Abstract
Study Objectives:
To determine whether sleep-disordered breathing (SDB) is associated with cardiac arrhythmia in a clinic-based population with multiple cardiovascular comorbidities and severe SDB.
Methods:
This was a cross-sectional analysis of 697 veterans who underwent polysomnography for suspected SDB. SDB was categorized according to the apnea-hypopnea index (AHI): none (AHI < 5), mild (5 ≥ AHI < 15), and moderate-severe (AHI ≥ 15). Nocturnal cardiac arrhythmias consisted of: (1) complex ventricular ectopy, (CVE: non-sustained ventricular tachycardia, bigeminy, trigeminy, or quadrigeminy), (2) combined supraventricular tachycardia, (CST: atrial fibrillation or supraventricular tachycardia), (3) intraventricular conduction delay (ICD), (4) tachyarrhythmias (ventricular and supraventricular), and (5) any cardiac arrhythmia. Unadjusted, adjusted logistic regression, and Cochran-Armitage testing examined the association between SDB and cardiac arrhythmias. Linear regression models explored the association between hypoxia, arousals, and cardiac arrhythmias.
Results:
Compared to those without SDB, patients with moderate-severe SDB had almost three-fold unadjusted odds of any cardiac arrhythmia (2.94; CI 95%, 2.01–4.30; p < 0.0001), two-fold odds of tachyarrhythmias (2.16; CI 95%,1.47–3.18; p = 0.0011), two-fold odds of CVE (2.01; 1.36–2.96; p = 0.003), and two-fold odds of ICD (2.50; 1.58–3.95; p = 0.001). A linear trend was identified between SDB severity and all cardiac arrhythmia subtypes (p value linear trend < 0.0001). After adjusting for age, BMI, gender, and cardiovascular diseases, moderate-severe SDB patients had twice the odds of having nocturnal cardiac arrhythmias (2.24; 1.48–3.39; p = 0.004). Frequency of obstructive respiratory events and hypoxia were strong predictors of arrhythmia risk.
Conclusions:
SDB is independently associated with nocturnal cardiac arrhythmias. Increasing severity of SDB was associated with an increasing risk for any cardiac arrhythmia.
Citation:
Selim BJ, Koo BB, Qin L, Jeon S, Won C, Redeker NS, Lampert RJ, Concato JP, Bravata DM, Ferguson J, Strohl K, Bennett A, Zinchuk A, Yaggi HK. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: the DREAM study. J Clin Sleep Med 2016;12(6):829–837.
Keywords: sleep-disordered breathing, obstructive sleep apnea, sudden cardiac death, cardiac arrhythmias, atrial fibrillation, autonomic nervous system, hypoxemia
INTRODUCTION
Sleep-disordered breathing (SDB) affects 17% of adults in the general U.S. population.1 The prevalence of SDB among U.S. veterans is estimated to be as high as four times that of the general population due to major risk factors for SDB that are common among veterans, including male gender, increasing age, smoking, obesity, and cardiovascular comorbidity.2 SDB has been linked to a number of cardiovascular outcomes, including hypertension, myocardial infarction, congestive heart failure, cardiovascular mortality, and sudden death.3–6 Importantly, a circadian pattern has been observed in the occurrence of sudden death in SDB, such that the risk for sudden death doubles from midnight to 6 AM among patients with SDB compared to those without.5 A putative association between SDB and nocturnal cardiac arrhythmias may, in part, explain this increased risk for nocturnal sudden death.7,8
BRIEF SUMMARY
Current Knowledge/Study Rationale: The association between sleep-disordered breathing (SDB) and cardiac arrhythmias has been examined in community-based with self-reported morbidities but not in clinic-based populations that tend to have more severe sleep-disordered breathing and higher rates of cardiovascular comorbidity.
Study Impact: The association between SDB and nocturnal cardiac arrhythmia may, in part, explain the observed increased risk in nocturnal sudden death among patients with sleep apnea. Understanding the nature of the association between sleep apnea and cardiac arrhythmias also has the potential to change the practice of polysomnographic interpretation, leading to a more systematic approach to the detection and quantifications of cardiac events.
SDB is associated with a number of physiologic perturbations that may plausibly increase the risk of nocturnal cardiac arrhythmias including: repetitive episodes hypoxia and nocturnal sympathetic hyperactivity which in turn have been associated with ventricular ectopy, non-sustained ventricular tachycardia.7,9 Additionally, obstructive sleep apnea (OSA), a common form of SDB consisting of repetitive upper airway obstruction, leads to significant intrathoracic pressure swings contributing to increased atrial and ventricular cardiac wall stress. This increase in ventricular afterload will predispose to transient acute ischemia in the short term and to cardiac remodeling in the long term, increasing the risk for cardiac arrhythmias.10–12
Surprisingly, few studies have been conducted to clarify the association between SDB and nocturnal cardiac arrhythmias. Data are limited regarding the magnitude of risk from clinic-based populations who tend to have multiple cardiovascular comorbidities and more severe SDB than population-based studies. We analyzed data from a clinic-based prospective observational cohort of veterans to further understand the relationship between SDB and nocturnal cardiac arrhythmia. Our objective was to determine whether SDB is independently associated nocturnal cardiac arrhythmias and whether increasing severity of SDB is associated with an increasing risk of these events. As a secondary analysis, we explored which selected polysomnographic (PSG) measures of SDB best predict risk of arrhythmias.
