Abstract
Background
We here examined whether plasma desmosterol-to-cholesterol ratio (DES/CHO) is decreased in patients with Alzheimer's disease (AD) and investigated the association between plasma DES/CHO and longitudinal cognitive decline.
Methods
Plasma DES/CHO of AD patients and age-matched controls in a Japanese cross-sectional cohort was determined. Plasma DES/CHO at baseline and follow-up visits was assessed in relation to cognitive decline in Japanese and Swedish longitudinal cohorts.
Results
Plasma DES/CHO was significantly reduced in Japanese AD patients and significantly correlated with Mini-Mental State Examination (MMSE) score. The longitudinal analysis revealed that plasma DES/CHO in AD patients shows a significant decrease at follow-up intervals. The decline in plasma DES/CHO is larger in the AD group with rapid progression than in that with slow progression. The changes in plasma DES/CHO significantly correlated with changes in the MMSE score.
Conclusion
Plasma DES/CHO is decreased in AD patients and may serve as a longitudinal surrogate marker associated with cognitive decline.
Keywords: Alzheimer's disease, Mild cognitive impairment, Blood-based biomarker, Desmosterol, Longitudinal biomarker
1. Introduction
Alzheimer's disease (AD) is one of the most common and debilitating neurodegenerative disorders of the aging population. AD manifests itself as a progressive decline in memory accompanied by other cognitive and functional disabilities [1]. From the viewpoint of clinical practice and therapeutic clinical trials in AD, biomarkers are becoming increasingly important particularly when disease-modifying drugs will become available. Numerous studies have shown that tau, phosphorylated tau, and amyloid-β (Aβ) 42 in cerebrospinal fluid (CSF) are reliable biomarkers for AD diagnosis [2], [3], [4]. However, the CSF examination of AD patients has not been broadly applied in general clinical practice because lumbar puncture to obtain CSF is relatively invasive and time consuming. Moreover, these CSF markers do not seem to be associated with longitudinal cognitive decline in patients with AD [5]. Thus, there is a compelling need to establish a noninvasive biomarker for AD that follows the disease progression. Efforts to find reliable blood-based biomarkers for AD have met with little success [6]. Several reports have been published describing altered levels of proteins, peptides, or metabolites in patients with AD, but those blood-based biomarkers have proven difficult to replicate in independent studies [6], highlighting the importance of multiple validations.
In our previous report, we found that the plasma desmosterol-to-cholesterol ratio (DES/CHO) is significantly decreased in Caucasian patients with AD and subjects with mild cognitive impairment (MCI) [7]. Desmosterol is the most abundant precursor but rarely exceeds 1% of total brain sterols because the conversion from desmosterol to cholesterol is tightly regulated by the enzyme 3-hydroxysterol 24-reductase (DHCR24) [8]. A substantially higher desmosterol concentration in the hippocampus could be attributed to neurogenesis and synaptic plasticity that take place in the adult dentate gyrus [9]. Conversely, a decrease in desmosterol level in the hippocampus could at least in part correlate with the reduced number of progenitor cells differentiating into neurons [10]. These reports suggest an important role of desmosterol in the brain.
With this background, we here measured plasma DES/CHO of samples from a large Japanese cohort to extend our previous result that plasma DES/CHO is decreased in patients with AD in a different ethnic group. Furthermore, we performed longitudinal studies to determine the association between plasma DES/CHO and cognitive decline in patients with AD over time.
2. Materials and methods
2.1. Subjects
For cross-sectional analysis, plasma samples of 200 patients with AD and 201 age-matched cognitively normal elderly individuals (older than 65 years) were collected from seven clinical institutions in Japan (Table 1). The diagnosis of AD was made on the basis of the criteria of the National Institute of Neurological and Communicative Diseases and the Stroke–Alzheimer's Disease and Related Disorders Association [11]. Each participant was asked to complete the Mini-Mental State Examination (MMSE) [12]. APOE genotyping was performed as previously reported [13].
