The detection of hypoglycemia stems from sensors located in both the periphery and within the central nervous system and likely involves a complex circuit that incorporates information from both peripheral and central components. Glucose sensors located in each of these regions play a unique role in hypoglycemia detection that appears to be at least in part dependent on the rate of fall of blood glucose levels. Sensors that are located within the brain, particularly those located within the ventromedial hypothalamus (VMH), appear to dominate when glucose levels fall rapidly (1–3). While still speculative, it has been proposed that this may serve as a protective fail-safe mechanism that is put in place to prevent a sudden and potentially catastrophic depletion of fuel supply to the brain. On the other hand, glucose sensors located in the portal-mesenteric vein (PMV) have been proposed to be more important for detecting a gradual decline in blood glucose levels (4–6). Interestingly, it has recently been suggested that central and peripheral sensors appear to operate independently from one another as lesions that are made to PMV sensors do not influence the ability of central sensors to detect rapid-onset hypoglycemia but did prevent the detection of slow-onset hypoglycemia (7). This implies that these two sets of hypoglycemia sensors may exert differential roles in glucose sensation and glucose counterregulation and that they are not merely redundant mechanisms. Hypoglycemic signals from PMV glucose sensors that are transmitted to the central nervous system appear to use central pathways that are independent from those used by hypothalamic centers. Although much of our current understanding of glucose-sensing mechanisms stems from work that was conducted in the VMH of the brain (8), the existence of a relay circuit between PMV sensors, the brain, and the sympathoadrenal system has been postulated for some time (9–11). Moreover, the reliance of PMV sensors on central nervous system networks to relay their message to peripheral targets has not been explored to date. This brings into question which brain regions are crucial for integrating and transmitting glucoprivic signals derived from PMV glucose sensors to regulate sympathoadrenal responses during slow-onset hypoglycemia and whether these systems overlap with neural circuits generated in the VMH that regulate the sympathoadrenal responses during rapid-onset hypoglycemia.
In this issue, there are two articles presented by the research groups of Donovan and Watts at the University of Southern California that explore the PMV circuit in more detail (12,13). The first article by Bohland et al. (12) identified the crucial PMV sensor-brain arm of the circuit using some elegant denervation studies. Here, the authors either used capsaicin to denervate PMV sensors or they eliminated spinal afferents from the portal and superior mesenteric veins using celiac-superior mesenteric ganglionectomy (CSMG) or they removed vagal afferents from the PMV using a total subdiaphragmatic vagotomy. These three different forms of lesions helped to identify the role of PMV glucose sensors and establish whether hypoglycemic signals from the PMV are carried to the hindbrain via spinal or vagal afferents, respectively. They then subjected the animals to either a slow- or rapid-onset hypoglycemic clamp to identify the effect of these manipulations on the counterregulatory hormone responses. They demonstrated that PMV denervation blunts the counterregulatory responses to slow-onset hypoglycemia, and this was associated with a substantial reduction in Fos-labeled nuclei within hindbrain centers, including the area postrema, the nucleus of the solitary tract, and the dorsal motor vagal complex, that receive PMV projections. Lesioning of the spinal afferents with CSMG reduced counterregulatory hormone responses to slow-onset hypoglycemia, and this was associated with a reduction in Fos-labeled nuclei in the same regions as seen with the PMV denervation. In contrast, total subdiaphragmatic vagotomy had no effect on either the hormonal responses or Fos expression. Taken together, these data suggest that hypoglycemic signals detected by PMV glucose sensors are relayed to hindbrain centers within the central nervous system via the CSMG spinal afferents and not through vagal afferent pathways. Of particular interest, none of these lesions affected the counterregulatory responses to rapid-onset hypoglycemia, supporting the hypothesis that the rate of fall of glucose exerts differential effects on the capacity of central and peripheral glucose sensors to detect and respond to decrements in circulating glucose.
Once activated, the hindbrain centers communicate through catecholaminergic neurons, which project to various hypothalamic nuclei to regulate neuroendocrine responses as well as to autonomic networks to enhance counterregulatory hormone release (14). These outputs are crucial for conveying information that drives hypothalamic–hypophyseal function and feeding responses (15–18), but the role of these projections in promoting glucose counterregulation is less well understood.