METHODS
Subjects/Study Design
The study sample was derived from a parent prospective observational cohort study named the Determining Risk of vascular Events by Apnea Monitoring (DREAM) study. The rationale and methods of this parent study has been previously published.13 In this study, 1,840 patients underwent comprehensive sleep testing for SDB at 3 VA medical centers (VA Connecticut Healthcare System, West Haven, CT; Richard L. Rhoudebush VAMC, Indianapolis, IN; Louis Stokes VAMC, Cleveland, OH) and were followed for cardiovascular outcomes. The current study is a cross-sectional analysis of the first 982 consecutive veteran patients referred for PSG, a subgroup of DREAM patients who also underwent additional detailed cardiac arrhythmia evaluation. Subjects were excluded if diagnostic PSG data were < 2 h duration, referral for PSG was for reasons other than suspected sleep-disordered breathing (e.g., narcolepsy or movement disorder), or referral was for therapeutic PSG study only (e.g., continuous positive airway pressure titration). The study was approved by the Human Investigation Committee at all 3 VA medical centers.
Polysomnographic Data Collection
Raw PSG data were acquired at the 3 clinical centers, processed, and rescored by trained polysomnologists at a central reading center at the Clinical Epidemiology Research Center, West Haven, CT. The PSG montage included recording of electroencephalogram (F3/4-M1, C3/4-M1, O1/2-M1), bilateral electro-oculogram, submental, and anterior tibialis electromyo-gram, electrocardiogram (a single modified electrocardiograph lead II, sampled at 500 Hz), nasal pressure transduction, oronasal thermistry, thoracic and abdominal respiratory inductance plethysmography, and a finger probe for oxygen saturation (sampling frequency of 25 Hz). Apneas were defined by a decrease of peak thermal sensor ≥ 90% of baseline for ≥ 10 s, and were classified as obstructive or central, if respiratory effort was present or absent, respectively. Mixed apneas were scored when apneas were associated with absent inspiratory effort in the initial portion of the event, followed by resumption of inspiratory effort in the second portion of the event. Hypopneas were defined as a nasal pressure drop > 30% from baseline, lasting ≥ 10 s, and associated with oxygen desaturation ≥ 4%.14 The apnea-hypopnea index (AHI) was enumerated as the sum of all apneas and hypopneas divided by total sleep time in hours. Participants were categorized according to severity of SDB by the apnea-hypopnea index (AHI): (1) no SDB (AHI < 5); (2) mild SDB (5 ≤ AHI < 15); and (3) moderate-severe SDB (AHI ≥ 15). The obstructive apnea-hypopnea index (OAHI) is defined as the number of apneas (obstructive and mixed) and hypopneas per hour of sleep. Time spent below 90% of oxygen saturation (T < 90%) represented the percentage of the total sleep time spent below 90% oxygen saturation. Oxygen desaturation index 4% (ODI 4%) was described as the number of oxygen desaturations ≥ 4% per hour of sleep. Finally, arousals were scored when an abrupt shift of EEG frequency occurred (including alpha, theta, or frequencies > 16 Hz) for ≥ 3 s, with ≥ 10 s of preceding stable sleep. Scoring arousals during REM sleep required additional increases in submental electromyogram (EMG) lasting at least one second.14,15 Arousal index represented the number of respiratory and nonrespiratory arousals per hour of sleep.
Cardiac Arrhythmia Outcomes
Raw data from PSG studies were exported into European Data Files (EDF), a standard file format designed for exchange and storage of biological time series data. The PSG electrocardiogram (ECG) data and the respiratory wave forms from these files were analyzed by Somte ECG interpretation software (Somte, Compumedics). This software displayed the electrocardiographic tracing of the comprehensive sleep study in a “Holter-type” visual format, allowing manual as well as an automatic ECG analysis, including QRS complex classification, and arrhythmia detection/classification.
All ECG data were reviewed manually for specific arrhythmia types; events were quantified, and categorized by one of the investigators (BJS), blinded to respiratory events and clinical data, with arbitration by a cardiac electrophysiologist (RL) when necessary. Categories included supraventricular arrhythmias, ventricular arrhythmias and conduction delay arrhythmias. Supraventricular arrhythmias included: (1) premature atrial contraction (PAC ≥ 5 events per hour, measured in studies without atrial fibrillation); (2) atrial fibrillation or flutter (occurring intermittently or continuously); (3) supraventricular tachycardia, and (4) and a composite variable of combined supraventricular tachycardia (combination of atrial fibrillation and supraventricular tachycardia, excluding PACs). Ventricular arrhythmias included: (1) isolated premature ventricular contractions (PVC ≥ 5 events/h); (2) bigeminy/trigeminy/ quadrigeminy; (3) nonsustained ventricular tachycardia; and (4) a composite variable of complex ventricular ectopy (i.e., bigeminy, trigeminy, quadrigeminy or nonsustained ventricular tachycardia, excluding isolated PVCs). Conduction delay arrhythmias included: (1) sinus pause (> 3 s), (2) second degree atrioventricular block, (3) third degree atrioventricular block, (4) intraventricular conduction delay (QRS > 120 msec), and (5) tachyarrhythmia (ventricular or supraventricular). Finally, any nocturnal cardiac arrhythmias included the following arrhythmias: complex ventricular ectopy, or combined supraventricular tachycardia, or intraventricular conduction delay.
