Abstract
Herbal medicines and natural herb extracts are widely used as alternative treatments for various parasitic diseases, and such extracts may also have potential to decrease the side effects of the standard regimen drugs used to treat toxoplasmosis (sulfadiazine-pyrimethamine combination). We evaluated how effective the Thai piperaceae plants Piper betle, P. nigrum and P. sarmentosum are against Toxoplasma gondii infection in vitro and in vivo. Individually, we extracted the piperaceae plants with ethanol, passed them through a rotary evaporator and then lyophilized them to obtain crude extracts for each one. The in vitro study indicated that the P. betle extract was the most effective extract at inhibiting parasite growth in HFF cells (IC50 on RH-GFP: 23.2 μg/mL, IC50 on PLK-GFP: 21.4 μg/mL). Furthermore, treatment of experimental mice with the P. betle extract for 7 days after infection with 1,000 tachyzoites of the T. gondii PLK strain increased their survival (survival rates: 100% in 400 mg/kg-treated, 83.3% in 100 mg/kg-treated, 33.3% in 25 mg/kg-treated, 33.3% in untreated mice). Furthermore, treatment with 400 mg/kg of the P. betle extract resulted in 100% mouse survival following infection with 100,000 tachyzoites. The present study shows that P. betle extract has the potential to act as a medical plant for the treatment of toxoplasmosis.
Introduction
Toxoplasma gondii, an obligate intracellular protozoan, causes toxoplasmosis. Infection with T. gondii threatens one-third of the global human population [1]. Toxoplasma infections have nonspecific symptoms, but can be associated with several clinical syndromes and cause serious complications and severe life-threatening disease in congenitally infected and immunocompromised hosts. Ingestion of raw or undercooked meat containing T. gondii tissue cysts is the main route of infection for this parasite [2,3]. Currently, sulfonamide drugs and pyrimethamine used in combination are the gold-standard medicines for treating toxoplasmosis [4]. These drugs have a synergistic activity against tachyzoites, but have limited efficacy in eliminating T. gondii encysted bradyzoites [5], and severe side effects and adverse drug reactions such as hematological reactions, embryopathies, bone marrow supression, hypersensitivity, and gastrointestinal disorders have been noted [6,7]. Therefore, the development of novel efficacious drugs with low toxicities is urgently needed.
Piperaceae plants comprise approximately 1,000 species of herbs, and are found in tropical areas of India, Southeast Asia and Africa [8]. Forty species of such plants have been identified in Thailand [9]. These plants are used as active ingredients in Thai traditional medicine and have many uses, such as ameliorating stress, improving digestion and nutrient absorption, and balancing general health; they also have antimalarial properties and are used for cancer treatment [10,11,12,13]. Pharmacologically, the properties of piperaceae plants have been shown to be antibacterial, antioxidant, gastro protective, and anticancer [14]. Furthermore, such plants have been shown to have anti-leishmanial activity [15] and anti-malarial activity [16,17]. Because herbal medicines and natural herb extracts are widely used as alternative treatments for various parasitic diseases and some have been tested on T. gondii in vitro [18], we were interested in exploring whether piperaceae plants possess anti-Toxoplasma activity. Thus, the aim of this study was to evaluate the effects of ethanol extracts from Thai piperaceae plants (P. betle, P. nigrum and P. sarmentosum) on T. gondii infections in vitro and in vivo. Our data indicate that of the three plants that we tested, P. betle extract has the potential to act as a medical plant for treating toxoplasmosis.
Materials and Methods
Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Obihiro University of Agriculture and Veterinary Medicine (Permit number 27–30, 28–46). All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize animal suffering.
Animals
Experiments were performed using female C57BL/6 mice (6–8 weeks old) obtained from Clea Japan, Inc. Six mice per cage were kept in the animal facility at the National Research Center for Protozoan Diseases (Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan) under standard laboratory conditions with commercial food and water available ad libitum.
Parasites and cell cultures
The two strains of T. gondii used for the experiments, RH-GFP (a green fluorescent protein expressing-RH strain) [19] and PLK-GFP [20] (a PLK strain), were maintained in African green monkey kidney (Vero) cells cultured in minimum essential medium eagle (MEM, Sigma, St. Louis, MO, USA) supplemented with 8% heat-inactivated fetal bovine serum (FBS) and 100 U/mL penicillin, 10 mg/mL streptomycin at 37°C in a 5% CO2 atmosphere. Parasites were propagated every three days. Parasites were purified by washing them in cold phosphate-buffered saline (PBS), and the final pellet was resuspended in cold PBS and passed three times through a 27-gauge needle syringe. Next, the parasites were filtered through a 5.0 μm pore filter (Millipore, Bedford, MA), washed twice with 10 mL of PBS, and centrifuged at 1,000 × g for 10 min. The parasites were refiltered and their numbers counted on a hemacytometer for each experiment. Human foreskin fibroblast (HFF) cells were maintained in Dulbecco's modified Eagle’s medium (DMEM, Sigma) supplemented with 10% FBS, 100 U/mL penicillin, and 10 mg/mL streptomycin at 37°C in a 5% CO2 atmosphere.
Plant materials
Three types of piperaceae plants were used in this study. The fresh leaves of Piper betle L. 8,955 g and P. sarmentosum Roxb. 11,310 g were purchased from Don Wai Floating market in Nakhon Pathom province, and the 1,000 g dried seeds of Piper nigrum L., were purchased from herbal drug stores (Vejpong Pharmacy Co., Ltd) Thailand. All plant materials were identified by the Faculty of Pharmacy, Mahidol University. The plant serial numbers for P. betle L. are as follows: PBM05160, P. nigrum L.: PBM05159, P. sarmentosum Roxb.: PBM05161 (S1 Fig). The fresh leaves were cleaned with water. Only sound leaves were dried in a hot air oven at 70°C for 48 h, after which they were ground into small pieces. The extraction methods were modified from Choochote et al [21]. Plant materials were extracted once with 97% ethanol (Sigma-Aldrich, St. Louis, MO, USA) at room temperature (RT) over a 3-day period. The solution was filtrated through sterile gauze and cotton, the filtrate was evaporated to dryness under reduced pressure at 40°C with a rotary evaporator (Rotavapor R-200/205, BÜCHI, Flawil, Switzerland), and then lyophilized using a Freeze Dry Vacuum System (Labconco, Kansas City, MO USA). The weights of the final crude extracts of P. betle, P. sarmentosum and P. nigrum were 43.42 g, 39.07 g and 51.18 g, respectively, and the yields of the extracts based on their dry weights [22] were 3.05%, 3.97% and 3.93%, respectively. The final crude extracts were dissolved in dimethyl sulfoxide (DMSO) at 100 mg/mL and kept at −30°C.
