Abstract
Intervertebral disc regeneration field is rapidly growing since disc disorders represent a major health problem in industrialized countries with very few possible treatments. Indeed, current available therapies are symptomatic, and surgical procedures consist in disc removal and spinal fusion, which is not immune to regardable concerns about possible comorbidities, cost-effectiveness, secondary risks and long-lasting outcomes. This review paper aims to share recent advances in stem cell therapy for the treatment of intervertebral disc degeneration. In literature the potential use of different adult stem cells for intervertebral disc regeneration has already been reported. Bone marrow mesenchymal stromal/stem cells, adipose tissue derived stem cells, synovial stem cells, muscle-derived stem cells, olfactory neural stem cells, induced pluripotent stem cells, hematopoietic stem cells, disc stem cells, and embryonic stem cells have been studied for this purpose either in vitro or in vivo. Moreover, several engineered carriers (e.g., hydrogels), characterized by full biocompatibility and prompt biodegradation, have been designed and combined with different stem cell types in order to optimize the local and controlled delivery of cellular substrates in situ. The paper overviews the literature discussing the current status of our knowledge of the different stem cells types used as a cell-based therapy for disc regeneration.
Keywords: Stem cells, Intervertebral disc degeneration, Spine, Tissue engineering, Cell therapy
Core tip: This review paper aims to share recent advances in stem cell therapy for the treatment of intervertebral disc degeneration. The paper overviews the literature discussing the current status of our knowledge of the different stem cells types used as a cell-based therapy for disc regeneration. Intervertebral disc regeneration field is rapidly growing since disc disorders represent a major health problem in industrialized countries with very few possible treatments. Indeed, current available therapies are symptomatic, and surgical procedures consist in disc removal and spinal fusion.
INTRODUCTION
Low back pain (LBP) is a common musculoskeletal symptom referred by more than 80% of the general population at least once in their life[1]. It results in a relevant social and economic problem, affecting above all the productive population in developed countries. In fact, it presents a maximum rate of incidence in people between the ages of 45 and 64[2] and it is the most frequent cause of disability and loss of days of activity in people under 45 years of age[3,4].
The wide majority of LBP related to degenerative changes of the intervertebral disc (IVD). The IVD is a complex structure consisting of three specialized tissues: The annulus fibrosus (AF), the nucleus pulposus (NP) and the cartilaginous end-plate (CEP) which coats the adjacent vertebral body.
The AF is a fibro-cartilaginous ring providing the outer part of IVD. It is composed of collagen type I fibers, oriented radially and in opposite directions throughout concentric lamellae[5], associated with an interlamellar matrix consisting of proteoglycans and non-collagenous proteins (such as elastin), in which mesenchymal cells, with a fibroblast-like morphology and phenotype[6], are present. This matrix leads to an efficient interlamellar cohesion[7].
The NP is a less structured gelatinous matrix rich in proteoglycans, mainly aggrecan, and type II collagen fibers randomly oriented. Aggrecan comprises a great number of negatively charged sulfated glycosaminoglycans that attract and imbibe water. The high level of hydration helps to maintain disc height and contributes to the load-bearing ability of the IVD[8]. Small chondrocyte-like cells are scattered within the NP and are responsible for synthesizing and maintaining the matrix[9].
The embryonic human IVD consists in two different structures: The NP, derived from aggregation of notochordal cells within a proteoglycan matrix, forming the gelatinous centre of the disc; the AF, which is derived from the perichordal mesenchyme, forming organized fibers surrounding the nucleus. During the sixth embryonic month, a mucoid degeneration of notochordal cells takes place in the NP and mesenchymal IVD cells invade the fibrocartilage. However, some notochordal remnants can be found in the IVD up to adulthood[10].
The IVD provides support for vertebrae, shock absorber function and allows movements of flex-extension, lateral bending and rotation. The NP, surrounded by the annulus fibers, resists compressive stress, whereas AF resists primarily tensile, circumferential, longitudinal and torsional stresses[10,11].
Intervertebral disc degeneration (IDD) is an age-related chronic process characterized by a progressive decline of proteoglycans and water content in NP with loss of the disc ability to resist compressive loads[12]. The first symptom of IDD is often LBP, that may lead to disc herniation, degenerative spondylolisthesis, instability and spinal stenosis associated with neurological symptoms such as radiculopathy and/or myelopathy. Current treatments for LBP and IDD range from conservative to surgical procedures[13]. However, these treatment modalities have limited efficacy and do not produce predictable and reliable outcomes. In fact, they target the clinical symptoms instead of the pathophysiology involved in the degenerative process. An effective early treatment for LBP that may prevent, slow down or reverse the degenerative changes of the IVD is the goal of many researchers in the spine field. Exciting advances in tissue engineering have led spine researchers to develop novel regenerative techniques in order to alter the course of IDD and possibly lead to disc repair and recovery of function.
PATHOPHYSIOLOGY OF IDD
Although the increasing interest in biological treatments for IDD, its pathological basis is still not completely understood. The degenerative pathway is related to aging, starting from the second decade of life[14], and to certain genetic profile expressions as well as environmental factors[15]. Moreover, the IVD is the largest avascular tissue in the body, in which nutrition takes place by diffusion through the CEP, maintaining the viability of NP cells[16].
The main structural changes in NP during IDD consist in a progressive reduction of proteoglycan content, first of all aggrecan[17]. Morphological modifications are related to metabolic imbalance between anabolic and catabolic processes, regulated by multiple factors, such as anabolic growth factors [e.g., insulin-like growth factor-1 (IGF1)[18], transforming growth factors β (TGFβ), bone morphogenetic proteins (BMPs)[19]] and catabolic enzymes [matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) resulting in changes in NP cells function[20].
Extracellular matrix changes are associated with alterations of disc cell viability. The progressive reduction of cell density results in the inability of the IVD to revert degenerative changes by producing and maintaining a functional extracellular matrix[21]. The decrease of NP proteoglycan content leads to progressive dehydration of gel-like nuclear matrix, decreasing disc height and altering its load-bearing capacity[22]. The inefficiency of NP to absorb compressive stresses and to transmit forces circumferentially to the AF, leads to deterioration of the lamellar architecture of the AF itself, consisting of internal fissures spreading outward to the periphery[23]. In addition to cracking and fissuring of the AF, disc herniation, subchondral sclerosis, CEP ossification and osteophyte formation[24] may take place. The inherent avascularity, isolation, and low metabolic activity of the IVD may explain its apparent inability for self-repair following injury and degeneration[25].
POTENTIAL BIOLOGICAL TREATMENTS
The therapeutic approach to treat or prevent disc degeneration could consist in recovering the disc ability to synthesize extracellular matrix, rich in proteoglycans, in order to re-establish disc hydration and NP visco-elastic features, thus restoring biomechanical properties of the IVD.
Several genes and growth factors have been found to influence the anabolic and catabolic processes, regulating the extracellular matrix homeostasis within the IVD. In this way recombinant growth factors or gene therapy technologies could be applied to treat the IDD[26,27]. Intradiscal injection of growth factors, such as BMP-7, BMP-2 or IGF-1, has been shown to increase the proteoglycan level within the disc[28-30].
The possibility to synthesize recombinant growth factors and to inject them with a percutaneous approach represents interesting advantages. However, the short half-life of exogenous growth factors has led to increasing interest for gene therapy in the treatment of IDD.
Gene therapy modifies the pattern of gene expression resulting in an in situ sustained production of specific gene products. In literature, numerous anabolic factors (such as TGF-β, BMP-2, BMP-7 or IGF-1), anti-catabolic factors (such as TIMP-1), and gene regulators (such as SOX-9 and LMP-1) have been found able to modulate the metabolic activity of disc cells, increasing proteoglycans disc content[31-34]. However, side effects related to this emerging technology, such as inflammatory reactions of nerve roots and/or dura, have been described[35]. More efficient and safe systems of transfection and transduction may be performed before clinical application of gene therapy[27,36].
According to pathological findings in IVD aging and IDD, characterized by a progressive disc cell loss, cell therapy has been proposed in order to restore the disc cell population by introducing exogenous cells. A cell therapy approach can be performed by using different types of differentiated cells, such as NP cells[37,38], AF cells[39], cartilaginous chondrocytes[40] and progenitor cells[41-43]. The autologous disc-derived chondrocyte transplantation (ADTC) is a treatment based on autologous NP cells to replace the tissue loss caused by disc herniation and disc surgery[44]. Although clinical data seems to report back pain improvement and prevention of disc height reduction after the treatment[45]. ADTC procedure presents the following limits: (1) it is only applicable when discectomy is required; (2) it is a two-steps procedure, because discectomy and cell transplantation are performed in two different times; and (3) disc cells lose their phenotypic characteristics when expanded in monolayer cell culture[46].
Therefore, stem cell therapy is more attractive due to low harvest site morbidity, ease of ex vivo cell expansion, and favorable modulation of the cell phenotype before or after transplantation. In this review we will discuss about the potential use of different types of stem cells employed for disc repair.
