Abstract
Objective
Many things can impact the reproducibility of results from laboratory to laboratory. For example food from various sources can vary markedly in composition. We examined the effects of two different food sources, the Teklad Global Soy Protein-Free Extruded Rodent Diet (irradiated diet) and the Teklad Sterilizable Rodent Diet (autoclaved diet), on central nervous system disease.
Research Methods & Procedures
Three preclinical models for human disease: two different experimental autoimmune encephalomyelitis (EAE) models (multiple sclerosis) and the Theiler’s murine encephalomyelitis virus (TMEV)-induced seizure model (epilepsy), were examined for the effects of two different food sources on disease.
Results
We found that mice fed the irradiated diet had more severe clinical disease and enhanced seizures compared to animals provided the autoclaved diet in both EAE models examined and in the TMEV-induced seizure model, respectively.
Conslusions
Therefore, just altering the source of food (lab chow) can have marked effects on disease severity and outcome.
Keywords: Experimental autoimmune encephalomyelitis, Theiler’s murine encephalomyelitis virus, Seizures, Diet
Introduction
The effects of food and nutrition on the initiation and pathogenesis of neurological diseases of the CNS have not been extensively examined. Such variables likely contribute to differences observed among laboratories using similar preclinical models of human disease. Here we examine the effects of two different food sources on TMEV-induced seizures and two different EAE models.
TMEV, a non-enveloped, positive-sense, single-stranded RNA picornavirus, is a naturally occurring enteric pathogen of the mouse [1,2]. I.c. infection of C57BL/6 mice with the DA strain of TMEV induces acute behavioral seizures in about 50–80% of the mice [3,4]. The seizures occur at a frequency of one per mouse per 2 hour observation period and typically last 1–2 minutes [3,5]. Seizures start on day 3 p.i., peak on day 6 p.i. and resolve by day 10 p.i. The severity of the seizures are typically observed to be a Racine scale seizure score 3 (forelimb clonus) and above (score 4, rearing; score 5, rearing and falling) [3,6]. Following a latent period many of these animals go on to develop epilepsy [7]. The TMEV-induced seizure model is a new animal model [3] that is currently being examined by just a few labs [8–11]. Therefore, lab-to-lab variability has not been examined. However, we noticed variability in seizure susceptibility upon change of animal facilities in conjunction with a change in diet.
EAE is a commonly used animal model of MS (reviewed in [12]). EAE can be induced in SJL/J or C57BL/6 mice through inoculation with CNS myelin peptides, from PLP or MOG, emulsified in CFA. The clinical course of EAE varies depending on the particular myelin peptide and strain of mouse (reviewed in [12]). Weight loss, ataxia, incontinence and flaccid or spastic hind limb paralysis are common clinical signs of EAE [13]. Researchers have notice that variability in day of onset, severity and incidence of EAE can occur within an experimental group, from experiment to experiment, and from lab to lab even when using the same peptide and reagents in genetically identical mice [14–16]. The reasons for the variability are unknown, but differences in diet may be one factor accounting for some of the lab-to-lab variability.
We performed a TMEV-induced seizure experiment and two EAE experiments, where disease was induced with peptides from either PLP or MOG, to examine the effects of two different food sources, the Teklad Global Soy Protein-Free Extruded Rodent Diet (irradiated diet) and the Teklad Sterilizable Rodent Diet (autoclaved diet), on CNS disease.
Methods
Animals
Four-week-old, male C57BL/6 and female SJL/J mice were obtained from the Jackson Laboratory. All animal experiments were reviewed and approved by the University of Utah Institutional Animal Care and Use Committee and conducted in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council. Animals were maintained on a 12 hour light/12 hour dark cycle at 72°F. All efforts were made to minimize suffering. For each of the three experiments, mice were randomly divided into two groups: one received Teklad Global Soy Protein-Free Extruded Rodent Diet (irradiated diet) (cat. #2920X; Harlan Laboratories) and the other received Teklad Sterilizable Rodent Diet (autoclaved diet) (cat. #8656; Harlan Laboratories). Food and water was available ad libitum. A comparison of the nutrient composition of the two varieties of food is given in Table 1. One physical difference between the two types of food is that the irradiated diet is extruded whereas the autoclaved diet is a pellet.
Table 1.
Nutrient Composition of Irradiated and Autoclaved Diets.