Covariate Data Collection
A number of clinical variables were collected at baseline: (1) demographic and anthropometric variables included age, gender, race, body mass index; (2) cardiovascular disease risk factors included diabetes, hypertension, (determined by either physician diagnosis or receiving appropriate medication), smoking history (≥ 20 pack year history), high density lipo-protein levels (HDL), and low density lipoprotein levels; and (3) cardiovascular diseases included stroke, angina, coronary artery disease, myocardial infarction, congestive heart failure, angioplasty, coronary artery bypass graft (CABG), pacemaker or implantable cardioverter defibrillator.
Statistical Analysis
Baseline demographic and clinical characteristics of patients were described using mean ± standard deviation for continuous variables, and frequency counts for categorical variables. ANOVA testing was used to compare mean values, and χ2 testing to compare frequency of categorical values across SDB severity groups. Kruskal-Wallis testing was used to compare median polysomnographic values among groups. All subtypes of arrhythmias were analyzed as dichotomous variables. We used Cochran-Armitage testing for linear trend to examine whether increased severity of SDB is associated with increased risk of nocturnal cardiac arrhythmias.
We used logistic regression analysis to examine the association between SDB and nocturnal cardiac arrhythmias subtypes (dichotomous outcome). Multivariable adjustment in a forward stepwise modeling approach was first used with demographic variables, adding later cardiovascular risk factors, followed by cardiovascular diseases.
Finally, as a secondary analysis, we examined the association between selected polysomnographic measures of sleep-disordered breathing and nocturnal cardiac arrhythmias using linear regression models.
Assuming an estimated arrhythmia prevalence of 40% in the reference group (patients without SDB), the study had more than 95% power to detect a minimum of 20% increase in prevalence of nocturnal cardiac arrhythmias in our sample size (PASS software, 2008). All the analyses were performed using the SAS (9.2 version) statistical package, and a p value < 0.05 was considered statistically significant.
RESULTS
After screening 982 consecutive veteran patients referred for PSG between 2000 and 2004, 697 patients fulfilled these inclusion and exclusion criteria (Figure 1). Among these 697 participants; 94.5% were male and 79.1% Caucasian with a mean age of 58.7 ± 12.1 years. Table 1 shows the baseline characteristics of patients without SDB (reference group), with mild SDB, and with moderate-severe SDB. As expected, increasing age, BMI, and male gender, were associated with increase severity of SDB. Diabetes mellitus, lower levels of high-density lipoproteins, and smoking tended to have increased prevalence in patients with more severe SDB. The distribution of prevalent cardiovascular diseases (such as myocardial infarction, coronary artery disease, coronary artery bypass graft, stroke, and congestive heart failure) did not vary significantly among patients with and without sleep apnea.
Figure 1. Consort patient flow diagram.
Table 1.
baseline characteristics by sleep-disordered breathing severity.

Table 2 demonstrates that nocturnal cardiac arrhythmia is common in this clinic-based population, affecting 58.5% (n = 86) of patients with mild SDB and 65.6% (n = 256) of patients with moderate-severe SDB, compared to 39.4% (n = 63) of those without SDB. Subjects with moderate-severe SDB had almost 3 times the unadjusted risk of any cardiac arrhythmias (OR 2.94; CI 95%, 2.01–4.30), 2-fold risk of tachyarrhythmias (OR 2.16; CI 95%,1.47–3.18; p = 0.0011), 2 times the risk of complex ventricular ectopy (OR 2.01; CI 95%, 1.36–2.96; p = 0.002), 4 times the risk of combined supraventricular tachycardia (OR 4.21; CI 95%, 1.48–12.00; p = 0.03), and 2 times the risk of intraventricular conduction delay (OR 2.50; CI 95%, 1.58–3.95; p = 0.001). Within these subtypes of arrhythmias, a nonsignificant trend towards a 2- to 3-fold higher prevalence of atrial fibrillation was observed among patients with moderate-severe SDB than those without SDB. The frequency of any cardiac arrhythmias and each individual sub-type of arrhythmia (complex ventricular ectopy, combined supraventricular tachycardia, and intraventricular conduction delay) was greater with increasing SDB severity (p for linear trend, < 0.0001; Figure 2)
Table 2.
Unadjusted association of sleep-disordered breathing (categorized by severity) with cardiac arrhythmias.
