Abstract
Objective
A common cause of failure in laminectomy surgery is when epidural, peridural, or perineural adhesion occurs postoperatively. The purpose of this study is to examine the efficacy of a temperature-sensitive, anti-adhesive agent (TSAA agent), Guardix-SG®, as a mechanical barrier for the prevention or reduction of peridural scar adhesion in a rabbit laminectomy model.
Methods
Twenty-six mature rabbits were used for this study. Each rabbit underwent two separate laminectomies at lumbar vertebrae L3 and L6, left empty (the control group) and applied 2 mL of the TSAA agent (the experimental group), respectively. Invasive scar formation or inflammation after laminectomy was quantitatively evaluated by measuring the thickness of the dura, the distance from the surface of dura to the scar tissues, the number of inflammatory cells in the scar tissues at the laminectomy site, and the concentration of collagen in histological sections.
Results
At 6 weeks postsurgery, the dura was significantly thinner and the distance from the surface of dura to the scar tissues was greater in the experimental group than in the control group (p=0.04 and p=0.01). The number of inflammatory cells was not significantly different in the two groups (p=0.08), although the mean number of inflammatory cells was relatively lower in the experimental group than in the control group.
Conclusion
The current study suggests that the TSAA agent, Guardix-SG®, could be useful as an interpositional physical barrier after laminectomy for the prevention or reduction of adhesion.
Keywords: Laminectomy, Epidural fibrosis, Epidural adhesion, Anti-adhesive agent
INTRODUCTION
Postoperative epidural fibrosis and adhesion are common occurrences during the healing process of injured tissue after a spinal operation. Postoperative epidural, peridural, or perineural adhesions after a laminectomy frequently results in failed spinal surgeries1,22). A large number of patients also experience a recurrence of pain after spinal decompression surgery7,20,29,32). Consequently, approximately 19% of surgical patients undergo at least one additional spine operation within 5 years after the initial spinal operation4,5), while as few as 65% of patients return to normal daily activities and only 61% return to work after spinal surgery3). Most of these patients undergo additional surgical procedures because of the development of new musculoskeletal and radicular symptoms29). The operation time length and the incidence of iatrogenic nerve root damage and dural tears are often increased in these patients due to technical difficulties arising from the presence of epidural fibrosis or adhesion tissue from their initial surgeries21,24,49).
Many factors have been suggested as triggers of adhesion formation, including foreign materials (e.g., starch, surgical gloves, and suture materials), infection, and duration of surgery9,52). However, even the careful management of these factors has not reduced the rate of adhesion occurrence. Tissue hypoxia can also induce adhesion formation40); a routine spinal surgery incision that interrupts blood circulation can produce hypoxia52), which alters the phenotype of normal fibroblasts to adhesion fibroblasts39) and triggers superoxide production and inflammation19), thus leading to postoperative adhesion.
Surgeons rely on several classic strategies for adhesion prevention or reduction. For example, they avoid the use of powdered gloves; minimize tissue handling and trauma; use sufficient irrigation; minimize the use of electro-coagulation; perform meticulous hemostasis; use small, biocompatible suture materials; and avoid desiccation of the tissue27,34,47). However, these surgical strategies are not completely effective. Various treatments, such as the use of low-dose irradiation, non-steroid anti-inflammatory drugs (NSAIDs), and steroids, have been tested for their ability to reduce peridural adhesion after spinal surgery12,14,17). The use of physical barriers has also been reported, including nonbiological synthetic membranes and forms, as well as free or pedicle fat grafts6,10,11,28,42,48).
Physical barriers rely on a separation of the injured tissue surface from adjacent tissues as the basic mechanism for the prevention of epidural adhesion41). Numerous studies have employed physical barrier methods to prevent postoperative tissue adhesion10,11,16,25,28,29,35,36,37,48), but sufficient anti-adhesion effects can only be achieved if the barrier is placed between the dura and its surrounding tissues for an adequate amount of time, and after the healing process is complete, the barrier must be resolved or absorbed naturally into the body, rather than remaining as a foreign body. The barrier material must also be nontoxic while it is in the body25,51).
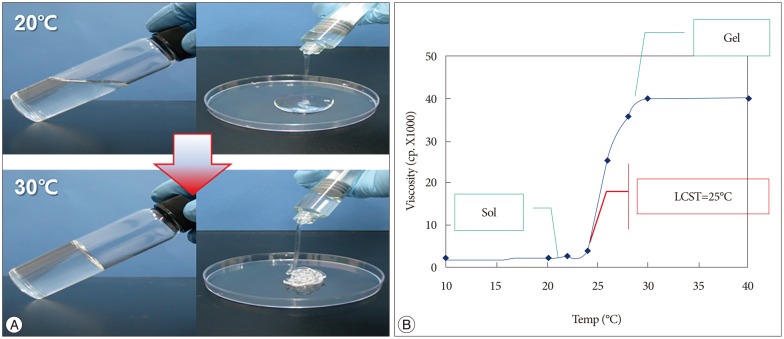
Guardix-SG® (Genewel, Dongsung Company, Seongnam, Korea) is a poloxamer-based, temperature-sensitive, anti-adhesive agent (TSAA agent)18,35,36). This TSAA agent is a complex consisting of crosslinked poloxamer, alginate, and CaCl2, that overcomes the most critical fault of poloxamer as a barrier material-absorption by the body before an anti-adhesion effect can occur43). Notably, this TSAA agent also has the ability to transform from a solution to a gel form at body temperature, which enhances its properties as a physical barrier (Fig. 1). The purpose of this study is to examine the efficacy of the TSAA agent as a mechanical barrier for the prevention or reduction of peridural scar adhesion in a rabbit laminectomy model.
