Abstract
Dendritic polymers are highly branched polymers with controllable structures, which possess a large population of terminal functional groups, low solution or melt viscosity, and good solubility. Their size, degree of branching and functionality can be adjusted and controlled through the synthetic procedures. These tunable structures correspond to application-related properties, such as biodegradability, biocompatibility, stimuli-responsiveness and self-assembly ability, which are the key points for theranostic applications, including chemotherapeutic theranostics, biotherapeutic theranostics, phototherapeutic theranostics, radiotherapeutic theranostics and combined therapeutic theranostics. Up to now, significant progress has been made for the dendritic polymers in solving some of the fundamental and technical questions toward their theranostic applications. In this review, we briefly summarize how to control the structures of dendritic polymers, the theranostics-related properties derived from their structures and their theranostics-related applications.
Keywords: Dendritic polymers, theranostics
Introduction
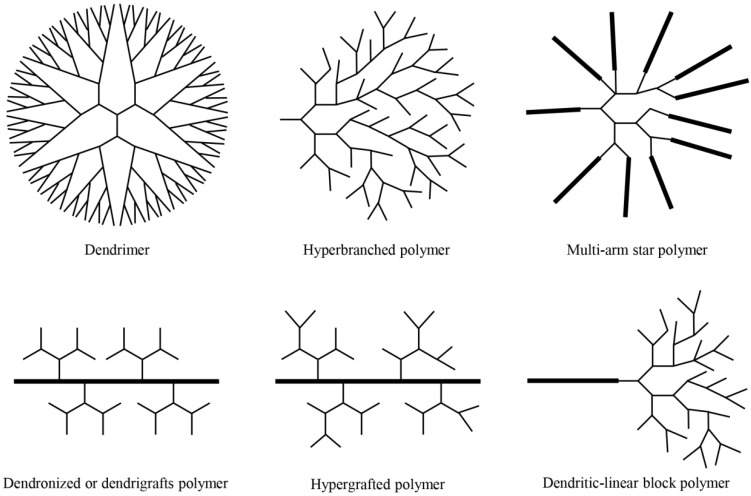
Dendritic polymers are highly branched polymers with three-dimensional architectures, which consist of at least six subclasses: (a) dendrimers; (b) hyperbranched polymers; (c) multi-arm star polymers; (d) dendronized or dendrigrafts polymers; (e) hypergrafts or hypergrafted polymers; (f) dendritic-linear block polymers (Fig. 1) 1. The history of dendritic polymers can be dated back to the end of 19th century, when Berzelius fabricated a hyperbranched resin by tartaric acid and glycerol 1. Subsequently, Flory developed the concept of degree of branching (DB). One of the typical dendritic polymers, hyperbranched polymer, was first coined by Kim and Webster 1; while the other representative, dendrimer, was successfully synthesized and characterized through both divergent and convergent approaches in 1980s 2,3. Since then, numerous research works focus on synthesizing different kinds of dendritic polymers and exploring their applications in diverse areas to promote significant progress in this field.
Figure 1.
Schematic description of six subclasses of dendritic polymers.
The pervasive applications of dendritic polymers can be attributed to their unique chemical/physical properties derived from their three-dimensional dendritic architecture. Compared with linear polymers, dendritic polymers possess a large number of terminal functional groups, low solution or melt viscosity, and good solubility 4. The size, DB and functionality of dendritic polymers can be facilely adjusted and controlled through the synthetic procedures, which could satisfy the diverse requirements of applications. Moreover, amphiphilic dendritic polymers can self-assemble into various delicate supramolecular structures with different morphologies and functions. As two major subclasses of dendritic polymers, dendrimers are perfectly branched and monodisperse, while hyperbranched polymers have a unique advantage of facile one-pot fabrication. Considering their tunable properties and excellent performance, dendritic polymers are promising candidates for various kinds of applications in biological and biomedical fields.
Up to now, some excellent reviews have been published on the bioapplications of dendritic polymers, including drug delivery, gene transfection, protein delivery, bioimaging, biomineralization, tissue engineering, etc. 5. Dendritic polymers are one of the most important research topics nowadays in material science, and are of great significant in biomedical science. Especially, the applications of dendritic polymers in theranostics arouse the great interest of researchers in recent years. Dendritic polymers have exhibited attractive properties including favorable physicochemical properties, excellent biodegradability and biocompatibility, versatile functionalization and smart responsiveness. These advantages pave the way for the diagnosis and treatment of disease. In the past few years, a rapidly increasing number of research works related to the theranostic applications have been reported. Till now, the applications of dendrimers for theranostics have been summarized. However, a systematic review of all kinds of dendritic polymers for theranostic application has not yet been published.
This review summarizes how to control the structures of dendritic polymers, the theranostics-related properties derived from their structures and various theranostic applications. We outline these exciting achievements of dendritic polymers for various kinds of theranostic applications, and hope to inspire continued endeavors in this promising research area.
Synthesis and Structure of Dendritic Polymers
Over the past decades, dendritic polymers, including dendrimers and hyperbranched polymers, have received much attention 1,6. The ways to prepare dendritic polymers are summarized below. For dendrimers, two complementary general routes, the divergent and the convergent, have been widely used in recent years 2. The divergent approach was first proposed by Tomalia and coworkers, which means initiating growth at the core of the dendrimer and continuing outward by repetition of coupling and activation steps 2. In contrast, the convergent growth approach, raised by Hawker and Fréchet, refers to starting from the periphery of the polymer and progressing inward 3. For hyperbranched polymers, they can be prepared by means of single-monomer methodology (SMM) and double-monomer methodology (DMM) 1. By utilizing these synthetic approaches, we can adjust and control the size, DB and functionality of dendritic polymers.
Size
Size is an important parameter for theranostic applications of dendritic polymers. Dendritic polymers with well-defined structure (eg. dendrimers) can meet specific requirement for theranostics. The size of dendrimers is determined by the number of generations. Table 1 presents the hydrodynamic diameter of polyamidoamine (PAMAM) dendrimers from core to generation G = 7, which shows the linear increase in diameter and exponential growth of the number of surface groups with the increase of the generation 7,8. The precisely controlled size of dendrimers through adjusting the number of generations makes them promising materials for researches focusing on the size influence of biomaterials. Besides, due to their defined structure, dendrimers are compatible for reproducible manufacture to meet the batch-to-batch requirements for clinical application of theranostics. While for the dendritic polymers with ill-defined structure (eg. hyperbranched polymers), the size is closely related to their molecular weights. For example, the higher molecular weight is, the larger hydrodynamic volume is for a certain hyperbranched polymer. More importantly, the structure of hyperbranched polymers can be further adjusted via their synthetic procedures. Different from the precisely controlled size of dendrimers, the size of hyperbranched polymers can just be roughly regulated.
Table 1.
| Generation | Molecular formula | Hydrodynamic diameter (nm) |
|---|---|---|
| 0 | C24H52N10O4S2 | 1.5 |
| 1 | C64H132N26O12S2 | 2.2 |
| 2 | C144H292N58O28S2 | 2.9 |
| 3 | C304H612N122O60S2 | 3.6 |
| 4 | C624H1252N250O124S2 | 4.5 |
| 5 | C1264H2532N506O252S2 | 5.4 |
| 6 | C2544H5092N1018O508S2 | 6.7 |
| 7 | C5104H10212N2042O1020S2 | 8.1 |
Degree of branching (DB)
The branching structure of polymers can be described by an important parameter DB. According to the definition of DB, the DB values of linear polymers and dendrimers are 0 and 1, respectively. For hyperbranched polymers, the DB value is between 0 and 1 (normally, 0.4-0.6), which depends on the synthesis routes and can be controlled via different synthesis strategies in a predetermined synthesis route. For polycondensation of ABx monomers in one pot, when X = 2, the maximum DB value is 0.5 at full conversion of A groups. When X = 3, DB value approaches 0.44 at the same conversion 9. DB grows linearly or nearly linearly with conversion of A groups. Besides, polymerization of dendrons having prefabricated dendritic unites can increase DB values. The increase in DB is mainly attributed to the decrease of linear units having one unreacted B group. DB value can also be enhanced via two strategies: polymerization of ABx monomers in the presence of core molecules (Bf) and enhancement of the reactivity of linear units formed during the polymerization 9. The calculated DB value of the self-condensing vinyl polymerization is 0.465. The DB value can be improved by slowly adding monomers 10. For the couple-monomer methodology, the DB value of A2 + CBn approach can be calculated with Eq. (1)
| DB = (D + T)/(D + T + L) ≈ 1/(1 + L/2T) | (1) |
The calculated value ranges between 0.42 and 0.56. If the hyperbranched polymers are made from A2, B2 and BB′2 approach, the DB values can be adjusted at least from 0.5 to 0 by the feed ratio of the monomers (B2 to BB′2) 1,11.
Functionality
Different from traditional linear polymers, dendritic polymers have a large population of terminal functional groups. The number and species of functionalities can be controlled through the reaction process, mole ratio of reactants and post-modification of dendritic polymers. The number of functionalities corresponds to the generation of dendrimers and molecular weight of hyperbranched polymers. For dendrimers, the number of surface group grows exponentially due to their well-defined structure. Besides, different reaction processes relate to different functionalities of hyperbranched polymers. For AA* + Bn approach, hyperbranched polyester with the carboxylic acid-terminated groups or ester-terminated group can be prepared even the original reactants are the same 1. For AA′ + B′B2 and AB + Cn approach, the terminal functional groups can be adjusted by the feed ratio of monomers. When the feed ratio of AA′ to B′B2 is lower than 1/1, in the range of 1/1 and 2/1 or greater than 2/1, products with B groups, both A and B functional groups or A groups can be fabricated, respectively. Yan and coworkers prepared hyperbranched poly(sulfone-amine)s by using different feed ratio of divinyl sulfone (DV) to 1-(2-aminoethyl)piperazine (AP). When the feed ratio of DV to AP was 1/1, the hyperbranched poly(sulfone-amine)s with amino groups were fabricated. When the feed ratio of DV to AP was set at 3/2, the hyperbranched polymers contained both amino and vinyl groups 12. Moreover, the functionalities of dendritic polymers can also be modified through post-modification, such as end-capping and terminal grafting. In the end-capping process, dendritic polymers can be conveniently modified with small organic molecules. Gao and coworkers prepared water-soluble inverse-vulcanized hyperbranched polymers through end-capping by sequential click chemistry of thiol-ene and Menschutkin quaternization reactions 13. Huang and coworkers synthesized hyperbranched PAMAM end-capped by 1-adamantylamine with thermo-/pH-responsive properties 14. Core-shell multi-arm star polymers or hyperstars with tailored properties, such as polarity and solubility, can be prepared through terminal grafting modification. Zhu and coworkers prepared star-conjugated copolymers with a dendritic conjugated core and many linear poly(ethylene glycol) (PEG) arms for real-time monitoring of anticancer drug release 15.
Properties of Dendritic Polymers
The size, DB and functionality of dendritic polymers can be precisely adjusted through the synthetic procedures. These tunable structures correspond to some application-related properties, such as biodegradability, biocompatibility, stimuli-responsiveness and self-assembly ability, which are the key points for theranostic applications.
