Abstract
There is an urgent need to develop reliable strategies for the rapid assembly of complex oligosaccharides. We demonstrate here that a set of strategically selected orthogonal protecting groups, glycosyl donors modified by a (S)-phenylthiomethylbenzyl ether at C-2 and a glycosyl acceptor containing a fluorous tag, make it possible to rapidly prepare complex branched oligosaccharides of biological importance. The C-2 auxiliary controlled the 1,2-cis anomeric selectivity of the various galactosylations. The orthogonal protecting groups, 2-naphthylmethyl ether (Nap) and levulinic ester (Lev), made it possible to generate glycosyl acceptors and allowed the installation of a crowded branching point. After the glycosylations, the chiral auxiliary could be removed using acidic conditions, which was compatible with the presence of the orthogonal protecting groups Lev and Nap, thereby allowing the efficient installation of 1,2-linked glycosides. The light fluorous tag made it possible to purify the compounds by a simple filtration method using silica gel modified by fluorocarbons. The set of building blocks was successfully employed for the preparation of the carbohydrate moiety of the GPI anchor of Trypanosoma brucei, which is a parasite causing sleeping sickness in humans and similar diseases in domestic animals.
Keywords: stereoselective glycosylations, fluorous tag, auxiliary, sulfonium ion
A set of strategically selected orthogonal protecting groups, glycosyl donors modified by a C-2 chiral auxiliary and a glycosyl acceptor containing a fluorous tag, made it possible to rapidly prepare the carbohydrate moiety of the GPI anchor of Trypanosoma brucei, which is a parasite causing sleeping sickness in humans and similar diseases in domestic animals.
Introduction
It is now well established that a dense layer of complex carbohydrates covers the surface of all prokaryotic and eukaryotic cells. These carbohydrates have been implicated in a wide range of biological processes such as protein folding, fertilization, embryogenesis, host-guest interactions, and cell differentiation and mobility.[1] In addition, overwhelming data supports the relevance of glycosylation in pathogen recognition, inflammation, innate immunity and the development of autoimmunity and cancer.[2] Although the importance of cell surface carbohydrates in health and disease is widely appreciated, advances in glycoscience have been slow due to the staggering complexity of the glycome.[3] This complexity makes it difficult to define glycan structures expressed by a given cell type and complicates the identification of specific glycan recognition determinants of glycan-binding proteins.[4] Libraries of well-defined glycans will make it possible to address these difficulties.
The need for diverse collections of complex glycans has stimulated the development of fast and convenient methods for their synthesis.[5] For example, several synthetic strategies make it possible to assembly complex oligosaccharides from carefully selected monosaccharide building blocks using a minimal number of chemical steps.[6] Among these strategies, one-pot multi-step glycosylations, in which several glycosyl donors are sequentially reacted in the same flask, are particularly attractive and can furnish target oligosaccharides without the need for protecting group manipulations and isolation and purification of synthetic intermediates.[6c] Within the past few years, automated solid-phase oligosaccharide synthesis has also substantially advanced.[7] A host of glycosylating agents, new linker systems, different solid supports and a variety of protecting groups have been carefully evaluated and these efforts have resulted in the first commercially available glycan synthesizer.
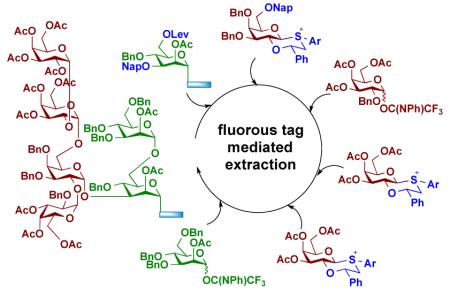