| Ingredient | Irradiated | Autoclaved | Unit of Measure | |
|---|---|---|---|---|
| Macronutrients | Crude Protein | 19.1 | 24.5 | % |
| Fat | 6.5 (acid hydrolysis) | 4.3 (ether extract) | % | |
| Carbohydrate (available) | 47.0 | 38.2 | % | |
| Crude Fiber | 2.7 | 3.7 | % | |
| Neutral Detergent Fiber | 12.3 | 14.0 | % | |
| Ash | 5.1 | 8.0 | % | |
| Energy Density | 3.1 (13.0) | 2.9 (12.1) | kcal/g (kJ/g) | |
| Calories from Protein | 24 | 34 | % | |
| Calories from Fat | 16 | 13 | % | |
| Calories from Carbohydrate | 60 | 53 | % | |
| Minerals | Calcium | 0.9 | 1.2 | % |
| Phosphorus | 0.7 | 1.0 | % | |
| Non-Phytate Phosphorus | 0.4 | 0.7 | % | |
| Sodium | 0.1 | 0.3 | % | |
| Potassium | 0.4 | 0.9 | % | |
| Chloride | 0.4 | 0.4 | % | |
| Magnesium | 0.2 | 0.2 | % | |
| Zinc | 60 | 84 | mg/kg | |
| Manganese | 80 | 106 | mg/kg | |
| Copper | 15 | 23 | mg/kg | |
| Iodine | 6 | 2 | mg/kg | |
| Iron | 200 | 300 | mg/kg | |
| Selenium | 0.23 | 0.35 | mg/kg | |
| Amino Acids | Aspartic Acid | 1.1 | 2.3 | % |
| Glutamic Acid | 3.5 | 4.2 | % | |
| Alanine | 1.2 | 1.4 | % | |
| Glycine | 0.7 | 1.3 | % | |
| Threonine | 0.6 | 0.9 | % | |
| Proline | 1.9 | 1.7 | % | |
| Serine | 0.9 | 1.6 | % | |
| Leucine | 2.3 | 1.9 | % | |
| Isoleucine | 0.7 | 1.0 | % | |
| Valine | 0.9 | 1.2 | % | |
| Phenylalanine | 1.0 | 1.1 | % | |
| Tyrosine | 0.5 | 0.9 | % | |
| Methionine | 0.5 | 0.4 | % | |
| Cystine | 0.3 | 0.4 | % | |
| Lysine | 0.9 | 1.4 | % | |
| Histidine | 0.4 | 0.6 | % | |
| Arginine | 0.8 | 1.6 | % | |
| Tryptophan | 0.2 | 0.3 | % | |
| Vitamins | Vitamin A | 15.0 | 37.0 | IU/g |
| Vitamin D3 | 1.5 | 3.0 | IU/g | |
| Vitamin E | 110 | 170 | IU/kg | |
| Vitamin K3 (menadione) | 50 | 100 | mg/kg | |
| Vitamin B1 (thiamin) | 17 | 120 | mg/kg | |
| Vitamin B2 (riboflavin) | 15 | 17 | mg/kg | |
| Niacin (nicotinic acid) | 75 | 129 | mg/kg | |
| Vitamin B6 (pyridoxine) | 18 | 21 | mg/kg | |
| Pantothenic Acid | 33 | 107 | mg/kg | |
| Vitamin B12 (cyanocobalamin) | 0.08 | 0.13 | mg/kg | |
| Biotin | 0.40 | 0.93 | mg/kg | |
| Folate | 4 | 8 | mg/kg | |
| Choline | 1200 | 2380 | mg/kg | |
| Fatty Acids | C 16:0 Palmitic | 0.6 | 0.7 | % |
| C 18:0 Stearic | 0.1 | 0.1 | % | |
| C 18:1ω9 Oleic | 1.1 | 0.8 | % | |
| C 18:2ω6 Linoleic | 2.6 | 1.8 | % | |
| C 18:3ω3 Linolenic | 0.3 | 0.1 | % | |
| Total Saturated | 0.8 | 0.9 | % | |
| Total Monounsaturated | 1.1 | 1.0 | % | |
| Total Polyunsaturated | 2.9 | 1.9 | % | |
| Other | Cholesterol | -- | 50 | mg/kg |
TMEV infection
C57BL/6 mice were anesthetized with isoflurane by inhalation and infected i.c. with 3 × 10 5 pfu of the DA strain of TMEV at a final volume of 20 µl per mouse. TMEV was propagated as described [17].
Seizure scoring
DA-infected C57BL/6 mice were weighed and monitored daily for seizures through day 21 p.i. The monitoring of seizure activity was performed as described [18]. Briefly, mice were observed for 2 hours each day. Seizure activity was graded using the Racine scale: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling [6,19].
EAE induction
SJL/J mice were injected subQ in the flanks with 200 µl of 1 mM PLP139–151 peptide (DNA/Peptide Synthesis Core Facility, University of Utah) emulsified with reconstituted CFA, composed of Freund’s incomplete adjuvant (Pierce Biotechnology) containing Mycobacterium tuberculosis H37 Ra (2 mg/ml) (Difco Laboratories). Mice were i.v. injected with 100 µl BP toxin (List Biological Laboratories, Inc.) at 0.2 µg per mouse on days 0 and 2 following sensitization. Mice developed a RR-EAE clinical course.
C57BL/6 mice were injected subQ in the flanks with 200 µl of 1 mM MOG35–55 peptide (DNA/Peptide Synthesis Core Facility) emulsified as above. Mice were injected i.v. with 100 µl BP toxin as above. Mice developed a monophasic disease course.
EAE scoring
SJL/J mice injected with PLP139–151 and C57BL/6 mice injected with MOG35–55 were weighed and scored daily for clinical signs through day 48 p.s. Clinical scoring was: 0, no clinical disease; 1, loss of tail tonicity; 2, mild hind leg paralysis with no obvious gait disturbance; 3, mild leg paralysis with gait disturbance and paralysis; 4, hind limb paralysis; and 5, moribund or dead. If the mice were paralyzed to the point where they could not feed or groom themselves (moribund), or they lost 20% of their body weight, the mice were euthanized via inhaled anesthetic.
Histology
Mice were euthanized through an overdose of isoflurane, on day 21 p.i. for the seizure experiment and on day 48 p.s. for the EAE experiments, and perfused with PBS, followed by 4% paraformaldehyde phosphate-buffered solution. For seizure mice, brains were harvested, divided into 5 coronal slabs, embedded in paraffin and cut into 4 µm thick tissue sections. To visualize neuronal loss and inflammation within the hippocampus, sections were stained with Luxol fast blue. Neuronal cell loss in the pyramidal cell layer of the hippocampus, from CA1 to CA3, was scored as follows: score 0, no damage; score 1, 10 to 29% cell loss (10 to 29% of the pyramidal cell layer from CA1 to CA3 was missing); score 2, 30 to 59% cell loss; and score 3, >60% cell loss [4,20]. An undamaged (intact) control brain was assigned a score of 0 with no cell loss (100% of the cells present). A score was given for each of the two hippocampi present in a brain, and then the scores were summed; the highest possible score for cell loss per brain was 6 (i.e., the sum of the highest score, 3, for each of two regions of the brain). PVC, comprised of infiltrating mononuclear cells, were enumerated over the entire brain section containing the hippocampus.
For EAE mice, spinal cords were harvested, divided into 12 transverse portions, embedded in paraffin and cut into 4 µm thick tissue sections. To visualize myelin, sections were stained with Luxol fast blue. For scoring of inflammation (PVC), meningitis and demyelination in spinal cord sections, each spinal cord segment was divided into four quadrants: the anterior and posterior funiculi, and each lateral funiculus. Any quadrant containing PVC, meningitis or demyelination was given a score of 1 in that pathologic class. The overall score was determined by giving a quadrant a score of 1 if the quadrant had any PVC, meningitis or demyelination within the quadrant. The total number of positive quadrants for each pathologic class and overall was determined, then divided by the total number of quadrants scored on the slide and multiplied by 100 to give the percent involvement for each pathologic score and overall.