Biodegradability and biocompatibility
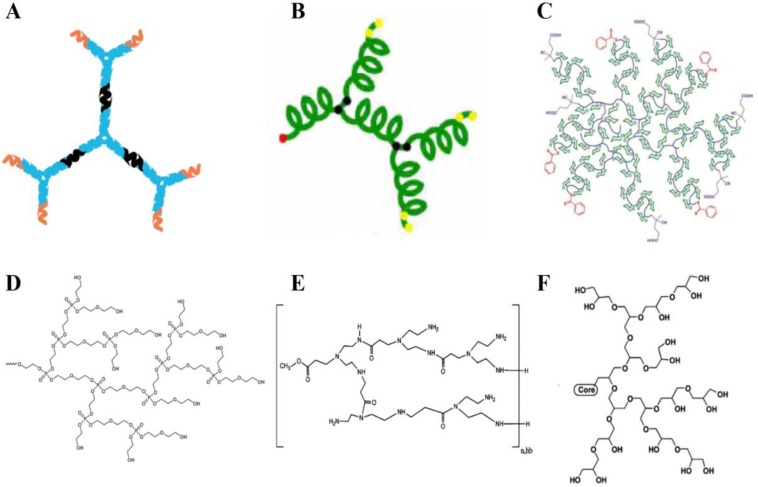
Biodegradability and biocompatibility are of great significance for the bioapplications of dendritic polymers. Especially, in the in vivo applications, biocompatibility is essential during the blood circulation and biodegradability is crucial after they accomplish their mission to avoid serious side effects. So far, many biocompatible dendritic polymers, such as dendritic DNA, dendritic polypeptide, dendritic polyglycerol, dendritic polyphosphate, dendritic polyamide, dendritic polysaccharide, etc. have been constructed by using biocompatible monomers (Fig. 2). Till now, traditional dendritic polymers with biocompatibility have been successfully synthesized. For example, Yan and coworkers prepared hyperbranched polyphosphates, hyperbranched copolyphosphates and functional hyperbranched polyphosphates as drug delivery platforms for cancer therapy 16-20. In addition, with the development of biotechnology, more and more molecules derived from organisms, such as DNA and polypeptide, have been used as building units to fabricate superiorly biocompatible dendritic materials. DNA dendrimer prepared by Yang and coworkers shows excellent biocompatibility 21. Dong and Chang developed a hyperbranched polypeptide-based drug delivery system presenting an α-helix conformation 22. With the rapid development of chemistry and biology, more and more biodegradable and biocompatible dendritic polymers will be prepared for bioapplications.
Figure 2.
Schematic structures of biodegradable or biocompatible dendritic polymers. (a) DNA dendrimer 21, (b) hyperbranched peptide 22, (c) hyperbranched glycopolymer 23, (d) hyperbranched polyphosphate 18, (e) hyperbranched polyamide 24, (f) dendritic polyglycerol 25.
Stimuli-responsiveness
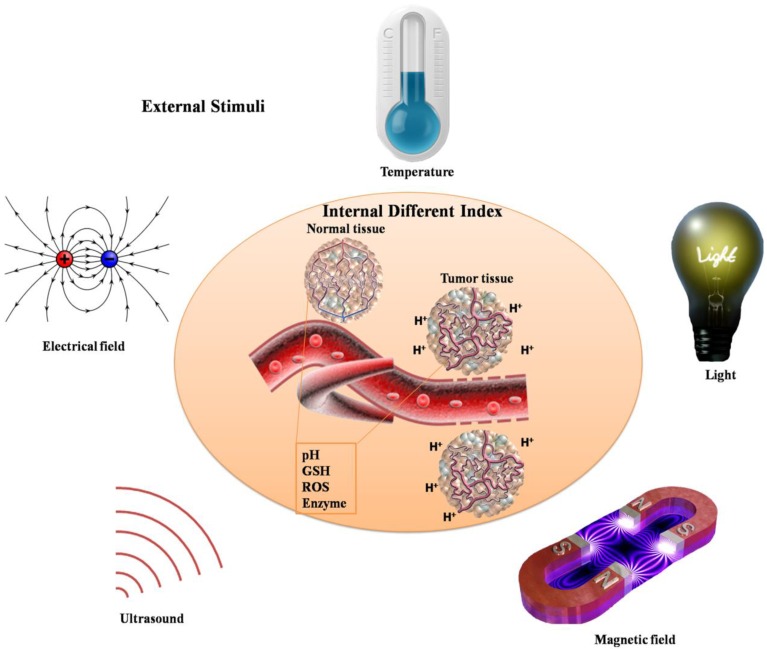
Biomaterials with on-demand responsive properties to release cargoes will lead to significant enhancement of in vitro and in vivo therapeutic efficacy, especially in cancer therapy. For the special tumor microenvironment in vivo, various stimuli are used to stimulate the responsive behaviors, which include extracellular pH, the concentration of glutathione (GSH), oxidative stress, enzyme overexpression, etc. (Fig. 3). Moreover, external stimuli can also be utilized to achieve responsive properties, such as light, magnetic field, electrical field, temperature, ultrasound, etc.. Dendritic polymers can be prepared with stimuli-responsive ability through elaborate design or post-modification. These stimuli-responsive dendritic polymers encapsulated with anticancer drugs will respond to corresponding stimuli rapidly and thus release the drugs with enhanced therapeutic efficacy and weak side effect. Till now, numerous stimuli-responsive dendritic polymers have been designed and synthesized (Table 2). In tumor site, pH-responsive, reduction-responsive, oxidation-responsive and enzyme-responsive dendritic polymers can respond to lower pH (extracellular pH: ~ 6.8), higher concentration of GSH (in the cytosol: ~10 mM), enhanced intrinsic oxidative stress, and elevated enzyme expression of tumor tissue, respectively. Zhu and coworkers described a drug carrier, PEGylated hyperbranched polyacylhydrazone through a pH-responsive acylhydrazone linkage, which could self-assemble into nanoscale micelles as anticancer drug carriers with pH-controlled drug release 26. Besides, Yan and coworker synthesized a novel kind of amphiphilic hyperbranched multiarm copolyphosphates with disulfide bonds in the backbone, which was used to construct a nanosized redox-responsive drug delivery system 20. Degradation of biomaterials can be triggered by enzymes, which is also very interesting. Shabat and coworkers came up with novel self-immolative dendritic prodrugs by catalytic antibody 38C2 27. Furthermore, delicate design will endow dendritic polymers with external stimuli-responsive ability. Almost all kinds of light including ultraviolet light (UV), visible light and near-infrared light can be used to construct stimuli-responsive dendritic polymers. Even temperature changes will lead to responsive behaviors of dendritic polymers. But few works focus on magnetic field, electrical field or ultrasound responsive dendritic polymers. Moreover, in contrast to the abovementioned single stimuli-responsive dendritic polymers, multi-stimuli responsive ones can simultaneously respond to multiple external stimuli corresponding to the complicated physiological environment in vivo, thereby exhibiting great potential in clinical theranostics.
Figure 3.
Schematic illustration of various stimuli including internal different index and external stimuli.
Table 2.
Examples of stimuli-responsive dendritic polymers
| Stimuli | Responsive moiety | Reference | |
|---|---|---|---|
| Internal stimuli | pH | Acylhydrazone | [26] |
| Phenylboronate | [28] | ||
| Hydrazide | [29] | ||
| Acetal | [30] | ||
| Reduction | Disulfide | [20,31] | |
| Diselenide | [32] | ||
| Oxidation | Selenide | [33,34] | |
| Enzyme | Enzyme substrate | [27,33,35] | |
| External stimuli | Light | Spiropyran | [33,36] |
| Azobenzene | [37, 38] | ||
| Aminomethylpyrene | [39] | ||
| Temperature | PDMAEMA, | [40] | |
| PEGMEMA-PPGMA-EGDMA | [41] | ||
| PNIPAM | [42] | ||
| Collagen | [43] | ||
| Magnetic field | Magnetic nanoparticles | [44] | |
| Multi-stimuli | Combination | Combination | [14,45-47] |
Note: Poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA). A copolymer composed of poly(ethylene glycol) methyl ether methylacrylate (PEGMEMA) copolymerized, poly(propylene glycol) methacrylate (PPGMA) and ethylene glycol dimethacrylate (EGDMA), (PEGMEMA-PPGMA-EGDMA).
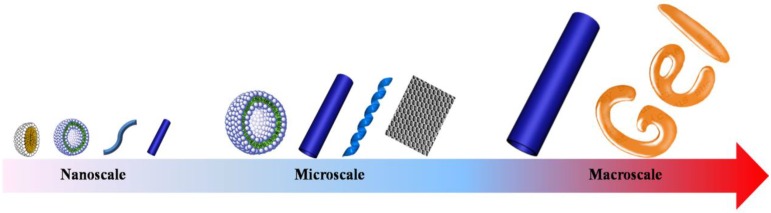
Self-assembly
Similar to the self-assembly of amphiphilic linear copolymers, dendritic polymers can also self-assemble into various morphologies with different size scales including nanoscale, microscale and even macroscale (Fig. 4). Since Zimmerman and coworkers' pioneering research on self-assembly of dendrimers through hydrogen bond 48, the self-assembly of dendrimers has experienced a rapid development in the past few decades. In 2004, Yan and coworkers observed the macroscopic self-assembly of amphiphilic hyperbranched polymers in acetone for the first time, in spite of their irregular structures 49. From then on, numerous works focusing on self-assembly of hyperbranched polymers through various interactions such as host-guest interaction, hydrogen bonding interaction or electrostatic adsorption have been reported. The topological structures of assemblies can be controlled through the self-assembling process.
Figure 4.
Schematic illustration of various topological structures self-assembled from dendritic polymers with different size scales.
These controllable topological structures consist of micelles 50-52, vesicles 53-57, nanofibers 52, nanometer/micrometer-sized tubes 58,59, giant vesicles 60,61, ribbons 62,63, films 64,65, macroscopic tubes 49, gels 66, etc.. For instance, the hyperbranched poly[3-ethyl-3-(hydroxymethyl)oxetane]-star-poly(propylene oxide) (PEHO-star-PPO) prepared by Yan and coworkers can aggregate into large regular spherical micelles with controlled size via the alteration of the molar ratios of PPO arms to PEHO core 50. Dendritic polymers can also self-assemble into vesicles with narrow size distribution. As reported by Zhou and coworkers, unilamellar bilayer vesicles were obtained through a Janus hyperbranched polymer constructed by the host-guest interactions between a hydrophobic hyperbranched poly(3-ethyl-3-oxetanemethanol) with an apex of an azobenzene (AZO) group and a hydrophilic hyperbranched polyglycerol with an apex of a β-cyclodextrin (CD) group 56. The unimolecular micelles could further self-assemble into thread-like nanofibers 52. Moreover, the size of giant polymer vesicles can be easily controlled by adjusting the hydrophilic fraction of the copolymer 60. In addition, supramolecular architectures, such as micrometer-sized tubes and ribbon can be obtained through rational molecular design. Molecular design is a crucial parameter for ionic bent-core dendrimers to control the morphology of the aggregation 62. Beyond these microscopic topological structures, macroscopic tube and gel were made by using different dendritic polymers 49,66.
Theranostic applications
As mentioned above, by controlling their structure and functional groups, dendritic polymers have exhibited specific properties and versatile functions, such as stimuli-responsive ability and self-assembly behavior, which makes them excellent candidates for theranostic applications. In this section, we present the advanced progress of dendritic polymers for theranostics, including chemotherapy, biotherapy, phototherapy, radiotherapy, and combined therapy. These theranostic systems usually refer to various modes of imaging, for example, magnetic resonance imaging (MRI), computed tomography (CT), nuclear imaging and fluorescence imaging.
Chemotherapy
At present, drug delivery systems based on dendritic polymers have been widely reported for theranostics, which could improve therapeutic efficacy and reduce side effects of the parent drug 67. Rational design of dendritic polymer-based delivery systems can meet the criteria of theranostics. Firstly, dendritic polymers have a large amount of cavities and surface functional groups which are suitable for loading or conjugating drugs, targeting ligands and imaging agents to construct theranostic platforms. Secondly, appropriate surface modifications of dendritic polymers facilitate the development of stealth nano-delivery systems with minimal non-specific interaction between cells or blood-proteins, which could improve pharmacokinetic properties. Thirdly, structural control over the shape and size of dendritic polymers can enhance the bioavailability of drugs. For example, nano-delivery theranostic systems (20-200 nm) could passively target solid tumors via the enhanced permeability and retention (EPR) effect. Finally, by controlling the dendritic polymer/drug interaction (such as association, complexation and encapsulation), various stimuli-responsive systems can be achieved. In order to monitor the drug delivery process and evaluate the therapeutic efficacy, theranostic platforms based on various modes of imaging have been developed in recent years. Dendritic polymers have exhibited unique advantages in the theranostic applications because of their highly branched architectures, numerous terminal functional groups, adequate spatial cavities and versatile functionalization, which could combine imaging and therapeutic function together. To date, numerous researches have been reported, focusing on dendritic polymer-based theranostic systems with combined chemotherapy with various imaging modalities such as MRI, CT, nuclear imaging and fluorescence imaging.