Fig. 1. Phase transition. A : The temperature-sensitive, anti-adhesive agent (TSAA agent), Guardix-SG® is a solution at 20℃ and a gel at 30℃. B : Warming (above 25℃) induces the phase transition to the gel form. Sol : solution, LCST : lower critical solution temperature.
MATERIALS AND METHODS
Animals
Because the rabbit laminectomy model is convenient and cost-effective for evaluating postoperative adhesion using macroscopic and microscopic methods, many previous studies have used these models to evaluate various biological and nonbiological barrier materials.
This study was supported by the research fund of Genewel, Donsung Company. The Animal Care and Ethics Committee of Dongsung Company and Hanyang Medical Collage have approved all experimental protocols used in this study.
Sample size calculations were performed using STATA/IC 10 (Stata Corp., College Station, TX, USA). This study predicted a normalized mean effect size of 14.4% on the basis of a previous study that reported that the incidence of peridural adhesion was 48%13). With a significance level of 0.05 and a desired power of the experiment of 0.8, the minimal sample size needed to detect the difference of the effects between two groups was 23 rabbits. Therefore, by taking an attrition rate of 10% into consideration, this study used a total sample size of 26 mature New Zealand White rabbits (males, 10 weeks of age, and weighting 2 kg).
Surgical procedure
All rabbits were sedated with intramuscular Zoletil 50 (60 mg/kg; tiletamine chlorohydrate/zolazepam chlorohydrate, Vibac Laboratories, Seoul, Korea) and Rumpun (18.64 mg/kg; xylazine HCL, Bayer, Seoul, Korea). Before beginning the surgical procedure, the lower back area of each rabbits was shaved, and the operative field was sterilized with povidone. The paraspinal muscles were retracted subperiostically through a L2–S1 midline incision, and two separate laminectomies (10×5 mm2) were performed at lumbar vertebrae L3 and L6 in each rabbit. The ligamentum flavum and peridural fat tissue were cleared away from the surgical site. Hemostasis was obtained using a bipolar coagulator. Each of the two laminectomy sites was treated differently : 1) At the lumbar vertebra L3 laminectomy site, the defect was left empty and covered by a re-approximation of soft tissue and skin (the control group), and 2) at the lumbar vertebra L6 laminectomy site, 2 mL of the TSAA agent was applied to the dura (the experimental group). The fascia was then closed with 3-0 Surgifit, and the skin was closed with 3-0 nylon sutures. After the operation, an intramuscular injection of antibiotics (Gentamicin sulfate 0.05 mL) was administered, and the rabbits were raised without any intervention. The rabbits were postoperatively housed in individual cages, received a normal diet, and were allowed normal activity.
Histological examination
Six weeks following the operation, the experimental animals were killed by an injection of a lethal dose of intramuscular Zoletil 50 (120 mg/kg) and Rumpun (37.28 mg/kg).
The lumbar vertebrae, together with their adjacent muscles, were removed en bloc at each of the two laminectomy sites. Specimens were fixed in a 10% buffered formalin solution for further histological examination a week later. Lumbar spine specimens were then decalcified in formic acid. After paraffin sections were obtained, each block was cut into 6 µm transverse sections. To ensure that evaluating various factors were performed using specimens that were as similar as possible, the middle section from each paraffin block was selected. The histological sections were then stained with Masson trichrome and hematoxylin and eosin.
Quantitative histological analyses were performed with a semi-automated image system, including a computer with image analysis software (Image J, National Institutes of Health, Bethesda, MD, USA) and a high-resolution color monitor. Invasive scar formation or inflammation after the laminectomy was evaluated quantitatively by measuring 1) the thickness of the dura (µm) (Fig. 2), 2) the distance from the surface of dura to the scar tissues (µm) (Fig. 3), 3) the number of inflammatory cells in the scar tissues at the site of laminectomy (No. of inflammatory cells/mm2) (Fig. 4), and 4) the concentration of collagen (%/mm2) (Fig. 5). All examinations were performed by two observers. Each observer performed the evaluations twice and then averaged their results. The values presented here are the averages of the data from the two observers.
Fig. 2. Measurement of the thickness of the dura (µm) using a histological section (hematoxylin and eosin staining) from a laminectomy site six weeks after operation.
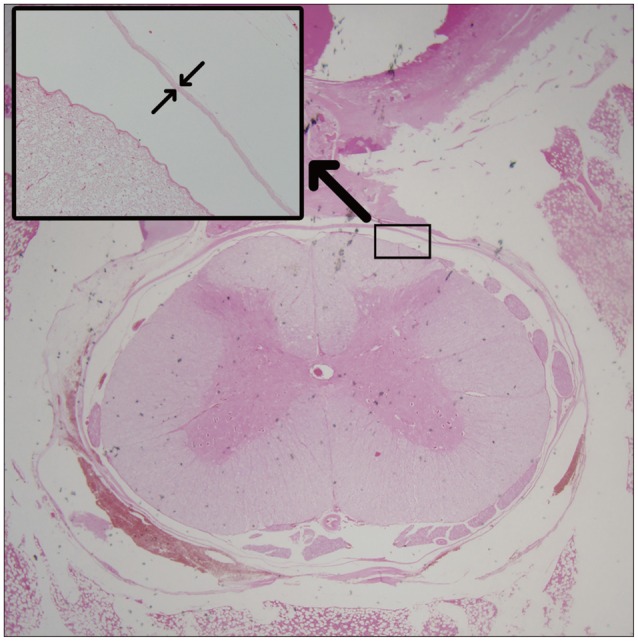
Fig. 3. Measurement of the distance from the surface of dura to the scar tissues (µm) using a histological section (hematoxylin and eosin staining) of a laminectomy site six weeks after operation.