Cytotoxicity tests
Cytotoxicity analysis of the three piperaceae crude ethanol plant extracts (P. betle, P. nigrum, P. sarmentosum) and sulfadiazine (Sigma-Aldrich) were conducted on HFF cells. Sulfadiazine was dissolved in 1-M NaOH (stock solution 200 mg/mL) according to the manufacturer’s recommendations. Because 0.01 M NaOH did not inhibit HFF cell growth, we used 1 mg/mL sulfadiazine at the highest concentration in our study. HFF cells were plated at 100 μl/well in 96-well plates (cell suspensions 1 × 105 cells/mL in DMEM supplemented with 10% FBS), and then incubated at 37°C in a 5% CO2 atmosphere for 48 h. Next, the cells were exposed to the piperaceae extracts at final concentrations of 1, 5, 10, 25, 50, 100 μg/mL, sulfadiazine (at 10 ng/mL to 1 mg/mL), and culture medium was used as a control. After 24 h, the cell viability was measured by adding cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, Inc. Japan) to the cultures. The absorbance of the supernatant was measured at 450 nm using an MTP-120 micro plate reader (Corona Electric, Ibaraki, Japan). HFF cell viability (%) is expressed as [(the absorbance of cells treated with the extracts / (the absorbance of cells cultured with medium alone) × 100].
Indirect fluorescent antibody test (IFAT)
Vero cells, plated at 1 mL/well in 12-well plates (cell suspensions 1 × 105 cells/mL in MEM supplemented with 8% FBS), were incubated at 37°C in a 5% CO2 atmosphere for 24 h. Coverslips were collected at 24 h after parasite inoculation, washed twice with PBS containing 1 mM CaCl2 and 1 mM MgCl2 (PBS++), and then fixed with 3% paraformaldehyde in PBS++. After washing twice with PBS++, the cells were permeabilized with 0.3% Triton X-100 in PBS++ for 5 min at RT. After washing, the coverslips were incubated with 3% bovine serum albumin (BSA) in PBS++ at RT for 30 min. To count the number of parasites in parasitophorous vacuoles (PVs), the coverslips were incubated with an anti-SAG1 monoclonal antibody (clone TP3; Advanced ImmunoChemical Inc., Long Beach, CA, USA) diluted 1:100 in 3% BSA in PBS++ for 1 h at RT. After washing three times with PBS++, the coverslips were incubated with Alexa Fluor 594-conjugated goat anti-mouse IgG (Sigma) diluted 1:1,000 in 3% BSA in PBS++ for 1 h at RT, and then washed again with PBS++. Nuclear DNA was labelled with Hoechst 33342 (1:10,000 dilution, Thermo Fisher Scientific Inc., MA, USA) for 30 min. The coverslips were placed on a glass slide coated with Mowiol (Calbiochem, San Diego, CA, USA), and the slides were examined using an All-in-one Fluorescence Microscope (BZ-9000, Keyence, Tokyo, Japan).
Effects of piperaceae extracts on intracellular T. gondii in vitro
HFF cells, plated at 100 μl/well in 96-well plates (cell suspensions 1 × 105 cells/mL in DMEM supplemented with 10% FBS), were incubated at 37°C in a 5% CO2 atmosphere for 48 h. To examine the effects of this treatment on the intracellular parasites, RH-GFP and PLK-GFP (5 × 104 tachyzoites per well) were added to the wells for 4 h and the extracellular parasites were washed away. Then, the piperaceae extracts at final concentrations of 1, 5, 10, 25, 50, and 100 μg/mL (100 μl/well of media) were added for 72 h. Sulfadiazine (1 mg/mL, Sigma) and control media were used as positive and negative controls, respectively. The fluorescence intensity of RH-GFP and PLK-GFP were measured using a microplate reader (SH-900, Corona Electric Co., Ltd, Ibaraki, Japan). The correlation coefficient between the fluorescence intensity of GFP and the number of parasites (a two-fold serial dilution ranging from 1,000,000 to 7812.5 parasites) was calculated using the Pearson correlation coefficient and a positive correlation was confirmed (RH-GFP: r = 0.992, PLK-GFP: r = 0.969). The growth inhibition of RH-GFP and PLK-GFP (%) was expressed as follows: [(average fluorescence intensity of GFP with medium alone) − (the fluorescence intensity of GFP treated with either of the extracts or sulfadiazne) / (average fluorescence intensity of GFP with medium alone)] × 100. The half maximal inhibitory concentration (IC50) values of the plant extracts and sulfadiazine on T. gondii were calculated based on three independent experiments performed together by GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). Additionally, to measure T. gondii replication in vero cells, the PV sizes for RH-GFP expressed as a percentage (%) were determined by counting the number of parasites per PV (a total of 25 randomly selected vacuoles) at 24 h after infection (post-treatment with the extract as described above) based on the SAG1 signal measured by IFAT, as described above.
Effects of piperaceae extracts on extracellular T. gondii in vitro
RH-GFP tachyzoites (2 × 105) were pretreated with either of the three piperaceae extracts (25 μg/mL), sulfadiazine (1 mg/mL), or MEM alone (1 mL/tube) for 1 h at 37°C. Then, the pretreated parasites were added to vero cells at 1 mL/well in a 12-well plate (parasites per host cell ratio = 2:1). At 2–3 h post-infection, the extracellular parasites were washed away and MEM supplemented with 8% FBS was added. To determine the percentage inhibition of the T. gondii tachyzoites, the infection rates at 24 h post-infection were calculated by IFAT as follows: [(number of SAG1-positive vero cells) / (100 randomly selected vero cells)] × 100. The percentage inhibition of the tachyzoites was expressed as follows: [(average infection rate after treatment with medium alone) − (infection rate after treatment with either of the extracts) / (average infection rate after treatment with the medium alone)] × 100.