Statistics
The program StatView (SAS Institute Inc.) was used for all statistical analyses performed. The Student’s t test was performed for pairwise comparison (clinical score, weight change). The Chi-Square test was utilized for nominal data (seizures: yes or no). ANOVA, followed by the Fisher’s PLSD post hoc test, was performed to determine group differences for continuous data (PVC). The unpaired two-group Mann-Whitney U test was performed for all nonparametric analyses (neuronal cell loss, pathology score).
Results
TMEV-induced seizures
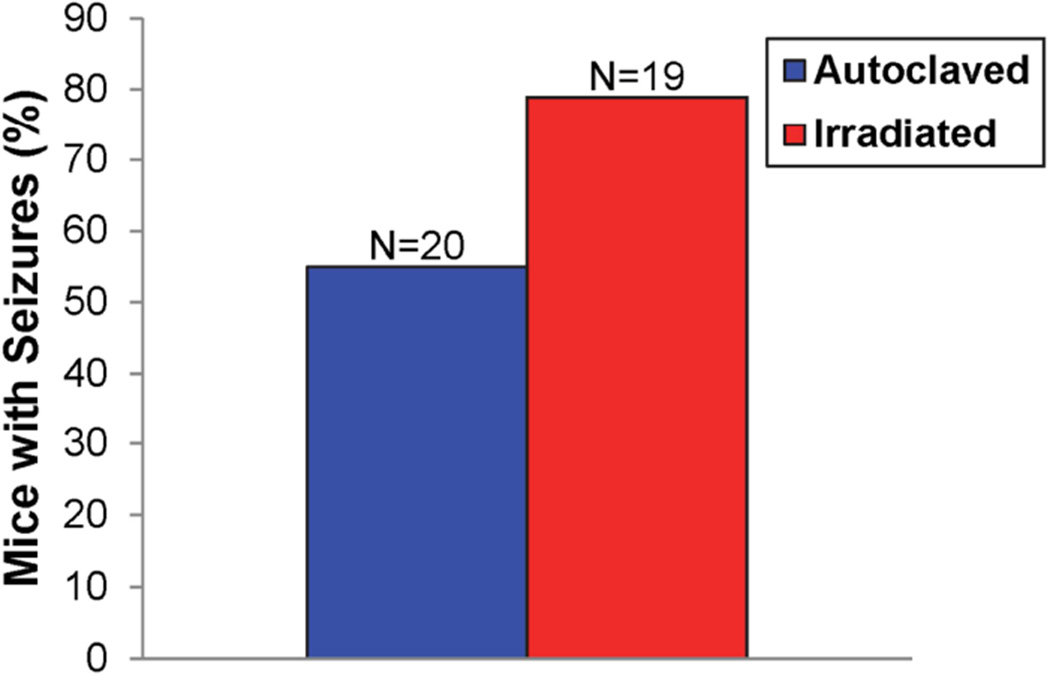
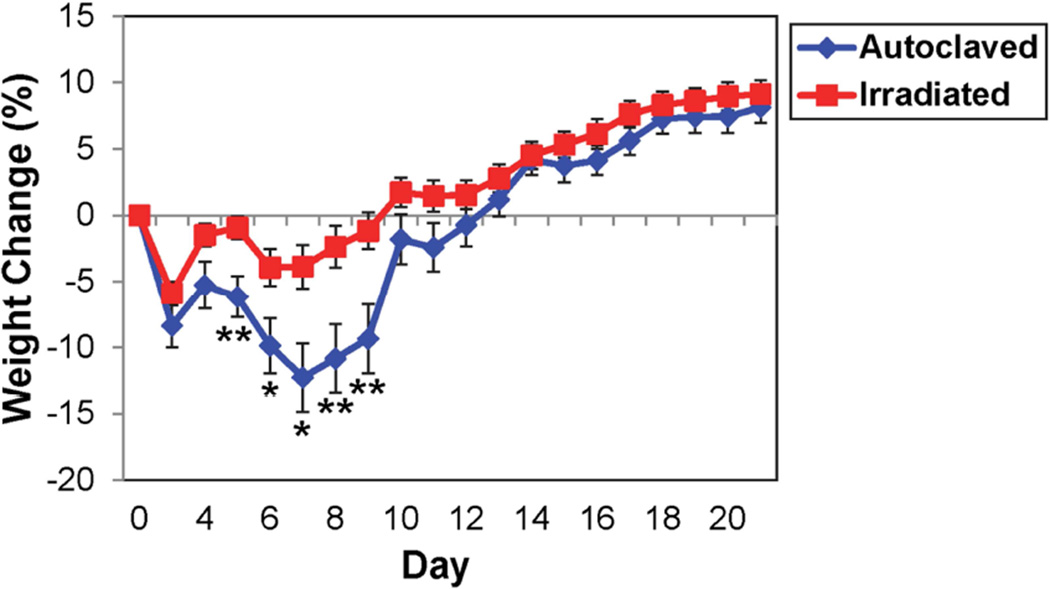
The effects of diet on the severity and outcome of disease were compared using the TMEV-induced seizure model. We found no significant difference in the number of mice experiencing seizures between DA-infected C57BL/6 mice maintained on the autoclaved diet compared to mice on the irradiated diet (Fig. 1). However, for mice that were experiencing seizures, a greater percentage of mice on the irradiated diet experienced Racine scale stage 5 seizures, compared to mice on the autoclaved diet (Table 2). Analysis of weight change in the two groups of animals, a general assessment of animal health, demonstrated that animals on the autoclaved diet had significantly greater weight loss for days 5–9 p.i., compared to animals on the irradiated diet (Fig. 2). Therefore animals on the irradiated diet did not display any significantly increased incidence of disease or increased weight loss in the seizure model; however, there was a shift to higher maximal seizure scores in these mice, suggesting more severe seizures.
Fig. 1.
