Abstract
The evolutionary mechanisms generating the tremendous biodiversity of islands have long fascinated evolutionary biologists. Genetic drift and divergent selection are predicted to be strong on islands and both could drive population divergence and speciation. Alternatively, strong genetic drift may preclude adaptation. We conducted a genomic analysis to test the roles of genetic drift and divergent selection in causing genetic differentiation among populations of the island fox (Urocyon littoralis). This species consists of 6 subspecies, each of which occupies a different California Channel Island. Analysis of 5293 SNP loci generated using Restriction-site Associated DNA (RAD) sequencing found support for genetic drift as the dominant evolutionary mechanism driving population divergence among island fox populations. In particular, populations had exceptionally low genetic variation, small Ne (range = 2.1–89.7; median = 19.4), and significant genetic signatures of bottlenecks. Moreover, islands with the lowest genetic variation (and, by inference, the strongest historical genetic drift) were most genetically differentiated from mainland gray foxes, and vice versa, indicating genetic drift drives genome-wide divergence. Nonetheless, outlier tests identified 3.6–6.6% of loci as high FST outliers, suggesting that despite strong genetic drift, divergent selection contributes to population divergence. Patterns of similarity among populations based on high FST outliers mirrored patterns based on morphology, providing additional evidence that outliers reflect adaptive divergence. Extremely low genetic variation and small Ne in some island fox populations, particularly on San Nicolas Island, suggest that they may be vulnerable to fixation of deleterious alleles, decreased fitness, and reduced adaptive potential.
Keywords: population divergence, genetic drift, divergent selection, effective population size, conservation genomics
Introduction
Islands are global centers of biodiversity and endemism (Stuart et al. 2012), but the evolutionary mechanisms generating this diversity are typically poorly understood (with some notable exceptions, e.g., Losos et al. 1998; Grant & Grant 2002; Jordan & Snell 2008). Isolation is a common feature associated with divergence and speciation among island populations (Grant 1998), but ultimately, the processes of genetic drift and/or divergent selection acting on standing genetic variation and new mutations is required for differences to accumulate (Fisher 1930; Wright 1931, 1951). To date, most research on island biodiversity has largely focused on those examples where divergent selection has generated striking cases of adaptive evolution (Losos et al. 1998; Grant & Grant 2002). However, much less attention has been given to the role of genetic drift in generating differences among isolated islands (Jordan & Snell 2008). Thus, investigating the joint roles of genetic drift and divergent selection is key for understanding how island populations diverge, thereby generating island biodiversity and endemism.
Genetic drift is expected to be strong in island populations for several reasons. First, many island populations are founded by a small number of individuals whose genetic composition may differ significantly from the source (often continental) population due to random chance (Martínez-Solano & Lawson 2009; Kolbe et al. 2012). Second, island populations often have small effective population sizes (Ne). Especially if an island is limited in size, it may have a low carrying capacity and, as a result, a small Ne (Frankham 1998; Eldridge et al. 1999). The third reason is bottlenecks. Like many populations, island populations are likely to experience fluctuations in population size in the course of their history, sometimes resulting in significant population size reductions or bottlenecks (Frankham 1998; Heber et al. 2013). Yet unlike continental populations, isolated island populations may not receive an infusion of genetic variation through gene flow after bottlenecks, resulting in a permanent reduction in genetic variation (or at least a long-term reduction, as mutation may eventually partially replenish lost genetic variation; Eldridge et al. 1999).
Divergent selection is also expected to be strong among island populations due to high environmental heterogeneity among islands and between islands and the mainland (Weigelt et al. 2013). One potential source of environmental variation on islands is climate, including temperature, precipitation, and fog (Fischer & Still 2007). The specific position of islands relative to ocean currents can have profound effects on their climate (Spalding et al. 2007), as do differences in elevation and topography. Another source of environmental variation among islands is community composition. Island biogeography theory makes the simple but important prediction that larger islands have more species (MacArthur & Wilson 1967), but even adjacent islands that are the same size may have different species due to climate, microhabitat availability, and random chance of which species end up on which islands (Burns 2007). Thus, both genetic drift and divergent selection can be strong in island populations, which means either or both processes could drive genetic differentiation and population divergence among islands. However, if drift is strong, it may overwhelm selection, precluding adaptive divergence (Wright 1931, 1951).
One of the most iconic island species in the world is the island fox (Urocyon littoralis), a species whose origin, evolution, and divergence has fascinated evolutionary biologists for decades (Grinnell et al. 1937; Gilbert et al. 1990; Wayne et al. 1991; Collins 1993; Goldstein et al. 1999; Aguilar et al. 2004). This species is a diminutive, endemic fox sister to the mainland gray fox (Urocyon cinereoargenteus) and is found on 6 of the 8 Channel Islands off the coast of southern California (Fig. 1) (Coonan et al. 2010). Recent archeological and mitogenomic studies have demonstrated that island foxes diverged from their mainland progenitor ~9200–7100 years ago and have been on the northern Channel Islands (San Miguel [SMI], Santa Rosa [SRI], and Santa Cruz [SCI]) for at least 7100 years and the southern Channel Islands (Santa Catalina [SCA], San Clemente [SCL], and San Nicolas [SNI]) for at least 5000 years (Rick et al. 2009; Hofman et al. 2015). Foxes may have been transported to the Channel Islands by rafting on debris flows or by Native Americans as part of ritual practices, as they are found in formal cemeteries and burials, for their pelts which were used as clothing, and for helping reduce pests (e.g., mice populations) (Hofman et al. 2015; Rick et al. 2009). These studies also suggest a prehistoric, human translocation of island foxes from the northern to the southern Channel Islands based on AMS radiocarbon dates. Earlier morphological and genetic studies described island foxes on each island as a separate subspecies (Grinnell et al. 1937; Gilbert et al. 1990; Wayne et al. 1991; Collins 1993; Goldstein et al. 1999). Although high levels of genetic differentiation have been documented among island fox populations (Aguilar et al. 2004), there is no evidence to date that observed morphological differences among islands are genetically based or adaptive.
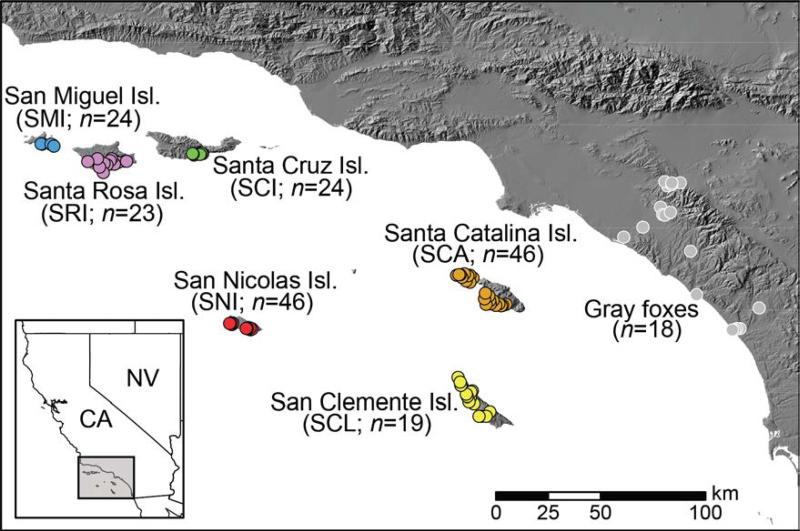
Fig. 1.
Map of island fox and gray fox individuals included in genomic analyses. Abbreviations and sample sizes are shown in parentheses. Inset shows location of study area in southern California, USA.
Solving the puzzle of high genetic differentiation among island fox populations requires an understanding of their recent population crashes and recovery. In the late 1990s, subspecies on four islands went through severe population bottlenecks (Table 1), leading to their listing as endangered in 2004 under the U.S. Endangered Species Act (U.S. Fish and Wildlife Service 2004). The three northern island populations crashed because of predation by golden eagles (Aquila chrysaetos), which had colonized the islands due to abundant food provided by feral pigs and sheep (Roemer et al. 2001; Coonan et al. 2010). On SCA, declines were caused by canine distemper virus (CDV), likely introduced by raccoons (Procyon lotor) or dogs (Timm et al. 2009). San Nicolas Island foxes may have dropped to as low as 20 individuals in the 1970s for unknown reasons (Coonan et al. 2010). The only island that has not experienced a recent bottleneck based on intensive island fox population monitoring efforts on all islands is SCL. Populations on each of the northern islands and SCA have recovered over the past decade as the result of intensive and rapid management efforts, including removal of golden eagles, feral pigs, and ungulates, CDV vaccinations, and separate captive breeding programs on each of these islands (Table 1) (Coonan et al. 2010). However, the SNI population of island foxes is small and currently declining (Table 1; Coonan 2015).
Table 1.
Population bottleneck year and sizes, current population sizes, sample sizes (n) before and after SNP quality filters for mainland gray foxes and each island fox population
| Site | Bottleneck year | Bottleneck size | Current size in 2014 | n (total sampled) | n (after filters) |
|---|---|---|---|---|---|
| gray foxes | NA | NA | Unknown | 18 | 16 |
| SMI | 1999-2000 | 15 | 470 | 24 | 21 |
| SRI | 1999-2000 | 15 | 826 | 23 | 23 |
| SCI | 1999-2000 | 50-60 | 2466 | 24 | 24 |
| SCA | 1999 | (> 90% decline) | 1624 | 46 | 43 |
| SCL | NA | NA | 1230 | 19 | 17 |
| SNI | 1970s | 20? | 263 | 46 | 44 |
Island fox bottleneck year and bottleneck population size estimates from Coonan et al. (2010). No estimate of the population size is available for Santa Catalina Island during its 1999 bottleneck, but this population is estimated to have declined by > 90%. San Nicolas island foxes may have dropped to as low as 20 individuals in the 1970s. Current adult population size estimates for 2014 from Coonan (2015).
Strong genetic drift caused by founder effects and the bottlenecks described above could play an important role in causing high genetic differentiation among island fox populations. In addition, island fox populations are exposed to varying environmental conditions across the Channel Islands archipelago, likely generating divergent selection on top of genetic drift. In particular, SMI and SRI are substantially cooler and wetter than SCI, SCA, and SCL because they are nested within different marine ecoregions (Spalding et al. 2007). In addition, island foxes on different islands have significantly different prey bases and diets (Cypher et al. 2014). Finally, exposure to pathogens varies among islands, e.g., CDV in the late 1990s on SCA (Timm et al. 2009). Genetic drift and divergent selection therefore could both be important drivers of divergence among island fox populations, or genetic drift may overwhelm selection, preventing adaptation.