Effects of the piperaceae extracts on T. gondii infections in vivo
Mice were intraperitoneally inoculated with T. gondii (PLK strain, 1 × 103 tachyzoites/mouse). At 24 h post-infection, the mice were intraperitoneally injected with the P. betle extract at either 25 (n = 6), 100 (n = 12), and 400 mg/kg/24 h (n = 12), or PBS (n = 12) for 7 days. To further evaluate the anti-Toxoplasma activity of the P. betle extract, the mice infected with 1,000 and 100,000 PLK strain T. gondii tachyzoites (n = 6 per group) were treated with 400 mg/kg of P. betle extract or PBS via the intraperitoneal route, or 400 mg/L sulfadiazine via the drinking water as a standard treatment [23] for 7 days. The mice were observed daily for 30 days post-infection. Daily observations such as body weight, morbidity, mortality and clinical signs were noted, as were the clinical scores. Most of the signs recorded were assessed by the criteria used in other infection studies with protozoan parasites [24,25]. The scores varied from 0 (no signs) to 10 (all signs). The clinical signs recorded included hunching, piloerection, worm-seeking behavior, ptosis, sunken eyes, ataxia, latency of movement, deficient evacuation and touch reflexes, and lying on belly. The brains from the surviving mice were collected to determine the parasite burden by quantitative PCR, as described previously [26].
Statistical analysis
GraphPad Prism 5 software (GraphPad Software Inc.) was used. Data represent the mean ± SD. Statistical analyses were performed using Student’s t-test, a one-way or two-way analysis of variance (ANOVA) followed by the Tukey–Kramer test for group comparisons. Survival curves were generated by the Kaplan–Meier method, and statistical comparisons were made using the log-rank method. The levels of statistical significance are shown as asterisks or letters and defined in each figure legend together with the name of the statistical test that was used. A p value of P < 0.05 was considered statistically significant.
Results
Cytotoxicity of piperaceae extracts and sulfadiazine
To analyze the toxicity of each piperaceae extract on HFF cells in vitro, we examined cell proliferation using a CCK-8 cell counting kit. When exposed to 100 μg/mL of P. betle extract for 24 h, the cell proliferation rate was 66.19%, while the proliferation rates of HHF cells treated with either 1, 5, 10, 25 or 50 μg/mL of P. betle extract were more than 100% (S2A Fig). Therefore, the safe concentration of P. betle extract was considered to be < 50 μg/mL in this study. However, the proliferation rates of the HFF cells treated with 100 μg/mL of P. nigrum or P. sarmentosum extract were more than 100% (S2A Fig), indicating that these extracts had lower cytotoxicity than that of the P. betle extract. The IC50 value of the P. betle extract against HFF cell growth was 180.2 μg/mL (S2B Fig). There was no obvious cytotoxic effect of sulfadiazine on the HFF cells, even at the highest concentration (1 mg/mL) (S3C Fig).
Effects of the piperaceae extracts and sulfadiazine on T. gondii growth in vitro
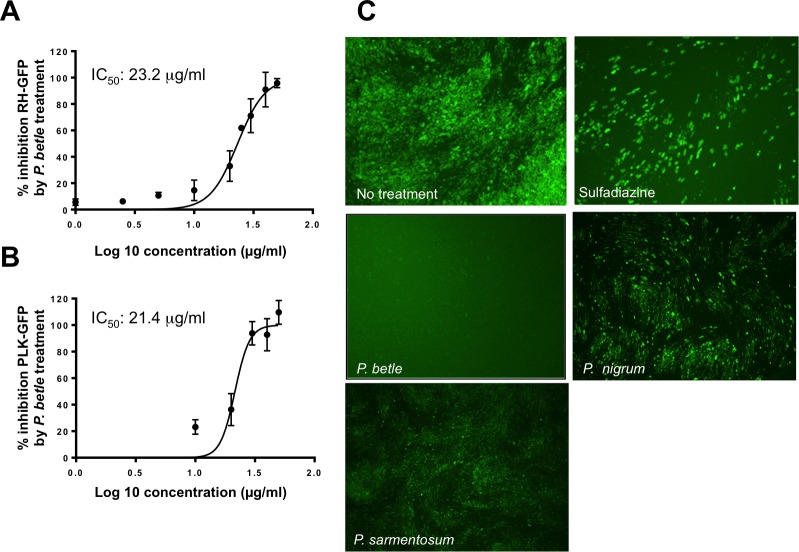
To analyze the anti-Toxoplasma effects of each piperaceae extract in vitro, we examined the fluorescence intensity of RH-GFP. At 72 h post-treatment, the P. betle extract inhibited RH-GFP growth at concentrations 25 and 50 μg/mL showing 63.4 ± 16.9% and 96.8 ± 7.2% inhibition, respectively (Fig 1A). However, the P. nigrum and P. sarmentosum extracts had no effects on RH-GFP growth (Fig 1C). The IC50 of the P. betle extract on RH-GFP was 23.2 μg/mL, while those of the P. sarmentosum and P. nigrum extracts were > 100 μg/mL. Futhermore, the P. betle extract also inhibited the growth of PLK-GFP (IC50: 21.4 μg/mL) (Fig 1B). Although sulfadiazine inhibited the growth of T. gondii (IC50 on RH-GFP: 99.4 μg/mL, IC50 on PLK-GFP: 22.3 μg/mL), the GFP signal was still observed, even at the highest concentration of sulfadiazine (1 mg/mL) (S3 Fig).
Fig 1. Anti-Toxoplasma activity of P. betle extract on RH-GFP and PLK-GFP.
Anti-Toxoplasma activity of the P. betle extract on intracellular parasites RH-GFP (A) and PLK-GFP (B). The RH-GFP and PLK-GFP-infected HFF cells were treated with the P. betle extract for 72 h at different concentrations from 0 to 50 μg/mL. Data represent the mean values ± SD for three independent experiments. The IC50 values of the P. betle extract on RH-GFP and PLK-GFP were 23.2 g/mL and 21.4 μg/mL, respectively. (C) Representative images of T. gondii RH-GFP-infected HFF cells treated with sulfadiazine (1 mg/mL), or either P. betle (50 μg/mL), P. nigrum (50 μg/mL) or P. sarmentosum (50 μg/mL) extract.