Table 1.
Demographic characteristics of AD patients and age-matched cognitively normal controls in the Japanese cross-sectional cohort
| Variable | Control (n = 201) | AD (n = 200) |
|---|---|---|
| Female (%) | 72 | 75 |
| Age, mean (SD) | ||
| Age at examination (y) | 77.6 (4.7) | 77.6 (5.4) |
| Age at onset (y) | n/a | 73.5 (5.0) |
| MMSE, mean (SD) | 28.7 (1.5) | 17.0 (5.2)∗∗ |
| APOE genotype | ||
| 2*3 | 15 | 6 |
| 2*4 | 2 | 1 |
| 3*3 | 151 | 71 |
| 3*4 | 32 | 99 |
| 4*4 | 1 | 23 |
| DES/CHO (10−6), mean (SD) | 456 (119) | 357 (134)∗∗ |
Abbreviations: AD, Alzheimer's disease; n/a, not available; MMSE, Mini-Mental State Examination; DES/CHO, desmosterol-to-cholesterol ratio.
∗∗P < .01.
For longitudinal analysis, we used 17 subjects with AD (Japanese longitudinal cohort collected at Niigata University Hospital) and 28 subjects (Swedish longitudinal cohort consisting of 6 control, 12 MCI, and 10 AD subjects collected at Uppsala University Hospital), whose blood was drawn at two different time points (Table 2). Additional longitudinal plasma samples of 30 subjects at least at 3 different time points were obtained from Uppsala University Hospital (AD, n = 6; MCI, n = 6; control, n = 2) or purchased from PrecisionMed, Inc. (AD, n = 12; control, n = 4) (San Diego, CA, USA). The criteria of Petersen et al. [14], [15] were used for the diagnosis of MCI. To be considered as having MCI, the patients had to be free of significant underlying medical, neurologic, or psychiatric illness and meet the following criteria: (1) subjective memory complaint, (2) objective signs of decline in any cognitive domain, (3) intact activities of daily living, and (4) clinical features not fulfilling the DSM-IV/ICD-10 criteria for dementia [16]. The two AD/MCI groups with slow and rapid progression were classified on the basis of the mean change in MMSE score (change from the baseline) as threshold. Written informed consent was obtained from each of the participants (or the respective legal guardian); the study was approved by the appropriate university, hospital, and company institutional ethics committees.
Table 2.
Demographic characteristics of subjects in the longitudinal study
| Variable | Japanese cohort, AD (n = 17) | Swedish cohort |
Combined cohort, MCI/AD (n = 39) | ||
|---|---|---|---|---|---|
| Control (n = 6) | MCI (n = 12) | AD (n = 10) | |||
| Female (%) | 71 | 75 | 33 | 30 | 56 |
| Age, mean (SD) | 68 (8) | 67 (9) | 62 (8) | 66 (10) | 66 (9) |
| Follow-up time, y, mean (SD) | 2.0 (1.0) | 2.7 (0.8) | 1.8 (0.9) | 2.2 (0.9) | 2.1 (1.0) |
| MMSE, mean (SD) | |||||
| Baseline | 20 (5) | 30 (1) | 28 (2) | 25 (3) | 24 (6) |
| Follow-up | 16 (7) | 30 (0) | 28 (2) | 22 (3) | 21 (7) |
| ΔMMSE | −4 (4)∗∗ | 0 (1) | 0 (2) | −3 (3)∗ | −2 (4)∗∗ |
| DES/CHO (10−6), mean (SD) | |||||
| Baseline | 329 (103) | 654 (146) | 660 (200) | 556 (232) | 489 (225) |
| Follow-up | 290 (79) | 661 (130) | 607 (237) | 487 (276) | 438 (237) |
| % Change | −10 (14)∗ | 1.7 (5.1) | −8.3 (21) | −18 (27) | −11 (20)∗∗ |
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; DES/CHO, desmosterol-to-cholesterol ratio.