Determining the roles of genetic drift and divergent selection in causing genetic differentiation in island foxes also has direct bearing on conservation and management of this high-profile species of conservation concern. First, understanding the relative importance of these mechanisms is directly relevant to their current legal designation and management as distinct subspecies. Under most definitions of subspecies and Evolutionarily Significant Units (ESUs)—which can receive legal protection under the U.S. Endangered Species Act (ESA), as in the case of island foxes on SMI, SRI, SCI, and SCA—adaptive differences, in addition to genome-wide genetic differentiation and phenotypic differences, are required (Ryder 1986; Crandall et al. 2000; Funk et al. 2012; Robertson et al. 2014). Thus, knowing whether or not there are adaptive differences among island fox populations is directly relevant to their legal protection as subspecies.
Second, understanding the strength of genetic drift in island fox populations is important for knowing whether loss of genetic variation is a significant threat to their persistence. If extreme bottlenecks have substantially reduced genetic variation in island foxes, this could make these populations vulnerable to both inbreeding depression (Ralls et al. 1979; Ralls & Ballou 1983; Lacy 1997; Newman & Pilson 1997; Saccheri et al. 1998) and a reduced ability to adapt to future environmental change (Bürger & Lynch 1995), such as the predicted warming and drying of southern California in the coming century (Cayan et al. 2008; LaDochy & Witiw 2012; Cook et al. 2015).
Lastly, understanding divergent selection and adaptive differentiation among island fox populations is relevant to future consideration of genetic rescue as a management strategy (Tallmon et al. 2004; Whiteley et al. 2015). Outbreeding depression rather than genetic rescue could result if a source population is maladapted to the target population (Edmands 2007; Frankham et al. 2011). Thus, understanding patterns of adaptive differentiation among islands will inform predictions about the likelihood that genetic rescue would increase fitness and population sizes.
Our goal here was to use a population genomic approach with single-nucleotide polymorphism (SNP) data generated from Restriction-site Associated DNA (RAD) sequencing to investigate the roles of genetic drift and divergent selection in causing population divergence among island fox populations. Genomics greatly improves our ability to address this question compared to traditional population genetics approaches with small numbers of markers by increasing power to identify loci under selection, providing enough variability in small populations to estimate Ne and test for bottlenecks, and improving statistical power and precision. We had four specific aims. First, we characterize the genetic population structure of island foxes. Second, we test the hypothesis that genetic drift contributes to genetic differentiation among populations. Third, we test the hypothesis that divergent selection caused by environmental differences among islands contributes to genetic differentiation among populations. Fourth, we characterize patterns of population divergence at neutral vs. any detected adaptive loci. We conclude with a discussion of the conservation implications of our findings.
Methods
RAD sequencing and genotyping
We used Restriction-site Associated DNA (RAD) sequencing (Baird et al. 2008) to genotype 182 island foxes and 18 outgroup gray foxes (Goldstein et al. 1999; Hofman et al. 2015) collected between 2008-2011. DNA was extracted from blood samples or muscle tissue from road-killed foxes using DNeasy blood and tissue extraction kits (Qiagen, Valencia, CA) following the manufacturer's protocol. We sequenced 18–46 individuals (median = 24 individuals) per island (or from the mainland, in the case of gray foxes; Fig. 1 and Table 1).
As there is currently no island fox reference genome, we used a two-step RAD sequencing approach. First, we assembled reference contigs using paired-end sequences from eight individuals at high coverage depth (~100 X) (Etter & Johnson 2012; Hohenlohe et al. 2013). DNA from these individuals was prepared in an individually barcoded RAD library following the method of Etter et al. (2011) using the restriction enzyme SbfI. Next, we selected fragments corresponding to insert sizes of 230-400 bp and sequenced this library in a single 150 bp paired-end Illumina HiSeq lane. After sequencing, we filtered for read quality and presence of a correct barcode and SbfI recognition site, identified and removed PCR duplicates, pooled the data from all individuals, identified loci using stacks software (Catchen et al. 2011, 2013), and assembled consensus RAD contigs from the overlapping paired-end reads separately at each locus following methods and parameters outlined in Hohenlohe et al. (2013).
We next prepared individually barcoded RAD libraries for the remaining individuals (n = 192) as above and sequenced them on an Illumina HiSeq with single-end 100 bp reads at lower depth (~20 X) in a total of four lanes with 47–49 individuals per lane. After filtering reads as above, we aligned these “clean” reads against the reference RAD contigs, removing those loci that did not align uniquely, and called diploid genotypes along the 100 bp stretch using a maximum likelihood statistical method (Hohenlohe et al. 2010a; Catchen et al. 2013). The forward reads from the eight individuals used to assemble contigs were also aligned and genotyped.
This two-step procedure of first assembling reference contigs using paired-end sequencing, followed by aligning single-end reads to reference contigs provides multiple advantages over single-end sequencing alone. First, assembling reference contigs provides a high-confidence reference “genome” for the RAD loci. Longer paired-end reads and contig assembly better distinguish paralogous and duplicate loci and allow for a greater chance of finding functional information about high FST outliers with blasting than shorter, single-end contigs (Hohenlohe et al. 2013). Second, alignment of subsequent single-end reads to these reference contigs provides higher-confidence clustering of reads to the correct loci and an additional layer of filtering for the single-end read data (e.g., removing non-RAD sequence, quality filtering, etc.).
After calling SNPs, we performed several additional quality filters. First, we removed any loci for which more than half of the individuals had missing data (Hohenlohe et al. 2010a). Second, for those RAD tags with more than 1 SNP per contig, we only used the first (most 5’) SNP per contig, as the choice of which SNP to use per contig did not affect results. For example, there were no biases or statistically significant differences in genetic differentiation or within population genetic variation based on the first vs. latter SNPs. Third, we removed loci with minor allele frequencies less than 0.10, as low frequency alleles may represent PCR errors. Fourth, we removed any individuals with genotypes for less than 50% of loci. Finally, we removed loci with exceptionally high coverage (coverage greater than 2 SD above the mean), as these loci could be paralogs (Emerson et al. 2010).
Data analysis
Aim 1: Characterize population structure
We used several analyses to characterize population structure among island fox and gray fox populations. First, we estimated two different indices of genetic differentiation among all island fox populations and mainland gray foxes: pairwise FST and Jost's D (Jost 2008; Verity & Nichols 2014). Pairwise FST estimates and their significance were calculated using 1000 permutations in arlequin 3.5 (Excoffier et al. 2005) and Jost's D estimates were calculated in GenAlEx 6.5 (Peakall & Smouse 2006, 2012). In addition, we tested the sensitivity of FST estimates to our threshold for the allowed level of missing genotypes (< 50%; see “RAD sequencing and genotyping” section of Methods above) by calculating FST again using a more stringent threshold (< 20%) and then estimating the correlation coefficient between pairwise FST estimates calculated with these different thresholds. We also calculated the correlation coefficient between pairwise FST and Jost's D estimates.
Secondly, we inferred the number of island fox and gray fox populations using the Bayesian clustering algorithm implemented in program structure 2.3.4 (Pritchard et al. 2000). structure infers the best-supported number of clusters (K) in the sample and the proportion of each individual's genome assigned to each cluster (qk). We ran structure with an MCMC burn-in of 100,000 steps followed by 100,000 steps for inference of clustering (Willing et al. 2010) and used the admixture model with correlated allele frequencies. We tracked LnP(D), the probability of the data given K, over the course of the run to ensure that these values had stabilized at the end of the burn-in period. structure was run for K = 1–10 with 10 replicates for each value of K. We inferred the best-supported value of K using a combination of mean LnP(K) and the ΔK method of Evanno et al. (2005) as implemented in structure harvester (Earl & Vonholdt 2012).
Third, we examined patterns of genetic divergence and similarity using two different analyses. Principal Components Analysis (PCA) was performed with the ‘prcomp’ package in program R (R Development Core Team 2010). Neighbor-net trees were then inferred using program splitstree4 (Bryant & Moulton 2004; Huson & Bryant 2006). For the Neighbor-net tree analysis, all heterozygous SNPs were coded according to International Union of Pure and Applied Chemistry (IUPAC), uncorrected_P distance was used as the metric, and ambiguous states were treated as average matches. These last two analyses were performed with and without gray foxes to test the sensitivity of patterns of genetic differentiation to inclusion of the outgroup.
Aim 2: Test the contribution of genetic drift to genetic differentiation
Two main predictions stem from the hypothesis that genetic drift due to small effective population sizes (Ne), founder effects, and/or bottlenecks is a significant cause of high genetic differentiation among island fox populations: (1) island fox populations will have low genetic variation (indicating strong historical genetic drift), small Ne, and/or evidence of bottlenecks; and (2) pairwise genetic differentiation between island fox populations and the mainland (gray foxes) will be negatively correlated with indices of historical genetic drift (estimates of within population genetic variation). In other words, following Jordan and Snell (2008) and Whiteley et al. (2010), we expected high FST values when within-population genetic variation was low, and vice versa.
To test the first prediction, we estimated several indices of within-population genetic variation, estimated Ne, and tested for bottlenecks. We used four measures to characterize within-population genetic variation for island foxes and gray foxes: observed heterozygosity (Ho), expected heterozygosity (He), allelic richness (Ar), and nucleotide diversity (π). Ho, He, and π were estimated directly from stacks output and Ar was estimated using hp-rare 1.0 (Kalinowski 2005). Importantly, estimates of Ho, He, and π are relatively insensitive to sample size, especially when the number of loci used is large (Nei 1978); Ar uses a rarefaction approach to correct for variation in sample sizes.
We estimated effective population size (Ne) of island fox populations using the linkage disequilibrium (LD) method implemented in program NeEstimator 2.01 (Do et al. 2014). This method is based on theory showing that the amount of linkage (i.e., gametic) disequilibrium at independent loci in randomly mating, isolated populations is purely a function of the magnitude of genetic drift and can therefore be used to estimate Ne (Hill 1981). The LD method provides a contemporary estimate of Ne in the previous generation, although it can also be affected by LD generated over several generations (Waples 2005). Because island foxes have overlapping generations, the LD method estimates the effective number of breeders (Nb) that produced the sampled cohort(s), which may or may not be the per-generation estimate of Ne. However, the relationship between Ne and Nb for single-sample estimates of Ne remains unclear (Waples 2010), so here, we refer to our estimates as Ne.