Effects of the piperaceae extracts on intracellular and extracellular T. gondii in vitro
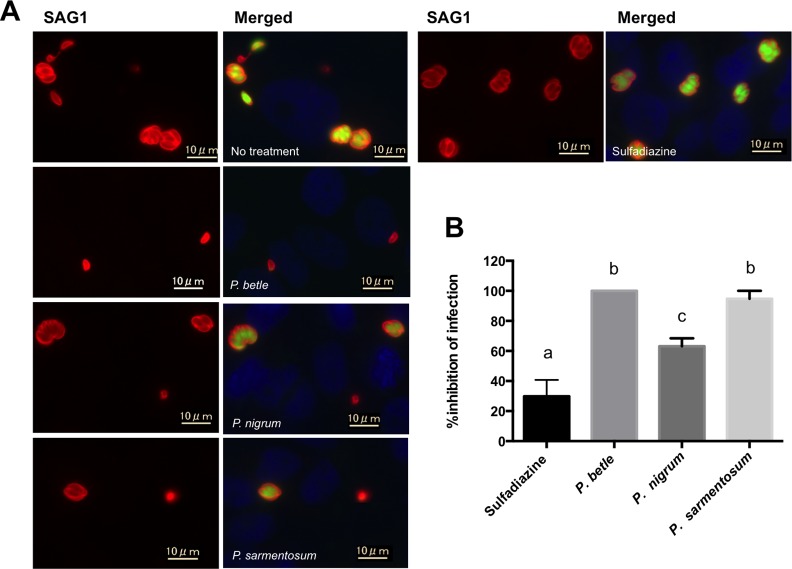
To examine the effect of each piperaceae extract on extracellular T. gondii, purified extracellular parasites pretreated with 25 μg/mL of either extract were used to infect vero cells (Fig 2A). Pretreatment with the P. betle extract resulted in 100% inhibition of the parasite infection while pretreatment with extracts from P. sarmentosum or P. nigrum resulted in 94.7 ± 5.3% and 63.2 ± 5.3% inhibition, respectively (Fig 2B). Treatment with the P. betle extract resulted in no GFP signal from the RH-GFP parasites (Fig 2A), suggesting that destruction of the parasite cell membrane and release of GFP from the cytosol had occurred. All extracts were more effective at parasite inhibition than 1 mg/mL of sulfadiazine (29.8 ± 10.9%) (Fig 2B).
Fig 2. Effects of the three piperaceae extracts at 25 μg/mL and sulfadiazine at 1 mg/mL on extracellular T. gondii.
The RH-GFP line, pre-treated with either of the extracts (P. betle, P. nigrum or P. sarmentosum) or sulfadiazine for 1 h, was then used to infect vero cells. After 24 h, the infected cells were analyzed by IFAT to measure the infection rates. (A) Representative images of T. gondii RH-GFP-infected vero cells. The cells were treated with either P. betle (25 μg/mL), P. nigrum (25 μg/mL), P. sarmentosum (25 μg/mL) extract or sulfadiazine (1 mg/mL). SAG1, red; GFP, green; nucleus, blue. (B) The % inhibition of infection for RH-GFP was measured by counting the number of SAG1-positive vero cells per 100 vero cells. Each bar represents the mean ± SD of three wells per group. The results represent two independent experiments. The different letters above the data bars in the graphs indicate statistically significant differences as determined by a one-way ANOVA plus Tukey–Kramer post-hoc analysis (P < 0.05).
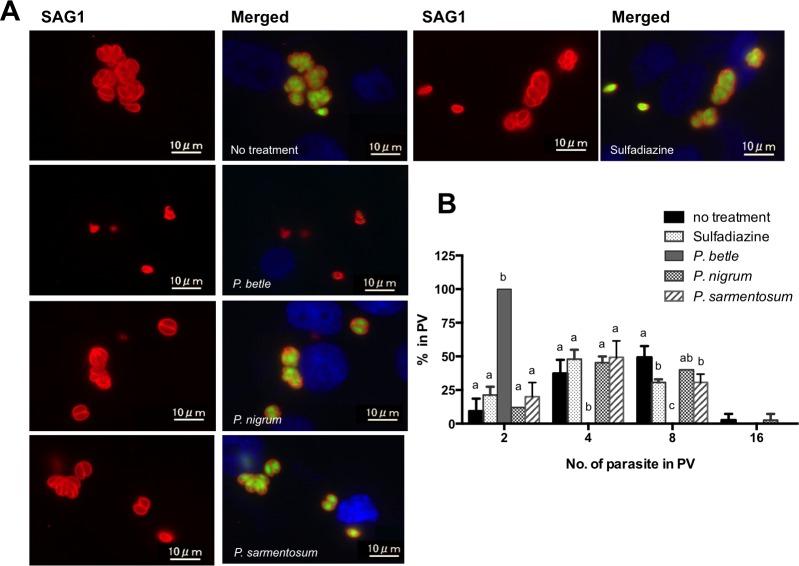
To test the effect of each piperaceae extract on intracellular T. gondii, RH-GFP-infected vero cells were treated with 25 μg/mL of either extract or 1 mg/mL of sulfadiazine (Fig 3A). Only two parasites per PV were found in the cells treated with the P. betle extract at 24 h post-infection (Fig 3B). Additionally, the GFP signal from the parasites was lower in cells treated with the P. betle extract (Fig 3A), indicating the anti-Toxoplasma effect of this extract. Sulfadiazine or P. sarmentosum treatment caused only slight inhibition of parasite replication (Fig 3B).
Fig 3. Effects of the three piperaceae extracts at 25 μg/mL and sulfadiazine at 1 mg/mL on intracellular T. gondii.
The RH-GFP-infected vero cells were treated with either of the three piperaceae extracts or sulfadiazine for 72 h, and were then analyzed by IFAT to measure the parasitophorous vacuole (PV) sizes. (A) Representative images of T. gondii RH-GFP-infected vero cells. The cells were treated with either P. betle (25 μg/mL), P. nigrum (25 μg/mL), P. sarmentosum (25 μg/mL) extract, or sulfadiazine (1 mg/mL). SAG1, red; GFP, green; nucleus, blue. (B) The number of parasites in PVs was measured by counting the number of SAG1-positive parasites per PV. Each bar represents the mean ± SD of three wells per group. Results represent two independent experiments. The different letters above the data bars in the graphs indicate statistically significant differences in the number of parasites in PVs as determined by two-way ANOVA plus Tukey–Kramer post-hoc analysis (P < 0.05).