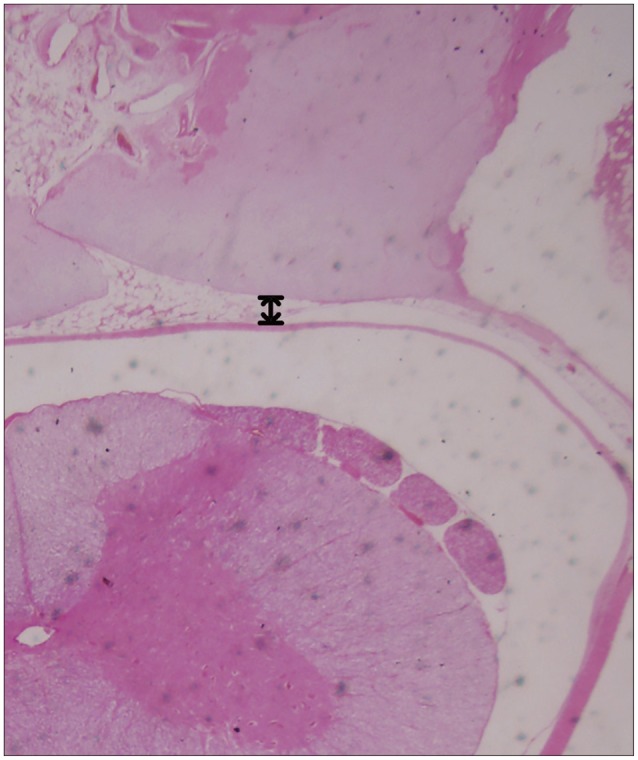
Fig. 4. Counting the number of inflammatory cells in the scar tissues (No. of inflammatory cells/mm2) using a histological section (hematoxylin and eosin staining) of a laminectomy site six weeks after operation (black arrow indicates inflammatory cells).
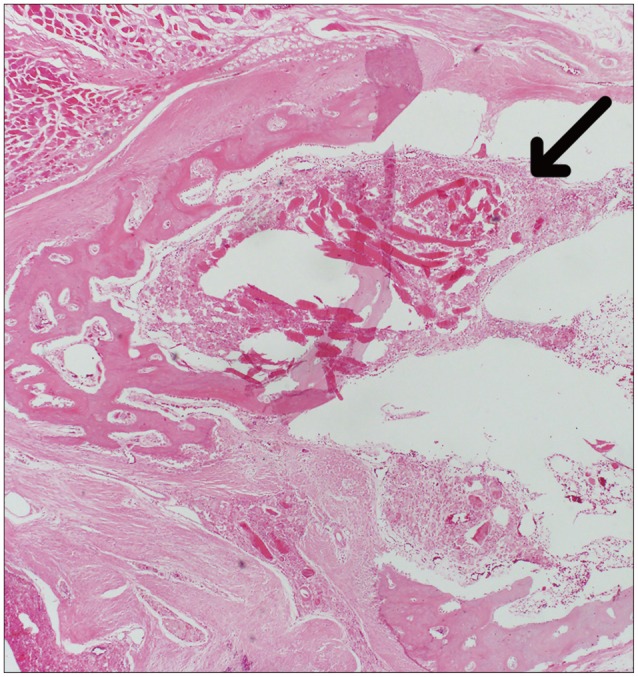
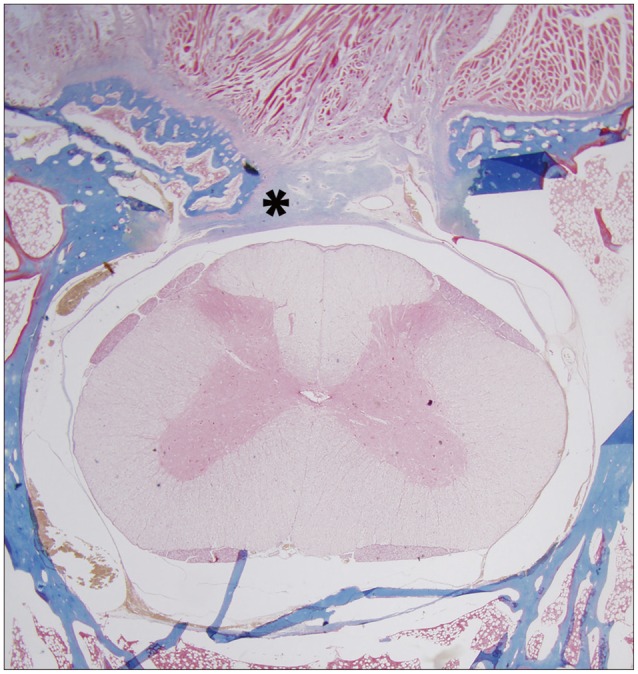
Fig. 5. Measurement of the concentration of collagen (%/mm2) using a histological section (Masson trichrome staining) of a laminectomy site six weeks after operation (Asterisk indicates fibrosis by collagen).
Statistical analysis
The anti-adhesion effects of the TSAA agent were evaluated by comparing the measurements of the four factors between the experimental group and the control group using the Student's t-test (SPSS for Windows, version 17.0; SPSS, Chicago, IL, USA). A statistical difference was considered significant when the p-value was less than 0.05.