Soluble light fluorous tags offer another attractive means to simplify the process of oligosaccharide synthesis. In this case, tagged carbohydrates can easily be separated from nonfluorous-tagged side products by solid phase extraction using silica gel modified by fluorocarbons.[8] This generic procedure, which more closely resembles filtration than chromatography, depends primarily on the presence or absence of a fluorous tag and not on the polarity or other molecular features of the compound. Unlike solid phase supported synthesis, light fluorous technology does not require large excesses of reagents to drive the reactions to completion. Fluorous-tagged compounds can easily be analyzed by standard spectroscopic methods, thereby providing control over the synthesis. Furthermore, efforts are underway to develop a liquid handler to automate fluorous supported oligosaccharide synthesis.[9] Several fluorous versions of protecting groups have been developed for a variety of functional groups, and thus tags can easily be installed.[10] Additionally, it is possible to array fluorous-tagged glycans, thereby eliminating the necessity to install reactive functional groups for glycan immobilization.[11]
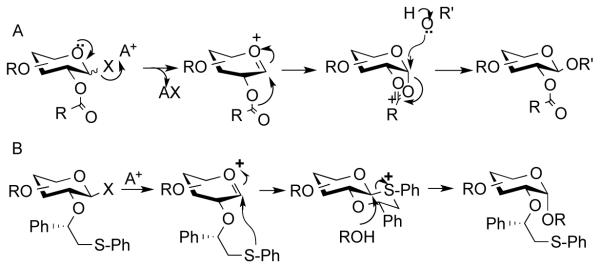
Despite the promise of fluorous supported oligosaccharide synthesis, it has mainly been employed for the preparation of relatively simple linear compounds.[10] This limited application is most likely due to the difficulties of controlling anomeric selectivities in glycosylations and challenges to install branching points in high yield.[5a, 6a, 11] In this respect, many complex oligosaccharides are branched and due to steric crowding, the corresponding glycosylations are often low yielding. Furthermore, 1,2-trans-glycosides, such as β-glucosides and β-galactosides, can reliably be introduced by neighboring group participation of an ester-protecting group at C-2 of a glycosyl donor (Scheme 1A). On the other hand, the installation of 1,2-cis glycosidic linkages, such as α-glucosides and α-galactosides, requires glycosyl donors that have a non-assisting functionality at C-2, and often these coupling reactions result in mixtures of anomers.[5a, 11] Low yielding glycosylations and the formation of anomers defeat the purpose of fluorous support synthesis that relies on simple filtration protocols for purification.
Scheme 1.
Control of anomeric selectivity in glycosylations. A) Neighboring group by C-2 esters to give a five membered ring oxocarbenium ion intermediate to form selectively 1,2-trans glycosides. B) Neighboring group participation by chiral auxiliary to give a trans-decalin anomeric sulfonium ion intermediate to provide 1,2-cis glycosides.
Recently, we introduced a stereoselective glycosylation approach based on neighboring group participation by a (S)-phenylthiomethylbenzyl moiety at C-2 of a glycosyl donor, which can readily provide 1,2-cis-glycosides (Scheme 1B).[12] Upon activation of the donor and formation of an oxacarbenium ion, the thiophenyl moiety of the C-2 auxiliary participates resulting in the formation of an intermediate sulfonium ion having a trans-decalin configuration. This stereoisomer is strongly favored because of the absence of unfavorable gauche interactions. Furthermore, the alternative cis-decalin system places the phenyl-substituent in an axial position thereby inducing unfavorable steric interactions. Displacement of the anomeric sulfonium ion by a sugar alcohol then results in the formation of a 1,2-cis-glycoside.
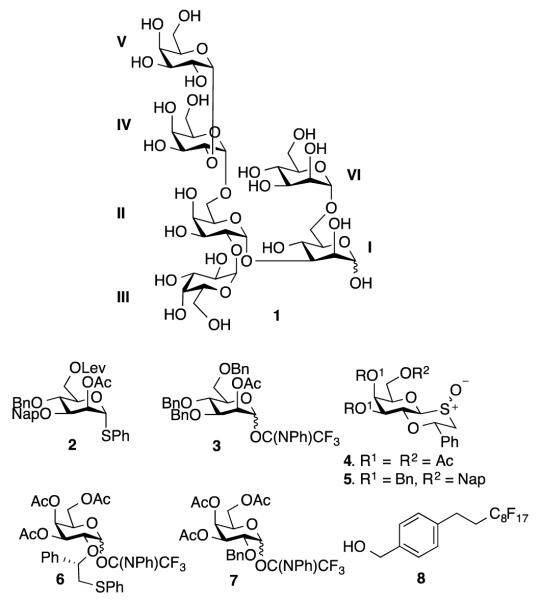
We describe here that the use of glycosyl donors modified by a C-2 (S)-phenylthiomethylbenzyl ether or ester-protecting group to stereoselectively introduce 1,2-cis or 1,2-trans glycosides, respectively and glycosyl acceptors modified by a fluorous tag can readily provide highly complex branched oligosaccharides of biological importance. The strategy was applied to the preparation of the carbohydrate moiety of the GPI anchor of Trypanosoma brucei (Figure 1), which is the parasite causing sleeping sickness in humans and similar diseases in domestic animals.[13] The oligosaccharide is composed of a branched tri-mannoside core, which is a structurally conserved motif of GPI anchors of many different organisms. It is further elongated by α-galactosides that are unique to T. brucei. It is expected that synthetic carbohydrates of different compositions will aid in the development of therapeutics and diagnostic for infections caused by this pathogen.[14] Previous attempts to prepare such oligosaccharides entailed low yielding galactosylations and provided anomeric mixtures.[15]
Figure 1.
