Abstract
Background
The nuclear magnetic resonance (NMR) lipoprotein profile provides additional cardiovascular risk information beyond traditional lipids in high risk adults. Similar studies have not been conducted in youth.
Objective
To evaluate the relationship between the NMR profile and pre-clinical vascular measures in youth.
Methods
We studied 96 obese youth with pre-diabetes (mean age 18.1±3.6 years, 63% female, 78% African-American) and 118 obese normoglycemic controls (mean age 18.0±3.1 year, 75% female, 62% African American) cross-sectionally. Traditional lipids (triglycerides, total, HDL and LDL cholesterol), NMR particle size (particle concentration (P) and size) and vascular thickness (carotid IMT) and stiffness (pulse wave velocity (PWV)) were measured. Independent associations between lipoproteins with carotid IMT and PWV after adjustment for group, age, race, sex, BMI z score, blood pressure, HOMA-IR and A1c were studied.
Results
NMR analysis revealed youth with pre-diabetes exhibited a more atherogenic profile with higher levels of small LDL-P and HDL-P and lower levels of intermediate and large HDL-P (p<0.03). In addition, lower intermediate HDL-P was associated with a higher carotid IMT while higher small HDL-P was associated with a higher PWV (p<0.01). Traditional lipids were not significantly different between groups and were not associated with either vascular outcome.
Conclusions
NMR lipoprotein subclasses have improved sensitivity compared to traditional lipids to detect lipoprotein abnormalities in normoglycemic and pre-diabetic obese youth and are independently associated with pre-clinical vascular thickness and stiffness. Whether this enhances future cardiovascular risk in youth needs to be determined.
Keywords: lipids, lipoproteins, vasculature, pediatrics, obesity, pre-diabetes
Introduction
In adults, pre-diabetes, a precursor to type 2 diabetes, is associated with an increased risk to develop future cardiovascular events including myocardial infarction, stroke (1) and death (2). Youth with pre-diabetes appear to be on the same trajectory for similar complications. Youth with pre-diabetes have higher body weight, blood pressure, insulin levels (3, 4) and higher vascular stiffness and thickness (3), known predictors of future myocardial infarction and stroke (5, 6) compared to their normoglycemic obese peers.
Elevated total and low density lipoprotein (LDL) cholesterol levels are well-established risk factors for cardiovascular disease (CVD) in adults (7, 8). In fact, as part of the Framingham Risk Score, traditional lipids have long been used to assess CVD risk in adults (9). However, the role of these traditional lipid measurements (total, HDL, and LDL cholesterol and triglycerides) in adolescents and young adults is less clear as previous work has demonstrated that traditional lipids are not different in pre-diabetic and normoglycemic obese youth (3, 4) and are not associated with early markers of CVD (3). These findings may be explained by the fact that traditional lipid measurements are not sensitive enough to detect subtle lipoprotein changes because they only quantify the cholesterol within HDL and that associated with LDL is calculated.
NMR (nuclear magnetic resonance) lipoprotein measurements, on the other hand, have the ability to quantitate specific particle concentrations and sizes which may provide more information about the atherogenic properties of the lipoprotein (10–15). As a result, NMR profile has been shown to enhance CVD risk stratification (10–15). Whether the NMR profile improves CVD risk assessment in high risk youth has not been established.
Given youth with pre-diabetes appear to have a higher CVD risk, it is important to clarify the role of modifiable risk factors such as lipids and lipoproteins on markers of early atherosclerosis. As such, we sought to determine if lipoproteins derived from NMR spectroscopy could detect lipoprotein changes between obese normoglycemic and pre-diabetic youth. Additionally, we sought to determine if lipoprotein particle concentrations and/ or size were independently associated with measures of vascular thickness and stiffness. We hypothesized that the NMR profile would be superior to traditional lipids to detect associations with measures of vascular thickness and stiffness.
Materials and Methods
Participants
Participants in this analyses were recruited as part of a larger cross sectional study designed to assess the effects of obesity and type 2 diabetes on the heart and the vasculature between 2006–2010 at Cincinnati Children’s Hospital Medical Center (16–18). Only obese (body mass index (BMI) ≥ 95th percentile for sex and age) participants are included here as the research question here is an extension of our previous work that found no difference in traditional lipids between obese and normoglycemic youth and no association between traditional lipids and pre-clinical vascular measurements (3). Obese participants in this study were defined as having pre-diabetes using the American Diabetes Association Criteria, either 1) impaired fasting glucose defined as a fasting plasma glucose level ≥100–125mg/dl; 2) impaired glucose tolerance 2-hour plasma glucose concentration, measured by a 75-g oral glucose tolerance test (OGTT), ≥140–199 mg/dL; or 3) hemoglobin A1c (A1c) value of ≥5.7–6.4% (19). Normal glucose tolerance was defined as a normal fasting glucose level defined as <100mg/dl, normal glucose tolerance defined as 2 hour oral glucose tolerance plasma glucose concentration of <140mg/dl and A1c <5.7%. Any obese participant using metformin was excluded. Any participant with missing data was excluded.