RESULTS
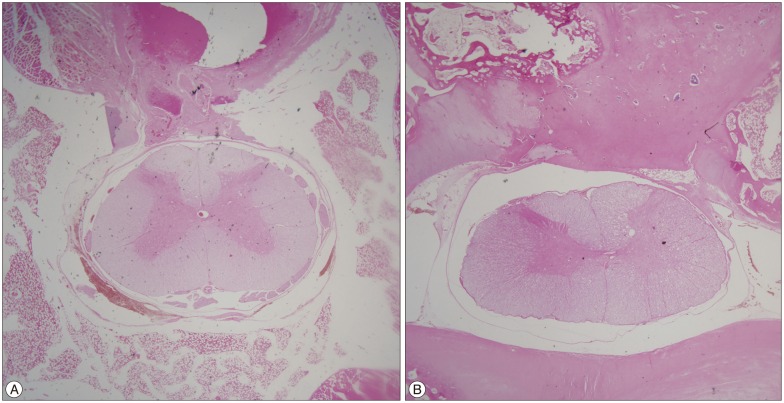
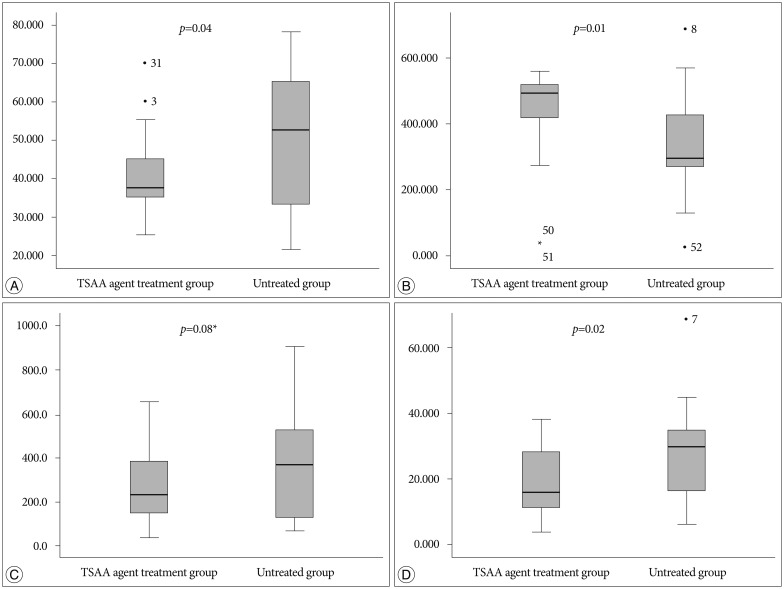
The general condition of all rabbits remained good after surgery, with no cases of postoperative paralysis or infection noted. As a result, the control group (for whom the defect was left empty and covered by a re-approximation of soft tissue and skin at the laminectomy site) and the experimental group (for whom 2 mL of the TSAA agent was applied to the dura) each consisted of 26 samples (Fig. 6, 7, Table 1).
Fig. 6. Histological sections (hematoxylin and eosin staining) of the laminectomy site six weeks after the operation showing the dura and adhesion scar tissue in the TSAA agent treatment group (A) and untreated group (B). TSAA : temperature-sensitive, anti-adhesive agent.
Fig. 7. Differences in postoperative adhesion between the TSAA agent, Guardix-SG® treatment group and untreated group for the thickness of the dura (µm) (A), the distance from the surface of dura to the scar tissues (µm) (B), the number of inflammatory cells in the scar tissues at the site of laminectomy (No. of inflammatory cells/mm2) (*no significant difference) (C), and the concentration of collagen (%/mm2) (D). TSAA : temperature-sensitive, anti-adhesive agent.
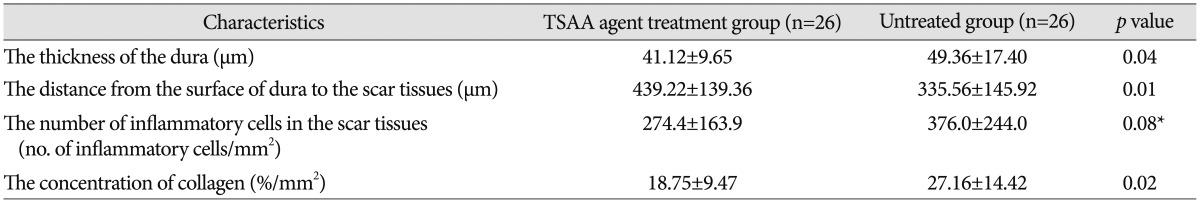
Table 1. Quantitative histological analysis at postoperative 6 weeks in a rabbit laminectomy model.
*No significant difference
The thickness of the dura
The dura at postoperative 6 weeks was significantly thinner in the experimental group than in the control group (p=0.04). The mean thicknesses of the dura were 41.12 µm in the experimental group and 49.36 µm in the control group, retrospectively. The normal thickness of the dura in a rabbit is approximately 40 µm, so the thickness of the dura in the experimental group was indistinguishable from the normal thickness. This result suggests that inflammation in the dura after surgery was reduced by the TSAA agent.
The distance from the surface of dura to the scar tissues
The distance from the surface of dura to the scar tissues at postoperative 6 weeks was significantly greater in the TSAA agent treatment group than in the untreated group (p=0.01). The mean distances from the surface of dura to the scar tissues were 439.22 µm in the experimental group and 335.56 µm in the control group, retrospectively. The TSAA agent therefore appeared to prevent invasion by the surrounding scar tissue even under a potent inflammatory condition.
