It is quite possible that if she had seen but a glimpse of her young country’s future while writing “The New Colossus” in late 19th-century America, Emma Lazarus might have modified her famous line to read “Give me your…huddled masses yearning to breathe free,” well-nourished and long-lived. And indeed, at a time when food shortage and famine were sources of great suffering throughout much of the world, such a persuasive line would have seemed very compelling to the “masses.” How strange might it also have been for people at that time to be told of what we now know, which is that overnourishment typically prohibits a person from living a long and healthy life. Evidence of this fact is manifested in the rapidly rising rates of obesity-related pathologies, particularly cardiovascular disease and metabolic syndrome/type 2 diabetes, which of course are hardly unique to the U.S. as recent reports show that rates of obesity and related cardiometabolic diseases are escalating worldwide, with the highest rates in developing countries across Africa and the Middle East (1,2). Lifestyle interventions to combat this epidemic are increasingly being prioritized by health care professionals who recognize that, unless action is taken, the tidal wave of associated costs and resource demands placed on hospitals and primary care providers will wash over us all with devastating consequences.
Caloric restriction (CR) is an intervention that consistently leads to improved cardiometabolic outcomes (3–5). Yet despite its clear benefits, CR remains difficult to implement as a long-term therapy in obese patients for many legitimate reasons (e.g., challenges with dietary adherence, perceived decreases in quality of life). Understanding the mechanisms by which CR improves cardiometabolic fitness would be immensely valuable as it would illuminate novel therapeutic targets and allow for better treatment strategies. However, identifying one singular pathway is likely to prove difficult as CR is known to exert beneficial effects throughout every organ system in the body. It is therefore important for investigators to focus on CR within a specific disease context, at the cell and molecular level in the relevant organ system, to more clearly delineate underlying mechanisms.
In this issue of Diabetes, Johnson et al. (6) provide insight into the mechanism by which CR improves whole-body insulin sensitivity. The authors used the hyperinsulinemic-euglycemic clamp method to evaluate whole-body insulin sensitivity before and after a 16-week CR intervention in obese individuals. They also comprehensively evaluated a number of metabolic and molecular end points in the skeletal muscle and blood of the CR group and compared these values with a normal lifestyle control group. A substantial improvement in systemic insulin sensitivity was observed in the CR group, corresponding with a greater than twofold increase in nonoxidative glucose disposal rate in the skeletal muscle. Interestingly, muscle glucose and lipid oxidation capacity were unaffected by CR, as were mitochondrial respiratory capacity and reactive oxygen species (ROS) emission supported by carbohydrate-linked substrates. There were also no changes in muscle ceramide or diacylglycerol content with CR. The most striking observation was that the expression of thioredoxin-interacting protein (TXNIP) in the skeletal muscle decreased with CR and the degree of TXNIP downregulation was associated with the rate of glucose disposal during clamp measurements.
TXNIP belongs to the arrestin superfamily of proteins and was named for its ability to bind and negatively regulate thioredoxin via a disulfide bond (7). A critical role for TXNIP in regulating glucose metabolism in humans was revealed in a report where TXNIP was shown to strongly inhibit glucose uptake, and this effect was abrogated by insulin (8). In a recent study, TXNIP deficiency led to the upregulation of glycolysis in the skeletal muscle (9), a finding that nicely complements the findings of Johnson et al. (6). Although the exact mechanisms by which TXNIP acts in this manner are still unclear, one study reported that TXNIP negatively regulates Akt, and subsequent glucose uptake, by maintaining PTEN in a reductive (i.e., active) state (10). An obvious theory initially emerged among investigators that perhaps TXNIP was the “unifying mechanism” connecting nutrient overload to oxidative stress and insulin resistance. This theory was largely repudiated by a study showing that a Cys-247-Ser mutant of TXNIP, which does not bind thioredoxin and is insensitive to cellular redox status, is just as effective at blunting glucose uptake in cells as wild-type TXNIP is (11). A redox-independent role for TXNIP is supported by the findings of Johnson et al. (6) in that no changes in mitochondrial ROS emission occurred with CR. The authors did not measure global redox status (e.g., reduced/oxidized glutathione) of the skeletal muscle before and after CR, however, and that may prove to be the more important trigger for induction of TXNIP, as shown by others (12,13).
Another interesting outcome of this study is that many of the “usual suspects” that previously have been associated with muscle insulin sensitivity (e.g., mitochondrial respiration/ROS, ceramide, diacylglycerol) did not change with CR in this study. Despite this fact, the comprehensive analysis of muscle metabolism and related parameters presented by the authors will undoubtedly be useful for establishing future studies. For example, levels of carnosine, anserine, and taurine in the skeletal muscle increased with CR, and these dipeptides and amino acid metabolites are potent scavengers of lipid- and sugar-derived reactive carbonyl species (RCS) (14). A causal role for RCS in obesity-related cardiometabolic diseases is starting to emerge (15–17). Autophagy is another well-characterized process known to enhance cellular “metabolic fitness” in response to CR (18,19). This pathway was not examined by Johnson et al. (6), although temporal aspects of the study design likely prohibited the investigators from properly examining the skeletal muscle autophagic flux before and after CR.
