate the physiological significance of epithelial expression from those of the adjacent mesenchymal compartment in the etiopathology of dentoalveolar defects caused by the absence of MT1-MMP.
HERS is a bilayer structure derived from the epithelial enamel organ, and its directed growth defines the size and shape of the tooth root [7, 14]. In MT1-MMP−/− mice, HERS displayed a progressive alteration in shape and angle suggestive of a functional defect related to the lack of root elongation, in association with an accumulated mass of mesenchymal cells surrounding the defective HERS. Conditional K14-Cre-mediated ablation of MT1-MMP in the epithelium, however, did not recapitulate the altered HERS structure and root formation. Normal HERS structure and root formation in K14-MT1-MMP cKO mice suggested that the underlying defect is in the surrounding mesenchyme of the papilla and/or dental follicle, and reproduction of the HERS defect in Osx-MT1-MMP cKO mice confirmed this observation. Based on these data, we propose that lack of remodeling and associated cellular defects due to collagen accumulation in these mesenchymal compartments physically impairs proper growth and function of HERS. This hypothesis is supported by previous findings that MT1-MMP−/− PDL fibroblasts accumulate massive amounts of collagen, which is routed into phagolysosomes to compensate for the lack of MT1-MMP-mediated pericellular matrix degradation [13].
During root growth, HERS cells proliferate, and a portion undergo apoptosis, while the inner enamel epithelium layer (IEE) of HERS induces adjacent dental papilla cells to differentiate into odontoblasts. We analyzed these functions of HERS in MT1-MMP−/− mice and identified no alterations in proliferation or apoptosis either in HERS, or in surrounding cells. Epithelial-mesenchymal signaling between HERS and papilla is further involved with root formation, and two critical signaling events are canonical Wnt activation [18] and odontoblast expression of the transcription factor, NFIC, as a result of epithelial SMAD4-induced sonic hedgehog (SHH) signaling [19, 42, 43]. Immunostaining for ß-catenin, an indicator of Wnt activation, did not document altered Wnt activity in roots of MT1-MMP−/− mice. However, reduced NFIC localization in the nuclei of mesenchymal cells surrounding HERS suggests a signaling defect that may be related to the root growth defect. While MT1-MMP has been demonstrated to process several types of signaling molecules, including those affecting Wnt and Notch signaling pathways [5], it is presently unclear whether there is a direct effect of MT1-MMP proteolytic activity on processing of signaling molecules in the dental mesenchyme, or whether the accumulated collagen surrounding HERS negatively affects secreted signals between cells.
MT1-MMP mRNA is abundant in odontoblasts, and loss of MT1-MMP activity caused defects in both crown-associated and root dentin. The crown dentin of MT1-MMP−/− mice featured regions of dystrophic matrix secretion associated with disrupted odontoblast organization and embedding of cells in ECM. Moreover, thin dentin, interglobular mineralization patterns, ectopic matrix accumulation and mineralization, abnormal induction of COL XII expression, and decreased collagen organization, all suggested an important function for MT1-MMP in proper maintenance of the pulp-dentin border during dentinogenesis Consistent with this notion, older MT1-MMP−/− mice display overt fibrosis of the dental pulp. Molar roots of MT1-MMP−/− mice presented thinner dentin and wider predentin, although odontoblast differentiation and early function appeared grossly normal, as indicated by histological analysis and expression of markers (TNAP and DSP). In contrast, the reduced NFIC induction, especially in root odontoblasts, would be expected to negatively impact odontoblast function, and as such could contribute to the shortened roots. Observations of severe defects in molar crown and root dentin in Osx-MT1-MMP cKO mice support an important function for odontoblast-expressed MT1-MMP in dentinogenesis. The discrepancy in severity of defects in the cKO versus the systemic knockout mouse however raises questions about how Osx-negative cells affect dentin synthesis and pulp homeostasis.
3.2 Failure of tooth eruption in MT1-MMP−/− mice
Coincident with root formation, teeth erupt from their bony crypts into their functional (occlusal) positions within the oral cavity. Failure of eruption in mice and humans can result from dysfunction in either coronal bone resorption or apical bone formation [11, 26, 44-59]. Micro-CT imaging and TRAP staining of histological sections from MT1-MMP−/− mice indicated no defect in osteoclast activation or function that would explain failure of eruption, pointing towards other causes. Formation of bone was severely affected by loss of MT1-MMP, showing persistent disorganization and woven appearance throughout the mandible, strikingly reduced alveolar bone formation, and an adynamic appearance and lack of alveolar bone apposition adjacent to the tooth root. Pockets of fibrotic cells, excessive ECM and aberrant osteoblasts were further identified at the alveolar bone surface. Together these data point towards a major diminution in bone formation and bone organization as being a significant contributor to lack of molar eruption. Conditionally ablating MT1-MMP in osteoblasts in Osx-MT1-MMP cKO mice also affected bone formation and remodeling, but to a lesser extent than complete gene-knock-out. Greater alveolar bone formation was evident and molar tooth eruption occurred in Osx-MT1-MMP cKO compared to MT1-MMP−/− mice, suggesting that non-Osx-expressing cells (e.g., pulp and PDL cells) significantly affect the root formation and tooth eruption.
