Abstract
Unconventional myosins are proteins that bind actin filaments in an ATP-regulated manner. Because of their association with membranes, they have traditionally been viewed as motors that function primarily to transport membranous organelles along actin filaments. Recently, however, a wealth of roles for myosins that are not obviously related to organelle transport have been uncovered, including organization of F-actin, mitotic spindle regulation and gene transcription. Furthermore, it has also become apparent that the motor domains of different myosins vary strikingly in their biophysical attributes. We suggest that the assumption that most unconventional myosins function primarily as organelle transporters might be misguided.
Introduction
Myosins are proteins that bind to actin filaments (F-actin) in an ATP-regulated manner. Binding of F-actin promotes ATP hydrolysis by the myosin, which can power movement of actin filaments or movement of the myosin along actin filaments. The ‘motor’ activity is contained within the N-terminal ‘head’ portion of the myosin heavy chain, whereas the C-terminal ‘tails’ of the heavy chains are quite divergent, binding to a diverse array of partners [1].
Myosins form >30 distinct classes, based on sequence comparisons of the heavy chains [2–4]. Traditionally, the myosins-2, the first myosin class discovered, are considered ‘conventional’ and all other classes are considered ‘unconventional’. The conventional myosins form large bipolar filaments via tail-directed homo-oligomerization (Box 1). The unconventional myosins do not form filaments, although some can dimerize. Rather, their tails typically direct membrane binding and binding to other proteins.
Box 1. Is myosin-2 really all that conventional?
Our view of myosins has been shaped by the history of their discovery – myosin-2 was the first class of myosins to be identified, >50 years ago, owing to their role in muscle contraction (for a review, see Ref. [86]). When new classes of myosins began to be uncovered from the 1970s onwards (e.g. see Ref. [87]), they were described as unconventional myosins, whereas myosins-2 were called conventional, thus implying that myosin-2 is the paradigm myosin and all other myosins are adaptations of this. However, as described here, myosin-2 is a highly specialized myosin and recent evolutionary studies indicate that it is not the most ancient of the myosin superfamily.
Myosins-2 are the only myosins capable of forming bipolar filaments, a feature that is key to their roles both in muscle and non-muscle cells. They are able to form filaments owing to their extended coiled-coil domains, which make up most of their tail [86] (Figure I). Muscle myosin-2 forms very large filaments containing many myosin molecules. The bipolar arrangement of myosin-2 filaments means that, as the myosin heads at each end of a filament interact with F-actin, they will slide the actin filaments in opposite directions (Figure I). This action underlies the ‘sliding filament hypothesis’ of muscle contraction – whereby bipolar filaments of myosin ratchet anti-parallel actin filaments together, causing contraction of the muscle [5]. In non-muscle cells, it is not known if myosin-2 works in exactly the same way, although it can generate a contractile force, as is seen in its role in the cytokinetic furrow [88].
In keeping with the position of myosin-2 as the conventional myosin, it had been thought that this class, along with myosins-1, represented the most ancient of myosins [89]. However, two recent studies of myosin evolution, which are based on a much wider range of genomes than previous investigations, indicate that this is not the case [2,4]. Instead, it seems that the last common eukaryotic ancestor is likely to have possessed three myosins: a class 1 myosin, a myosin containing a dilute domain (found in myosins-5 and -11) and a MyTH4–FERM myosin, but no myosin-2 [4]. The myosin-2 class is predicted to have evolved at a slightly later stage from the ancestral dilute domain myosins [4]. Therefore, in a very real sense, the ‘conventional’ myosins are probably much more specialized than is widely thought.
Figure I.
Myosin-2 and the sliding filament hypothesis. Myosins-2 can form bipolar filaments through their extended coiled-coil domains. Here, just two dimers are shown in a filament, but muscle myosin-2 can form large filaments containing many myosin molecules. When the myosin-2 heads ratchet along the actin filaments towards the barbed ends (+), the actin filaments will slide in opposite directions.
Our view of myosins has been colored by the fact that the first myosin understood in detail was skeletal muscle myosin-2, which powers F-actin sliding in sarcomeres during muscle contraction [5]. Thus, when unconventional myosins were found to comprise the motor, fused to a variety of membrane-binding tails (e.g. see Ref. [6]), it was naturally proposed that unconventional myosins function to move membranous organelles along actin filaments. This view spawned the ‘highways and local roads’ model in which microtubules serve as long range tracks for organelle transport powered by kinesins or dyneins, whereas F-actin serves as short range transport tracks powered by unconventional myosins [7]. This model made perfect sense at the time, based not only on the background provided by skeletal muscle myosins-2, but also on the finding that organelles could move along actin filaments in extruded squid axoplasm [8]. Once it became clear that some unconventional myosins were necessary for organelle trafficking, this idea gained considerable traction and has since then been a fixture in both textbooks and the primary cell biology literature [5]. We argue that the evidence for unconventional myosins serving as point-to-point organelle transporters in animal cells is not particularly strong and that the organelle transporter idea obscures other important functions for these fascinating proteins.
Unconventional myosins as organelle transporters?