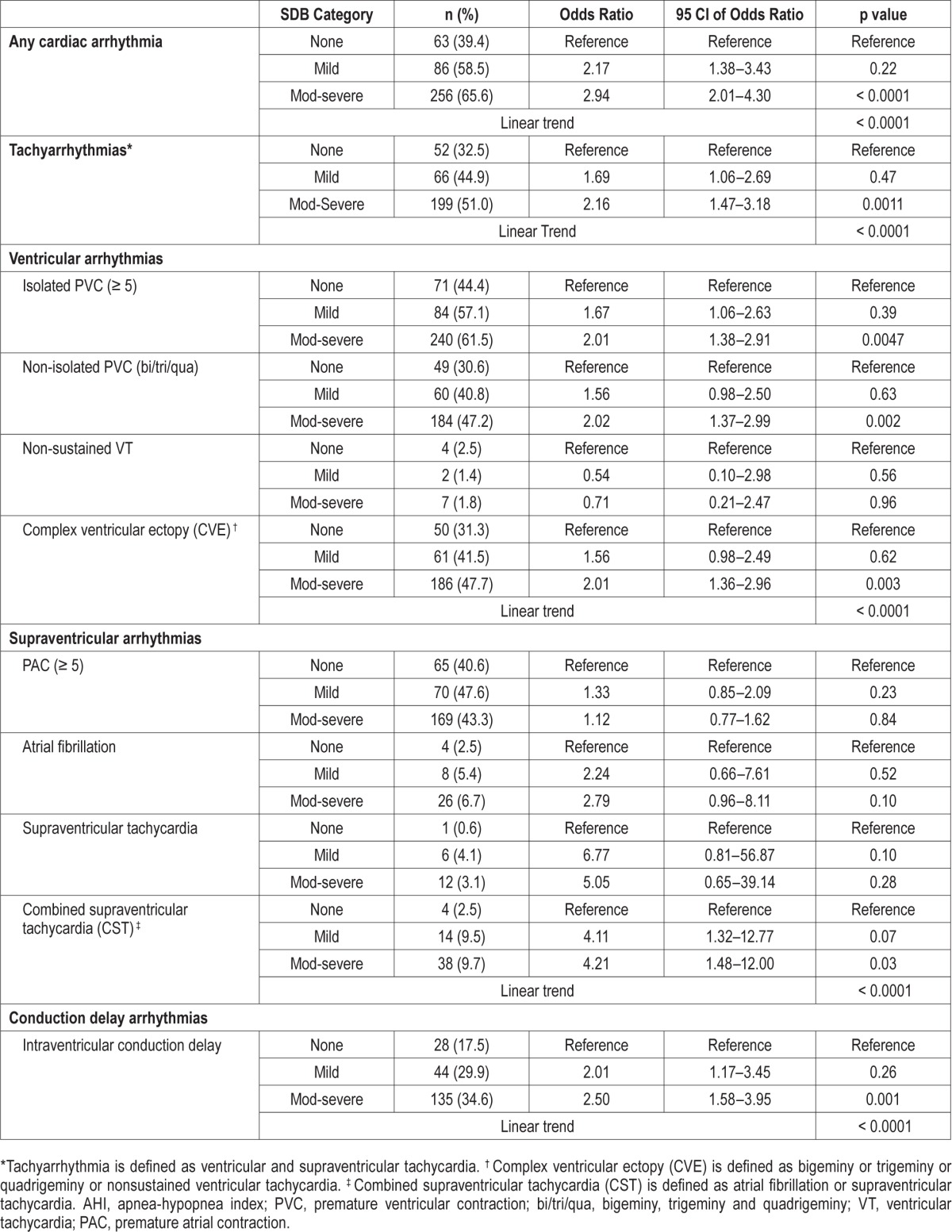
Figure 2. Unadjusted association of sleep-disordered breathing (categorized by severity) with cardiac arrhythmias.
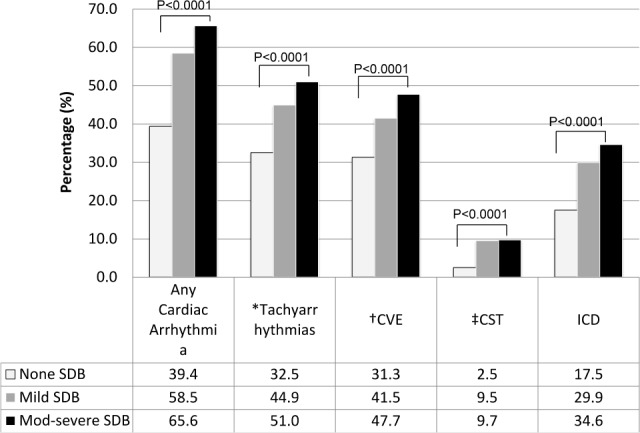
*Tachyarrhythmia is defined as ventricular and supraventricular tachycardia. † Complex ventricular ectopy (CVE) is defined as bigeminy or trigeminy or quadrigeminy or nonsustained ventricular tachycardia. ‡ Combined supraventricular tachycardia (CST) is defined as atrial fibrillation or supraventricular tachycardia. ICD, intraventricular conduction delay.
As shown in Table 3, after adjusting for several domains of covariates (demographics, cardiovascular risk factors, and cardiovascular disease), patients with moderate-severe SDB had twice the odds of having any cardiac arrhythmias (OR 2.24; CI 95% 1.48–3.40; p = 0.004). The association between moderate-severe SDB category and cardiac arrhythmia persisted for some but not all types of arrhythmia. For example, patients with moderate-severe SDB had twice the odds of intraventricular conduction delay (OR 2.39; CI 95% 1.44- 3.97; p = 0.007). Adjusting for the same covariates, patients with mild SDB had 4 times the odds of having combined supraventricular arrhythmia (OR 4.39; CI 95% 1.19–16.13; p = 0.039), in comparison to those without SDB. There were significant linear trends for the association between sleep-disordered severity and any cardiac arrhythmia as well as with intraventricular conduction delay. (Figure 3)
Table 3.