The negative effects of loss of MT1-MMP on bone formation and mineralization are likely manifold. While an osteopenic skeletal phenotype was apparent in the original description of MT1-MMP−/− mice [6], subsequent work has identified regulatory roles for MT1-MMP in osteoblast differentiation, osteocyte function, and osteogenesis-related signaling pathways [5, 60-65]. A more direct effect on mineralization may result from enzymatic activity of MT1-MMP on ECM-modifying factors such as transglutaminase 2 (TG2), present in bone, teeth, and the PDL [66, 67]. Cleavage of TG2 by MT1-MMP was shown to alter its cross-linking and ATPase activity in osteoblasts, and inhibition of MT1-MMP decreased osteoblast mineralization, in vitro [68], though the function of TG2 in skeletal mineralization remains unclear [69].
Considering the reduced bone formation and excess matrix accumulation in MT1-MMP-deficient mice, we may ask whether defective collagen metabolism in the PDL is responsible for the lack of tooth eruption. A functional periodontium depends on stable insertion of Sharpey's fibers into the acellular cementum and alveolar bone, organization of PDL collagen fibrils to accommodate and disperse forces arising from occlusion, as well as ability of the PDL-bone complex to remodel in response to changing physiological cues and functional demands [70]. Nearly all of these attributes of a functional periodontium are defective in MT1-MMP−/− mice, which exhibit lack of directed PDL collagen fibrils, reduced Sharpey's insertion into bone, and adynamic bone. The PDL is comprised of a complex and dynamic ECM featuring predominantly collagenous fibers, especially types I and III, with lesser amounts of types IV, V, VI, and XII [9]. Collagens I and III are known substrates for MT1-MMP [5], therefore it is perhaps not surprising that this tissue is severely affected by deletion of MT1-MMP activity, which is essential for collagen cleavage, and in turn, in the rapid remodeling of the PDL. This concept is supported by previous observations of age-dependent accumulation of phagocytosed collagen fibrils in PDL fibroblasts in MT1-MMP−/− mice [13]. In contrast to the defective PDL-bone interface in MT1-MMP−/− mice, tooth root cementum formed and had normal fringe collagen fiber organization and insertion of Sharpey's fibers. This contrasting outcome likely results from lack of remodeling of the cementum under normal circumstances, compared to active remodeling required for the PDL-bone interface, especially during tooth movement such as eruption [9, 11].
Despite this dramatic failure of PDL development caused by lack of MT1-MMP, current prevailing theories do not hold that PDL plays a significant role in tooth eruption. This is based in part on experiments demonstrating eruption of rootless teeth and replacement of tooth germs with inanimate objects [11, 71]. However, changes occurring in the newly organizing PDL may contribute to tooth eruption. For example, large-scale migration of progenitor cells from the coronal to apical follicle region [72] may contribute to the motive force of tooth eruption by their apical dislocation, as well as by contributing to osteoblast populations synthesizing newly forming alveolar bone. Migratory capabilities of follicle cells would be expected to be disturbed in the absence of MT1-MMP, as collagen content increases dramatically in the follicle during root formation and tooth eruption [73]. MMPs have been shown to aid cell migration in several contexts, and MMP activity is associated with cell protrusions involved in directing cell motility, with MMPase activity especially essential in densely cross-linked three dimensional ECM [74-77]. Findings in the Osx-MT1-MMP cKO mice support this concept because deletion of MT1-MMP in committed osteoblasts was not sufficient to inhibit tooth eruption, allowing for an important contribution to tooth eruption of non-Osx expressing cells in the periodontium, e.g. subsets of cells in the PDL.
3.3 MT1-MMP, vanishing bone diseases, and failure of tooth eruption
A missense mutation in MT1-MMP in humans is associated with the rare condition, Winchester syndrome, one of a group of “vanishing bone” syndromes featuring marked osteolysis and joint destruction [40]. Case reports on Winchester syndrome have reported delayed or lack of tooth eruption [78-80], consistent with our findings in MT1-MMP−/− mice. However, further dental manifestations, such as effects on tooth structures, have not been reported. To date, dental effects have not been reported in closely related vanishing bone diseases, such as multicentric osteolysis with nodulosis and arthropathy (MONA), associated with mutations in MMP-2 [81].