As this method assumes that markers are selectively neutral, we estimated Ne using only presumably neutral loci not identified as high FST outliers (which may be under divergent selection). For the purposes of this analysis, we identified high FST outliers as those loci with the top 5% of global FST values (Hohenlohe et al. 2010a) and then removed these outlier loci before running NeEstimator, leaving 4615 loci for analysis. This simple non-model-based approach for identifying high FST outlier loci may incorrectly identify some loci as outliers that in reality are not under divergent selection (i.e., false positives) (Bierne et al. 2013). Thus, this is a conservative approach because it errs on the side of removing too many loci.
We tested for evidence of population bottlenecks in each of the six island fox subspecies and the mainland gray fox species using the program Bottleneck 1.2.02 (Cornuet & Luikart 1996; Piry et al. 1999). This analysis is based on the loss of rare alleles predicted in recently bottlenecked populations, resulting in heterozygosity excess. As this method assumes that markers are selectively neutral, we only used non-outlier loci, identified using the “top 5% method” as explained above. We used the infinite alleles model (IAM) as the most appropriate evolutionary model for SNP loci. To test for significant heterozygosity excess compared to the level predicted under mutation-drift equilibrium, we used a one-tailed Wilcoxon signed rank test implemented in R v3.1.3. Stringent filters on minor allele frequency (MAF) could bias this analysis in favor of detecting bottlenecks because rare alleles are removed. Therefore, we carried out two sets of analyses, the first using all non-outlier loci that met the standard MAF filter of > 0.1, and the second using all non-outlier loci that met a less stringent filter of MAF > 0.02.
To test the second prediction that genetic differentiation between island fox populations and the mainland will be negatively correlated with the magnitude of genetic drift, we conducted linear regression analyses with pairwise estimates of FST between each island fox population vs. gray foxes as the response variable and Ho, He, Ar, or π as the predictor variable (in four separate regression analyses). This analysis assumes that within island genetic variation is a reasonable index of the magnitude of historical genetic drift, which should be a valid assumption, due to minimal gene flow among island fox populations and gray foxes after initial colonization of the islands (Hofman et al. 2015). Therefore, gene flow would not be expected to contribute significantly to within-island genetic variation.
Aim 3: Test the contribution of divergent selection to genetic differentiation
Divergent selection could contribute to genetic differentiation of a subset of loci or genome-wide. In the early stages of adaptive divergence, selection is predicted to target specific loci underlying traits involved in local adaptation, causing a relatively small subset of loci to be identified as high FST outliers with higher genetic differentiation than background, neutral levels (Beaumont & Nichols 1996; Beaumont & Balding 2004; Hohenlohe et al. 2010a, b). If adaptive divergence proceeds to the point of causing reproductive isolation in the process of ecological speciation, “isolation-by-adaption” can result whereby genome-wide genetic differentiation is correlated with environmental differences among populations (Nosil et al. 2008). We tested both of these possible outcomes of divergent selection in island foxes.
First, we identified high FST outliers among island fox populations (without gray foxes) using three outlier tests: a non-model-based method (loci with the highest 5% of FST values, described above); a maximum likelihood test implemented in FDist2 (Beaumont & Nichols 1996; Beaumont & Balding 2004); and a Bayesian approach implemented in BayesFST (Beaumont & Balding 2004). Both of these methods have been shown to be relatively robust to deviations from assumed population structure (Beaumont & Balding 2004). FDist2 was run assuming either a simple island model or a hierarchical island model with islands nested in separate northern (SMI, SRI, SCI) and southern (SCA, SCL, SNI) groups. To standardize the false positive rate between FDist2 and BayesFST, we set the critical P-value to 0.01 for FDist2 to compare with the Bayesian 10% level, as recommended by Beaumont and Balding (2004). With a critical P-value of 0.01, 1% of loci should be identified as high FST outliers by chance. Thus, following previous studies (Beaumont & Balding 2004; Willing et al. 2010), if > 1% of loci were identified as high FST outliers, we interpreted this as evidence of true divergent selection and adaptive divergence. We also corrected for type I errors from multiple testing in FDist2 using a false discovery rate (FDR) correction (Benjamini & Hochberg 1995). BayesFST already addresses the problem of multiple testing through the prior distribution of the regression parameter for the locus effect (Beaumont & Balding 2004). We then ran a Gene Ontology (GO) term enrichment analysis (Mi et al. 2013) for those high FST outliers that blasted to genes in the dog (Canis lupus familiaris) genome (canFam3.1; Hoeppner et al. 2014). The GO analysis tested for overrepresentation of genes associated with specific biological processes relative to the full set of dog genes.
To complement our high FST outlier tests, we tested for loci associated with environmental variation with BayeScEnv (Villemereuil & Gaggiotti 2015). This method considers a model incorporating environmental data from each collection site, and compares that to both the null F-model and standard α-model to identify FST outlier loci that show variation associated with environmental differentiation. We considered five environmental variables hypothesized to be related to adaptive divergence among island fox populations: mean annual temperature and precipitation (Weigelt et al. 2013) and three dietary variables that reflect differences in prey availability among islands (proportion of insects, fruit, or mice in the diet; Cypher et al. 2014).
BayeScEnv was run for each of the environmental variables with the parameter settings of: g (upper bound) = 10; α (mean prior) = −1.0; p = 0.50; and π = 0.10. After 20 pilot runs of 2,000 iterations each and a burn-in of 50,000 iterations, 5,000 MCMC samples were taken with 10 steps between each sample. Diagnostics of the log likelihoods and FST values for the 5,000 sampled iterations were checked using the ‘coda’ package in R to confirm convergence and sample sizes of at least 2,500.
Second, we tested for relationships between genome-wide genetic differentiation and environmental differences among populations using multiple regression on distance matrices (MRDM; Legendre et al. 1994; Balkenhol et al. 2009). MRDM regresses multiple predictor matrices against a response matrix of genetic distances, and uses permutation to assess statistical significance. The MRM function in the R package ‘ecodist’ (Goslee & Urban 2007) was run with 1,000 permutations using the genetic distance matrices (FST or Jost's D) as the response variable. Each of three matrices representing climatic differences among the islands (mean temperature and precipitation; Weigelt et al. 2013) and dietary differences (Horn's similarity index; Cypher et al. 2014), along with the geographic distance matrix were used as the predictor variables. Prior to running MRDM, we used variance inflation factors (VIF) to assess multicollinearity among predictor variables. Geographic distance and temperature were highly correlated (>80%) but had VIF values < 4; thus, both factors were retained for the subsequent analyses. To obtain a best-reduced model, stepwise regression with both forward and backward selection was implemented with the ‘step’ function in R. A full MRDM model that included all predictor variables was then run, as well as a model that considered geographic distance alone.
Aim 4: Characterize population divergence at neutral vs. adaptive loci
Lastly, we used the results of the above outlier tests to partition our SNP dataset into non-outlier (presumably neutral) and high FST outlier (presumable adaptive) loci for examining patterns of genetic divergence and similarity using PCA and Neighbor-net trees (Funk et al. 2012). In addition, we used the previously described non-model-based method of identifying outliers, in which loci with the highest 5% of FST values were designated as outliers, as a third way to partition our dataset.
Results
RAD sequencing and genotyping
After filtering for read quality and presence of a correct barcode and SbfI recognition site, a total of 93,314,044 “clean” read pairs were generated across the eight individuals included in the paired-end Illumina HiSeq lane. Of these, 69% were identified as PCR duplicates and were removed, leaving 29,357,186 read pairs from which we assembled a total of 126,264 unique consensus RAD contigs.
Single-end sequencing yielded 494,418,159 clean sequence reads across 192 individuals. These were added to 55,262,507 clean forward reads from the eight individuals included in the paired-end sequencing lane for a total of 549,680,666 clean reads from 200 individuals that were aligned to the above 126,264 RAD contigs. After all quality filters, a total of 4858 variable SNP loci were available for analysis when the gray fox outgroup was included and 5293 SNPs were available without gray foxes (Table 2). Mean coverage per locus (averaged across individuals) ranged from 5–40 (median = 20; Fig. S1, Supporting information) and the number of loci per individual (for the dataset including gray foxes) ranged from 2381–4854 (median = 4419 [= 91% of all variable SNPs]; Fig. S2, Supporting information).
Table 2.
Counts of SNP loci after each step of filtering with or without gray foxes included
| With gray foxes included | Without gray foxes included | |||
|---|---|---|---|---|
| Filtering step | Count | % of total | Count | % of total |
| (1) Total contigs | 126264 | 100.0 | 126264 | 100.0 |
| (2) SNPs w/ genotypes for ≥ 50% individuals | 50135 | 39.7 | 20153 | 16.0 |
| (3) 1 SNP per contig | 30719 | 24.3 | 15291 | 12.1 |
| (4) Minor allele frequency (MAF) ≥ 0.10 | 4997 | 4.0 | 5404 | 4.3 |
| (5) Coverage ≤ mean coverage + 2SD | 4858 | 3.8 | 5293 | 4.2 |
Aim 1: Characterize population structure
We found exceptionally high genetic differentiation among island fox populations. Pairwise FST values between most islands were extremely high, ranging from 0.463–0.963 (median = 0.749), and all values were statistically significant (P < 0.00001; Table 3). FST values were insensitive to the threshold used for the allowed level of missing genotypes, as revealed by a high correlation between pairwise FST values calculated using our standard threshold of < 50% missing genotypes vs. a more stringent threshold of < 20% missing genotypes (r = 0.999, P < 0.00001). Pairwise Jost's D values were also significantly correlated with pairwise FST values (r = 0.711, P = 0.0003), although SNI was more similar to SCL with Jost's D than with FST (Table 3).
Table 3.
Pairwise FST estimates below diagonal and Jost's D estimates above diagonal between all pairs of island fox and gray fox populations
| Gray foxes | SMI | SRI | SCI | SCA | SCL | SNI | |
|---|---|---|---|---|---|---|---|
| Gray foxes | - | 0.376 | 0.345 | 0.346 | 0.282 | 0.357 | 0.384 |
| SMI | 0.664 | - | 0.136 | 0.325 | 0.457 | 0.460 | 0.603 |
| SRI | 0.589 | 0.515 | - | 0.199 | 0.368 | 0.392 | 0.547 |
| SCI | 0.623 | 0.773 | 0.584 | - | 0.291 | 0.362 | 0.527 |
| SCA | 0.462 | 0.676 | 0.596 | 0.558 | - | 0.204 | 0.237 |
| SCL | 0.629 | 0.884 | 0.749 | 0.778 | 0.463 | - | 0.239 |
| SNI | 0.814 | 0.963 | 0.902 | 0.919 | 0.646 | 0.914 | - |
Pairwise FST and Jost's D estimates were calculated from 4858 SNP loci using arlequin 3.5 (Excoffier et al. 2005) and GenAlEx 6.5 (Peakall & Smouse 2006, 2012), respectively. All pairwise FST estimates were statistically significant. See Fig. 1 for full names of islands abbreviated here.