Written informed consent was obtained from participants older than 18 years or from a parent or guardian with written assent for participants less than 18 years according to the guidelines established by the local institutional review board and in accordance with the Declaration of Helsinki.
Anthropometrics
Trained personnel measured height and weight twice with the average used in the analysis. Body mass index (BMI) was calculated as kg/m2 with a z score generated according to the U.S. Centers for Disease Control and Prevention reference criteria (20). Blood pressure was measured three times manually with a mercury sphygmomanometer (Baum Desktop model with V-Lok cuffs, Copiague, NY) according to the Fourth Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (21) and averaged.
Blood Analysis
Fasting blood was drawn for traditional lipids, glucose, insulin, A1c and an additional plasma aliquot was stored at −80 degree °C. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides were analyzed in a National Institute of Health and Centers for Disease Control and Prevention standardized laboratory using a Roche reagent on a Hitachi Modular P autoanalyzer (Roche Diagnostics, Indianapolis, IN, USA). Low density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald equation for individuals with triglyceride levels<400 mg/dL and by Lipid Research Clinics Beta Quantification for those with triglyceride levels ≥400 mg/dL. Glucose was measured using a Hitachi model 704 glucose analyzer. Fasting insulin was measured by radioimmunoassay with an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St Louis, MO), and a double antibody method to separate bound from free tracer. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated (glucose *insulin) /22.5. Hemoglobin A1c was measured in red blood cells by using high-performance liquid chromatography. C-reactive protein (CRP) was measured with the use of a high-sensitivity ELISA (Linco Research, Inc).
NMR lipoproteins were analyzed using LipoProfile-3 algorithm by LipoScience, Inc (Raleigh, North Carolina) on plasma that had been stored at −80 °C. Previous studies have shown NMR lipoprotein particle analyses are unaffected by frozen storage (22). To summarize NMR technology utilizes the concept that each size lipoprotein in plasma emits its own NMR signal characteristic of its lipid methyl group with intensity proportional to its abundance. Different particles concentrations are therefore derived from the measured amplitudes. The amplitude of each signal is proportional to the quantity of the lipoprotein subclass and is converted to a particle concentration (nmol/L). The size of the particles in nanometers (nm) is then calculated by the sum of the diameter of each subclass multiplied by its relative mass percentage as estimated from the amplitude of its NMR methyl signal (23, 24).
Outcome Variables (Vascular Measurements)
Carotid intima media thickness (IMT) was measured using B mode ultrasonography with a high resolution linear array transducer centered at 7.5MHz (GE Vivid 7, GE Medical Systems, Wauwatosa WI). For each participant, three independent measurements of bilateral far walls of the common carotid artery were measured in millimeters (mm). Digital images were transmitted and read offline by a single sonographer using a manual trace technique to measure the maximum carotid thickness. The right and left carotid segments were averaged in the analysis. Coefficients of variation for repeat readings are 5.4–8.0% (18).
Pulse wave velocity (PWV) was measured with a SphygmoCor SCORPVx System (AtcorMedical). For each participant, the distance (in mm) from the carotid artery to the suprasternal notch (using a tape measure) and from the suprasternal notch to the femoral artery (using calipers, Cardiovascular Engineering, Norwood MA) was measured three times, and averaged. Additionally, a tonometer was used to collect proximal and distal arterial waveforms at both sites using a simultaneously recorded electrocardiogram. PWV then was calculated as the distance from the carotid-to-femoral artery divided by the time delay measured between the carotid and femoral waveforms reported in meters per second (5). Pulse wave velocity is based on the principle that the pressure pulse generated by the left ventricular ejection travels at a speed determined by the size, shape, and properties of the artery (25) with a higher pulse wave velocity indicating increased vascular stiffness. PWV is the gold standard measurement of arterial stiffness in both adults and children (5, 26) and has been shown to predict future cardiovascular events and mortality (27). Three measurements were obtained on each participant and averaged. Repeat measures show a coefficient of variation of <5.2%.
Statistics
All analyses were performed using SAS Version 9.3 (SAS, Cary North Carolina). Values are reported as mean ± standard deviation or median (1st quartile, 3rd quartile). Group comparisons were made by t-tests for normally distributed variables and by Wilcoxon rank-sum test for non-normally distributed variables with a p value of <0.05 indicating significance. Variance stabilizing procedures were employed as needed. Predictors of carotid IMT and PWV were sought after using backward elimination linear regression analysis to allow us to assess the most predictive variables of each outcome. The criterion for variables to remain in the model was set to <0.05. Variables considered in each model included group (pre-diabetes vs. obese), age, race, sex, systolic and diastolic blood pressure, body mass index z score, HOMA-IR, hemoglobin A1c and CRP. These variables were chosen since each has been previously found to be associated with one or both of the outcomes of interest. Model 1 evaluated the role of traditional lipid measures (LDL-C, HDL-C and triglycerides) on the outcomes of interest. Model 2 excluded LDL-C, HDL-C and triglycerides and included total particle concentration (VLDL-P, LDL-P and HDL-P) and size. Model 3 sought to determine whether specific particle subtypes (small, intermediate and large VLDL, LDL, HDL) were associated with carotid IMT or PWV. Model 3 therefore excluded LDL-C, HDL-C and triglycerides, total particle concentration (VLDL-P, LDL-P and HDL-P) and size and only including subparticle concentrations. This multistep modelling was taken 1) to decrease the number of lipids variables in each model, and 2) to allow for each of the lipoprotein classes (LDL, VLDL and HDL) to compete with one another to determine, if any, were independently associated with the vascular outcomes. This approach has been shown to be a valid way to detect meaningful disease associations (15). Collinearity amongst the lipoproteins in each model was assessed using a variance inflation factor (VIF). Group by lipid interactions were tested on PWV and carotid IMT. Given that the sample size for this study was fixed as the cohort was recruited from a previous cross sectional study, sample size calculations were not performed. Analyses were done without correction of for multiple comparisons given the exploratory nature study.