Ultimately, most causes for primary failure of tooth eruption in humans remain unidentified and poorly understood [82, 83]. These studies on tooth formation and eruption in the absence of MT1-MMP point to a role for collagen metabolism in tooth eruption, possibly through effects on bone formation, as well as remodeling and organization of the follicle/PDL region. Further studies will elucidate functions of MT1-MMP and other regulators of ECM remodeling on tooth formation and eruption, and enhance diagnosis and interventions into cases of failure of eruption in human patients.
4. EXPERIMENTAL PROCEDURES
4.1 Mice
Generation and genotyping of MT1-MMP deficient (MT1-MMP−/−) mice have been described previously [6]. MT1-MMP−/− mice and control littermates were euthanized at 5, 14, and 26 days postnatal (dpn) and skulls and mandibles were collected. For tissue-specific ablation, a Cre-recombinase recognition target (LoxP)-mediated gene excision strategy was used to conditionally knock out MT1-MMP. Keratin 14 (K14)-Cre mice [84] were crossed with mice harboring a floxed MT1-MMP allele [85] to ablate MT1-MMP from the oral epithelium and its derived tissues. These K14-Cre+; MT1-MMP flox/flox (K14-MT1-MMP cKO) mice were compared to control littermates (MT1-MMP flox/flox and MT1-MMP flox/+) at 14 and 26 dpn (n=3-5 samples each per age). Osterix (Osx)-Cre mice [86] were crossed with MT1-MMP flox/flox mice to ablate MT1-MMP from mesenchymal cells including osteoblasts and odontoblasts. These Osx-Cre+; MT1-MMP flox/flox (Osx-MT1-MMP cKO) mice were compared to control littermates (including Osx-Cre+; MT1-MMP flox/+, MT1-MMP flox/flox, and MT1-MMP flox/+) at 10 and 76-79 dpn (n=3 cKO samples per age and n=1-3 of the various control genotypes per age, for a total of 9 controls).
4.2 Radiography and microcomputed tomography
Conventional radiography was performed using a Faxitron cabinet x-ray (Faxitron Bioptics, LLC, Tucson, AZ) Kodak PPL film was exposed at 30 kV for 40 sec. For microcomputed tomography (microCT), mandibles were scanned at a 10 μm voxel resolution, 70keV, 80μA, 300 ms exposure time in a Scanco Medical μCT 50 (Scanco Medical AG, Brüttisellen, Switzerland). Z-stacks were exported as DICOM files and reoriented using ImageJ software (1.48r), with identical sectioning planes chosen for comparison. DICOM stacks were rendered as 3D isoimages using Amira software (version 5.6.0; FEI, Hillsboro, OR).
4.3 Histology and staining
Dissected mandibles were fixed with 4% formaldehyde in PBS, demineralized in 20% EDTA at 4°C, processed for paraffin embedding, and serial sectioned at a thickness of 5 μm. Hematoxylin and eosin (H&E) and picrosirius red staining were performed as described previously [22].
Non-decalcified hemi-mandibles were processed and embedded in methyl methacrylate for von Kossa and Goldner's trichrome staining, as described previously [70]. Tartrate resistant acid phosphatase (TRAP) staining (Wako Chemicals, Japan) was used to identify osteoclast-like cells [70], while TdT-mediated dUTP-biotin nick end-labeling (TUNEL) assay (Promega, Madison, WI, USA) was used to visualize apoptotic cells.
4.4 In situ hybridization and immunohistochemistry
In situ hybridization (ISH) for MT1-MMP mRNA was performed as described previously [87]. Immunohistochemistry (IHC) was performed on paraffin sections using an avidin-biotinylated peroxidase enzyme complex (ABC) based kit (Vector Labs, Burlingame, CA) with 3-amino-9-ethylcarbazole (AEC) or 3, 3’-diaminobenzidine (DAB) chromogenic substrates (Vector Labs), as described in more detail previously [22]. Primary antibodies included: Rabbit polyclonal anti-cytokeratin 14 (Covance, Berkeley, CA, USA), rabbit polyclonal anti-Proliferating Cell Nuclear Antigen (PCNA) (Santa Cruz, CA, USA), rabbit polyclonal anti-ß-catenin (H-102, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-nuclear factor IC (NFIC; a gift from Dr. J.C. Park, Seoul National University, Korea) [88], rabbit polyclonal anti-dentin sialoprotein (DSP) LF-153 (a gift from Dr. Larry Fisher, NIDCR/NIH, Bethesda, MD, USA)[89], rat anti-tissue nonspecific alkaline phosphatase (TNAP, R&D Systems, Minneapolis, MN, USA)[90], rabbit polyclonal anti-collagen type 1a1 (COL1A1) LF-68 (a gift from Dr. Larry Fisher)[91], rabbit polyclonal anti-collagen type 12 (COLXII) KR-33 (a gift from Dr. Manual Koch, University of Cologne, Germany)[92], rabbit polyclonal anti-bone sialoprotein (BSP; a gift from Dr. Renny Franceschi, University of Michigan, Ann Arbor, MI, USA)[70], polyclonal rabbit anti-osteopontin (OPN) LF-175 (a gift from Dr. Larry Fisher, NIDCR/NIH)[70], and rabbit polyclonal anti-periostin (POSTN; Abcam, Cambridge, MA).