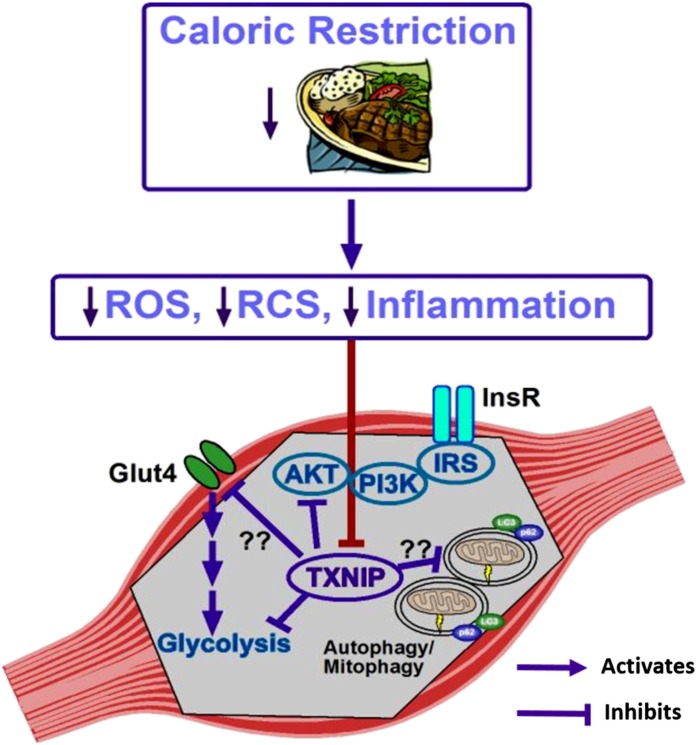
To conclude, although more work is clearly necessary to elucidate all the pathways involved, Johnson et al. (6) present compelling new evidence that the downregulation of TXNIP plays a role in the insulin-sensitizing effects of CR in the muscle (Fig. 1). In addition, they establish an excellent basis for hypothesis-driven studies to better understand and improve CR and for the development of novel therapeutics to mitigate obesity-related cardiometabolic diseases.
Figure 1.
Depiction of the known and proposed mechanisms by which CR normalizes skeletal muscle insulin sensitivity via TXNIP downregulation. In response to CR and subsequent decrease in adipose mass, oxidative stress, and inflammation, a signal is transmitted to cause the downregulation of TXNIP in the skeletal muscle. This drop in TXNIP eliminates the inhibitory effect of this protein on glycolysis and insulin-stimulated Akt activation. The pathways denoted with question marks (??) are those in which a role for TXNIP has yet to be defined. Glut4, glucose transporter type 4; InsR, insulin receptor; IRS, insulin receptor substrate; PI3K, phosphatidylinositol 3-kinase.
Article Information
Funding. Research in the laboratory of E.J.A. is funded by the National Institutes of Health National Heart, Lung, and Blood Institute (R01HL122863).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 74.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al.; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 3.Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res 2014;164:302–311 [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura E, Kumahara H, Tobina T, et al. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes 2014;2014:197216 [DOI] [PMC free article] [PubMed]
- 5.Bales CW, Kraus WE. Caloric restriction: implications for human cardiometabolic health. J Cardiopulm Rehabil Prev 2013;33:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson ML, Distelmaier K, Lanza IR, et al. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes 2016;65:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Radic Biol Med 2007;43:861–868 [DOI] [PubMed] [Google Scholar]
- 8.Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBalsi KL, Wong KE, Koves TR, et al. Targeted metabolomics connects thioredoxin-interacting protein (TXNIP) to mitochondrial fuel selection and regulation of specific oxidoreductase enzymes in skeletal muscle. J Biol Chem 2014;289:8106–8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui ST, Andres AM, Miller AK, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A 2008;105:3921–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patwari P, Chutkow WA, Cummings K, et al. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J Biol Chem 2009;284:24996–25003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 2004;279:30369–30374 [DOI] [PubMed] [Google Scholar]
- 13.Hui TY, Sheth SS, Diffley JM, et al. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem 2004;279:24387–24393 [DOI] [PubMed] [Google Scholar]
- 14.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev 2013;93:1803–1845 [DOI] [PubMed] [Google Scholar]
- 15.Katunga LA, Gudimella P, Efird JT, et al. Obesity in a model of Gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol Metab 2015;4:493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frohnert BI, Long EK, Hahn WS, Bernlohr DA. Glutathionylated lipid aldehydes are products of adipocyte oxidative stress and activators of macrophage inflammation. Diabetes 2014;63:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes 2010;59:1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest 2015;125:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinowitz JD, White E. Autophagy and metabolism. Science 2010;330:1344–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]