Effects of the piperaceae extracts on T. gondii infections in vivo
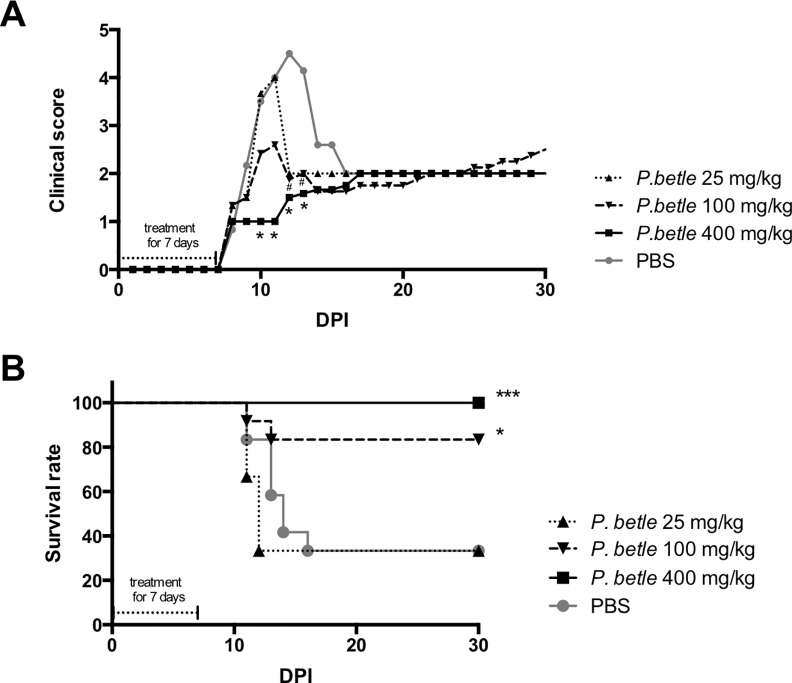
Because the P. betle extract had anti-T. gondii activity in vitro, we evaluated the effects of the P. betle extract on T. gondii in vivo (Fig 4). Although higher clinical scores were seen in the PBS-injected mice from 10 to 13 days post infection (dpi), treatment with P. betle extract at 400 and 100 mg/kg reduced the clinical signs from 10 to 13 dpi and from 12 to 13 dpi, respectively (Fig 4A). Furthermore, treating the infected mice with 400 and 100 mg/kg of the P. betle extract resulted in 100% and 83.3% mouse survival, respectively (Fig 4B). However, the survival rates of the mice treated with 25 mg/kg of P. betle extract or PBS was 33.3% (Fig 4B). This result indicates that treatment with 400 and 100 mg/kg of P. betle extract ameliorated toxoplasmosis in mice during the acute infection.
Fig 4. Clinical scores and survival of T. gondii-infected mice treated with P. betle extract.
Mice were intraperitoneally administrated with P. betle extracts at 25, 100 and 400 mg/kg/day or PBS from 1 to 7 days post-infection with 103 PLK tachyzoites per mouse. Clinical scores (A) and survival (B) were monitored for 30 days post-infection in the mice. The clinical scores represent the mean total values for all mice used in this study. Data represent the mean values of all the mice used in two independent experiments performed together (P. betle extract at 100 and 400 mg/kg/day, PBS, n = 6 + 6; P. betle extract at 25 mg/kg/day, n = 6). The clinical scores were analyzed by two-way ANOVA plus Tukey–Kramer post-hoc analysis at the time points indicated (*Difference between PBS and P. betle extract at 400 mg/kg/day, P < 0.05; # Difference between PBS and P. betle extract at 100 mg/kg/day, P < 0.05). Survival curves were generated with the Kaplan–Meier method. According to the log-rank test, the differences between the PBS and P. betle extracts were significant (*Difference between PBS and P. betle extract at 100 mg/kg/day, P < 0.05; *** Difference between PBS and P. betle extract at 400 mg/kg/day, P < 0.001).
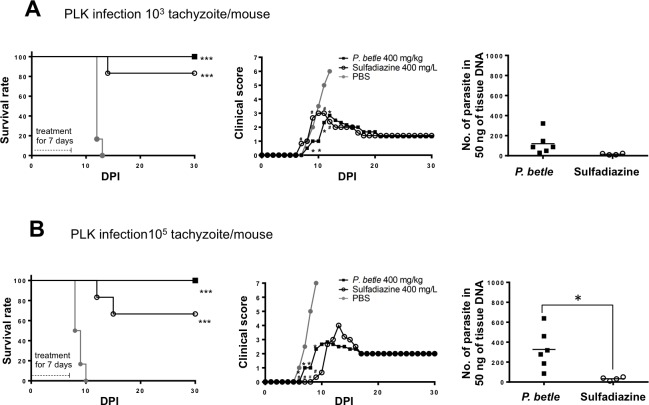
To evaluate further the anti-Toxoplasma activity of P. betle extract, we performed additional experiments to compare the extract-treated group of mice with the sulfadiazine-treated mouse group (Fig 5). In the case of infection with 1,000 tachyzoites, the survival rate of the P. betle–treated mouse group was 100%, while one mouse died at 14 dpi in sulfadiazine-treated group and all mice died within 13 dpi in the PBS-injected group (Fig 5A). The clinical scores of the P. betle- and sulfadiazine-treated mice against infection with 1,000 T. gondii were significantly lower than those of the PBS-injected mice from 9 to 12 dpi and 11 to 12 dpi, respectively (Fig 5A). There was no significant difference in the parasite numbers in the brains of the surviving mice between the P. betle–treated and sulfadiazine-treated groups (Fig 5A). Furthermore, all mice in the P. betle–treated group survived the infection with 100,000 tachyzoites, but two mice died at 12 and 15 dpi in the sulfadiazine-treated group, and all mice died within 10 dpi in the PBS-injected group (Fig 5B). Treatment of the infected mice with P. betle or sulfadiazine decreased the clinical signs during the acute phase of the infection from 6 to 9 dpi (Fig 5B). The clinical score from 7 to 9 dpi and the number of parasites in the brains of the surviving mice in the sulfadiazine-treated group were significantly lower than those of P. betle–treated group (Fig 5B). Altogether, treatment with the P. betle extract controlled acute toxoplasmosis in the mice, although some parasites were detectable in their brains.
Fig 5. Survival, clinical score and parasite burden of mice infected with 1,000 and 100,000 T. gondii tachyzoites under treatment with P. betle extract or sulfadiazine.