Supplementary Material
HIGHLIGHTS.
MT1-MMP is expressed in multiple cell populations during tooth development
Lack of MT1-MMP causes defective epithelial root sheath structure and reduced root formation
Reduced bone formation in mice deficient for MT1-MMP is associated with lack of molar tooth eruption
Dentin formation and mineralization is aberrant in the absence of MT1-MMP
Selective ablation of MT1-MMP in osteoblasts and odontoblasts recapitulates the major dentoalveolar defects of global knock-out
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; to BLF) and the National Institute for Dental and Craniofacial Research (NIDCR; to KH) of the National Institutes of Health (NIH, Bethesda, MD), grant AR066110 from NIAMS/NIH (to BLF), by Overseas Research and Personal Training Fund from Peking University School and Hospital of Stomatology (to HX), and by the NIH Medical Research Scholars Program (MRSP)(to TNS). MRSP is a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as alumni of student research programs and other individual supporters. The authors thank Dr. Pamela G. Robey (NIDCR/NIH) for her support and critical review of the manuscript.
ABBREVIATIONS USED
- BSP
bone sialoprotein
- COL1A1
collagen type Ia1
- COL XII
collagen type 12
- DSP
dentin sialoprotein
- ECM
extracellular matrix
- HERS
Hertwig's epithelial root sheath
- K14
cytokeratin 14
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type 1 matrix metalloproteinase
- NFIC
nuclear factor 1C
- OPN
osteopontin
- OSX
osterix
- PCNA
proliferating cell nuclear antigen
- PDL
periodontal ligament
- POSTN
periostin
- TNAP
tissue nonspecific alkaline phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
BLF and KH designed the project, BLF, HX, TNS, SSY, and TY performed experiments. BLF, KH, HX, and TNS analyzed data. BLF, HX, and TNS wrote the manuscript. All authors read, contributed to discussion, and approved the final manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Holmbeck K, Bianco P, Birkedal-Hansen H. MT1-mmp: a collagenase essential for tumor cell invasive growth. Cancer Cell. 2003;4:83–4. doi: 10.1016/s1535-6108(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 4.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200:11–9. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 5.Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015:44–46. 207–23. doi: 10.1016/j.matbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 7.Foster BL, Nociti FH, Jr., Somerman MJ. Tooth Root Formation. In: Huang GTJ, Thesleff I, editors. Stem Cells, Craniofacial Development and Regeneration. 1 ed Wiley-Blackwell; 2013. pp. 153–177. [Google Scholar]
- 8.Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontol. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. 2000. [DOI] [PubMed] [Google Scholar]
- 9.Beertsen W, McCulloch C, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol. 1997;13:20–40. doi: 10.1111/j.1600-0757.1997.tb00094.x. 2000. [DOI] [PubMed] [Google Scholar]
- 10.Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 2009;312B:309–19. doi: 10.1002/jez.b.21280. [DOI] [PubMed] [Google Scholar]
- 11.Wise GE, King GJ. Mechanisms of Tooth Eruption and Orthodontic Tooth Movement. Journal of Dental Research. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho S, Kurylo M, Fong T, Lee S, Wagner H, Ryder M, Marshall G. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials. 2010;31:6635–46. doi: 10.1016/j.biomaterials.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beertsen W, Holmbeck K, Niehof A, Bianco P, Chrysovergis K, Birkedal-Hansen H, Everts V. On the role of MT1-MMP, a matrix metalloproteinase essential to collagen remodeling, in murine molar eruption and root growth. Eur J Oral Sci. 2002;110:445–51. doi: 10.1034/j.1600-0722.2002.21384.x. [DOI] [PubMed] [Google Scholar]