The number of inflammatory cells in the scar tissues at the site of laminectomy
The number of inflammatory cells at postoperative 6 weeks was not significantly different between the two groups (p=0.08), although the mean number of inflammatory cells was relatively lower in the experimental group than in the control group. The mean number of inflammatory cells was 274.3 cells/mm2 in the experimental group and 376.0 cells/mm2 in the control group.
The concentration of collagen
The concentration of collagen at postoperative 6 weeks was significantly less in the experimental group than in the control group (p=0.02). The mean concentration of collagen was 18.75 %/mm2 in the experimental group and 27.16 %/mm2 in the control group, retrospectively. The concentration of collagen is closely related to peridural adhesion because the collagen concentration increases during fibrosis. Therefore, the TSAA agent appeared to reduce peridural adhesion and fibrosis after surgery.
DISCUSSION
Postoperative peridural adhesion causing compression or tethering of the nerve root has been proposed as one of the possible factors leading to a poor clinical outcome after a successful laminectomy32,38). After surgery, some patients show increased susceptibility to recurrent disc herniation or stenosis because of scar tissues that may restrict nerve root mobility42). The relationship between clinical symptoms and adhesion formation is still unclear, but peridural scar adhesion is attributed to as many as 24% of all failed back surgery cases38).
The precise mechanism of postoperative peridural adhesion is not clear. LaRocca and McNabb26) have reported that epidural and peridural adhesion, forming a membrane called the laminectomy membrane, is caused by a posterior invasion of fibroblasts from the erector spinal muscles. Songer et al.45) have proposed that epidural fibrosis arises from replacement of the epidural fat by a hematoma. The absorption of the hematoma then leads to replacement with granulation tissues, which mature into dense fibrotic tissue. However, regardless of the exact mechanism of formation of postoperative peridural adhesions, preventing or limiting fibroblast proliferation from coming into contact with the exposed dura in the early healing phase seems to be a key factor in preventing adhesion formation8,42). In other words, postoperative peridural adhesion occurs following a proliferation of fibroblasts and biosynthesis of collagen and the extracellular matrix.
Many previous studies have used experimental animal models to determine the efficacy of various anti-adhesive agents for the prevention or reduction of adhesion10,11,16,25,28,29,35,36,37,48). For example, Songer et al.44,45) have shown that the anti-adhesive agent sodium hyaluronic acid solution greatly reduced scar formation after a laminectomy and discectomy in a dog model. They found less fibrosis and thinner collagen layering around the nerve roots and dura in histological sections. The current study assessed the effects of the TSAA agent, Guardix-SG® compared with an untreated group by utilizing the rabbit laminectomy model used in several previous studies and found that the thickness of the dura was significantly less and the distance from the surface of dura to the scar tissues was greater in the TSAA agent treatment group than in the untreated group.
Peridural adhesion after spinal surgery ultimately results in the release of vasoactive factors at the damage site and induces accumulation of inflammatory cells, thereby inducing the formation of collagen-rich vessels and infiltration of fibroblasts, macrophages, and giant cells. Gradually, the normal tissue is substituted by angiogranular tissue and the adhesion becomes permanent16). The current study has identified a significantly lower concentration of collagen in the experimental group than in the control group. The number of inflammatory cells was not significantly different in the two groups at postoperative 6 weeks, although the mean number of inflammatory cells was relatively lower in the experimental group than in the control group. These results suggest that the swelling of the dura or spinal cord due to inflammation was reduced by the TSAA agent treatment and that the TSAA agent effectively prevented invasion by scar tissue. Additionally, based on the observations of the rabbit histological sections, this study has indicated that treatment with the TSAA agent has several advantages. First, because the TSAA agent is a sterile, absorbable gel matrix, it is non-toxic and does not induce granuloma formation. Second, the anti-fibrotic properties of the TSAA agent are localized to the discrete area to which it is applied, so healing of the adjacent tissue is not significantly affected. No damage to the skin, the subcutaneous tissue, or the muscle was identified in the present study-the use of the TSAA agent primarily resulted in the prevention scar formation and adhesion adjacent to the dura at the laminectomy site.
As previously stated, histological sections were obtained at postoperative 6 weeks in this study. An experimental period of 6 weeks is short for evaluating the degree of peridural adhesion, but the formation of dense fibrosis at the laminectomy site at least 4 weeks postoperatively in rabbits is a reliable finding46). In addition, previous studies have reported that significant bone regeneration can be seen about 9 weeks postoperatively in rabbit models46). Therefore, this study limited the experimental period of peridural adhesion to only 6 weeks to eliminate the confounding bias of bone growth. In addition, in current study, the L3 laminectomy site only was used in the control group and the L6 laminectomy site only was used in the experimental group. Rabbits are quadrupeds with seven vertebrae. Because the difference is at least between the upper lumbar and lower lumbar in the quadrupeds, which is different from bipeds, different levels within same animal were used for the control and experimental groups23).
In general, physical barriers, such as anti-adhesive agents, prevent adhesion after a laminectomy by a basic mechanism that involves the separation of the surrounding injured tissue surface from the adjacent dura membrane41). Many kinds of biological and nonbiological materials have been tested since LaRocca and McNabb first published the use of the laminectomy membrane in 19746,10,11,16,25,28,29,35,36,37,42,48). Among these, suitable materials for clinical use are film, solution, gel, and the recently developed sol-gel transition-type barriers. The film-type barrier is easily handled and can be cut into a shape that fits the form of the exposed area. However, the most critical disadvantage of the film barrier is potential reduction of the anti-adhesive effects because this type of barrier cannot ensure complete coverage of nerves in the marginal area after laminectomy and can cause the proliferation of fibrous tissue in gaps between the barrier and the tissues15,31). The solution-type barrier can be easily applied to surgery irrespective of the shape of the exposed laminectomy site, and it overcomes the concerns regarding incomplete coverage of the nerves in the marginal area. However, it cannot be fixed onto the surgical site due to flow to other regions and can be drained too early by drainage catheters before adhesion can be prevented2,33). The gel-type barrier can cover almost the whole site of the nerves in the marginal area regardless of the shape of the exposed laminectomy site because of its high shape-molding capacity or shape-filling effect. The disadvantage of the gel type is that the material is relatively difficult to handle2,51).