Adjusted model of association between SDB and nocturnal cardiac arrhythmias.
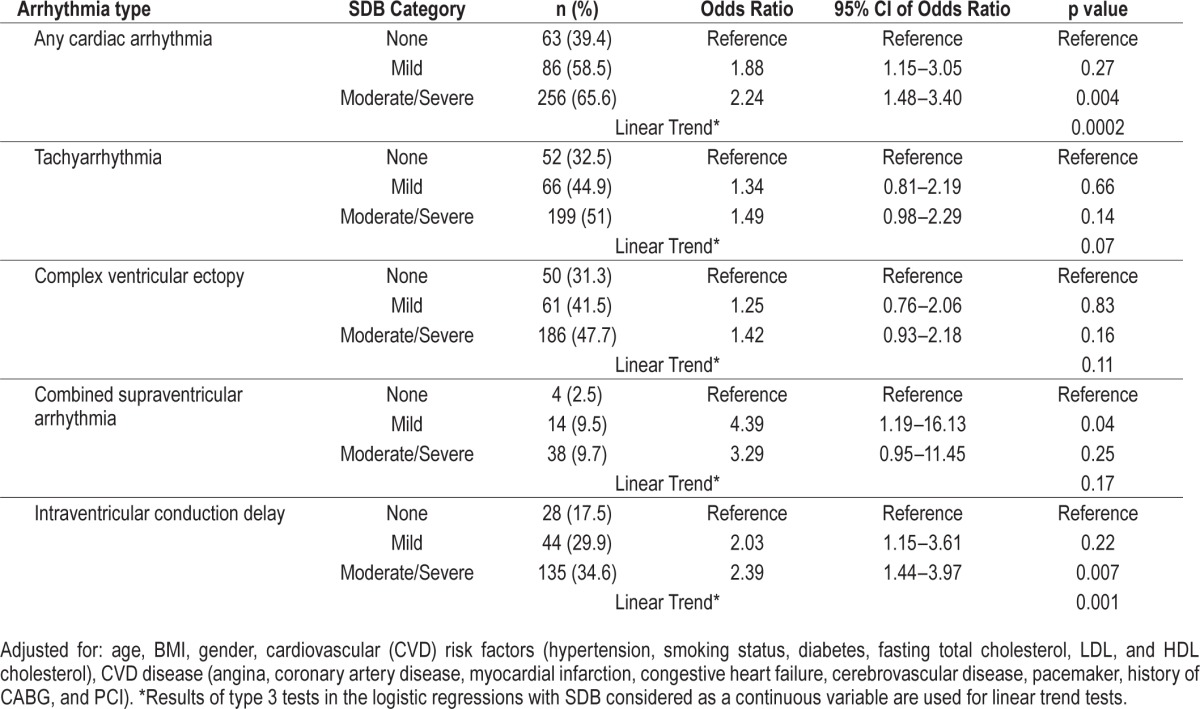
Figure 3. Adjusted model of association between SDB and nocturnal cardiac arrhythmias.
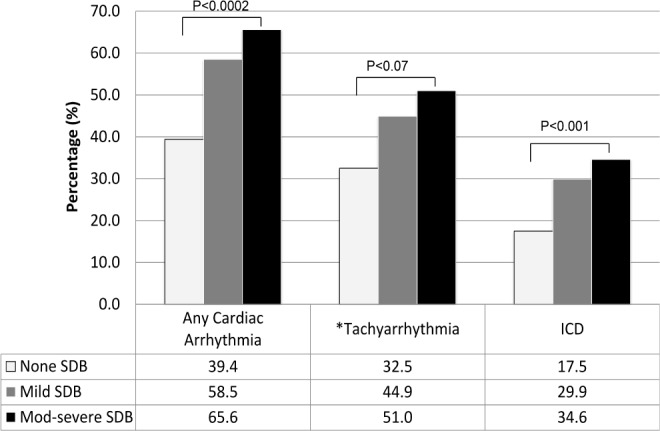
Adjusted for: age, BMI, gender, cardiovascular (CVD) risk factors (hypertension, smoking status, diabetes, fasting total cholesterol, LDL, and HDL cholesterol), CVD disease (angina, coronary artery disease, myocardial infarction, congestive heart failure, cerebrovascular disease, pacemaker, history of CABG, and PCI). *Tachyarrhythmia is defined as ventricular and supraventricular tachycardia. ICD, intraventricular conduction delay.
Table 4 describes a secondary analysis examining the association between selected polysomnographic measures of SDB and their association with nocturnal cardiac arrhythmias. SDB as measured by the overall AHI was the strongest predictor of nocturnal cardiac arrhythmias, such that the risk of nocturnal arrhythmias increased by 10% per every 10-unit increase in AHI (OR 1.10; CI 95% 1.04–1.15; p = 0.0005). Obstructive respiratory events were stronger predictors of nocturnal cardiac arrhythmias than were central respiratory events. Finally, various measures of hypoxemia (i.e., ODI, lowest oxygen saturation) were significantly associated with nocturnal cardiac arrhythmias, whereas the measure of arousal from sleep was not.