- 14.Luan X, Ito Y, Diekwisch T. Evolution and development of Hertwig's epithelial root sheath. Dev Dyn. 2006;235:1167–80. doi: 10.1002/dvdy.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara N, Tabata MJ, Endoh M, Ishizeki K, Nawa T. Insulin-like growth factor-I stimulates cell proliferation in the outer layer of Hertwig's epithelial root sheath and elongation of the tooth root in mouse molars in vitro. Cell Tissue Res. 2005;320:69–75. doi: 10.1007/s00441-004-1065-5. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Matsuzaka K, Yamada S, Shimono M, Abiko Y, Inoue T. Morphology of Malassez's epithelial rest-like cells in the cementum: transmission electron microscopy, immunohistochemical, and TdT-mediated dUTP-biotin nick end labeling studies. J Periodontal Res. 2006;41:280–7. doi: 10.1111/j.1600-0765.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Yamada T, Yamamoto T, Hasegawa T, Hongo H, Oda K, Amizuka N. Hertwig's Epithelial Root Sheath Fate during Initial Cellular Cementogenesis in Rat Molars. Acta Histochem Cytochem. 2015;48:95–101. doi: 10.1267/ahc.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. β-catenin is Required in Odontoblasts for Tooth Root Formation. J Dent Res. 2013 doi: 10.1177/0022034512470137. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Xu X, Bringas P, Hung YP, Chai Y. Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J Bone Miner Res. 2010;25:1167–78. doi: 10.1359/jbmr.091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster BL, Nagatomo KJ, Tso HW, Tran AB, Nociti FH, Jr., Narisawa S, Yadav MC, McKee MD, Millan JI, Somerman MJ. Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J Bone Miner Res. 2013;28:271–82. doi: 10.1002/jbmr.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–80. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 22.Foster BL. Methods for studying tooth root cementum by light microscopy. Int J Oral Sci. 2012;4:119–28. doi: 10.1038/ijos.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacNeil R, Berry J, Strayhorn C, Shigeyama Y, Somerman M. Expression of type I and XII collagen during development of the periodontal ligament in the mouse. Arch Oral Biol. 1998;43:779–87. doi: 10.1016/s0003-9969(98)00054-5. [DOI] [PubMed] [Google Scholar]
- 24.Rios H, Koushik SV, Wang H, Wang J, Zhou H-M, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. periostin Null Mice Exhibit Dwarfism, Incisor Enamel Defects, and an Early-Onset Periodontal Disease-Like Phenotype. Molecular and Cellular Biology. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos- Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 26.Wise GE, Frazier-Bowers S, D'Souza RN. Cellular, Molecular, and Genetic Determinants of Tooth Eruption. Critical Reviews in Oral Biology & Medicine. 2002;13:323–335. doi: 10.1177/154411130201300403. [DOI] [PubMed] [Google Scholar]
- 27.Bosshardt D, Zalzal S, McKee M, Nanci A. Developmental appearance and distribution of bone sialoprotein and osteopontin in human and rat cementum. Anat Rec. 1998;250:13–33. doi: 10.1002/(SICI)1097-0185(199801)250:1<13::AID-AR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Bringas PJ, Slavkin H, Chai Y. Fate of HERS during tooth root development. Dev Biol. 2009;334:22–30. doi: 10.1016/j.ydbio.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Jung J, Liu Y, Yuan B, Lu Y, Feng JQ, Qin C. The specific role of FAM20C in amelogenesis. J Dent Res. 2013;92:995–9. doi: 10.1177/0022034513504588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Cox MK, Coricor G, Macdougall M, Serra R. Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation. Dev Biol. 2013;382:27–37. doi: 10.1016/j.ydbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakian A, Yang WC, Gluhak-Heinrich J, Cui Y, Harris MA, Villarreal D, Feng JQ, Macdougall M, Harris SE. Bone morphogenetic protein-2 gene controls tooth root development in coordination with formation of the periodontium. Int J Oral Sci. 2013;5:75–84. doi: 10.1038/ijos.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata A, Sugahara T, Nakamura H. Localization of runx2, osterix, and osteopontin in tooth root formation in rat molars. J Histochem Cytochem. 2009;57:397–403. doi: 10.1369/jhc.2008.952192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao T, Xiao Y, de Crombrugghe B, Somerman MJ, Feng JQ. Genetic evidence for the vital function of Osterix in cementogenesis. J Bone Miner Res. 2012;27:1080–92. doi: 10.1002/jbmr.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JY, Kim BI, Jue SS, Park JH, Shin JW. Localization of osteopontin and osterix in periodontal tissue during orthodontic tooth movement in rats. Angle Orthod. 2012;82:107–14. doi: 10.2319/030911-173.