Seizure frequency (Racine scale stages 3–5) in mice maintained on either irradiated or autoclaved diet. DA-infected C57BL/6 mice were monitored for seizures through day 21 p.i. The total numbers of mice infected (N) is shown. The percentages of mice with seizures were calculated: (number of mice with seizures/total number of mice infected) × 100.
Table 2.
Seizures, Racine Scale – Maximum severity of disease.
| Diet | Number of mice |
% of mice with maximum clinical stages | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Autoclaved | 11 | 0 | 0 | 0 | 9 | 91 |
| Irradiated | 15 | 0 | 0 | 0 | 0 | 100 |
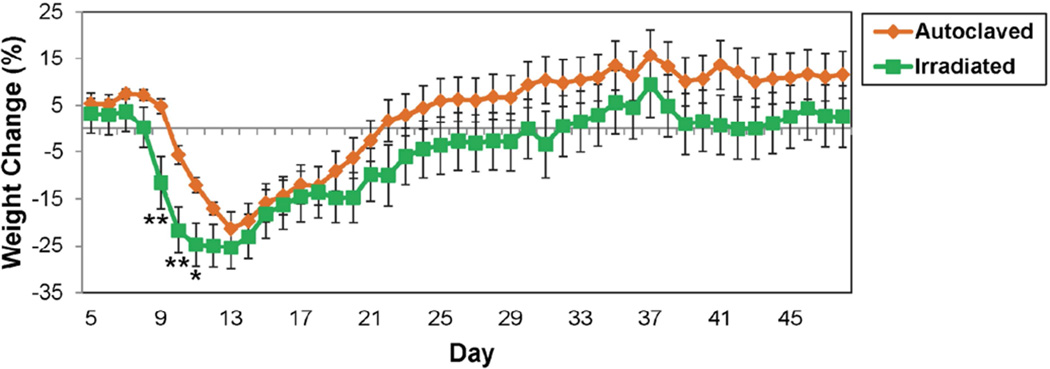
Fig. 2.
Weight change in DA-infected C57BL/6 mice maintained on irradiated or autoclaved diet. Data represents percent of daily weight compared to weight at day 0, given as mean ± SEM for groups of 20 (autoclaved) and 19 (irradiated) mice. *p<0.05, **p<0.01, t-test.
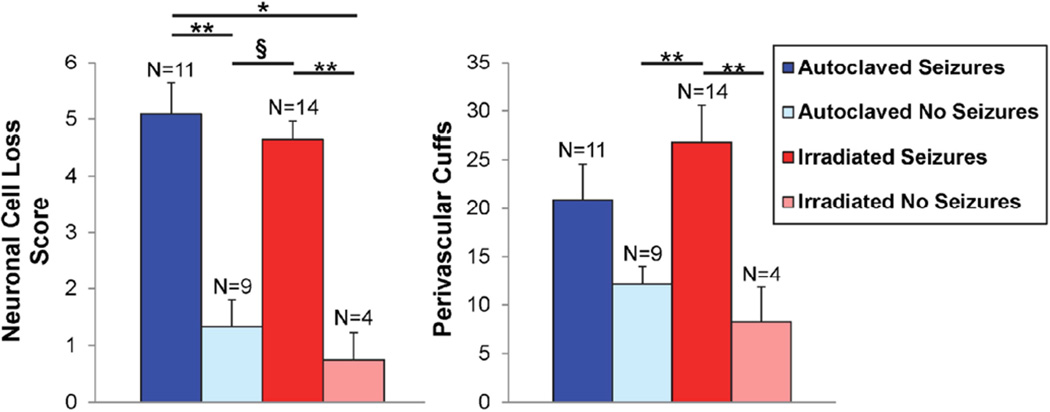
The hippocampus of the brain has been shown to be targeted in mice that experience seizures [20]. Therefore, brains were examined for neuronal cell loss within the pyramidal layer of the hippocampus and for the presence of PVC, an indication of inflammation. As expected, mice that experienced seizures had greater neuronal cell loss and more PVC compared to mice not experiencing seizures irrespective of the diet (Fig. 3). For neuronal cell loss, these differences reached significance (Fig. 3). For PVC, the only differences that reached significance were between mice on the irradiated diet that experienced seizures compared to mice not experiencing seizures irrespective of the diet (Fig. 3). There were no significant differences in the neuronal cell loss scores or PVC between mice that experienced seizures or between mice that did not experience seizures when comparing the autoclaved diet to the irradiated diet (Fig. 3). Therefore the animals on the irradiated diet are very similar to the animals on the autoclaved diet in terms of brain pathology in the seizure model. This was not unexpected as we previously determined that in mice that develop seizures, the pathologic changes in the mice are very similar [21].
Fig. 3.
Brain pathology in DA-infected C57BL/6 mice maintained on irradiated or autoclaved diet. The food groups were divided into mice experiencing seizures and mice not experiencing seizures. The neuronal cell loss was scored as described in the methods. Data represents mean ± SEM. The total numbers of mice (N) is shown. *p<0.05, **p<0.01, §p<0.001, Mann-Whitney U (neuronal cell loss), ANOVA (PVC).