Table 4.
Selected polysomnographic measures of SDB and their association with any cardiac arrhythmia.

DISCUSSION
Using laboratory-based, objective measures of SDB, combined with a comprehensive method to ascertain nocturnal cardiac arrhythmias, we observed an independent association between SDB and nocturnal cardiac arrhythmias after adjusting for relevant clinical variables. Increasing severity of SDB, using common clinical cut-points, was associated with an increased risk of all three subtypes of nocturnal cardiac arrhythmias; supraventricular, ventricular, and conduction delay. Obstructive respiratory events were stronger predictors of nocturnal cardiac arrhythmias than were central respiratory events. Various measures of hypoxemia (i.e., ODI, lowest oxygen saturation) were significantly associated with nocturnal cardiac arrhythmia, whereas the arousal index, a measure of arousal from sleep (and presumably sympathetic activity), was not. These associations were found in a sleep clinic-based population of U.S. veterans at risk of having both SDB and cardiac arrhythmias.
Our results are consistent with the small number of previous studies in this domain that have demonstrated a strong and independent association between SDB and nocturnal cardiac arrhythmias.9,16,17 In addition, our study adds to the current understanding of SDB and arrhythmias in several important ways. First, we targeted a clinic-based population, which is likely more representative of patients referred for sleep testing. Second, we accounted for numerous cardiovascular risk factors, as well as established cardiovascular disease. Third, we observed a strong dose-response relationship between increasing severity of SDB, using accepted clinical cut-points and the three main subtypes of nocturnal cardiac arrhythmia: supraventricular, ventricular, and conduction delay. Finally, we observed that obstructive respiratory events, rather than central respiratory events, and measures of hypoxia, rather than arousal, were stronger predictors of nocturnal arrhythmia risk.
Limited research has characterized the association between SDB and nocturnal cardiac arrhythmias. One of the first studies to examine the SDB-arrhythmia relationship reported that 193 of 400 patients with severe OSA demonstrated cardiac arrhythmias (non-sustained ventricular tachycardia, sinus arrest, and second-degree atrioventricular conduction block) after evaluation by PSG and 24-h Holter monitoring. In that study, only patients with severe OSA were included; no reference group was used, and analyses did not adjust for potential confounders.16 Comparing patients with severe SDB to those with no SDB, a previous analysis from the Sleep Heart Health Study (SHHS) demonstrated an association between severe SDB and prevalent nocturnal cardiac arrhythmias.17 A subsequent study, by the same investigators, using data from the Outcomes of Sleep Disorders in Older Men (MrOS Sleep), focused specifically on atrial fibrillation and complex ventricular ectopy. The results suggested that central respiratory events had more significant association with atrial fibrillation, whereas obstructive respiratory events had a stronger linkage with ventricular arrhythmias.9 As these were population-based studies, they tended to include participants with less severe SDB and fewer cardiovascular comorbidities. Our results in large part are consistent with these previous findings, but show association between OSA and arrhythmia in a U.S. veteran population with high cardiovascular comorbidity rates.
Hypoxia has a complex association with cardiac arrhythmias. Chronic intermittent hypoxia may be cardio-protective and associated with “preconditioning,” while acute intermittent hypoxia could be pro-arrhythmogenic by inducing release of reactive oxygen species (ROS) and by increasing sympathetic nerve activity.18–23 In line with these mechanisms, our findings suggest that hypoxia frequency (ODI 4%) was associated with increased nocturnal arrhythmias. Mechanistically, intermittent hypoxia in OSA patients may directly cause ventricular ectopy by inducing the release of ROS, which pathologically stimulate cardiac ion channels and predispose to arrhythmia.24
There are other potential mechanisms why arrhythmia may arise in the presence of OSA. During an obstructive apnea, inspiratory effort against a collapsed upper airway generates negative intrathoracic force on the order of −60 mm Hg,25,26 stimulating cardiac mechanoreceptors and increasing cardiac transmural pressure, thereby predisposing to arrhythmia.27–29 Negative intrathoracic pressure has been shown to increase supraventricular premature beats and also to prolong the QTc interval, which in turn may explain the increased risk of dysrhythmias and sudden cardiac death.30–32
At the same time, the frequent co-stimulation of sympathetic and parasympathetic systems during sleep-disordered breathing may predispose to atrial arrhythmia.33 Atrial fibrillation (AF), in particular, has been associated with OSA.34 Experimental manipulations of the autonomic nervous system perturb the electrical system of the atrium, shortening the effective refractory period, increasing heterogeneity of conduction and repolarization, facilitating atrial fibrillation.33,35 Ambulatory monitoring studies analyzing heart rate variability have also shown a primary adrenergic increase followed by a vagal response may precede atrial arrhythmia supporting this concept.36
Despite the well documented association of AF with SDB, our study showed a non-significant trend towards a 2- to 3-fold higher prevalence of AF among patients with moderate-severe SDB in comparison to those without SDB. This finding could be explained by the small representation of patients with predominant central sleep apnea (CAI > 50% of total AHI) in our SDB group (16 of 537 patients), and the finding in previous studies that prevalent AF is more highly associated with central rather than obstructive sleep apnea.9