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Mishina Y, Liu F. Osterix-Cre transgene causes craniofacial bone development defect. Calcif Tissue Int. 2015;96:129–37. doi: 10.1007/s00223-014-9945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W, Olsen BR. Skeletal defects in Osterix-Cre transgenic mice. Transgenic Res. 2015;24:167–72. doi: 10.1007/s11248-014-9828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, Anderson PH, Zajac JD. Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res. 2012;21:885–93. doi: 10.1007/s11248-011-9581-z. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Ma J, Zhu G, Jules J, Wu M, McConnell M, Tian F, Paulson C, Zhou X, Wang L, Li YP. Cbfbeta deletion in mice recapitulates cleidocranial dysplasia and reveals multiple functions of Cbfbeta required for skeletal development. Proc Natl Acad Sci U S A. 2014;111:8482–7. doi: 10.1073/pnas.1310617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shekaran A, Shoemaker JT, Kavanaugh TE, Lin AS, LaPlaca MC, Fan Y, Guldberg RE, Garcia AJ. The effect of conditional inactivation of beta 1 integrins using twist 2 Cre, Osterix Cre and osteocalcin Cre lines on skeletal phenotype. Bone. 2014;68:131–41. doi: 10.1016/j.bone.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans BR, Mosig RA, Lobl M, Martignetti CR, Camacho C, Grum-Tokars V, Glucksman MJ, Martignetti JA. Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am J Hum Genet. 2012;91:572–6. doi: 10.1016/j.ajhg.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakano M, Otsu K, Fujiwara N, Fukumoto S, Yamada A, Harada H. Cell dynamics in cervical loop epithelium during transition from crown to root: implications for Hertwig's epithelial root sheath formation. J Periodontal Res. 2013;48:262–7. doi: 10.1111/jre.12003. [DOI] [PubMed] [Google Scholar]
- 42.Lee DS, Park JT, Kim HM, Ko JS, Son HH, Gronostajski RM, Cho MI, Choung PH, Park JC. Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J Biol Chem. 2009;284:17293–303. doi: 10.1074/jbc.M109.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JC, Herr Y, Kim HJ, Gronostajski RM, Cho MI. Nfic gene disruption inhibits differentiation of odontoblasts responsible for root formation and results in formation of short and abnormal roots in mice. J Periodontol. 2007;78:1795–802. doi: 10.1902/jop.2007.060363. [DOI] [PubMed] [Google Scholar]
- 44.Wise GE, He H, Gutierrez DL, Ring S, Yao S. Requirement of alveolar bone formation for eruption of rat molars. Eur J Oral Sci. 2011;119:333–8. doi: 10.1111/j.1600-0722.2011.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao S, Pan F, Wise GE. Chronological gene expression of parathyroid hormone-related protein (PTHrP) in the stellate reticulum of the rat: implications for tooth eruption. Arch Oral Biol. 2007;52:228–32. doi: 10.1016/j.archoralbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Wise GE, Yao S, Henk WG. Bone formation as a potential motive force of tooth eruption in the rat molar. Clin Anat. 2007;20:632–9. doi: 10.1002/ca.20495. [DOI] [PubMed] [Google Scholar]
- 47.Wise GE, Yao S. Regional differences of expression of bone morphogenetic protein-2 and RANKL in the rat dental follicle. Eur J Oral Sci. 2006;114:512–6. doi: 10.1111/j.1600-0722.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 48.Wise GE, Yao S, Odgren PR, Pan F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J Dent Res. 2005;84:837–41. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D, Yao S, Pan F, Wise GE. Chronology and regulation of gene expression of RANKL in the rat dental follicle. Eur J Oral Sci. 2005;113:404–9. doi: 10.1111/j.1600-0722.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 50.Yao S, Ring S, Henk WG, Wise GE. In vivo expression of RANKL in the rat dental follicle as determined by laser capture microdissection. Arch Oral Biol. 2004;49:451–6. doi: 10.1016/j.archoralbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Wise GE, Huang H, Que BG. Gene expression of potential tooth eruption molecules in the dental follicle of the mouse. Eur J Oral Sci. 1999;107:482–6. doi: 10.1046/j.0909-8836.1999.eos107610.x. [DOI] [PubMed] [Google Scholar]