Due to its high spatial resolution and deep tissue penetration, MRI is one of the most powerful imaging techniques for theranostics 68. In addition, the overriding shortcoming for MRI, relatively low sensitivity, can be overcome by introducing MRI contrast agents (CAs) 69,70, such as T1-weighted CAs (gadolinium complex 71-73, manganese complex 74, etc.) or T2-weighted CAs (iron oxide particles 75, etc.). Nevertheless, most small molecular weight CAs suffer from low contrast enhancement, non-specificity, and fast renal excretion, which severely limits the application of these materials for molecular MRI. One important approach to increase the contrast and prolong blood circulation is to attach MRI CAs into a certain polymer scaffold. As an important subclass of polymers, dendritic polymers are optimal materials to provide large numbers of surface functional groups to attach MRI CAs. Therefore, dendritic polymer-based delivery system is suitable for the development of integrated platform loading MRI CAs and drugs with combined imaging and therapeutic function to monitor the drug delivery process. For instance, Liu and coworkers fabricated hyperbranched polymeric micelles for cancer targeted drug delivery and MR imaging 76. Derived from the hyperbranched core, amphiphilic multi-arm star block copolymer was obtained, in which the anticancer drugs were encapsulated in the hydrophobic layer and hydrophilic MRI CA was conjugated in the outer corona. This unimolecular micelle theranostic system synergistically integrated cancer drug delivery with MRI function.
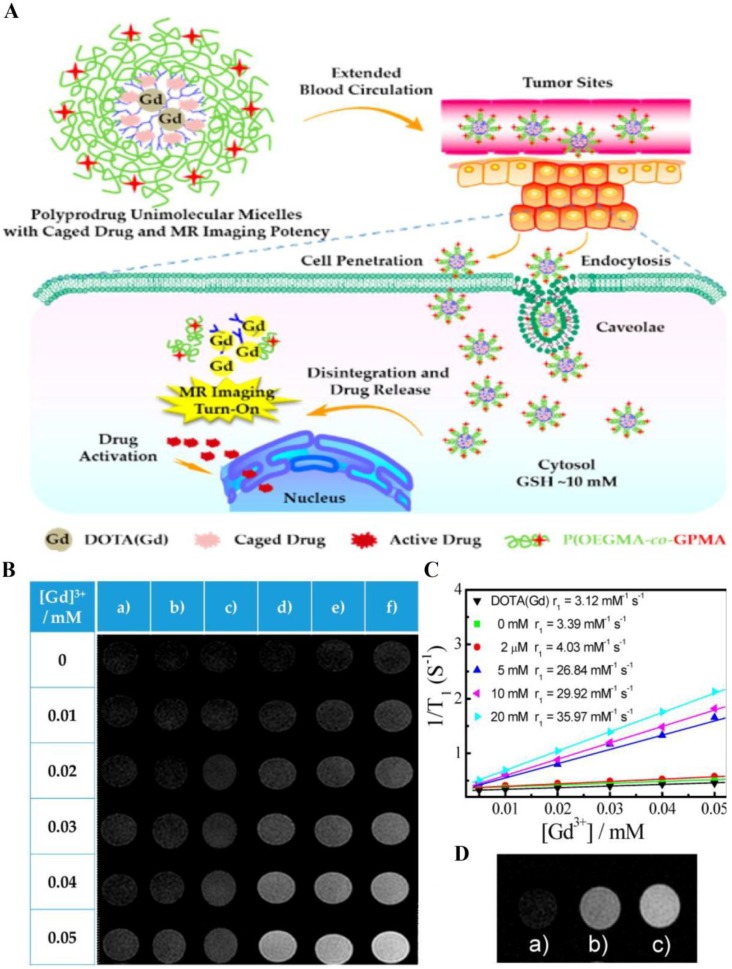
Besides, rational design of dendritic polymeric delivery system can improve the sensitivity of CAs, resulting in prominent changes in MRI signal when responding to the external pathological milieu. These responsive theranostic systems are widely reported for monitoring both drug release and therapeutic feedback. For example, Liu and coworkers constructed self-reporting theranostic system based on MRI, which exhibited excellent cell-penetrating ability, tumor milieu-actuated drug release property and responsive turn-on of MRI signal (Fig. 5) 77. This system was composed of hyperbranched hydrophobic cores carrying anticancer drug camptothecin (CPT) and MRI CA (gadolinium complex), along with hydrophilic coronas modified with guanidine residues. Guanidine-functionalized surface contributed to prolonged blood circulation time and enhanced tumor cell penetration efficiency. After internalization, the reductive milieu in cancer cell accelerated the drug release and induced a turn-on MRI signal. The dendritic polymeric delivery platform provides a framework to achieve synergistic imaging/chemotherapy and enhanced tumor uptake.
Figure 5.
(A) Schematic illustration of polyprodrug unimolecular micelles with hyperbranched cores conjugated with gadolinium complex, reductive milieu-cleavable camptothecin prodrugs and hydrophilic coronas functionalized with guanidine residues. (B) T1-weighted spin-echo MR images of (a) small molecule alkynyl-DOTA(Gd) (DOTA, tetraazacyclododecanetetraacetic acid) complex and hyperbranched polyprodrug amphiphilies (HPA) after incubating with various concentrations of DL-dithothreito (DTT) for 12 h. (C) Water proton longitudinal relaxation rates (1/T1) of small molecule alkynyl-DOTA(Gd) complex and HPA after treating with DTT (0-20 mM). (D) MR images recorded for (a) untreated HepG2 cells, (b) HepG2 cells treated with HPA for 12 h, and (c) HepG2 cells pretreated with 10 mM GSH-OEt (GSH reduced ethyl ester) for 2 h to elevate intracellular GSH level, and then coincubated with HPA for 12 h. Reprinted with permission from ref. 77. Copyright 2015, American Chemical Society.
CT is another widely used anatomical imaging modality with excellent tissue penetration and high spatial resolution 78. However, considering the imaging principle of measuring the X-ray beam attenuation through the body, CT obtains poor soft-tissue contrast and hence CAs are necessary to complete the mission of theranostics, such as measuring therapy response and monitoring therapeutic process 79. CT CAs must have high atomic number, including iodinated compounds 80, barium complex 81, gold nanoparticles (Au NPs) 82, and so on. Through encapsulation or conjugation, dendritic polymers are promising delivery vehicles for carrying CT CAs along with drugs. Shi and coworkers reported a dendrimer-entrapped Au NPs conjugated with anticancer drug α-tocopheryl succinate (α-TOS) as a multifunctional platform for targeted cancer CT imaging and therapy 83. The α-TOS and targeting ligand folic acid (FA) were covalently linked to the amine-terminated dendrimer, which was afterward used as templates to synthesize and encapsulate Au NPs. This system displayed effective targeted CT imaging as well as specific therapy effect in vitro and in vivo.
Nuclear imaging techniques, such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET), are powerful tools to quantify the distribution of radioactive compounds in the whole body with high penetration depth 84. As mostly performed molecular imaging, SPECT detects gamma rays during radioisotope compound (99mTc, 111In, 123I, 177Lu etc.) decay process, and PET counts the photons produced by the isotopes (11C, 18F, 64Cu, 68Ga, et al.). However, most of these radioactive compounds are small molecules, which may undergo rapid renal excretion and possess non-specificity in vivo. To solve these problems, dendritic polymers can be utilized to deliver radioactive compounds along with anticancer drugs to form theranostic nanodevices 85,86. Gong and coworkers fabricated unimolecular micelles self-assembled from hyperbranched amphiphilic block copolymer for cancer-targeted drug delivery and PET imaging 87. This polymeric nanoplatform consisted of hyperbranched core Boltorn H40 (a 4th generation hyperbranched polyester), hydrophobic segments poly(L-glutamate) conjugated with anticancer drug doxorubicin (DOX) via pH-labile hydrazone bond, and hydrophilic shell PEG with targeting ligand cyclo(Arg-Gly-Asp-D-Phe-Cys) peptides (cRGD) and PET probe 64Cu. The uniform-sized unimolecular theranostic micelles exhibited high cancer cell uptake ability as well as pH-sensitive drug release property. In tumor-bearing mice, these unimolecular micelles achieved high tumor accumulation confirmed by the PET imaging and ex vivo fluorescence imaging. In this theranostic system, non-invasive PET facilitated quantitative measurement of tumor-targeting efficiency and in vivo biodistribution, which would benefit for personalized therapy.
For its high sensitivity and spatial resolution, fluorescence imaging has been widely used in diagnostics and therapy 88. There are various kinds of fluorescence probes, such as organic fluorescent dyes 51,89,90, inorganic fluorescent agents (quantum dots 91,92, silicon NPs 93, carbon dots 94 and up-conversion NPs 95). Nevertheless, the bioapplication of small molecular organic fluorescent probes has been hampered by some shortcomings, such as short blood circulation time, lack of specificity and poor membrane permeability. On the other hand, some inorganic probes possess concerns for biocompatibility and specificity. To overcome these problems, dendritic polymers have been utilized to be the delivery vehicles or surface-modified layer to improve the imaging performance of fluorescence probes. Considering the numerous cavities and terminal functional groups, dendritic polymers are also excellent candidates for the construction of theranostic systems combining chemotherapy with fluorescence imaging. Till now, a large number of researches focusing on this field have been reported 96-100. As one example, Baker and coworkers developed PAMAM dendrimer-based multifunctional system combining fluorescence imaging and chemotherapy 101. Besides, Radosz and coworkers fabricated a linear-dendritic polymer-based nano-drug delivery system, which achieved tunable shape and size corresponding to the dendritic structure of polymers 96. Subsequently, the authors evaluated the biodistribution and tumor targeting effect of these nanostructures with different shape via fluorescence imaging.
Generally, fluorescence imaging faces severe challenges in clinical use: high background autofluorescence and limited depth penetration. Nevertheless, near-infrared (NIR) imaging probes could overcome these problems and have received great attention. NIR imaging possesses enhanced light penetration depth through living tissues because of reduced absorbance by tissue pigments and hemoglobin in the NIR region (700-900 nm) 102. Hence, NIR imaging has high sensitivity and offers a unique advantage for bioimaging application. However, many NIR imaging probes still suffer from the low solubility and non-specificity. Dendritic polymers can be designed rationally to deliver NIR imaging probes along with targeting ligand or therapeutic drugs, paving the way for NIR-based theranostic systems. For example, Zhuo and coworkers fabricated a site and time dual-controlled chemotherapeutic system utilizing a linear-hyperbranched polymer. This dendritic polymer was composed of hyperbranched diol-enriched polycarbonate and linear PEG. This nanostructure self-assembled from the linear-hyperbranched polymer was stable in simulated physiological conditions, and could accumulate in tumor site as revealed by NIR imaging technique 103. After cell internalization, this nanostructure underwent a pH-labile destruction in response to acidic microenvironment and simultaneously released the loaded drugs in cancer cells.