The structure of hexasaccharide 1 of the GPI anchor of T. Brucei and monosaccharide building blocks for its assembly.
Results and discussion
The synthesis of building blocks
We envisaged that building blocks 2-7 and fluorous tag modified benzyl alcohol 8 (Figure 1) would make it possible to assemble target compound 1. Levulinic ester (Lev)[16] and 2-Naphthylmethyl ether (Nap)[17] were employed as a convenient set of orthogonal protecting groups for glycosyl acceptor formation and branching point installation. The donors 2 and 3, having participating esters at C-2, were used to install the mannosyl moieties. Furthermore, it was anticipated that galactosyl donors 4-6, having a chiral auxiliary at C-2, could be employed for the stereoselective introduction of the challenging α-galactosides.
First, attention was focused on the preparation of galactosyl donors 4-6 (Scheme 2). It was expected that activation of a trifluoro-N-phenyl imidate of 6 would result in the formation of an oxacarbenium ion which will undergo neighboring group participation by the (S)-(phenylthiomethyl)benzyl ether leading to a 1,2-trans anomeric sulfonium ion. Nucleophilic displacement of the anomeric sulfonium ion by a sugar alcohol will then provide an α-galactoside.[12a] Alternatively, arylation of the 1,2-oxathiane of compounds such as 4 and 5 will also provide anomeric sulfonium ions and such a transformation can easily be accomplished by activation the sulfoxide with triflic anhydride followed by reaction with 1,3,5-trimethoxybenzene.[18] An attractive feature of the 2-oxathianes is that they can be converted into compounds such as 7 by treatment with benzyne which leads to a derivative having a (S)-(phenylthiomethyl)benzyl ether at C-2 and an acetate at the anomeric center.[19] Standard procedures can then be employed to install an anomeric imidate for glycosylations.[20] Thus, it was anticipated that 2-oxathiane 11 would be an appropriate precursor for the synthesis of glycosyl donors 4-6.
Scheme 2.
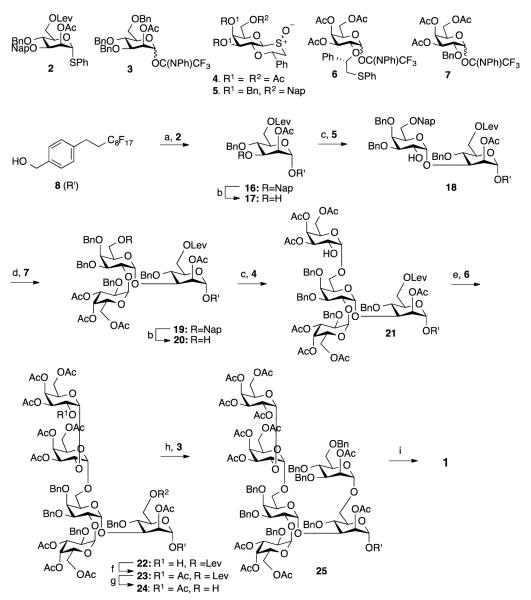
Preparation of building blocks for the GPI anchor carbohydrate moiety of T. brucei. Reagents and conditions: a) MeONa, MeOH, rt, 1 h, then p-TSA, MeOH, rt, 18 h; then acetic anhydride, pyridine, rt, 3 h, 73% (for 3 steps) then TiCl4, Et3SiH, DCM, 0 °C, 8 h, 83%; b) m-CPBA, DCM, −15 °C, 30 min, 96%; c) NaOMe, MeOH, rt, 1 h, then TBDPSCl, Imidazole, DMF, 0 °C, 2 h, 98%; d) BnBr, NaH, DMF, 0 °C, 1 h, 75%; e) HF-pyridine in pyridine, rt, 18 h, 61%; f) NaH, NapBr, DMF, 0 °C, 5 h, 95%; g) m-CPBA, DCM, −15 °C, 30 min, 72%; h) Pb(AcO)4, 1-aminobenzotriazole, DCM, −78 °C, 1 h, 95%; i)NH2NH2-AcOH, DMF, 50 °C, 4 h; then 2,2,2-trifluoro-N-phenyl-acetimidoyl chloride, DBU, DCM, rt, 1 h, 71%.