STEM CELLS BASED THERAPY
Stem cells are unspecialized cells characterized by a high proliferation rate. They can reside in a quiescent state, in which they self-renew; during the proliferation process, they perform an asymmetric division producing two daughter populations: One of them constituted by identical stem cells and the second ones formed by progenitor cells committed to a lineage-specific differentiation program[47]. Different types of human stem cells, ranging form embryonic to adult stem cells, have been found. Although embryonic stem (ES) cells are considered to be totipotent, legal and ethical controversies limit their use for clinical application in regenerative medicine[48]. Adult stem cells represent a reservoir of progenitor cells harbored within specialized niches of the adult organism, suggesting the potential for therapeutic application in their host tissues. Adult stem cells have been discovered and characterized in tissues such as bone marrow[49], adipose tissue[50], peiosteum[51,52], synovial membrane[53], muscle[54], skin[55], pericytes[56,57], blood[58] and trabecular bone[59,60]. Their function consists in maintenance of the anatomical and functional features of each specialized tissue. Because they are committed to a lineage-specific differentiation pathway, these cells are able to produce a limited range of specialized cells according to the embryonic origin of the tissue itself. The application of adult stem cells in regenerative medicine does not raise any ethical problems, as they can be directly isolated from the patient.
The potential application in IVD regeneration has been described for some types of adult stem cells, including bone marrow-derived mesenchymal stem cells (MSCs)[42,61,62], adipose tissue-derived stem cells (ASCs)[41], muscle-derived stem cells (MdSCs)[43], hematopoietic stem cells, olfactory membrane stem cells and synovial stem cells (Figure 1). Adult stem cell types are committed to differentiate following the lineage of mesenchymal tissues, including bone, cartilage, fat, and muscle[49,50,54,63]. Moreover, according to recent findings[64,65], MSCs, such as bone marrow MSCs, ASCs and MdSCs, seem to derive from the perivascular wall[66] of the tissue the stem cells are from.
Figure 1.
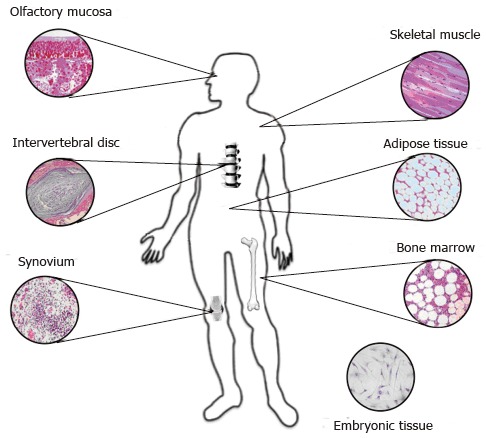
Major sources for harvesting stem cells used for disc regeneration.
Several therapeutic strategies for IVD regeneration based on stem cells have been described that direct injection into the IVD of undifferentiated or predifferentiated cells (cell therapy). Application of constructs derived by conjunction of stem cells with a visco-elastic hydrogel (tissue engineering strategy); genetic modification by transfection of target genes followed by injection of transfected stem cells into the IVD (ex vivo gene therapy)[27,67] (Figure 2).
Figure 2.
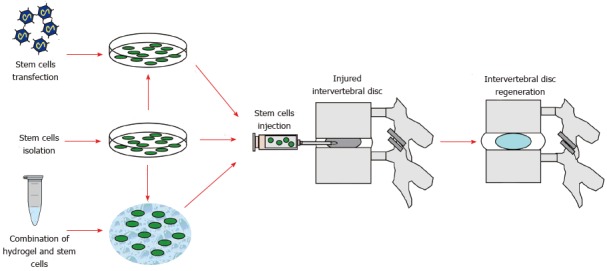
Flow chart summarizing the main steps of disc regeneration stem cell-based therapy. Stem cells can be either directly isolated and expanded, or combined with biocompatible carriers (e.g., hydrogels) or transfected with target genes, then injected into the nucleus pulposus of the injured intervertebral disc, potentially leading to disc regeneration.
Recently, a specific population of progenitor cells has been identified in the degenerated human IVD[68]. This finding has been confirmed by Blanco et al[69], who demonstrated that progenitor stem cells are quite similar to mesenchymal stromal cells derived from bone marrow. Therefore, an alternative approach for treatment consists in recruiting endogenous progenitors to orchestrate IVD repair by administration of suitable drugs/growth factors.
BONE MARROW MSCS
In the past few years several in vitro studies have been conducted to evaluate the use of MSCs for the treatment of IDD.
The actual capacity of adult human MSCs to differentiate towards NP cells has been one of the first steps in the evaluation of their utilization as a cell source for IVD regeneration. MSCs can differentiate into chondrocyte-like cells phenotypically similar to NP cells in chondrogenic conditions[68,70]. These methods have been considered a preconditioning system to direct MSCs into NP-like cells before they are implanted into the IVD.
The therapeutic effects of stem cells have been extensively studied in vitro. Several studies suggested that the regenerative potential of MSCs may result from MSCs and NP cells interactions that up-regulate extracellular matrix protein synthesis in terms of proteoglycans. Le Visage et al[71] cocultured bone marrow MSCs with NP cells at a 50:50 ratio in 3-dimensional pellet culture system for 2 wk showing that, although there was a trend of increase in glycosaminoglycan (GAG) production, the difference was not significant. On the contrary, Sobajima et al[62] using a similar pellet coculture system at a different ratio, revealed a synergistic effect between NP cells and MSCs at 75:25 and 50:50 NP:MSC ratio, yielding a significant increase of proteoglycan synthesis rate and GAG content compared with culture of NP cell and MSCs alone. Recently, Svanvik et al[72] confirmed that coculture of MSCs with degenerated NP cells increases proteoglycan and collagen-type II production.
In order to delineate the effects, several studies have utilized the coculture model systems to understand whether the interaction between MSCs and IVD cells leads to MSC differentiation to an NP-like phenotype and/or whether MSCs promote regeneration through stimulation of native NP cells. Stem cells undergo differentiation under stimuli that come from the surrounding microenvironment. However, adult stem cells contribute to tissue repair and regeneration by not only differentiating into the phenotypes of the host cells[49] but also creating a microenvironment that promotes the local regeneration of endogenous cells[73]. Richardson et al[74] cocultured human MSCs with normal NP cells in monolayer with or without cell-to-cell contact. After 1 wk of culture, fluorescent-labeled MSCs separated by fluorescence-activated cell sorting and gene expression was evaluated. MSCs underwent a change in gene expression profile similar to NP-like cells as demonstrated by an increased expression of aggrecan and collagen type II genes[74]. However, the low cell density in the NP[75] makes direct cell-to-cell contact between MSCs and NP cells a rare event if stem cells are implanted into the disc. Therefore, our group studied the mechanisms of the interaction between human NP cells from degenerating discs and MSCs in 3-dimensional culture, a system that allows short distance paracrine interactions typical of the NP tissue, hence miming its architecture[76]. Using a double labeling cell system, changes in gene expression profile were analyzed on the MSCs or NP cells populations isolated from the coculture. MSCs acquired a more chondrogenic gene expression profile and influenced mRNA levels within the human NP cells.
Paracrine stimulation in the interaction between MSCs and IVD cells was also assessed in other studies. Yang et al[77] used a noncontact coculture system to elucidate the interaction between NP cells and MSCs mediated by soluble factors. These authors showed that secreted factors in the coculture were able to induce collagen type II expression by uncommitted MSCs when cultured with a higher number of NP cells. Korecki et al[78] studied the effect of conditioned media from notochordal cell cultures on MSCs showing differentiation toward a potentially NP-like phenotype with some characteristics of the developing IVD.
MSCs secrete a variety of cytokines and growth factors that are able to stimulate mitosis and tissue-intrinsic reparative potential of the host cells[79]. Accordingly, Yamamoto et al[80] reported increased cell viability, proliferation and proteoglycan synthesis of rabbit NP cells induced by cell-to-cell contact with MSCs in a coculture monolayer system, supporting a trophic effect. Watanabe et al[81] further confirmed these data by using human cells. The trophic effect only partially reflected in our gene expression study. Although we observed an increase in collagen type II by NP cells after coculture with MSCs, aggrecan gene expression was down-regulated[76]. This data reflected only a modest trophic effect of MSCs on degenerate NP cells. This finding is in agreement with Strassburg et al[82] who investigated differences in the interaction between human MSCs and NP cells from both nondegenerate and degenerate discs during in vitro coculture with direct cell-cell contact. They concluded that, although both nondegenerate and degenerate NP cells are able to stimulate MSCs differentiation to an NP-like phenotype, MSCs were only able to stimulate degenerate NP cells to increase their matrix-associated gene expression to levels comparable to nondegenerate NP cells.