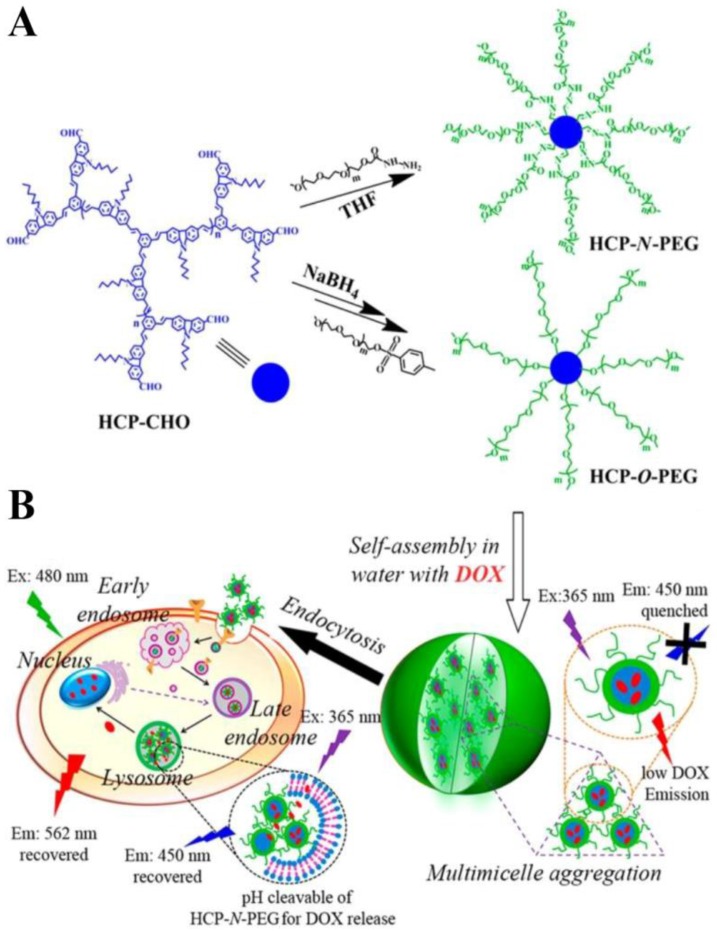
Fluorescent imaging can also give information about intracellular drug release kinetics. Calderón reported a fluorescence resonance energy transfer (FRET)-based theranostic macromolecular prodrug (TMP) 104. TMP was constructed from dendritic polyglycerol (PG), in which DOX and indodicarbocyanine dye (IDCC) were conjugated via pH-sensitive hydrazone. The fluorescence of DOX was quenched due to the FRET effect between IDCC and DOX. When the hydrazone bond was cleaved at acidic pH in cancer cells, the fluorescence of DOX was recovered. The intracellular drug release could be monitored in real time for quantitative analysis. In addition, dendritic polymers can be employed as optical imaging agents for themselves to trace drug release process. For instance, Zhu and coworkers developed a versatile fluorescent polymer-based real-time monitoring system for intracellular drug release (Fig. 6) 15. The star-conjugated copolymer was synthesized from hyperbranched conjugated polymer (HCP) core and linear PEG arms. The amphiphilic copolymer could further self-assemble into NPs in aqueous solution, which could encapsulate DOX, resulting in fluorescent quenching of copolymer and DOX. In vitro biological studies revealed that drug release in cancer cells could lead to fluorescent intensity activation of both star-conjugated copolymer and DOX. Hence, the fluorescent star-conjugated dendritic copolymer was hopeful to serve as delivery system, which could monitor the drug release process in real time to treat diseases.
Figure 6.
(A) Synthesis route of HCP-N-PEG (pH-responsive polymer, in which HCP and PEG were conjugated with acyldydrazone linkage) and HCP-O-PEG (non-responsive polymer, in which HCP and PEG were conjugated with ether linkage) star-conjugated copolymers. (B) Self-assembly of star-conjugated copolymer and their endocytosis in the tumor cells. (C, D) Time-dependent fluorescence microscope images of MCF-7 cells incubated with DOX-loaded HCP-N-PEG (C) and HCP-O-PEG (D) micelles. Reproduced with permission from ref. 15. Copyright 2014, American Chemical Society.
Biotherapy
In recent years, biotherapy has emerged as one of the most promising therapeutic methods. Compared with the abovementioned physically or chemically based therapies, biologically based therapy is more selective. Biotherapy utilize biological agents such as nucleic acids (DNA, RNA), proteins or peptides (eg. monoclonal antibodies, tumor antigens, cytokines), cells (eg. activated killer cells, immune T cells, mesenchymal stem cells etc.) and others to treat disease 105. It can be a therapy of targeting the disease site or stimulating the body response such as immune response. Here, we present some remarkable advances in dendritic polymer-based theranostic systems for biotherapy, such as gene therapy, protein therapy and cell therapy, which are promoted by various methods of imaging modalities.
The principle of gene therapy is transferring genetic materials into specific cells to treat disease 106. The therapeutic genes can regulate the amount of proteins, adjust existing gene expression, or generate cytotoxic proteins. To deliver the nucleic acids to target sites effectively and safely, various kinds of gene vectors have been developed, including viral and nonviral vector systems. Compared to viral vectors, synthetic nonviral vectors are safer and more flexible to be designed and manufactured, including cationic lipids, polymers, peptides, and so on 107. These vector systems must overcome a series of obstacles in vivo: 1) The gene delivery system should be chemically and physically stable during blood circulation to protect nucleic acid from nuclease degradation and serum albumin adsorption. 2) Cell-specific targeting and efficient internalization are absolutely necessary for the delivery system to transfer genes into disease cells. 3) After internalized by endocytosis, endolysosomal escape and gene release from vectors play a key role in therapy. Considering these obstacles, synthetic nonviral vectors may suffer from insufficient gene-transfer efficiency. Nevertheless, cationic dendritic polymers, such as PAMAM and poly(propyleneimine) (PPI), can be afforded to complex nucleic acids through electrostatic interactions and be optimized by chemical modification to increase the transfection efficacy apparently 108,109. Moreover, dendritic polymers can also encapsulate imaging agents through complexation or conjugation to help visualize and evaluate the therapy process.
For instance, in order to navigate biological obstacles, Minko and coworkers caged the PPI dendrimers/siRNA complex with a dithiol-contained cross-linker molecules and PEG followed by targeting ligands modification and fluorescent label 110. The strategy of layer-by-layer protection and targeting approach provided the vector system with enhanced stability in plasma and specific accumulation in tumor cells. Therefore, in vivo distribution and optical imaging results confirmed that the gene delivery system had high specificity to tumor tissues.
Apart from cancer, dendritic polymer-based gene delivery system can treat other diseases 111. As one example, Jiang and coworkers prepared gene nanovehicles crossing the blood-brain barrier (BBB) to fight against cerebral ischemia reperfusion injury 112. This theranostic platform was achieved by decorating dendrigraft poly-L-lysine (DGL) with dermorphin (a μ-opiate receptor agonist) through PEG. After loading anti-Ask1 shRNA plasmid DNA, these brain-targeted nanoparticles exhibited increased accumulation in brain according to the fluorescent images. This nano-delivery system revealed high transfection efficiency and excellent neuroprotection ability, which was useful for the treatment of cerebral ischemia reperfusion injury. Moreover, other imaging modalities, for instance, MRI 113-115 and SPECT 116 are also widely used in gene therapy-based theranostic systems, benefiting from their high penetration ability.
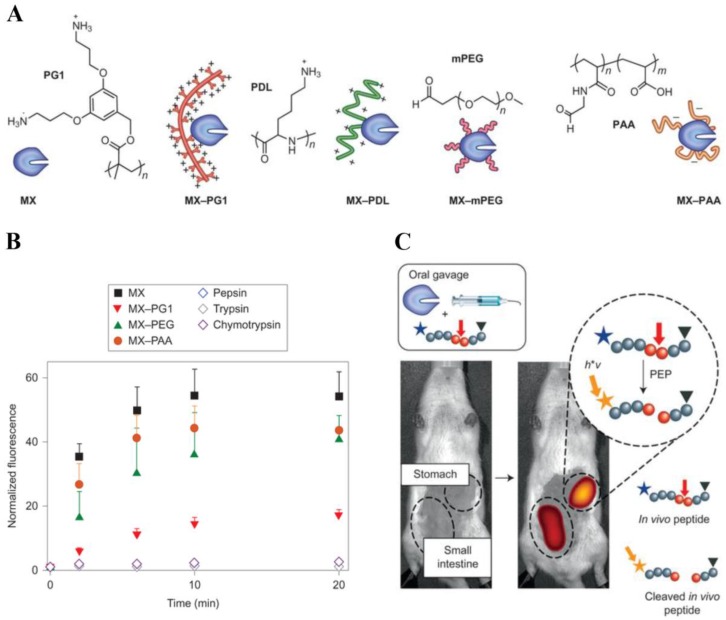
Over the past decades, due to their high specificity and activity, therapeutic proteins have tremendous impacts in the treatment of various disorders, such as cancer, autoimmune and metabolic disease 117. However, the effective use of proteins is hampered by these limitations: 1) The sensitive and flexible proteins are easy to be degraded by enzyme in the body. 2) Proteins may undergo rapid clearance via kidney excretion or uptake by non-target organs during systemic circulation. 3) Protein therapeutics may result in immune response. To overcome these problems, dendritic polymers have been widely used in protein delivery systems to improve its stability and circulation time as well as decrease its immune response 118. For example, Leroux and coworkers developed the strategy of polymer-enzyme conjugation to stabilize and retain the enzyme activity in gastrointestinal tract after oral administration (Fig. 7) 119.
Figure 7.
(A) Chemical structures of the four polymers examined: a cationic dendronized poly-(3,5-bis(3-aminopropoxy)benzyl)-methacrylate (PG1), cationic ɑ-poly(D-lysine) (PDL), neutral methoxy PEG (mPEG) and anionic poly(acrylic acid) (PAA). (B) The uncleaved peptide substrate was incubated in vitro with MX, MX-polymer conjugates or endogenous enzymes. All MX-polymer conjugates induced a significant increase in fluorescence intensity after peptide cleavage. (C) The activity of the individual MX-polymer conjugates was measured using an in vivo fluorescence assay. Reproduced with permission from ref. 119. Copyright 2013, Nature Publishing Group.
They chose architecturally and functionally diverse polymers to protect model enzyme Myxococcus xanthus (MX), and use fluorescence imaging in vivo to monitor the enzyme activity in stomach after polymer protection. A fluorescence-quenched peptide probe was used as the enzymatic substrate and fluorescence recovery was quantified by in vivo imaging to assess the location and activity of MX proteolysis. Using this approach, they found that conjugation to a polycationic dendronized poly-(3,5-bis(3-aminopropoxy)benzyl)-methacrylate was a highly efficient method to sustain gastrointestinal activity.
The use of cell therapy has been further expanded in recent years 120,121. For example, mesenchymal stem cells (MSCs) are multipotent stem cells for the treatment of various diseases, such as liver cirrhosis 122, amyotrophic lateral sclerosis 123 and Parkinson's disease 124. The therapeutic effect of stem cells can be evaluated by monitoring cell distribution, survival and function after transplantation. Several imaging modalities have been introduced into cell therapy system, such as optical imaging, MRI and nuclear imaging 125,126. For example, Hashida and coworkers chose optical imaging with QDs to label MSCs and monitor their in vivo distribution 127. The QDs were modified with PAMAM dendrimer, which facilitated cellular internalization through electrostatic interaction. In addition, PAMAM dendrimer enhanced endosomal escape into cytoplasm, which could solve the dilemma of fluorescence quench in acidic endosomal environment. Hence, PAMAM facilitated cellular uptake and promoted endosomal escape, which was beneficial for tracking MSCs via QDs in vivo.
Phototherapy
As a noninvasive clinical approach, phototherapy is emerging as one of the most promising therapeutic methods for the treatment of cancer 128 and dermatosis 129 for its high efficiency and low side effect. Phototherapy is based on photoactive agents illuminated with specific light to induce cell apoptosis or death. Meanwhile, the photoactive drug can also emit fluorescence under light excitation to endow the system with theranostic ability easily. According to the therapeutic mechanism, phototherapy can be classified into two major categories: photodynamic (PDT) 130 and photothermal (PTT) 131.
PDT utilizes nontoxic photosensitizer (eg. porphyrins), which can generate cytotoxic reactive oxygen species (ROS) after light activation to treat disease 130,132. Nevertheless, clinical application of PDT is mainly limited by the poor water solubility, aggregation tendency and non-selectivity of photosensitizers. To solve these problems, the dendritic polymers have been used to deliver hydrophobic photosensitizer to disease site. As one example, Taratula and coworkers designed a theranostic dendrimer platform loading phthalocyanines (Pc) to achieve fluorescence image-guided drug delivery and noninvasive cancer treatment of PDT 133. They chose a 4th generation (G4) PPI dendrimer to physically encapsulate Pc molecules with a hydrophobic linker. In order to improve the tumor-targeting ability and biocompatibility, the Pc-PPIG4 complexes were modified with PEG and luteinizing hormone-releasing hormone (LHRH) peptide on the surface. This formulation exhibited low dark cytotoxicity as well as prominent PDT effect and fluorescence imaging ability. In addition, other imaging technologies, such as MRI, are also widely used for PDT-based theranostic system 134.
As another category of phototherapy, PTT is based on light absorbing agents (eg. gold nanomaterials) to transform the energy into heat generating localized hyperthermia 131,135. However, some of these photothermal agents suffer from biocompatibility concern. For instance, the clinical use of gold nanomaterials is hampered by the toxicity of surfactant cetyltrimethylammonium bromides (CTAB) on their surface to stabilize these nanomaterials. Therefore, Cui and coworkers applied biocompatible PAMAM dendrimer to replace CTAB on the surface of gold nanorods and introduced targeting ligand (Arg-Gly-Asp-D-Phe-Cys) peptides (RGD) into the system 136. The results demonstrated that gold nanorods modified with RGD-conjugated dendrimer provided an excellent theranostic platform for tumor targeting, imaging and selective photothermal therapy.