The current study investigated the effectiveness of the TSAA agent, Guardix-SG® developed to remedy the shortcomings of the existing types of anti-adhesive agents. This TSAA agent is a complex of CaCl2, alginate, and poloxamer. Alginate, a hydrogel form extracted from seaweed, is commonly used clinically36). A previous study by Nagakura et al.30) demonstrated that a pure viscous injectable alginate solution reduced scar formation by forming a physical barrier against fibroblast invasion and that it also stimulated wound healing in the surrounding tissue. Poloxamer, a complex of nonionic triblock copolymers of central hydrophobic polypropylene and side hydrophilic polyethylene, is used clinically as a surfactant because of its amphiphilic characteristics. Poloxamer exists in the solution form at room temperature but transforms to a gel form at body temperature. Therefore, it provides a convenient and efficient means for forming a firm physical barrier during a laminectomy procedure36). Reigel et al.37) have demonstrated that poloxamer had an approximately 50% anti-adhesion effect in a rabbit laminectomy model. Furthermore, the gel that adhered to the tissue was hydrolyzed and, absorbed and was then excreted renally after several weeks50). Thus, the TSAA agent does not remain as a foreign body at the application site.
Limitations
Because the experimental period for this study was short, it was not possible to identify how the empty space between the surface of dura and the scar tissues, especially in the TSAA agent treatment group, would change over a longer time period postsurgery. Though 6 weeks are enough time for tissue adhesion and also disappearance of TSAA agent, tissue recovering after tissue adhesion was not possible to describe. To focus on this point, a longer-term experimental period is required for future studies.
A control group of this study was just irrigated with saline before the wound closure. Further grouping with NSAIDs, steroid, gelatin sponge, fibrinolytics or other anti-adhesive agents would make more various data and informations about peridural scar formation.
The methods used in the current study included a quantitative histological analysis to assess differences in postoperative peridural adhesion. The use of a semi-automatic analysis simplified surface measurements, including the thickness of the dura and the distance from the surface of dura to the scar tissues and provided good interobserver correlation. Nevertheless, determination of the number of inflammatory cells in the scar tissues and the concentration of collagen was poor because counting and measuring were difficult with the semi-automatic procedure and because of variations in different section areas despite attempts to ensure that inflammatory cell counts and collagen concentration measurements were performed using equivalent sections of the specimens. In addition, no discectomies were performed in the current study, so the possible correlation between the TSAA agent use and peridural adhesion at a discectomy site in terms of annular ligament healing processes could not be evaluated.
CONCLUSION
The TSAA agent, Guardix-SG® is easily applied to a laminectomy site after spinal surgery, it ensures complete coverage of the dura, and it localizes to the desired area due to its gel-forming capacity at body temperature. This experimental study assessed the efficacy of the TSAA agent in the prevention of postoperative adhesions in a rabbit laminectomy model. The findings of this study show that the TSAA agent appears to be useful as an interpositional physical barrier after laminectomy for the prevention or reduction of adhesions. Further experimental studies are needed to confirm its clinical value.
Acknowledgements
This work was supported by the research fund of Hanyang University (HY-2015-MC).
References
- 1.Akeson WH, Massie JB, Huang B, Giurea A, Sah R, Garfin SR, et al. Topical high-molecular-weight hyaluronan and a roofing barrier sheet equally inhibit postlaminectomy fibrosis. Spine J. 2005;5:180–190. doi: 10.1016/j.spinee.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Alkalay RN, Kim DH, Urry DW, Xu J, Parker TM, Glazer PA. Prevention of postlaminectomy epidural fibrosis using bioelastic materials. Spine (Phila Pa 1976) 2003;28:1659–1665. doi: 10.1097/01.BRS.0000083161.67605.40. [DOI] [PubMed] [Google Scholar]
- 3.Asch HL, Lewis PJ, Moreland DB, Egnatchik JG, Yu YJ, Clabeaux DE, et al. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm. J Neurosurg. 2002;96(1 Suppl):34–44. doi: 10.3171/spi.2002.96.1.0034. [DOI] [PubMed] [Google Scholar]
- 4.Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, et al. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine (Phila Pa 1976) 1996;21:1777–1186. doi: 10.1097/00007632-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, et al. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21:1787–1794. doi: 10.1097/00007632-199608010-00012. discussion 1794-1795. [DOI] [PubMed] [Google Scholar]