Thus, thioglycoside 9 was prepared by sequential treatment of per-O-acetyl-galactose with thiourea and 2-bromoacetophenone. The acetyl esters of 9 were cleavage with sodium methoxide in methanol and the resulting tetraol was treated with methanol in the presence of camphorsulfonic acid (CSA) to form a 1,2-oxathiane ketal. Due to the poor solubility of the latter compound, it was not purified and immediately treated with trimethylsilyl trifluoromethanesulfonate (TMSOTf) or BF3OEt2 in the presence Et3SiH to reduce the ketal to a 1,2-oxathiane ether. Although the latter reaction proceeded smoothly for glucose derivatives,[18a, 21] in the case of galactose no reaction occurred. Fortunately, the use of TiCl4 as the Lewis acid in the presence of Et3SiH gave, after O-acetylation with acetic anhydride in pyridine, the target compound 10 in a yield of 83%. Oxidation of compound 10 using meta-chloroperoxybenzoic acid (m-CPBA) in dichloromethane (DCM) at −15 °C gave the galactosyl donor 4. Compound 11 was readily prepared by treatment of 10 with 1-aminobenzotriazole and Pb(OAc)4 to generate benzyne for arylation of the 1,2-oxathiane. The latter compound was treated with hydrazine acetate to remove the anomeric acetate and the resulting lactol was converted into an N-phenyl trifluoroacetimidate (6) using 2,2,2,-trifluoro-N-phenylacetimidoyl chloride in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).[22]
The selectively protected galactosyl donor 5 was synthesized by removal of the acetyl esters of 10 followed by selective silylation of the primary hydroxyl using tert-butyl(chloro)diphenylsilane (TBDPSCl) in the presence of imidazole in DMF to give 12. The latter compound was benzylated under standard conditions (→13) followed by removal of the TBDPS ether using HF-pyridine to give 14, which was converted into Nap ether 15 by alkylation with NapBr in the presence of sodium hydride in dimethylformamide (DMF). Prior to glycosylation, the 1,2-oxathiane 15 was oxidized to the corresponding sulfoxide 5 using m-CPBA. The mannosyl donors 2 and 3 were prepared by standard protecting group manipulations as detailed in the supporting information.
Assembly of the carbohydrate moiety of the GPI anchor of T. brucei
First, target compound 1 was prepared by a conventional purification protocol using silica gel or size exclusion column chromatography (Scheme 3). In this case, each intermediate was carefully characterized by two-dimensional NMR spectroscopy and mass spectrometry. After establishing an appropriate synthetic protocol, the target compound was resynthesized in a rapid manner by employing fluorous solid phase extraction and in this case only the fully assembled oligosaccharide was characterized. The attraction of this approach is that a streamlined synthetic protocol for 1 can easily be adapted for the preparation of many analogs.
Scheme 3.
The assembly of the GPI anchor moiety of T. brucei. Reagents and conditions: a) NIS, TfOH, DCM, −25 °C, 30 min, 89%; b) DDQ, DCM: H2O = 10: 1, rt, 2 h, 17: 82%; 20: 77%; c) Tf2O, TMB, DTBMP −40 °C to rt then 10% TFA in DCM, rt, 1 h; (18: 87%, α only; 21: 67%, α-only); d) TfOH, DCM, −25 °C to rt, 3 h, 71%, α:β > 20:1; e) TfOH, DTBMP, DCM, −60 °C to rt, 18 h, then 10% TFA in DCM, rt, 1 h, 76%, α-only; f) Ac2O, pyridine, DMAP, rt, 4 h; g) NH2NH2-AcOH, pyridine, rt, 1 h; h) TMSOTf, DCM, −25 °C to rt, 1 h, 51% over three steps; i) H2, Pd/C, AcOH, MeOH, rt, 24 h, then MeONa, MeOH, rt, 1 h, 65%.
Thus, glycosyl donor 2 was coupled with 4-(1H, 1H, 2H, 2H-perfluorodecyl)benzyl alcohol (8) using N-Iodosuccinimide (NIS) and triflic acid (TfOH) as the activator[23] at −25 °C to give, after a reaction time of 30 min, fluorous tagged mannoside 16 in high yield. As expected, only the α-anomer was formed due to neighboring group participation of the acetyl ester of 2. Next, the Nap ether of 16 was removed by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in the mixture of DCM and water to give glycosyl acceptor 17, which was coupled with glycosyl donor 5 to provide, after acid mediated removal of the C-2 auxiliary, disaccharide 18. In this glycosylation, 5 was arylated by treatment with a stoichiometric amount of triflic anhydride (Tf2O) and 1,3,5-trimethoxybenzene (TMB) in the presence of 2,6-di-tert-butyl-4-methylpyridine (DTBMP) in DCM at −40 °C to form a sulfonium ion intermediate. Next, glycosyl acceptor 17 was added and the reaction mixture was allowed to warm to room temperature and after a reaction time of 11 h and purification by silica gel column chromatography, a glycoside product was obtained having a (trimethoxyphenylthiomethyl)benzyl ether moiety at C-2. The latter functionality was cleaved by treatment with 10% trifluoroacetic acid (TFA) in DCM to give glycosyl acceptor 18. Careful analysis by 1H NMR spectroscopy confirmed that only the expected α-anomer had formed.