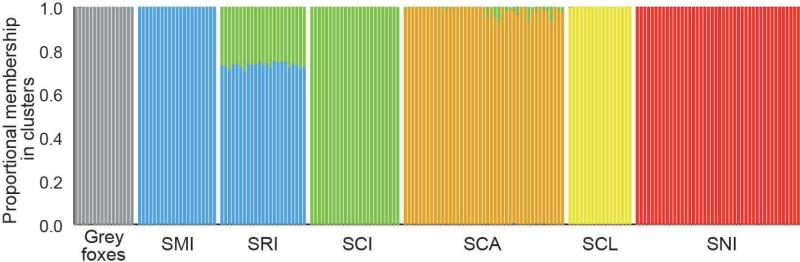
The best-supported value of K in our structure analysis was K = 7 based on mean LnP(K) and K = 2 based on the ΔK method. However, K = 2 was clearly an underestimate based on our FST, Jost's D, PCA, and Neighbor-net results. Interestingly, although K = 7 was best-supported based on mean LnP(K), no individuals had any measurable portion (to the thousandths place) of their genome assigned to the seventh cluster, meaning K = 6 effectively had the highest support. With K = 7, individuals were generally assigned to a single island (or to the mainland, in the case of gray foxes; Fig. 2). However, approximately 73% of the genomes of individuals from SRI were assigned to SMI and approximately 27% to SCI, indicating SRI has an intermediate genetic relationship to SMI and SCI. Several individuals on SCA also had a small proportion of their genomes (mean = 1.3%) assigned to SCI.
Fig. 2.
Results from Bayesian individual clustering with Structure for K = 7. Each color corresponds to a distinct genetic cluster and each bar corresponds to the proportion of an individual's genotype assigned to each cluster. Note that although K = 7 was the best-supported number of K, no individuals had any measurable portion (to the thousandths place) of their genome assigned to the seventh cluster, meaning K = 6 effectively had the highest support.
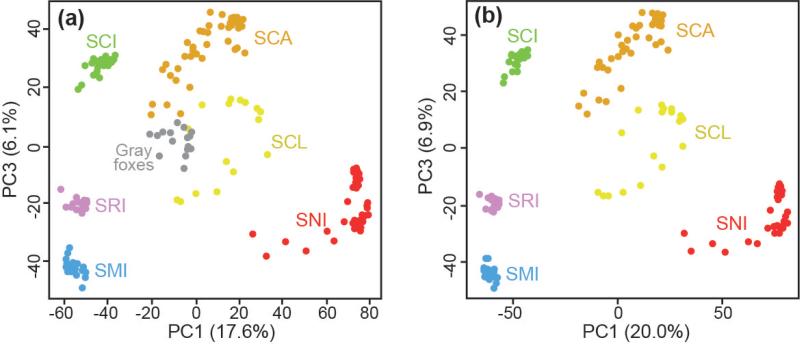
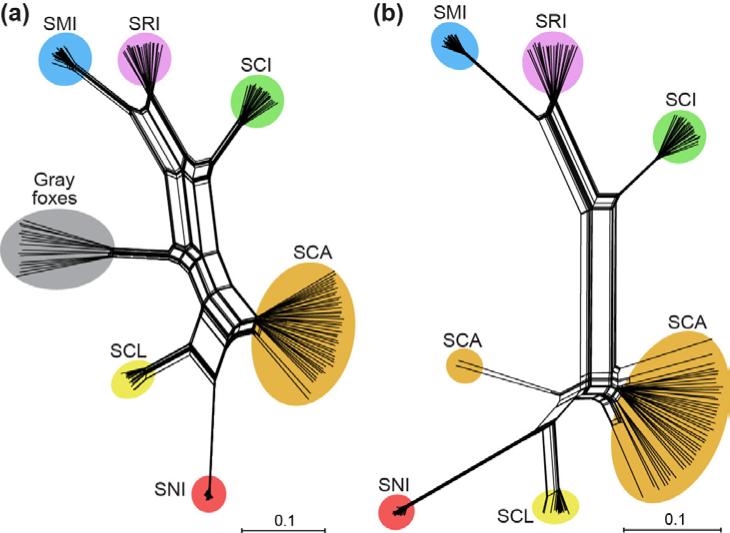
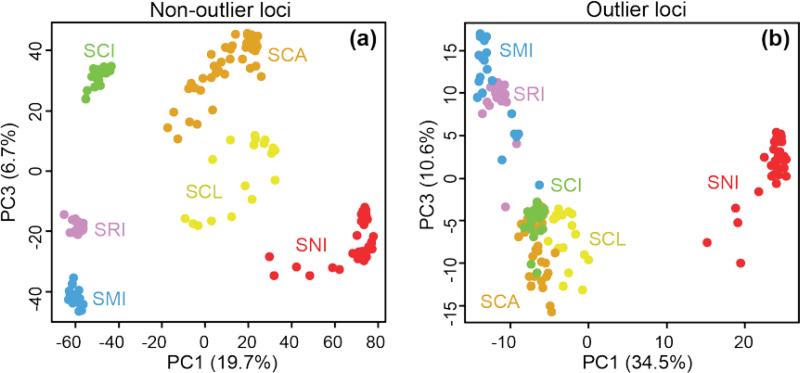
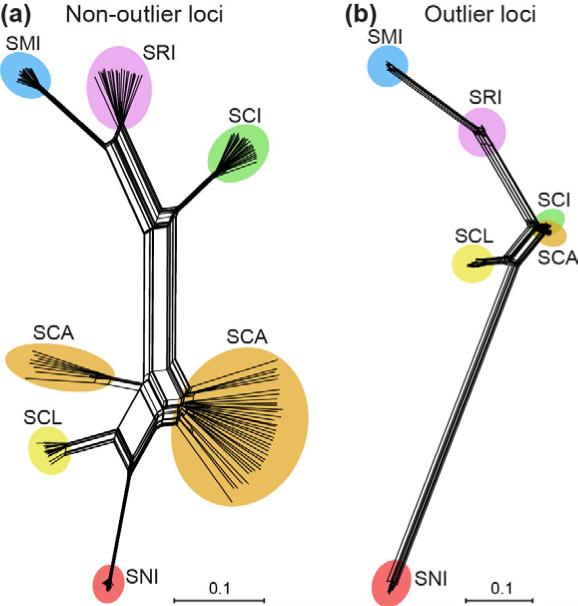
As expected, all island foxes grouped by island in the PCA (Fig. 3) and Neighbor-net tree (Fig. 4). Removal of gray foxes did not change this result (Figs. 3b and 4b). Island fox populations grouped geographically, with two broad clusters representing northern island (SMI, SRI, and SCI) and southern island (SCA, SCL, SNI) populations.
Fig. 3.
Principal component analysis (PCA) to characterize genetic differentiation among island fox populations using SNP loci with (a) or without (b) the gray fox outgroup. As PC2 primarily reflected the amount of missing data, we used PC1 and PC3 to visualize genetic divergence among individuals. Colors and abbreviations correspond to different islands as shown in Fig. 1.
Fig. 4.
Neighbor-net tree to characterize genetic differentiation among island fox populations using SNP loci with (a) or without (b) the gray fox outgroup. Colors and abbreviations correspond to different islands as shown in Fig. 1.
Aim 2: Test the contribution of genetic drift to genetic differentiation
Island fox populations had low within-population genetic variation compared to mainland gray foxes (Table 4). This pattern was evident for all four measures of genetic variation estimated (observed heterozygosity [Ho], expected heterozygosity [He], allelic richness [Ar], nucleotide diversity [π]), but was most pronounced for π, which is based on invariant sites as well as SNPs (whereas the other three measures are only based on SNPs). Effective population sizes (Ne) estimated using NeEstimator were also generally small on islands, ranging from 2.1–89.7 (median = 19.4), relative to an Ne of 109.2 for our mainland gray fox population (Table 4). Finally, Bottleneck found overwhelming evidence for historical bottlenecks in all populations (island foxes and gray foxes), regardless of whether a MAF filter of > 0.10 or > 0.02 was used (Wilcoxon signed rank test, P < 0.000001).
Table 4.
Observed heterozygosity (Ho), expected heterozygosity (He), allelic richness (Ar), nucleotide diversity (π), and effective population size estimates (Ne, with 95% confidence intervals) for mainland gray foxes and each island fox population
| Site | H o | H e | A r | π | Ne (95% confidence intervals) |
|---|---|---|---|---|---|
| Gray foxes | 0.238 | 0.261 | 1.73 | 0.00296 | 109.2 (105.2–113.6) |
| SMI | 0.060 | 0.059 | 1.16 | 0.00027 | 13.7 (13.2–14.1) |
| SRI | 0.147 | 0.148 | 1.39 | 0.00054 | 13.6 (13.5–13.7) |
| SCI | 0.114 | 0.112 | 1.30 | 0.00045 | 25.1 (24.6–25.5) |
| SCA | 0.231 | 0.251 | 1.65 | 0.00082 | 47.0 (46.7–47.4) |
| SCL | 0.065 | 0.064 | 1.17 | 0.00033 | 89.7 (77.1–107.0) |
| SNI | 0.016 | 0.011 | 1.03 | 0.00012 | 2.1 (2.0–2.2) |
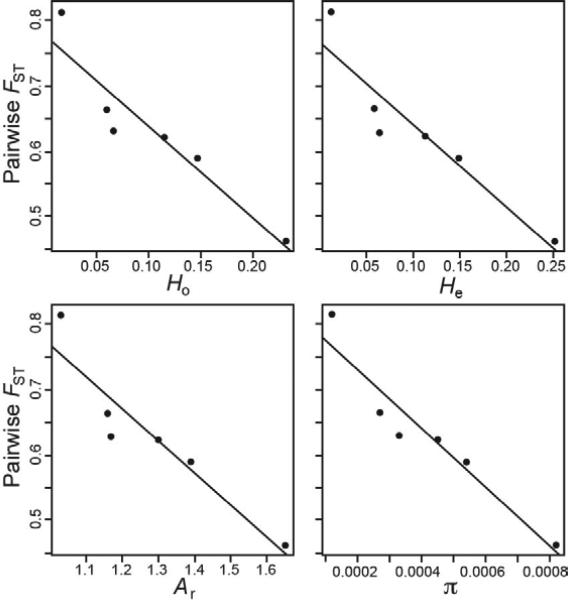
Also as predicted, pairwise FST values between gray foxes vs. island fox populations were significantly predicted by within-island genetic variation (Fig. 5; Ho: F1,4 = 28.35, P = 0.006; He: F1,4 = 28.44, P = 0.006; Ar: F1,4 = 29.56, P = 0.006; π: F1,4 = 42.45, P = 0.003). For example, gray foxes were most genetically similar to island foxes on SCA, which had the highest within-population genetic variation, and were most genetically divergent from island foxes on SNI, which had the lowest genetic variation.