- 6.Bryant MS, Bremer AM, Nguyen TQ. Autogeneic fat transplants in the epidural space in routine lumbar spine surgery. Neurosurgery. 1983;13:367–370. doi: 10.1227/00006123-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Cauchoix J, Ficat C, Girard B. Repeat surgery after disc excision. Spine (Phila Pa 1976) 1978;3:256–259. doi: 10.1097/00007632-197809000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Choi MY, Auh SJ, Choi DG, Chang BL. Effect of ADCON-L on adjustable strabismus surgery in rabbits. Br J Ophthalmol. 2001;85:80–84. doi: 10.1136/bjo.85.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond MP, Wexner SD, diZereg GS, Korell M, Zmora O, Van Goor H, et al. Adhesion prevention and reduction : current status and future recommendations of a multinational interdisciplinary consensus conference. Surg Innov. 2010;17:183–188. doi: 10.1177/1553350610379869. [DOI] [PubMed] [Google Scholar]
- 10.DiFazio FA, Nichols JB, Pope MH, Frymoyer JW. The use of expanded polytetrafluoroethylene as an interpositional membrane after lumbar laminectomy. Spine (Phila Pa 1976) 1995;20:986–991. doi: 10.1097/00007632-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Einhaus SL, Robertson JT, Dohan FC, Jr, Wujek JR, Ahmad S. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L) Spine (Phila Pa 1976) 1997;22:1440–1446. doi: 10.1097/00007632-199707010-00003. discussion 1446-1447. [DOI] [PubMed] [Google Scholar]
- 12.Foulkes GD, Robinson JS., Jr Intraoperative dexamethasone irrigation in lumbar microdiskectomy. Clin Orthop Relat Res. 1990;(261):224–228. [PubMed] [Google Scholar]
- 13.Ganzer D, Giese K, Völker L, Pietzner U, Follak N, Merk H. Two-year results after lumbar microdiscectomy with and without prophylaxis of a peridural fibrosis using Adcon-L. Arch Orthop Trauma Surg. 2003;123:17–21. doi: 10.1007/s00402-002-0455-y. [DOI] [PubMed] [Google Scholar]
- 14.Gerszten PC, Moossy JJ, Bahri S, Kalend A, Martínez AJ. Inhibition of peridural fibrosis after laminectomy using low-dose external beam radiation in a rat model. Neurosurgery. 1999;44:597–602. doi: 10.1097/00006123-199903000-00090. discussion 602-603. [DOI] [PubMed] [Google Scholar]
- 15.Gill GG, Sakovich L, Thompson E. Pedicle fat grafts for the prevention of scar formation after laminectomy. An experimental study in dogs. Spine (Phila Pa 1976) 1979;4:176–186. doi: 10.1097/00007632-197903000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74:203–212. doi: 10.1016/s0015-0282(00)00656-7. [DOI] [PubMed] [Google Scholar]
- 17.Hinton JL, Jr, Warejcka DJ, Mei Y, McLendon RE, Laurencin C, Lucas PA, et al. Inhibition of epidural scar formation after lumbar laminectomy in the rat. Spine (Phila Pa 1976) 1995;20:564–570. doi: 10.1097/00007632-199503010-00011. discussion 579-580. [DOI] [PubMed] [Google Scholar]
- 18.Hong JH, Choe JW, Kwon GY, Cho DY, Sohn DS, Kim SW, et al. The effects of barrier materials on reduction of pericardial adhesion formation in rabbits : a comparative study of a hyaluronan-based solution and a temperature sensitive poloxamer solution/gel material. J Surg Res. 2011;166:206–213. doi: 10.1016/j.jss.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZL, Fletcher NM, Diamond MP, Abu-Soud HM, Saed GM. Hypoxia regulates iNOS expression in human normal peritoneal and adhesion fibroblasts through nuclear factor kappa B activation mechanism. Fertil Steril. 2009;91:616–621. doi: 10.1016/j.fertnstert.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jönsson B, Strömqvist B. Repeat decompression of lumbar nerve roots. A prospective two-year evaluation. J Bone Joint Surg Br. 1993;75:894–897. doi: 10.1302/0301-620X.75B6.8245078. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Haro H, Komori H, Shinomiya K. Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J. 2005;5:479–488. doi: 10.1016/j.spinee.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Key JA, Ford LT. Experimental intervertebral-disc lesions. J Bone Joint Surg Am. 1948;30A:621–630. [PubMed] [Google Scholar]
- 23.Kim DW, Chun HJ, Lee SK. Percutaneous needle puncture technique to create a rabbit model with traumatic degenerative disk disease. World Neurosurg. 2015;84:438–445. doi: 10.1016/j.wneu.2015.03.066. [DOI] [PubMed] [Google Scholar]
- 24.Kim SS, Michelsen CB. Revision surgery for failed back surgery syndrome. Spine (Phila Pa 1976) 1992;17:957–960. doi: 10.1097/00007632-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Kwon SW, Lim SH, Lee YW, Lee YG, Chu BY, Lee JH, et al. Anti-adhesive effect of poloxamer/alginate/CaCl2 mixture in the rat model. J Korean Surg Soc. 2006;71:280–287. [Google Scholar]
- 26.LaRocca H, Macnab I. The laminectomy membrane. Studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br. 1974;56B:545–550. [PubMed] [Google Scholar]
- 27.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions : etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Boutrand JP, Bittoun J, Tadie M. A collagen-based sealant to prevent in vivo reformation of epidural scar adhesions in an adult rat laminectomy model. J Neurosurg. 2002;97(1 Suppl):69–74. doi: 10.3171/spi.2002.97.1.0069. [DOI] [PubMed] [Google Scholar]
- 29.Massie JB, Schimizzi AL, Huang B, Kim CW, Garfin SR, Akeson WH. Topical high molecular weight hyaluronan reduces radicular pain post laminectomy in a rat model. Spine J. 2005;5:494–502. doi: 10.1016/j.spinee.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Nagakura T, Hirata H, Tsujii M, Sugimoto T, Miyamoto K, Horiuchi T, et al. Effect of viscous injectable pure alginate sol on cultured fibroblasts. Plast Reconstr Surg. 2005;116:831–838. doi: 10.1097/01.prs.0000176894.70848.98. [DOI] [PubMed] [Google Scholar]