PLP139–151-induced EAE
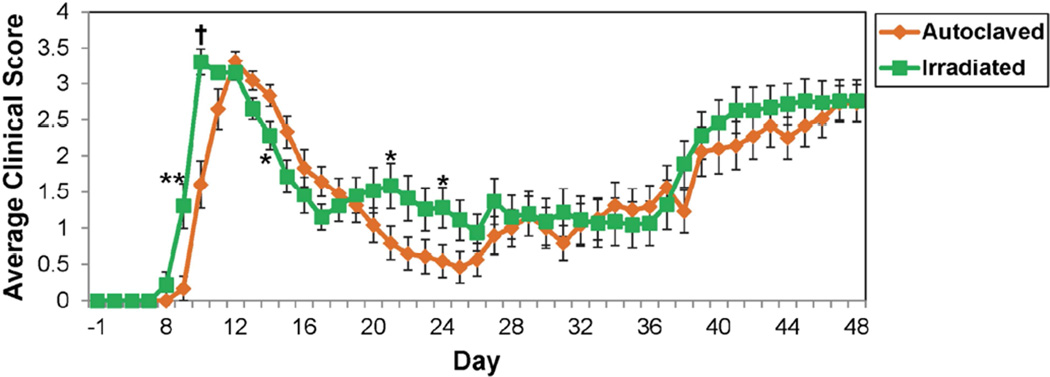
The effects of diet on disease were also compared using the SJL/J PLP139–151-induced EAE model. We found that the onset of disease occurred one day earlier in animals on the irradiated diet compared to animals on the autoclaved diet (Fig. 4). In addition the average clinical scores for mice on the irradiated diet were significantly higher on days 9–10, 21 and 24 p.s. and significantly lower on day 14 p.s., compared to animals on the autoclaved diet (Fig. 4). The percentage of mice that were either found dead or were moribund and needed to be sacrificed was 2-fold greater for mice on the irradiated diet compared to mice on the autoclaved diet (Table 3). Also, animals on the irradiated diet had significantly greater weight loss on days 9–11 p.s. (Fig. 5). Additionally, although the differences did not reach significance for any other days, animals given the irradiated diet had greater weight loss over the entire time course of the experiment, compared to animals on the autoclaved diet (Fig. 5). Therefore animals on the irradiated diet showed increased severity of disease in this EAE model. Despite this apparent increase in severity of disease, examination of spinal cord pathology: demyelination, PVC, meningitis and overall pathology, failed to demonstrate any significant differences between mice on the autoclaved diet and mice on the irradiated diet (Fig. 6). However, overall pathology was assessed at the end of the study when clinical disease was similar.
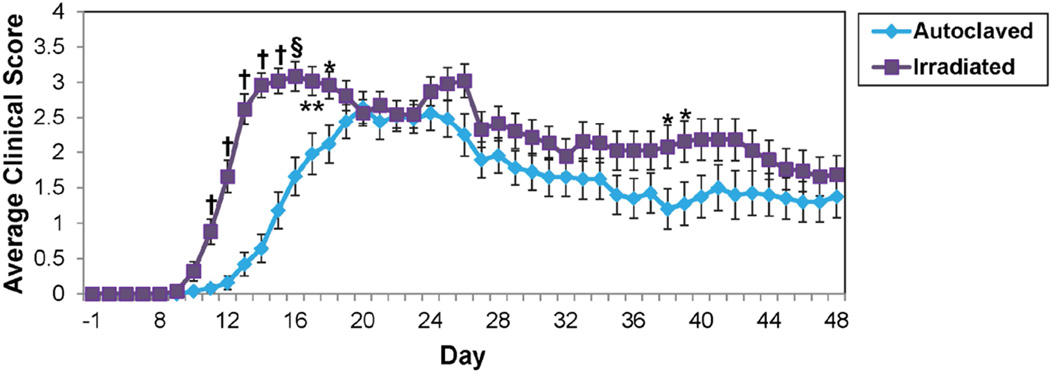
Fig. 4.
Clinical scores in PLP139–151-sensitized SJL/J mice maintained on irradiated or autoclaved diet. Mice were monitored for signs of disease through day 48 p.s. Data is given as the mean ± SEM for groups of 24 (autoclaved) and 23 (irradiated) mice. *p<0.05, **p<0.01, †p<0.0001, t-test.
Table 3.
EAE, Clinical Scores – Maximum severity of disease.
| Induction Peptide |
Diet | Number of mice |
% of mice with maximum clinical scores | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5(# Moribund/Dead) | |||
| PLP139–151 | Autoclaved | 25 | 0 | 0 | 20 | 76 | 4(1) |
| Irradiated | 25 | 0 | 0 | 16 | 76 | 8(2) | |
| MOG35–55 | Autoclaved | 25 | 8 | 0 | 40 | 32 | 20(5) |
| Irradiated | 25 | 4 | 4 | 28 | 40 | 24(6) | |
Fig. 5.
Weight change in PLP139–151-sensitized SJL/J mice maintained on irradiated or autoclaved diet. Data represents percent of daily weight compared to weight at day −1, given as mean ± SEM for groups of 24 (autoclaved) and 23 (irradiated) mice. *p<0.05, **p<0.01, t-test.
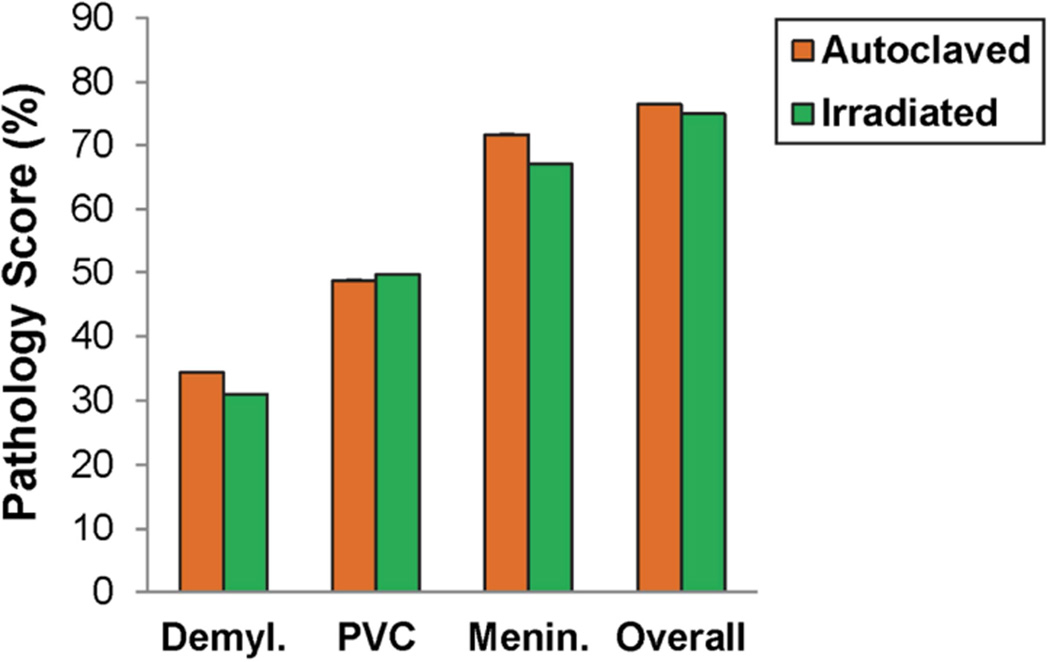
Fig. 6.