Mice infected with 1,000 (A) and 100,000 tachyzoites (B) of the T. gondii PLK strain (n = 6) were treated with 400 mg/kg P. betle extract, PBS via the intraperitoneal route, or 400 mg/L sulfadiazine via drinking water as a standard treatment from 1 to 7 days post-infection (dpi). Survival and clinical scores were monitored for 30 dpi and the brains from the surviving mice were collected to determine the parasite burden at 30 dpi. Survival curves were generated by the Kaplan–Meier method. According to the log-rank test, the differences between the PBS and P. betle extracts were significant (***Difference between PBS and P. betle extract or sulfadiazine treatment, P < 0.001). The clinical scores represent the mean total values for all the mice used in this study. Data represent the mean values of all the mice. The clinical scores were analyzed by two-way ANOVA plus Tukey–Kramer post-hoc analysis at the time points indicated (*Difference between PBS and P. betle extract, P < 0.05; # Difference between PBS and sulfadiazine, P < 0.05). The number of parasites in 50 ng of tissue DNA per individual (symbols) and the mean levels (horizontal lines) are indicated. A significant difference between the two groups was observed by a student’s t-test. (*Difference between treatment with sulfadiazine and P. betle extract, P < 0.05)
Discussion
Toxoplasmosis is one of the most important and challenging diseases in public health. To control T. gondii infection and the toxoplasmosis caused by it, herbal medicine and natural herb extracts are of growing interest. There are many traditional herbal medicines with antimicrobial and antihelminthic properties, and some have anti-Toxoplasma activities such as Curcuma longa [27], Eurycoma longifolia Jack [28,29], and Myristica fragrans Houtt [30]. Moreover, the anti-T. gondii activity of Artemisia annua L. [5,31,32], Dichroa febrifuga [33], herbal extracts from South Korea (Sophora flavescens, Sinomenium acutum, Pulsatilla koreana, Ulmus macrocarpa and Torilis japonica) [34], and Eurycoma longifolia [29] have been reported. However, piperaceae extracts have not been tested for their potential anti-Toxoplasma effects even though they have many pharmacological properties including activities against fungi [35], insects [8], protozoa [11,17,36], helminthes [37,38] and cancer cells [12]. Only extracts of P. nigrum have been shown to possess in vivo activity, with a reported T. gondii growth inhibition of 78.3% and 86.3% with treatment doses 100 and 200 mg/kg/day, respectively [27], but there is no in vitro information for this extract. Herein, we evaluated the effects of ethanol extracts of P. betle, P. nigrum and P. sarmentosum from Thailand on T. gondii growth in vitro and in vivo.
Importantly, the P. betle extract had anti-Toxoplasma activity both in vitro and in vivo. In the in vitro tests, 25 μg/mL of the P. betle extract eradicated extracellular and intracellular parasites. The selective activity of the treatment was considered through the selectivity index (SI). Both P. betle extract and sulfadiazine were calculated as the ratio between cytotoxic IC50 values and parasitic IC50 values [39]. The IC50 values of the P. betle extract for HFF cells, RH-GFP and PLK-GFP were 180.2 μg/mL, 23.2 μg/mL and 21.4 μg/mL, respectively. Therefore, each SI for RH-GFP and PLK-GFP was 7.77 and 8.42, respectively. Because there was no obvious cytotoxic effect of sulfadiazine on the HFF cells, even at the highest concentration (1 mg/mL), the SI was not able to be used in our study. In a previous study, de Oliveira et al [5] reported that the IC50 value of sulfadiazine on T. gondii (RH strain) in HFF cells was 70 μg/mL, and the viability of HFF cells in the presence of 200 μg/mL of sulfadiazine decreased by 28% (no SI value was reported). Schoondermark-van de Ven et al [40] reported that the IC50 of sulfadiazine against the growth of T. gondii in human epithelial type 2 (HEp-2) cells could not be determined because the drug was toxic to HEp-2 cells at concentrations above 1000 μg/mL. Thus, in comparision with sulfadiazine, P. betle extract should be effective at controlling the growth of T. gondii in vitro.
Furthermore, treatment of T. gondii-infected mice with the P. betle extract increased the survival rates of the mice, particularly at the highest concentration (400 mg/kg) of extract that we used. When compared with sulfadiazine treatment, treatment with P. betle extract (400 mg/kg) produced better mouse survival rates, although T. gondii DNA was still detectable in the mouse brains. Thus, treatment with P. betle extract was effective at controling acute toxoplasmosis in mice. In this study, we did not test the toxicity of P. betle extract in vivo, but a previous study showed that an ethanol extract of P. betle leaves (after oral administration of 1,500 mg/kg/day for 40 consecutive days) produced no significant side-effects to cross-bred male albino rats in terms of clinical signs, food intake, percent weight gain and serum parameters [41], indicating that our regimen should be reliable.
Extracts of P. nigrum and P. sarmentosum were also tested in vitro in our study. P. nigrum and P. sarmentosum each showed an inhibitory effect against extracellular T. gondii, but not against intracellular parasites. Our results indicate that P. nigrum and P. sarmentosum extracts affected the extracellular parasites, while the P. betle extract was effective against extracellular and intracellular parasites alike. The main chemical classes found in P. betle leaves are alkaloids, terpenes, anthraquinones, flavonoids, tannins, saponins and steroids [17]; more specifically, chavibetol, chavibetol acetate, allylpyrocatechol diacetate, eugenol, safrole, quercetin, a-Pinene, f-pinene, u-limonene, and saprobe [14,17,42,43]. Furthermore, phytochemical compounds such as alkaloids and flavonoids have antiplasmodial activities [17]. Therefore, any one or a combination of these compounds might affect T. gondii growth. In a future study, we will test the effects of the phytochemical compounds that were found in P. betle extract for their anti-Toxoplasma activities.
Conclusion
In the present study, we found that P. betle had little toxicity to HFF cells and effectively inhibited T. gondii in vitro and in vivo. These results indicate that P. betle extract contains active ingredients with potential for treating toxoplasmosis.
Supporting Information
(A) Piper betle L., (B) P. nigrum L., and (C) P. sarmentosum Roxb. were identified and transferred to the herbarium by the Faculty of Pharmacy, Mahidol University, 447 Sri-Ayuthaya Road. Rajathevi Bankok 10400, Thailand. The serial numbers given were as follows: P. betle L.: PBM05160, P. nigrum L.: PBM05159, P. sarmentosum Roxb.: PBM05161.