Results
Table 1 shows there were no differences in age and sex distribution among obese youth with and without pre-diabetes. There was a higher proportion of African Americans in the prediabetes group compared with the normoglycemic group, p<0.006. The pre-diabetes group also had a higher body mass index (and z scores), fasting insulin, HOMA-IR levels and evidence of more dysglycemia (higher fasting and 2 hour oral glucose tolerance test glucose and A1c), all p<0.05. For details of how pre-diabetes was diagnosed, see Table 2.
Table 1.
Characteristics of the Study Population
| Variable | Obese Normoglycemic n=118 |
Obese Pre-diabetic n=96 |
p value |
|---|---|---|---|
| Age, years | 18.0± 3.1 | 18.1± 3.6 | 0.92 |
| Sex (female), n (%) | 88 (75%) | 60 (63%) | 0.06 |
| Race | 0.014 | ||
| Caucasian, n (%) | 45(38%) | 20(21%) | |
| African American, n (%) | 73(62%) | 75(78%) | |
| Hispanic, n (%) | 0(0%) | 1(1%) | |
| Smokers, n (%) | 5 (4%) | 2 (2%) | 0.38 |
| Weight (kg) | 100.3± 20.3 | 106.7± 22.6 | 0.030 |
| Body mass index (kg/m2) | 36.2± 6.4 | 38.3± 7.8 | 0.036 |
| BMI z score | 2.1± 0.4 | 2.2± 0.3 | 0.014 |
| Systolic blood pressure (mm Hg) | 116± 12 | 118.4± 9.8 | 0.06 |
| Diastolic blood pressure (mm Hg) | 66± 13 | 66± 11 | 0.94 |
| Fasting insulin (mIU/mL) | 17.4 (12.7, 22.8) | 20.2 (15.3, 29.6) | 0.003 |
| Fasting glucose (mg/dL) | 90.0 (86.5, 93.5) | 95.1 (89.1, 100.9) | <0.001 |
| 2-h OGTT glucose (mg/dL) | 103.4 (90.4, 117.2) | 114.8 (98.5, 132.8) | <0.001 |
| Hemoglobin A1c (%) | 5.3 (5.1, 5.5) | 5.7 (5.6, 5.9) | <0.001 |
| HOMA-IR | 3.9(2.8, 5.2) | 5.7 (5.6, 5.9) | <0.001 |
| C reactive protein (mg/dL) | 2.8(1.1, 6.8) | 2.8 (1.4, 6.8) | 0.53 |
| Carotid intima media thickness (mm) | 0.48 (0.43, 0.54) | 0.50 (0.45, 0.55) | 0.13 |
| Pulse Wave Velocity (m/s) | 6.2 (5.6, 6.8) | 6.3 (5.5, 7.1) | 0.29 |
Smoking was self-report data. HOMA-IR= homeostatic model assessment of insulin resistance.
Values are mean ± SD or median (Q1, Q3).
Table 2.
Classification of Study Population by Pre-diabetes Group
| Diagnosis of Pre-diabetes | N (%) |
|---|---|
| Hemoglobin A1c only | 50 (52%) |
| Impaired fasting glucose (IFG) only | 18 (19%) |
| Impaired glucose tolerance (IGT) only | 6 (6%) |
| IFG and Hemoglobin A1c | 12 (13%) |
| IFG and IGT | 2 (2%) |
| IGT and Hemoglobin A1c | 7 (7%) |
| IFG, IGT and Hemoglobin A1c | 1 (1%) |
| Total | 96 (100%) |
There were no significant differences in total cholesterol, LDL-C, HDL-C or triglycerides between groups (Table 3). The NMR lipid profile revealed the pre-diabetes group had a significantly higher concentration of small LDL-P and significantly smaller average LDL-P size, p<0.05. Similarly, the pre-diabetes group had a higher concentration of small HDL-P but a lower concentration of large and intermediate sized HDL-P with an overall a smaller average HDL-P size, p<0.05. There were no differences in IDL or VLDL (total, large, intermediate, small) concentrations or size. The above analyses were repeated excluding participants with pre-diabetes diagnosed by A1c only and the data were unchanged.