Cell fusion has been found to be potentially responsible for the plasticity and tissue regeneration potential of adult stem cells[83,84]. The possible occurrence of cell fusion in the interaction between male MSCs and female NP cells has been studied by our group[76]. Cell fusion was examined in a pellet coculture system to accentuate cell-to-cell interactions, a necessary condition for inducing cell fusion[83]. Fluorescence in situ hybridization assay for the X and Y chromosomes demonstrated that cell fusion does not occur in the interaction between MSCs and NP cells[76]. This result is in contrast with the data of Strassburg et al[82] who also studied cells fusion in the pellet coculture system. These authors were able to detect binucleated cells among cocultured cells during histologic observation, thus raising the possibility of cell fusion. Therefore, further studies needed to determine if the exposure of NP cells to MSCs leads to spontaneous cell fusion.
Organ culture systems were also used to study the potential effect of MSCs for IVD regeneration. Le Maitre et al[85] transplanted human MSCs in a bovine caudal disc and cultured them in vitro up to 4 wk. These authors showed that MSCs from older individuals differentiate spontaneously into chondrocyte-like NP cells upon insertion into NP tissue. Chen et al[86] studied the effect of porcine MSCs injected in an IVD degeneration model showing a regenerative potential. Therefore the ex vivo degenerative IVD organ culture system has the potential to contribute to a better comprehension of alternative IVD regeneration strategies.
MSCs engraftment and long term survival in the harsh environment of the normal disc tissue has been demonstrated by several in vivo study in small animals[62,87,88]. Crevensten et al[88] have shown that MSCs injected into rat discs with hyaluronan gel as a carrier, maintained viability over 28 d with cell proliferation. Zhang et al[87] have shown that allogeneic MSCs injected in a healthy disc increased the total proteoglycan content in the NP of rabbit discs. Sobajima et al[62] evaluated the long-term survival of allogeneic MSCs in healthy rabbit lumbar IVDs. The MSCs, retrovirally transduced with LacZ marker gene, were identified up to 6 mo after transplantation, observing MSCs migration from the NP to the inner AF.
However, in order to determine the efficacy of a stem cell therapy in preventing or delaying the progression of IDD, it is critical to rigorously test stem cell therapy in animal models of IDD. The first efficacy study published in the field is dated back to 2003: Sakai et al[89] elegantly showed that autologous bone marrow MSCs embedded in a collagen type II based carrier (Atelocollagen®) and injected in an NP aspiration model of IDD enhanced proteoglycan content and hydration. The same authors, using a similar study design, showed that the MSCs injected disc maintained disc height of 97% and magnetic resonance image (MRI) signal intensity of about 81% in a normal control group discs, while the degenerating disc group that did not received MSCs injection demonstrated a disc height value of about 67% and MRI signal intensity reduction of about 60%. Moreover, Sakai et al[90] demonstrated that undifferentiated MSCs transplanted into degenerated discs in rabbits proliferated and differentiated into cells expressing some of the major phenotypic characteristics of NP cells, suggesting that these MSCs may have undergone site-dependent differentiation.
Ho et al[91] studied the influence of the degenerative grade on the therapeutic potential of MSCs. These authors induced IDD in a rabbit model by stabbing and injecting MSCs at 1 or 7 mo. They observed that MSCs appear to be more effective in arresting degeneration at a relatively later stage of disc degeneration.
Jeong et al[92] used a xenogeneic transplantation model to study the effect of human MSCs injected into the coccygeal rat IVDs 2 wk after a blade stabbing. Disc height and MRI signal intensity of the MSCs transplanted disc increased compared to the degenerated control group at 2 wk after injection.
Yang et al[93] performed a study on a rabbit model of IDD induced by nucleus aspiration. These authors injected into the IVD a mixture of fibrin glue, TGF-β1 and rabbit MSCs using as a control the carrier alone with the growth factor or the sham. These authors have shown that MSCs injection led to a reduced height loss associated with IDD and an increased quantity of collagen type II content and a decrease in the rate of cell apoptosis.
Hee et al[94] performed a study on a rabbit model of IDD induced through a compression device. Allogeneic MSCs embedded in Atelocollagen were transplanted into the compressed disc followed by unload or distraction. Controls underwent just distraction or unload period. This animal study showed that the transplanted IVDs performed better with respect to disc height, morphological grading, histological scoring and average dead cell count and that distraction increased the regenerative effect of MSC transplantation.
Allon et al[95] explored the potential of the use of bilaminar coculture pellets (BCPs) of MSCs in a shell of NP cells for IVD regeneration.
The pellets were transplanted in vivo in a rat tail nucleotomy model of disc degeneration.
BCPs were transplanted in a fibrin sealant (FS) carrier using as a control the FS with a pellet of just MSCs or NP cells, MSCs and NP cells randomly mixed or the FS only; and surgery only. This study showed that the proteoglycan and cytokine levels were not significantly different among groups. The BCP group had higher cell retention, disc height and increased disc grade over time than controls[95].
Henriksson et al[96] performed a study using a xenotransplantation model in minipigs. IDD was induced in lumbar IVD by nucleoaspiration and 2 wk later human MSCs were injected in F12 media suspension (cell/med) or with a hydrogel carrier (cell/gel). The animals were sacrificed after 1, 3, or 6 mo. At MRI all injured discs demonstrated degenerative signs with fewer positive changes in the cell/gel group compared with cell/med discs and injured only discs. The authors concluded that transplanted human MSCs survived in the porcine spinal disc up to 6 mo and expressed SOX-9 and Collagen II, thus indicating differentiation. The hydrogel carrier has shown to facilitate the differentiation of transplanted hMSCs.
Recently, MSCs injection has tested in a larger animal model. Hiyama et al[97] evaluated the effect of MSCs transplantation on the suppression of IDD and preservation of immune privilege in a canine model of IDD. MSCs injection effectively led to the regeneration of degenerated discs and contributed to the maintenance of IVD immune privilege by the differentiation of transplanted MSCs into cells expressing FasL.
Serigano et al[98] used a canine IDD model to perform a dose-escalation study to assess the optimal number of cells to transplant into the degenerated IVD. Four weeks after nucleoaspiration, autologous MSCs transplanted at 105, 106, or 107 cells per disc. Unoperated and untransplanted disc were used as a control. MSCs-transplantation groups showed preservation of disc height and annular structure compared to the operated control group. The analysis of the survival rate of both transplanted and MSCs as well as NP cells demonstrated the better performance of 106 MSCs, when compared to 105 or 107, producing the best maintenance of the structure of IVDs and best inhibited IVD degeneration.
The goat study conducted by Zhang et al[99] performed using a disc degeneration model induced by stabbing the disc with a number 15 blade. One month after injury, allogeneic MSCs were injected with a hydrogel into the IVDs. Degenerating IVDs injected with MSCs showed significantly increased proteoglycan content within the disc. However, collagen content, MRI imaging, and histology did not show statistically significant differences between the cell-treated and control IVDs.
Subhan et al[100] transplanted allogeneic MSCs embedded in a hyaluronan based hydrogel (HyStem) into degenerate discs by fluoroscopy assisted minimally invasive delivery in a rabbit model. Animals were divided into three groups: Group I treated with MSCs coupled with Hystem, group II injected with Hystem alone and group III was left without any intervention. At 8 wk after transplantation, histological assay and MRI T2 mapping of NP showed higher T2 signal intensity, disc height index and type II collagen and aggrecan content in group I compared to other groups; similar results were reported by Cai et al[101] as well.
In a pilot study, Orozco et al[102] injected autologous expanded bone marrow MSCs into the nucleus pulposus of 10 patients diagnosed with lumbar disc degeneration. One year follow-up investigated evaluation of back pain, disability and quality of life, whereas disc height and fluid content were assessed through MRI. Patients reported prompt improvement of pain and disability at 3 mo and increased disc water content at 12 mo, even if disc height did not restore.
In a similar study, Yoshikawa et al[103] harvested autologous bone marrow MSCs from the ilium of two patients diagnosed with spinal stenosis: Cells cultured in an autogenous serum media and then transplanted percutaneously into the stenosed spinal canal within a collagen sponge graft. At 2 years after surgery, patients reported symptoms improvement, while X-ray, Rontgen kymography and CT showed decreased instability and T2-weighted magnetic resonance indicated high moisture contents.
Overall, MSCs show a great capacity to differentiate towards the NP phenotype, especially when exposed to the chondrogenic molecular signaling within the injured disc, thus potentially restoring its physiological microenvironment and biomechanical properties. MSCs can be readily harvested from multiple sites, even if the procedure itself is not immune to secondary risks. Moreover, MSCs are the only stem cell type that have been transplanted in human disc proving to be safe and being able to reduce LBP in patient affected by IDD.
ADIPOSE STEM CELLS
Adipose stem cells (ASCs) are an alternative source of stem cells, instead of bone marrow MSCs, to regenerate the IVD. These cells can easily expand under standard tissue culture conditions and show a pluripotent mesenchymal differentiation capacity. Their therapeutic effect has been tested using coculture system with other adult stem cell types[104].