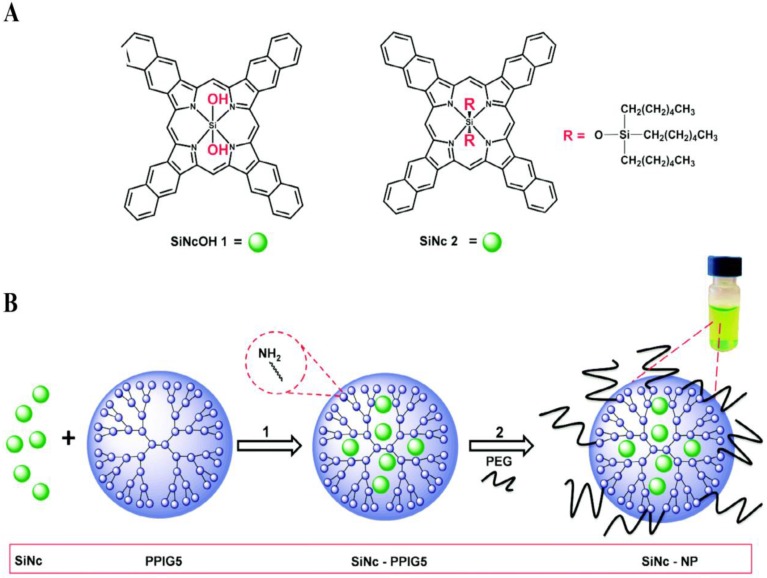
Considering that PDT and PTT act via different mechanisms, their rational combination may overcome the drug resistance, improve the therapeutic efficiency, and decrease the dosage-limiting toxicity 137. Taratula and coworkers integrated the two therapeutic methods into one theranostic nanoplatform using a single agent silicon naphthalocyanine (SiNc) (Fig. 8) 138. They used a generation 5 (G5) PPI dendrimer to encapsulate SiNc to provide aqueous solubility and protect its NIR fluorescence, PDT and PTT properties. This method impressively increased the photostability of SiNc during the therapy procedure. By regulating the laser, they can switch the therapeutic modality and efficiently eradicate chemotherapy resistant ovarian cancer cells.
Figure 8.
(A) Chemical structures of the parent silicon naphthalocyanine. (B) Schematic illustration of the fabrication of SiNc-loaded theranostic nanoplatform. Reproduced with permission from ref. 138. Copyright 2015, Royal Society of Chemistry.
Radiotherapy
Radiotherapy is one of the most commonly used nonsurgical therapeutic approach, which applies radiosensitizers (protons, neutrons, etc.) to kill cells at the disease site under ionizing radiation 139,140. The ultimate goal of radiotherapy is to induce greatest damage to the lesion as well as slightest injury of normal tissue. However, this goal is primarily limited by the difficulties in delivering radiosensitizers to the disease site efficiently and specifically 141. Several solutions based on developing radiosensitizers have been proposed to find a way out of this dilemma: 1) Targeted delivery of therapeutic agents to the lesion by a biocompatible and reasonable platform. 2) Utilization of theranostic system to precisely define the targeting efficiency and supervise drug distribution. As an ideal delivery platform, dendritic polymers can play a critical role in the optimization of radiotherapy due to the fact that the targeting ligands and imaging agents can be easily introduced into the backbone or cavity of dendritic polymers 142-147.
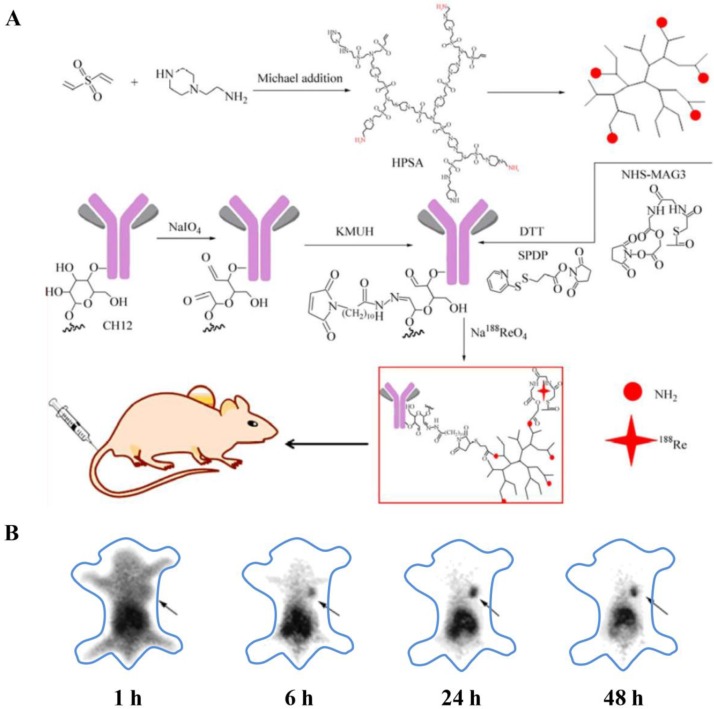
For instance, Zhu and coworkers fabricated 188Re-labeled hyperbranched polysulfonamine (HPSA) aiming to develop a targeted cancer diagnosis and radioimmunotherapy system (Fig. 9) 148. HPSA was modified with targeting ligands, monoclonal antibody CH12, which selectively recognized epidermal growth factor receptor vIII (EGFRvIII). In addition, HPSA was conjugated with N-hydroxysuccinimidyl S-acetylmercaptoacetyltriglycinate (NHS-MAG3) for labeling 188Re to perform SPECT imaging and radioimmunotherapy. This CH12-HPSA-188Re theranostic system could effectively and specially target EGFRvIII-positive human hepatocarcinoma cells and thus facilitate both tumor detection and targeted radioimmunotherapy.
Figure 9.
(A) Synthetic route of CH12-HPSA-188Re. (B) The biodistribution of CH12-HPSA-188Re in nude mice was determined by SPECT. The gamma images were acquired at 1, 6, 24, 48 h after tail vein injection, respectively. (Black arrow: tumor area). Reproduced from ref. 148.
For another example, Backer and coworkers developed boronated dendrimers, VEGF-BD/Cy5, which contained Cy5 and targeting ligands, for NIR imaging and potential boron neutron capture therapy (BNCT) 149. A fifth-generation (G5) PAMAM dendrimer was utilized in this system to offer a large number of terminal functional groups for boron decoration. Besides, the dendrimers were conjugated with vascular endothelial growth factor to target tumor neovasculature. NIR imaging revealed selective accumulation of VEGF-BD/Cy5 in 4T1 mouse breast carcinoma, particularly at the tumor periphery where angiogenesis was most active. These results indicated that VEGF-BD/Cy5 was a promising theranostic platform for BNCT.
Combined therapy
Combined therapy, which uses different therapeutic approaches to treat disease, may produce additive or synergistic antitumor activity 150. Considering the different physical and chemical characteristics of various therapeutic agents, dendritic polymers are ideal delivery vehicles to achieve an integrated platform because of their tailored property and plenty of functional end-groups. For instance, Ma and coworkers fabricated nanoparticles of PAMAM-grafted gadolinium-functionalized nanographene oxide (Gd-NGO) as effective carriers to deliver both chemotherapeutic drugs and highly specific gene-targeting agents such as microRNAs (miRNAs) to cancer cells 151. PAMAM-modified Gd-NGO surface was positively charged and was capable of simultaneously adsorbing anticancer drug epirubicin (EPI) and complex with negatively charged Let-7g miRNA. Furthermore, this Gd-NGO/Let-7g/EPI theranostic system could act as contrast agent for MRI to monitor the location and extent of BBB opening and quantitate tumor drug uptake. These results indicated that Gd-NGO/Let-7g/EPI was promising to become a non-viral vector for chemogene combined therapy and molecular imaging diagnosis.
Moreover, dendritic polymers are suitable vehicles to build promising platforms for combined photothermal chemotherapy. For instance, Kono and coworkers fabricated pH-sensitive drug-dendrimer conjugate-hybridized gold nanorods, which could be utilized to treat cancer through synergistic photothermal therapy and chemotherapy 152. PEG-attached PAMAM G4 dendrimers (PEG-PAMAM) were first linked to mercaptohexadecanoic acid-functionalized gold nanorods (AuNR). Afterwards, DOX was conjugated to the dendrimer layer through an acid-labile hydrazone linkage to afford PEG-DOX-PAMAM-AuNR particles. These particles exhibited high therapeutic efficacy both in vitro and in vivo, which could be attributed to the synergistic hyperthermia ablation and chemotherapy.
Besides, various combined therapy strategies have been raised, such as combinatorial phototherapy with dual PDT and PTT 153, combination of gene therapy and radiotherapy 154. It is hopeful for dendritic polymers to be vehicles for various combined therapy and exhibit great potential in theranostics.
Conclusions and Perspective
In this review we summarized the current progress in the exploitation of dendritic polymer-based theranostic systems, including the synthetic strategies and structure design, biological properties and applications in the theranostic field. During the past decades, dendritic polymers have shown great potential to be ideal candidates for theranostics, and numerous researches on dendritic polymer-based theranostic systems have been reported 155. Dendritic polymer-based theranostic systems can integrate various kinds of therapies and diagnoses effectively, such as chemotherapy, biotherapy, phototherapy, radiotherapy, and combined therapy, as well as MRI, CT, SPECT, PET, fluorescence imaging and NIR imaging156,157. This dramatic progress is mainly attributed to the unique properties of dendritic polymers. Firstly, dendritic polymers possess three-dimensional branched structure with large quantities of cavities and terminal functional groups. Their physical and chemical structures are beneficial for encapsulating or conjugating therapeutic drugs and imaging agents. Besides, dendritic polymers can be endowed with favorable properties, such as biocompatibility, biodegradability, stimuli-responsive ability and self-assembly behaviors, to satisfy the requirement of theranostics. In addition, different categories of dendritic polymers possess their unique advantages. For instance, dendrimers are monodisperse and perfectly branched, which are compatible for reproducible manufacture to meet the batch-to-batch requirements for clinical applications. On the other hand, hyperbranched polymers have convenient synthetic procedure, such as one-pot fabrication, which benefits for large-scale production and industrial transformation. Hence, these attractive features contribute to the wide application of dendritic polymers in theranostics.
Up to now, great progress has been achieved for dendritic polymers in the theranostic application. However, this area still faces several key challenges and a number of discoveries still lie ahead. One of the major concerns is about the mass-production of dendritic polymers with scalability and reproducibility. For instance, dendrimers possess complicated synthetic procedures and low cost-effectiveness, which have hampered their large-scale production. On the other hand, hyperbranched polymers have ill-defined structure and lack reproducibility, which impede their practical applications. Hence, more efforts are needed urgently to achieve mass-production of dendritic polymers. Moreover, challenges still remain to be addressed for the application of dendritic polymers in theranostic systems. Firstly, how to balance the ratio of the diagnostic probes and therapeutic agents in dendritic polymers is still a great concern for individualized application. Secondly, it is necessary to investigate the in vivo behavior of dendritic polymers, such as biodistribution, bioavailability and controlled release of therapeutic agents. These issues would be solved through structural parameter design and performance optimization of dendritic polymers. Thirdly, a systematic in vivo study is highly desired to address the safety issues of dendritic polymers, such as potential toxicity, bioelimination and long-term effect. As reported previously, some dendritic polymers, particularly cationic dendritic polymers, may exhibit cytotoxicity, haemolytic toxicity and haematological toxicity 158-161. These toxicities are strongly influenced by the nature of terminal groups and the cationic properties of dendritic polymers. For instance, cationic dendritic polymers interact nonspecifically with negative cell membranes, which may cause membrane disruption, leakage of cytosolic enzymes and even cell death. Hence, there is great need to reduce the inherent toxicities of cationic dendritic polymers. One of the efficient strategies is surface modification with functional groups to shield the cationic charge of dendritic polymers. Another rewarding strategy is developing biodegradable and biocompatible dendritic polymers, such as biomimetic polymers. These strategies are important to the exploration of non-toxic dendritic polymer-based theranostic systems.