(PDF)
(A) Cytotoxicity testing of HFF cells after treatment with either one of three piperaceae extracts (P. betle, P. nigrum, or P. sarmentosum) at concentrations of 0 to 100 μg/mL for 24 h. Data represent the mean values ± SD for three independent experiments. (B) Inhibition HFF cell growth by the P. betle extract. HFF cells were exposed to P. betle extract at concentrations 0 to 500 μg/mL. Data represent the mean values ± SD for three independent experiments. The IC50 value of the P. betle extract on HFF cells was calculated based on three independent experiments performed together.
(PDF)
Anti-Toxoplasma activity of sulfadiazine against RH-GFP (A) and PLK-GFP parasites (B). The RH-GFP and PLK-GFP-infected HFF cells were treated with sulfadiazine for 72 h at concentrations of 10 ng/mL to 1 mg/mL and the IC50 values were calculated for RH-GFP and PLK-GFP. Data represent the mean values ± SD for three independent experiments. (C) Inhibition of HFF cell growth by sulfadiazine. HFF cells were exposed to sulfadiazine at the highest concentration of 1 mg/mL. Data represent the mean values ± SD (n = 3). (D) Representative images of T. gondii RH-GFP- and PLK GFP-infected HFF cells treated with sulfadiazine (1 mg/mL) and culture medium alone (no treatment).
(PDF)
Acknowledgments
We thank Dr. Parntep Patanakorn (Faculty of Veterinary Science, Mahidol University) for providing the crude extracts from piperaceae plants and the Faculty of Pharmacy, Mahidol University for their help with identification of plant materials.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363 (9425):1965–76. 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- 2.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39 (8):895–901. 10.1016/j.ijpara.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsheikha HM. Congenital toxoplasmosis: Priorities for further health promotion action. Public Health. 2008;122(4):335–53. 10.1016/j.puhe.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 4.McLeod R, Lykins J, Gwendolyn Noble A, Rabiah P, Swisher C, Heydemann P, et al. Management of Congenital Toxoplasmosis. Curr Pediatr Rep. 2014;2:166–94. 10.1007/s40124-014-0055-7 [DOI] [Google Scholar]
- 5.de Oliveira TC, Silva DA, Rostkowska C, Béla SR, Ferro EA, Magalhães PM, et al. Toxoplasma gondii: Effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp Parasitol. 2009;122 (3):233–41. 10.1016/j.exppara.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 6.Furtado JM, Smith JR, Belfort R, Gattey D, Winthrop KL. Toxoplasmosis: A Global Threat. J Glob Infect Dis. 2011;3 (3):281–4. 10.4103/0974-777X.83536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47 (4):554–66. 10.1086/590149 [DOI] [PubMed] [Google Scholar]
- 8.Scott IM, Jensen HR, Philogène BJR, Arnason JT. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochemistry Reviews. 2007;7(1):65–75. 10.1007/s11101-006-9058-5 [DOI] [Google Scholar]
- 9.Runglawan S, Tawatchai T, Varima W, Nat B, Arunrat C. Ethnobotany and species specific molecular markers of some medicinal sakhan (Piper, Piperaceae). J Med Plants Res. 2012;6(7):1168–75. 10.5897/JMPR11.807 [DOI] [Google Scholar]
- 10.Chaveerach A, Mokkamul P, Sudmoon R, Tanee T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobotany Research and Applications. 2006;4:223–31. [Google Scholar]
- 11.Thiengsusuk A, Chaijaroenkul W, Na-Bangchang K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol Res. 2013;112 (4):1475–81. 10.1007/s00436-013-3294-6 [DOI] [PubMed] [Google Scholar]
- 12.Ruangnoo S, Itharat A, Sakpakdeejaroen I, Rattarom R, Tappayutpijam P, Pawa KK. In vitro cytotoxic activity of Benjakul herbal preparation and its active compounds against human lung, cervical and liver cancer cells. J Med Assoc Thai. 2012;95:127–34. [PubMed] [Google Scholar]
- 13.Suthanurak M, Sakpakdeejaroen I, Rattarom R, Ithara A. Formulation and stability test of Benjakul extract tablets: a preliminary study.Thai J Pharmacol. 2010;32(1):160–3. [Google Scholar]
- 14.Rekha VP, Kollipara M, Gupta BRSS, Bharath Y, Pulicherla K. A Review on Piper betle L.: Nature’s Promising Medicinal Reservoir. American Journal of Ethnomedicine. 2014;1(5):276–89. [Google Scholar]
- 15.Misra P, Kumar A, Khare P, Gupta S, Kumar N, Dube A. Pro-apoptotic effect of the landrace Bangla Mahoba of Piper betle on Leishmania donovani may be due to the high content of eugenol. JMed Microbiol.2009;58(8):1058–66. 10.1099/jmm.0.009290-0 [DOI] [PubMed] [Google Scholar]
- 16.Bagatela BS, Lopes AP, Fonseca FL, Andreo MA, Nanayakkara DN, Bastos JK, et al. Evaluation of antimicrobial and antimalarial activities of crude extract, fractions and 4-nerolidylcathecol from the aerial parts of Piper umbellata L. (Piperaceae). Nat Prod Res. 2013;27:2202–9. 10.1080/14786419.2013.821123 [DOI] [PubMed] [Google Scholar]
- 17.Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules. 2011;16(1):107–18. 10.3390/molecules16010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sepúlveda-Arias JC, Veloza LA, Mantilla-Muriel LE. Anti-Toxoplasma Activity of Natural Products: A Review. Recent Pat Antiinfect Drug Discov. 2014;9(3):186–194. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa Y, Xuenan X, Makala L, Vielemeyer O, Joiner KA, Nagasawa H. Characterisation of Toxoplasma gondii engineered to express mouse interferon-gamma. Int J Parasitol. 2003;33(13):1525–35. 10.1016/S0020-7519(03)00204-2 [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa Y, Zhang H, Ibrahim HM, Ui F, Ogiso A, Xuan X. Construction of Toxoplasma gondii bradyzoite expressing the green fluorescent protein. Parasitol Int. 2008;57(2):219–22. 10.1016/j.parint.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Choochote W, Chaithong U, Kamsuk K, Rattanachanpicha E, Jitpakdi A, Tippawangoso P, et al. ADULTICIDAL ACTIVITY AGAINST Stegomyia aegypti (DIPTERA: CULICIDAE) OF THREE Piper spp. Rev Inst Med trop S Paulo. 2006;48(1):33–7. [DOI] [PubMed] [Google Scholar]
- 22.Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–18. 10.1016/j.foodchem.2005.11.032 [DOI] [Google Scholar]