∗P < .05, ∗∗P < .01.
2.2. Blood sampling and laboratory measurement
Peripheral blood samples were obtained from each participant using the commercially available blood collection tubes (Terumo Venoject for Japanese cohort and BD Vacutainer for Swedish cohort) containing EDTA as the anticoagulant. Plasma was separated by centrifugation at 1500 g for 15 minutes at room temperature before being aliquoted and stored at −80°C until analysis.
The concentrations of desmosterol and cholesterol were measured with a liquid chromatography mass spectrometer (LC/MS), as described previously [7]. Briefly, 25 μL of plasma was spiked with cholesterol-25,26,26,26,27,27,27-D7 and desmosterol-26,26,26,27,27,27-D6 as internal standards. Fifty percent potassium hydroxide was then added to the solution, which was then mixed thoroughly and incubated at 70°C for 60 minutes. After the incubation, 2 mL of hexane and 0.5 mL of phosphate-buffered saline (pH 6.8) were added and mixed well. The solution was centrifuged for 10 minutes at 2000 g, and the upper organic phase was transferred to a new tube. The lower layer was extracted with an additional 1 mL of hexane, which was also added to the organic-phase extract. The solvents were evaporated to dryness under a nitrogen gas stream at 40°C, and the obtained pellet was reconstituted in ethanol and the resulting solution was subjected to liquid chromatography/atomospheric pressure chemical ionization-mass spectrometer analysis as described previously [7].
2.3. Statistical analysis
Values are shown as mean ± standard deviation. Correlations between different variables were assessed using the Pearson correlation coefficient on log-transformed data. The t test or analysis of variance was carried out to determine differences between two or more groups. Nonparametric tests (Mann-Whitney U test) were carried out when the variables were not normally distributed. The paired t test was carried out to see the difference in the individual values between baseline and follow-up visits. The statistical significance was set at P < .05.
3. Results
3.1. Cross-sectional study of plasma desmosterol
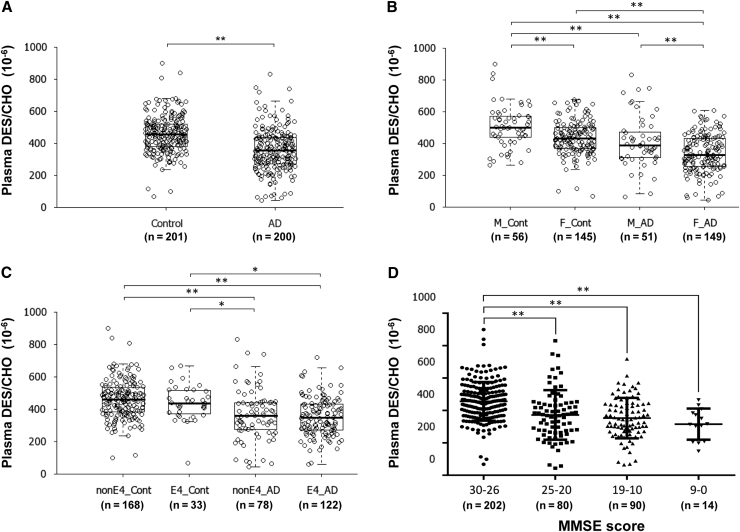
The characteristics of the AD and age-matched control subjects included in this cross-sectional study are listed in Table 1. A significant decline in plasma DES/CHO was observed in patients with AD compared with control subjects (P < .01; Fig. 1A). The decline in plasma DES/CHO in AD patients was significant regardless of gender or APOE ε4 status (Fig. 1B and C). There was a significant decline in plasma DES/CHO in AD patients with both the APOE ε3/3 and ε3/4 genotypes (data not shown; see Supplementary Fig. 1A and B for review). Plasma DES/CHO of female subjects in both the AD and control groups was significantly lower than that of male subjects (Fig. 1B, P < .01). No significant correlation of plasma DES/CHO with age was observed (data not shown). A significant correlation between plasma DES/CHO and MMSE score in both males and females was observed (data not shown; see Supplementary Fig. 1C and D). We divided subjects of this cohort into four MMSE score groups: high MMSE score (30 ≧ MMSE ≧ 26), middle MMSE score (25 ≧ MMSE ≧ 20), low MMSE score (19 ≧ MMSE ≧ 10), and very low MMSE score (9 ≧ MMSE ≧ 0). We then compared DES/CHO among these groups. A significant difference between the high MMSE score group and the other MMSE score groups was observed (Fig. 1D). The linear trend analysis revealed that there was also a significant change showing that groups with lower MMSE scores had lower DES/CHO (trend t test: P < .01).