Table 3.
Lipids and Lipoprotein Values in the Study Population
| Variable | Obese Normoglycemic n=118 |
Obese Pre-diabetic N=96 |
p value |
|---|---|---|---|
| Lipids (mg/dl) | |||
| Total cholesterol | 172 (147, 193) | 169 (149, 192) | 0.85 |
| LDL cholesterol | 102 (85, 121) | 102 (86, 128) | 0.62 |
| HDL cholesterol | 47 (42, 54) | 45 (41, 51) | 0.25 |
| Triglycerides | 81 (60, 111) | 86 (62, 113) | 0.44 |
| Lipoprotein Particle Concentrations | |||
| LDL-P (nmol/L) | |||
| Total | 1037 (834, 1266) | 1098 (898, 1353) | 0.10 |
| Large | 527 (398, 649) | 484 (393, 648) | 0.46 |
| Small | 426 (247,559) | 468 (339, 673) | 0.010 |
| IDL-P (nmol/L) | 124.5 (72.0, 177.0) | 106.5 (63.5, 160.5) | 0.19 |
| HDL-P (µmol/L) | |||
| Total | 32.2 (28, 38.8) | 31.9 (29.5, 36.6) | 0.75 |
| Large | 4.4 (3.1, 6.1) | 4.0 (2.4, 5.2) | 0.027 |
| Intermediate | 12.5 (9.2, 15.5) | 10.8 (8.3, 13.0) | 0.007 |
| Small | 15.6 (12.7, 18.3) | 17.2 (14.5, 19.7) | 0.004 |
| VLDL_P (nmol/L) | |||
| Total | 2.4 (1.3, 4.0) | 2.5 (1.5, 4.2) | 0.72 |
| Large | 43.2 (26.5, 60.6) | 41.8 (25.1, 56.8) | 0.71 |
| Intermediate | 13.7 (7.9, 26.8) | 11.8 (7.5, 23.4) | 0.64 |
| Small | 23.7 (14.6, 33.9) | 21.7 (14.7, 32.3) | 0.53 |
| Lipoprotein Particle Size (nm) | |||
| LDL | 21.1 (20.8, 21.4) | 21 (20.6, 21.3) | 0.033 |
| HDL | 9.0 (8.7, 9.3) | 8.8 (8.6, 9.1) | 0.010 |
| VLDL | 47.2 (44.1, 51.3) | 47.8 (44.1, 51.6) | 0.58 |
Values are median (Q1, Q3).
Multiple linear regression analyses found that LDL-C, HDL-C and triglycerides were not independently associated with either carotid IMT or PWV (model 1, Table 4). Variables that were significantly associated with a higher carotid IMT included older age, male sex and non-Caucasian race (majority African American). Older age, female sex, non-Caucasian race, body mass index z score and systolic and diastolic blood pressure were significantly associated with a higher PWV.
Table 4.
Determinants of Vascular Thickness and Stiffness
| Carotid Intimal Media Thickness (mm) | Pulse Wave Velocity (m/s) | |||||
|---|---|---|---|---|---|---|
| Variables | Model 1- CVRFs + traditional lipids |
Model 2- CVRFs + total particle number and size |
Model 3-CVRFs + subparticle concentrations |
Model 1- CVRFs + traditional lipids |
Model 2- CVRFs + total particle number and size |
Model 3-CVRFs + subparticle concentrations |
| Age (years) | 0.225 (0.0008) | 0.223 (0.0009) | 0.226 (0.0006) | 0.472 (0.0000) | 0.450 (0.0000) | 0.445 (0.0000) |
| Sex (Male) | 0.180 (0.0068) | 0.195 (0.0035) | 0.201 (0.0025) | −0.211 (0.0003) | −0.227 (0.0001) | −0.199 (0.0005) |
| Race (African American) | 0.133 (0.0457) | 0.181 (0.0009) | 0.195 (0.0003) | 0.188 (0.0004) | 0.133 (0.0457) | |
| BMI z score | 0.268 (0.0000) | 0.234 (0.0001) | 0.238 (0.0000) | |||
| Systolic BP (mmHg) | 0.154 (0.0217) | 0.166 (0.0121) | 0.144 (0.0277) | |||
| Diastolic BP (mmHg) | 0.163 (0.0109) | 0.153 (0.0154) | 0.173 (0.0059) | |||
| HbA1c (%) | 0.180 (0.0068) | 0.144 (0.0333) | ||||
| HDL-P | −0.143 (0.0317) | |||||
| Intermediate HDL-P | −0.161 (0.0179) | |||||
| HDL size | −0.138 (0.0116) | |||||
| Small HDL-P | 0.165 (0.0018) | |||||
| R2 | 0.10 | 0.11 | 0.13 | 0.51 | 0.52 | 0.53 |
BMI=body mass index; BP=blood pressure; HbA1c=hemoglobin A1c. Data are presented as standardized parameter estimate with p values in parentheses. Only significant variables (p<0.05) were retained in the final model. In all models, the following CVRFs variables were considered: group (pre-diabetes vs. obese), age, race, sex, systolic and diastolic blood pressure, body mass index z score, HOMA-IR, hemoglobin A1c, hs-CRP. Model 1 included LDL-C, HDL-C, triglycerides and CVRFs. Model 2 included total LDL, VLDL, and HDL particle number and size + CVRFs and excluded LDL-C, HDL-C and triglycerides. Model 3 included subparticle concentrations + CVRFs and excluded LDL-C, HDL-C and triglycerides, total particle number and size.