There are some cases in which unconventional myosins could be viewed as functioning as organelle transporters, although direct evidence is very often lacking. For a given process to be accepted as an example of unconventional myosin-powered organelle transport on actin filaments, it must be shown that the organelle is indeed transported on actin filaments, that the unconventional myosin associates with the organelle during transport along actin filaments, and that inhibiting or depleting the myosin stops transport of the organelle [1]. To date, the following examples satisfy these criteria: (i) budding yeast myosin-5 transports organelles along F-actin cables [9]; (ii) myosin-11 transports organelles along F-actin cables in alga and plants [10]; and (iii) myosin-5 in amphibian melanophores can transport pigment granules on actin filaments [11,12]. There are also two other potential cases: myosin-6 is needed for transport of uncoated endocytotic vesicles toward the cell interior in cultured mammalian cells [13] and myosin-5 is needed for transport of endosomes toward the cell interior in cultured astrocytes [14]. In neither of these examples have microtubules been excluded as a potential track because although a short treatment with colchicine did not prevent transport in astrocytes, the effect of this treatment on microtubules was not assessed and, based on other work, it is unlikely to have depolymerized the heavily acetylated microtubules found in these cells [15]. Thus, the evidence for point-to-point transport along actin filaments in these examples must be considered inconclusive.
This list of examples is surprisingly short and could be even shorter than it seems: in the amphibian melanophore system, the major role of myosin-5 could be dynamic alteration of microtubule-based movement, which occurs by engaging in a tug-of-war with the microtubule-based motors rather than by any extensive displacement of the pigment along F-actin tracks [16]. Because this tug-of-war results in changes in microtubule-based movement, these results imply that what seems to be transport on F-actin by an unconventional myosin (such as the astrocyte example earlier) might actually reflect transport on microtubules that is modulated by F-actin and an unconventional myosin.
In addition to relatively few examples of unconventional myosins having a ‘conventional’ role of organelle transporters, the highways and local roads model is not consistent with the fact that, in the typical animal cell, nearly all of the stable, uniformly polarized F-actin is found in cell surface projections such as filopodia and microvilli, which do not contain membranous organelles [5]. By contrast, the cell types that represent the clear-cut cases of unconventional myosins acting as organelle transporters – budding yeast, alga and plants – do contain large, internal arrays of uniformly polarized F-actin [5]. Even here, however, the local roads idea does not really work because the arrays in question actually run the length of the cell (in yeast) or run around the perimeter of the cell (plants and alga) and apparently have long-range transport roles.
A further line of evidence argues that most unconventional myosins might not function as organelle transporters. That is, most of the unconventional myosins so-far characterized are relatively slow motors when assayed for their ability to transport F-actin in vitro, having transport rates typically between ~0.015–0.4 μm/sec [17]. This is striking when compared with plant myosin-11 and yeast myosin-5, the two motors responsible for the examples of organelle transport described earlier, which have rates an order of magnitude faster, at ~4.5 μm/s [17]. These latter rates are similar to those of organelle transport on microtubules in animal cells, implying that unless animals have evolved a special need for slow organelle transport along actin filaments, the unconventional myosins are unlikely to be serving this role.
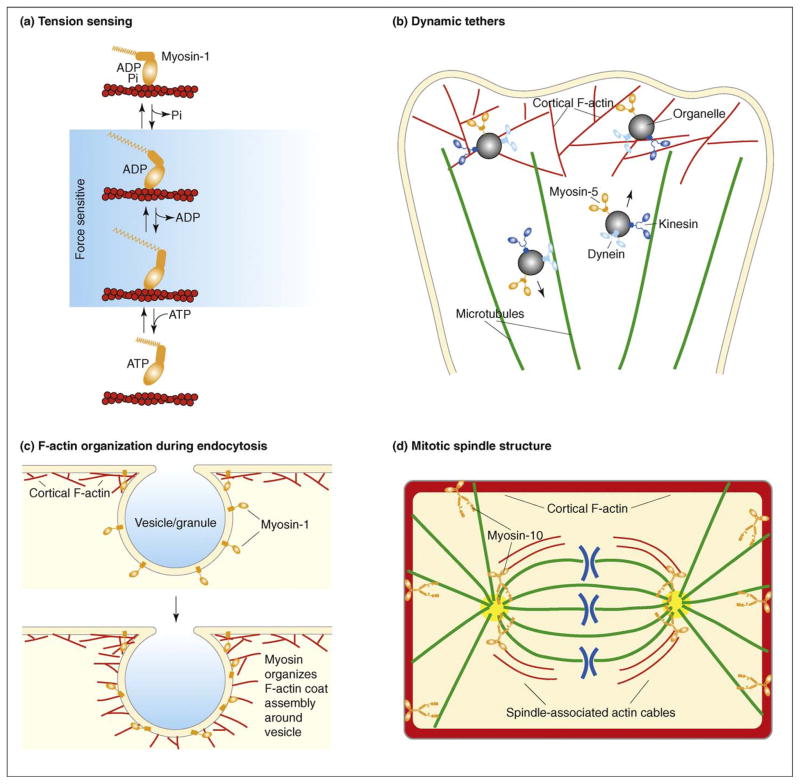
In addition to being slow, some unconventional myosins have a motor feature that is not easy to reconcile with a simple organelle motor function, namely, the ability to respond to increased load with a reduction in the kinetics of the ATP cycle. This includes myosins-5 [18,19] and myosin-6 [20] and is particularly apparent in myosins-1 [21]. A recent single-molecule study of myosin-1 elegantly demonstrated that the application of a small force greatly increased the time the myosin remained bound to actin [21] (Figure 1a). It is difficult to imagine how an organelle transporter would benefit from a feature that greatly reduces the efficiency of transport exactly when it is needed (i.e. when it is carrying cargo). However, the extraordinary sensitivity of myosins-1 to force fits well with a role in which they act as tension-sensitive tethers. Curiously, the amount of force needed to stall a myosin-1 is approximately equivalent to that exerted by a polymerizing actin filament [22], which could explain how myosins-1 couple assembling actin to force production on membranes (see later).