Li et al[41] evaluated changes in the gene expression pattern of rabbit fat-derived mesenchymal cells when exposed to NP and AF cells in vitro. Authors demonstrated an increase in expression of type II collagen and aggrecan genes from rabbit ASCs cocultured in 3-dimensional alginate beads with NP cells, compared to ASCs cocultured with AF cells and NP cells alone. These data have been also confirmed by Lu et al[105], who investigated the ability of ASCs to differentiate when exposed to stimuli secreted by NP cells in vitro. Authors performed a transwell co-culture system of human NP cells and human ASCs, employing both monolayer and micromass configurations, in order to evaluate the sole effects of soluble signals. Lu et al[106] demonstrated that the transwell co-culture of NP cells and ASCs, both cultured under micromass conditions, induces gene expression of both aggrecan and collagen type II, with concomitant down-regulation of osteopontin, collagen type I and PPAR-c in ASCs. Moreover, Lu et al[106] also evaluated the gene pattern expression of human ASCs cultured in collagen type I or type II hydrogels alone, or cocultured in transwells with micromass human NP cells. They demonstrated that ASCs differentiation along the cartilaginous lineage is characterized by up-regulation of collagen type IIA, type IIB and aggrecan gene expression and it closely related to cocultures with NP cells and type II hydrogel. Collagen type II represents an appropriate scaffold for the attachment of ASCs and a favorable microenvironment in combination with soluble factors secreted by NP cells inducing the differentiation along cartilage/NP lineage.
Disc regeneration strategies based on adipose stem cells have also evaluated in small and large size animal models. Jeong et al[107] investigated the adipose-tissue-derived stromal cell (ADSC) implantation to restore disc in a rat degenerated IVD model. The IVD damaged by needle injection and, after two weeks, injected with ADSCs or saline (as control). At 6 wk after transplantation, authors demonstrated the ability of ADSCs to restore degenerated IVDs, according to reduced disc height loss and restoration of disc signal intensity on MRI. The histological analysis with hematoxylin and eosin staining confirmed a greater IVD restoration in discs transplanted with ADSCs. In addition, positive findings in immunohistochemical staining for collagen type II and aggrecan have also revealed.
Ganey et al[108] investigated ADSCs-based cell therapy in degenerated IVD using a dog model obtained by performing a partial nucleotomy on lumbar discs. Six weeks after surgery, authors randomized discs to receive: ADSCs loaded in hyaluronic acid carrier (cells/HA) or HA without cells or nothing. Dogs were killed at 6 mo or at 12 mo. Disc analysis has performed with MRI, radiography, histology and biochemistry. No significant differences between the three different approaches have found in MRI signal intensity and radiographic disc height. However, gene expression of type II collagen and aggrecan demonstrated a statistically significant increase of expression in discs transplanted with ADSCs when compared to discs receiving either the HA only or no treatments. ADSCs are able to provide a regenerative stimulation in the injured IVD. Moreover, the histological analysis showed an abundant extracellular matrix surrounding the cells and cell clustering or cloning within NP. AF fibers were tight and laminated according to the normal IVD morphology. Because of the evidence of injected ADSCs survival, the histology suggested their responsibility for the observed morphology resembling the healthy IVD. Ganey et al[108] reported that ADSCs were effective in promoting disc regeneration in an animal injured disc model.
Sun et al[109] assessed the influence of ADSCs on NP cells in a compressive load culture: Unphysiological mechanical stimulation was set in order to mimic the stressful conditions leading to IDD. ADSCs protected NP cells from apoptosis through caspase-9 and caspase-3 inhibition, increasing ECM gene expression while diminishing metalloproteinases synthesis inhibiting production of pro-inflammatory factors.
ADSCs have proven to give rise to a chondrogenic lineage and to increase aggrecan and type II collagen synthesis, hence favoring disc regeneration. While they can easily harvested without significant risks, ADSCs actual efficacy has not established yet.
SYNOVIAL MSCS
In the last years, synovial MSCs aroused an increasing interest about their application in cell therapy strategies for disc regeneration. They could be a potential source of stem cells because they present a proliferative rate greater than other types of MSCs, such as bone marrow MSCs[110]. In addition, they show a high chondrogenic potential, demonstrated by the ability to synthesize extracellular matrix after transplantation into articular cartilage defects in a rabbit model[111].
Miyamoto et al[112] assessed the effect of intradiscal transplantation of synovial MSCs by using an IVD degeneration rabbit model. After allogeneic synovial MSCs transplantation, researchers performed imaging analyses, including X-ray, MRI and histological analysis. Moreover, they performed an in vitro study in order to investigate the interaction between synovial MSCs and NP cells, by producing a co-culture system of human synovial MSCs and rat NP cells. The results showed that synovial MSCs injected into the disc were able to stimulate the remaining NP cells to synthesize type II collagen and to inhibit the expression of matrix degradative enzymes and inflammatory cytokines. These data were confirmed by in vivo findings, showing that IVD height in the MSCs group was higher than disc height in the degeneration group.
Synovial MSCs exhibit a notable proliferative and regenerative potential, which confirmed by pilot studies in articular and disc degenerative models. Further studies needed to support these findings, in order to plan an appropriate therapeutical protocol.
MDSCS
MdSCs have shown to reside within skeletal muscles and to be characterized by typical stem cells features, such as self-renewal a multilineage differentiation. Indeed, they are capable of giving rise to other mesodermal cell types, including hematopoietic, osteogenic, chondrogenic, adipogenic and skeletal myogenic cells[113].
As Adachi et al[114] reported appreciable healing of cartilage defects using muscle-derived cells embedded in collagen gels in a rabbit model, it has been hypothesized that the chondrogenic lineage commitment of MdSCs might therefore provide a prompt source for generating and expanding NP cells as well.
In this regard, our group investigated the role of MdSCs as a source of chondroprogenitor NP cells using an in vitro coculture system. NP cells were isolated from human IVD specimens and then cocultured with MdSCs harvested from the hind limb skeletal muscle of three mice. Proteoglycan synthesis and total GAG content were subsequently analyzed to assess eventual changes in extracellular matrix production, while DNA content measured as an index of cell proliferation. Each of these parameters was significantly increased in the coculture compared to NP cells monoculture, hence suggesting a promising role of MdSCs for disc regeneration[115].
MdSCs have been only recently discovered as a novel stem cell population residing within muscles: As mesodermal progenitors, they can differentiate into different cell types, including chondrocytes, thence showing a potential role in disc regeneration. In vivo studies are needed to evaluate the factual MdSCs regenerative potential for disc regeneration cell-based therapy.
OLFACTORY NEURAL STEM CELLS
Human olfactory neural stem cells are multipotent stem cells showing the ability to differentiate along both neural lineage, leading to neurons, astrocytes and oligodendrocytes formation[116], and non-neural lineages[117].
Murrell et al[118] investigated the differentiation of olfactory stem cells (OSCs) into NP chondrocyte-like cells both in in vitro and in vivo settings. The in vitro study has performed coculturing OSCs derived from rat olfactory mucosa with rat IVD biopsies. The in vivo study consisted in transplanting genetically engineered OSCs, which were able to express green fluorescent protein, into a rat model of injured IVD, without any pre-differentiation in vitro. Authors showed that olfactory mucosa-derived progenitor cells could induce to differentiate into NP chondrocyte-like cells, as demonstrated by cellular morphology at the microscopy and by expression of proteins suggestive of NP chondrocyte phenotype (collagen Type II - CT2 and aggrecan - CSPG) at the immunochemistry. These findings have been confirmed by both OSCs inducted in vitro with medium conditioned with NP environment and OSCs transplanted into injured rat NP.
OSCs can surprisingly differentiate into NP-like chondrocytes when exposed to the injured IVD environment and produce NP matrix constitutive elements. However, major concerns related to the invasive approach to harvest OSCs from the olfactory bulb can not be disregarded.
INDUCED PLURIPOTENT STEM CELLS
Induced pluripotent stem cells (iPSCs) are somatic cells which have been genetically reprogrammed in order to forcedly express such genes and factors that lead to an embryonic stem cell-like state. Mouse iPSCs were firstly reported in 2006: These cells have been proven to act like pluripotent cells, given that they express stem cells peculiar markers, generate tumours containing cells from all three germ layers and are able to differentiate into different cytotypes when injected in mouse embryos[119]. Human iPSCs, isolated in 2007 for the first time, seem to show similar properties[120,121].
Due to their pluripotency and patient-specificity, human iPSCs have been proposed as a source for generating notochordal cells in order to re-establish disc homeostasis.