The installation of the α(1,2)-linked galactoside of 1 proved challenging. Preactivation of 4 followed by the addition of acceptor 18 did not lead to glycoside formation. A TMSOTf mediated coupling of 6 with 18 gave only a trace amount of product as shown by MALDI-TOF mass spectrometry. The use of 5 equivalents of 6 provided the corresponding trisaccharide in a disappointing yield of 25%. We reasoned that the failures of these glycosylations was due to the rather low reactivity of C-2 hydroxyl of 18 and the bulky nature of the C-2 auxiliary of glycosyl donors 4 and 6.[24] Therefore, a smaller and more reactive glycosyl donor was required for this glycosylation. Indeed, a triflic acid mediated coupling of 7 with 18 led to the formation of trisaccharide 19 in an isolated yield of 71% and fortunately only a trace amount of the unwanted β-anomer was detected. Removal of Nap ether of 19 to give glycosyl acceptor 20 was accomplished by oxidation with DDQ in a mixture of DCM and water. In this reaction, care had to be taken to avoid oxidative removal of one of the benzyl ethers and in particular the use of only a small excess of recrystallized DDQ was critical to avoid overoxidation.[25] α-Galactosylation of 20 was easily accomplished by preactivation of 4 using Tf2O and TMB in the presence of DTBMP in DCM at −40 °C followed by the addition of glycosyl acceptor 20. The remnant of the auxiliary of the resulting tetrasaccharide was cleaved by treatment with 10% trifluoroacetic acid (TFA) in DCM to give glycosyl acceptor 21 in an overall yield of 67% as only the α-anomer. Surprisingly, a glycosylation of 21 with 4 gave a pentasaccharide a disappointing yield of 20%. Fortunately, a TMSOTf mediated glycosylation of 21 with 6 in DCM gave, after cleavage of the auxiliary, pentasaccharide 22 in an overall yield of 76% as only the α-anomer. The HSQC data of 22 showed that all H1-C1 coupling constants were in the range of 171 to 176 confirming the α-configurations of the glycosidic linkages.
The hydroxyl of 22 was acetylation and the Lev ester of the resulting compound (23) was removed using hydrazine acetate to give glycosyl acceptor 24, which was coupled with mannosyl donor 3 using TMSOTf as the catalyst to provide hexasaccharide 24 in an excellent overall yield of 51% (three steps). In this case, only the α-anomeric product was formed due to neighboring group participation of the acetyl ester at C-2 of the glycosyl donor. The overall yield of the assembly of the hexasaccharide, starting from the monomeric building blocks, was 9%. Finally, hexasaccharide 24 was converted into target compound 1 by hydrogenation over Pd/C followed by removal of the acetyl esters using sodium methoxide in methanol.
Fluorous assisted target glycan assembly
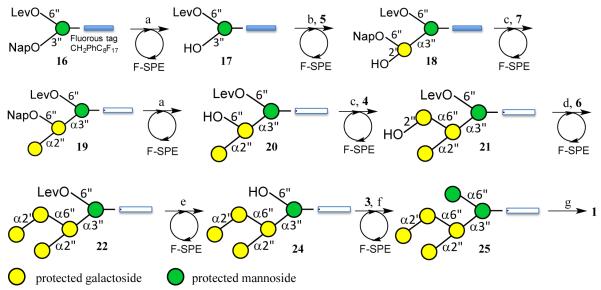
Having established a robust synthetic approach for the preparation of 1, the synthesis of this compound was performed using a purification protocol based fluorous solid phase extraction (Scheme 4). In this case, each glycosylation was performed twice to ensure completion of these critical reactions. Thus, the Nap ether of 16 was oxidatively removed with DDQ and the resulting acceptor 17 was isolated by fluorous solid phase extraction (F-SPE) using 20% water in methanol as the eluant to remove untagged compounds and the desired compound was isolated by elution with acetone. Next, acceptor 17 was coupled with 5 using the standard preactivation protocol and, as expected, aqueous workup and solid phase extraction resulted in the removal of hydrolyzed donor and other non-fluorous by-products. The glycosylation was repeated and the remnant of the auxiliary was removed using 10% TFA in DCM to give, after standard fluorous solid phase extraction, disaccharide 18. The latter compound was coupled twice with donor 4 using triflic acid as the promoter to provide trisaccharide 19, which was subjected to DDQ oxidation to remove the NAP ether to provide acceptor 20. Next, the α(1-6)-galactoside was installed by preactivation of 4 using Tf2O, TMB and DTBMP followed by glycosylation with 20 and, after repeating the coupling protocol, the remnant of the auxiliary was removed by treatment with 10% TFA in DCM to give tetrasaccharide acceptor 21. This compound was coupled twice with donor 6 using a standard preactivation protocol to give, after removal of the C-2 auxiliary and passing the material through a F-SPE cartridge, pentasaccharide 22. The hydroxyl of 22 was acetylated and the resulting compound was treated with hydrazine acetate to remove the Lev ester to give an acceptor which was subjected to a double coupling with mannosyl donor 2. After each step, the product was isolated by solid phase extraction and immediately used in the next reaction step. Homogeneous hexasaccharide 25 was obtained after purification by silica gel and LH-20 size exclusion column chromatography. This compound was obtained in an overall yield of 16.7%, which corresponds to an 85% yield per reaction step. The assembly of the hexasaccharide could be completed within 6 days. Standard deprotection of 25 gave target compound 1, the analytical data of which were identical to the compound prepared by the conventional approach described above.
