Abstract
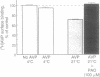
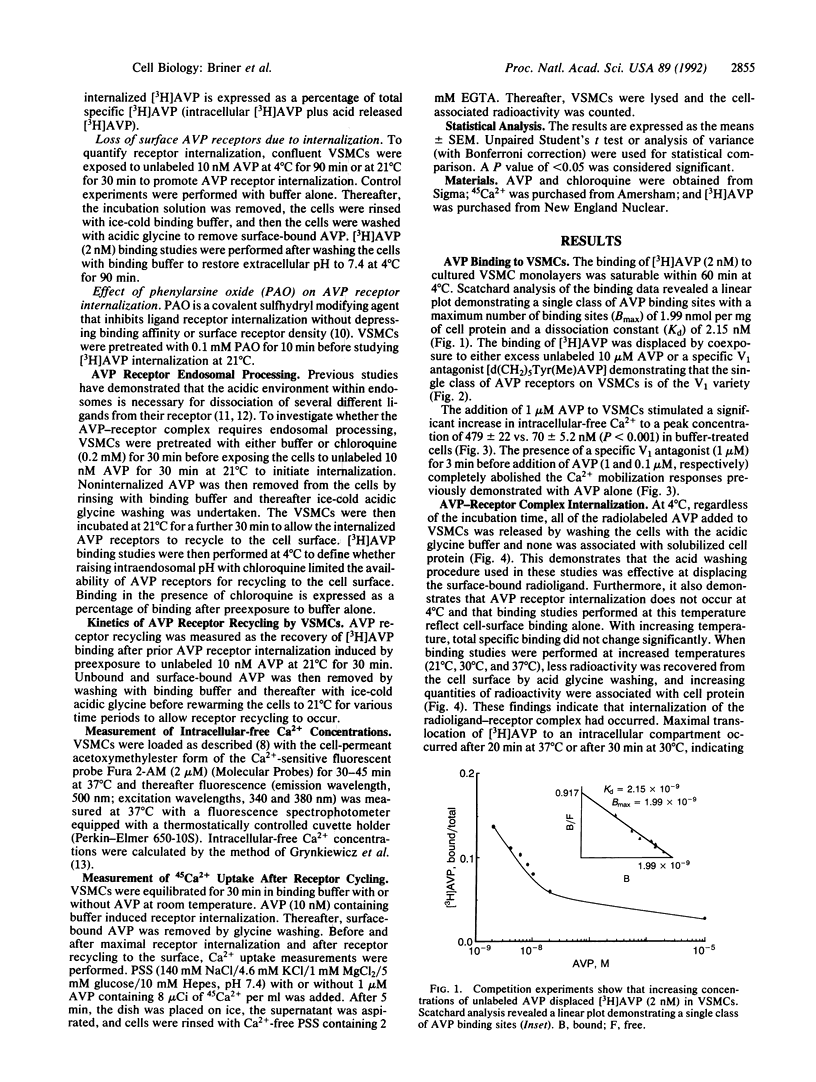
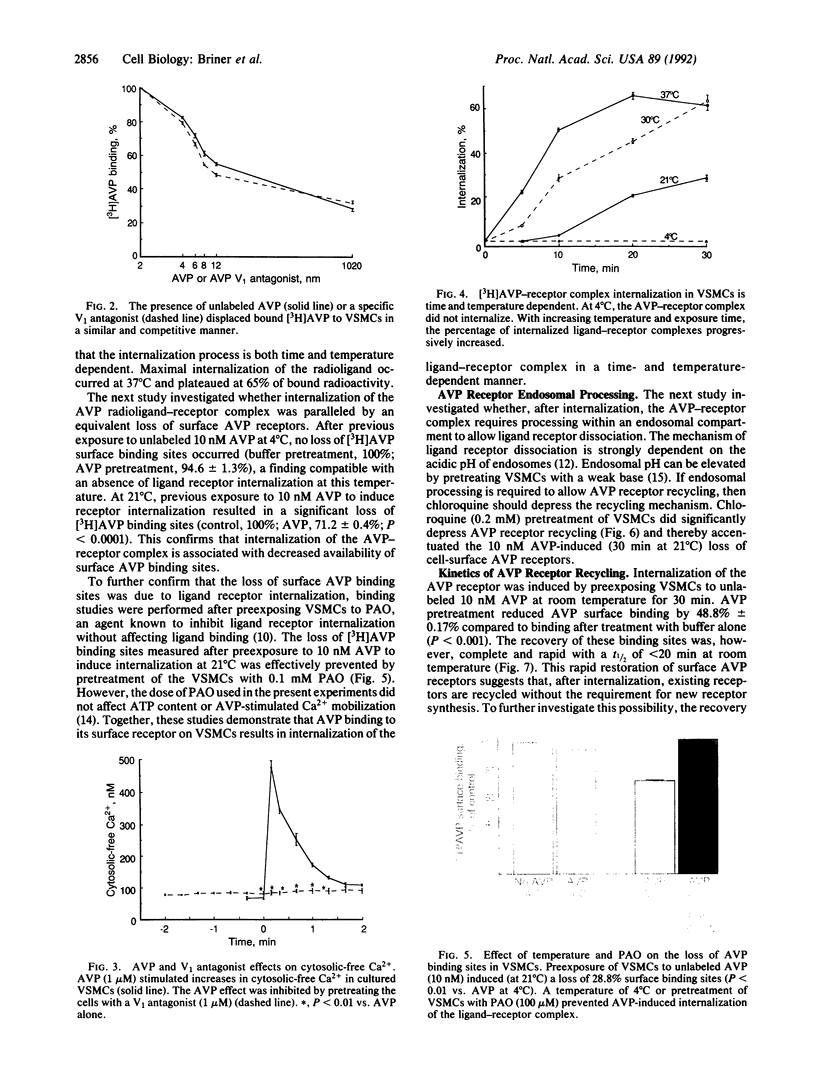
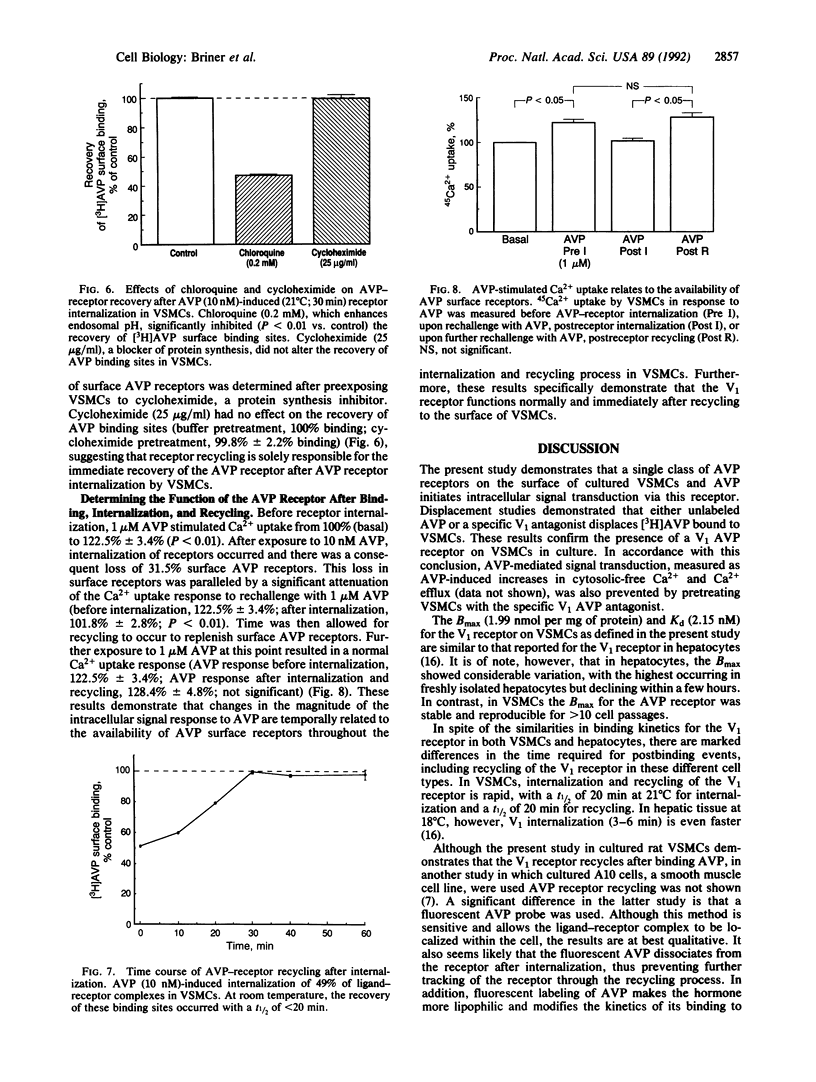
The present study examines the binding and postbinding cellular processing and recycling of the V1 arginine vasopressin (AVP) receptor in cultured vascular smooth muscle cells (VSMCs). The surface binding of AVP to VSMCs was temperature dependent and reached equilibrium within 60 min at 4 degrees C. Displacement studies with unlabeled AVP or a specific V1 AVP antagonist revealed a single class of V1 receptors (Bmax, 1.99 pmol [corrected] per mg of protein; Kd, 2.15 nM). Incubation of VSMCs with unlabeled 10 nM AVP to promote receptor internalization resulted in a time- and temperature-dependent loss of AVP surface binding. At 37 degrees C, maximum loss of binding sites (65%) occurred within 20 min. Recovery of AVP binding occurred rapidly (t1/2, 15-20 min at room temperature) and was uninfluenced by inhibiting protein synthesis with cycloheximide. Pretreating VSMCs with chloroquine prevented AVP receptor recycling, indicating that the AVP-receptor complex requires endosomal processing. The biological competence of the recycled AVP receptor was shown by AVP-induced Ca2+ uptake. The results of these