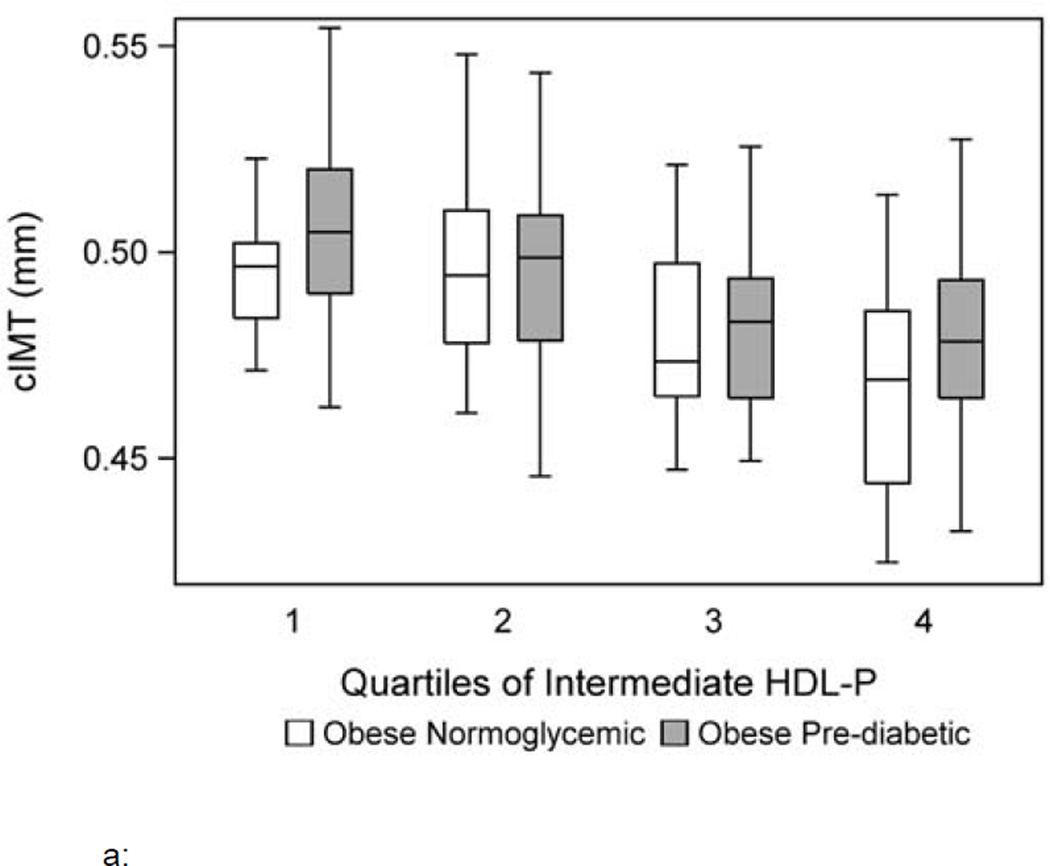
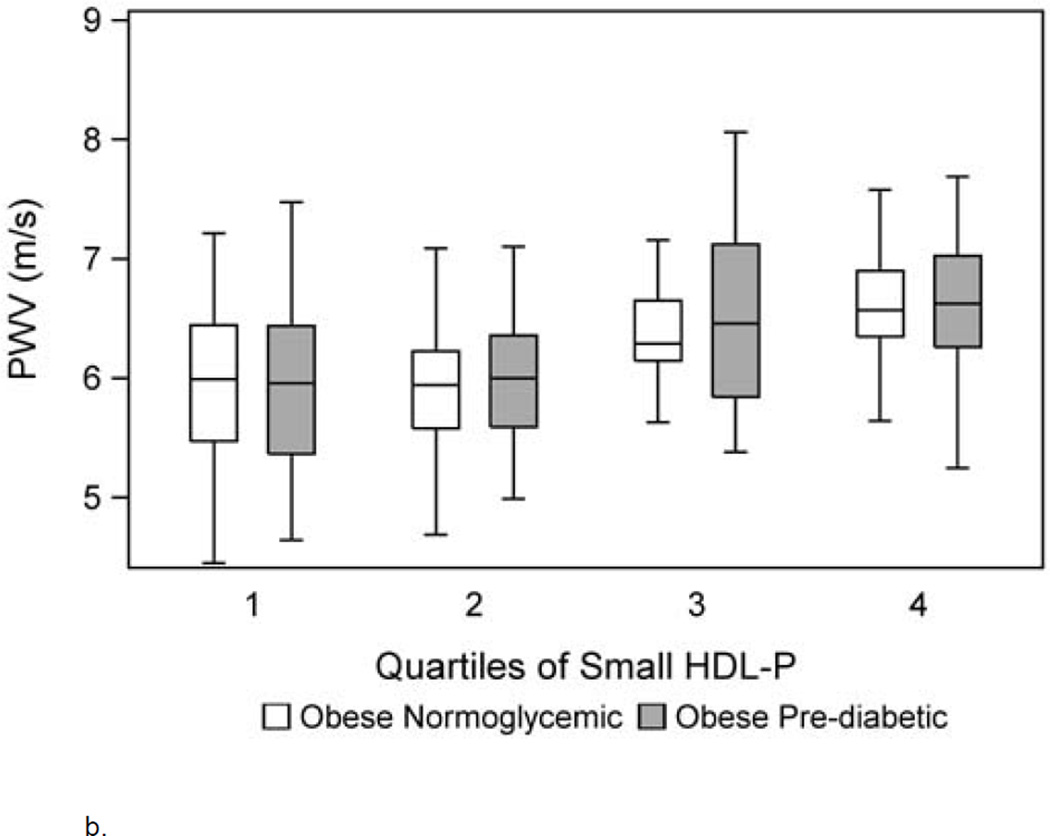
When total lipoprotein particle number and size were evaluated, total HDL-P and HDL size were independently and inversely associated with carotid IMT and PWV, respectively (model 2). To determine which lipoprotein subtypes may be responsible for the associations seen in model 2, model 3 was evaluated using only particle subtypes. Model 3 shows a lower intermediate HDL-P was associated with a higher carotid IMT while a higher small HDL-P was associated with a higher PWV (p<0.01). Figure 1a and b depicts carotid IMT and PWV for each quartile of intermediate and small HDL-P, respectively, adjusted for other risk factors.
Figure 1.
a: Carotid IMT by group per quartile of intermediate HDL-P adjusted for covariates including age, race, sex, systolic and diastolic blood pressure, body mass index z score, HOMA-IR, hemoglobin A1c and hs-CRP.
b. Carotid femoral PWV by group per quartile of small HDL-P adjusted for covariates including age, race, sex, systolic and diastolic blood pressure, body mass index z score, HOMA-IR, hemoglobin A1c and hs-CRP.
Model 3 for carotid IMT and PWV was adjusted for LDL-P and the results were unchanged. Collinearity was assessed among the lipid parameters included in each model using VIF and no collinearity was detected. The maximum VIF for any model was 2, where a value of >5 suggests collinearity among the variables in each model (28). Group by lipid interactions were not significant in the final models suggesting the association of intermediate and small HDL-P on thickness and stiffness was not different between normoglycemic and pre-diabetic obese youth. Finally, all models were repeated first by removing group and then by replacing A1c and HOMA-IR with fasting glucose and insulin and the results were unchanged. Fasting glucose and insulin were not associated with carotid IMT and PWV and did not change the associations between lipids and lipoproteins and the outcomes of interest.
Discussion