It has been suggested that hindbrain catecholaminergic projections to the VMH are enhanced by hypoglycemia, but the necessity of these neuronal inputs for the initiation of counterregulatory responses has not been established. In this issue, Jokiaho et al. (13) addressed this question. This follow-up study used a dopamine-β-hydroxylase conjugated form of saporin, a cytotoxin specific for catecholaminergic neurons, so as to lesion catecholaminergic terminals proximal to the paraventricular nucleus of the hypothalamus (PVH). Subsequently, plasma glucose was lowered either rapidly or slowly, and immunohistochemistry was used to determine the number of activated catecholaminergic neurons in the hindbrain. Saporin-lesioned animals showed a substantial loss of hindbrain catecholaminergic neurons but no significant impairments in counterregulatory responses to rapid-onset hypoglycemia, suggesting that the majority of the sensing mechanisms required for detecting rapid-onset hypoglycemia lie within the VMH. In contrast, responses to slow-onset hypoglycemia were diminished in saporin-lesioned rats, suggesting that during slower falls in blood glucose levels, the PVH serves as an important relay for signals derived from peripheral glucose sensors. Of particular note was the fact that compromising the PVH-VMH–projecting catecholaminergic neurons produced the same outcome as PMV denervation, suggesting this brain circuit forms a crucial component in the PMV glucose-sensing mechanism. In this model, slowly developing hypoglycemic signals detected by PMV sensors are conveyed via spinal afferents to the hindbrain and then project to the PVH and VMH, which, in turn, modulate sympathoadrenal responses.
While the data from these two studies (12,13) support the idea of two separate mechanisms that process hypoglycemia sensory information differently depending on the rate of fall of glucose, it is surprising that these studies failed to show the presence of cross talk between these two systems. Jokiaho et al. (13) speculate that catecholaminergic neurons projecting to the VMH may provide additional facilitative inputs to diminish VMH GABA release during slow-onset hypoglycemia (19–21). However, this remains to be established. While the establishment of sensors that detect a gradual fall in blood glucose (as in starvation) is important from an evolutionary perspective, it is surprising that these two systems appear to operate completely independently of one another. It remains to be seen whether the PMV and VMH glucose sensors communicate with one another through central circuits and respond to declining glucose levels over a broader range and time course than we might anticipate. Catecholaminergic inputs, therefore, may form a crucial communicatory link between the two systems (Fig. 1). As circulating glucose levels fall more rapidly than central nervous system glucose levels, it is conceivable that PMV sensors may provide the initial input to the brain, which helps to prepare it for an impending drop in glucose. Should brain glucose levels continue to decline or decline more rapidly, hypothalamic centers may then provide additional input to autonomic centers to further enhance counterregulatory output. What is clearly evident from these studies (12,13) is that catecholaminergic neurons within the hypothalamus are important for modulating counterregulatory hormone release during slow-onset hypoglycemia.
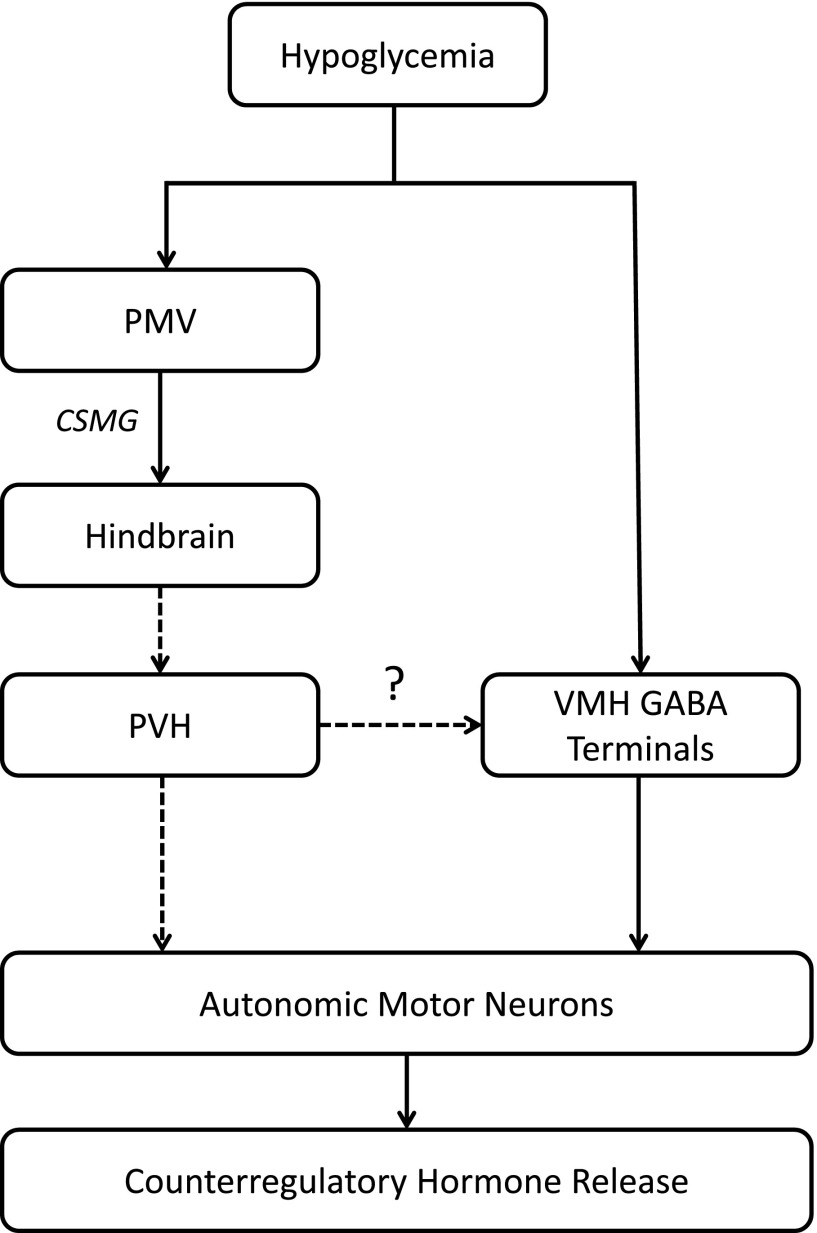
Figure 1.