Fig. 5.
Scatterplots of pairwise FST values between gray foxes and each island fox population vs. different measures of within population genetic variation (Ho, observed heterozygosity; He, expected heterozygosity; Ar, allelic richness; π, nucleotide diversity). All four relationships were statistically significant (P < 0.05; indicated by solid black regression lines).
Aim 3: Test the contribution of divergent selection to genetic differentiation
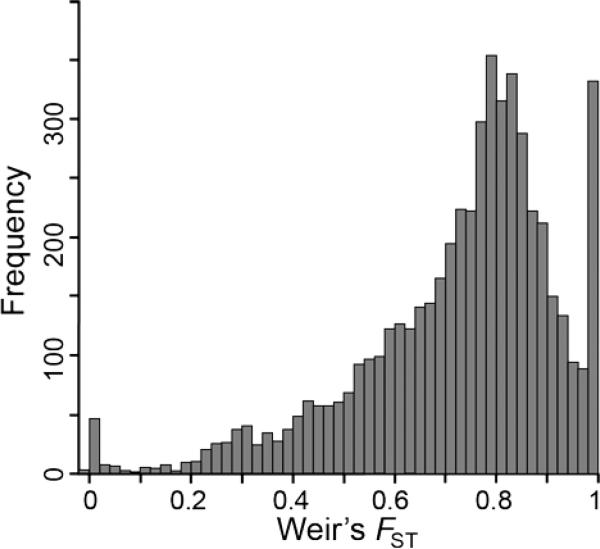
Mean FST among all populations was 0.726, but many loci had FST values of one or close to one, suggesting they may be high FST outliers (Fig. 6). We used three different tests to identify high FST outlier loci with a signature of divergent selection and adaptive divergence among the six island fox populations: a non-model-based method (loci with the highest 5% of FST values); a likelihood model-based method (implemented in FDist2); and a Bayesian model-based method (implemented in BayesFST). As we set the critical P-value to 0.01 for the two model-based approaches, we expected 53 loci (1% of the total 5293 loci) to be identified as significant high FST outliers by chance. However, 351 (6.6%) and 188 (3.6%) loci were actually identified as high FST outliers by FDist2 and BayesFST, respectively, suggesting that many of these loci (or linked loci) are under divergent selection. Moreover, the FDist2 outlier test assuming a hierarchical island model (rather than the simple island model assumed above) still identified 325 (6.1%) loci as high FST outliers, indicating the results were insensitive to the model. Similarly, 297 (5.6%) loci were still identified by FDist2 as high FST outliers even after the false discovery rate (FDR) correction.
Fig. 6.
Histogram of Weir's FST values among all island fox populations at 5293 SNP loci.
In addition, 437 out of 439 loci identified as high FST outliers by at least one of the above three tests blasted to the dog genome (median E-value = 0; range = 0–1.41 × 10−24) and 195 out of these 437 loci (44.6%) blasted to genes (Table S1, Supporting information), indicating many high FST outliers are functional. Gene Ontology (GO) term enrichment analysis of the outliers that blasted to dog genes uncovered three categories of gene function that were statistically overrepresented relative to the full set of dog genes. These included genes involved in regulation of catalytic activity (Bonferroni-corrected P = 0.044), cellular protein modification (P = 0.046), and regulation of molecular function (P = 0.050). However, no loci were significantly associated with the climatic or diet variables tested in BayeScEnv (P > 0.05).
We did not find any evidence of an effect of environmental differences among islands on genome-wide genetic distance. Matrix regression results (Table 5) using geometric distance as a predictor were not significant for either genetic distance measure (FST: F1,13 = 0.236, r2 = 0.018, P = 0.60; Jost's D: F1,13 = 0.021, r2 = 0.002, P = 0.90), nor was the full model that also incorporated all three habitat distance matrices as predictors (FST: F5,9 = 0.406, r2 = 0.140, P = 0.89; Jost's D: F5,9 = 0.285, r2 = 0.102, P = 0.84). In addition, model selection based on AIC did not identify any of the habitat distance matrices as significant predictors.
Table 5.
Results of multiple regression on distance matrices (MRDM)
| Genetic distance | MRM model | Coef | P-value | r2 | F | P-value | |
|---|---|---|---|---|---|---|---|
| F ST | pFST ~ Geo | Int | 0.547 | 0.70 | 0.018 | 0.236 | 0.60 |
| Geo | 0.000 | 0.60 | |||||
| Full model | Int | 0.447 | 0.77 | 0.140 | 0.406 | 0.89 | |
| Geo | 0.001 | 0.69 | |||||
| Diet | 0.322 | 0.59 | |||||
| Temp | −0.112 | 0.66 | |||||
| Precip | 0.000 | 0.90 | |||||
| Jost's D | pD ~ Geo | Int | 0.362 | 0.65 | 0.002 | 0.021 | 0.90 |
| Geo | 0.000 | 0.90 | |||||
| Full model | Int | 0.330 | 0.63 | 0.102 | 0.285 | 0.84 | |
| Geo | 0.000 | 0.74 | |||||
| Diet | 0.210 | 0.56 | |||||
| Temp | −0.053 | 0.70 | |||||
| Precip | 0.000 | 0.49 |
Int = y-intercept; Geo = geographic distance; Diet = Horn's similarity index for diet; Temp = difference in mean annual temperature; Precip = difference in mean annual precipitation.
Aim4: Characterize population divergence at neutral vs. adaptive loci
Patterns of population divergence based on high FST outlier (presumably adaptive) vs. non-outlier (presumably neutral) loci differed in two different ways. The main consistent difference as seen with both PCA (Fig. 7; Figs. S3 and S4, Supporting information) and Neighbor-net trees (Fig. 8; Figs. S5 and S6, Supporting information) is that SCI is more similar to SCA when using outliers vs. non-outliers. Moreover, SNI is very divergent from all other populations based on outlier loci identified using the top 5% method and FDist2, but more similar to SCL based on outlier loci identified using BayesFST.
Fig. 7.
Principle component analysis (PCA) to characterize genetic differentiation among island fox populations using high FST outlier SNPs or non-outliers. (a) PCA based on 5028 presumably neutral SNPs not identified as high FST outliers or (b) 265 presumably adaptive SNPs identified as high FST outliers. Here, outlier loci were identified as the highest 5% of FST values. As PC2 primarily reflected the amount of missing data, we used PC1 and PC3 to visualize genetic divergence among individuals. Colors and abbreviations correspond to different islands as shown in Fig. 1. See Figs. S3 and S4 for PCA using four different methods for identifying outlier loci (highest 5% of FST values, FDist2, FDist2 with the false discovery rate correction, or BayesFST) with (Fig. S3) or without (Fig. S4) gray foxes.
Fig. 8.
Neighbor-net trees to characterize genetic differentiation among island fox populations using high FST outlier SNPs or non-outliers. (a) Neighbor-net trees based on 5028 presumably neutral SNPs not identified as high FST outliers or (b) 265 presumably adaptive SNPs identified as high FST outliers. Here, outlier loci were identified as the highest 5% of FST values. Colors and abbreviations correspond to different islands as shown in Fig. 1. See Figs. S5 and S6 for Neighbor-net trees using four different methods for identifying outlier loci (highest 5% of FST values, FDist2, FDist2 with the false discovery rate correction, or BayesFST) with (Fig. S5) or without (Fig. S6) gray foxes.
Discussion
Our analysis of over 5000 SNPs revealed that genetic drift is the dominant evolutionary force driving genetic differentiation among island fox populations. Three lines of evidence support this conclusion. First, genetic variation, particularly nucleotide diversity (π), was much lower in island fox populations than in their sister species, the gray fox, or other species with published data on π. Second, most island fox populations have low (in some cases, extremely low) effective population sizes (Ne), and all have genetic signatures of historical bottlenecks. Third, the significant negative relationship between pairwise FST (between each island fox population and mainland gray foxes) and measures of within population genetic variation suggests that the strength of genetic drift determines the degree of divergence.
Nonetheless, we also uncovered evidence for adaptive divergence among island fox populations based on high FST outlier tests, indicating that divergent selection may have contributed to divergence despite strong genetic drift (McKay et al. 2001). No loci were associated with variation in climate or diet. However, patterns of population similarity at high FST outlier loci mirrored patterns of morphological similarity (discussed below), suggesting genetically-based, adaptive differences exist among populations, supporting subspecies designation.
Alone, the finding of adaptive divergence among island fox populations suggests that they should continue to be managed separately. However, extremely low genetic variation and Ne found on some populations, particularly SNI, indicate that they are vulnerable to negative inbreeding effects and loss of genetic variation. These populations might therefore benefit from genetic rescue using source individuals from another island. These opposing management options—managing islands separately to maintain adaptive differences vs. supplementing small, declining populations with individuals from another island to boost fitness through genetic rescue—create a management conundrum. We argue that this uncertainty could best be resolved by research to determine the severity of inbreeding depression, if any, and the potential benefits/costs of genetic rescue. Below, we discuss these and other results in more detail.
High genetic differentiation
Our finding of high genetic differentiation among island fox populations using a large number of genome-wide markers is in agreement with the results of previous studies that used traditional molecular markers, including allozymes, minisatellites, mtDNA, and microsatellites (Wayne et al. 1991; Goldstein et al. 1999). We found that in some cases, genetic differentiation was exceptionally high, particularly between SNI and other island populations, with pairwise FST values ranging from 0.646 to 0.963. Importantly, the measure of genetic differentiation we used did not affect our conclusion that island fox populations were highly divergent from each other. Genetic differentiation measured using FST and Jost's D were highly concordant (r = 0.711, P < 0.00001). The main exception to this concordance was that SNI was more similar to SCL using Jost's D than FST. Although some have recently argued that some measures of genetic differentiation are superior to others (Verity & Nichols 2014), our results were insensitive to the measure used.