Scheme 4.
The assembly of the GPI anchor moiety of T. brucei by fluorous solid phase extraction. Reagents and conditions: a) DDQ, DCM:H2O = 10:1, 2 h, b) Tf2O, TMB, DTBMP −40 °C to rt then 10% TFA in DCM, 1 h; c) TfOH, DCM, −25 °C to rt, 3 h; d) TfOH, DTBMP, DCM, −60 °C to rt, 18 h, then 10% TFA in DCM, 1 h; e) Ac2O, pyridine, DMAP, 4 h, then NH2NH2-AcOH, pyridine, 1 h; f) TMSOTf, DCM, −25 °C to rt, 1 h; g) H2, Pd/C, AcOH, MeOH, 24 h, then NaOMe, MeOH, 1 h.
After establishing a protocol for the efficient fluorous supported synthesis of 1, it could easily be adapted to the preparation of structurally related compounds and for example, a pentasaccharide was assembled by appropriate protecting group manipulations and sequential coupling of 2 with 8 to give a product that was further extended with 5, 4, 4 and 3, respectively. The preparation of this compound was completed within 5 days.
Conclusion
We demonstrate here that a set of strategically selected orthogonal protecting groups, glycosyl donors modified by a chiral auxiliary and glycosyl acceptors containing a fluorous tag, make it possible to rapidly prepare complex branched oligosaccharides of biological importance. After the glycosylations, the chiral auxiliary could be removed using moderately strong acidic conditions, which were compatible with the presence of the orthogonal protecting groups Lev and Nap, thereby allowing efficient installation of 1,2-cis-linked glycosides. Previously, the auxiliary mediated methodology was employed for the installation of α-glucosides,[12a, 12c, 18] and it is shown here that it can easily be extended to other monosaccharides such as galactosides. An exploratory study was required to identify potential synthetic problems. For example, due to the bulky nature of the auxiliary, a glycosylation of a sterically hindered acceptor site was challenging and in this case, a conventional donor had to be used. The attraction of the fluorous supported methodology is that after establishing a successful synthetic approach, target compounds can rapidly be resynthesized by routine procedures. Also, it allows for fast preparation of structural analogs and for example the approach for fluorous supported synthesis of 1 could easily be adapted to the preparation of structurally related compounds. Efforts are underway to develop a liquid handling system to automate fluorous supported synthesis,[9] which will make it possible to further speedup the process of oligosaccharide assembly.
Experimental Section
General procedure for the preparation of sulfoxide donors 4 and 5 from their corresponding oxathianes 10 and 15
m-CPBA (≤ 77%, 1.05 eq) was dissolved in DCM and the resulting solution was slowly added to a cooled (−78 °C) solution of oxathiane in DCM. The reaction mixture was stirred at −78 °C for 30 min, diluted with DCM (20 mL) and then poured into 10% Na2S2O3 aqueous solution. The organic layer was washed with aq. saturated NaHCO3, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography.
General glycosylation procedure for oxathiane donors with various acceptors
Oxathiane donor (1.2 eq), 1,3,5 trimethoxybenzene (2.5 eq) and 2,6-di-tert-butyl-4-methylpyridine (3.0 eq) were dissolved in DCM. Molecular sieves (4 Å) were added and the resulting suspension was cooled to −15 °C. Trifluoromethanesulfonic anhydride (1.2 eq) was added dropwise to the solution and stirring was continued for 10 min. The reaction mixture was further cooled to −40 °C and a solution of acceptor (1.0 eq) in DCM, which was dried over molecular sieves (4 Å) was added dropwise. After a reaction time of 30 min, the reaction was quenched with aq. saturated NaHCO3 (30 mL). The organic phase was washed with brine (30 mL), dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography or Sephadex LH-20 size exclusion chromatography (DCM/MeOH = 1:1, 0.2 mL/min).