Using a mouse model, Chen et al[122] isolated autologous embryonic fibroblasts which were then epigenetically reprogrammed into iPSCs through a polycystronic lentiviral vector. CD24+ iPSCs subpopulation was further detached using magnetic activated cell sorting and subsequently cultured in a laminin-rich media in order to drive NP-like cell differentiation and matrix synthesis which appreciably resembled native NP tissue. Culturing iPSCs in a hypoxic notochordal cell-conditioned medium (NCCM) led to similar outcomes.
Liu et al[115] harvested porcine NP tissue, which was pulverized and added to a culture plate loaded with human iPSCs, in order to induce notochordal cell-like differentiation, which was highlighted by the expression of three notochordal marker genes: Brachyury T, cytokeratin-8 and cytokeratin-18. Most notably, these cells showed the ability to generate NP-like tissue in vitro, which was characterized by NP phenotypic markers such as type II collagen, aggrecan and GAGs.
In spite of their capacity to induce chondrogenic differentiation, iPSCs might potentially lead to tumorigenesis due to their pluripotency. In addition, genetic engineering reprogrammation techniques are characterized by notable costs that might be unlikely borne.
HEMATOPOIETIC STEM CELLS
Adult bone marrow includes two different kinds of stem cell populations: Non-hematopoietic stem cells [non-hematopoietic stem cells (HSCs)], including MSCs, which do not express CD34 and HSCs which express CD34. Wei et al[123] evaluated xenogeneic transplantation of human bone marrow cells, both non-HSCs and HSCs, in a rat degenerated disc model in order to find out which population could be used to obtain disc-like cells. The human bone-marrow (CD34+ and CD34-) cells have been injected into rat coccygeal discs, after isolation and labeling with a fluorescent marker. Authors performed histological analysis, immunochemistry and survival rate analysis in all groups at different time points (at 1, 10, 21, and 42 d). They demonstrated that CD34- cells were able to survive in the NP of host discs until 42 d, whereas CD34+ cells detected only up to 21 d. Moreover, only CD34- cells presented a gene expression pattern similar to chondrocyte cells (positive for Collagen II and SOX-9).
Wei et al[123] registered data providing evidence that HSCs should not be used to treat IDD, because they are not able to differentiate in chondrocyte-like cells and restore degenerated NP.
The inefficacy of HSCs transplantation in the regenerative cell-based that strategies to treat disc degeneration was also demonstrated by Haufe et al[124] in a clinical study, in which autologous HSCs derived from pelvic bone marrow were injected into degenerated discs of patients affected by low back pain. This study presents an important methodological bias, because any evidence, both in vitro and in vivo, supporting HSCs transplantation in degenerated disc, has been reported in literature. In fact, authors concluded that HSCs transplantation do not produce any clinical improvement in treated patients[124].
DISC STEM CELLS
To date, several studies have successfully reported the isolation of disc stem cells from the IVD, namely cartilage end plate-derived stem cells (CESCs), annulus fibrosus-derived stem cells (AFSCs) and nucleus pulposus-derived stem cells (NPSCs), according to their localization within the disc.
These cells exhibit typical stem cell markers and are able to differentiate in vitro along the mesengenic pathway into various cytotypes belonging to osteogenic, chondrogenic and adipogenic lineages. In addition, disc stem cells notably resemble bone marrow MSCs immunophenotype, gene expression profile and self-renewal capacity[125,126]: It is thought that these cells are remnants of multipotent mesendodermal embryonic cells, while they might, in a smaller proportion, derive from adjacent vertebrae bone marrow[127].
A real stem cell niche was identified in the pericondrium and in the ligament side of the AF: Henriksson et al[128] proposed that cells from the niche are promptly recruited to NP and AF, along with MSCs from bone marrow, to undergo differentiation in case of tissue injury.
This assumption is further confirmed by the expression of migration and epithelial-mesenchymal transition markers (e.g., SNAIl, SLUG, ITGB1) within the niche itself[129].
However, lack of standardization in both characterization and isolation methodology makes disc stem cells inner potential difficult to be evaluated. Nonetheless, as reported by Sakai et al[130] disc progenitors number seems to decrease progressively with aging and degeneration, thus limiting the possibility to perform autologous re-implantation in older patients. The same study individuated hTIE-2 and GD2 as markers of disc stem cells committed to differentiate into NP progenitor cells. Quantification of these markers might thus correlate with the actual number of progenitor cells present in the disc, in order to assess the extent of disc degeneration.
Wang et al[131] compared human bone marrow MSCs, AFSCs, NPSCs and CECs regenerative properties in a rabbit model of IDD. The abovementioned cells harvested from patients undergoing posterior lumbar interbody fusion and isolated from iliac crest bone marrow and discectomy specimens, respectively. Stem cells were then cultured, expanded and seeded in alginate gel to subsequently injected into rabbit IVDs after NP aspiration. At 6 mo after implantation, animals sacrificed and discs analyzed; morphological evaluation demonstrated that CESCs yielded the highest regenerative potential, followed by NPSCs, BM-MSCs and AFSCs, that showed the lowest potency.
To date, eleven preclinical animal studies investigated outcomes subsequent to IVD tissue or cells transplantation, which demonstrated to delay disc degeneration and, in some models, to favor NP regeneration. However, some of the aforementioned studies reported an increased synthesis of type II collagen while proteoglycan production - and correspondingly disc hydration - was not restored to physiological rates[132].
Disc stem cells seem to reside within the inner disc niche, as remnants of mesendodermal embryonic stem cells. According to conflicting results, further studies needed to assess their actual usability for disc degeneration cell-based therapy and to establish standardized protocols regarding harvesting techniques, isolation and identification.
EMBRYONIC STEM CELLS
Embryonic stem cells (ESCs) represent another possible source of stem cells for disc regeneration, basing on their ability to differentiate along different cell lineages, including notochordal cells.
Notochordal cells are the first cells forming the NP during the embryogenesis of IVD. Moreover, it is also known that the adult NP host chondrocyte-like cells. According to their ability to differentiate along the chondrogenic lineage with opportune culture conditions[133], they could differentiate into cells able to produce extracellular matrix restoring the inner disc material. Sheikh et al[134] investigated the ESC-derived chondroprogenitors transplantation into a degenerated disc in a rabbit model. Researchers performed a pre-conditionating culture of murine ESCs in order to induce differentiation toward a chondrocyte lineage. In addition, ESC-derived cells have been labeled prior to implantation with a green fluorescent protein. After 8 wk from the implantation, H&E staining, confocal fluorescent microscopy and immunohistochemical analysis have performed on disc samples. Comparing with control non-punctured discs and control degenerate punctured discs, IVDs subdued to implantation of chondroprogenitor cells showed islands of notochordal cells at H&E histological analysis and immunofluorescence staining. These authors demonstrated the proliferation of notochordal-like cells, which are responsible of proteoglycan matrix production, into degenerated IVDs transplanted with ESCs.
However, ESCs show notable tumorigenic properties: They characterized by high telomerase activity (which leads to potentially infinite proliferation) and formation of teratoma. Nonetheless, ESCs handling is surrounded by several ethical issues due to their embryonic provenance, thus making improbable their use in IDD treatment[135].
TISSUE ENGINEERING APPROACHES
The choice of a suitable scaffold for stem cells remains an important issue in the development of this new therapy. Injectable viscoelastic scaffolds are more desirable for IVD tissue engineering to minimize the annular damage and to favor the implantation in a high-pressure structure. Sakai et al[42,89,90] compared cell viability after injection into rabbit NPs of a pure cell suspension compared to a soluble cell-augmented polymer such as fibrin glue that can polymerize in situ, providing the evidence that matrix-assisted cell transfer allows efficient augmentation of IVD. Besides fibrin glue, other scaffolds have been used in IVD tissue engineering such as collagen gels, hyaluronan gel[88], and genipin cross-linked chitosan[136]. The architectural and mechanical properties of the scaffold are also important. New micro- or nano-scale dimension scaffold as well as new signal release technologies may provide new perspective in IVD stem cell based tissue engineering.
Mercuri et al[137] explored the use of a chemical stabilized elastin-glycosaminoglican-collagen hydrogel as a scaffold capable of resembling NP resilient, mechanical and hydrophilic properties. This material proved to induce chondrogenic differentiation of human-derived adipose tissue stromal cells (hADSCs), resulting into increased aggrecan and type II collagen synthesis in vitro. In vivo evaluation performed by transplantation of the hydrogel into subdermal pockets of the dorsal mid-line of rats; at 4 wk after injection, the material showed full biocompatibility, cell infiltration and evident remodeling.
Tsaryk et al[138] investigated the use of a collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres in order to resemble NP main features, such as gel-like consistency, high hydration rate and appreciable biomechanical strength. Moreover, this fully biocompatible material has proved to easily inject inside the NP and to favor proliferation and chondrogenic differentiation of bone marrow MSCs and nasal chondrocytes, both in vitro and in vivo.