As a final remark, the process of converting dendritic polymers into marketable products for theranostic application can be long and hard. But we firmly believe that the rapid development of this field will resolve the problem from large-scale production to clinical application.
Acknowledgments
The authors thank the National Basic Research Program (2015CB931801) and the National Natural Science Foundation of China (51473093).
References
- 1.Gao C, Yan D. Hyperbranched polymers: from synthesis to applications. Prog Polym Sci. 2004;29:183–275. [Google Scholar]
- 2.Grayson SM, Frechét JM. Convergent dendrons and dendrimers: from synthesis to applications. Chem Rev. 2001;101:3819–67. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]
- 3.Hawker CJ, Frechét JM. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112:7638–47. [Google Scholar]
- 4.Zhou Y, Huang W, Liu J, Zhu X, Yan D. Self-assembly of hyperbranched polymers and its biomedical applications. Adv Mater. 2010;22:4567–90. doi: 10.1002/adma.201000369. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Zhao T, Zhu X, Yan D, Wang W. Bioapplications of hyperbranched polymers. Chem Soc Rev. 2015;44:4023–71. doi: 10.1039/c4cs00229f. [DOI] [PubMed] [Google Scholar]
- 6.Fischer M, Vӧgtle F. Dendrimers: from design to application - a progress report. Angew Chem Int Ed. 1999;38:884–905. doi: 10.1002/(SICI)1521-3773(19990401)38:7<884::AID-ANIE884>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Svenson S, Tomalia DA. Dendrimers in biomedical applications - reflections on the field. Adv Drug Del Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Tomalia DA. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic organic chemistry. Aldrichimica Acta. 2004;37:39–57. [Google Scholar]
- 9.Jikei M, Kakimoto MA. Hyperbranched polymers: a promising new class of materials. Prog Polym Sci. 2001;26:1233–85. [Google Scholar]
- 10.Yan D, Gao C, Frey H. Hyperbranched polymers: synthesis, properties, and applications: John Wiley & Sons; 2011.
- 11.Gao C, Yan D. Polyaddition of B2 and BB'2 type monomers to A2 type monomer. 1. Synthesis of highly branched copoly (sulfone-amine)s. Macromolecules. 2001;34:156–61. [Google Scholar]
- 12.Yan D, Gao C. Hyperbranched polymers made from A2 and BB'2 type monomers. 1. Polyaddition of 1-(2-aminoethyl) piperazine to divinyl sulfone. Macromolecules. 2000;33:7693–9. [Google Scholar]
- 13.Wei Y, Li X, Xu Z, Sun H, Zheng Y, Peng L. et al. Solution processible hyperbranched inverse-vulcanized polymers as new cathode materials in Li-S batteries. Polym Chem. 2015;6:973–82. [Google Scholar]
- 14.Guo Z, Zhang Y, Huang W, Zhou Y, Yan D. Terminal modification with 1-adamantylamine to endow hyperbranched polyamidoamine with thermo-/pH-responsive properties. Macromol Rapid Commun. 2008;29:1746–51. [Google Scholar]
- 15.Qiu F, Wang D, Zhu Q, Zhu L, Tong G, Lu Y. et al. Real-time monitoring of anticancer drug release with highly fluorescent star-conjugated copolymer as a drug carrier. Biomacromolecules. 2014;15:1355–64. doi: 10.1021/bm401891c. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Huang W, Pang Y, Zhu X, Zhou Y, Yan D. Hyperbranched polyphosphates for drug delivery application: design, synthesis, and in vitro evaluation. Biomacromolecules. 2010;11:1564–70. doi: 10.1021/bm100188h. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Huang W, Pang Y, Zhu X, Zhou Y, Yan D. Self-assembled micelles from an amphiphilic hyperbranched copolymer with polyphosphate arms for drug delivery. Langmuir. 2010;26:10585–92. doi: 10.1021/la1006988. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Huang W, Zhou Y, Yan D. Synthesis of hyperbranched polyphosphates by self-condensing ring-opening polymerization of HEEP without catalyst. Macromolecules. 2009;42:4394–9. [Google Scholar]
- 19.Liu J, Pang Y, Huang W, Zhu X, Zhou Y, Yan D. Self-assembly of phospholipid-analogous hyperbranched polymers nanomicelles for drug delivery. Biomaterials. 2010;31:1334–41. doi: 10.1016/j.biomaterials.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Pang Y, Huang W, Zhu Z, Zhu X, Zhou Y. et al. Redox-responsive polyphosphate nanosized assemblies: a smart drug delivery platform for cancer therapy. Biomacromolecules. 2011;12:2407–15. doi: 10.1021/bm2005164. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Ma Y, Xie Y, An Y, Huang Y, Zhu Z. et al. A controllable aptamer-based self-assembled DNA dendrimer for high affinity targeting, bioimaging and drug delivery. Sci Rep. 2015;5:10099–106. doi: 10.1038/srep10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang X, Dong C-M. Synthesis of biomacromolecules block copolymer by consecutive thiol-yne chemistry. Biomacromolecules. 2013;14:3329–37. doi: 10.1021/bm400951m. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M, Lai BF, Kizhakkedathu JN, Narain R. Hyperbranched glycopolymers for blood biocompatibility. Bioconjate Chem. 2012;23:1050–8. doi: 10.1021/bc3000723. [DOI] [PubMed] [Google Scholar]
- 24.Sangoro J, Turky G, Abdel Rehim M, Iacob C, Naumov S, Ghoneim A. et al. Charge transport and dipolar relaxations in hyperbranched polyamide amines. Macromolecules. 2009;42:1648–51. [Google Scholar]
- 25.Frey H, Haag R. Dendritic polyglycerol: a new versatile biocompatible material. Rev Mol Biotechnol. 2002;90:257–67. doi: 10.1016/s1389-0352(01)00063-0. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Deng H, Xie F, Chen W, Zhu B, Xu Q. The potential of pH-responsive PEG-hyperbranched polyacylhydrazone micelles for cancer therapy. Biomaterials. 2014;35:3132–44. doi: 10.1016/j.biomaterials.2013.12.074. [DOI] [PubMed] [Google Scholar]
- 27.Shamis M, Lode HN, Shabat D. Bioactivation of self-immolative dendritic prodrugs by catalytic antibody 38C2. J Am Chem Soc. 2004;126:1726–31. doi: 10.1021/ja039052p. [DOI] [PubMed] [Google Scholar]
- 28.Jia HZ, Zhang W, Zhu JY, Yang B, Chen S, Chen G. et al. Hyperbranched-hyperbranched polymeric nanoassembly to mediate controllable co-delivery of siRNA and drug for synergistic tumor therapy. J Control Release. 2015;216:9–17. doi: 10.1016/j.jconrel.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Saito K, Lee H-R, Lee MJ, Shibasaki Y, Oishi Y. et al. Hyperbranched double hydrophilic block copolymer micelles of poly(ethylene oxide) and polyglycerol for pH-responsive drug delivery. Biomacromolecules. 2012;13:1190–6. doi: 10.1021/bm300151m. [DOI] [PubMed] [Google Scholar]
- 30.Gillies ER, Jonsson TB, Fréchet JMJ. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J Am Chem Soc. 2004;126:11936–43. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 31.Kojima C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010;7:307–19. doi: 10.1517/17425240903530651. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Pang Y, Chen J, Huang P, Huang W, Zhu X. et al. Hyperbranched polydiselenide as a self assembling broad spectrum anticancer agent. Biomaterials. 2012;33:7765–74. doi: 10.1016/j.biomaterials.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Zhang G, Hu J, Wang X, Zhu M, Liu S. Hyperbranched self-immolative polymers (hSIPs) for programmed payload delivery and ultrasensitive detection. J Am Chem Soc. 2015;137:11645–55. doi: 10.1021/jacs.5b05060. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Pang Y, Zhu Z, Wang D, Li C, Huang W. et al. Therapeutic nanocarriers with hydrogen peroxide-triggered drug release for cancer treatment. Biomacromolecules. 2013;14:1627–36. doi: 10.1021/bm4002574. [DOI] [PubMed] [Google Scholar]
- 35.Satoh T, Tamaki M, Kitajyo Y, Maeda T, Ishihara H, Imai T. et al. Synthesis of unimolecular reversed micelle consisting of a poly(L-lactide) shell and hyperbranched D-mannan core. J Polym Sci Part A. 2006;44:406–13. [Google Scholar]
- 36.Son S, Shin E, Kim B-S. Light-responsive micelles of spiropyran initiated hyperbranched polyglycerol for smart drug delivery. Biomacromolecules. 2014;15:628–34. doi: 10.1021/bm401670t. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Yu C, Jin H, Jiang B, Zhu X, Zhou Y. et al. A supramolecular janus hyperbranched polymer and its photoresponsive self-assembly of vesicles with narrow size distribution. J Am Chem Soc. 2013;135:4765–70. doi: 10.1021/ja3122608. [DOI] [PubMed] [Google Scholar]
- 38.Dong R, Liu Y, Zhou Y, Yan D, Zhu X. Photo-reversible supramolecular hyperbranched polymer based on host-guest interactions. Polym Chem. 2011;2:2771–4. [Google Scholar]
- 39.Amir RJ, Pessah N, Shamis M, Shabat D. Self-immolative dendrimers. Angewandte Chemie. 2003;115:4632–7. doi: 10.1002/anie.200351962. [DOI] [PubMed] [Google Scholar]
- 40.Qiu F, Wang D, Wang R, Huan X, Tong G, Zhu Q. et al. Temperature-induced emission enhancement of star conjugated copolymers with poly (2-(dimethylamino) ethyl methacrylate) coronas for detection of bacteria. Biomacromolecules. 2013;14:1678–86. doi: 10.1021/bm4003317. [DOI] [PubMed] [Google Scholar]
- 41.Tai H, Wang W, Vermonden T, Heath F, Hennink WE, Alexander C. et al. Thermoresponsive and photocrosslinkable PEGMEMA-PPGMA-EGDMA copolymers from a one-step ATRP synthesis. Biomacromolecules. 2009;10:822–8. doi: 10.1021/bm801308q. [DOI] [PubMed] [Google Scholar]
- 42.Kimura M, Kato M, Muto T, Hanabusa K, Shirai H. Temperature-sensitive dendritic hosts: synthesis, characterization, and control of catalytic activity. Macromolecules. 2000;33:1117–9. [Google Scholar]
- 43.Kojima C, Tsumura S, Harada A, Kono K. A collagen-mimic dendrimer capable of controlled release. J Am Chem Soc. 2009;131:6052–3. doi: 10.1021/ja809639c. [DOI] [PubMed] [Google Scholar]
- 44.Yunfeng S, Linzhu Z, Ruibin W, Yan P, Wangchuan X, Huiqin L. et al. In situ preparation of magnetic nonviral gene vectors and magnetofection in vitro. Nanotechnology. 2010;21:115103. doi: 10.1088/0957-4484/21/11/115103. [DOI] [PubMed] [Google Scholar]
- 45.Pu WF, Liu R, Li B, Jin FY, Peng Q, Sun L. et al. Amphoteric hyperbranched polymers with multistimuli-responsive behavior in the application of polymer flooding. RSC Adv. 2015;5:88002–13. [Google Scholar]
- 46.Chen QB, You YZ. Multistimuli-responsive hydrogel particles prepared via the self-assembly of PEG-based hyperbranched polymers. Chem Lett. 2015;44:677–9. [Google Scholar]
- 47.Liu B, Wang D, Liu Y, Zhang Q, Meng L, Chi H. et al. Hydrogen peroxide-responsive anticancer hyperbranched polymer micelles for enhanced cell apoptosis. Polym Chem. 2015;6:3460–71. [Google Scholar]
- 48.Zimmerman SC, Zeng F, Reichert DEC, Kolotuchin SV. Self-assembling dendrimers. Science. 1996;271:1095–8. doi: 10.1126/science.271.5252.1095. [DOI] [PubMed] [Google Scholar]
- 49.Yan D, Zhou Y, Hou J. Supramolecular self-assembly of macroscopic tubes. Science. 2004;303:65–7. doi: 10.1126/science.1090763. [DOI] [PubMed] [Google Scholar]
- 50.Mai Y, Zhou Y, Yan D. Synthesis and size-controllable self-assembly of a novel amphiphilic hyperbranched multiarm copolyether. Macromolecules. 2005;38:8679–86. [Google Scholar]
- 51.Yu S, Dong R, Chen J, Chen F, Jiang W, Zhou Y. et al. Synthesis and self-assembly of amphiphilic aptamer-functionalized hyperbranched multiarm copolymers for targeted cancer imaging. Biomacromolecules. 2014;15:1828–36. doi: 10.1021/bm5002203. [DOI] [PubMed] [Google Scholar]
- 52.Mao J, Ni P, Mai Y, Yan D. Multicompartment micelles from hyperbranched star-block copolymers containing polycations and fluoropolymer segment. Langmuir. 2007;23:5127–34. doi: 10.1021/la063576w. [DOI] [PubMed] [Google Scholar]
- 53.Zhang D, Fan Y, Li H, Li K, Yao Y, Zhou Y. et al. A dumbbell-like supramolecular triblock copolymer and its self-assembly of light-responsive vesicles. RSC Adv. 2015;5:47762–5. [Google Scholar]