Figure 1.
Examples of unconventional roles for unconventional myosins. (a) Tension sensing: members of the myosin-1 family can act as tension sensors owing to their structural and kinetic properties. Myosins-1 have a two-step working stroke, the second step of which is sensitive to applied force [21]. When tension is applied to the myosin, it is less likely to release ADP and remains bound to the actin filament for longer periods of time. (b) Dynamic tethers: rather than acting to transport organelles along F-actin tracks, there is growing evidence that unconventional myosins (such as myosins-5a and -5b) instead function to tether organelles to cortical F-actin after their delivery to the cell periphery along microtubules by kinesin [23,25]. These myosin tethers are proposed to be very dynamic and their effect reversible, such that organelles or vesicles can be released and sent back down microtubules to the cell interior via dynein-mediated transport [27]. (c) F-actin organization during endocytosis: myosins-1c and -1e have been shown to organize the assembly of F-actin during endo- and exocytosis [27,35,36]; here, compensatory endocytosis is shown. Myosin-1 localizes to the vesicle or granule before the formation of the F-actin coat and is required to organize the F-actin to facilitate its endocytosis. (d) Mitotic spindle structure: myosins-10 and -15 have been found to be involved in maintaining mitotic spindle structure [55–57]; here, for simplicity, just myosin-10 is shown. Myosin-10 localizes to the actin-rich cell cortex and to the mitotic spindle and functions in spindle length regulation, spindle pole structure and spindle anchoring [57]. These functions could be partly mediated through possible connections between cortical myosin-10 and astral microtubules or through separate functions by myosin-10 in the spindle interior.
Unconventional functions for unconventional myosins
Based on the aforementioned evidence, we suggest that the highways and local roads model is not in fact well supported by the available data and we further suggest that although unconventional myosins serve as organelle transporters in yeast, alga and plants, this is unlikely to be their main function in animal cells. What then do the unconventional myosins do in animal cells? Here, we consider several functions for these proteins, including organization of dynamic actin, modulation of microtubule–actin interactions and regulation of transcription, all of which have recently received strong support.
Dynamic tethers for organelles or membrane-associated proteins
Several unconventional myosins have now been shown to act as tethers for organelles or proteins, a fact that explains why these myosins are thought to function as point-to-point transporters. Indeed, the first evidence for tethering came from work on myosin-5a, which was initially promoted as a transporter of pigment and secretory granules in mice, but was subsequently found to act instead as a tether for these organelles [23–25]. In both cases, after being transported to the plasma membrane on microtubules, the organelles bind to cortical F-actin via myosin-5a (Figure 1b). Similarly, myosin-5b is needed for proper localization of endosomes in many systems, leading to the assertion that it serves as an organelle transporter. The hypothesis that myosin-5b transports endosomes from the perinuclear region to the plasma membrane predicts that induction of tight binding of this motor to F-actin will result in accumulation of endosomes near the nucleus. This prediction was tested using a chemical genetic approach [26] and shown to be incorrect. Furthermore, studies based on chemical-genetic, dominant-negative and overexpression all supported a dynamic tether model [27]. These studies are particularly important in that they not only explain how myosin-5b contributes to an organelle trafficking process, they also make it clear why previous results were misinterpreted to reflect an organelle transport model. Although it remains to be seen whether or not the dynamic tether model functions in other contexts, it has recently been reported that myosin-5b is needed for endosome trafficking in neuronal spines [28]. Because it was thought that microtubules do not penetrate spines, this was interpreted to reflect myosin-5a-powered transport along spine F-actin. However, shortly after that work was published, two new studies revealed that not only do microtubules penetrate spines, they are triggered to do so under the same conditions that induce endosome transport [29,30].
The load-dependent activity described for myosins-1 gives them the potential to reversibly anchor membrane proteins in a tension-dependent manner. That myosins-1 have such a role has been strongly supported for myosin-1c, which is thought to control the anchoring of a calcium channel in hair cell stereocilia [31]. At least one other myosin-1 also has a role in tethering a membrane protein: myosin-1a, a component of enterocyte microvilli, is essential for retention of sucrase isolmaltase in the microvillus [32].