- 31.Neel HB., 3rd Implants of Gore-Tex. Arch Otolaryngol. 1983;109:427–433. [PubMed] [Google Scholar]
- 32.North RB, Campbell JN, James CS, Conover-Walker MK, Wang H, Piantadosi S, et al. Failed back surgery syndrome : 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28:685–690. discussion 690-691. [PubMed] [Google Scholar]
- 33.Oh SH, Kim JK, Song KS, Noh SM, Ghil SH, Yuk SH, et al. Prevention of postsurgical tissue adhesion by anti-inflammatory drug-loaded pluronic mixtures with sol-gel transition behavior. J Biomed Mater Res A. 2005;72:306–316. doi: 10.1002/jbm.a.30239. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Jeong SH, Lee YJ, Choi SK, Hong SC, Jung EJ, et al. Current status of the use of antiadhesive agents for gastric cancer surgery : a questionnaire survey in South Korea. J Korean Surg Soc. 2013;84:160–167. doi: 10.4174/jkss.2013.84.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JS, Cha SJ, Kim BG, Choi YS, Kwon GY, Kang H, et al. An assessment of the effects of a hyaluronan-based solution on reduction of postsurgical adhesion formation in rats : a comparative study of hyaluronan-based solution and two film barriers. J Surg Res. 2011;168:49–55. doi: 10.1016/j.jss.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Park SO, Han J, Minn KW, Jin US. Prevention of capsular contracture with Guardix-SG (®) after silicone implant insertion. Aesthetic Plast Surg. 2013;37:543–548. doi: 10.1007/s00266-013-0087-3. [DOI] [PubMed] [Google Scholar]
- 37.Reigel DH, Bazmi B, Shih SR, Marquardt MD. A pilot investigation of poloxamer 407 for the prevention of leptomeningeal adhesions in the rabbit. Pediatr Neurosurg. 1993;19:250–255. doi: 10.1159/000120740. [DOI] [PubMed] [Google Scholar]
- 38.Ross JS, Robertson JT, Frederickson RC, Petrie JL, Obuchowski N, Modic MT, et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy : magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38:855–861. discussion 861-863. [PubMed] [Google Scholar]
- 39.Rout UK, Saed GM, Diamond MP. Expression pattern and regulation of genes differ between fibroblasts of adhesion and normal human peritoneum. Reprod Biol Endocrinol. 2005;3:1. doi: 10.1186/1477-7827-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saed GM, Diamond MP. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2003;79:164–168. doi: 10.1016/s0015-0282(02)04557-0. [DOI] [PubMed] [Google Scholar]
- 41.Seifer DB, Diamond MP, DeCherney AH. An appraisal of barrier agents in the reduction of adhesion formation following surgery. J Gynecol Surg. 1990;6:3–10. doi: 10.1089/gyn.1990.6.3. [DOI] [PubMed] [Google Scholar]
- 42.Shih HN, Fang JF, Chen JH, Yang CL, Chen YH, Sung TH, et al. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71:421–428. doi: 10.1002/jbm.b.30106. [DOI] [PubMed] [Google Scholar]
- 43.Shim HS, Lee YW, Lee YM, Oh YH, Kwon SW, Kim JH, et al. Evaluation of resorbable materials for preventing surgical adhesion on rat experiment. J Korean Surg Soc. 2002;63:179–186. [Google Scholar]
- 44.Songer MN, Ghosh L, Spencer DL. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine (Phila Pa 1976) 1990;15:550–554. doi: 10.1097/00007632-199006000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Songer MN, Rauschning W, Carson EW, Pandit SM. Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in dogs. Spine (Phila Pa 1976) 1995;20:571–580. doi: 10.1097/00007632-199503010-00012. discussion 579-580. [DOI] [PubMed] [Google Scholar]
- 46.Temel SG, Ozturk C, Temiz A, Ersozlu S, Aydinli U. A new material for prevention of epidural fibrosis after laminectomy : oxidized regenerated cellulose (interceed), an absorbable barrier. J Spinal Disord Tech. 2006;19:270–275. doi: 10.1097/01.bsd.0000203946.11546.d9. [DOI] [PubMed] [Google Scholar]
- 47.Ward BC, Panitch A. Abdominal adhesions : current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Welch WC, Thomas KA, Cornwall GB, Gerszten PC, Toth JM, Nemoto EM, et al. Use of polylactide resorbable film as an adhesion barrier. J Neurosurg. 2002;97(4 Suppl):413–422. doi: 10.3171/spi.2002.97.4.0413. [DOI] [PubMed] [Google Scholar]
- 49.Yong-Hing K, Reilly J, de Korompay V, Kirkaldy-Willis WH. Prevention of nerve root adhesions after laminectomy. Spine (Phila Pa 1976) 1980;5:59–64. doi: 10.1097/00007632-198001000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Young RL, Cota J, Zund G, Mason BA, Wheeler JM. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril. 1991;55:624–628. doi: 10.1016/s0015-0282(16)54197-1. [DOI] [PubMed] [Google Scholar]
- 51.Yu CH, Lee JH, Baek HR, Nam H. The effectiveness of poloxamer 407-based new anti-adhesive material in a laminectomy model in rats. Eur Spine J. 2012;21:971–979. doi: 10.1007/s00586-011-2098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zavala-Rodriguez JM, Correa Rovelo JM, Martinez-Morales N, Muñoz-Arce C, Bobadilla-Lugo RA, Kross RD, et al. Oxychlorine species suppress postsurgical adhesions in rats. J Surg Res. 2014;186:164–169. doi: 10.1016/j.jss.2013.07.043. [DOI] [PubMed] [Google Scholar]