Fig. 1.
Decreased plasma DES/CHO in patients with AD. (A) Comparison of plasma desmosterol-to-cholesterol ratio (DES/CHO) between 201 control and 200 AD subjects from a large Japanese cohort. (B) Comparison of plasma DES/CHO among male control (M_Cont), female control (F_Cont), male AD (M_AD), and female AD (F_AD) subjects. (C) Comparison of plasma DES/CHO between control subjects without APOE ε4 (nonE4_Cont) and with APOE ε4 (E4_Cont), and AD patients without APOE ε4 (nonE4_AD) and with APOE ε4 (E4_AD). (D) Comparison of plasma DES/CHO among groups classified as MMSE groups. ∗P < .05; ∗∗P < .01.
3.2. Longitudinal analysis
Longitudinal plasma samples were collected from two clinical institutes, namely, Niigata University Hospital (Japanese cohort) and Uppsala University Hospital Memory Clinic (Swedish cohort). Forty-seven participants composed of 17 subjects in the Japanese cohort and 30 subjects in the Swedish cohort were included, and the demographic characteristics of these subjects are listed in Table 2. At baseline, DES/CHO in Japanese AD patients was significantly lower than that of Swedish AD patients (P < .01).
In the Japanese longitudinal cohort, the average change in the MMSE score (ΔMMSE) between the baseline and follow-up visits was −4 ± 4 with a change in DES/CHO of −10 ± 14% (Table 2). Both MMSE score and plasma DES/CHO significantly decreased between the two visits in the Japanese cohort (paired t test, P < .05). In the Swedish cohort, the MMSE score (−3 ± 3) decreased significantly in AD patients between the baseline and follow-up visits (P < .05). However, in this cohort, there was no significant change in DES/CHO in both the AD patients and MCI subjects (Table 2). In the combined AD/MCI cohort, MMSE score (−2 ± 4) and plasma DES/CHO (−11 ± 20%) decreased significantly between the two visits (Table 2).
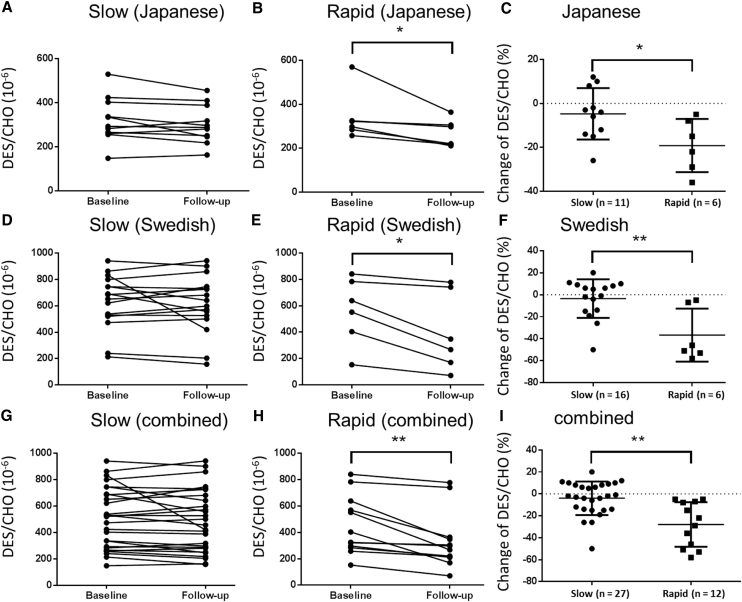
Next, we divided the AD/MCI subjects into two groups, namely, those with slow or rapid progression, on the basis of their mean ΔMMSE in the Japanese (cutoff score, −4) and Swedish cohorts (cutoff score, −2) and compared the longitudinal change in plasma DES/CHO between groups (Fig. 2). Although the AD/MCI group with slow progression did not show any significant change in plasma DES/CHO (Fig. 2A, D, and G), groups with rapid progression showed a significant decrease in plasma DES/CHO between the baseline and follow-up visits (Fig. 2B, E, and H). In addition, the change in plasma DES/CHO was significantly larger in the AD/MCI group with rapid progression than in the group with slow progression in the Japanese, Swedish, and combined cohorts (Fig. 2C, F, and I).