Using NMR lipoprotein analysis we show youth lipoprotein abnormalities exist in youth with pre-diabetes and they have higher levels of small LDL-P and small HDL-P and lower levels of intermediate and large HDL-P. We also show that higher concentrations of small HDL-P and lower concentrations of intermediate HDL-P are independently associated with vascular thickness and stiffness. These results suggest that NMR subparticle analysis is a more sensitive measure of lipoprotein changes that occur in pre-diabetic youth and may be a better predictor of early vascular thickness and stiffness.
Obese youth with pre-diabetes had a more atherogenic lipid profile (16, 29–31) with an enrichment of smaller particles (higher small LDL-P and HDL-P) and lower intermediate and large HDL-P compared to normoglycemic youth. This was seen despite no significant difference in the TC, LDL-C, HDL-C or triglycerides quantified by the traditional lipid profile. This finding is in agreement with prior work in youth published by Magge et al in 2012 where they showed in a younger and smaller cohort of n=21 pre-diabetics and n=76 controls higher concentrations of small LDL-P and HDL-P in youth with pre-diabetes without changes in total cholesterol, HDL-C or LDL-C. This enrichment of smaller particles with less intermediate and large particles in pre-diabetes is likely driven by inefficient insulin action in the muscle (32) and liver (33) that promotes increased VLDL production from the liver and increased cholesterol ester transfer protein (CETP) transfer of triglyceride to LDL and HDL particles. The resulting triglyceride enriched HDL and LDL particles become a good substrate for increased action by hepatic and lipoprotein lipase which generates smaller HDL and LDL particles (34).
In high risk youth with obesity, the association between the NMR lipoprotein profile and non-invasive arterial stiffness and thickness has not been demonstrated. Thus, our work extends on the current literature. This observation lends itself to three additional points that will each be discussed separately below 1) different HDL subtypes appear to have different effects on the vasculature; 2) HDL-P is independently associated with higher arterial thickness and stiffness; and 3) the lipoprotein subclass profile may be a better predictor of early vascular thickness and stiffness.