F-actin organization and dynamics
Myosins-1 in budding yeast, Acanthamoeba and Dictystelium can regulate actin assembly by interacting with proteins that stimulate the Arp2/3 complex. In budding yeast, this interaction is important for endocytosis because the power for endocytosis is provided by rapidly assembling actin, which is organized by the myosins-1 such that the force drives PM invagination [33]. Although the motor activity of the myosins is also important [33], this is apparently not because it transports the vesicle along the actin, but rather because it transports the F-actin associated with the nascent endosome away from the plasma membrane.
Myosins-1 also control F-actin during both exo- and endocytosis of cortical granule membranes in Xenopus eggs [34–36] (Figure 1c). Disruption of myosin-1c function results in abnormal actin assembly during exocytosis, which limits fusion-pore expansion. Disruption of its function during endocytosis impairs the F-actin coat that normally forms on and compresses the exocytosing granule surface; instead of enclosing the granule, the F-actin extends outward from the apical portion of the granule surface into the cytoplasm [35]. By contrast, when myosin-1e function is disrupted, coat F-actin concentrates on the basal surface of the exocytosing cortical granule [36]. The findings for Xenopus myosin-1c could be relevant to other systems; although it was initially proposed that mammalian myosin-1c transports vesicles that carry the GLUT4 glucose transporter during GLUT4 exocytosis [36], it was subsequently found to function more proximally to the membrane fusion step and to do so in a manner consistent with it controlling rapidly assembling actin [37].
Myosin-6 has also been firmly linked to control of actin dynamics and organization. During Drosophila spermatid individualization, myosin-6 localizes to the leading edge of a moving, F-actin-rich structure called the cone. Myosin-6 mutants have reduced levels of F-actin in cones and, furthermore, this F-actin is disorganized [38]. Somewhat surprisingly, dimerization of the myosin-6 heavy chain is not required for myosin-6 function in cones [39], which implies that transport activity is not likely to be important because dimerization is required for normal motor function in vitro. This finding is also consistent with the observation that the rate of cone movement does not differ between wild-type and mutant embryos. Based on these and other results, the authors propose that myosin-6 does not have any kind of transport role in this process but instead functions to stabilize F-actin branches within the cones, by either recruiting the Arp2/3 complex or protecting filaments from depolymerizing factors [38,39].
Myosin-6 has also been implicated in control of adherens-junction assembly in mammalian cells and, again, the conclusion is that this reflects not a transport role, but a role in F-actin organization [40]. In particular, myosin-6 knockdown reduces F-actin packing at junctions [40]. It was also found that myosin-6 forms a complex with E-cadherin, a key adherens-junction protein, and demonstrated that myosin-6 works, at least in part, via binding to vinculin, a protein that organizes F-actin at junctions [40].
Myosin-10 not only prefers bundled F-actin as a substrate [41], it is also capable of promoting F-actin bundling in vivo. The first hint of this came from the demonstration that myosin-10 overexpression increases the number of filopodia in mammalian cells [42]. It was subsequently shown that reduction of myosin-10 expression suppressed filopodia formation [43]. Although in principle filopodium induction might reflect some other activity of myosin-10, such as transport of actin-regulatory proteins bound to its tail, a role for the bundling activity was revealed in a clever experiment in which isolated myosin-10 heads were experimentally induced to dimerize [44]. After dimerization, the heads directed formation of actin bundles at the plasma membrane, giving rise to short plasma-membrane protrusions.
Myosins and microtubules and microtubule-based structures
A growing body of work indicates that unconventional myosins both associate with and have important roles in microtubule-based structures (Table 1). Although there are no known examples of myosins that can use microtubules as a motor substrate, several unconventional myosins nevertheless associate with microtubules in other ways, as demonstrated by biochemical and yeast-two hybrid approaches. Both myosin-5a [45] and myosin-10 [46] can bind directly to microtubules via their tails and myosin-5a heads can undergo 1D diffusion along microtubules in vitro [47]. The association with microtubules might also be indirect: myosin-5 has been reported to bind to kinesin [48]; myosin-6 binds to CLIP-190 [49], a protein that binds to EB1 and thereby associates with the plus ends of growing microtubules; myosin-7 binds to MAP2B [50]; and myosin-15 binds to α-tubulin, EB1 and katanin, a microtubule-severing protein [51].
Table 1.