Fig. 2.
Change in plasma DES/CHO between baseline and follow-up visits. AD/MCI subjects were classified into two groups, namely, those with slow progression (A, D, G) and those with rapid progression (B, E, H) on the basis of the mean ΔMMSE between baseline and follow-up visits in a Japanese cohort (A–C), a Swedish cohort (D–F), and a combined cohort (G–I). Plasma DES/CHO remains stable in the group with slow progression (A, D, G), whereas in the group with rapid progression, a significant decline over time was found (B, E, H). The change in plasma DES/CHO was significantly larger in the group with rapid progression than in the group with slow progression (C, F, I). ∗P < .05; ∗∗P < .01.
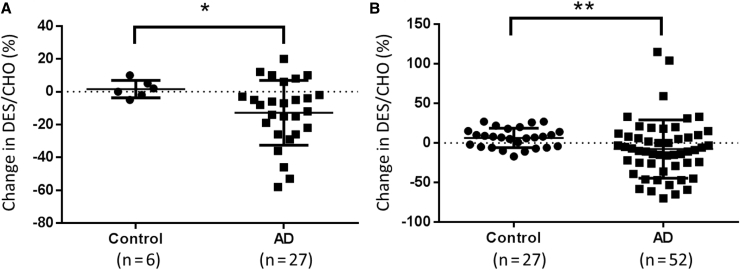
We further compared the longitudinal change in plasma DES/CHO in control subjects and AD patients (Fig. 3). The change in DES/CHO between the two visits was −12.8 ± 19.7 in the AD patients, which was significantly larger than that in the control subjects (1.7 ± 5.3; Fig. 3A). These results suggest that although plasma DES/CHO in normal subjects remained stable, plasma DES/CHO in AD patients tended to decline over time.
Fig. 3.
Comparison of longitudinal change in plasma DES/CHO between control and AD subjects. (A) Longitudinal changes in plasma DES/CHO at two different points were compared between control subjects and AD patients from the combined cohort. ∗P < .05. (B) Longitudinal changes in plasma DES/CHO at multiple points were compared between control subjects and AD patients from the Swedish cohort and a commercially available resource. ∗∗P < .01.