Higher small HDL-P were found to be associated with a higher PWV while lower intermediate HDL-P were associated with a higher carotid IMT. This suggests that HDL subtypes are not all equal or atheroprotective. We have previously shown similar results utilizing a gel filtration chromatography system to separate HDL subspecies by size. We found that while a depletion of large HDL subspecies was significantly inversely associated with higher PWV in adolescents with type 2 diabetes, the opposite was seen for small HDL particles, there was a significant direct correlation with PWV (35). The reason for the differential associations between HDL subtypes and vascular measures is not clear but may be due to impaired function in small HDL particles. Nobecourt et al has previously found antioxidant activity is impaired in small HDL particles (HDL 3b, 3c) in adults with type 2 diabetes (36).
We found no relationship with LDL-P. Lack of association between LDL-P and carotid IMT and PWV may be because the mean LDL-P for this group falls close to the low risk reference range for adults. It is therefore possible that with worsening LDL-P that an independent association would be observed similar to adult studies (12, 13). Studies that include other racial/ethnic distributions, larger sample size, and additional vascular endpoints such as brachial ankle PWV or femoral ankle PWV is also important as previous work has detected associations between these vascular measurements and LDL-P but not carotid femoral PWV (37).
Instead, we found measures of HDL-P to have an association with carotid IMT and PWV after adjustment for LDL-P. HDL-P has been shown to predict coronary events and death in adults (14, 22, 38–41). Specifically, the Multi-Ethnic Study of Atherosclerosis (MESA) cohort found HDL-P predicted cardiovascular risk better than HDL-C even after adjusting for LDL-C and LDL-P (14). Whether therapies aimed at improving intermediate HDL-P while lowering small HDL-P will improve future CVD risk remains to be established.
Traditional lipid measurements were not independently associated with arterial thickness and stiffness. This finding is discordant with work in adults (42) and previous work in lean, obese and youth with type 2 diabetes where HDL-C and TG/HDL-C ratio have been shown to associate with carotid IMT and arterial stiffness, respectively (43, 44). Lack of associations between traditional lipids and preclinical vascular thickness and stiffness in obese youth with and without pre-diabetes may be due to the smaller number of individuals evaluated here but also point to the improved sensitivity of NMR lipids over the traditional lipid panel.
Data evaluating the association between lipoprotein subclasses and early arterial thickness and stiffness in youth is limited. Gallo et al showed no relationship between lipoprotein subclasses and augmentation index (by radial tonometry), in youth with type 1 diabetes (n=35) concluding that in type 1 diabetes, other risk factors besides lipids may play a more important role in arterial stiffness (45). Potential explanations for why we detected associations between lipoprotein subclasses and vascular thickness and stiffness include a larger sample size here, a more atherogenic lipoprotein profile as a result of concomitant obesity and insulin resistance, and clear differences in the pathophysiology of the two different forms of diabetes. Given the many studies in adults demonstrating enhanced CVD risk stratification using the NMR profile over traditional lipids, our data suggest this may also be the case for high risk adolescents with obesity. However, it should be noted though that the improved variance in the vascular outcomes explained by the NMR lipid profile are small. Thus, the cost benefit analysis of this testing need to be considered before decisions of utility in youth can be decided.
This study has some limitations. It is cross sectional study and cannot demonstrate causality. We also collected lipids at one point in time and the NMR lipoproteins were performed on frozen samples. We lacked pubertal staging, other inflammatory markers, oxidized LDL particles, and acute glycation end products all of which could be important confounders to explain the low R2 in carotid IMT that need to be addressed in future work. We also lack apo-A1 and apoB levels and therefore could not compare these measures to the NMR lipid profile. Additionally, a large majority of participants studied were diagnosed with pre-diabetes by hemoglobin A1c at a single time point. While the results in Table 3 were unchanged with this group excluded, there are limitations of the hemoglobin A1c measurement and future work should include additional metrics to rule out spurious results (46). These analyses were performed on a previous recruited study cohort therefore our sample was fixed. Thus, whether these results can be extended to a non-obese cohort or other race/ethnicity (that are not majority African American) needs to be confirmed. Finally, we performed statistical tests on multiple outcomes thus caution must be exercised in interpreting these findings.
In conclusion, the NMR lipoprotein analysis has improved sensitivity to detect early lipoprotein changes in pre-diabetic youth. Additionally, the NMR lipoprotein analysis can detect associations with measures of vascular thickness and stiffness not seen with traditional lipids.
Highlights.
Traditional lipids are not different between normoglycemic and pre-diabetic youth
Traditional lipids are not associated with carotid IMT and pulse wave velocity
NMR analysis shows pre-diabetic youth have a more atherogenic lipid profile
NMR lipoproteins are associated higher carotid IMT and pulse wave velocity
NMR lipoproteins have improved sensitivity compared to traditional lipids
Acknowledgments
Funding: This work was supported by the NIH (R01 HL076269, K23HL118132 and UL1 RR026314).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author Contributions: ASS designed the study and wrote the manuscript, WSD and EMU designed the study and edited the manuscript, ZG conducted the statistical analysis and edited the manuscript and LMD and TRK edited the manuscript.