As expected based on high FST values and previous work, Structure identified each island as a distinct genetic cluster, with the exception of SRI, in which each individual had an average of 73% of its genome assigned to SMI and 27% assigned to SCI, indicating a genetic composition intermediate to these two populations (Fig. 2). The intermediate position of SRI between SMI and SCI was also apparent in the PCA (Fig. 3) and Neighbor-net trees (Fig. 4). Interestingly, a small proportion of some individual's genomes on SCA were assigned to SCI, which is in agreement with the finding of a SCI mtDNA haplotype in the SCA population, suggesting a recent human movement of island foxes from SCI to SCA (Hofman et al. 2015). This result is interesting in light of the potential use of genetic rescue in island foxes (see “Conservation implications” below); introduction of these individuals did not have any detectable, long-term negative consequences for the SCA population. SCA maintained its genetic distinctness despite anthropogenically-mediated gene flow.
The PCA and Neighbor-net analyses showed the same overall pattern of genetic relationships among island fox populations (Figs. 3, 4). Both clustered individuals by their island of origin, as expected based on high FST and Jost's D values among populations, and both grouped the northern islands (SMI, SRI, and SCI) together and the southern islands (SCA, SCL, and SNI) together. These results, once again, are in general agreement with patterns uncovered from previous genetic studies (Wayne et al. 1991; Goldstein et al. 1999), suggesting that when genetic structure is pronounced, as in the case of island foxes, relatively small numbers of traditional markers may be sufficient for inferring population structure.
Contribution of genetic drift to genetic differentiation
We found overwhelming evidence for strong genetic drift in island fox populations. Genetic variation was much lower in island fox populations than in mainland gray foxes based on all four measures of genetic variation examined. In particular, nucleotide diversity (π), which is based on invariant sites in addition to SNPs (and therefore more comparable among populations and species), was 3.6–24.7 times higher in gray foxes than island fox populations. Similarly, π was approximately an order of magnitude higher in two bumble bee species (mean π = 0.0025–0.0041 for Bombus impatiens and 0.0027–0.0042 for B. pensylvanicus; Lozier 2014), sticklebacks (0.00203–0.00268 in Gasterosteus aculeatus; Hohenlohe et al. 2010a), and endangered European eels (0.00529 for Anguilla anguilla; Pujolar et al. 2013), indicating island foxes are among the most genetically depauperate populations of sexually reproducing animals analyzed with SNPs to date.
Effective population sizes (Ne) were small in 5 out of 6 island fox populations analyzed (SMI, SRI, SCI, SCA, and SNI), which had effective population sizes ranging from 2.1 to 47.0. In contrast, Ne was significantly higher (89.7) in the one population, SCL, which has not experienced any recent documented bottlenecks. These Ne estimates, which reflect Ne from the last one to several generations, generally mirror the severity of known bottlenecks (compare known bottlenecks in Table 1 to Ne estimates in Table 4). The Ne estimate of 2.1 from SNI is the lowest such value for any population of a sexually reproducing animal species of which we are aware.
We detected a genetic signal of population bottlenecks in all island fox populations, regardless of the minor allele frequency filter used. We were somewhat surprised by the discovery of a bottleneck in gray foxes on the mainland. However, in retrospect, this could reflect declines in this population, which is negatively affected by urbanization in southern California (Ordeñana et al. 2010).
Finally, the statistically significant negative relationship we found between pairwise FST (between each island fox population and the mainland gray fox population) and all measures of genetic variation strongly suggests that historical genetic drift has caused most variation in genetic differentiation among island fox populations. This same analysis has previously proven effective to test the effects of genetic drift and bottlenecks on population divergence (Jordan & Snell 2008; Whiteley et al. 2010).
Contribution of divergent selection to genetic differentiation
Although the multiple regression on distance matrices (MRDM) analysis failed to find evidence for a genome-wide association between genetic divergence and environmental factors (temperature, precipitation, and diet), likelihood (FDist2) and Bayesian (BayesFST) outlier tests found evidence for high FST outlier loci that may be under divergent selection and involved in adaptive divergence. Many of these loci, as well as outliers identified using a non-model based approach (loci with the top 5% of FST values), blasted to genes in the dog genome, which were enriched for genes involved in regulation of catalytic activity, cellular protein modification, and regulation of molecular function. However, no loci were associated with variation in the climatic or diet indices we used, suggesting that the high FST outlier loci identified must be involved in adaptation to other environmental factors.
Several recent studies have warned about the limitations of FST outlier tests for identifying loci under divergent selection, indicating that the results of these tests should be interpreted carefully. Other explanations for high FST outliers besides divergent selection include neutral factors, such as demographics (Lotterhos & Whitlock 2014), recombination rate heterogeneity (Roesti et al. 2012), or background selection within populations (Cruickshank & Hahn 2014). In addition, the assumptions of model-based outlier tests are rarely completely upheld. For example, like many outlier tests, FDist2 and BayesFST assume an island model in which migration is equally liked among all populations, an assumption that may not hold for island fox populations due to erratic and rare dispersal events among populations (for example, the human-mediated dispersal from SCI to SCA described above). Moreover, as these populations have become more and more divergent over time, even neutral loci may drift to fixation by chance, resulting in FST values equal to one. Nonetheless, the sensitivity of FDist2, BayesFST, and other model-based outlier tests to their assumptions is poorly understood, so the high FST outliers identified may still be valid. This conclusion is supported by concordance between patterns of population similarity based on high FST outlier loci and patterns based on morphometric traits, discussed next.
Population divergence at presumably neutral vs. adaptive loci
Overall, patterns of population divergence and similarity based on high FST outlier (presumably adaptive) vs. non-outlier (presumably neutral) loci were similar, as seen in both PCA plots and Neighbor-net trees (Figs. 7, 8), but there were some notable exceptions. The principal consistent difference was the position of SCI. In particular, SCI was more similar to SCA based on outliers than non-outliers, suggesting adaptive similarity between these populations. SNI was also very divergent from all other populations based on outliers identified using the top 5% method and FDist2 (Figs. 7, 8). Given extremely low genetic variation and small Ne in SNI, this result could be an artifact of strong genetic drift causing fixation of alleles at many loci in SNI, such that these loci are identified as high FST outliers and SNI appears highly divergent from all other populations based on the outlier dataset. In contrast, SNI was more similar to SCL based on outlier loci identified using BayesFST (Figs. S3–S6, Supporting information), suggesting SNI is most adaptively similar to SCL.
Interestingly, patterns of population similarity at high FST outlier loci were similar to patterns of similarity based on a suit of 29 morphometric (cranial and dentition) traits (Wayne et al. 1991). In particular, Wayne et al. (1991) found that, using these morphometric traits, SCI was most similar to SCA (the same pattern found here with high FST outliers) and that SNI was most similar to SCL (the same pattern found with high FST outliers identified using BayesFST). The concordance between population similarity based on high FST outliers and morphology provides an independent line of evidence that high FST outliers or linked loci are under divergent selection and involved in adaptation. These high FST outlier loci or linked loci could underlie the actual morphological differences, or they could underlie other unmeasured, but correlated, traits also involved in adaptation to environmental heterogeneity among islands.
Conservation implications
Our results have several implications for island fox conservation and management. First, the high genetic differentiation we document, particularly at potentially adaptive, functional loci, coupled with previous studies documenting morphological differences among island fox populations (Grinnell et al. 1937; Wayne et al. 1991; Collins 1993), supports the current designation of each island fox population as a distinct subspecies. However, as we argue below, some circumstances might make it prudent for managers to consider supplementing a severely threatened subspecies with individuals from another subspecies, as has been done for Florida panthers (Puma concolor coryi; Hedrick 1995; Johnson et al. 2010).
Second, despite population rebounds on the northern islands and SCA following population crashes in the late 1990s, all island fox populations except SCL have very low genetic variation and small Ne, suggesting that they remain vulnerable to the increase in frequency and expression of deleterious recessive alleles and to the loss of additive genetic variation. Managers should therefore strive to maintain large populations (e.g., at or close to carrying capacity) and avoid future population crashes, which could exacerbate these negative genetic effects. In addition, low genetic variation and small Ne estimates documented here mean some populations may already be suffering from inbreeding depression (Ralls et al. 1979; Ralls & Ballou 1983; Lacy 1997; Newman & Pilson 1997; Saccheri et al. 1998). In particular, SNI has < 300 adults (Table 1; Coonan 2015), a rapidly declining population (λ = 0.77), incredibly low genetic variation, and an extremely low Ne of 2.1; this population is highly vulnerable to extinction due to both demographic and genetic factors. We strongly recommend adjusting island fox monitoring programs to include tests for potential negative genetic effects in all subspecies. For example, genomic screening (e.g., Schwartz et al. 2007) would enable testing for variation in fitness related to inbreeding coefficients, average individual heterozygosity, and genotypes at specific loci.
The adaptive differentiation among island fox populations that we document here and evidence that outbreeding depression is most likely in crosses between adaptively divergent populations (Edmands 2007; Frankham et al. 2011) might suggest that genetic rescue would have the unintended consequence of decreasing fitness, rather than the desired effect of reducing extinction risk. However, population genetic theory demonstrates that when Ne is small, the threat of swamping out locally adapted alleles is low because high genetic drift precludes the maintenance of many of these alleles in the first place (Wright 1931, 1951). Selection would have to be very strong for an adaptive allele to be maintained by selection with Ne as small as observed in those island fox populations that would be the most likely candidates for genetic rescue. For example, on SNI with an Ne of 2.1, population genetic theory predicts that the selection coefficient would have to be s > 1/(2Ne) = 0.24 to maintain an adaptive allele (Conner & Hartl 2004), which is high relative to most empirical estimates of selection coefficients, at least for phenotypes (Kingsolver et al. 2001; Conner 2001). Moreover, if selection is this strong for a native, adaptive allele, then it is unlikely that a new, foreign, maladaptive allele will successfully “invade” and persist in the population.
Thus, when Ne is extremely small, as observed in some island fox populations, concerns about inbreeding depression may be more important than concerns about outbreeding depression. If a population has an unacceptably high probability of going extinct, inbreeding depression significantly contributes to this high extinction risk, and genetic rescue is predicted to reduce inbreeding depression, then genetic rescue should be considered as a viable management option (Tallmon et al. 2004; Hedrick & Fredrickson 2010; Frankham 2015; Whiteley et al. 2015). Research is therefore critically needed to determine the viability of island fox populations, the magnitude of inbreeding depression, and potential fitness effects of inter-population crosses and genetic rescue to determine if and when genetic rescue would be an effective management strategy. This research should be pursued as soon as possible so that these important management decisions can be made before population recovery is unlikely. Lessons from other systems—such as Isle Royale wolves (Canis lupus), Vancouver Island marmots (Marmota vancouverensis), and greater gliders (Petauroides volans)—highlight that waiting too long to make management decisions (and waiting too long to test the efficacy of management options) can cause imperiled populations to decline beyond the point of recovery (Lindenmayer et al. 2013; Marris 2015; Mlot 2015).