Rapid-onset hypoglycemia is detected by glucose-sensing mechanisms within the VMH, which, in turn, stimulates autonomic output to enhance counterregulatory hormone release. Slow-onset hypoglycemia is detected by PMV glucose sensors, and this signal is then transmitted to hindbrain catecholaminergic centers via CSMG where they are integrated. Catecholaminergic neurons (dotted lines) then project to the PVH, where they activate neuroendocrine and autonomic centers to stimulate counterregulatory hormone responses. In addition, catecholaminergic neurons may project to VMH GABAergic terminals to provide additional input to reduce GABA release during slow-onset hypoglycemia to stimulate counterregulatory responses.
Although these are some of the first studies to identify a PMV-hypothalamic-sympathoadrenal relay, it will be important to establish the contribution of hypothalamic centers, and in particular the VMH, to glucose counterregulation during slow-onset hypoglycemia via lesion studies or direct recordings of these circuits in intact animals during slower declines in glucose levels. In addition, what remains to be elucidated from these studies is the neurocircuitry of the catecholaminergic projections that are involved in hypoglycemia detection—that is to say, what neurons they form synapses with and how they affect the downstream output signals that regulate counterregulatory hormone secretion. It will be important to identify the neural networks that regulate these sensing mechanisms in greater detail as a better understanding will help identify suitable therapeutic targets, whether they lie in the periphery or within the brain, to prevent future hypoglycemic attacks.
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997;99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 1994;93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 1995;44:180–184 [DOI] [PubMed] [Google Scholar]
- 4.Donovan CM. Portal vein glucose sensing. Diabetes Nutr Metab 2002;15:308–312; discussion, 313–314 [PubMed] [Google Scholar]
- 5.Hevener AL, Bergman RN, Donovan CM. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes 2000;49:8–12 [DOI] [PubMed] [Google Scholar]
- 6.Matveyenko AV, Bohland M, Saberi M, Donovan CM. Portal vein hypoglycemia is essential for full induction of hypoglycemia-associated autonomic failure with slow-onset hypoglycemia. Am J Physiol Endocrinol Metab 2007;293:E857–E864 [DOI] [PubMed] [Google Scholar]
- 7.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes 2008;57:1380–1386 [DOI] [PubMed] [Google Scholar]
- 8.Chan O, Sherwin R. Influence of VMH fuel sensing on hypoglycemic responses. Trends Endocrinol Metab 2013;24:616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niijima A. Nervous regulation of metabolism. Prog Neurobiol 1989;33:135–147 [DOI] [PubMed] [Google Scholar]
- 10.Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J Auton Nerv Syst 1984;10:359–372 [DOI] [PubMed] [Google Scholar]
- 11.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev 1987;3:185–206 [DOI] [PubMed] [Google Scholar]
- 12.Bohland M, Matveyenko AV, Saberi M, Khan AM, Watts AG, Donovan CM. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes 2014;63:2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jokiaho AJ, Donovan CM, Watts AG. The rate of fall of blood glucose determines the necessity of forebrain-projecting catecholaminergic neurons for male rat sympathoadrenal responses. Diabetes 2014;63:2854–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 2010;31:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AM, Kaminski KL, Sanchez-Watts G, et al. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci 2011;31:18479–18491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull 1988;21:905–912 [DOI] [PubMed] [Google Scholar]
- 17.Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 2001;22:502–548 [DOI] [PubMed] [Google Scholar]
- 18.Hudson B, Ritter S. Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav 2004;82:241–250 [DOI] [PubMed] [Google Scholar]
- 19.Beverly JL, de Vries MG, Beverly MF, Arseneau LM. Norepinephrine mediates glucoprivic-induced increase in GABA in the ventromedial hypothalamus of rats. Am J Physiol Regul Integr Comp Physiol 2000;279:R990–R996 [DOI] [PubMed] [Google Scholar]
- 20.Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS. ATP-sensitive K(+) channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 2007;56:1120–1126 [DOI] [PubMed] [Google Scholar]
- 21.de Vries MG, Lawson MA, Beverly JL. Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. Am J Physiol Regul Integr Comp Physiol 2005;289:R977–R981 [DOI] [PubMed] [Google Scholar]