Unconventional myosins–microtubule connections
| Myosin | Organism | Interaction | Function | Citation |
|---|---|---|---|---|
| Myosin-5a | Mammalian | Centrosome localization | Unknown | [52,53] |
| Microtubule localization | Unknown | [79] | ||
| Chicken | Microtubule binding | Unknown | [45] | |
|
| ||||
| Myosin-6 | Mammalian | Centrosome and midbody localization | Mitotic progression and cytokinesis | [54] |
| Drosophila | CLIP-190 binding | Unknown | [49] | |
|
| ||||
| Myosin-7a | Mammalian | MAP4A binding | Unknown | [50] |
| Cilia localization | Unknown | [80] | ||
|
| ||||
| Myosin-10 | Mammalian | Cortical microtubules | Spindle positioning | [56] |
| Mammalian cancer cells | Unknown | Centrosome clustering at spindle poles | [55] | |
| Xenopus | Microtubule binding | Spindle assembly and organization | [46] | |
| Meiotic spindle localization | ||||
| Mitotic spindle pole | Spindle length, spindle pole integrity, mitotic progression | [57] | ||
| Spindle anchoring | ||||
|
| ||||
| Myosin-15 | Drosophila | Tubulin-, EB1-, Katanin-binding | Centrosomes clustering at spindle poles | [51,55] |
The potential importance of such interactions is suggested by the following: myosin-5a localizes to centrosomes in a variety of cell types [52,53], and myosin-6 localizes to spindle poles in mitotic cells [54]. The role of myosin-5a in the centrosome is unknown, although a small but significant fraction of dilute-mouse-derived fibroblasts fail cell division [53]. Similarly, in myosin-6 knockdown cell lines, a delay in metaphase progression and a higher than normal rate of cytokinesis failure are observed [54]. Additionally, myosin-6 is required for proper spindle orientation in Drosophila neuroblasts [54]. Collectively, these results suggest that myosin-5 and -6 contribute to spindle functions and/or cell division in several different systems.
Recent evidence indicates that two members of the MyTH4–FERM subfamily of unconventional myosins (myosin-10 and myosin-15) are particularly important for proper spindle formation and function [55–57]. Myosin-10 localizes to the meiotic spindle in Xenopus eggs and inhibition of myosin-10 function disrupts meiotic spindle assembly and orientation [46]. Myosin-10 also localizes to mitotic spindle poles in Xenopus embryos and knockdown of this myosin results in spindle elongation, improper spindle anchoring, spindle pole fragmentation and mitotic delay [57]. Myosin-15 localizes to the chromosomes in Drosophila mitotic spindles [51]. Moreover, disruption of myosin-15 function in Drosophila S2 cells inhibits clustering of centrosomes during spindle assembly, producing spindles with fragmented poles, a phenotype also seen after knockdown of myosin-10 in mammalian cancer cells [55].
With myosins localizing to the spindle and having roles in spindle function, the obvious question is: where is the actin? Previous work has emphasized the role of cortical F-actin in spindle formation and orientation [58] and the potential localization and role of F-actin in the spindle has been the subject of heated debate over many years. However, in the past year, direct imaging of living mammalian oocytes [59–61] and Xenopus embryos [57] has shown that dynamic F-actin does indeed associate with spindles. Thus, it is possible that at least some of the roles for myosins in the spindle might rely on interactions with spindle actin (Figure 1d), although this will require further investigation to confirm.
Nuclear myosins
Several years ago, De Lanerolle and colleagues demonstrated that myosin-1c is targeted to the nucleus [62] and then showed that it has a role in transcription [63]. This pioneering work opened a new area of research into nuclear myosin and, to date, at least five different myosins (myosin-1c, myosin-5a, myosin-6, myosin-10 and myosin-16; Table 2 and references therein) have been reported to localize to the nucleus. These results seem surprising because many previous studies of the same myosins failed to detect them in the nucleus. Although in most cases the basis for this discrepancy is unclear, a particular splice form of myosin-1c is targeted to the nucleus [62], whereas myosin-5a is apparently targeted after phosphorylation [64].
Table 2.
Unconventional myosins in the nucleus
| Myosin | Organism | Function in nucleus | Citation |
|---|---|---|---|
| Myosin-1c | Mammalian | Pol I transcription | [63,81] |
| Chromatin reorganization | [65,66,82] | ||
| Myosin-5a | Mammalian | Unknown | [64] |
| Myosin-6 | Mammalian | Pol II transcription | [67] |
| P53 activation | [83] | ||
| Myosin-10 | Xenopus | Unknown | [57] |
| Myosin-15 | Drosophila | Unknown | [51] |
| Myosin-16b | Mammalian | Unknown, possibly S-phase progression | [84] |
| Myosin-18b | Mammalian | Unknown | [85] |
What do the myosins do within the nucleus? Myosin-1c associates with and is necessary for efficient transcription by RNA polymerase (Pol) I [63]. Myosin-1c is also involved in chromatin remodeling [65,66]. Both of these functions depend on actin as well, and the polymerases bind actin, but precisely how myosin-1 and actin contribute to these processes is quite mysterious. Myosin-6 is also apparently involved in transcription, but by RNA Pol II [67]. Again, the details are poorly understood but with further application of in vitro and in vivo approaches it should be possible to distinguish between models in which the myosins serve to move the polymerases and models in which they serve to anchor the polymerases on the chromatin.