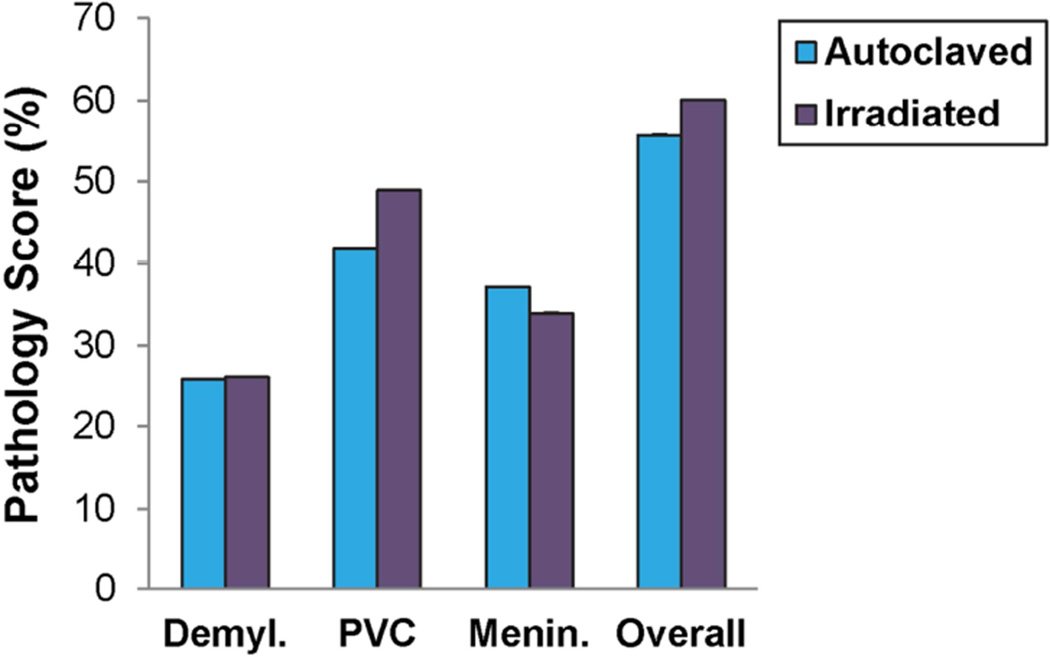
Spinal cord pathology in PLP139–151-sensitized SJL/J mice maintained on irradiated or autoclaved diet. Demyl., PVC, Menin. and Overall pathology were scored as described in the methods. Data is given as the mean ± SEM for groups of 24 (autoclaved) and 23 (irradiated) mice.
MOG35–55-induced EAE
Finally, the effects of diet on disease were compared using the C57BL/6 MOG35–55-induced EAE model. We found that the onset of disease occurred 2–3 days earlier in animals on the irradiated diet compared to animals on the autoclaved diet (Fig. 7). In addition the average clinical scores for mice given the irradiated diet were significantly higher on days 11–18, 38–39 p.s., compared to animals fed the autoclaved diet (Fig. 7). Although the differences did not reach significance for any other days, animals given the irradiated diet had greater average clinical scores for most days of the time course, compared to animals fed the autoclaved diet (Fig. 7). The percentage of mice that were either found dead or were moribund and needed to be sacrificed was greater for the mice on the irradiated diet compared to the mice on the autoclaved diet; likewise, the percentage of mice that scored a maximum of 4 over the course of the disease was greater for the mice on the irradiated diet compared to the mice on the autoclaved diet (Table 3). Also, the percentage of mice that scored a maximum of 1 over the course of the disease was 2-fold less for the mice on the irradiated diet compared to the mice on the autoclaved diet (Table 3). In terms of weight change, animals on the irradiated diet had a precipitous and significant loss in weight over days 12–16 p.s., after having had a significantly smaller loss in weight for day 5–10 p.s., compared to animals on the autoclaved diet (Fig. 8). Therefore animals on the irradiated diet showed increased severity of disease in the C57BL/6 MOG35–55-induced EAE model. However, similar to the other EAE model, despite this apparent increase in severity of disease, examination of spinal cord pathology: demyelination, PVC, meningitis and overall pathology, failed to demonstrate any significant differences between mice on the autoclaved diet and mice on the irradiated diet (Fig. 9). However, the spinal cord pathology was not performed until day 48 p.s. when the clinical signs were very similar.
Fig. 7.
Clinical scores in MOG35–55-sensitized C57BL/6 mice maintained on irradiated or autoclaved diet. Mice were monitored for signs of disease through day 48 p.s. Data is given as the mean ± SEM for groups of 20 (autoclaved) and 19 (irradiated) mice. *p<0.05, **p<0.01, §p<0.001, †p<0.0001, t-test.
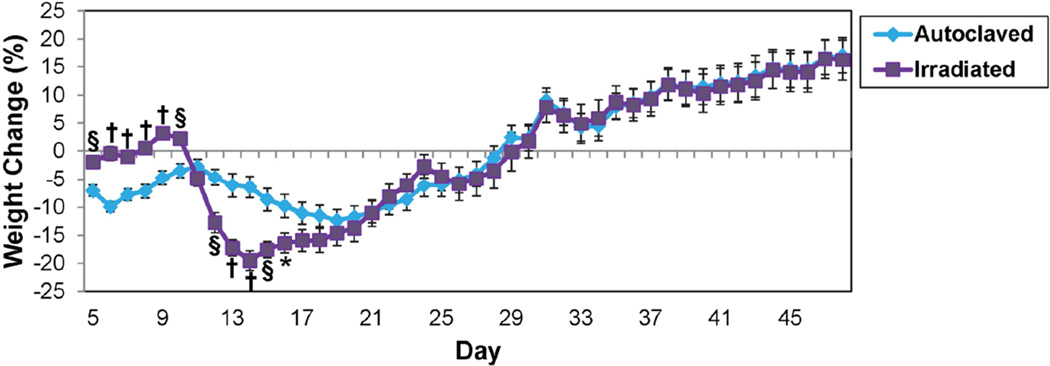
Fig. 8.