- 52.Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC. Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci U S A. 1998;95:11846–51. doi: 10.1073/pnas.95.20.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris SE, MacDougall M, Horn D, Woodruff K, Zimmer SN, Rebel VI, Fajardo R, Feng JQ, Gluhak-Heinrich J, Harris MA, Abboud Werner S. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50:42–53. doi: 10.1016/j.bone.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyata A, Baba O, Oda T, Ishikawa I, Takano Y. Diverse effects of c-src deficiency on molar tooth development and eruption in mice. Arch Histol Cytol. 2007;70:63–78. doi: 10.1679/aohc.70.63. [DOI] [PubMed] [Google Scholar]
- 55.Helfrich MH. Osteoclast diseases and dental abnormalities. Arch Oral Biol. 2005;50:115–22. doi: 10.1016/j.archoralbio.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 56.Tiffee JC, Xing L, Nilsson S, Boyce BF. Dental abnormalities associated with failure of tooth eruption in src knockout and op/op mice. Calcif Tissue Int. 1999;65:53–8. doi: 10.1007/s002239900657. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, McCauley LK. Osteoclasts and odontoclasts: signaling pathways to development and disease. Oral Dis. 2011;17:129–42. doi: 10.1111/j.1601-0825.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- 58.Ahmad S, Bister D, Cobourne MT. The clinical features and aetiological basis of primary eruption failure. Eur J Orthod. 2006;28:535–40. doi: 10.1093/ejo/cjl033. [DOI] [PubMed] [Google Scholar]
- 59.Huang H, Wise GE. Delay of tooth eruption in null mice devoid of the type I IL-1R gene. Eur J Oral Sci. 2000;108:297–302. doi: 10.1034/j.1600-0722.2000.108004297.x. [DOI] [PubMed] [Google Scholar]
- 60.Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118:147–56. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 61.Kulkarni RN, Bakker AD, Gruber EV, Chae TD, Veldkamp JB, Klein-Nulend J, Everts V. MT1-MMP modulates the mechanosensitivity of osteocytes. Biochem Biophys Res Commun. 2012;417:824–9. doi: 10.1016/j.bbrc.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 62.Karsdal M, Andersen T, Bonewald L, Christiansen C. Matrix metalloproteinases (MMPs) safeguard osteoblasts from apoptosis during transdifferentiation into osteocytes: MT1-MMP maintains osteocyte viability. DNA Cell Biol. 2004;23:155–65. doi: 10.1089/104454904322964751. [DOI] [PubMed] [Google Scholar]
- 63.Tang Y, Rowe RG, Botvinick EL, Kurup A, Putnam AJ, Seiki M, Weaver VM, Keller ET, Goldstein S, Dai J, Begun D, Saunders T, Weiss SJ. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Dev Cell. 2013;25:402–16. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manduca P, Castagnino A, Lombardini D, Marchisio S, Soldano S, Ulivi V, Zanotti S, Garbi C, Ferrari N, Palmieri D. Role of MT1-MMP in the osteogenic differentiation. Bone. 2009;44:251–65. doi: 10.1016/j.bone.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 65.Chan KM, Wong HL, Jin G, Liu B, Cao R, Cao Y, Lehti K, Tryggvason K, Zhou Z. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev Cell. 2012;22:1176–90. doi: 10.1016/j.devcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 66.Kaartinen M, Sun W, Kaipatur N, McKee M. Transglutaminase crosslinking of SIBLING proteins in teeth. J Dent Res. 2005;84:607–12. doi: 10.1177/154405910508400705. [DOI] [PubMed] [Google Scholar]
- 67.Trombetta-eSilva J, Rosset EA, Hepfer RG, Wright GJ, Baicu C, Yao H, Bradshaw AD. Decreased Mechanical Strength and Collagen Content in SPARC-Null Periodontal Ligament Is Reversed by Inhibition of Transglutaminase Activity. J Bone Miner Res. 2015;30:1914–24. doi: 10.1002/jbmr.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakano Y, Forsprecher J, Kaartinen MT. Regulation of ATPase activity of transglutaminase 2 by MT1-MMP: implications for mineralization of MC3T3-E1 osteoblast cultures. J Cell Physiol. 2010;223:260–9. doi: 10.1002/jcp.22034. [DOI] [PubMed] [Google Scholar]
- 69.Cordell PA, Newell LM, Standeven KF, Adamson PJ, Simpson KR, Smith KA, Jackson CL, Grant PJ, Pease RJ. Normal Bone Deposition Occurs in Mice Deficient in Factor XIII-A and Transglutaminase 2. Matrix Biol. 2015;43:85–96. doi: 10.1016/j.matbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Foster BL, Soenjaya Y, Nociti FH, Holm E, Zerfas PM, Wimer HF, Holdsworth DW, Aubin JE, Hunter GK, Goldberg HA, Somerman MJ. Deficiency in acellular cementum and periodontal attachment in bsp null mice. J Dent Res. 2013;92:166–72. doi: 10.1177/0022034512469026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marks SC, Jr., Cahill DR. Experimental study in the dog of the non-active role of the tooth in the eruptive process. Arch Oral Biol. 1984;29:311–22. doi: 10.1016/0003-9969(84)90105-5. [DOI] [PubMed] [Google Scholar]