- 54.Tan H, Wang W, Yu C, Zhou Y, Lu Z, Yan D. Dissipative particle dynamics simulation study on self-assembly of amphiphilic hyperbranched multiarm copolymers with different degrees of branching. Soft Matter. 2015;11:8460–70. doi: 10.1039/c5sm01495f. [DOI] [PubMed] [Google Scholar]
- 55.Tao W, Liu Y, Jiang B, Yu S, Huang W, Zhou Y. et al. A linear-hyperbranched supramolecular amphiphile and its self-assembly into vesicles with great ductility. J Am Chem Soc. 2012;134:762–4. doi: 10.1021/ja207924w. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Yu C, Jin H, Jiang B, Zhu X, Zhou Y. et al. A supramolecular janus hyperbranched polymer and its photoresponsive self-assembly of vesicles with narrow size distribution. J Am Chem Soc. 2013;135:4765–70. doi: 10.1021/ja3122608. [DOI] [PubMed] [Google Scholar]
- 57.Fan Y, Zhang D, Wang J, Jin H, Zhou Y, Yan D. Preparation of anion-exchangeable polymer vesicles through the self-assembly of hyperbranched polymeric ionic liquids. Chem Commun. 2015;51:7234–7. doi: 10.1039/c5cc01802a. [DOI] [PubMed] [Google Scholar]
- 58.Wei Z, Zhang L, Yu M, Yang Y, Wan M. Self-assembling sub-micrometer-sized tube junctions and dendrites of conducting polymers. Adv Mater. 2003;15:1382–5. [Google Scholar]
- 59.Gao C, Zheng X. Facile synthesis and self-assembly of multihetero-arm hyperbranched polymer brushes. Soft Matter. 2009;5:4788–96. [Google Scholar]
- 60.Zhou Y, Yan D. Supramolecular self-assembly of giant polymer vesicles with controlled sizes. Angew Chem Int Ed. 2004;43:4896–9. doi: 10.1002/anie.200460325. [DOI] [PubMed] [Google Scholar]
- 61.Cheng H, Wang S, Yang J, Zhou Y, Yan D. Synthesis and self-assembly of amphiphilic hyperbranched polyglycerols modified with palmitoyl chloride. J Colloid Interf Sci. 2009;337:278–84. doi: 10.1016/j.jcis.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 62.Cano M. et al. Supramolecular architectures from bent-core dendritic molecules. Angew Chem Int Ed. 2014;126:13667–71. doi: 10.1002/anie.201407705. [DOI] [PubMed] [Google Scholar]
- 63.Percec V, Wilson DA, Leowanawat P, Wilson CJ, Hughes AD, Kaucher MS. et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science. 2010;328:1009–14. doi: 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C, Pan D, Luo K, Li N, Guo C, Zheng X. et al. Dendrimer-doxorubicin conjugate as enzyme-sensitive and polymeric nanoscale drug delivery vehicle for ovarian cancer therapy. Polym Chem. 2014;5:5227–35. [Google Scholar]
- 65.Liu C, Gao C, Yan D. Honeycomb-patterned photoluminescent films fabricated by self-assembly of hyperbranched polymers. Angew Chem Int Ed. 2007;46:4128–31. doi: 10.1002/anie.200604429. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Huang W, Zhou Y, Yan D. A physical gel made from hyperbranched polymer gelator. Chem Commun; 2007. pp. 2587–9. [DOI] [PubMed] [Google Scholar]
- 67.Sk UH, Kojima C. Dendrimers for theranostic applications. Biomol Concept. 2015;6:205–17. doi: 10.1515/bmc-2015-0012. [DOI] [PubMed] [Google Scholar]
- 68.Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41:2575–89. doi: 10.1039/c1cs15248c. [DOI] [PubMed] [Google Scholar]
- 69.Werner EJ, Datta A, Jocher CJ, Raymond KN. High-relaxivity MRI contrast agents: where coordination chemistry meets medical imaging. Angew Chem Int Ed. 2008;47:8568–80. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 70.Lee D-E, Koo H, Sun I-C, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41:2656–72. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 71.Kim KS, Park W, Hu J, Bae YH, Na K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials. 2014;35:337–43. doi: 10.1016/j.biomaterials.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Garimella PD, Datta A, Romanini DW, Raymond KN, Francis MB. Multivalent, high-relaxivity MRI contrast agents using rigid cysteine-reactive gadolinium complexes. J Am Chem Soc. 2011;133:14704–9. doi: 10.1021/ja204516p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boros E, Polasek M, Zhang Z, Caravan P. Gd(DOTAla): a single amino acid Gd-complex as a modular tool for high relaxivity MR contrast agent development. J Am Chem Soc. 2012;134:19858–68. doi: 10.1021/ja309187m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun M, Zhang HY, Liu BW, Liu Y. Construction of a supramolecular polymer by bridged bis(permethyl-β-cyclodextrin)s with porphyrins and its highly efficient magnetic resonance imaging. Macromolecules. 2013;46:4268–75. [Google Scholar]
- 75.Zhu L, Wang D, Wei X, Zhu X, Li J, Tu C. et al. Multifunctional pH-sensitive superparamagnetic iron-oxide nanocomposites for targeted drug delivery and MR imaging. J Control Release. 2013;169:228–38. doi: 10.1016/j.jconrel.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Qian Y, Liu T, Hu X, Zhang G, You Y. et al. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials. 2011;32:6595–605. doi: 10.1016/j.biomaterials.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 77.Hu X, Liu G, Li Y, Wang X, Liu S. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J Am Chem Soc. 2015;137:362–8. doi: 10.1021/ja5105848. [DOI] [PubMed] [Google Scholar]
- 78.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–21. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hahn MA, Singh AK, Sharma P, Brown SC, Moudgil BM. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal Bioanal Chem. 2011;399:3–27. doi: 10.1007/s00216-010-4207-5. [DOI] [PubMed] [Google Scholar]
- 80.Dekrafft KE, Xie Z, Cao G, Tran S, Liqing M, Zhou OZ. et al. Iodinated nanoscale coordination polymers as potential contrast agents for computed tomography. Angew Chem Int Ed. 2009;48:9901–4. doi: 10.1002/anie.200904958. [DOI] [PubMed] [Google Scholar]
- 81.Arifin DR, Manek S, Call E, Arepally A, Bulte JWM. Microcapsules with intrinsic barium radiopacity for immunoprotection and X-ray/CT imaging of pancreatic islet cells. Biomaterials. 2012;33:4681–9. doi: 10.1016/j.biomaterials.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J, Fu F, Xiong Z, Shen M, Shi X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surf B Biointerfaces. 2015;133:36–42. doi: 10.1016/j.colsurfb.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 83.Zhu J, Zheng L, Wen S, Tang Y, Shen M, Zhang G. et al. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2014;35:7635–46. doi: 10.1016/j.biomaterials.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 84.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–16. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y. et al. Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials. 2013;34:8323–32. doi: 10.1016/j.biomaterials.2013.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao W, Luo J, Jain T, Riggs JW, Tseng HP, Henderson PT. et al. Biodistribution and pharmacokinetics of a telodendrimer micellar paclitaxel nanoformulation in a mouse xenograft model of ovarian cancer. Int J Nanomed. 2012;7:1587–97. doi: 10.2147/IJN.S29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao Y, Hong H, Javadi A, Engle JW, Xu W, Yang Y. et al. Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging. Biomaterials. 2012;33:3071–82. doi: 10.1016/j.biomaterials.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheuer W, van Dam GM, Dobosz M, Schwaiger M, Ntziachristos V. Drug-based optical agents: infiltrating clinics at lower risk. Sci Transl Med. 2012;4:134–9. doi: 10.1126/scitranslmed.3003572. [DOI] [PubMed] [Google Scholar]
- 89.Chen G, Wang L, Cordie T, Vokoun C, Eliceiri KW, Gong S. Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging. Biomaterials. 2015;47:41–50. doi: 10.1016/j.biomaterials.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng H, Liu B, Yang C, Li G, Zhuang Y, Li B. et al. Multi-color cell imaging under identical excitation conditions with salicylideneaniline analogue-based fluorescent nanoparticles. RSC Adv. 2014;4:62021–9. [Google Scholar]
- 91.Akin M, Bongartz R, Walter JG, Demirkol DO, Stahl F, Timur S. et al. PAMAM-functionalized water soluble quantum dots for cancer cell targeting. J Mater Chem. 2012;22:11529–36. [Google Scholar]
- 92.Zhu L, Shi Y, Tu C, Wang R, Pang Y, Qiu F. et al. Construction and application of a pH-sensitive nanoreactor via a double-hydrophilic multiarm hyperbranched polymer. Langmuir. 2010;26:8875–81. doi: 10.1021/la9046275. [DOI] [PubMed] [Google Scholar]
- 93.Zhong Y, Peng F, Bao F, Wang S, Ji X, Yang L. et al. Large-scale aqueous synthesis of fluorescent and biocompatible silicon nanoparticles and their use as highly photostable biological probes. J Am Chem Soc. 2013;135:8350–6. doi: 10.1021/ja4026227. [DOI] [PubMed] [Google Scholar]
- 94.Tong G, Wang J, Wang R, Guo X, He L, Qiu F. et al. Amorphous carbon dots with high two-photon fluorescence for cellular imaging passivated by hyperbranched poly(amino amine) J Mater Chem B. 2015;3:700–6. doi: 10.1039/c4tb01643b. [DOI] [PubMed] [Google Scholar]
- 95.Dai Y, Xiao H, Liu J, Yuan Q, Ma P, Yang D. et al. In vivo multimodality imaging and cancer therapy by near-infrared light-triggered trans-platinum pro-drug-conjugated upconverison nanoparticles. J Am Chem Soc. 2013;135:18920–9. doi: 10.1021/ja410028q. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Z, Ma X, Jin E, Tang J, Sui M, Shen Y. et al. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials. 2013;34:5722–35. doi: 10.1016/j.biomaterials.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Mugabe C, Matsui Y, So AI, Gleave ME, Baker JHE, Minchinton AI. et al. In vivo evaluation of mucoadhesive nanoparticulate docetaxel for intravesical treatment of non-muscle-invasive bladder cancer. Clin Cancer Res. 2011;17:2788–98. doi: 10.1158/1078-0432.CCR-10-2981. [DOI] [PubMed] [Google Scholar]
- 98.Kolhe P, Khandare J, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials. 2006;27:660–9. doi: 10.1016/j.biomaterials.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Chen T, Xu S, Zhao T, Zhu L, Wei D, Li Y. et al. Gold nanocluster-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. ACS Appl Mater Interfaces. 2012;4:5766–74. doi: 10.1021/am301223n. [DOI] [PubMed] [Google Scholar]
- 100.Baker J.R Jr, Quintana A, Piehler L, Banazak-Holl M, Tomalia D, Raczka E. The synthesis and testing of anti-cancer therapeutic nanodevices. Biomed Microdevices. 2001;3:61–9. [Google Scholar]
- 101.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker Jr JR. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7:572–9. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 102.Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin αvβ3 in brain tumor xenografts. Cancer Res. 2004;64:8009–14. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- 103.Jia H, Zhu J, Wang X, Cheng H, Chen G, Zhao Y. et al. A boronate-linked linear-hyperbranched polymeric nanovehicle for pH-dependent tumor-targeted drug delivery. Biomaterials. 2014;35:5240–9. doi: 10.1016/j.biomaterials.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 104.Krüger HR, Schütz I, Justies A, Licha K, Welker P, Haucke V. et al. Imaging of doxorubicin release from theranostic macromolecular prodrugs via fluorescence resonance energy transfer. J Control Release. 2014;194:189–96. doi: 10.1016/j.jconrel.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 105.Schirrmacher V, Hagmüller E, Lehnert T, Pomer S, Ahlert T, Ockert D. et al. Biotherapy of cancer - perspectives of immunotherapy and gene therapy. J Cancer Res Clin Oncol. 1995;121:443–51. doi: 10.1007/BF01218359. [DOI] [PubMed] [Google Scholar]
