Abstract
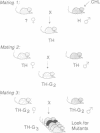
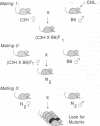
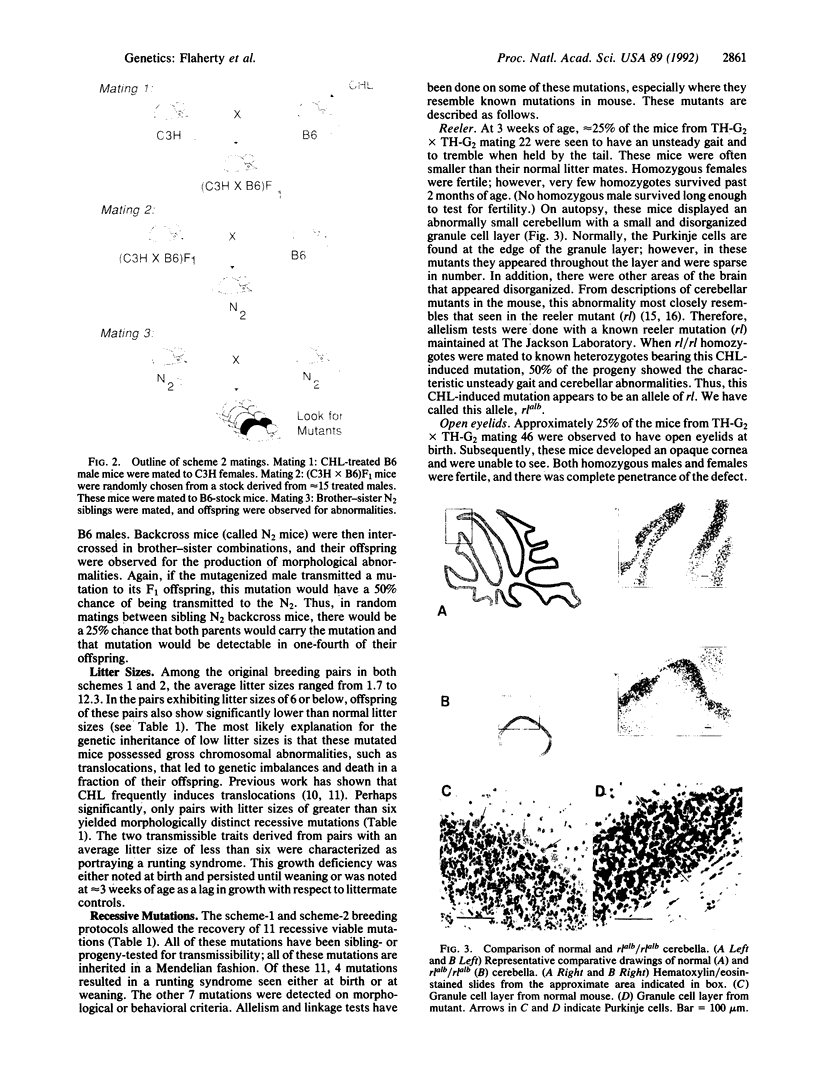
Chlorambucil induced a number of recessive visible mutations in the mouse. Induction of these mutations was studied in two mating schemes, each designed to recover mutations after two intercrosses. In scheme 1, 10 mutations were detected in 82 mice; in scheme 2, 1 mutation was detected in 19 mice. We have estimated that the proportion of gametes carrying a recessive visible mutation may be as high as 25% after a dose of 10 mg of chlorambucil per kg to early spermatids. Seven of these mutations caused morphologically distinct abnormalities, including (i) a cerebellar abnormality similar to that expressed in homozygotes for the reeler (rl) mutation; (ii) open eyelids at birth; (iii) a rostral head hemangioma; (iv) abnormally small spleens, anemia, and umbilical hemorrhages; (v) immobility at birth; (vi) polycystic kidneys; and (vii) a circling behavior. Four additional mutations resulted in growth retardation and a runting syndrome. Because, in earlier studies, all molecularly characterized mutations induced by chlorambucil in poststem cells have proved to be deletions, these recessive visible mutations are probably deletions as well. These mutations may be useful in isolating and characterizing the genes responsible for the observed phenotypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitman T. J., Hearne C. M., McAleer M. A., Todd J. A. Mononucleotide repeats are an abundant source of length variants in mouse genomic DNA. Mamm Genome. 1991;1(4):206–210. doi: 10.1007/BF00352326. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Weiss R., Xu G. F., Viskochil D., Culver M., Stevens J., Robertson M., Dunn D., Gesteland R., O'Connell P. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990 Jul 13;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- Hearne C. M., McAleer M. A., Love J. M., Aitman T. J., Cornall R. J., Ghosh S., Knight A. M., Prins J. B., Todd J. A. Additional microsatellite markers for mouse genome mapping. Mamm Genome. 1991;1(4):273–282. doi: 10.1007/BF00352339. [DOI] [PubMed] [Google Scholar]
- Johnson D. K., Hand R. E., Jr, Rinchik E. M. Molecular mapping within the mouse albino-deletion complex. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8862–8866. doi: 10.1073/pnas.86.22.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Larin Z., Monaco A. P., Lehrach H. Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4123–4127. doi: 10.1073/pnas.88.10.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Niswander L., Yee D., Rinchik E. M., Russell L. B., Magnuson T. The albino-deletion complex in the mouse defines genes necessary for development of embryonic and extraembryonic ectoderm. Development. 1989 Jan;105(1):175–182. doi: 10.1242/dev.105.1.175. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. L. X-ray-induced mutations in mice. Cold Spring Harb Symp Quant Biol. 1951;16:327–336. doi: 10.1101/sqb.1951.016.01.024. [DOI] [PubMed] [Google Scholar]
- Rinchik E. M., Bangham J. W., Hunsicker P. R., Cacheiro N. L., Kwon B. S., Jackson I. J., Russell L. B. Genetic and molecular analysis of chlorambucil-induced germ-line mutations in the mouse. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1416–1420. doi: 10.1073/pnas.87.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M. Chemical mutagenesis and fine-structure functional analysis of the mouse genome. Trends Genet. 1991 Jan;7(1):15–21. doi: 10.1016/0168-9525(91)90016-j. [DOI] [PubMed] [Google Scholar]
- Russell L. B., Hunsicker P. R., Cacheiro N. L., Bangham J. W., Russell W. L., Shelby M. D. Chlorambucil effectively induces deletion mutations in mouse germ cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3704–3708. doi: 10.1073/pnas.86.10.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskochil D., Buchberg A. M., Xu G., Cawthon R. M., Stevens J., Wolff R. K., Culver M., Carey J. C., Copeland N. G., Jenkins N. A. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990 Jul 13;62(1):187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Marchuk D. A., Andersen L. B., Letcher R., Odeh H. M., Saulino A. M., Fountain J. W., Brereton A., Nicholson J., Mitchell A. L. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990 Jul 13;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Welsh J., Petersen C., McClelland M. Polymorphisms generated by arbitrarily primed PCR in the mouse: application to strain identification and genetic mapping. Nucleic Acids Res. 1991 Jan 25;19(2):303–306. doi: 10.1093/nar/19.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. C., Mason J. M., Valencia R., Zimmering S. Chemical mutagenesis testing in Drosophila. V. Results of 53 coded compounds tested for the National Toxicology Program. Environ Mutagen. 1985;7(5):677–702. doi: 10.1002/em.2860070507. [DOI] [PubMed] [Google Scholar]