Liu et al[139] designed aligned fibrous polyurethane scaffolds using electrospinning to culture rabbit AFSCs in order to perform functional AF replacement. Random fibrous scaffolds were used as a control: Both showed comparable cell attachment and proliferation features, while AFSCs cultured on aligned scaffolds exhibited more natural morphology, higher gene expression activity and increased type I collagen and aggrecan synthesis.
Peroglio et al[140] designed a thermoversible hyaluronan-based hydrogel [hyaluronan-poly(N-isopropylacrylamide)] to induce human MSCs differentiation into the disc phenotype and to evaluate the effects of preconditioning. Cells conducted in the hydrogel or alginate for 1 wk under hypoxic conditions in a chondropermissive media alone or with TGF-β1 or GDF-5. Then, the cells suspended ex vivo in the gel and supplied to bovine IVDs. The HA-pNIPAM gel led to disc phenotype differentiation more promptly than alginate: Higher GAG/DNA ratio, type II collagen, SOX9 and other markers reported in vitro. In addition, preconditioning seemed to induce a lower degree of differentiation if compared to direct combination of the cells with the gel into the disc environment.
TOWARDS CLINICAL TRIALS
Though many issues remain unresolved[141-143], exciting progress certainly has been made toward the realization of a stem cell therapy as a potential therapeutic option for the treatment of IDD.
In a systematic review and meta-analysis, Wang et al[144] assessed the efficacy of cell therapy in IDD treatment in 22 animal controlled trials: Stem cells transplantation was significantly associated with increased disc height index, T2 weighted MRI signal intensity, type II collagen synthesis and diminished degeneration grade. Therefore, these promising results provide a solid basis for testing the effects of cell therapy on humans.
In order to move toward successful human clinical trials, it is critical to rigorously test the long-term effects of stem cell-mediated strategies in animal models of disc degeneration that closely simulate the human condition on disc biology, nutrition and biomechanical functions such as larger animal models or primates. In fact, animal models of IDD are all of relatively short duration, induced in young and previously healthy animal discs rich in notochordal cells, where the effects of the induced degeneration on disc nutrition are unknown[145-147].
The patients that could expect to benefit from stem cell-mediated therapy are those with mild or moderate grades of IDD, in whom the structural integrity of the disc remains preserved. Because nutrition supply to many degenerated discs is poor[25], there is theoretical concern over the added nutritional demands arising from the increased number of metabolic active cells into the disc after transplantation. Therefore, evaluation of the nutrition transport into the IVD, using microelectrodes able to evaluate oxygen or nitrous oxide diffusion[148,149], may be useful in order to select the patients that could benefit from the treatment (Table 1).
Table 1.
Summary of studies sorted by stem cells types and experimental setting
| Cell type | Ref. | Years |
In vitro study |
Pre-clinical study |
Clinical study | ||
| Cell culture | Organ culture | Small animal | Large animal | ||||
| Bone marrow MSC | Sakai et al[42] | 2003 | x | ||||
| Crevensten et al[88] | 2004 | x | |||||
| Yamamoto et al[80] | 2004 | x | |||||
| Risbud et al[61] | 2004 | x | |||||
| Zhang et al[87] | 2005 | x | |||||
| Steck et al[70] | 2009 | x | |||||
| Sakai et al[90] | 2005 | x | |||||
| Sakai et al[89] | 2006 | x | |||||
| Richardson et al[74] | 2006 | x | |||||
| Le Visage et al[71] | 2006 | x | |||||
| Ho et al[91] | 2008 | x | |||||
| Vadala et al[67] | 2008 | x | |||||
| Hiyama et al[97] | 2008 | x | |||||
| Yang et al[77] | 2008 | x | |||||
| Sobajima et al[62] | 2008 | x | x | ||||
| Jeong et al[92] | 2009 | x | |||||
| Henriksson et al[96] | 2009 | x | |||||
| Le Maitre et al[85] | 2009 | x | |||||
| Chen et al[86] | 2009 | x | |||||
| Wei et al[123] | 2009 | x | |||||
| Svanvik et al[72] | 2010 | x | |||||
| Watanabe et al[81] | 2010 | x | |||||
| Yoshikawa et al[103] | 2010 | x | |||||
| Hee et al[94] | 2010 | x | |||||
| Korecki et al[78] | 2010 | x | |||||
| Yang et al[93] | 2010 | x | |||||
| Strassburg et al[82] | 2010 | x | |||||
| Serigano et al[98] | 2010 | x | |||||
| Allon et al[95] | 2012 | x | |||||
| Zhang et al[99] | 2011 | x | |||||
| Di Martino et al[143] | 2012 | x | |||||
| Subhan et al[100] | 2014 | x | |||||
| Cai et al[101] | 2015 | x | |||||
| Tsaryk et al[138] | 2015 | x | x | ||||
| Orozco et al[102] | 2011 | x | |||||
| Vadalà et al[147] | 2015 | x | |||||
| Adipose tissue MSC | Lee et al[54] | 2000 | x | ||||
| Lu et al[105] | 2007 | x | |||||
| Lu et al[106] | 2008 | x | |||||
| Jeong et al[107] | 2010 | x | |||||
| Ganey et al[108] | 2009 | x | |||||
| Mercuri et al[137] | 2014 | x | x | ||||
| Muscle-derived SC | Vadalà et al[76] | 2008 | x | ||||
| Olfactory SC | Murrell et al[118] | 2009 | x | ||||
| iPSCs | Chen et al[122] | 2013 | x | x | |||
| Liu et al[115] | 2014 | x | x | ||||
| Synovial MSC | Miyamoto et al[112] | 2010 | x | ||||
| Hematopoietic SC | Haufe et al[124] | 2006 | x | ||||
| Disc stem cells | Liu et al[125] | 2011 | x | ||||
| Sakai et al[130] | 2012 | x | |||||
| Wang et al[131] | 2014 | x | x | ||||
| Liu et al[139] | 2015 | x | |||||
| Shi et al[126] | 2015 | x | |||||
| Embryonic SC | Sheikh et al[134] | 2009 | x | ||||
| Wharton’s Jelly SC | Liu et al[125] | 2011 | x | ||||
MSC: Mesenchymal stem cells; SC: Stem cells; iPSCs: Induced pluripotent stem cells.
CONCLUSION
Thanks to the recent research efforts aimed at further developing our knowledge of the biology and biochemistry of the IVD, our understanding of the process of IDD is rapidly growing. While there is still much to learn, some key factors involved in disc breakdown have become evident. Identification of the importance of cell loss within the disc has led to a focus on novel treatments aimed at regenerating the degenerating tissue. With its unique ability to differentiate into different cell types and to secrete a wide range of trophic cytokines, adult stem cell therapy has received considerable interest showing much promise with regard to treating chronic conditions such as IDD. Multiple studies have determined the feasibility of adult stem cell therapy for IDD, and recent studies have demonstrated proof of efficacy of autologous bone marrow MSCs transplantation in reproducible animal models as well as to be safe in human clinical trials. Other stem cells populations are still under evaluation with few proofs of efficacy in animals. Nonetheless, adult stem cell therapy has shown promise in becoming a powerful tool in the future treatment of IDD.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 4, 2015
First decision: November 30, 2015
Article in press: February 16, 2016
P- Reviewer: Deng WP, Gantenbein-Ritter B S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Lively MW. Sports medicine approach to low back pain. South Med J. 2002;95:642–646. [PubMed] [Google Scholar]
- 2.Weiner DK, Sakamoto S, Perera S, Breuer P. Chronic low back pain in older adults: prevalence, reliability, and validity of physical examination findings. J Am Geriatr Soc. 2006;54:11–20. doi: 10.1111/j.1532-5415.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 3.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976) 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- 4.Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine (Phila Pa 1976) 2006;31:3052–3060. doi: 10.1097/01.brs.0000249521.61813.aa. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 6.Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208:317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban JP, McMullin JF. Swelling pressure of the inervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22:145–157. doi: 10.3233/bir-1985-22205. [DOI] [PubMed] [Google Scholar]
- 9.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 10.Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 11.Best BA, Guilak F, Setton LA, Zhu W, Saed-Nejad F, Ratcliffe A, Weidenbaum M, Mow VC. Compressive mechanical properties of the human anulus fibrosus and their relationship to biochemical composition. Spine (Phila Pa 1976) 1994;19:212–221. doi: 10.1097/00007632-199401001-00017. [DOI] [PubMed] [Google Scholar]
- 12.Boni M, Denaro V. Anatomo-clinical correlations in cervical spondylosis. In: Kehr P, Weidner A, editors. Cervical spine i. New York: Springer-Verlag; 1987. pp. 3–20. [Google Scholar]
- 13.Di Martino A, Vaccaro AR, Lee JY, Denaro V, Lim MR. Nucleus pulposus replacement: basic science and indications for clinical use. Spine (Phila Pa 1976) 2005;30:S16–S22. doi: 10.1097/01.brs.0000174530.88585.32. [DOI] [PubMed] [Google Scholar]
- 14.Haefeli M, Kalberer F, Saegesser D, Nerlich AG, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 2006;31:1522–1531. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- 15.Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88 Suppl 2:3–9. doi: 10.2106/JBJS.E.01313. [DOI] [PubMed] [Google Scholar]