- 23.Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun. 2005;73(2):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gomez ND, Taylor T, Haldar K. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One. 2010;5:e13124 10.1371/journal.pone.0013124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48–84. 10.1186/1742-2094-5-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, Nishimura M, Ihara F, Yamagishi J, Suzuki Y, Nishikawa Y. Transcriptome analysis of mouse brain infected with Toxoplasma gondii. Infect Immun. 2013;81(10):3609–19. 10.1128/IAI.00439-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Zanbagi NA. In vivo effect of some home spices extracts on the toxoplasma gondii tachyzoites. J Family Community Med. 2009;16(12):59–65. [PMC free article] [PubMed] [Google Scholar]
- 28.Khanam Z, Wen CS, Bhat IUH. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). Journal of King Saud University–Science. 2015;27(1):23–30. 10.1016/j.jksus.2014.04.006 [DOI] [Google Scholar]
- 29.Kavitha N, Noordin R, Chan KL, Sasidharan S. In vitro Anti-Toxoplasma gondii Activity of Root Extract/Fractions of Eurycoma longifolia Jack. BMC Complement Altern Med. 2012;12:91–8. 10.1186/1472-6882-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillai S, Mahmud R, Lee WC, Perumal S. Anti-Parasitic Activity of Myristica Fragrans Houtt. Essential Oil Against Toxoplasma Gondii Parasite. APCBEE Procedia. 2012; 2:92–6. 10.1016/j.apcbee.2012.06.017 [DOI] [Google Scholar]
- 31.El Zawawy LA. Effect of artesunate on Toxoplasma gondii: in vitro and in vivo studies. J Egypt Soc Parasitol. 2008;38(1):185–201. [PubMed] [Google Scholar]
- 32.Nagamune K, Beatty WL, Sibley LD. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell. 2007;6(11): 2147–56. 10.1128/EC.00262-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain V, Yogavel M., Oshima Y, Kikuchi H, Touquet B, Hakimi MA, et al. Structure of Prolyl-tRNA Synthetase-Halofuginone Complex Provides Basis for Development of Drugs against Malaria and Toxoplasmosis. Structure. 2015;23(5):819–29. 10.1016/j.str.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 34.Youn HJ, Lakritz J, Kim DY, Rottinghaus GE, Marsh AE. Anti-protozoal efficacy of medicinal herb extracts against Toxoplasma gondii and Neospora caninum. Vet Parasitol. 2003;116(1):7–14. 10.1016/S0304-4017(03)00154-7 [DOI] [PubMed] [Google Scholar]
- 35.Ali I, Khan FG, Suri KA, Gupta BD, Satti NK, Dutt P, et al. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann Clin Microbiol Antimicrob. 2010;9:7–15. 10.1186/1476-0711-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fazal F, Mane PP, Rai MP, Thilakchand KR, Bhat HP, Kamble PS, et al. The phytochemistry, traditional uses and pharmacology of Piper Betel. linn (Betel Leaf): A pan-asiatic medicinal plant. Chin J Integr Med. 2014;1–11. [DOI] [PubMed] [Google Scholar]
- 37.Atjanasuppat K, Wongkham W, Meepowpan P, Kittakoop P, Sobhon P, Bartlett A, et al. In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. J Ethnopharmacol. 2009;123(3):475–82. 10.1016/j.jep.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 38.Philip HE, William SB, Evangeline JF. Identification of Fungicidal and Nematocidal Components in the Leaves of Piper betle (Piperaceae). J Agric Food Chem. 1984;32(6):1254–1256. 10.1021/jf00126a011 [DOI] [Google Scholar]
- 39.Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasaiof Kenya. J Ethnopharmacol. 2005;101(1–3):95–9. 10.1016/j.jep.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 40.Schoondermark-van de Ven E, Vree T, Melchers W, Camps W, Galama J. In vitro effects of sulfadiazine and its metabolites alone and in combination with pyrimethamine on Toxoplasma gondii. Antimicrob Agents Chemother. 1995;39(3):763–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. J Ethnopharmacol. 2005;102(2): 239–45. 10.1016/j.jep.2005.06.016 [DOI] [PubMed] [Google Scholar]
- 42.Dwivedi V and Tripathi S. Review study on potential activity of Piper betle. J Pharmacogn Phytochem. 2014;3(4)93–8. [Google Scholar]
- 43.Bhalerao SA, Verma DR, Gavankar RV, Teli NC, Rane YY, Didwana VS, et al. Phytochemistry, Pharmacological Profile and Therapeutic Uses of Piper Betle Linn.–An Overview. J Pharmacogn Phytochem. 2013;1(2):10–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Piper betle L., (B) P. nigrum L., and (C) P. sarmentosum Roxb. were identified and transferred to the herbarium by the Faculty of Pharmacy, Mahidol University, 447 Sri-Ayuthaya Road. Rajathevi Bankok 10400, Thailand. The serial numbers given were as follows: P. betle L.: PBM05160, P. nigrum L.: PBM05159, P. sarmentosum Roxb.: PBM05161.
(PDF)
(A) Cytotoxicity testing of HFF cells after treatment with either one of three piperaceae extracts (P. betle, P. nigrum, or P. sarmentosum) at concentrations of 0 to 100 μg/mL for 24 h. Data represent the mean values ± SD for three independent experiments. (B) Inhibition HFF cell growth by the P. betle extract. HFF cells were exposed to P. betle extract at concentrations 0 to 500 μg/mL. Data represent the mean values ± SD for three independent experiments. The IC50 value of the P. betle extract on HFF cells was calculated based on three independent experiments performed together.
(PDF)
Anti-Toxoplasma activity of sulfadiazine against RH-GFP (A) and PLK-GFP parasites (B). The RH-GFP and PLK-GFP-infected HFF cells were treated with sulfadiazine for 72 h at concentrations of 10 ng/mL to 1 mg/mL and the IC50 values were calculated for RH-GFP and PLK-GFP. Data represent the mean values ± SD for three independent experiments. (C) Inhibition of HFF cell growth by sulfadiazine. HFF cells were exposed to sulfadiazine at the highest concentration of 1 mg/mL. Data represent the mean values ± SD (n = 3). (D) Representative images of T. gondii RH-GFP- and PLK GFP-infected HFF cells treated with sulfadiazine (1 mg/mL) and culture medium alone (no treatment).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.