- 72.Diekwisch T. Pathways and fate of migratory cells during late tooth organogenesis. Connect Tissue Res. 2002;43:245–56. [PubMed] [Google Scholar]
- 73.Gorski JP, Marks SC, Jr., Cahill DR, Wise GE. Developmental changes in the extracellular matrix of the dental follicle during tooth eruption. Connect Tissue Res. 1988;18:175–90. doi: 10.3109/03008208809016806. [DOI] [PubMed] [Google Scholar]
- 74.Itoh Y. MT1-MMP: a key regulator of cell migration in tissue. IUBMB Life. 2006;58:589–96. doi: 10.1080/15216540600962818. [DOI] [PubMed] [Google Scholar]
- 75.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 76.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–9. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 77.Fraley SI, Wu PH, He L, Feng Y, Krisnamurthy R, Longmore GD, Wirtz D. Three- dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci Rep. 2015;5:14580. doi: 10.1038/srep14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winchester P, Grossman H, Lim WN, Danes BS. A new acid mucopolysaccharidosis with skeletal deformities simulating rheumatoid arthritis. Am J Roentgenol Radium Ther Nucl Med. 1969;106:121–8. doi: 10.2214/ajr.106.1.121. [DOI] [PubMed] [Google Scholar]
- 79.Prapanpoch S, Jorgenson RJ, Langlais RP, Nummikoski PV. Winchester syndrome. A case report and literature review. Oral Surg Oral Med Oral Pathol. 1992;74:671–7. doi: 10.1016/0030-4220(92)90363-u. [DOI] [PubMed] [Google Scholar]
- 80.Matthiesen G, Pedersen VF, Helin P, Jacobsen GK, Nielsen NS. Winchester syndrome. Int Orthop. 2001;25:331–3. doi: 10.1007/s002640100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castberg FC, Kjaergaard S, Mosig RA, Lobl M, Martignetti C, Martignetti JA, Myrup C, Zak M. Multicentric osteolysis with nodulosis and arthropathy (MONA) with cardiac malformation, mimicking polyarticular juvenile idiopathic arthritis: case report and literature review. Eur J Pediatr. 2013;172:1657–63. doi: 10.1007/s00431-013-2102-8. [DOI] [PubMed] [Google Scholar]
- 82.Frazier-Bowers SA, Puranik CP, Mahaney MC. The etiology of eruption disorders - further evidence of a 'genetic paradigm'. Semin Orthod. 2010;16:180–185. doi: 10.1053/j.sodo.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Proffit WR, Frazier-Bowers SA. Mechanism and control of tooth eruption: overview and clinical implications. Orthod Craniofac Res. 2009;12:59–66. doi: 10.1111/j.1601-6343.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 84.Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–85. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 85.Szabova L, Son MY, Shi J, Sramko M, Yamada SS, Swaim WD, Zerfas P, Kahan S, Holmbeck K. Membrane-type MMPs are indispensable for placental labyrinth formation and development. Blood. 2010;116:5752–61. doi: 10.1182/blood-2009-10-249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 87.Szabova L, Yamada SS, Birkedal-Hansen H, Holmbeck K. Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J Cell Physiol. 2005;205:123–32. doi: 10.1002/jcp.20385. [DOI] [PubMed] [Google Scholar]
- 88.Bae CH, Kim TH, Chu JY, Cho ES. New population of odontoblasts responsible for tooth root formation. Gene Expr Patterns. 2013;13:197–202. doi: 10.1016/j.gep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Chu E, Fong H, Blethen F, Tompkins K, Foster B, Yeh K, Nagatomo K, Matsa-Dunn D, Sitara D, Lanske B, Rutherford R, Somerman M. Ablation of systemic phosphate-regulating gene fibroblast growth factor 23 (Fgf23) compromises the dentoalveolar complex. Anat Rec (Hoboken) 2010;293:1214–26. doi: 10.1002/ar.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zweifler LE, Patel MK, Nociti FH, Jr., Wimer HF, Millan JL, Somerman MJ, Foster BL. Counter-regulatory phosphatases TNAP and NPP1 temporally regulate tooth root cementogenesis. Int J Oral Sci. 2014 doi: 10.1038/ijos.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fisher LW, Stubbs JT, 3rd, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–5. [PubMed] [Google Scholar]
- 92.Veit G, Hansen U, Keene DR, Bruckner P, Chiquet-Ehrismann R, Chiquet M, Koch M. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem. 2006;281:27461–70. doi: 10.1074/jbc.M603147200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.