Regarding conduction delay arrhythmias, the prevalence of sinus pauses, atrioventricular block, and asystole has been reported in up to 18% in patients with SDB, in comparison to just 3% among a healthy population.16 In OSA patients, the occurrence of conduction delay arrhythmias has been associated with OSA severity, the extent of hypoxemia, and REM sleep.37,38 Our study found a significant relationship of hypoxemia with frequency of intraventricular conduction delay. Other studies have yielded different results. Mehra et al. reported no significant difference in conduction delay arrhythmia between individuals with SDB and those without.17,39 This difference in observational data may be explained by difference in study design and study population, as well as degree of oxygen desaturation. Mechanistically, the combination of an apneic event and hypoxemia are necessary to trigger a vagally mediated apneic arrhythmia.37,38,40
Our study has several strengths, including: (1) a detailed individually scored full in-laboratory polysomnography with coding and abstraction of various measures of hypoxia, arousals, and sleep architecture; (2) a rigorous, standardized collection of demographic information, cardiovascular risk factors, and established cardiovascular disease, with limited amount of missing data from a robust VA electronic medical record, and finally (3) the use of validated methodology for detailed collection and quantification of various subtypes of nocturnal cardiac arrhythmia. There are also some important limitations to consider. The study used a cross-sectional analytic design, which limits the ability to draw conclusions about temporal relationship. In addition, the population was predominantly male. However, such a sample is also likely more consistent with common clinical referral populations to sleep centers, and provides an opportunity to examine the impact of more severe SDB given the known heightened prevalence of risk factors for SDB among veterans (e.g., age, male gender, obesity).2 Although part of a recommended standard PSG recording, a single ECG bipolar lead (e.g., lead II) prevented us from diagnosing bundle branch blocks, changes in electrical axis, and ST segment abnormalities.13 As in most clinical epidemiologic studies, residual confounding is also a potential limitation. At the same time, it is also possible that our multivariable adjustments for a variety of covariates, including prevalent cardiovascular disease, may represent an over-adjustment for the effects of confounding, given that cardiovascular disease itself may be on the causal pathway between sleep apnea and nocturnal cardiac arrhythmias. Particular clinical characteristics of our cohort, such as cardiovascular risk factors and prevalent cardiovascular disease, should be noted when considering the results. For example, the relationship between SDB and hypertension has been well established by comprehensive longitudinal population-based studies.3,41 Though, the prevalence of hypertension in our study is not different between patients with SDB and those without. This could be attributed to the following factors: (1) it being a referral population with inherent sampling bias, (2) age, the association between AHI and hypertension, seemed stronger among those who are younger than 60 years, but not for those who are older, and (3) the sharing of common cofounding factors, as SDB and hypertension are exceedingly common and share similar risk factors (e.g., obesity, male sex, older age).42
In all, this study adds to the current understanding of the increased risk of cardiac arrhythmia associated with OSA. A heightened awareness of this association is essential to case identification that will lead to appropriate testing and treatment for sleep apnea. Further work is needed to determine the prognostic significance of these various subtypes of nocturnal cardiac arrhythmia on cardiovascular outcomes and mortality, as well as randomized controlled trials examining the impact of OSA treatment.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by the T-32 fellowship research training grant awarded to Yale Pulmonary and Critical Care Section, by the VA CSR&D Merit Review Award Program granted to Dr. Henry K. Yaggi for the Determining Risk of Vascular Events by Apnea Monitoring study (DREAM), as well as by the National Institute of Nursing Research grant (1P20NR014126) awarded to Dr. Yaggi and Nancy S. Redeker, PhD, RN, FAHA, FAAN, for the Yale Center for Sleep Disturbance in Acute and Chronic Conditions. Dr. Strohl has received research support from and consulted for Inspire Medical Systems. Dr. Lampert has received research support from GE Medical and Medtronic. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AF
atrial fibrillation
- AHI
apnea-hypopnea index
- BMI
body mass index
- CABG
coronary artery bypass graft
- CAI
central apnea index
- CST
combined supraventricular tachycardia
- CVE
complex ventricular ectopy
- ECG
electrocardiogram
- EEG
electroencephalogram
- EMG
electromyogram
- HDL
high density lipoprotein
- ICD
intraventricular conduction delay
- OAHI
obstructive apnea-hypopnea index
- ODI
oxygen desaturation index 4%
- OSA
obstructive sleep apnea
- PAC
premature atrial contraction
- PSG
polysomnography
- PVC
premature ventricular contractions
- ROS
reactive oxygen species
- SDB
sleep-disordered breathing
- VT
ventricular tachycardia
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of veterans health administration beneficiaries. Sleep Med. 2004;5:345–50. doi: 10.1016/j.sleep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–95. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 6.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–6. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 7.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 8.Zeidan-Shwiri T, Aronson D, Atalla K, et al. Circadian pattern of life-threatening ventricular arrhythmia in patients with sleep-disordered breathing and implantable cardioverter-defibrillators. Heart Rhythm. 2011;8:657–62. doi: 10.1016/j.hrthm.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (mros sleep) study. Arch Intern Med. 2009;169:1147–55. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119:1827–35. doi: 10.1378/chest.119.6.1827. [DOI] [PubMed] [Google Scholar]