- 16.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila Pa 1976) 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Gruber HE, Norton HJ, Hanley EN. Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, Hahn SB, Lee HM. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine (Phila Pa 1976) 2003;28:2679–2684. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 20.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber HE, Hanley EN. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine (Phila Pa 1976) 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine (Phila Pa 1976) 1990;15:111–113. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1995;20:2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 24.Vernon-Roberts B. The biology of the intervertebral disc. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- 25.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 26.Masuda K, Oegema TR, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine (Phila Pa 1976) 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 27.Vadalà G, Sowa GA, Kang JD. Gene therapy for disc degeneration. Expert Opin Biol Ther. 2007;7:185–196. doi: 10.1517/14712598.7.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine (Phila Pa 1976) 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17:423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [DOI] [PubMed] [Google Scholar]
- 30.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976) 2005;30:25–31; discussion 31-32. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 31.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976) 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 32.Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976) 2003;28:755–763. [PMC free article] [PubMed] [Google Scholar]
- 33.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, Gilbertson LG, Kang JD. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine (Phila Pa 1976) 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 34.Yoon ST, Park JS, Kim KS, Li J, Attallah-Wasif ES, Hutton WC, Boden SD. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine (Phila Pa 1976) 2004;29:2603–2611. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- 35.Wallach CJ, Kim JS, Sobajima S, Lattermann C, Oxner WM, McFadden K, Robbins PD, Gilbertson LG, Kang JD. Safety assessment of intradiscal gene transfer: a pilot study. Spine J. 2006;6:107–112. doi: 10.1016/j.spinee.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Vadalà G, Sowa GA, Smith L, Hubert MG, Levicoff EA, Denaro V, Gilbertson LG, Kang JD. Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine (Phila Pa 1976) 2007;32:1381–1387. doi: 10.1097/BRS.0b013e3180601215. [DOI] [PubMed] [Google Scholar]
- 37.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976) 2003;28:2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine (Phila Pa 1976) 1998;23:1531–1538; discussion 1539. doi: 10.1097/00007632-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, Fujikawa K. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine (Phila Pa 1976) 2003;28:548–553. doi: 10.1097/01.BRS.0000049909.09102.60. [DOI] [PubMed] [Google Scholar]
- 40.Gorensek M, Jaksimović C, Kregar-Velikonja N, Gorensek M, Knezevic M, Jeras M, Pavlovcic V, Cör A. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9:363–373. [PubMed] [Google Scholar]
- 41.Li X, Lee JP, Balian G, Greg Anderson D. Modulation of chondrocytic properties of fat-derived mesenchymal cells in co-cultures with nucleus pulposus. Connect Tissue Res. 2005;46:75–82. doi: 10.1080/03008200590954104. [DOI] [PubMed] [Google Scholar]
- 42.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 43.Vadalà G, Sobajima S, Lee JY, Huard J, Denaro V, Kang JD, Gilbertson LG. In vitro interaction between muscle-derived stem cells and nucleus pulposus cells. Spine J. 2008;8:804–809. doi: 10.1016/j.spinee.2007.07.394. [DOI] [PubMed] [Google Scholar]
- 44.Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15 Suppl 3:S397–S405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Maldonado BA, Oegema TR. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 47.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function. Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 48.Wobus AM. Potential of embryonic stem cells. Mol Aspects Med. 2001;22:149–164. doi: 10.1016/s0098-2997(01)00006-1. [DOI] [PubMed] [Google Scholar]
- 49.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 50.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 51.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 52.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 53.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 54.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 56.Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992;(275):287–299. [PubMed] [Google Scholar]
- 57.Reilly TM, Seldes R, Luchetti W, Brighton CT. Similarities in the phenotypic expression of pericytes and bone cells. Clin Orthop Relat Res. 1998;(346):95–103. [PubMed] [Google Scholar]
- 58.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nöth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 60.Osyczka AM, Nöth U, Danielson KG, Tuan RS. Different osteochondral potential of clonal cell lines derived from adult human trabecular bone. Ann N Y Acad Sci. 2002;961:73–77. doi: 10.1111/j.1749-6632.2002.tb03054.x. [DOI] [PubMed] [Google Scholar]
- 61.Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, Shapiro IM. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976) 2004;29:2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 62.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, Cummins J, Fu FH, Huard J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 64.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 65.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Caplan AI. All MSCs are pericytes. Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Hubert MG, Vadala G, Sowa G, Studer RK, Kang JD. Gene therapy for the treatment of degenerative disk disease. J Am Acad Orthop Surg. 2008;16:312–319. doi: 10.5435/00124635-200806000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 69.Blanco JF, Graciani IF, Sanchez-Guijo FM, Muntión S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado MV, Cruz G, Gutierrez-Cosío S, Herrero C, San Miguel JF, Briñon JG, del Cañizo MC. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 70.Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 71.Le Visage C, Kim SW, Tateno K, Sieber AN, Kostuik JP, Leong KW. Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesis. Spine (Phila Pa 1976) 2006;31:2036–2042. doi: 10.1097/01.brs.0000231442.05245.87. [DOI] [PubMed] [Google Scholar]
- 72.Svanvik T, Henriksson HB, Karlsson C, Hagman M, Lindahl A, Brisby H. Human disk cells from degenerated disks and mesenchymal stem cells in co-culture result in increased matrix production. Cells Tissues Organs. 2010;191:2–11. doi: 10.1159/000223236. [DOI] [PubMed] [Google Scholar]
- 73.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, Hoyland JA. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 75.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- 76.Vadalà G, Studer RK, Sowa G, Spiezia F, Iucu C, Denaro V, Gilbertson LG, Kang JD. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine (Phila Pa 1976) 2008;33:870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 77.Yang SH, Wu CC, Shih TT, Sun YH, Lin FH. In vitro study on interaction between human nucleus pulposus cells and mesenchymal stem cells through paracrine stimulation. Spine (Phila Pa 1976) 2008;33:1951–1957. doi: 10.1097/BRS.0b013e31817e6974. [DOI] [PubMed] [Google Scholar]
- 78.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto Y, Mochida J, Sakai D, Nakai T, Nishimura K, Kawada H, Hotta T. Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: significance of direct cell-to-cell contact in coculture system. Spine (Phila Pa 1976) 2004;29:1508–1514. doi: 10.1097/01.brs.0000131416.90906.20. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe T, Sakai D, Yamamoto Y, Iwashina T, Serigano K, Tamura F, Mochida J. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28:623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 82.Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5:701–711. doi: 10.2217/rme.10.59. [DOI] [PubMed] [Google Scholar]
- 83.Chen EH, Olson EN. Unveiling the mechanisms of cell-cell fusion. Science. 2005;308:369–373. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- 84.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 85.Le Maitre CL, Baird P, Freemont AJ, Hoyland JA. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res Ther. 2009;11:R20. doi: 10.1186/ar2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, Hsiao SH, Wu CH, Chiu WT, Chen BJ, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30:5523–5533. doi: 10.1016/j.biomaterials.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 87.Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005;(430):219–226. doi: 10.1097/01.blo.0000146534.31120.cf. [DOI] [PubMed] [Google Scholar]
- 88.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32:430–434. doi: 10.1023/b:abme.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 89.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, Nakai T, Ando K, Hotta T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 90.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 91.Ho G, Leung VY, Cheung KM, Chan D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res. 2008;49:15–21. doi: 10.1080/03008200701818595. [DOI] [PubMed] [Google Scholar]
- 92.Jeong JH, Jin ES, Min JK, Jeon SR, Park CS, Kim HS, Choi KH. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59:55–64. doi: 10.1007/s10616-009-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, Tang T, Ebraheim NA. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10:802–810. doi: 10.1016/j.spinee.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 94.Hee HT, Ismail HD, Lim CT, Goh JC, Wong HK. Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc. J Bone Joint Surg Br. 2010;92:726–736. doi: 10.1302/0301-620X.92B5.23015. [DOI] [PubMed] [Google Scholar]