Supplementary Material
Acknowledgements
We thank Lisa Lyren, Erin Boydston, Francesca Ferrara, and many field assistants who helped collect blood and tissue samples, Tamara Max for helping prepare RAD libraries, and Brian Cypher for providing island fox diet data. We also thank Lisette Waits, Andrew R. Whiteley, and three anonymous reviewers for providing helpful suggestions on the manuscript. This research was funded by the U.S. Department of Defense Legacy Resource Management Program, The Nature Conservancy, and the U.S. National Institutes of Health (grant number P30GM103324). This project was approved by the Institutional Animal Care and Use Committee at Colorado State University. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Author contributions
W.C.F., R.E.L., P.A.H., C.A.H. S.A.M., T.S.S., C.K.G., J.E.M., and T.C.R: Research concept and design; T.J.C., K.R.C., A.D., D.K.G., J.L.K., C.L.B., N.G., and W.F.A: Sample collection; W.C.F., P.A.H., M.D.D. N.R.P., and S.W.F: Data analysis and interpretation; W.C.F: Writing the article; All authors: Critical revision and final approval of the article.
Data accessibility
Raw sequence data, RAD contig sequences, and genotype data: Dryad doi:10.5061/dryad.2kn1v
Supporting information
Additional supporting information may be found in the online version of this article.
References
- Aguilar A, Roemer G, Debenham S, et al. High MHC diversity maintained by balancing selection in an otherwise genetically monomorphic mammal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3490–3494. doi: 10.1073/pnas.0306582101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Etter PD, Atwood TS, et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE. 2008;3:e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkenhol N, Waits LP, Dezzani RJ. Statistical approaches in landscape genetics: an evaluation of methods for linking landscape and genetic data. Ecography. 2009;32:818–830. [Google Scholar]
- Beaumont MA, Balding DJ. Identifying adaptive genetic divergence among populations from genome scans. Molecular Ecology. 2004;13:969–980. doi: 10.1111/j.1365-294x.2004.02125.x. [DOI] [PubMed] [Google Scholar]
- Beaumont MA, Nichols RA. Evaluating loci for use in the genetic analysis of population structure. Proceedings of the Royal Society B-Biological Sciences. 1996;263:1619–1626. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- Bierne N, Roze D, Welch JJ. Pervasive selection or is it...? why are FST outliers sometimes so frequent? Molecular Ecology. 2013;22:2061–2064. doi: 10.1111/mec.12241. [DOI] [PubMed] [Google Scholar]
- Bryant D, Moulton V. Neighbor-Net: An agglomerative method for the construction of phylogenetic networks. Molecular Biology & Evolution. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Bürger R, Lynch M. Evolution and extinction in a changing environment - a quantitative-genetic analysis. Evolution. 1995;49:151–163. doi: 10.1111/j.1558-5646.1995.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Burns KC. Patterns in the assembly of an island plant community. Journal of Biogeography. 2007;34:760–768. [Google Scholar]
- Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. STACKS: Building and genotyping loci de novo from short-read sequences. Genes, Genomes, & Genetics (G3) 2011;1:171–182. doi: 10.1534/g3.111.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. STACKS: an analysis tool set for population genomics. Molecular Ecology. 2013;22:3124–3140. doi: 10.1111/mec.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayan DR, Maurer EP, Dettinger MD, Tyree M, Hayhoe K. Climate change scenarios for the California region. Climatic Change. 2008;87:S21–S42. [Google Scholar]
- Collins PW. Third California Islands Symposium: Recent Advances in Research on the California Islands. Santa Barbara Museum of Natural History; Santa Barbara, California: 1993. Taxonomic and biogeographic relationships of the island fox (Urocyon littoralis) and gray fox (Urocyon cinereoargenteus) from western North America. pp. 351–390. [Google Scholar]
- Conner JK. How strong is natural selection? Trends in Ecology & Evolution. 2001;16:215–217. doi: 10.1016/s0169-5347(01)02138-3. [DOI] [PubMed] [Google Scholar]
- Conner JK, Hartl DL. A Primer of Ecological Genetics. Sinauer Associates, Inc.; Sunderland, Massachusetts: 2004. [Google Scholar]
- Cook BI, Ault TR, Smerdon JE. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Science Advances. 2015;1:e1400082. doi: 10.1126/sciadv.1400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonan TJ. Seventeenth annual meting, Island Fox Working Group, June 16-17, 2015, summary report. 2015 Available online at http://www.mednscience.org/reports.
- Coonan TJ, Schwemm CA, Garcelon DK. Decline and Recovery of the Island Fox: A Case Study for Population Recovery. Cambridge University Press; Cambridge: 2010. [Google Scholar]
- Cornuet JM, Luikart G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics. 1996;144:2001–2014. doi: 10.1093/genetics/144.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall KA, Bininda-Emonds ORP, Mace GM, Wayne RK. Considering evolutionary processes in conservation biology. Trends in Ecology & Evolution. 2000;15:290–295. doi: 10.1016/s0169-5347(00)01876-0. [DOI] [PubMed] [Google Scholar]
- Cruickshank TE, Hahn MW. Reanalysis suggests that genomic islands of speciation are due to reduced diversity, not reduced gene flow. Molecular Ecology. 2014;23:3133–3157. doi: 10.1111/mec.12796. [DOI] [PubMed] [Google Scholar]
- Cypher BL, Madrid AY, Van Horn Job CL, et al. Multi-population comparison of resource exploitation by island foxes: implications for conservation. Global Ecology & Conservation. 2014;2:255–266. [Google Scholar]
- Do C, Waples RS, Peel D, et al. NEESTIMATOR v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Molecular Ecology Resources. 2014;14:209–214. doi: 10.1111/1755-0998.12157. [DOI] [PubMed] [Google Scholar]
- Earl DA, Vonholdt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2012;4:359–361. [Google Scholar]
- Edmands S. Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Molecular Ecology. 2007;16:463–475. doi: 10.1111/j.1365-294X.2006.03148.x. [DOI] [PubMed] [Google Scholar]
- Eldridge MDB, King JM, Loupis AK, et al. Unprecedented low levels of genetic variation and inbreeding depression in an island population of the black-footed rock-wallaby. Conservation Biology. 1999;13:531–541. [Google Scholar]
- Emerson KJ, Merz CR, Catchen JM, et al. Resolving postglacial phylogeography using high-throughput sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16196–16200. doi: 10.1073/pnas.1006538107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter PD, Johnson EA. RAD paired-end sequencing for local de novo assembly and SNP discovery in non-model organisms. In: Pompanon F, Bonin A, editors. Data Production and Analysis in Population Genomics: Methods and Protocols. Humana Press; New York: 2012. pp. 135–151. [DOI] [PubMed] [Google Scholar]
- Etter PD, Bassham S, Hohenlohe PA, Johnson EA, Cresko WA. SNP discovery and genotyping for evolutionary genetics using RAD sequencing. In: Orgogozo V, Rockamn MV, editors. Molecular Methods for Evolutionary Genetics. Springer Science + Business Media, LLC.; 2011. pp. 157–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Laval G, Schneider S. ARLEQUIN (version 3.0): An integrated software package for population genetics data analysis. Evolutionary Bioinformatics. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Fischer DT, Still CJ. Evaluating patterns of fog water deposition and isotopic composition on the California Channel Islands. Water Resources Research. 2007;43 [Google Scholar]
- Fisher RA. The Genetical Theory of Natural Selection. Clarendon Press; Oxford, UK: 1930. [Google Scholar]
- Frankham R. Inbreeding and extinction: Island populations. Conservation Biology. 1998;12:665–675. [Google Scholar]
- Frankham R. Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Molecular Ecology. 2015;24:2610–2618. doi: 10.1111/mec.13139. [DOI] [PubMed] [Google Scholar]
- Frankham R, Ballou JD, Eldridge MDB, et al. Predicting the probability of outbreeding depression. Conservation Biology. 2011;25:465–475. doi: 10.1111/j.1523-1739.2011.01662.x. [DOI] [PubMed] [Google Scholar]
- Funk WC, McKay JK, Hohenlohe PA, Allendorf FW. Harnessing genomics for delineating conservation units. Trends in Ecology & Evolution. 2012;27:489–496. doi: 10.1016/j.tree.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DA, Lehman N, Obrien SJ, Wayne RK. Genetic fingerprinting reflects population differentiation in the California Channel Island fox. Nature. 1990;344:764–767. doi: 10.1038/344764a0. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Roemer GW, Smith DA, et al. The use of microsatellite variation to infer population structure and demographic history in a natural model system. Genetics. 1999;151:797–801. doi: 10.1093/genetics/151.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslee SC, Urban DL. The ecodist package for dissimilarity-based analysis of ecological data. Journal of Statistical Software. 2007;22:1–19. [Google Scholar]
- Grant PR. Evolution on Islands. Oxford University Press; Oxford: 1998. [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Grinnell J, Dixon JS, Linsdale JM. Fur-Bearing Mammals of California: Their Natural History, Systematic Status, and Relations to Man. University of California Press; Berkeley, California: 1937. [Google Scholar]
- Heber S, Varsani A, Kuhn S, et al. The genetic rescue of two bottlenecked South Island robin populations using translocations of inbred donors. Proceedings of the Royal Society B- Biological Sciences. 2013;280:2012–2228. doi: 10.1098/rspb.2012.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick PW. Gene flow and genetic restoration: the Florida panther as a case study. Conservation Biology. 1995;9:996–1007. doi: 10.1046/j.1523-1739.1995.9050988.x-i1. [DOI] [PubMed] [Google Scholar]
- Hedrick PW, Fredrickson R. Genetic rescue guidelines with examples from Mexican wolves and Florida panthers. Conservation Genetics. 2010;11:615–626. [Google Scholar]
- Hill WG. Estimation of effective population size from data on genetic linkage disequilibrium. Genetical Research. 1981;38:209–216. [Google Scholar]