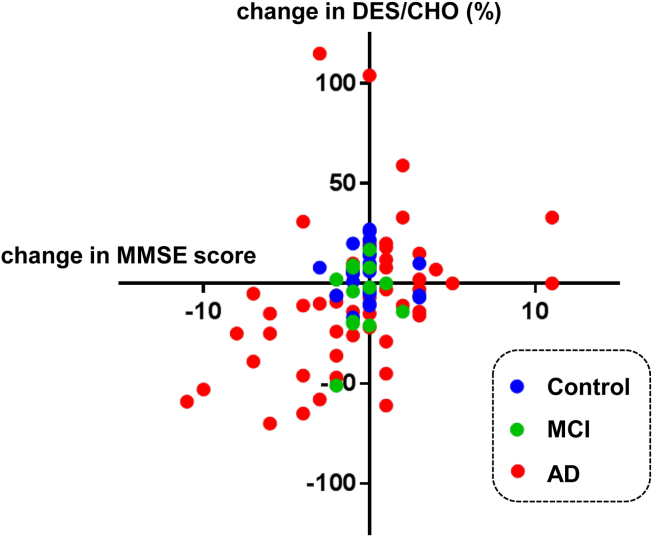
Finally, we performed another longitudinal study to determine the association between plasma DES/CHO and ΔMMSE in 30 participants, including 18 AD, 6 MCI, and 6 control subjects from either the Swedish cohort or from PrecisionMed. Blood samples were collected every year or every 6 months from the participants. There were 122 points for blood collection, consisting of 30 baselines and 92 follow-ups. The correlation between the ΔMMSE and the change in plasma DES/CHO (compared with baseline) determined using all 92 follow-up points is shown in Fig. 4. There was a significant correlation between the longitudinal ΔMMSE and the change in DES/CHO from the baseline (P = .01, r = 0.37). All the changes in MMSE score and plasma DES/CHO in each of the participants are shown (see Supplementary Fig. 2). In addition, AD patients showed a significant decrease in plasma DES/CHO at follow-up intervals (−7.7 ± 36.8%) compared with the control subjects (6.4 ± 12.3%) in this cohort (Fig. 3B).
Fig. 4.
Correlation between longitudinal changes in MMSE and plasma DES/CHO (%) in AD patients, MCI patients, and normal subjects. There were 122 points for blood collection, consisting of 30 baselines and 92 follow-ups (AD, 52 follow-ups; MCI, 13 follow-ups; and control, 27 follow-ups). There was a significant correlation between changes in MMSE and plasma DES/CHO (r = 0.37, P < .01).
4. Discussion
The present cross-sectional study using a Japanese cohort was undertaken to replicate our previous finding that plasma DES/CHO is decreased in Caucasian patients with AD. The following points were confirmed in the present Japanese and previously reported Caucasian cross-sectional cohorts: (1) plasma DES/CHO was decreased in patients with AD in comparison with control subjects, (2) the decrease in plasma DES/CHO in AD patients was independent of gender and APOE genotype, (3) female subjects tended to have a lower plasma DES/CHO than male subjects, and (4) plasma DES/CHO correlated significantly with the MMSE score. Taken together, the results suggest that plasma DES/CHO may be a potential diagnostic biomarker reflecting cognitive dysfunction in AD patients.
Recently, Popp et al. [17] have reported that the plasma desmosterol level does not change in AD patients. The discrepancy in finding between that and the present study may be explained by differences in the analytical methods used to determine the concentration of desmosterol. We previously showed that the LC/MS method that we used in the present study enables the purification of desmosterol in plasma more efficiently than the gas chromatography method used in the study by Popp et al. [7], [17]. Thus, the LC/MS method is likely to be more suitable for the measurement of plasma desmosterol concentration.
Here, we determined for the first time the longitudinal change in plasma DES/CHO and examined a possible association with concurrent cognitive decline in AD/MCI patients. Our results revealed (1) that plasma DES/CHO was relatively stable over time in cognitively normal controls, whereas it significantly decreased in AD patients; (2) a more pronounced decline in plasma DES/CHO in the AD/MCI group with rapid progression than in the group with slow progression; and (3) that the longitudinal change in plasma DES/CHO positively correlated with the change in the MMSE score. These results suggest that the plasma DES/CHO change is associated with the cognitive decline in AD and might be used to monitor the progression of cognitive decline in patients with AD. It will be interesting to clarify the usefulness of monitoring plasma DES/CHO as a surrogate marker for evaluating the effects of clinical drug trials in patients with AD.