studies therefore indicate that, after surface binding, the AVP-receptor complex internalizes and dissociates in an endosomal compartment. It is demonstrated that in VSMCs biologically active V1 AVP receptors recycle back to the cell surface, thus attenuating the loss of AVP surface binding sites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Rodewald R. Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol. 1981 Oct;91(1):270–280. doi: 10.1083/jcb.91.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramelo C., Tsai P., Okada K., Briner V. A., Schrier R. W. Mechanisms of rapid desensitization to arginine vasopressin in vascular smooth muscle cells. Am J Physiol. 1991 Jan;260(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1991.260.1.F46. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gazzano H., Van Obberghen E., Fehlmann M., Freychet P., Orci L. Intracellular pathway followed by the insulin receptor covalently coupled to 125I-photoreactive insulin during internalization and recycling. J Cell Biol. 1986 Mar;102(3):989–996. doi: 10.1083/jcb.102.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J. B., Dickey B. F., Bucher N. L., Fine R. E. Internalization, recycling, and redistribution of vasopressin receptors in rat hepatocytes. J Biol Chem. 1985 Oct 15;260(23):12641–12646. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lutz W., Sanders M., Salisbury J., Kumar R. Internalization of vasopressin analogs in kidney and smooth muscle cells: evidence for receptor-mediated endocytosis in cells with V2 or V1 receptors. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6507–6511. doi: 10.1073/pnas.87.17.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Lovenberg W., Beaven M. A. Increase in cytosolic calcium and phosphoinositide metabolism induced by angiotensin II and [Arg]vasopressin in vascular smooth muscle cells. J Biol Chem. 1985 Apr 25;260(8):4661–4670. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Boulanger C., Weber E., Bühler F. R. Internalization of endothelin by cultured human vascular smooth muscle cells: characterization and physiological significance. Mol Pharmacol. 1990 Aug;38(2):244–252. [PubMed] [Google Scholar]
- Schrier R. W. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (2) N Engl J Med. 1988 Oct 27;319(17):1127–1134. doi: 10.1056/NEJM198810273191705. [DOI] [PubMed] [Google Scholar]
- Ullian M. E., Linas S. L. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J Clin Invest. 1989 Sep;84(3):840–846. doi: 10.1172/JCI114244. [DOI] [PMC free article] [PubMed] [Google Scholar]