- 95.Allon AA, Butcher K, Schneider RA, Lotz JC. Structured bilaminar coculture outperforms stem cells and disc cells in a simulated degenerate disc environment. Spine (Phila Pa 1976) 2012;37:813–818. doi: 10.1097/BRS.0b013e31823b055f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henriksson HB, Svanvik T, Jonsson M, Hagman M, Horn M, Lindahl A, Brisby H. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009;34:141–148. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 97.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 98.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267–1275. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Drapeau S, Howard SA, Thonar EJ, Anderson DG. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Phila Pa 1976) 2011;36:372–377. doi: 10.1097/BRS.0b013e3181d10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Subhan RA, Puvanan K, Murali MR, Raghavendran HR, Shani S, Abdullah BJ, Abbas AA, Mohamed JA, Kamarul T. Fluoroscopy assisted minimally invasive transplantation of allogenic mesenchymal stromal cells embedded in HyStem reduces the progression of nucleus pulposus degeneration in the damaged ntervertebral [corrected] disc: a preliminary study in rabbits. Scientific World Journal. 2014;2014:818502. doi: 10.1155/2014/818502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cai F, Wu XT, Xie XH, Wang F, Hong X, Zhuang SY, Zhu L, Rui YF, Shi R. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits. Int Orthop. 2015;39:149–159. doi: 10.1007/s00264-014-2481-0. [DOI] [PubMed] [Google Scholar]
- 102.Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822–828. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 103.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976) 2010;35:E475–E480. doi: 10.1097/BRS.0b013e3181cd2cf4. [DOI] [PubMed] [Google Scholar]
- 104.Hoogendoorn RJ, Lu ZF, Kroeze RJ, Bank RA, Wuisman PI, Helder MN. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med. 2008;12:2205–2216. doi: 10.1111/j.1582-4934.2008.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu ZF, Zandieh Doulabi B, Wuisman PI, Bank RA, Helder MN. Differentiation of adipose stem cells by nucleus pulposus cells: configuration effect. Biochem Biophys Res Commun. 2007;359:991–996. doi: 10.1016/j.bbrc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Lu ZF, Doulabi BZ, Wuisman PI, Bank RA, Helder MN. Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med. 2008;12:2812–2822. doi: 10.1111/j.1582-4934.2008.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir (Wien) 2010;152:1771–1777. doi: 10.1007/s00701-010-0698-2. [DOI] [PubMed] [Google Scholar]
- 108.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976) 2009;34:2297–2304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 109.Sun Z, Luo B, Liu ZH, Samartzis D, Liu Z, Gao B, Huang L, Luo ZJ. Adipose-derived stromal cells protect intervertebral disc cells in compression: implications for stem cell regenerative disc therapy. Int J Biol Sci. 2015;11:133–143. doi: 10.7150/ijbs.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 111.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 112.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, Sekiya I. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642–647. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 114.Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, Niyibizi C, Huard J. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920–1930. [PubMed] [Google Scholar]
- 115.Liu Y, Rahaman MN, Bal BS. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS One. 2014;9:e100885. doi: 10.1371/journal.pone.0100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roisen FJ, Klueber KM, Lu CL, Hatcher LM, Dozier A, Shields CB, Maguire S. Adult human olfactory stem cells. Brain Res. 2001;890:11–22. doi: 10.1016/s0006-8993(00)03016-x. [DOI] [PubMed] [Google Scholar]
- 117.Murrell W, Féron F, Wetzig A, Cameron N, Splatt K, Bellette B, Bianco J, Perry C, Lee G, Mackay-Sim A. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- 118.Murrell W, Sanford E, Anderberg L, Cavanagh B, Mackay-Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009;9:585–594. doi: 10.1016/j.spinee.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 120.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 121.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 122.Chen J, Lee EJ, Jing L, Christoforou N, Leong KW, Setton LA. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One. 2013;8:e75548. doi: 10.1371/journal.pone.0075548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei A, Tao H, Chung SA, Brisby H, Ma DD, Diwan AD. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res. 2009;27:374–379. doi: 10.1002/jor.20567. [DOI] [PubMed] [Google Scholar]
- 124.Haufe SM, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15:136–137. doi: 10.1089/scd.2006.15.136. [DOI] [PubMed] [Google Scholar]
- 125.Liu LT, Huang B, Li CQ, Zhuang Y, Wang J, Zhou Y. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One. 2011;6:e26285. doi: 10.1371/journal.pone.0026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi R, Wang F, Hong X, Wang YT, Bao JP, Cai F, Wu XT. The presence of stem cells in potential stem cell niches of the intervertebral disc region: an in vitro study on rats. Eur Spine J. 2015;24:2411–2424. doi: 10.1007/s00586-015-4168-7. [DOI] [PubMed] [Google Scholar]
- 127.Wang F, Shi R, Cai F, Wang YT, Wu XT. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015;24:2479–2495. doi: 10.1089/scd.2015.0158. [DOI] [PubMed] [Google Scholar]
- 128.Henriksson H, Thornemo M, Karlsson C, Hägg O, Junevik K, Lindahl A, Brisby H. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 129.Henriksson HB, Svala E, Skioldebrand E, Lindahl A, Brisby H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine (Phila Pa 1976) 2012;37:722–732. doi: 10.1097/BRS.0b013e318231c2f7. [DOI] [PubMed] [Google Scholar]
- 130.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang H, Zhou Y, Huang B, Liu LT, Liu MH, Wang J, Li CQ, Zhang ZF, Chu TW, Xiong CJ. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A. 2014;20:908–920. doi: 10.1089/ten.TEA.2012.0703. [DOI] [PubMed] [Google Scholar]
- 132.Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–256. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- 133.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 134.Sheikh H, Zakharian K, De La Torre RP, Facek C, Vasquez A, Chaudhry GR, Svinarich D, Perez-Cruet MJ. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009;10:265–272. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 135.Oehme D, Goldschlager T, Rosenfeld JV, Ghosh P, Jenkin G. The role of stem cell therapies in degenerative lumbar spine disease: a review. Neurosurg Rev. 2015;38:429–445. doi: 10.1007/s10143-015-0621-7. [DOI] [PubMed] [Google Scholar]
- 136.Mwale F, Iordanova M, Demers CN, Steffen T, Roughley P, Antoniou J. Biological evaluation of chitosan salts cross-linked to genipin as a cell scaffold for disk tissue engineering. Tissue Eng. 2005;11:130–140. doi: 10.1089/ten.2005.11.130. [DOI] [PubMed] [Google Scholar]
- 137.Mercuri J, Addington C, Pascal R, Gill S, Simionescu D. Development and initial characterization of a chemically stabilized elastin-glycosaminoglycan-collagen composite shape-memory hydrogel for nucleus pulposus regeneration. J Biomed Mater Res A. 2014;102:4380–4393. doi: 10.1002/jbm.a.35104. [DOI] [PubMed] [Google Scholar]
- 138.Tsaryk R, Gloria A, Russo T, Anspach L, De Santis R, Ghanaati S, Unger RE, Ambrosio L, Kirkpatrick CJ. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015;20:10–21. doi: 10.1016/j.actbio.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 139.Liu C, Zhu C, Li J, Zhou P, Chen M, Yang H, Li B. The effect of the fibre orientation of electrospun scaffolds on the matrix production of rabbit annulus fibrosus-derived stem cells. Bone Res. 2015;3:15012. doi: 10.1038/boneres.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peroglio M, Eglin D, Benneker LM, Alini M, Grad S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013;13:1627–1639. doi: 10.1016/j.spinee.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 141.Vadalà G, De Strobel F, Bernardini M, Denaro L, D’Avella D, Denaro V. The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J. 2013;22 Suppl 6:S972–S978. doi: 10.1007/s00586-013-3007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vadalà G, Russo F, Di Martino A, Denaro V. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med. 2015;9:679–690. doi: 10.1002/term.1719. [DOI] [PubMed] [Google Scholar]
- 143.Di Martino A, Papapietro N, Lanotte A, Russo F, Vadalà G, Denaro V. Spondylodiscitis: standards of current treatment. Curr Med Res Opin. 2012;28:689–699. doi: 10.1185/03007995.2012.678939. [DOI] [PubMed] [Google Scholar]
- 144.Wang Z, Perez-Terzic CM, Smith J, Mauck WD, Shelerud RA, Maus TP, Yang TH, Murad MH, Gou S, Terry MJ, et al. Efficacy of intervertebral disc regeneration with stem cells - a systematic review and meta-analysis of animal controlled trials. Gene. 2015;564:1–8. doi: 10.1016/j.gene.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 145.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration. Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vadalà G, Russo F, Pattappa G, Schiuma D, Peroglio M, Benneker LM, Grad S, Alini M, Denaro V. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976) 2013;38:E319–E324. doi: 10.1097/BRS.0b013e318285bc4a. [DOI] [PubMed] [Google Scholar]
- 147.Vadalà G, Russo F, Pattappa G, Peroglio M, Stadelmann VA, Roughley P, Grad S, Alini M, Denaro V. A Nucleotomy Model with Intact Annulus Fibrosus to Test Intervertebral Disc Regeneration Strategies. Tissue Eng Part C Methods. 2015;21:1117–1124. doi: 10.1089/ten.TEC.2015.0086. [DOI] [PubMed] [Google Scholar]
- 148.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976) 1998;23:1–7; discussion 8. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 149.Urban MR, Fairbank JC, Etherington PJ, Loh FRCA L, Winlove CP, Urban JP. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine (Phila Pa 1976) 2001;26:984–990. doi: 10.1097/00007632-200104150-00028. [DOI] [PubMed] [Google Scholar]