Our longitudinal study suggests that the plasma DES/CHO changes before the appearance of clinical symptoms, as determined by MMSE in some cases (see Supplementary Fig. 2 for review; subjects A, C, and H). The result obtained from the subject with MCI that converted to AD (see Supplementary Fig. 2 for review; subject S) may suggest that plasma DES/CHO is useful as a progression marker to monitor the conversion from MCI to AD. In this connection, recent lipidomic analysis showed that the quantification of several lipid metabolites in plasma, such as phosphatidylcholine and acylcarnitine, is useful for predicting phenoconversion to amnestic MCI or AD in cognitively normal subjects [18].
There is now accumulating evidence that cholesterol metabolism may be relevant to the production and clearance of Aβ and thus to the Aβ-related toxicity in the pathogenesis of AD [19]. The strongest genetic risk factor for sporadic AD is the ε4 allele of APOE, which encodes apolipoprotein E (apoE), with a crucial role in cholesterol metabolism [20]. The presence of APOE ε4 may contribute to the pathologic accumulation and deposition of cerebral Aβ at early preclinical disease stages [21]. A recent study has shown that CSF apoE levels are decreased in patients with AD and that MCI in subjects with a low CSF apoE level will more likely convert to AD [22]. An interaction between APOE genotype and plasma desmosterol level may be postulated because desmosterol is the immediate precursor of cholesterol. However, the plasma desmosterol level was not clearly associated with APOE genotype in this study.
It is of particular interest that the level of desmosterol in the AD brain was found to be lower than that of control brain [7], [23]. It has been demonstrated that the levels of steroid hormones (e.g., progesterone, pregnenolone, and 17αOH-progesterone) that exhibit inhibitory activity against DHCR24 are decreased in the AD brain, particularly in the vicinity of plaques and neurofibrillary tangles [24]. Notably, the concentration of desmosterol is 100-fold higher in the rat brain than in the rat liver [25], which implies that most of the desmosterol in the blood might originate from the brain. Taken together, it could be speculated that brain desmosterol level may decrease with an increase in DHCR24 activity in the AD brain; this may subsequently result in a change in plasma DES/CHO. The question of why plasma DES/CHO decreases in patients with AD and is associated with longitudinal cognitive decline in the course of the disease warrants further investigation.
Although our findings, using samples from cross-sectional and longitudinal cohorts, are interesting, our study has some limitations. We did not analyze the samples from other types of dementia, including dementia with Lewy bodies, frontotemporal dementia, and vascular dementia. An additional cross-sectional study that includes samples from other types of dementia will be necessary. The number of samples from longitudinal cohorts in this study is relatively small. Longitudinal studies with a prospective design using a larger number of samples should be performed to confirm the utility of plasma DES/CHO as a longitudinal biomarker. Moreover, it is important to understand how early plasma DES/CHO starts to decline using longitudinal samples from asymptomatic AD subjects with amyloid deposition confirmed by amyloid-positron emission tomography imaging. Although our findings need to be validated in independent cohorts, our data suggest that the use of plasma desmosterol as a blood biomarker can be useful in the diagnosis of AD and also in monitoring disease progression.
Research in context.
-
1.
Systemic review: There is a compelling need to establish blood-based biomarker to diagnose Alzheimer's disease (AD) and monitor the disease progression. A previous study reported that plasma desmosterol-to-cholesterol ratio (DES/CHO) is significantly decreased in Caucasian patients with AD.
-
2.
Interpretation: We found that plasma DES/CHO was significantly reduced in Japanese AD patients. The longitudinal study revealed (1) that plasma DES/CHO was relatively stable in normal controls, whereas it significantly decreased in AD patients; (2) a more pronounced decline in plasma DES/CHO in the AD/MCI group with rapid progression than in that with slow progression; and (3) that the longitudinal change in plasma DES/CHO positively correlated with the change in MMSE score.
-
3.
Future directions: A future cross-sectional study that includes samples from other types of dementia and longitudinal studies with a prospective design using a larger number of samples need to be performed to confirm the utility of plasma DES/CHO.