- Hoeppner MP, Lundquist A, Pirun M, et al. An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PLoS ONE. 2014;9:e91172. doi: 10.1371/journal.pone.0091172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman CA, Rick TC, Hawkins MTR, et al. Mitochondrial genomes suggest rapid evolution of dwarf California Channel Islands foxes (Urocyon littoralis). PLoS ONE. 2015;10:e0118240. doi: 10.1371/journal.pone.0118240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, et al. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genetics. 2010a;6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Phillips PC, Cresko WA. Using population genomics to detect selection in natural populations: key concepts and methodological considerations. International Journal of Plant Sciences. 2010b;171:1059–1071. doi: 10.1086/656306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Day MD, Amish SJ, et al. Genomic patterns of introgression in rainbow and westslope cutthroat trout illuminated by overlapping paired-end RAD sequencing. Molecular Ecology. 2013;22:3002–3013. doi: 10.1111/mec.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Molecular Biology & Evolution. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Onorato DP, Roelke ME, et al. Genetic restoration of the Florida panther. Science. 2010;329:1641–1645. doi: 10.1126/science.1192891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Snell HL. Historical fragmentation of islands and genetic drift in populations of Galápagos lava lizards (Microlophus albemarlensis complex). Molecular Ecology. 2008;17:1224–1237. doi: 10.1111/j.1365-294X.2007.03658.x. [DOI] [PubMed] [Google Scholar]
- Jost L. GST and its relatives do not measure differentiation. Molecular Ecology. 2008;17:4015–4026. doi: 10.1111/j.1365-294x.2008.03887.x. [DOI] [PubMed] [Google Scholar]
- Kalinowski ST. HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Molecular Ecology Notes. 2005;5:187–189. [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, et al. The strength of phenotypic selection in natural populations. American Naturalist. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Kolbe JJ, Leal M, Schoener TW, Spiller DA, Losos JB. Founder effects persist despite adaptive differentiation: a field experiment with lizards. Science. 2012;335:1086–1089. doi: 10.1126/science.1209566. [DOI] [PubMed] [Google Scholar]
- Lacy RC. Importance of genetic variation to the viability of mammalian populations. Journal of Mammalogy. 1997;78:320–335. [Google Scholar]
- LaDochy S, Witiw M. The continued reduction in dense fog in the southern California region: possible causes. Pure & Applied Geophysics. 2012;169:1157–1163. [Google Scholar]
- Legendre P, Lapointe FJ, Casgrain P. Modeling brain evolution from behavior: a permutational regression approach. Evolution. 1994;48:1487–1499. doi: 10.1111/j.1558-5646.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Lindenmayer DB, Piggott MP, Wintle BA. Counting the books while the library burns: why conservation monitoring programs need a plan for action. Frontiers in Ecology and the Environment. 2013;11:549–555. [Google Scholar]
- Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. [DOI] [PubMed] [Google Scholar]
- Lotterhos KE, Whitlock MC. Evaluation of demographic history and neutral parameterization on the performance of FST outlier tests. Molecular Ecology. 2014;23:2178–2192. doi: 10.1111/mec.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier JD. Revisiting comparisons of genetic diversity in stable and declining species: assessing genome-wide polymorphism in North American bumble bees using RAD sequencing. Molecular Ecology. 2014;23:788–801. doi: 10.1111/mec.12636. [DOI] [PubMed] [Google Scholar]
- MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton, N.J.; Princeton University Press: 1967. [Google Scholar]
- Marris E. Wolf decline threatens iconic island study. Nature. 2015;520:415. doi: 10.1038/nature.2015.17263. [DOI] [PubMed] [Google Scholar]
- Martínez-Solano I, Lawson R. Escape to Alcatraz: evolutionary history of slender salamanders (Batrachoseps) on the islands of San Francisco Bay. BMC Evolutionary Biology. 2009;9:38. doi: 10.1186/1471-2148-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JK, Bishop JG, Lin JZ, et al. Local adaptation across a climatic gradient despite small effective population size in the rare sapphire rockcress. Proceedings of the Royal Society B-Biological Sciences. 2001;268:1715–1721. doi: 10.1098/rspb.2001.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi HY, Muruganujan A, Thomas PD. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Research. 2013;41:D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlot C. Inbred wolf population on Isle Royale collapses. Science. 2015;348:383. doi: 10.1126/science.348.6233.383. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D, Pilson D. Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution. 1997;51:354–362. doi: 10.1111/j.1558-5646.1997.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Nosil P, Egan SP, Funk DJ. Heterogeneous genomic differentiation between walking-stick ecotypes: “Isolation by adaptation” and multiple roles for divergent selection. Evolution. 2008;62:316–336. doi: 10.1111/j.1558-5646.2007.00299.x. [DOI] [PubMed] [Google Scholar]
- Ordeñana MA, Crooks KR, Boydston EE, et al. Effects of urbanization on carnivore species distribution and richness. Journal of Mammalogy. 2010;91:1322–1331. [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piry S, Luikart G, Cornuet JM. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. Journal of Heredity. 1999;90:502–503. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujolar JM, Jacobsen MW, Frydenberg J, et al. A resource of genome-wide single-nucleotide polymorphisms generated by RAD tag sequencing in the critically endangered European eel. Molecular Ecology Resources. 2013;13:706–714. doi: 10.1111/1755-0998.12117. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: 2010. Available at http://www.Rproject.org/ [Google Scholar]
- Ralls K, Ballou J. Extinction: lessons from zoos. In: Schonewald-Cox C, Chambers S, MacBryde B, Thomas L, editors. Genetics and Conservation. Benjamin/Cummings; Menlo Park, California: 1983. pp. 164–184. [Google Scholar]
- Ralls K, Brugger K, Ballou J. Inbreeding and juvenile mortality in small populations of ungulates. Science. 1979;206:1101–1103. doi: 10.1126/science.493997. [DOI] [PubMed] [Google Scholar]
- Rick TC, Erlandson JM, Vellanoweth RL, et al. Origins and antiquity of the island fox (Urocyon littoralis) on California's Channel Islands. Quaternary Research. 2009;71:93–98. [Google Scholar]
- Robertson JM, Langin KM, Sillett TS, Morrison SA, Ghalambor CK, Funk WC. Identifying Evolutionarily Significant Units and prioritizing populations for management on islands. Monographs of the Western North American Naturalist. 2014;7:397–411. [Google Scholar]
- Roemer GW, Coonan TJ, Garcelon DK, Bascompte J, Laughrin L. Feral pigs facilitate hyperpredation by golden eagles and indirectly cause the decline of the island fox. Animal Conservation. 2001;4:307–318. [Google Scholar]
- Roesti M, Hendry AP, Salzburger W, Berner D. Genome divergence during evolutionary diversification as revealed in replicate lake-stream stickleback population pairs. Molecular Ecology. 2012;21:2852–2862. doi: 10.1111/j.1365-294X.2012.05509.x. [DOI] [PubMed] [Google Scholar]
- Ryder OA. Species Conservation and systematics - the dilemma of subspecies. Trends in Ecology & Evolution. 1986;1:9–10. doi: 10.1016/0169-5347(86)90035-2. [DOI] [PubMed] [Google Scholar]
- Saccheri I, Kuussaari M, Kankare M, et al. Inbreeding depression and extinction in a butterfly metapopulation. Nature. 1998;392:491–494. [Google Scholar]
- Schwartz MK, Luikart G, Waples RS. Genetic monitoring as a promising tool for conservation and management. Trends in Ecology & Evolution. 2007;22:25–33. doi: 10.1016/j.tree.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Spalding MD, Fox HE, Halpern BS, et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience. 2007;57:573–583. [Google Scholar]
- Stuart YE, Losos JB, Algar AC. The island-mainland species turnover relationship. Proceedings of the Royal Society B-Biological Sciences. 2012;279:4071–4077. doi: 10.1098/rspb.2012.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallmon DA, Luikart G, Waples RS. The alluring simplicity and complex reality of genetic rescue. Trends in Ecology & Evolution. 2004;19:489–496. doi: 10.1016/j.tree.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Timm SF, Munson L, Summers BA, et al. A suspected canine distemper epidemic as the cause of a catastrophic decline in Santa Catalina Island foxes (Urocyon littoralis catalinae). Journal of Wildlife Diseases. 2009;45:333–343. doi: 10.7589/0090-3558-45.2.333. [DOI] [PubMed] [Google Scholar]
- U.S. Fish and Wildlife Service Listing of the San Miguel island fox, Santa Rosa island fox, Santa Cruz island fox, and Santa Catalina island fox as endangered; final rule. Federal Register. 2004;69:10335–10353. [Google Scholar]
- Verity R, Nichols RA. What is genetic differentiation, and how should we measure it: GST, D, neither or both? Molecular Ecology. 2014;23:4216–4225. doi: 10.1111/mec.12856. [DOI] [PubMed] [Google Scholar]
- Villemereuil P, Gaggiotti OE. A new FST-based method to uncover local adaptation using environmental variables. Methods in Ecology & Evolution. 2015 [Google Scholar]
- Waples RS. Genetic estimates of contemporary effective population size: to what time periods do the estimates apply? Molecular Ecology. 2005;14:3335–3352. doi: 10.1111/j.1365-294X.2005.02673.x. [DOI] [PubMed] [Google Scholar]
- Waples RS. Spatial-temporal stratifications in natural populations and how they affect understanding and estimation of effective population size. Molecular Ecology Resources. 2010;10:785–796. doi: 10.1111/j.1755-0998.2010.02876.x. [DOI] [PubMed] [Google Scholar]
- Wayne RK, George SB, Gilbert D, et al. A morphological and genetic study of the island fox, Urocyon littoralis. Evolution. 1991;45:1849–1868. doi: 10.1111/j.1558-5646.1991.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Weigelt P, Jetz W, Kreft H. Bioclimatic and physical characterization of the world's islands. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15307–15312. doi: 10.1073/pnas.1306309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley AR, Hastings K, Wenburg JK, et al. Genetic variation and effective population size in isolated populations of coastal cutthroat trout. Conservation Genetics. 2010;11:1929–1943. [Google Scholar]
- Whiteley AR, Fitzpatrick SW, Funk WC, Tallmon DA. Genetic rescue to the rescue. Trends in Ecology & Evolution. 2015;30:42–49. doi: 10.1016/j.tree.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Willing E-M, Bentzen P, van Oosterhout C, et al. Genome-wide single nucleotide polymorphisms reveal population history and adaptive divergence in wild guppies. Molecular Ecology. 2010;19:968–984. doi: 10.1111/j.1365-294X.2010.04528.x. [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution in Mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.