- 11.Shivalkar B, Van de Heyning C, Kerremans M, et al. Obstructive sleep apnea syndrome: more insights on structural and functional cardiac alterations, and the effects of treatment with continuous positive airway pressure. J Am Coll Cardiol. 2006;47:1433–9. doi: 10.1016/j.jacc.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992;86:968–78. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- 13.Koo BB, Won C, Selim BJ, et al. The determining risk of vascular events by apnea monitoring (dream) study: design, rationale, and methods. Sleep Breath. 2015 Dec 7; doi: 10.1007/s11325-015-1254-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson A, Quan SQ. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events. Rules, terminology and technical specifications. [Google Scholar]
- 15.EEG arousals: scoring rules and examples: a preliminary report from the sleep disorders atlas task force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 16.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 17.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the sleep heart health study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolar F, Ostadal B. Molecular mechanisms of cardiac protection by adaptation to chronic hypoxia. Physiol Res. 2004;53(Suppl 1):S3–13. [PubMed] [Google Scholar]
- 19.Asemu G, Papousek F, Ostadal B, Kolar F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial k(atp) channel. J Mol Cell Cardiol. 1999;31:1821–31. doi: 10.1006/jmcc.1999.1013. [DOI] [PubMed] [Google Scholar]
- 20.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–8. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghias M, Scherlag BJ, Lu Z, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54:2075–83. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Jeong EM, Liu M, Sturdy M, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52:454–63. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asemu G, Neckar J, Szarszoi O, Papousek F, Ostadal B, Kolar F. Effects of adaptation to intermittent high altitude hypoxia on ischemic ventricular arrhythmias in rats. Physiol Res. 2000;49:597–606. [PubMed] [Google Scholar]
- 24.Benditt JO, Boitano LJ. Pulmonary issues in patients with chronic neuromuscular disease. Am J Respir Crit Care Med. 2013;187:1046–55. doi: 10.1164/rccm.201210-1804CI. [DOI] [PubMed] [Google Scholar]
- 25.Tolle FA, Judy WV, Yu PL, Markand ON. Reduced stroke volume related to pleural pressure in obstructive sleep apnea. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1718–24. doi: 10.1152/jappl.1983.55.6.1718. [DOI] [PubMed] [Google Scholar]
- 26.Buda AJ, Pinsky MR, Ingels NB, Jr., Daughters GT, Jr., Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301:453–9. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 27.Koshino Y, Villarraga HR, Orban M, et al. Changes in left and right ventricular mechanics during the mueller maneuver in healthy adults: a possible mechanism for abnormal cardiac function in patients with obstructive sleep apnea. Circ Cardiovasc Imaging. 2010;3:282–9. doi: 10.1161/CIRCIMAGING.109.901561. [DOI] [PubMed] [Google Scholar]
- 28.Orban M, Bruce CJ, Pressman GS, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol. 2008;102:1557–61. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–7. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Camen G, Clarenbach CF, Stowhas AC, et al. The effects of simulated obstructive apnea and hypopnea on arrhythmic potential in healthy subjects. Eur J Appl Physiol. 2013;113:489–96. doi: 10.1007/s00421-012-2457-y. [DOI] [PubMed] [Google Scholar]
- 31.de Bruyne MC, Hoes AW, Kors JA, Hofman A, van Bemmel JH, Grobbee DE. QTC dispersion predicts cardiac mortality in the elderly: the Rotterdam Study. Circulation. 1998;97:467–72. doi: 10.1161/01.cir.97.5.467. [DOI] [PubMed] [Google Scholar]
- 32.Straus SM, Kors JA, De Bruin ML, et al. Prolonged qtc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 33.Tomita T, Takei M, Saikawa Y, et al. Role of autonomic tone in the initiation and termination of paroxysmal atrial fibrillation in patients without structural heart disease. J Cardiovasc Electrophysiol. 2003;14:559–64. doi: 10.1046/j.1540-8167.2003.02462.x. [DOI] [PubMed] [Google Scholar]
- 34.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 35.Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 36.Huang JL, Wen ZC, Lee WL, Chang MS, Chen SA. Changes of autonomic tone before the onset of paroxysmal atrial fibrillation. Int J Cardiol. 1998;66:275–83. doi: 10.1016/s0167-5273(98)00241-1. [DOI] [PubMed] [Google Scholar]
- 37.Zwillich C, Devlin T, White D, Douglas N, Weil J, Martin R. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest. 1982;69:1286–92. doi: 10.1172/JCI110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koehler U, Fus E, Grimm W, et al. Heart block in patients with obstructive sleep apnoea: pathogenetic factors and effects of treatment. Eur Respir J. 1998;11:434–9. doi: 10.1183/09031936.98.11020434. [DOI] [PubMed] [Google Scholar]
- 39.Olmetti F, La Rovere MT, Robbi E, Taurino AE, Fanfulla F. Nocturnal cardiac arrhythmia in patients with obstructive sleep apnea. Sleep Med. 2008;9:475–80. doi: 10.1016/j.sleep.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Sleep-induced apnea syndrome. Prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am J Med. 1977;63:348–58. doi: 10.1016/0002-9343(77)90272-8. [DOI] [PubMed] [Google Scholar]
- 41.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep heart health study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 42.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]