References
- 1.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br Med J (Clin Res Ed) 1983;287:867–870. doi: 10.1136/bmj.287.6396.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 3.Shah AS, Gao Z, Urbina EM, Kimball TR, Dolan LM. Prediabetes: the effects on arterial thickness and stiffness in obese youth. J Clin Endocrinol Metab. 2014;99:1037–1043. doi: 10.1210/jc.2013-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magge SN, Prasad D, Koren D, Gallagher PR, Mohler ER, 3rd, Stettler N, Levitt Katz LE, Rader DJ. Prediabetic obese adolescents have a more atherogenic lipoprotein profile compared with normoglycemic obese peers. J Pediatr. 2012;161:881–886. doi: 10.1016/j.jpeds.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 6.Brohall G, Oden A, Fagerberg B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med. 2006;23:609–616. doi: 10.1111/j.1464-5491.2005.01725.x. [DOI] [PubMed] [Google Scholar]
- 7.Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47:4–24. doi: 10.2105/ajph.47.4_pt_2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner RM, Pearson TA. LDL-cholesterol: a risk factor for coronary artery disease--from epidemiology to clinical trials. Can J Cardiol. 1998;14(Suppl B):3B–10B. [PubMed] [Google Scholar]
- 9.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 10.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 11.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PW, D'Agostino RB. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study - Implications for LDL Management. J Clin Lipidol. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O'Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AS, Khoury PR, Dolan LM, Ippisch HM, Urbina EM, Daniels SR, Kimball TR. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011;54:722–730. doi: 10.1007/s00125-010-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119:2913–2919. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Standards of medical care in diabetes--2015: summary of revisions. Diabetes Care. 2015;38(Suppl: S4) doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 21.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 22.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 23.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48:171–180. [PubMed] [Google Scholar]
- 25.London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;138:220–224. doi: 10.1016/s0002-8703(99)70313-3. [DOI] [PubMed] [Google Scholar]
- 26.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus-Snyder M, Rocchini A, Steinberger J, McCrindle B H. American Heart Association Atherosclerosis, and Y. Obesity in Youth Committee of the Council on Cardiovascular Disease in the. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 27.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 28.Belsley DA, Kuh E, Welsch E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. 1980 [Google Scholar]
- 29.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109:III2–III7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 30.Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009;150:474–484. doi: 10.7326/0003-4819-150-7-200904070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cholesterol Education Program Expert Panel on Detection, E. and A. Treatment of High Blood Cholesterol. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 32.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108:3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon SM, Davidson WS, Urbina EM, Dolan LM, Heink A, Zang H, Lu LJ, Shah AS. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62:2958–2967. doi: 10.2337/db12-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobecourt E, Jacqueminet S, Hansel B, Chantepie S, Grimaldi A, Chapman MJ, Kontush A. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 2005;48:529–538. doi: 10.1007/s00125-004-1655-5. [DOI] [PubMed] [Google Scholar]
- 37.Vishnu A, Choo J, Masaki KH, Mackey RH, Barinas-Mitchell E, Shin C, Willcox BJ, El-Saed A, Seto TB, Fujiyoshi A, Miura K, Lee S, Sutton-Tyrrell K, Kuller LH, Ueshima H, Sekikawa A. Particle numbers of lipoprotein subclasses and arterial stiffness among middle-aged men from the ERA JUMP study. J Hum Hypertens. 2014;28:111–117. doi: 10.1038/jhh.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 39.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, Robinson JG, Lund B, Kuller LH G. Women's Health Initiative Research. Lipoprotein particle concentrations may explain the absence of coronary protection in the women's health initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–1671. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R G. Multiple Risk Factor Intervention Trial Research. Lipoprotein particles, insulin, adiponectin, Creactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of Black Race Between High Density Lipoprotein Cholesterol Content, High Density Lipoprotein Particles and Coronary Events (from the Dallas Heart Study) Am J Cardiol. 2015 doi: 10.1016/j.amjcard.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, Szklo M, Howard G, Evans GW. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 43.Shah AS, Urbina EM, Khoury PR, Kimball TR, Dolan LM. Lipids and lipoprotein ratios: contribution to carotid intima media thickness in adolescents and young adults with type 2 diabetes mellitus. J Clin Lipidol. 2013;7:441–445. doi: 10.1016/j.jacl.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbina EM, Khoury PR, McCoy CE, Dolan LM, Daniels SR, Kimball TR. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013;131:e1082–e1090. doi: 10.1542/peds.2012-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallo LM, Silverstein JH, Shuster JJ, Haller MJ. Arterial stiffness, lipoprotein particle size, and lipoprotein particle concentration in children with type 1 diabetes. J Pediatr Endocrinol Metab. 2010;23:661–667. doi: 10.1515/jpem.2010.23.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30:2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]