Weight change in MOG35–55-sensitized C57BL/6 mice maintained on irradiated or autoclaved diet. Data represents percent of daily weight compared to weight at day −1, given as mean ± SEM for groups of 20 (autoclaved) and 19 (irradiated) mice. *p<0.05, §p<0.001, †p<0.0001, t-test.
Fig. 9.
Spinal cord pathology in MOG35–55-sensitized C57BL/6 mice maintained on irradiated or autoclaved diet. Demyl., PVC, Menin. and Overall pathology were scored as described in the methods. Data is given as the mean ± SEM for groups of 20 (autoclaved) and 18 (irradiated) mice.
Discussion
The lab-to-lab reproducibility of results obtained from identical experimental procedures can be impacted by many different factors, one of which is the composition of food. Two food sources, irradiated and autoclaved diet, are available to us. A comparison of the ingredients in these two food sources is tabulated in Table 1. The only ingredients found in both diets at the exact same concentrations are the minerals chloride and magnesium and the C 18:0 stearic FA (Table 1). Of the ten macronutrients present in the two diets, they are evenly split with five at a higher concentration within the autoclaved diet (crude protein, crude fiber, neutral detergent fiber, ash, calories from protein) and five at a higher concentration within the irradiated diet (fat, carbohydrate, energy density, calories from fat, calories from carbohydrate). In the case of minerals, other than chloride and magnesium, all are present at a higher concentration within the autoclaved diet except iodine. Of the 18 amino acids, all are present at a higher concentration within the autoclaved diet except proline, leucine and methionine which are higher in the irradiated diet. The vitamins show the greatest difference in concentration of any of the ingredients and the concentration is higher within the autoclaved diet for all of the vitamins. For the FAs present in the two diets, other than C 18:0 stearic, all are present at a higher concentration within the irradiated diet except C 16:0 palmitic and total saturated FAs. Lastly, the autoclaved diet contains 50 mg/kg cholesterol, whereas the irradiated diet does not contain cholesterol. Any one of these differences in isolation or a certain combination of differences may be responsible for the effects of the change in diet on disease in the seizure and EAE models.
Studies examining the effects of dietary supplementation on EAE have been previously performed [22–26]. Dietary supplementation with C 18:2 linoleic FA was shown to suppress MBP-induced EAE in guinea pigs, based on clinical score, weight loss and histological changes in the CNS, but only when this FA was given during the time of clinical disease [22]. In addition, ex vivo MBP-specific reactivity of lymph node cells was reduced by linoleic acid supplementation [22]. In our two food sources, linoleic acid concentration is higher in the irradiated diet, however, the animals on the irradiated diet showed increased severity of disease in the two EAE models that we examined. This result is opposite to that found in MBP-induce EAE in guinea pigs. So either the EAE models are very different or other ingredients within the two diets have a greater effect on disease than any effect generated by the linoleic acid.
The effects of elevated dietary trace metals, nickel and zinc, on EAE have also previously been examined using the syngeneic spinal cord homogenate-induced EAE model in SJL mice [23]. Dietary supplementation with nickel resulted in a lower incidence and a later onset of disease [23]. Nickel was not included in our two food sources so no comparison can be made with our experiments. Dietary supplementation with zinc resulted in a more widespread inflammatory CNS disease [23]. Zinc was included as an ingredient in our two food sources and its concentration was higher in the autoclaved diet (Table 1). Our experimental model closest to the syngeneic spinal cord homogenate-induced EAE model in SJL mice is the PLP139–151-induced EAE model in SJL mice. Here again we saw the opposite in that there was increased severity of disease with the irradiated diet (lower zinc concentration) compared to the autoclaved diet. Therefore, it is possible that the amount of zinc in both of the two food sources that we used, irradiated and autoclaved diets, was negligible compared to the zinc supplementation achieved in the previous study or, again, other ingredients within the two diets have a greater effect on disease.
Various vitamins have also been examined for their effects on EAE [24,25]. One study showed that vitamin D3 supplementation significantly inhibited the incidence and severity of MBP-induced EAE in female B10.PL mice, compared to unsupplemented female mice and male mice with or without supplementation, a difference thought to be due to a gender difference in vitamin D3 metabolism within the CNS [24]. In our study, vitamin D3 was present at a 2-fold higher concentration in the autoclaved diet compared to the irradiated diet. Also, female mice were used in the PLP139–151-induced EAE model. Consistent with the earlier vitamin D3 supplementation study, the female mice with PLP139–151-induced EAE on the autoclaved diet had less severe disease than mice on the irradiated diet. Another study found that a deficiency of vitamin B1 exacerbated disease, in terms of higher incidence, earlier onset, more severe clinical signs and weight loss and greater cellular infiltration into the CNS, in the MOG35–55-induced EAE model [25]. In our study, vitamin B1 was present at a 7-fold higher concentration in the autoclaved diet compared to the irradiated diet and our MOG35–55-induced EAE mice on the irradiated diet had more severe disease, consistent with the earlier deficiency study. Therefore, the lower concentrations of vitamin D3 and vitamin B1, as well as other vitamins as yet unexamined, in the irradiated diet as compared to the autoclaved diet may contribute to the increased severity of the disease in the various EAE animals on the irradiated diet.
Finally, it has recently been shown that a high fat diet exacerbates EAE [26]. Using the C57BL/6 MOG35–55-induced EAE model, these researchers demonstrated increased disease severity and increased immune cell infiltration, oxidative stress and pro-inflammatory cytokines within the CNS of animals on a high fat diet (21% fat), compared to a normal diet [26]. Although our two food sources both contain much less fat, the irradiated diet (6.5% fat) had a higher percentage of fat and calories from fat than the autoclaved diet (4.3% fat). The animals on the irradiated diet showed increased severity of disease for both EAE models examined. Therefore the higher fat content of the irradiated diet may contribute, wholly or in part, to the effect of the diet on the severity of the EAE disease.