Retrograde flow and membrane extrusion
Retrograde flow is the process by which F-actin is pulled inward (that is, away from the cell periphery) and is powered, at least in part, by myosin. Retrograde flow is observed in lamellipodia [68] and also in plasma membrane extensions such as filopodia [69], microvilli [70] and stereocilia [71]. In all cases, it is thought to regulate membrane protrusion and it could also help actin cytoskeleton recycling. Myosin-1c has been directly shown to participate in retrograde flow in lamellipodia of mammalian cells [72] and, given the fact that filopodia, microvilli and sterocilia exclude myosin-2 but are rich in unconventional myosins, it is likely that the latter are also responsible for providing at least some of the force that powers retrograde flow of F-actin within these structures.
A potential consequence of a PM-associated unconventional myosin moving along a bundle of actin filaments in a PM projection is transport of membrane in the same direction (that is, to the tip of the projection). That such transport occurs was recently demonstrated in isolated brush borders [73]. Membrane is moved in the plus-end direction toward the tips of microvilli by myosin-1a. This results in the shedding of membranous vesicles from the microvillus tip, a process that also occurs in association with a wide variety of microvilli in vivo [73].
Cortical tension control
One of the early findings from studies of amoeboid cells subjected to systematic knockout of myosins-1 was a dramatic increase in the extension of ‘inappropriate’ pseudopodia [74]. Because this could potentially result from a loss of cortical cytoskeletal strength, it was proposed that one of the roles of myosins-1 in these systems was strengthening of the cortical actin cytoskeleton [75]. This point has been tested for several Dictyostelium myosins and, depending on the isoforms, knockdown of either a single or two or more myosins results in a significant reduction of cortical tension [76,77]. Whether myosins-1 are also involved in lending strength to cortices in mammalian cells is unknown, but it is consistent with the recent demonstration that podocytes in mouse myosin-1e knockouts are more prone to damage [78].
Conclusions
In summary, the number of cases in which unconventional myosins function is growing, as is the number in which the role does not seem to depend on transport at all. This includes examples in which the myosins control the force exerted by assembling actin and rather more surprising roles modulating interactions between the F-actin and microtubule cytoskeleton and controlling transcription. These findings are in keeping with the remarkable variation seen in the myosin motor domain across the different myosin classes and even within classes. A major goal of future studies should be understanding exactly how unconventional myosins work in these various processes and how variations in motor attributes equip the unconventional myosins for their jobs.
Acknowledgments
W.M.B. would like to thank the National Institutes of Health for funding. S.W. is supported by a Beit Memorial Fellowship.
References
- 1.Sokac AM, Bement WM. Regulation and expression of metazoan unconventional myosins. Int Rev Cytol. 2000;200:197–304. doi: 10.1016/s0074-7696(00)00005-x. [DOI] [PubMed] [Google Scholar]
- 2.Foth BJ, et al. New insights into myosin evolution and classification. Proc Natl Acad Sci U S A. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odronitz F, Kollmar M. Drawing the tree of eukaryotic life based on the analysis of 2,269 manually annotated myosins from 328 species. Genome Biol. 2007;8:R196. doi: 10.1186/gb-2007-8-9-r196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. doi: 10.1038/nature03949. [DOI] [PubMed] [Google Scholar]
- 5.Lodish H, et al. Molecular Cell Biology. 6. W.H. Freeman & Co; 2008. [Google Scholar]
- 6.Adams RJ, Pollard TD. Binding of myosin I to membrane lipids. Nature. 1989;340:565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- 7.Langford GM. Actin- and microtubule-dependent organelle motors: interrelationships between the two motility systems. Curr Opin Cell Biol. 1995;7:82–88. doi: 10.1016/0955-0674(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 8.Kuznetsov SA, et al. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- 9.Hoepfner D, et al. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol. 2001;155:979–990. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holweg C, Nick P. Arabidopsis myosin XI mutant is defective in organelle movement and polar auxin transport. Proc Natl Acad Sci U S A. 2004;101:10488–10493. doi: 10.1073/pnas.0403155101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Rodionov VI, et al. Functional coordination of microtubule-based and actin-based motility in melanophores. Curr Biol. 1998;8:165–168. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- 12.Rogers SL, Gelfand VI. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr Biol. 1998;8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- 13.Aschenbrenner L, et al. Uncoated endocytic vesicles require the unconventional myosin, Myo6, for rapid transport through actin barriers. Mol Biol Cell. 2004;15:2253–2263. doi: 10.1091/mbc.E04-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stachelek SJ, et al. Real-time visualization of processive myosin 5a-mediated vesicle movement in living astrocytes. J Biol Chem. 2001;276:35652–35659. doi: 10.1074/jbc.M103331200. [DOI] [PubMed] [Google Scholar]
- 15.Cambray-Deakin MA, et al. Colocalisation of acetylated microtubules, glial filaments, and mitochondria in astrocytes in vitro. Cell Motil Cytoskeleton. 1988;10:438–449. doi: 10.1002/cm.970100311. [DOI] [PubMed] [Google Scholar]