General procedure for the removal of a C-2 auxiliary
Trifluoroacetic acid was added dropwise to a solution of the glycosylation product in DCM at 0 °C adjusting the final concentration to 10% (v/v). The reaction mixture was stirred for 3 h until TLC indicated complete consumption of starting material. The reaction mixture was diluted with DCM and poured into saturated NaHCO3. The organic layer was dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography or sephadex LH-20 size exclusion chromatography (DCM/MeOH = 1:1, 0.2 mL/min).
General fluorous supported purification protocol
F-SPE cartridges (FluoroFlash® SPE Cartridges, 10 grams, 20 cc tube) were purchased from Fluorous Technologies. Inc. The fluorous tagged compound with (200 mg compound per 1 g resin) was loaded using a minimum amount of mixture of water and DMF (9:1, v:v). The order of elution was 20% water and methanol (3×20 mL), hexane (3×20 mL). The desired fluorous-tagged compound was obtained by elution with acetone (3×20 mL). The formation of the desired compound was determined by TCL and MALDI-TOF. The product containing fractions were concentrated in vacuo.
Supplementary Material
Acknowledgement
The research was supported by the National Institute of General Medical Sciences (NIGMS) of the U.S. National Institutes of Health (R01GM065248, G.-J.B.).
Footnotes
Supporting information for this article is available. Provided are full experimental procedures, characterizations of compounds and copies of 1H and 13C NMR spectra.
References
- [1]a).Cosgrove DJ. Nat. Rev. Mol. Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]; b) Huskens J. Curr. Opin. Chem. Biol. 2006;10:537–543. doi: 10.1016/j.cbpa.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [2]a).Dube DH, Bertozzi CR. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]; b) Ohtsubo K, Marth JD. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]; c) Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Science. 2014;343:1235681. doi: 10.1126/science.1235681. [DOI] [PubMed] [Google Scholar]; d) Hudak JE, Bertozzi CR. Chem. Biol. 2014;21:16–37. doi: 10.1016/j.chembiol.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cummings RD. Mol. BioSyst. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- [4]a).Hart GW, Copeland RJ. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cummings RD, Pierce JM. Chem. Biol. 2014;21:1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]a).Boltje TJ, Buskas T, Boons GJ. Nat. Chem. 2009;1:611–622. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yasomanee JP, Demchenko AV. Trends Glycosci. Glyc. 2013;25:13–42. [Google Scholar]
- [6]a).Codee JDC, Litjens R, van den Bos LJ, Overkleeft HS, van der Marel GA. Chem. Soc. Rev. 2005;34:769–782. doi: 10.1039/b417138c. [DOI] [PubMed] [Google Scholar]; b) Kaeothip S, Demchenko AV. Carbohydr. Res. 2011;346:1371–1388. doi: 10.1016/j.carres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yang L, Qin Q, Ye XS. Asian J. Org. Chem. 2013;2:30–49. [Google Scholar]
- [7]a).Seeberger PH. Chem. Commun. 2003:1115–1121. doi: 10.1039/b210230g. [DOI] [PubMed] [Google Scholar]; b) Hsu CH, Hung SC, Wu CY, Wong CH. Angew. Chem. Int. Ed. 2011;50:11872–11923. doi: 10.1002/anie.201100125. [DOI] [PubMed] [Google Scholar]
- [8].Zhang W, Curran DP. Tetrahedron. 2006;62:11837–11865. doi: 10.1016/j.tet.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang SL, Pohl NL. Org. Lett. 2015;17:2642–2645. doi: 10.1021/acs.orglett.5b01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]a).Jaipuri FA, Pohl NL. Org. Biomol. Chem. 2008;6:2686–2691. doi: 10.1039/b803451f. [DOI] [PubMed] [Google Scholar]; b) Ko KS, Park G, Yu Y, Pohl NL. Org. Lett. 2008;10:5381–5384. doi: 10.1021/ol802229b. [DOI] [PubMed] [Google Scholar]; c) Ko KS, Jaipuri FA, Pohl NL. J. Am. Chem. Soc. 2005;127:13162–13163. doi: 10.1021/ja054811k. [DOI] [PubMed] [Google Scholar]; d) Zhang F, Zhang W, Zhang Y, Curran DP, Liu G. J. Org. Chem. 2009;74:2594–2597. doi: 10.1021/jo9000993. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Liu L, Pohl NL. Org. Lett. 2011;13:1824–1827. doi: 10.1021/ol2003435. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Tanaka H, Tanimoto Y, Kawai T, Takahashi T. Tetrahedron. 2011;67:10011–10016. [Google Scholar]; g) Zong C, Venot A, Dhamale O, Boons GJ. Org. Lett. 2013;15:342–345. doi: 10.1021/ol303270v. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Cai C, Dickinson DM, Li LY, Masuko S, Suflita M, Schultz V, Nelson SD, Bhaskar U, Liu J, Linhardt RJ. Org. Lett. 2014;16:2240–2243. doi: 10.1021/ol500738g. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Hwang J, Yu H, Malekan H, Sugiarto G, Li YH, Qu JY, Nguyen V, Wu DY, Chen X. Chem. Commun. 2014;50:3159–3162. doi: 10.1039/c4cc00070f. [DOI] [PubMed] [Google Scholar]; j) Macchione G, de Paz JL, Nieto PM. Carbohydr. Res. 2014;394:17–25. doi: 10.1016/j.carres.2014.05.007. [DOI] [PubMed] [Google Scholar]; k) Roychoudhury R, Pohl NLB. Org. Lett. 2014;16:1156–1159. doi: 10.1021/ol500023y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Demchenko AV. Synlett. 2003:1225–1240. [Google Scholar]
- [12]a).Kim JH, Yang H, Park J, Boons GJ. J. Am. Chem. Soc. 2005;127:12090–12097. doi: 10.1021/ja052548h. [DOI] [PubMed] [Google Scholar]; b) Park J, Boltje TJ, Boons GJ. Org. Lett. 2008;10:4367–4370. doi: 10.1021/ol801833n. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Boltje TJ, Kim JH, Park J, Boons GJ. Org. Lett. 2011;13:284–287. doi: 10.1021/ol1027267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferguson MA, Homans SW, Dwek RA, Rademacher TW. Science. 1988;239:753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- [14].Nagamune K, Nozaki T, Maeda Y, Ohishi K, Fukuma T, Hara T, Schwarz RT, Sutterlin C, Brun R, Riezman H, Kinoshita T. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10336–10341. doi: 10.1073/pnas.180230697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]a).Murakata C, Ogawa T. Carbohydr. Res. 1992;235:95–114. doi: 10.1016/0008-6215(92)80081-b. [DOI] [PubMed] [Google Scholar]; b) Khiar N, Martinlomas M. J. Org. Chem. 1995;60:7017–7021. [Google Scholar]; c) Baeschlin DK, Chaperon AR, Green LG, Hahn MG, Ince SJ, Ley SV. Chem. Eur. J. 2000;6:172–186. doi: 10.1002/(sici)1521-3765(20000103)6:1<172::aid-chem172>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]; d) Ziegler T, Dettmann R, Duszenko M. Carbohydr. Res. 2000;327:367–375. doi: 10.1016/s0008-6215(00)00071-9. [DOI] [PubMed] [Google Scholar]; e) Dettmann R, Ziegler T. Carbohydr. Res. 2011;346:2348–2361. doi: 10.1016/j.carres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- [16].van Boom JH, Burgers PMJ. Tetrahedron Lett. 1976;17:4875–4878. [Google Scholar]
- [17]a).Gaunt MJ, Yu JQ, Spencer JB. J. Org. Chem. 1998;63:4172–4173. [Google Scholar]; b) Xia J, Abbas SA, Locke RD, Piskorz CF, Alderfer JL, Matta KL. Tetrahedron Lett. 2000;41:169–173. [Google Scholar]
- [18]a).Fang T, Mo KF, Boons GJ. J. Am. Chem. Soc. 2012;134:7545–7552. doi: 10.1021/ja3018187. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fascione MA, Kilner CA, Leach AG, Turnbull WB. Chem.-Eur. J. 2012;18:321–333. doi: 10.1002/chem.201101889. [DOI] [PubMed] [Google Scholar]
- [19].Fascione MA, Turnbull WB. Beilstein J. Org. Chem. 2010;6:19. doi: 10.3762/bjoc.6.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhu XM, Schmidt RR. Angew. Chem. Int. Ed. 2009;48:1900–1934. doi: 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- [21].Fascione MA, Adshead SJ, Stalford SA, Kilner CA, Leach AG, Turnbull WB. Chem. Commun. 2009:5841–5843. doi: 10.1039/b913308a. [DOI] [PubMed] [Google Scholar]
- [22].Yu B, Tao HC. Tetrahedron Lett. 2001;42:2405–2407. [Google Scholar]
- [23].Veeneman GH, van Leeuwen SH, van Boom JH. Tetrahedron Lett. 1990;31:1331–1334. [Google Scholar]
- [24].Boltje TJ, Zhong W, Park J, Wolfert MA, Chen W, Boons GJ. J. Am. Chem. Soc. 2012;134:14255–14262. doi: 10.1021/ja306274v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Crich D, Vinogradova O. J. Org. Chem. 2007;72:3581–3584. doi: 10.1021/jo062411p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.