Acknowledgments
The authors would like to thank the subjects who participated in this study. We would like to thank Dr. Makoto Ogo and Mr. Mamoru Yanagimachi who provided valuable comments. Special thanks also go to Mr. Kentaro Matsuura and Mr. Masayuki Ikawa whose support and information have helped us throughout this study. This study was funded by grants from Eisai Co. Ltd., Japanese Society of Promotion of Science, Ministry of Health, Labour and Welfare, Japan, and Uppsala University.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2014.11.009.
Supplementary data
References
- 1.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K., Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 3.Jack C.R., Jr., Holtzman D.M. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutphen C.L., Fagan A.M., Holtzman D.M. Progress update: Fluid and imaging biomarkers in Alzheimer's disease. Biol Psychiatry. 2014;75:520–526. doi: 10.1016/j.biopsych.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen C., Hansson O., Blennow K., Zetterberg H. Fluid biomarkers in Alzheimer's disease—current concepts. Mol Neurodegener. 2013;8:20. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y., Suzuki I., Nakamura T., Bernier F., Aoshima K., Oda Y. Identification of a new plasma biomarker of Alzheimer's disease using metabolomics technology. J Lipid Res. 2012;53:567–576. doi: 10.1194/jlr.M022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinse C.H., Shah S.N. The desmosterol reductase activity of rat brain during development. J Neurochem. 1971;18:1989–1998. doi: 10.1111/j.1471-4159.1971.tb09604.x. [DOI] [PubMed] [Google Scholar]
- 9.Couillard-Despres S., Iglseder B., Aigner L. Neurogenesis, cellular plasticity and cognition: the impact of stem cells in the adult and aging brain—a mini-review. Gerontology. 2011;57:559–564. doi: 10.1159/000323481. [DOI] [PubMed] [Google Scholar]
- 10.Jinno S. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol 2011;519:451-466. [DOI] [PubMed]
- 11.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Kuwano R., Miyashita A., Arai H., Asada T., Imagawa M., Shoji M. Dynamin-binding protein gene on chromosome 10q is associated with late-onset Alzheimer's disease. Hum Mol Genet. 2006;15:2170–2182. doi: 10.1093/hmg/ddl142. [DOI] [PubMed] [Google Scholar]
- 14.Petersen R.C., Morris J.C. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 7. [DOI] [PubMed] [Google Scholar]
- 15.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Giedraitis V., Sundelof J., Irizarry M.C., Garevik N., Hyman B.T., Wahlund L.O. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer's disease. Neurosci Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Popp J., Meichsner S., Kolsch H., Lewczuk P., Maier W., Kornhuber J. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer's disease. Biochem Pharmacol. 2013;86:37–42. doi: 10.1016/j.bcp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Mapstone M., Cheema A.K., Fiandaca M.S., Zhong X., Mhyre T.R., Macarthur L.H. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Paolo G., Kim T.W. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kok E., Haikonen S., Luoto T., Huhtala H., Goebeler S., Haapasalo H. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- 22.Toledo J.B., Da X., Weiner M.W., Wolk D.A., Xie S.X., Arnold S.E. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol. 2014;127:621–632. doi: 10.1007/s00401-013-1236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisniewski T., Newman K., Javitt N.B. Alzheimer's disease: brain desmosterol levels. J Alzheimers Dis. 2013;33:881–888. doi: 10.3233/JAD-2012-121453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenthal B., Holleran A.L., Aldaghlas T.A., Ruan B., Schroepfer G.J., Jr., Wilson W.K. Progestins block cholesterol synthesis to produce meiosis-activating sterols. FASEB J. 2001;15:775–784. doi: 10.1096/fj.00-0214com. [DOI] [PubMed] [Google Scholar]
- 25.Smiljanic K., Vanmierlo T., Djordjevic A.M., Perovic M., Loncarevic-Vasiljkovic N., Tesic V. Aging induces tissue-specific changes in cholesterol metabolism in rat brain and liver. Lipids. 2013;48:1069–1077. doi: 10.1007/s11745-013-3836-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.