- 106.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 107.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 108.Navarro G, Maiwald G, Haase R, Rogach AL, Wagner E, de Ilarduya CT. et al. Low generation PAMAM dendrimer and CpG free plasmids allow targeted and extended transgene expression in tumors after systemic delivery. J Control Release. 2010;146:99–105. doi: 10.1016/j.jconrel.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 109.Lemarié F, Croft DR, Tate RJ, Ryan KM, Dufès C. Tumor regression following intravenous administration of a tumor-targeted p73 gene delivery system. Biomaterials. 2012;33:2701–9. doi: 10.1016/j.biomaterials.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 110.Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP. et al. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release. 2009;140:284–93. doi: 10.1016/j.jconrel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzalo T, Clemente MI, Chonco L, Weber ND, Díaz L, Serramía MJ. et al. Gene therapy in HIV-infected cells to decrease viral impact by using an alternative delivery method. ChemMedChem. 2010;5:921–9. doi: 10.1002/cmdc.201000029. [DOI] [PubMed] [Google Scholar]
- 112.An S, Kuang Y, Shen T, Li J, Ma H, Guo Y. et al. Brain-targeting delivery for RNAi neuroprotection against cerebral ischemia reperfusion injury. Biomaterials. 2013;34:8949–59. doi: 10.1016/j.biomaterials.2013.07.060. [DOI] [PubMed] [Google Scholar]
- 113.Ardana A, Whittaker AK, Thurecht KJ. PEG-based hyperbranched polymer theranostics: optimizing chemistries for improved bioconjugation. Macromolecules. 2014;47:5211–9. [Google Scholar]
- 114.Pan B, Cui D, Sheng Y, Ozkan C, Gao F, He R. et al. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007;67:8156–63. doi: 10.1158/0008-5472.CAN-06-4762. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q, Li J, An S, Chen Y, Jiang C, Wang X. Magnetic resonance-guided regional gene delivery strategy using a tumor stroma-permeable nanocarrier for pancreatic cancer. Int J Nanomed. 2015;10:4479. doi: 10.2147/IJN.S84930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chisholm EJ, Vassaux G, Martin-Duque P, Chevre R, Lambert O, Pitard B. et al. Cancer-specific transgene expression mediated by systemic injection of nanoparticles. Cancer Res. 2009;69:2655–62. doi: 10.1158/0008-5472.CAN-08-2657. [DOI] [PubMed] [Google Scholar]
- 117.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 118.Santra S, Kaittanis C, Perez JM. Cytochrome c encapsulating theranostic nanoparticles: a novel bifunctional system for targeted delivery of therapeutic membrane-impermeable proteins to tumors and imaging of cancer therapy. Mol Pharm. 2010;7:1209–22. doi: 10.1021/mp100043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fuhrmann G, Grotzky A, Lukić R, Matoori S, Luciani P, Yu H. et al. Sustained gastrointestinal activity of dendronized polymer-enzyme conjugates. Nat Chem. 2013;5:582–9. doi: 10.1038/nchem.1675. [DOI] [PubMed] [Google Scholar]
- 120.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 122.Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F. et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 123.Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K. et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol. 2010;223:229–37. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Venkataramana NK, Kumar SKV, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A. et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Srinivas M, Aarntzen EHJG, Bulte JWM, Oyen WJ, Heerschap A, de Vries IJM. et al. Imaging of cellular therapies. Adv Drug Deliv Rev. 2010;62:1080–93. doi: 10.1016/j.addr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 126.Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–94. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Higuchi Y, Wu C, Chang KL, Irie K, Kawakami S, Yamashita F. et al. Polyamidoamine dendrimer-conjugated quantum dots for efficient labeling of primary cultured mesenchymal stem cells. Biomaterials. 2011;32:6676–82. doi: 10.1016/j.biomaterials.2011.05.076. [DOI] [PubMed] [Google Scholar]
- 128.Fraix A, Kandoth N, Manet I, Cardile V, Graziano ACE, Gref R. et al. An engineered nanoplatform for bimodal anticancer phototherapy with dual-color fluorescence detection of sensitizers. Chem Commun. 2013;49:4459–61. doi: 10.1039/c3cc40714d. [DOI] [PubMed] [Google Scholar]
- 129.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN. et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–49. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 131.Levi-Polyachenko NH, Stewart Iv JH. Clinical relevance of nanoparticle induced hyperthermia for drug delivery and treatment of abdominal cancers. Open Nanomed J. 2011;3:24–37. [Google Scholar]
- 132.Zhang Y, Pang L, Ma C, Tu Q, Zhang R, Saeed E. et al. Small molecule-initiated light-activated semiconducting polymer dots: an integrated nanoplatform for targeted photodynamic therapy and imaging of cancer cells. Anal Chem. 2014;86:3092–9. doi: 10.1021/ac404201s. [DOI] [PubMed] [Google Scholar]
- 133.Taratula O, Schumann C, Naleway MA, Pang AJ, Chon KJ, Taratula O. A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol Pharm. 2013;10:3946–58. doi: 10.1021/mp400397t. [DOI] [PubMed] [Google Scholar]
- 134.Yoon H-J, Lim TG, Kim J-H, Cho YM, Kim YS, Chung US. et al. Fabrication of multifunctional layer-by-layer nanocapsules toward the design of theragnostic nanoplatform. Biomacromolecules. 2014;15:1382–9. doi: 10.1021/bm401928f. [DOI] [PubMed] [Google Scholar]
- 135.Vonnemann J, Beziere N, Böttcher C, Riese SB, Kuehne C, Dernedde J. et al. Polyglycerolsulfate functionalized gold nanorods as optoacoustic signal nanoamplifiers for in vivo bioimaging of rheumatoid arthritis. Theranostics. 2014;4:629–41. doi: 10.7150/thno.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Z, Huang P, Zhang X, Lin J, Yang S, Liu B. et al. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol Pharm. 2010;7:94–104. doi: 10.1021/mp9001415. [DOI] [PubMed] [Google Scholar]
- 137.Taratula O, Patel M, Schumann C, Naleway MA, Pang AJ, He H. et al. Phthalocyanine-loaded graphene nanoplatform for imaging-guided combinatorial phototherapy. Int J Nanomed. 2015;10:2347–62. doi: 10.2147/IJN.S81097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taratula O, Schumann C, Duong T, Taylor KL, Taratula O. Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy. Nanoscale. 2015;7:3888–902. doi: 10.1039/c4nr06050d. [DOI] [PubMed] [Google Scholar]
- 139.Sancey L, Lux F, Kotb S, Roux S, Dufort S, Bianchi A, The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br J Radiol; 2014. p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raju MR. Particle radiotherapy: Historical developments and current status. Radiat Res. 1996;145:391–407. [PubMed] [Google Scholar]
- 141.Schiffer D. Radiotherapy by particle beams (hadrontherapy) of intracranial tumours: a survey on pathology. Neurol Sci. 2005;26:5–12. doi: 10.1007/s10072-005-0376-y. [DOI] [PubMed] [Google Scholar]
- 142.Wu G, Yang W, Barth RF, Kawabata S, Swindall M, Bandyopadhyaya AK. et al. Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin Cancer Res. 2007;13:1260–8. doi: 10.1158/1078-0432.CCR-06-2399. [DOI] [PubMed] [Google Scholar]
- 143.Yang W, Barth RF, Adams DM, Ciesielski MJ, Fenstermaker RA, Shukla S. et al. Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res. 2002;62:6552–8. [PubMed] [Google Scholar]
- 144.Yim CB, Dijkgraaf I, Merkx R, Versluis C, Eek A, Mulder GE. et al. Synthesis of DOTA-conjugated multimeric [Tyr3]octreotide peptides via a combination of Cu(I)-catalyzed click cycloaddition and thio acid/sulfonyl azide sulfo-click amidation and their in vivo evaluation. J Med Chem. 2010;53:3944–53. doi: 10.1021/jm100246m. [DOI] [PubMed] [Google Scholar]
- 145.Kobayashi H, Reijnders K, English S, Yordanov AT, Milenic DE, Sowers AL. et al. Application of a macromolecular contrast agent for detection of alterations of tumor vessel permeability induced by radiation. Clin Cancer Res. 2004;10:7712–20. doi: 10.1158/1078-0432.CCR-04-1175. [DOI] [PubMed] [Google Scholar]
- 146.Kobayashi H, Kawamoto S, Saga T, Sato N, Ishimori T, Konishi J. et al. Avidin-dendrimer-(1B4M-Gd)254: a tumor-targeting therapeutic agent for gadolinium neutron capture therapy of intraperitoneal disseminated tumor which can be monitored by MRI. Bioconjugate Chem. 2001;12:587–93. doi: 10.1021/bc010002o. [DOI] [PubMed] [Google Scholar]
- 147.Dubey R, Kushal S, Mollard A, Vojtovich L, Oh P, Levin MD. et al. Tumor targeting, trifunctional dendritic wedge. Bioconjugate Chem. 2015;26:78–89. doi: 10.1021/bc500436b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li N, Jin Y, Xue L, Li P, Yan D, Zhu X. 188Re-labeled hyperbranched polysulfonamine as a robust tool for targeted cancer diagnosis and radioimmunotherapy. Chin J Polym Sci. 2013;31:530–40. [Google Scholar]
- 149.Backer MV, Gaynutdinov TI, Patel V, Bandyopadhyaya AK, Thirumamagal BTS, Tjarks W. et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4:1423–9. doi: 10.1158/1535-7163.MCT-05-0161. [DOI] [PubMed] [Google Scholar]
- 150.Gasparini G, Longo R, Fanelli M, Teicher BA. Combination of antiangiogenic therapy with other anticancer therapies: Results, challenges, and open questions. J Clin Oncol. 2005;23:1295–311. doi: 10.1200/JCO.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 151.Yang H, Huang C, Lin C, Liu H, Huang C, Liao S. et al. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials. 2014;35:6534–42. doi: 10.1016/j.biomaterials.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 152.Li X, Takashima M, Yuba E, Harada A, Kono K. PEGylated PAMAM dendrimer-doxorubicin conjugate-hybridized gold nanorod for combined photothermal-chemotherapy. Biomaterials. 2014;35:6576–84. doi: 10.1016/j.biomaterials.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 153.Taratula O, Schumann C, Duong T, Taylor KL, Taratula O. Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy. Nanoscale. 2015;7:3888–902. doi: 10.1039/c4nr06050d. [DOI] [PubMed] [Google Scholar]
- 154.Grünwald GK, Vetter A, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R. et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J Nucl Med. 2013;54:1450–7. doi: 10.2967/jnumed.112.115493. [DOI] [PubMed] [Google Scholar]
- 155.Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660–77. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jin L, Zeng X, Liu M, Deng Y, He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 2014;4:240–55. doi: 10.7150/thno.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu L, Luderer M, Yang X, Swain C, Zhang H, Nelson K. et al. Surface passivation of carbon nanoparticles with branched macromolecules influences near infrared bioimaging. Theranostics. 2013;3:677–86. doi: 10.7150/thno.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shcharbin D, Janaszewska A, Klajnert-Maculewicz B, Ziemba B, Dzmitruk V, Halets I. et al. How to study dendrimers and dendriplexes III. biodistribution, pharmacokinetics and toxicity in vivo. J Control Release. 2014;181:40–52. doi: 10.1016/j.jconrel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 159.Mishra V, Gupta U, Jain NK. Surface-engineered dendrimers: a solution for toxicity issues. J Biomater Sci Polym Ed. 2009;20:141–66. doi: 10.1163/156856208X386246. [DOI] [PubMed] [Google Scholar]
- 160.Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: let's meet the challenge. Int J Pharm. 2010;394:122–42. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 161.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]