- 16.Gross SP, et al. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connell CB, et al. Myosin at work: motor adaptations for a variety of cellular functions. Biochim Biophys Acta. 2007;1773:615–630. doi: 10.1016/j.bbamcr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Purcell TJ, et al. A force-dependent state controls the coordination of processive myosin V. Proc Natl Acad Sci U S A. 2005;102:13873–13878. doi: 10.1073/pnas.0506441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veigel C, et al. Load-dependent kinetics of myosin-V can explain its high processivity. Nat Cell Biol. 2005;7:861–869. doi: 10.1038/ncb1287. [DOI] [PubMed] [Google Scholar]
- 20.Altman D, et al. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–749. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- 21.Laakso JM, et al. Myosin I can act as a molecular force sensor. Science. 2008;321:133–136. doi: 10.1126/science.1159419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berro J, et al. Attachment conditions control actin filament buckling and the production of forces. Biophys J. 2007;92:2546–2558. doi: 10.1529/biophysj.106.094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desnos C, et al. Myosin Va mediates docking of secretory granules at the plasma membrane. J Neurosci. 2007;27:10636–10645. doi: 10.1523/JNEUROSCI.1228-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provance DW, Jr, et al. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc Natl Acad Sci U S A. 1996;93:14554–14558. doi: 10.1073/pnas.93.25.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, et al. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provance DW, Jr, et al. Chemical-genetic inhibition of a sensitized mutant myosin Vb demonstrates a role in peripheral-pericentriolar membrane traffic. Proc Natl Acad Sci U S A. 2004;101:1868–1873. doi: 10.1073/pnas.0305895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provance DW, Jr, et al. Myosin-Vb functions as a dynamic tether for peripheral endocytic compartments during transferrin trafficking. BMC Cell Biol. 2008;9:44. doi: 10.1186/1471-2121-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu J, et al. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–12124. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, et al. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt JR, et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 32.Tyska MJ, Mooseker MS. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol. 2004;165:395–405. doi: 10.1083/jcb.200310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaksonen M, et al. Harnessing actin dynamics for clathrin-mediated endocytosis. Natl Rev. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 34.Schietroma C, et al. A role for myosin 1e in cortical granule exocytosis in Xenopus oocytes. J Biol Chem. 2007;282:29504–29513. doi: 10.1074/jbc.M705825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokac AM, et al. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev Cell. 2006;11:629–640. doi: 10.1016/j.devcel.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu HY, Bement WM. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol Biol Cell. 2007;18:4096–4105. doi: 10.1091/mbc.E06-11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bose A, et al. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol Cell Biol. 2004;24:5447–5458. doi: 10.1128/MCB.24.12.5447-5458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguchi T, et al. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell. 2006;17:2559–2571. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noguchi T, et al. Coiled-coil-mediated dimerization is not required for myosin VI to stabilize actin during spermatid individualization in Drosophila melanogaster. Mol Biol Cell. 2009;20:358–367. doi: 10.1091/mbc.E08-07-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddugoda MP, et al. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–540. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy S, et al. A myosin motor that selects bundled actin for motility. Proc Natl Acad Sci U S A. 2008;105:9616–9620. doi: 10.1073/pnas.0802592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 43.Bohil AB, et al. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tokuo H, et al. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J Cell Biol. 2007;179:229–238. doi: 10.1083/jcb.200703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao TT, et al. Myosin-Va binds to and mechanochemically couples microtubules to actin filaments. Mol Biol Cell. 2004;15:151–161. doi: 10.1091/mbc.E03-07-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber KL, et al. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–329. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- 47.Ali MY, et al. Myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc Natl Acad Sci U S A. 2007;104:4332–4336. doi: 10.1073/pnas.0611471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang JD, et al. Direct interaction of microtubule- and actin-based transport motors. Nature. 1999;397:267–270. doi: 10.1038/16722. [DOI] [PubMed] [Google Scholar]
- 49.Lantz VA, Miller KG. A class VI unconventional myosin is associated with a homologue of a microtubule-binding protein, cytoplasmic linker protein-170, in neurons and at the posterior pole of Drosophila embryos. J Cell Biol. 1998;140:897–910. doi: 10.1083/jcb.140.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todorov PT, et al. Myosin VIIA is specifically associated with calmodulin and microtubule-associated protein-2B (MAP-2B) Biochem J. 2001;354:267–274. doi: 10.1042/0264-6021:3540267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu R, et al. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development. 2008;135:53–63. doi: 10.1242/dev.011437. [DOI] [PubMed] [Google Scholar]