In terms of seizures and epilepsy, the ketogenic diet has been found to be effective in controlling seizures (reviewed in [27]). The ketogenic diet is a high fat diet in which the major source of calories is supplied in fat. This diet has also been shown to be effective in animal studies (reviewed in [27]). Neither of our two food sources is comparable to the ketogenic diet as both diets supply the majority of calories as carbohydrates (Table 1), therefore no comparison can be made with our experiments. Other than the ketogenic diet, we were unable to find other studies linking food and nutrition with seizures and epilepsy.
In conclusion, we found that animals treated identically can display different disease severities due to their diet. In general, it appears that the irradiated diet caused more severe disease than the autoclaved diet in both EAE models and caused more severe seizures in the seizure model. Therefore, differences in the composition of the animal’s diet may be one factor contributing to variability in results observed from lab-to-lab. Although this is a factor that may be difficult to control for, its presence should be taken into consideration when comparing results from different laboratories.
Highlights.
Varied sources of food can impact reproducibility of results from lab to lab.
Teklad irradiated rodent diet enhanced seizures in a mouse model of disease.
Mice fed the Teklad irradiated rodent diet had earlier onset of EAE.
Teklad irradiated rodent diet enhanced clinical disease in 2 EAE models.
Acknowledgments
We would like to thank Mitchell A. Wilson, Kelley M. Ingram and Samantha P. Duzy, for excellent technical assistance, F. Lynn Sonderegger, PhD, for many helpful discussions and Daniel J. Harper for the outstanding preparation of the manuscript.
This work was supported by NIH T32AI055434 (MFC), 5R01NS065714 (RSF) and 1R01NS082102 (RSF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Role of each author:
Conception and design: MFC, JLR, RSF
Generation, collection, analysis of data: JEL, DJD, JTS
Manuscript drafting: JEL, MFC, RSF
Manuscript approval: JEL, DJD, JTS, MFC, JLR, RSF
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- TMEV
Theiler’s murine encephalomyelitis virus
- CNS
central nervous system
- MS
multiple sclerosis
- i.c.
intracerebral
- DA
Daniels
- p.i.
post infection
- CFA
complete Freund’s adjuvant
- PLP
myelin proteolipid protein
- MOG
myelin oligodendrocyte glycoprotein
- MBP
myelin basic protein
- pfu
plaque forming unit
- subQ
subcutaneously
- i.v.
intravenously
- BP
Bordetella pertussis
- RR
relapsing-remitting
- p.s.
post sensitization
- PBS
phosphate-buffered saline
- PVC
perivascular cuffs
- ANOVA
analysis of variance
- PLSD
protected least significant difference
- SEM
standard error of the mean
- Demyl.
demyelination
- Menin.
Meningitis
- FA
fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theiler M, Gard S. Encephalomyelitis of mice. I. Characteristics and pathogenesis of the virus. J Exp Med. 1940;72:49–67. doi: 10.1084/jem.72.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, et al. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- 4.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Lack of correlation of central nervous system inflammation and neuropathology with the development of seizures following acute virus infection. J Virol. 2011;85:8149–8157. doi: 10.1128/JVI.00730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart K-AA, Wilcox KS, Fujinami RS, White HS. Theiler's virus infection chronically alters seizure susceptibility. Epilepsia. 2010;51:1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- 6.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 7.Stewart K-AA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler's virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating Macrophages are Key to the Development of Seizures Following Virus Infection. J Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, et al. Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler's virus model of temporal lobe epilepsy. Neurobiol Dis. 2014;64:98–106. doi: 10.1016/j.nbd.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler's murine encephalomyelitis virus. Future Virol. 2011;6:1339–1350. doi: 10.2217/fvl.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe CL, Lafrance-Corey RG, Sundsbak RS, Sauer BM, Lafrance SJ, Buenz EJ, et al. Hippocampal protection in mice with an attenuated inflammatory monocyte response to acute CNS picornavirus infection. Sci Rep. 2012;2:545. doi: 10.1038/srep00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsunoda I, Fujinami RS. Two models for multiple sclerosis: Experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Fujinami RS. Can virus infections trigger autoimmune disease? J Autoimmun. 2001;16:229–234. doi: 10.1006/jaut.2000.0484. [DOI] [PubMed] [Google Scholar]
- 14.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 15.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 16.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 17.Tsunoda I, McCright IJ, Kuang L-Q, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Once initiated, viral encephalitis-induced seizures are consistent no matter the treatment or lack of interleukin-6. J NeuroVirol. 2011;17:496–499. doi: 10.1007/s13365-011-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meade CJ, Mertin J, Sheena J, Hunt R. Reduction by linoleic acid of the severity of experimental allergic encephalomyelitis in the guinea pig. J Neurol Sci. 1978;35:291–308. doi: 10.1016/0022-510x(78)90010-2. [DOI] [PubMed] [Google Scholar]
- 23.Schiffer RB, Herndon RM, Eskin T. Effects of altered dietary trace metals upon experimental allergic encephalomyelitis. Neurotoxicology. 1990;11:443–450. [PubMed] [Google Scholar]
- 24.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119–4126. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 25.Ji Z, Fan Z, Zhang Y, Yu R, Yang H, Zhou C, et al. Thiamine deficiency promotes T cell infiltration in experimental autoimmune encephalomyelitis: the involvement of CCL2. J Immunol. 2014;193:2157–2167. doi: 10.4049/jimmunol.1302702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmermans S, Bogie JF, Vanmierlo T, Lutjohann D, Stinissen P, Hellings N, et al. High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the Renin Angiotensin system. J Neuroimmune Pharmacol. 2014;9:209–217. doi: 10.1007/s11481-013-9502-4. [DOI] [PubMed] [Google Scholar]
- 27.Zupec-Kania BA, Spellman E. An overview of the ketogenic diet for pediatric epilepsy. Nutr Clin Pract. 2008;23:589–596. doi: 10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]