- 52.Espreafico EM, et al. Localization of myosin-V in the centrosome. Proc Natl Acad Sci U S A. 1998;95:8636–8641. doi: 10.1073/pnas.95.15.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu X, et al. Myosin Va associates with microtubule-rich domains in both interphase and dividing cells. Cell Motil Cytoskeleton. 1998;40:286–303. doi: 10.1002/(SICI)1097-0169(1998)40:3<286::AID-CM7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 54.Arden SD, et al. Myosin VI is required for targeted membrane transport during cytokinesis. Mol Biol Cell. 2007;18:4750–4761. doi: 10.1091/mbc.E07-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 2007;26:1487–1498. doi: 10.1038/sj.emboj.7601599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woolner S, et al. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol. 2008;182:77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenblatt J, et al. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- 59.Azoury J, et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 60.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–1992. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Li H, et al. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat Cell Biol. 2008;10:1301–1308. doi: 10.1038/ncb1788. [DOI] [PubMed] [Google Scholar]
- 62.Pestic-Dragovich L, et al. A myosin I isoform in the nucleus. Science. 2000;290:337–341. doi: 10.1126/science.290.5490.337. [DOI] [PubMed] [Google Scholar]
- 63.Philimonenko VV, et al. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat Cell Biol. 2004;6:1165–1172. doi: 10.1038/ncb1190. [DOI] [PubMed] [Google Scholar]
- 64.Pranchevicius MC, et al. Myosin Va phosphorylated on Ser1650 is found in nuclear speckles and redistributes to nucleoli upon inhibition of transcription. Cell Motil Cytoskeleton. 2008;65:441–456. doi: 10.1002/cm.20269. [DOI] [PubMed] [Google Scholar]
- 65.Chuang CH, et al. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–831. doi: 10.1016/j.cub.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 66.Hu Q, et al. Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatingranules. ProcNatlAcadSciUSA. 2008;105:19199–19204. doi: 10.1073/pnas.0810634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vreugde S, et al. Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol Cell. 2006;23:749–755. doi: 10.1016/j.molcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Abercrombie M, et al. The locomotion of fibroblasts in culture. 3 Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res. 1970;62:389–398. doi: 10.1016/0014-4827(70)90570-7. [DOI] [PubMed] [Google Scholar]
- 69.Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82:1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rzadzinska AK, et al. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diefenbach TJ, et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158:1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol. 2007;177:671–681. doi: 10.1083/jcb.200701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Titus MA, et al. Theunconventional myosin encoded by the myoA gene plays a role in Dictyostelium motility. Mol Biol Cell. 1993;4:233–246. doi: 10.1091/mbc.4.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peterson MD, Titus MA. F-actin distribution of Dictyostelium myosin I double mutants. J Eukaryot Microbiol. 1994;41:652–657. doi: 10.1111/j.1550-7408.1994.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 76.Dai J, et al. Myosin I contributes to the generation of resting cortical tension. Biophys J. 1999;77:1168–1176. doi: 10.1016/s0006-3495(99)76968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwarz EC, et al. Dictyostelium myosin IK is involved in the maintenance of cortical tension and affects motility and phagocytosis. J Cell Sci. 2000;113:621–633. doi: 10.1242/jcs.113.4.621. [DOI] [PubMed] [Google Scholar]
- 78.Krendel M, et al. Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol. 2009;20:86–94. doi: 10.1681/ASN.2007111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waterman-Storer C, et al. Microtubules remodel actomyosin networks in Xenopus egg extracts via two mechanisms of F-actin transport. J Cell Biol. 2000;150:361–376. doi: 10.1083/jcb.150.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfrum U, et al. Myosin VIIa as a common component of cilia and microvilli. Cell Motil Cytoskeleton. 1998;40:261–271. doi: 10.1002/(SICI)1097-0169(1998)40:3<261::AID-CM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 81.Ye J, et al. Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes Dev. 2008;22:322–330. doi: 10.1101/gad.455908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavellan E, et al. The WSTF-SNF2h chromatin remodeling complex interacts with several nuclear proteins in transcription. J Biol Chem. 2006;281:16264–16271. doi: 10.1074/jbc.M600233200. [DOI] [PubMed] [Google Scholar]
- 83.Jung EJ, et al. Myosin VI is a mediator of the p53-dependent cell survival pathway. Mol Cell Biol. 2006;26:2175–2186. doi: 10.1128/MCB.26.6.2175-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cameron RS, et al. Myosin16b: The COOH-tail region directs localization to the nucleus and overexpression delays S-phase progression. Cell Motil Cytoskeleton. 2007;64:19–48. doi: 10.1002/cm.20162. [DOI] [PubMed] [Google Scholar]
- 85.Salamon M, et al. Human MYO18B, a novel unconventional myosin heavy chain expressed in striated muscles moves into the myonuclei upon differentiation. J Mol Biol. 2003;326:137–149. doi: 10.1016/s0022-2836(02)01335-9. [DOI] [PubMed] [Google Scholar]
- 86.Sellers JR. Fifty years of contractility research post sliding filament hypothesis. J Muscle Res Cell Motil. 2004;25:475–482. doi: 10.1007/s10974-004-4239-6. [DOI] [PubMed] [Google Scholar]
- 87.Pollard TD, Korn ED. Acanthamoeba myosin. I Isolation from Acanthamoeba castellanii of an enzyme similar to muscle myosin. J Biol Chem. 1973;248:4682–4690. [PubMed] [Google Scholar]
- 88.Maciver SK. Myosin II function in non-muscle cells. Bioessays. 1996;18:179–182. doi: 10.1002/bies.950180304. [DOI] [PubMed] [Google Scholar]
- 89.Thompson RF, Langford GM. Myosin superfamily evolutionary history. Anat Rec. 2002;268:276–289. doi: 10.1002/ar.10160. [DOI] [PubMed] [Google Scholar]